Abstract
Covering: September 1972 to December 2020
Explorations of complex symbioses have often elucidated a plethora of previously undescribed chemical compounds that may serve ecological functions in signalling, communication or defence. A case in point is the subfamily of termites that cultivate a fungus as their primary food source and maintain complex bacterial communities, from which a series of novel compound discoveries have been made. Here, we summarise the origins and types of 375 compounds that have been discovered from the symbiosis over the past four decades and discuss the potential for synergistic actions between compounds within the complex chemical mixtures in which they exist. We go on to highlight how vastly underexplored the diversity and geographic distribution of the symbiosis is, which leaves ample potential for natural product discovery of compounds of both ecological and medical importance.
Since the early 1970s, 375 natural products have been identified from members of the fungus-farming termite symbiosis, and this review summarises and discusses the ecological implications of the presence of this vast chemical repertoire.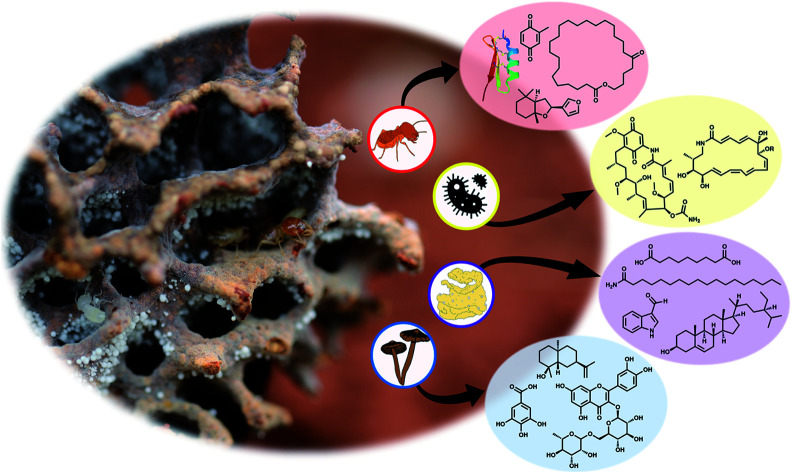
1. Introduction
Natural products represent structurally and functionally diverse molecules that exhibit a plethora of functional roles in signalling, communication, or defence in the natural context within which they are produced.1,2 Hosts may produce their own defensive compounds, while symbiont-derived natural products might serve as vital mediators between hosts and their antagonists by conferring protection directly through biological activity3 or indirectly by stimulating host immune systems to improve protection during infection.4 Despite increasing scientific interest, the precise functions of secreted natural products, their possible targets and modes of action, and synergies between compounds within complex chemical mixtures remain mostly unknown. In contrast, ecology and genome-mining driven exploration of complex host-symbiont associations using state-of-the-art analytical dereplication tools has proven to be a successful strategy for the discovery of novel chemical scaffolds and bioactivities.5–8 Amongst the many symbiosis-related model systems, fungus-farming insects have been intensively studied, both from a chemical ecology and pharmacological perspective. As both the fungus-growing termite and ant symbioses rely on the successful protection of a fungal cultivar, their primary food source, microbial symbionts are likely to act as defensive partners and thus as prolific natural product sources for novel chemistry.2,6–8
Fungus-farming termites (Macrotermitinae, Termitidae: Blattodea) engage in a symbiosis with a fungal cultivar (genus Termitomyces; Agaricales: Lyophyllaceae) that they have co-evolved with since the origin of fungiculture 30 mya.9–12 In addition, termite guts and fungus combs harbour diverse and co-adapted microbiomes that play roles in plant biomass decomposition and potentially prophylaxis.13–16 Symbiont complementarity ensures near-complete plant biomass decomposition with contribution from the termite-nurtured fungal garden and gut microbiomes.17,18 Consequently, the termites provide important ecological services as major decomposers of dead plant material, and translocation of water and nutrients that sustain vegetation growth in the vicinity of termite mounds.19,20
Foraging on decaying plant biomass should make the termites vulnerable to antagonists or competitors of their fungal crop entering colonies with the plant substrate.21 Remarkably, the symbiosis does not appear to suffer from specialised diseases despite maintaining fungal cultivars in monoculture within colonies that can become decades old.11,15 This suggests the presence of very effective defences, which extend to the use of natural products produced by the insect host22–24 and fungal and bacterial symbionts.25,26 However, despite receiving substantial – and increasing – attention, we lack a comprehensive overview of the natural products that have been identified in the fungus-growing termite symbiosis and reflection on their putative functions.
To do this, we screened the literature for natural products from fungus-farming termites and identified more than 60 sources that collectively report 375 natural products discovered from members of the termite symbiosis. For each compound, we subsequently identified known or putative activities reported in the literature. This overview allows us to discuss the identity of natural product classes and their activities, in addition to their origins. Lastly, by quantifying the vast under-sampling of the diversity and geographic distribution of the symbiosis, we outline opportunities for future studies on natural products of ecological and pharmacological importance.
2. The fungus-farming termite symbiosis
Fungus-farming in termites originated once ∼30MYA in the African rainforest in the subfamily Macrotermitinae.10 This monophyletic subfamily includes ∼404 described termite species in 13 genera that all cultivate specialised basidiomycete fungi in the genus Termitomyces.11,27 To date, 49 Termitomyces species have been described, all of which depend on association with termites. Co-phylogenetic patterns between the termites and Termitomyces indicate host-symbiont co-diversification,10,11 and over the course of time, the association has dispersed to inhabit most of sub-Saharan Africa and large parts of Southeast Asia.10 Most fungus-farming termites acquire their fungal mutualist horizontally as mushrooms growing from termite colonies produce basidiospores that are dispersed to the environment and collected by workers of new nests to establish incipient fungus garden.28 However, some degree of specialisation exists despite horizontal transmission, so that termite species preferentially associate with specific fungal species.11,29
Termitomyces is maintained within termite mounds in structures called fungus combs (gardens), within which the termites manure the fungus as a monoculture on decaying plant biomass that older workers bring to the nest. This biomass is passed to younger workers that ingest it along with asexual Termitomyces spores from mature parts of the fungus comb, and this process ensures efficient mixing and deposition of Termitomyces with the plant substrate as ‘fresh’ comb (Fig. 1).28Termitomyces proliferates while decomposing the plant substrate and after near-complete degradation of all plant biomass, comb biomass is consumed by older termites.14,28,29 The two gut passages (inoculation and final consumption) place the termite gut central in the symbiosis, and guts harbour diverse and distinct bacterial communities, containing 100s of bacterial lineages, predominantly in the phyla Firmicutes, Bacteroidetes, and Proteobacteria.13,30,31 Dominant members are distinct from those of other termites – and the ancestral cockroaches – implying that community shifts in compositions and functions have been associated with the transition from a plant to a fungal biomass-based diet of the termite host.30,31 Across termite species within the fungus-growing termite sub-family, conserved gut community compositions suggest specificities in association for reasons that remain unresolved.13 Gut contents are deposited during the inoculation of fungus combs, leading to an abundance of gut-inhabiting lineages within gardens; however, these bacterial communities fluctuate more in composition than those observed in guts.13
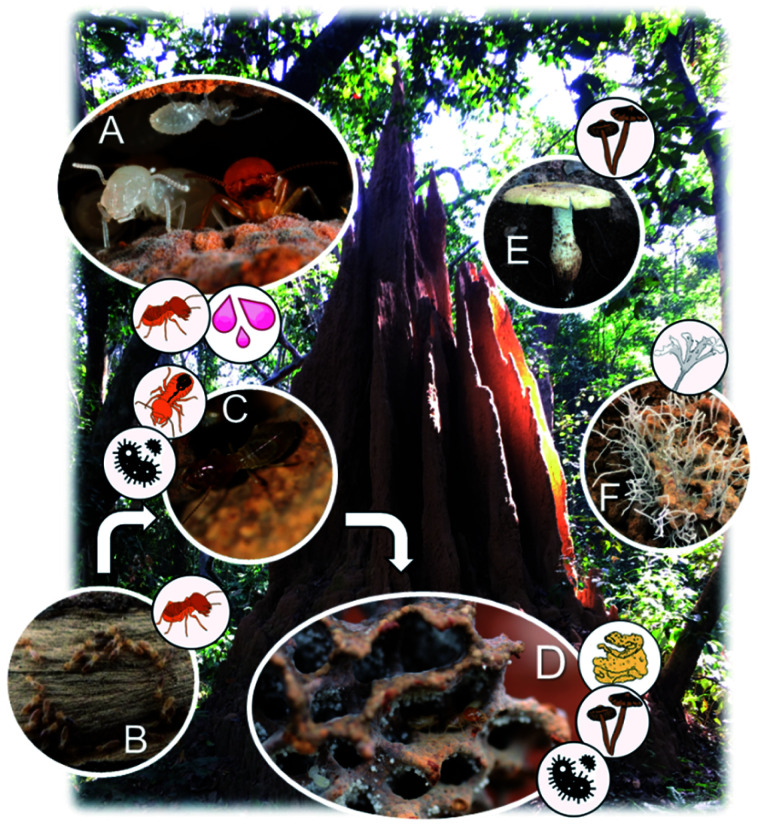
Fig. 1. The fungus-farming termite cooperation and origins of the natural products. (A) Nymph and worker termite from a Macrotermes bellicosus colony from Côte d'Ivoire (Nick Bos). A number of natural product discoveries have been made from extracts of entire termite bodies, which would imply either termite or symbiont origins. Oral secretions from the termites also contain a number of interesting natural products with antimicrobial activities. (B) Foraging termites, bringing back partly digested dead plant material for their fungal cultivar. (C) The termite gut hosts consistent and diverse bacterial communities, and it is central in the association, serving to inoculate plant substrate that passes through the gut along with asexual Termitomyces spores. (D) Termitomyces is maintained as a monoculture within fungus combs, which also comprise complex bacterial communities. (E) The fungal cultivar, Termitomyces, mushroom forming from an unknown fungus-farming termite species in Côte d'Ivoire. (F) Pseudoxylaria, a stowaway fungus of the symbiosis, seen in deteriorating comb material that produce and secrete antimicrobial compounds (Romen Palenzuela).
Maintaining a dense monoculture fungus15,32 within a colony in an environment that is optimised for fungal growth with high humidity,10 constant temperature,33 and continuous addition of plant biomass should make fungal gardens prone to infections. However, despite being in close contact with substrates containing potentially competing fungi,21 fungus combs appear free from actively growing competitors or antagonists.15 Only if colonies are compromised, e.g., due to the removal or death of workers, will combs rapidly get infested and overgrown by generalist (e.g., Trichoderma34) and specialist (Pseudoxylaria35) ascomycete fungi. A series of defences helps secure that fungus gardens are disease-free, including avoidance of antagonists by the termites,34 burial of unwanted fungi,36 and utilisation of antimicrobial compounds of termite, Termitomyces, and bacterial origins.23,24,37
3. Natural products reported from the termite symbiosis
Our extensive literature survey from 1972 to 2020 revealed that 375 natural products have been identified in the fungus-farming termite symbiosis. Subsequent literature screening of hundreds of scientific papers allowed us to generate a searchable database of all compounds (ESI, Table 1†) that includes molecular formulas, mass, compound name, natural product class, the producing organism and termite host (when known), geographic origin of discovery, method of identification, and known bioactivities or lack thereof. In addition to citing the articles that elucidated the presence of the compounds from the fungus-growing termite symbiosis, we also include references associated to bioactivities, modes of action, and synergies between compounds (ESI Table 1†).
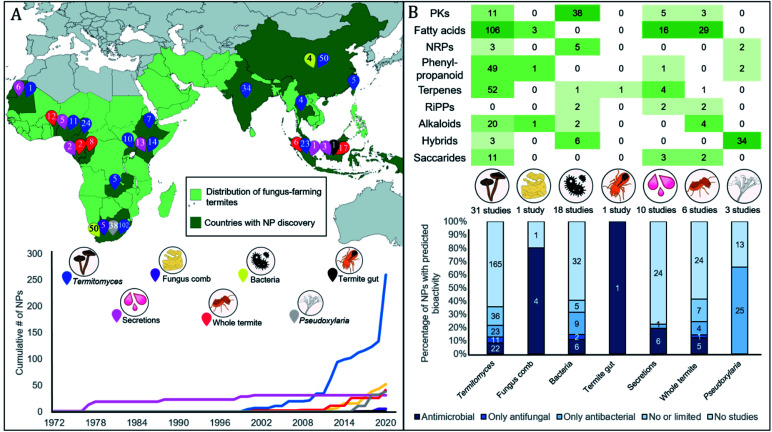
Below we summarise the discoveries of natural products from the symbiosis. While early reports focused on the termite host and particularly glandular defensive secretions, research from the late 1990s and onwards shifted towards analyses of symbiotic partners (Fig. 2A). Current reports include natural products from 14 termite and 11 Termitomyces species, five bacterial genera, and two studies on compound mixtures from the complex gut and comb environments (Fig. 2A). The natural products present are structurally diverse, often derived either from the phenylpropanoid pathway, or of polyketide, terpene or fatty acid origin (Fig. 2B). Unsurprisingly, their bioactivities also range in targets (antifungal and antibacterial) and levels (ESI Table 1† and Fig. 2B). We structure the sections below by organism, first discussing compounds of termite origin, thereafter Termitomyces, bacteria and Pseudoxylaria, and lastly symbiont communities.
Fig. 2. Thirty-five years of natural product discovery from the fungus-farming termite symbiosis. (A) Map showing in light green the distribution of the fungus-farming termites (Macrotermitinae) and in dark green countries and areas from which discoveries of natural products have been made. A total of 375 compounds have been discovered from the symbiosis, from across the symbiotic partners, as indicated with numbered pins of different colours. Below the map, we plot the timeline for the cumulative discovery of fungus-farming termite-associated natural products compounds by symbiosis source. (B) The top section provides a heatmap of compounds discovered across biosynthetic classes by symbiont origin. Two S-containing compounds from Termitomyces are not shown. The bottom section provides the percentage of natural products for each source where antimicrobial activities have been reported (MIC less than 128 μg μl−1 or ZOI more than 10 mm). The number of compounds that each bar represents is indicated within the stacked bar categories. For full details, see ESI Table 1.†.
3.1. The fungus-farming termite host
The insect host was the first source of termite-associated natural products to be exploited already in the 1970s, focusing on soldier oral and salivary gland secretions that serve in the chemical defence against e.g., ants.38 Since then, 73 natural products have been discovered, primarily being steroids and lipids/hydrocarbons, with 13 being tied to potential defensive or antimicrobial activities (ESI Table 1†).
3.1.1. Antimicrobial peptides
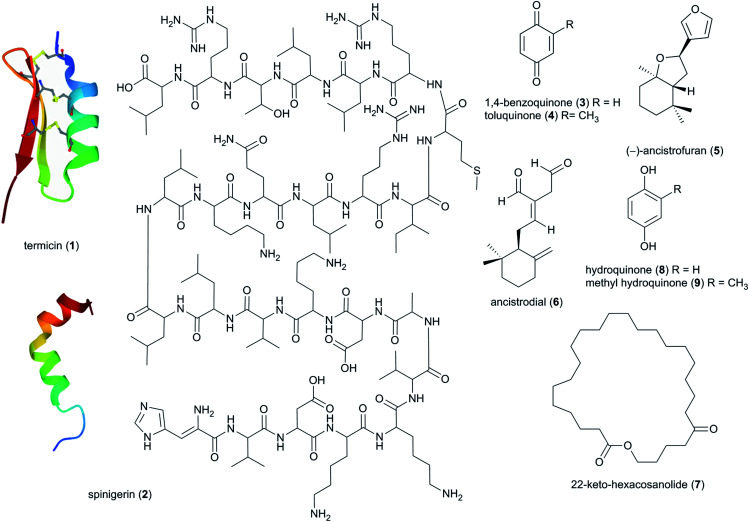
The presence of millions of individual termites within nests poses a risk of spread of infectious disease to which defences are essential. Among the first discoveries of defensive compounds of termite origin were two antimicrobial peptides, termicin (1) and spinigerin (2) from the salivary glands of Pseudacanthotermes spiniger (Fig. 3).39 Termicin is structurally related to the insect antimicrobial peptides defensins, which are important in host defence against pathogens,40 and they are built by a α-helical segment and a two-stranded antiparallel β-sheet forming a cysteine-stabilized α–β-motif. In contrast, spinigerin is devoid of cysteine and forms only the α-helical conformation. Both peptides are antifungal against ecologically relevant fungi, such as Trichoderma, Fusarium, Nectria, Neurospora and the entomopathogenic fungus Metarhizium anisopliae.41,42 The mode of action of termicin against M. anisopliae includes potential synergistic action with antifungal glucanases to break down β-glucans of the fungal cell wall and thereby fatally disrupt the cell membrane. Termicin may further limit pathogenic fungi by preventing the attachment and penetration of the fungus to the termite cuticle.40,41 Spinigerin also acts against Gram-positive and Gram-negative bacteria, and its mode of action involves induction of cell lyses through transmembrane pore formation.43
Fig. 3. Antimicrobial peptides (1, 2), benzoquinones (3, 4), sesquiterpenes (5, 6) and a macrolactone (7) identified in the termite hosts.
3.1.2. Benzoquinones and sesquiterpenoids
More recent analyses revealed that antimicrobial compounds in glandular secretions extend beyond peptides. Specifically, benzoquinones and benzoquinone mixtures with antifungal and antibacterial activities exist within salivary and labial gland secretions of Odontotermes, Macrotermes and Microtermes soldiers (Fig. 2 and 3). Of these, 1,4-benzoquinone (3) and toluquinone (4) show antifungal activity against entomopathogenic fungi, such as M. anisopliae and Beauveria brongniartii among other bioactivities.44 Notably, 1,4-benzoquinone has been reported as an inhibitor of the fungal aspartate semialdehyde dehydrogenase,45 an enzyme that is important for the biosynthesis of amino acids and thus crucial for viability. Other major chemical constituents of soldier salivary secretions include the sesquiterpenoids (−)-ancistrofuran (5) and ancistrodial (6), which have been isolated from Ancistrotermes cavithorax,46 and potentially-systemic macrolactone toxins against ants, including 22-keto-hexacosanolide (7) and analogues identified from P. spiniger major soldiers.23 A study from 2020 analysed extracts of Macrotermes bellicosus soldiers and found a significant abundance of hydroquinone (8) and methyl hydroquinone (9), both of which have antibacterial activities.47
3.2. Termitomyces
In the late 90s, the first reports of natural products from Termitomyces appeared, and now include work on 11 species from fourteen countries (Fig. 2A and B). By December 2020, 31 studies have identified 257 natural products from Termitomyces, of which at least 53 have antimicrobial properties (ESI Table 1† and Fig. 2B). Studies on Termitomyces mushrooms and cultures have revealed their prominent nutritional value, and several Termitomyces species are consumed also for medicinal purposes by indigenous communities.48 Some of the major bioactive compounds found in Termitomyces could have potential antioxidant, anti-tumour and antimicrobial effects.
3.2.1. Fatty acids
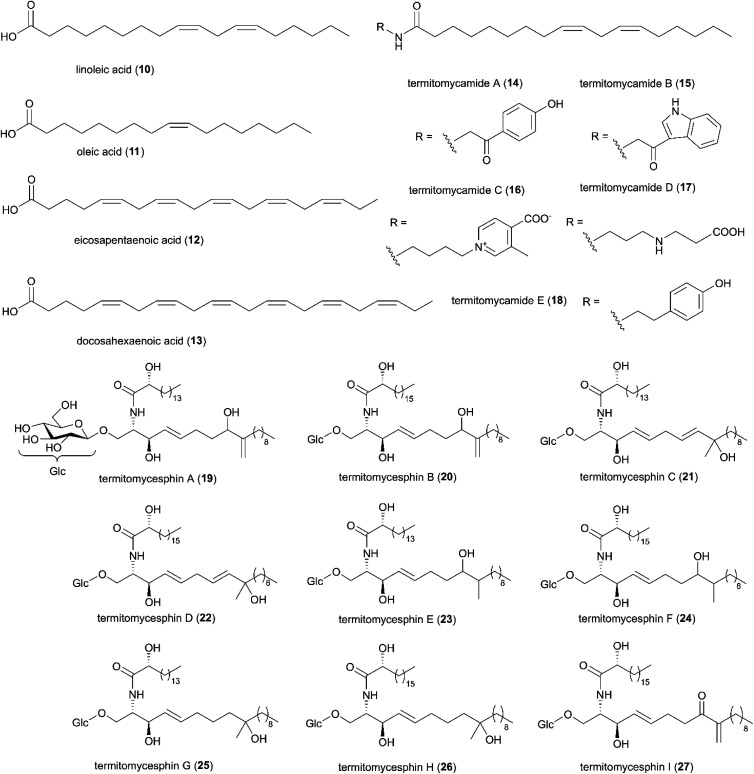
More than 100 of the natural products identified in Termitomyces are fatty acids, which are widely present in natural fats, play vital roles as metabolites in living organisms and in many cases display antimicrobial activities.49 Of these, unsaturated fatty acids, such as linoleic (10) and oleic acids (11) have been reported from Termitomyces heimii50 and other species (Fig. 4), and these strongly inhibit Gram-positive bacteria. Their mode of action is linked to the disruption of fatty acid synthesis through inhibition of type I fatty acid synthase,51,52 resulting in increased membrane permeability and formation of pores in the cell membrane.52–54 Although, they exhibit less antimicrobial activity than typical antimicrobial agents, such as erythromycin or streptomycin, it is notable that synergistic antibacterial activity with erythromycin have been reported.51 Other antibacterial fatty acids, such as eicosapentaenoic acid (12) and docosahexaenoic acid (13) (Fig. 4), inhibit both Gram-positive55 and Gram-negative56 bacteria, but no studies have investigated their potential synergies in naturally-occurring mixtures. In addition, five novel fatty acid amides (termitomycamides A–E (14–18)),57 and nine novel glycolipids (termitomycesphins A–I (19–27)),58–61 have been identified from Termitomyces mushrooms. Two of the fatty acid amides showed protective activity against stress-dependent cell death.57
Fig. 4. A selection of fatty acids identified in Termitomyces spp. Compound 19–27 were redrawn from original articles.58–61.
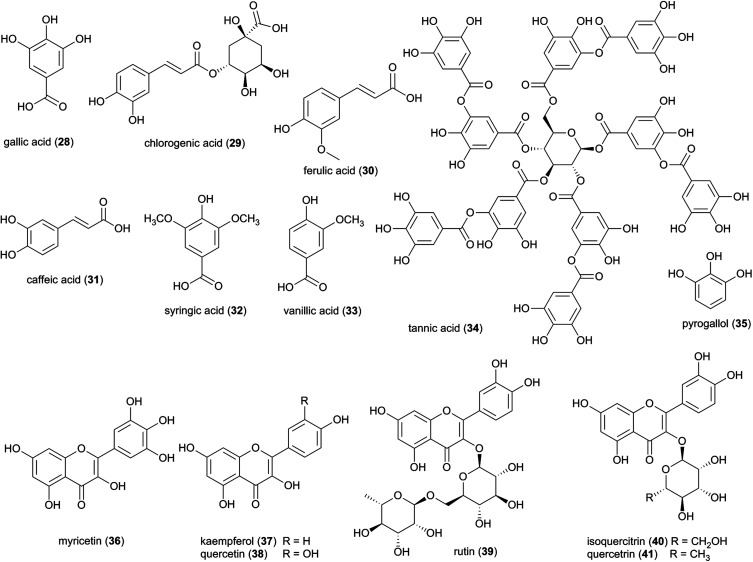
3.2.2. Phenolic acids
In addition to fatty acids, numerous phenolic natural products derived from the phenylpropanoid pathway have been identified from Termitomyces spp. mushrooms, including gallic acid (28), chlorogenic acid (29), ferulic acid (30), caffeic acid (31), syringic acid (32) and vanillic acid (33) (Fig. 5),62–64 all of which exhibit antimicrobial activity against bacteria and fungi.65–68 Further, gallic acid, caffeic acid and ferulic acid act synergistically with various aminoglycosides67 and quinolone-based antibiotics.69 Both of these antibiotic families act by entering the bacterial cell and inhibiting DNA or protein synthesis, and several studies have reported that the antimicrobial activity of the phenolic acids lies in the disruption of membrane integrity.66,68 This is consistent with another study which proposed that phenolic acids must have similar mode of actions based on their similar inhibitory effects of bacteria.67 Other phenols, such as the polymeric polyphenol tannic acid (34), have been found in culture extracts of T. heimii and T. mummiformis64 and their precursor molecule pyrogallol (35)62 exhibits strong antibacterial activity.65,70
Fig. 5. A selection of phenolic acids (28–35) and flavonoids (36–41) from Termitomyces spp.
3.2.3. Flavonoids
Eight flavonoids, six flavonols and two flavanols, were isolated from fruiting bodies from T. robustus, T. heimii, T. clypeatus and T. microcarpus (Fig. 5).62,71 Flavonoids are a large class of small secondary metabolites carrying a diverse substitution pattern and are commonly found in plant and fungi. These natural products are synthesized by the phenylpropanoid pathway and display a wide range of biological activities, including anti-inflammatory, antitumor, antimicrobial and antiviral, which have attracted the interest of the pharmaceutical industry.72
Characterized flavonoids include myricetin (36), kaempferol (37), quercetin (38) rutin (39), and isoquercetrin (40), which vary in their degree of antibacterial and antifungal activities (see ESI Table 1†)73 but have been proposed to exhibit complementary and synergistic activities (in vitro and in vivo) through various mode of actions and thus may be important in fungus garden defence. Specifically, synergistic activities of flavonoids in combination treatments have been observed for e.g., rutin, quercitrin (41), and quercetin, which revealed stronger inhibitory effects on Bacillus cereus and Salmonella enterica Serotype Enteritidis compared to treatment with either flavonoid alone.74 Notably, rutin (39) does not exhibit any antibacterial activity alone, but significantly enhances antibacterial effects of other flavonoids, including quercetin, quercitrin, kaempferol and myricetin.74 Causality for the complementary activities remains obscure, although modes of action for the various flavonoids allows for speculation. Rutin, might for example enhance the activity of other hydrophilic antibiotics by disrupting the bacterial cell walls of multi-drug-resistant Staphylococcus aureus (MRSA)75,76 and allowing its effective penetration. Despite not showing any effect alone, rutin have been shown to improves the antibiotic effect of quercetin,76 Quercetin (38) has been linked to multiple modes of action, including inhibition of DNA gyrase,77 inhibition of efflux pumps,78 membrane disruption79 and cell envelope synthesis.80 It has also both potentiating and synergistic antibacterial actions in combination with a broad range of antibiotics with different mode of actions, such as penicillins (ampicillin, amoxicillin and methicillin),76,81,82 cephalosporins (ceftriaxone, cefixime, and cephradine),76,83 tetracycline81 and aminoglycosides (tobramycin, gentamycin, amikacin).83 It is thus conceivable that combinations of multiple compounds play a significant role in termite nest defence through contributions to the antimicrobial activity observed in Termitomyces.24,84
Phenolic compounds are found widely throughout the plant kingdom, and it is possible that the phenolic compounds identified in Termitomyces could have been synthesized and absorbed from plants. However, the Shikimate pathway has been correlated to the presence of phenolic compounds in fungi85,86 and a recent study showed that despite flavonoids being well-known plant metabolites, all genes and protein sequences associated with the biosynthesis of flavonoids can be found in some fungi.87 Future genome analyses and molecular biological studies will thus be needed to confirm the putative fungal origin of these metabolites.
3.2.4. Terpenes and volatiles
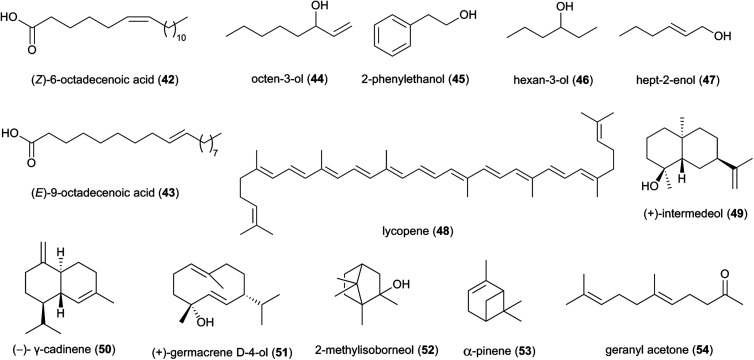
The enclosed nature of fungal combs within termite nests should allow for the build-up of volatile compounds that could play roles in inter-kingdom communication or suppress antagonist growth, provided they do not negatively affect Termitomyces or the termite themselves. Termites are well known to make use of volatile compounds to communicate among castes and in direction of division of labour within colonies.88 One study identified two novel compounds (Z)-6-octadecenoic acid (42) and (E)-9-octadecenoic acid (43) in Macrotermes gilvus, which appear to be involved in nestmate recognition.88
Studies based on head-space gas-chromatograph mass spectrometry (GC-MS) have allowed for the first insights into the volatilome of Termitomyces sp. and revealed several odorous compounds including typical C8 components (mushroom odours), such as octen-3-ol (44), 2-phenyl-ethanol (45), hexan-3-ol (46) and hept-2-enol (47) (Fig. 6), as well as benzaldehyde (almond odour), benzyl alcohol (sweet-spicy odour), phenylethanol (rose odour), and monoterpenes.16,89,90
Fig. 6. A selection of terpenes and volatiles produced by Termitomyces and Macrotermes gilvus.
More than 50 terpenes and their functional derivatives (terpenoids) have been reported from Termitomyces (ESI Table 1†). Both compound classes are found in most living organisms and contribute to flavour, scent and colour in plants and fungi, and often exhibit insecticidal and/or antimicrobial activities.91 Just to give an example: in 2016, the terpenoid lycopene (48), a carotenoid hydrocarbon, was detected in T. heimii and T. microcarpus63 and it exhibits strong antimicrobial activity (Fig. 6).
Terpenes and terpenoids are derived from the conversion of geranyl or farnesyl pyrophosphate catalysed by dedicated terpene synthases and/or terpene cyclases.92 A first bioinformatics analysis of the draft genome sequence of Termitomyces sp. J132 revealed more than 20 putative type I terpene cyclase genes, three of which were functionally characterized as (+)-intermedeol (49) synthase, (−)-γ-cadinene (50) synthase and (+)-germacrene D-4-ol (51) synthase.93 The cyclization reactions were surveyed for all three enzymes by incubation with isotopomers of FPP, yielding valuable insights into the stereochemical course of the ring closing mechanism, reprotonation steps and hydride shifts. A follow-up study in early 2021 identified 13 additional terpenoids from Termitomyces, including 2-methylisoborneol (52), α-pinene (53) and geranylacetone (54),16 which have been linked to antifungal and antibacterial activities.94–96 α-Pinene has also been observed to modulate the sensibility of antibiotic-resistant bacterial strains by preventing the efflux of multiple antibacterial agents.95,97
3.2.5. Alkaloids and N-containing heterocycles
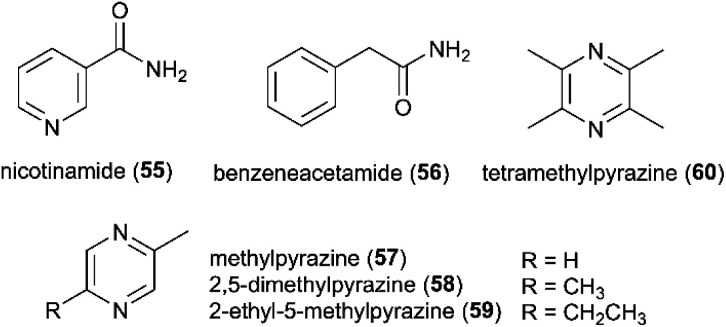
Seven studies have identified 20 alkaloids and nitrogen-containing heterocycles from Termitomyces, of which five have antimicrobial activities. These include the active form of vitamin B3, nicotinamide (55) and benzeneacetamide (56) isolated from fruiting bodies of T. heimii.50 Eight pyrazines, including methylpyrazine (57) 2,5-dimethylpyrazine (58), 2-ethyl-5-methylpyrazine (59) and tetramethylpyrazine (60), have been isolated from Termitomyces (Fig. 7). Pyrazines are the product of the condensation of α-aminocarbonyl compounds and represent a major class of volatile compounds produced and emitted from both bacteria and fungi.98–100 Their specific functions in Termitomyces remain poorly understood, but as other volatiles they could serve as intra- and inter-kingdom communication signals, growth promoters or inhibitors of other organisms.101 Pyrazines may also function as carbon or nitrogen sources, as shown in the case of Mycobacterium sp. DM-11 and Rhodococcus opacus, which can utilize 2,3-diethyl-5-methylpyrazine and tetramethylpyrazine as their sole carbon and nitrogen source, respectively.102,103 Pyrazines are also widely distributed among insects and terrestrial vertebrates, where they are used for intra- or interspecific communication, such as alarm or alerting pheromones.99 The high volatility and the low molecular weight are ideal conditions for quick temporary transfer of information.104
Fig. 7. Alkaloids and N-containing heterocycles isolated from Termitomyces.
3.3. Bacterial symbionts
The past decade has seen an increased focus on chemical analyses of termite-associated bacteria that have been isolated from the termite cuticle, termite gut or fungus comb. Most studies have been on termite species from South Africa (15 studies/50 compounds) and China (3 studies/4 compounds)105–107 (Fig. 2A). Termite guts and combs harbour diverse bacterial communities,13,15,108 however, chemical studies and novel natural products have only been reported from Bacillus, Streptomyces, Amycolatopsis and Actinomadura, of which three belong to the phylum Actinobacteria. Of the 54 natural products discovered from termite-associated bacteria, 17 have been reported to exhibit strong antimicrobial activities (ESI Table 1† and Fig. 2B).
3.3.1. Polyketides
Polyketides (PKs) are a large classes of structurally diverse natural product produced by many organisms and often exhibiting diverse biological activities, numerous of which have become clinically valuable drugs such as erythromycin and rapamycin.109 Polyketides are biosynthesized by mega-enzymes called polyketide synthases (PKS), which are related to fatty acid synthases, and act through a series of condensation reactions of dedicated starter units and their subsequent modifications.110
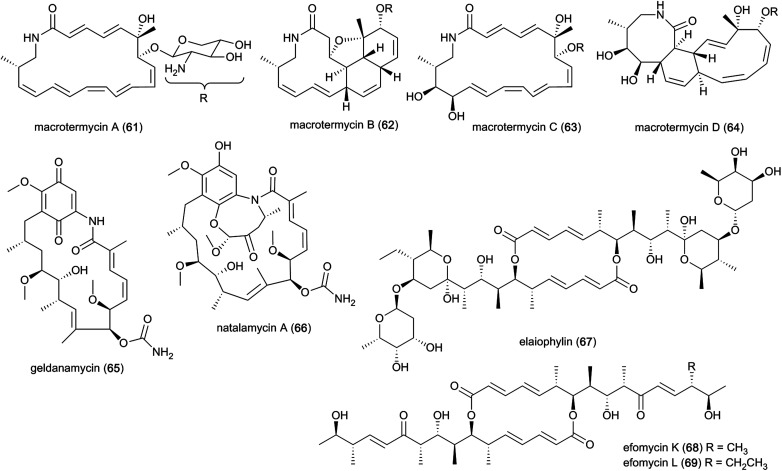
Ecological-driven co-cultivation studies of the M. natalensis-associated Amycolatopsis sp. with Pseudoxylaria sp. X802, which is often found dormant within fungus combs,35 lead to the isolation of four glycosylated macrolactams (macrotermycin A–D; 61–64). Macrotermycin A and C are 20-membered glycosylated polyene macrolactams, which are transformed by an intramolecular cycloaddition sequence leading to macrotermycin B and D. While polyene macrotermycin A and C exhibited strong antimicrobial activity, including selective inhibition of Pseudoxylaria, the cyclized derivatives were inactive (Fig. 8).26
Fig. 8. Polyketides from bacterial symbionts. Macrotermycin A–D (61–64) isolated from Amycolatopsis strain M39, and geldanamycin (65), natalamycin A (66), elaiophylin (67) and efomycin K–L (68–69) isolated from Streptomyces strain M56.
In a similar co-cultivation study, Streptomyces sp. M56 was also found to strongly inhibit Pseudoxylaria spp. (and Termitomyces) growth. This led to the isolation of the antifungal polyketide geldanamycin (65) along with the novel geldanamycin analogue, natalamycin A (66),111 which contains a rare C-5 unit on the 3-amino-5-hydroxybenzoic acid (AHBA) head group to form fused bicyclic[6.4.0]ansa macrolide. The same bacterium was also found to produce the antibacterial macrolactone elaiophylin (67), along with several analogues efomycins K and L (68–69), which are antibacterial towards Gram-positive bacteria.112 Efomycins are C2 symmetric 16-membered macrolides that are derived from two linear polyketide chains with an unsaturated enone moiety, while elaiophylins carry a hemiketal moiety and are glycosylated. However, neither of the compounds alone were found to be responsible for inhibition of Pseudoxylaria, and synergistic effects were postulated as geldanamycin has previously been reported to possess synergistic antifungal activities with e.g., triazoles, echinocandins and fluconazole.113,114 Indeed, elaiophylin enhances the activity of the co-produced antifungal macrolide rapamycin against Candida albicans, while lacking activity alone.115 Geldanamycin is a well-known potent inhibitor of the heat shock protein Hsp90,116 whereas the mode of action for elaiophylin has not yet been established, but may be associated with the alteration of membrane permeability by destabilization and formation of ion-penetrable channels.117 A study investigating the role of Hsp90 in the mTOR-signalling pathway reported that geldanamycin targets this pathway by suppressing mTOR activity, similar to the antifungal rapamycin (originally identified to target the TOR kinases in the yeast Saccharomyces cerevisiae), which induces dissociation of the mTOR-raptor complex. Both exhibit anti-cancer activities by targeting mTOR, but through different mechanisms of action.118 As TOR kinases are ubiquitously conserved in eukaryotic organisms, the same target may be responsible for the antifungal activity observed towards Candida.113 Geldanamycin and elaiophylin are often found to be co-produced, with the TetR-family regulator GdmRIII apparently having an inverse effect on the two compounds, positively regulating geldanamycin and to some extend repressing elaiophylin.119 No explanation for this possible co-production has been reported, but it has been hypothesized that reverse regulation is essential in controlling the flux of precursor metabolites for the two compounds.120 Further investigation into potential complementary activities of geldanamycin and elaiophylin against ecologically relevant fungal strains that challenge the symbiosis could help unravel their ecological importance.
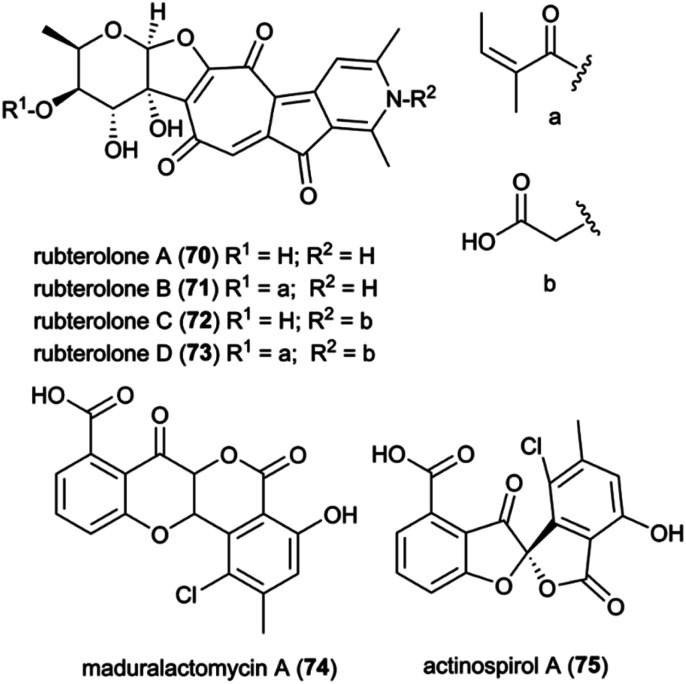
Recently, Actinomadura rubteroloni was found to produce two types of polyketide-derived, highly oxidized and rearranged types of natural products (Fig. 9). In a first study, a rare group of glycosylated tropolone-containing natural products, named rubterolones A–D (70–73), were identified and found to have anti-inflammatory activity.121 Rubterolones feature a tropolone moiety, a fused cyclopentanone ring, an O,C-condensated sugar and a highly substituted pyridine or pyridine inner salt moiety, and are structurally related to the recently identified rubrolone B122 and isarubrolones.123 Several biosynthetic studies and complementing isotope feeding experiments suggested that the tropolone and cyclopentanone containing carbon skeleton is of type II polyketide origin, but undergoes a series of complex oxidative rearrangements induced by the cluster-encoded oxygenases to yield first the later-identified pre-rubterolones, and then undergoes a spontaneous pyridine formation in the presence of amines.
Fig. 9. Polyketides isolated from Actinomadura rubteroloni.
While high biomass cultivation induced mostly the formation of rubterolones, growth from diluted spore cultures resulted in the dominant formation of chlorinated maduralactomycin (74) containing a rare bicyclic 4-chromanone fused with isocoumarin core structure and spirocyclic actinospirols (75).124 Intriguingly, the tested cultivation conditions likely simulate the natural ecological environment bacterial strains would face when entering the fungus comb after obligate gut passage of a termite worker. Based on comparative genome studies and HRMS2-based GNPS analyses, a putative biosynthetic mechanism was proposed that is based on a non-canonical angucycline biosynthesis and extensive oxidative modifications that ultimately result in the formation of the unique halogenated tetracyclic polyketides and after additional oxidative rearrangements in the formation of actinospirols. Bioactivity studies of maduralactomycin A revealed antibacterial activity towards both Vancomycin-Resistant Enterococcus faecalis and Mycobacterium vaccae.124
3.3.2. Non-ribosomally synthesized peptides
Non-ribosomally synthesized peptides (NRPs) refer to peptidic sequences that are assembled by non-ribosomal peptide synthases (NRPS) and seldom exceed more than 20 amino acids residues. NRP-derived molecules are often further modified by e.g. acylation, glycosylation or cyclisation.
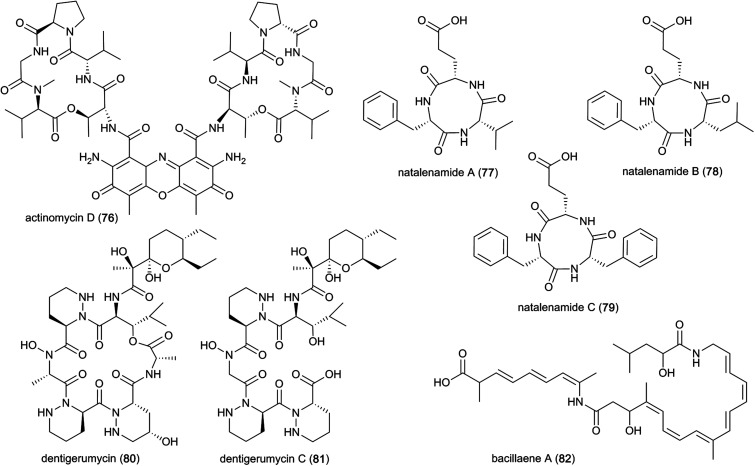
A well-known compound that has been discovered multiple times from termite-associated Streptomyces is the chromopeptide antibiotic actinomycin D (76). Actinomycin D belongs to a family of bicyclic chromopeptide lactones sharing the chromophoric phenoxazinone dicarboxylic acid attached to two cyclic pentapeptide lactones of non-ribosomal origin containing l-Thr, d-Val, l-Pro, Sar and l-MeVal. It appears to have broad-spectrum antibacterial activity and potent activity against several ecologically-relevant fungi, including Pseudoxylaria (Fig. 10).107,125 Actinomycin D may have this effect due to mode(s) of action that include DNA-dependent inhibition of RNA synthesis, and potentially targeting the fungal plasma membrane through a membrane splitting mechanism.126
Fig. 10. Non-ribosomally synthesized peptides and PKS–NRPS-hybrid molecules from symbiotic bacteria.
Interestingly, Actinomycins are also produced by Streptomyces isolated from fungus-farming ants, where they may be involved in fungistatic activity against Escovopsis spp. antagonists of the mutualistic fungus of the ants.127 Another study showed strong synergistic effects of mixtures of antifungals against E. weberi.128 However, strong antibacterial activities were also reported towards ant bacterial symbionts, suggesting that they may play roles in competition between bacteria.128 Actinomycin D has also been observed to be involved in synergistic effects with the antimicrobial agents amphotericin B and colistin,129,130 both of which disrupt the cell membrane, thereby facilitating actinomycin D access.
Lastly, a study directed at identifying novel pharmacologically relevant natural products elucidated three new cyclic tripeptides, containing l-Phe, l-Val/l-Leu and l-Glu, named natalenamides A–C (77–79).131 These compounds are presumably of non-ribosomal origin, and were obtained from Actinomadura sp. RB99 using an LC/MS/UV-based dereplication approach.131 Natalenamide A and B showed weak cytotoxicity against cancer cell lines HepG2 (liver) and HeLa (cervical), and A549 (lung), respectively. Natalenamide C (79) significantly inhibits the production of 3-isobutyl-1-methylxanthine induced melanin.
3.3.3. PKS–NRPS hybrids
PKS–NRPS hybrid natural products that are biosynthesized by a co-linear biosynthetic assembly line of interacting PKS and NRPS yielding natural products that feature peptide units as well extensions by C2-units. In 2013, a study revealed that Bacillus spp. from M. natalensis produce the polyene polyketide bacillaene A (82) as a major metabolite, which exhibits strong broad-spectrum antibacterial activity and antifungal activity against fungus-garden antagonists, e.g., Pseudoxylaria and Trichoderma.37 Bacillaene A is synthesized by a giant hybrid polyketide/non-ribosomal synthase and composed of a linear polyene containing two amide bonds: the first links an α-hydroxy carboxylic acid to a ω-amino carboxylic acid containing a conjugated hexaene, and the second links the hexaene-containing carboxylic acid to an (ω − 1) amino carboxylic acid containing a conjugated triene. While the polyene carboxylic acid moieties are biosynthesised by FAS/PKS modules containing unusual trans-AT domains, the two NRPS modules are responsible for the formation of the two peptide bonds (Fig. 10).132,133 The mode of action of bacillaene A has been reported as inhibition of bacterial protein synthesis,134 potentially by blocking translation elongation,135 but the mechanism for fungal inhibition is unknown.
In a subsequent study, dentigerumycins (80–81) were isolated from Streptomyces sp. M41 from the South African fungus-growing termite species Macrotermes natalensis, based on its unique metabolomic profile from principal component analysis (PCA) of 41 Actinobacteria isolates.136 Dentigerumycin is a 19-membered macrocyclic hexapeptide containing three piperazic acids, ester-forming Ala, N-OH-Ala, β-OH-Leu, and a pyran-bearing polyketide acyl chain, whereas dentigerumycin C is the linear form lacking the ester-forming alanine residue (Fig. 10).136–138 The identified derivatives are structurally related to the Pseudonocardia-derived antifungal dentigerumycin that inhibits the specialized mycopathogen Escovopsis spp. that invade and consume the ants' fungal cultivar.137
3.4. Pseudoxylaria stowaway fungus
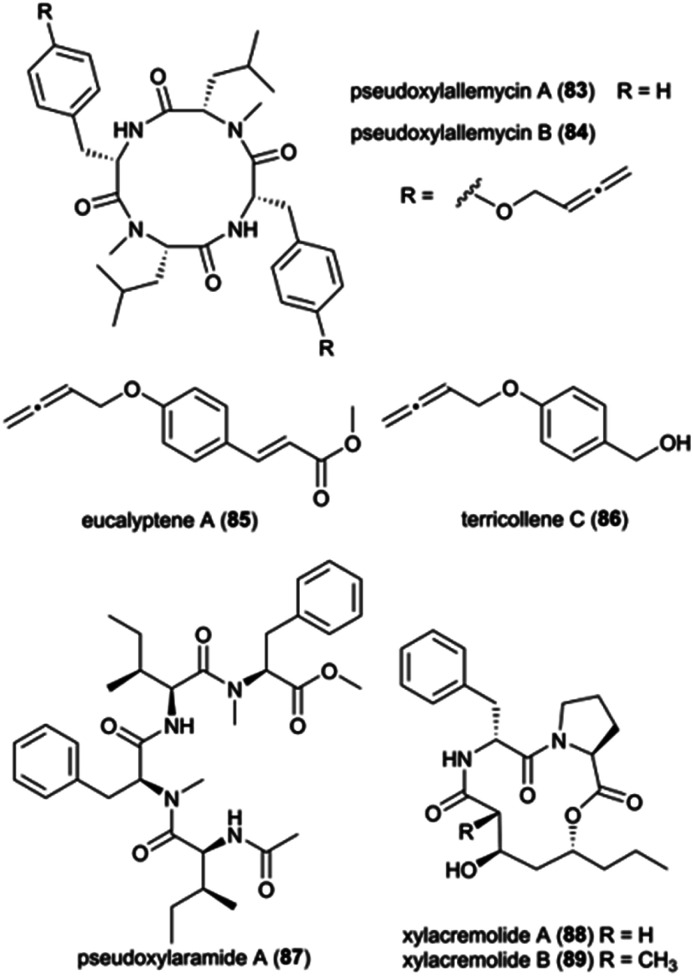
Pseudoxylaria spp. (Ascomycota: Xylariaceae) are frequently found on deteriorating comb material and is presently considered a stowaway fungus, waiting as a substrate specialist and opportunistic weed until conditions are favourable for outcompeting Termitomyces.21,35 Members of the Xylariaceae are ecologically important as saprotrophs, but they are also well-known for their biosynthetic capabilities to produce structurally diverse metabolites with a broad spectrum of biological activities.139 While the exact mechanisms of the interaction and role of Pseudoxylaria in the comb remains unknown, given the competitive nature of the association,35 it is conceivable that termite-associated Pseudoxylaria produce biologically active small molecules. Indeed, crude Pseudoxylaria extracts can be both antifungal and antibacterial, and a recent study discovered six novel compounds. Using an MS-based imaging and a dereplication strategy, Guo et al. discovered six new antimicrobial cyclic peptides, named pseudoxylallemycins A–F (83–84), and eucalyptene A (85) and terricollene C (86) from Pseudoxylaria sp. X802 (Fig. 11).140 Pseudoxylallemycins contain symmetric or asymmetric tetracyclic consisting l-Phe, NMe-l-Leu, and a very rare allenyl modification on tyrosine. More recently, high-resolution tandem mass spectrometry (HRMS2) based approaches led to the identification of chemical features unique to Pseudoxylaria sp. X187: four linear non-ribosomally synthesized peptides (NRPs) and two cyclic NRPS-polyketide synthase (PKS) derived natural products, pseudoxylaramides (87) and xylacremolides (88–89) (Fig. 11).141 Pseudoxylaramides are linear tetrapeptides containing l-Phe and l-Ile with methylation in the C-terminal and acylation in the N-terminal, while xylacremolides are cyclic polyketide-dipeptide hybrids composed of l-Pro and l-Phe and 3,5-dihydroxy octanoic acid (DHOA) (Fig. 11). The pseudoxylaramides share features with other antimicrobial fungal compounds,142 and the xylacremolides share some similarity to trapoxin, an antitumor cyclic tetrapeptide.143 None of the compounds identified in Pseudoxylaria showed activity towards the tested strains; thus, the bioactivity of these NRPS and NRPS–PKS hybrids still needs to be resolved. However, their structural similarities to other peptides point to ecologically-relevant roles as antimicrobials.
Fig. 11. Natural products from Pseudoxylaria spp.
3.5. Analysis of symbiont communities
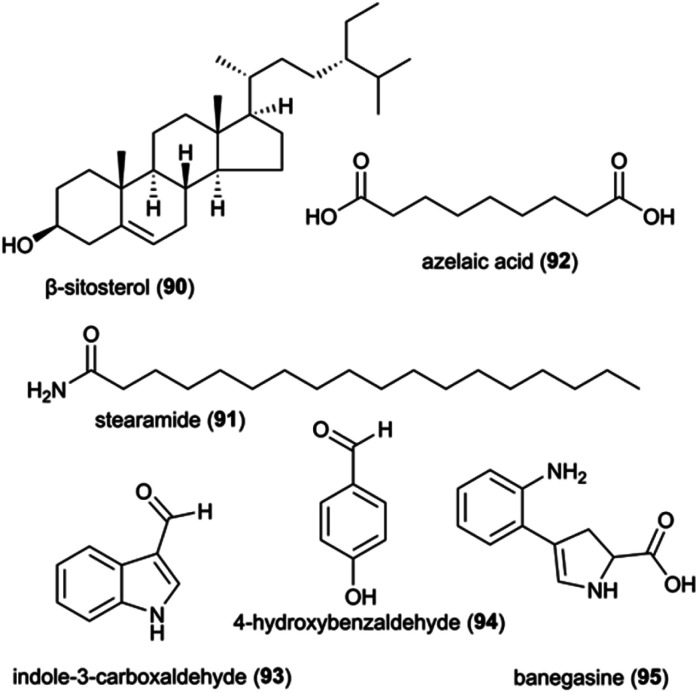
The identification and interactions of chemical compounds in the complex communities of organisms in termite guts and fungus combs hold particular promise for insights into ecologically-relevant functions. To date, only two studies have investigated the mixtures of natural products from the complex gut and fungus comb symbiont communities, one of which focused on putative defensive roles in Macrotermes and Odontotermes nests from South Africa15 and one on termite guts.144 The latter involved GC-MS analysis of dissected gut of Macrotermes gilvus workers, which led to the identification of the terpenoid β-sitosterol (90) (Fig. 12), which possesses selective antifungal and antibacterial activities.145,146 As β-sitosterol is one of the most abundant occurring phytosterols, its identification in the gut most likely stem from the plant material the termites gathered.
Fig. 12. A selection of natural product and small molecules identified from analyses on complex communities.
The chemical analysis of fungus combs led to the identification of five small molecules, of which stearamide (91), azelaic acid (92), indole-3-carboxaldehyde (93) and 4-hydroxybenzaldehyde (94) have reported antifungal and antibacterial activities (Fig. 12; ESI Table 1†). Indole-3-carboxaldehyde (93) was previously identified from the bacterium Janthinobacterium lividum on the skin of the red-backed salamander (Plethodon cinereus), where it appears to be involved in the chemical defence against the fungal pathogen Batrachochytrium dendrobatidis causing chytridiomycosis in amphibians.147 However, none of the identified compounds were verified to be responsible for the observed antifungal activity of chemical extracts of fungus comb against an ecological-relevant competitor of Termitomyces (Trichoderma) and the entomopathogen B. bassiana.15
Although comb metabolites may exhibit weak activity, it is conceivable that synergistic or additive antimicrobial effects exist. Synergistic interactions between multiple small molecules present in extracts of Termitomyces, fungus combs or Actinomadura, have been shown in other work to improve the antibiotic effect of multidrug resistant bacteria, like MRSA.148 These include phenolic acids of Termitomyces origin (see above) and the small molecule 4-hydroxybenzaldehyde (94) that was identified in antifungal fungus comb extracts.15 This compound appears to improve the sensitivity of Acinetobacter baumannii to amphenicol.149 Similarly, the two alkaloids, indole-3-carboxaldehyde and banegasine (95) enhance the efficacy of multiple antifungal agents against a diversity of fungal species.150–153 Instead of relying on one single metabolite, the combination of diverse molecules with synergistic or potentiating effects is likely essential for defence of the symbiosis, mirroring strategies employed by humans to overcome resistance evolution in target pathogens.154
4. Prospects for defence potential and novel natural product discovery
The fungus-farming termite symbiosis is extraordinarily successful in hindering the entry and spread of antagonists,15,21 likely through a multi-layer defence that integrates behaviours with sophisticated use of antimicrobial compounds. The roles of the vast majority of identified natural products from the symbiosis have yet to be established, including for general bioactivity and in ecologically-relevant contexts (ESI Table 1†). Current reports rarely provide evidence for importance in defence, leaving promising insights to come from elucidation of their concentrations, how and when compounds are applied, and their targets. Efforts to bridge objectives and methods of natural product discovery and elucidation of roles in defence are warranted to shed light on the implications of natural product use in defence in the environments within which they act.
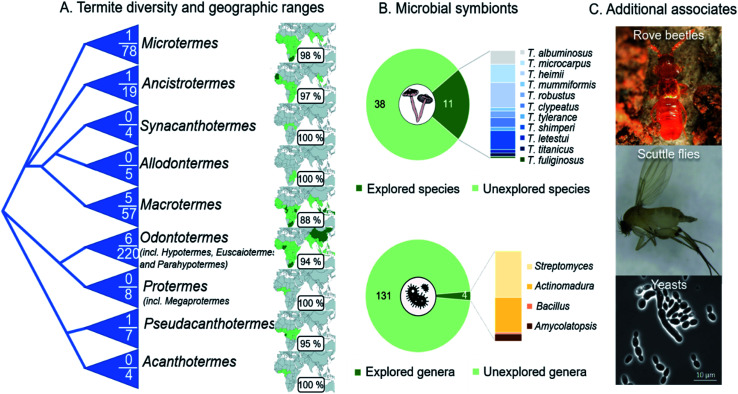
The natural product discovery potential from the symbiosis remains extraordinary, with only a small fraction of the geographical distribution and host and symbiont diversity, having been explored (Fig. 13A). Efforts so far have remained on the conspicuous termite genus Macrotermes (80% of studies), which accounts for only a fifth of the known termite species.27 Similarly, only a fourth of the described Termitomyces species, and a handful of gut and comb bacteria and antagonists of the symbiosis, have been explored (Fig. 13B). Among the bacteria, Actinobacteria dominate the focus,35,125,155 for good reason given their prolific potential,125,155,156 including as defensive symbionts in fungiculture.35,157 Recent genome analyses support that we are far from elucidating their full chemical potential: more than 100 biosynthetic gene clusters present within 16 actinobacterial genomes encode putative novel chemistry.155 It is thus undeniable that further investigation carries promising potential for novel discoveries. This potential extends to so-far unexplored organisms that inhabit termite mounds as guests, such as rove beetles and scuttle flies and their gut symbionts,158–160 and other conceivably beneficial symbionts (e.g., yeasts161). Understanding these multi-species interactions, and the compounds involved, remains a major challenge and an outstanding opportunity for future work on the symbiosis. To this end, comparison of the chemical repertoire that the farming termite symbiosis with other fungus-farming symbioses holds great potential to shed light on unique and convergently evolved utilisation of natural products in fungus farming.
Fig. 13. Outstanding opportunities for natural product discovery from the fungus-farming termite symbiosis. (A) A schematic phylogeny of the Macrotermitinae (based on ref. 11), indicating the number of termite species out of the total number of known species that have been explored for natural products. The geographic ranges of termite genera and the percentage of countries from which no work has been reported on natural product from any members of the symbiosis. (B) Eleven of the 49 described Termitomyces species have been explored for natural products, and the distribution of compounds discovered illustrated by species in the bar plot. Only four of the estimated 102–149 genus-level phylotypes (average 131 14) have been cultured and explored for natural product discoveries, not including metabolomic analyses of complex microbial communities in guts and fungus combs. (C) Natural product discoveries have largely overlooked guest species, including beetles (Tom Murray) and flies,158–160 and additional symbionts (e.g., yeast species161).
Bioactive natural products from the fungus-farming symbiosis occupy a unique chemical space and their bacterial and fungal symbiont represents an extraordinary discovery and studying potential from both ecological and chemical perspectives. Previous investigations have elucidated a large number of novel compounds and theorised their functions. Insights from reported activities show their potential to be used by the symbiosis in multi-target combination defences. In recent years, the importance of synergies between natural products has gained significant attention, including in the development of new methods and optimisation of approaches to identify mixture constituents, characterise the nature of compound interactions and unravel synergistic or potentiating modes of action. The continued studies on this topic are of the utmost importance and we are still only at the beginning of investigating the chemical potential of the fungus-farming termite symbiosis. If given proper attention, the symbiosis holds great promise for the future of natural product drug discovery.
5. Author contributions
SS: conceptualization, data curation, methodology, formal analysis, validation, visualization, writing – original draft, writing – review & editing. SK: conceptualization, data curation, methodology, formal analysis, visualization, writing – original draft, writing – review & editing. HG: data curation, methodology, validation, writing – review & editing. CB: conceptualization, funding acquisition, validation, visualization, writing – original draft, writing – review & editing. MP: conceptualization, funding acquisition, project administration, resources, supervision, validation, visualization, writing – voriginal draft, writing – review & editing.
6. Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We thank members of the Social and Symbiotic Evolution group at the University of Copenhagen for comments on a previous version of the manuscript. This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – EXC 2051 – Project-ID 390713860 and CRC 1127 – Project-ID 239748522 to CB; and a European Research Council Consolidator Grant (ERC-CoG-771349) to MP.
Biographies
Biography
Suzanne Schmidt.

Suzanne Schmidt has a BSc in Biology and in 2018 obtained her MSc in Human Biology from the University of Copenhagen, Denmark. She did her MSc thesis work at the Section of Forensic Genetics at the Department of Forensic Medicine. In 2019, she joined Prof. Michael Poulsen's group at the Section for Ecology and Evolution, Department of Biology, University of Copenhagen as a PhD student, and her PhD project focuses on natural products produced by the fungus-farming termite fungal crop, Termitomyces.
Biography
Sara Kildgaard.

Dr Sara Kildgaard holds an MSc in Engineering in Advanced and Applied Chemistry from The Technical University of Denmark (DTU), where she also did her PhD in analytical natural product chemistry as part of the EU-funded project PharmaSea, with the thesis entitled Discovery of Marine Bioactive Natural Products. After a short postdoc at the Technical University of Denmark, she joined Prof. Michael Poulsen's group at the Section for Ecology and Evolution, Department of Biology, University of Copenhagen in 2018 to investigate chemical compounds of bacterial and fungal origins in fungus-farming termites. In 2020, she began her current position as a Research Scientist at Lundbeck.
Biography
Huijuan Guo.

Dr Huijuan Guo studied biotechnology in China from 2000 to 2004, and natural product chemistry at Institute of Microbiology, Chinese Academy of Science from 2004 to 2009. In 2009, she joined the group of Prof. Wilhelm Boland at Max-Planck-Institute for Chemical Ecology (MPI-CE) for her doctoral thesis in Chemistry. In 2014, she joined the group of Dr Christine Beemelmanns at the Hans-Knöll Institute (HKI) for her postdoc research. Her main research focuses on chemical mediator(s) from ecosystems and their pharmaceutical applications.
Biography
Christine Beemelmanns.

Christine Beemelmanns received her diploma in chemistry at the RWTH Aachen in 2006. After a one-year research stay in the group of Prof. Mikiko Sodeoka at RIKEN (Wako-Shi, Japan) she then performed her PhD under the supervision of Prof. Hans-Ulrich Reissig with a fellowship of the Fonds der Chemischen Industrie and the Studienstiftung des Deutschen Volkes. After two postdoctoral stay in the group of Prof. Keisuke Suzuki at the Tokyo Institute of Technology (Tokyo, Japan) and Prof. Jon Clardy at Harvard Medical School (USA), supported by fellowships from the German Exchange Service and German National Academy of Sciences Leopoldina, she joined the Leibniz Institute for Natural Product Research and Infection Biology (Jena, Germany) as a junior research group leader in the end of 2013. Her research focuses of the isolation, characterization and total synthesis of microbial signalling molecules.
Biography
Michael Poulsen.

Michael Poulsen is a Professor in the Evolution of Microbial Symbiosis at the Department of Biology, University of Copenhagen, Denmark. Poulsen received his MSc in biology from the Department of Biology, Århus University (Denmark) in 2001, and his PhD from the University of Copenhagen in 2005. He subsequently did five years of postdoc work with Prof. Cameron R. Currie at the Department of Bacteriology, UW-Madison (USA), and returned to the University of Copenhagen in 2010, where he leads the Social and Symbiotic Evolution Group and heads the Section for Ecology and Evolution. His work focuses on the evolutionary origin and stability of symbiosis, including defensive associations that involve natural products for antimicrobial defence.
Electronic supplementary information (ESI) available: Table 1: a comprehensive overview of the 375 natural products that have been discovered from termites, Termitomyces, bacteria, complex communities and Pseudoxylaria. These natural products were identified by an extensive screening of literature and is the first in-depth investigation into all natural products associated with this termite symbiosis. The table includes molecular formula, mass, compound name, natural product class, termite host (when known), producing organism, country the source material was found in, method of identification and the articles that have discovered the compound from sources related to the fungus-growing termites symbiosis. Subsequently, hundreds of articles were screened for antimicrobial activities linked to each individual natural product, or lack thereof. If possible, the presumed mode of action(s) and reported synergies are indicated as are other known bioactivities. The references associated to bioactivities, mode of action and synergies are reported in the “Reference” column indicated by (no.). An asterisk (*) Indicates that the compound was found from multiple symbiosis sources. See DOI: 10.1039/d1np00022e
8 Notes and references
- Macheleidt J. Mattern D. J. Fischer J. Netzker T. Weber J. Schroeckh V. Valiante V. Brakhage A. A. Annu. Rev. Genet. 2016;50:371–392. doi: 10.1146/annurev-genet-120215-035203. [DOI] [PubMed] [Google Scholar]
- Scherlach K. Hertweck C. Annu. Rev. Microbiol. 2020;74:267–290. doi: 10.1146/annurev-micro-012420-081224. [DOI] [PubMed] [Google Scholar]
- Hrcek J. McLean A. H. Godfray H. C. J Anim Ecol. 2016;85:1605–1612. doi: 10.1111/1365-2656.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T. Ido A. Kusano K. Miura C. Miura T. PLoS One. 2014;9:e114823. doi: 10.1371/journal.pone.0114823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain A. L. Appl. Microbiol. Biotechnol. 1999;52:455–463. doi: 10.1007/s002530051546. [DOI] [PubMed] [Google Scholar]
- Van Arnam E. B. Currie C. R. Clardy J. Chem. Soc. Rev. 2018;47:1638–1651. doi: 10.1039/C7CS00340D. [DOI] [PubMed] [Google Scholar]
- Adnani N. Rajski S. R. Bugni T. S. Nat. Prod. Rep. 2017;34:784–814. doi: 10.1039/C7NP00009J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine D. Holmes N. A. Worsley S. F. Santos A. C. A. Innocent T. M. Scherlach K. Patrick E. H. Yu D. W. Murrell J. C. Vieria P. C. Boomsma J. J. Hertweck C. Hutchings M. I. Wilkinson B. Nat. Commun. 2018;9:2208. doi: 10.1038/s41467-018-04520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre T., Rouland-Lefèvre C. and Aanen D. K., in Biology of Termites: a Modern Synthesis, ed. D. E. Bignell, Y. Roisin and N. Lo, Springer Netherlands, Dordrecht, 2011, pp. 193–210 [Google Scholar]
- Aanen D. K. Eggleton P. Curr. Biol. 2005;15:851–855. doi: 10.1016/j.cub.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Aanen D. K. Eggleton P. Rouland-Lefevre C. Guldberg-Froslev T. Rosendahl S. Boomsma J. J. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14887–14892. doi: 10.1073/pnas.222313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Peppel L. N., Auxier M., Grum-Grzhimaylo B., Cárdenas A., de Beer M., Lodge Z. W., Smith D. J., Kuyper M. E., Franco-Molano T., Baroni T. and Aanen D. K., 2021, 10.2139/ssrn.3828201 [DOI]
- Otani S. Hansen L. H. Sorensen S. J. Poulsen M. Microb. Ecol. 2016;71:207–220. doi: 10.1007/s00248-015-0692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa R. R. Hu H. Li H. Poulsen M. Insects. 2019;10:87. doi: 10.3390/insects10040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S. Challinor V. L. Kreuzenbeck N. B. Kildgaard S. Krath Christensen S. Larsen L. L. M. Aanen D. K. Rasmussen S. A. Beemelmanns C. Poulsen M. Sci. Rep. 2019;9:8819. doi: 10.1038/s41598-019-45364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk F. Gostincar C. Kreuzenbeck N. B. Conlon B. H. Sommerwerk E. Rabe P. Burkhardt I. Kruger T. Kniemeyer O. Brakhage A. A. Gunde-Cimerman N. de Beer Z. W. Dickschat J. S. Poulsen M. Beemelmanns C. mBio. 2021;12:e0355120. doi: 10.1128/mBio.03551-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. J. Young S. E. Poulsen M. Currie C. R. Annu. Rev. Entomol. 2021;66:297–316. doi: 10.1146/annurev-ento-040920-061140. [DOI] [PubMed] [Google Scholar]
- Poulsen M. Hu H. F. Li C. Chen Z. S. Xu L. H. Otani S. Nygaard S. Nobre T. Klaubauf S. Schindler P. M. Hauser F. Pan H. L. Yang Z. K. Sonnenberg A. S. M. de Beer Z. W. Zhang Y. Wingfield M. J. Grimmelikhuijzen C. J. P. de Vries R. P. Korb J. Aanen D. K. Wang J. Boomsma J. J. Zhang G. J. Proc. Natl. Acad. Sci. U. S. A. 2014;111:14500–14505. doi: 10.1073/pnas.1319718111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erens H. Mujinya B. B. Mees F. Baert G. Boeckx P. Malaisse F. Van Ranst E. Geoderma. 2015;249:40–50. doi: 10.1016/j.geoderma.2015.03.003. [DOI] [Google Scholar]
- Sileshi G. W. Arshad M. A. Konate S. Nkunika P. O. Y. J. Veg. Sci. 2010;21:923–937. doi: 10.1111/j.1654-1103.2010.01197.x. [DOI] [Google Scholar]
- Bos N. Guimaraes L. Palenzuela R. Renelies-Hamilton J. Maccario L. Silue S. K. Kone N. A. Poulsen M. BMC Evol. Biol. 2020;20:163. doi: 10.1186/s12862-020-01727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobotnik J. Jirosova A. Hanus R. J. Insect Physiol. 2010;56:1012–1021. doi: 10.1016/j.jinsphys.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Plasman V. Daloze D. Braekman J. C. Connetable S. Robert A. Bordereau C. Tetrahedron Lett. 1999;40:9229–9232. doi: 10.1016/S0040-4039(99)01961-9. [DOI] [Google Scholar]
- Mahamat O. André-Ledoux N. Chrisopher T. Mbifu A. A. Kamanyi A. Clin. Phytosci. 2018;4:28. doi: 10.1186/s40816-018-0089-4. [DOI] [Google Scholar]
- Beemelmanns C. Guo H. J. Rischer M. Poulsen M. Beilstein J. Org. Chem. 2016;12:314–327. doi: 10.3762/bjoc.12.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemelmanns C. Ramadhar T. R. Kim K. H. Klassen J. L. Cao S. Wyche T. P. Hou Y. Poulsen M. Bugni T. S. Currie C. R. Clardy J. Org. Lett. 2017;19:1000–1003. doi: 10.1021/acs.orglett.6b03831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskov Y., Ower G., Orrell T., Nicolson D., Bailly N., Kirk P. M., Bourgoin T., DeWalt R. E., Decock W., van Nieukerken E. J. P. and Journal L., Species 2000 & ITIS Catalogue of Life, 2028th March 2018. Digital resource at, Species 2000: Naturalis, Leiden, the Netherlands, 2021, ISSN 2405-8858, https://www.catalogueoflife.org [Google Scholar]
- Sieber R. Leuthold R. H. Ins. Soc. 1981;28:371–382. doi: 10.1007/BF02224194. [DOI] [Google Scholar]
- da Costa R. R. Vreeburg S. M. E. Shik J. Z. Aanen D. K. Poulsen M. Fungal Ecol. 2019;38:54–61. doi: 10.1016/j.funeco.2018.08.009. [DOI] [Google Scholar]
- Dietrich C. Kohler T. Brune A. Appl. Environ. Microbiol. 2014;80:2261–2269. doi: 10.1128/AEM.04206-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S. Mikaelyan A. Nobre T. Hansen L. H. Kone N. A. Sorensen S. J. Aanen D. K. Boomsma J. J. Brune A. Poulsen M. Mol. Ecol. 2014;23:4631–4644. doi: 10.1111/mec.12874. [DOI] [PubMed] [Google Scholar]
- Aanen D. K. Licht H. H. D. Debets A. J. M. Kerstes N. A. G. Hoekstra R. F. Boomsma J. J. Science. 2009;326:1103–1106. doi: 10.1126/science.1173462. [DOI] [PubMed] [Google Scholar]
- Katariya L. Ramesh P. B. Borges R. M. Environ. Microbiol. 2018;20:971–979. doi: 10.1111/1462-2920.14026. [DOI] [PubMed] [Google Scholar]
- Bodawatta K. H. Poulsen M. Bos N. Insects. 2019;10:185. doi: 10.3390/insects10070185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser A. A. Kooij P. W. Debets A. J. M. Kuyper T. W. Aanen D. K. Fungal Ecol. 2011;4:322–332. doi: 10.1016/j.funeco.2011.05.003. [DOI] [Google Scholar]
- Katariya L. Ramesh P. B. Sharma A. Borges R. M. Insect Soc. 2018;65:561–569. doi: 10.1007/s00040-018-0644-5. [DOI] [Google Scholar]
- Um S. Fraimout A. Sapountzis P. Oh D. C. Poulsen M. Sci. Rep. 2013;3:3250. doi: 10.1038/srep03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich G. D. Bierl B. A. Devilbiss E. D. Chaudhury M. F. B. J. Chem. Ecol. 1977;3:579–590. doi: 10.1007/BF00989078. [DOI] [Google Scholar]
- Lamberty M. Zachary D. Lanot R. Bordereau C. Robert A. Hoffmann J. A. Bulet P. J. Biol. Chem. 2001;276:4085–4092. doi: 10.1074/jbc.M002998200. [DOI] [PubMed] [Google Scholar]
- Hegedus N. Marx F. Fungal Biol. Rev. 2013;26:132–145. doi: 10.1016/j.fbr.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton C. Bulmer M. S. Dev. Comp. Immunol. 2012;36:372–377. doi: 10.1016/j.dci.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hamilton C. Lay F. Bulmer M. S. J. Insect Physiol. 2011;57:1259–1266. doi: 10.1016/j.jinsphys.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Landon C. Meudal H. Boulanger N. Bulet P. Vovelle F. Biopolymers. 2006;81:92–103. doi: 10.1002/bip.20370. [DOI] [PubMed] [Google Scholar]
- Ruther J. Podsiadlowski L. Hilker M. Chemoecology. 2001;11:225–229. doi: 10.1007/PL00001855. [DOI] [Google Scholar]
- Dahal G. P. Viola R. E. SLAS Discov. 2018;23:520–531. doi: 10.1177/2472555218767844. [DOI] [PubMed] [Google Scholar]
- Baker R. Briner P. H. Evans D. A. J. Chem. Soc. Chem. Comm. 1978;9:410–411. doi: 10.1039/C39780000410. [DOI] [Google Scholar]
- Mahdi D. H. Hubert J. Renault J. H. Martinez A. Schubert A. Engel K. M. Koudogbo B. Vissiennon Z. Ahyi V. Nieber K. Vissiennon C. Molecules. 2020;25:5015. doi: 10.3390/molecules25215015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalapathi A. Paulsamy S. Mycosphere. 2016;7:118–130. doi: 10.5943/mycosphere/7/2/3. [DOI] [Google Scholar]
- Kabara J. J. Swieczkowski D. M. Truant J. P. Conley A. J. Antimicrob. Agents Chemother. 1972;2:23–28. doi: 10.1128/AAC.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek S. N. A. Kanagasabapathy G. Sabaratnam V. Abdullah N. Yaacob H. Int. J. Food. Prop. 2012;15:809–814. doi: 10.1080/10942912.2010.506017. [DOI] [Google Scholar]
- Dilika F. Bremner P. D. Meyer J. J. Fitoterapia. 2000;71:450–452. doi: 10.1016/S0367-326X(00)00150-7. [DOI] [PubMed] [Google Scholar]
- Zheng C. J. Yoo J. S. Lee T. G. Cho H. Y. Kim Y. H. Kim W. G. Febs Lett. 2005;579:5157–5162. doi: 10.1016/j.febslet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Parsons J. B. Yao J. Frank M. W. Jackson P. Rock C. O. J. Bacteriol. 2012;194:5294–5304. doi: 10.1128/JB.00743-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway D. L. Dyke K. G. J. Gen. Microbiol. 1979;115:233–245. doi: 10.1099/00221287-115-1-233. [DOI] [PubMed] [Google Scholar]
- Sun M. Dong J. Xia Y. Shu R. Microb. Pathog. 2017;107:212–218. doi: 10.1016/j.micpath.2017.03.040. [DOI] [PubMed] [Google Scholar]
- Sun M. Zhou Z. Dong J. Zhang J. Xia Y. Shu R. Microb. Pathog. 2016;99:196–203. doi: 10.1016/j.micpath.2016.08.025. [DOI] [PubMed] [Google Scholar]
- Choi J. H. Maeda K. Nagai K. Harada E. Kawade M. Hirai H. Kawagishi H. Org. Lett. 2010;12:5012–5015. doi: 10.1021/ol102186p. [DOI] [PubMed] [Google Scholar]
- Choi J. H. Maeda K. Hirai H. Harada E. Kawade M. Qi J. H. Ojika M. Kawagishi H. Biosci. Biotech. Bioch. 2012;76:1407–1409. doi: 10.1271/bbb.120130. [DOI] [PubMed] [Google Scholar]
- Qi J. H. Ojika M. Sakagami Y. Tetrahedron. 2000;56:5835–5841. doi: 10.1016/S0040-4020(00)00548-2. [DOI] [Google Scholar]
- Qi J. H. Ojika M. Sakagami Y. Bioorg. Med. Chem. 2001;9:2171–2177. doi: 10.1016/S0968-0896(01)00125-0. [DOI] [PubMed] [Google Scholar]
- Qu Y. Sun K. Y. Gao L. J. Sakagami Y. Kawagishi H. Ojika M. Qi J. H. Biosci. Biotech. Bioch. 2012;76:791–793. doi: 10.1271/bbb.110918. [DOI] [PubMed] [Google Scholar]
- Mitra P. Mandal N. C. Acharya K. J. Verbraucherschutz Lebensmittelsicherh. 2016;11:25–31. doi: 10.1007/s00003-015-0976-2. [DOI] [Google Scholar]
- Mitra P. Mandal N. C. Acharya K. Int. Food Res. J. 2016;23:2384–2389. [Google Scholar]
- Puttaraju N. G. Venkateshaiah S. U. Dharmesh S. M. Urs S. M. N. Somasundaram R. J. Agr. Food. Chem. 2006;54:9764–9772. doi: 10.1021/jf0615707. [DOI] [PubMed] [Google Scholar]
- Taguri T. Tanaka T. Kouno I. Biol. Pharm. Bull. 2006;29:2226–2235. doi: 10.1248/bpb.29.2226. [DOI] [PubMed] [Google Scholar]
- Campos F. M. Couto J. A. Figueiredo A. R. Toth I. V. Rangel A. O. S. S. Hogg T. A. Int. J. Food. Microbiol. 2009;135:144–151. doi: 10.1016/j.ijfoodmicro.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Saavedra M. J. Borges A. Dias C. Aires A. Bennett R. N. Rosa E. S. Simoes M. Med. Chem. 2010;6:174–183. doi: 10.2174/1573406411006030174. [DOI] [PubMed] [Google Scholar]
- Borges A. Ferreira C. Saavedra M. J. Simoes M. Microb. Drug Resist. 2013;19:256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- Lima V. N. Oliveira-Tintino C. D. M. Santos E. S. Morais L. P. Tintino S. R. Freitas T. S. Geraldo Y. S. Pereira R. L. S. Cruz R. P. Menezes I. R. A. Coutinho H. D. M. Microb. Pathogenesis. 2016;99:56–61. doi: 10.1016/j.micpath.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Tinh T. H. Nuidate T. Vuddhakul V. Rodkhum C. Procedia Chem. 2016;18:162–168. doi: 10.1016/j.proche.2016.01.025. [DOI] [Google Scholar]
- Borokini F. Lajide L. Olaleye T. Boligon A. Athayde M. Adesina I. J. Microb. Biotec. Food. 2016;5:416–423. [Google Scholar]
- Gorniak I. Bartoszewski R. Kroliczewski J. Phytochem. Rev. 2019;18:241–272. doi: 10.1007/s11101-018-9591-z. [DOI] [Google Scholar]
- Adamczak A. Ozarowski M. Karpinski T. M. J. Clin. Med. 2020;9:109. doi: 10.3390/jcm9010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima H. Ashida H. Danno G. Biosci. Biotech. Bioch. 2002;66:1009–1014. doi: 10.1271/bbb.66.1009. [DOI] [PubMed] [Google Scholar]
- Sanver D. Murray B. S. Sadeghpour A. Rappolt M. Nelson A. L. Langmuir. 2016;32:13234–13243. doi: 10.1021/acs.langmuir.6b02219. [DOI] [PubMed] [Google Scholar]
- Amin M. U. Khurram M. Khattak B. Khan J. BMC Complementary Altern. Med. 2015;15:59. doi: 10.1186/s12906-015-0580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriyanarayanan B. Shanmugam K. Santhosh R. S. Rom. Biotechnol. Lett. 2013;18:8587–8593. [Google Scholar]
- Suriyanarayanan B. Santhosh R. S. J. Biomol. Struct. Dyn. 2015;33:1819–1834. doi: 10.1080/07391102.2014.974211. [DOI] [PubMed] [Google Scholar]
- Biharee A. Sharma A. Kumar A. Jaitak V. Fitoterapia. 2020;146:104720. doi: 10.1016/j.fitote.2020.104720. [DOI] [PubMed] [Google Scholar]
- Zhang L. Kong Y. Wu D. Zhang H. Wu J. Chen J. Ding J. Hu L. Jiang H. Shen X. Protein Sci. 2008;17:1971–1978. doi: 10.1110/ps.036186.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu A. C. Serra S. C. Borges A. Saavedra M. J. McBain A. J. Salgado A. J. Simoes M. Microb. Drug Resist. 2015;21:600–609. doi: 10.1089/mdr.2014.0252. [DOI] [PubMed] [Google Scholar]
- Siriwong S. Teethaisong Y. Thumanu K. Dunkhunthod B. Eumkeb G. BMC Pharmacol. Toxicol. 2016;17:39. doi: 10.1186/s40360-016-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vipin C. Saptami K. Fida F. Mujeeburahiman M. Rao S. S. Athmika Arun A. B. Rekha P. D. PLoS One. 2020;15:e0241304. doi: 10.1371/journal.pone.0241304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreyohannes G. Nyerere A. Bii C. Sbhatu D. B. Sci. World J. 2019;2019:7357048. doi: 10.1155/2019/7357048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho P. L. N. Silva E. D. Chagas-Paula D. A. Luiz J. H. H. Ikegaki M. Mini-Rev. Med. Chem. 2016;16:259–271. doi: 10.2174/1389557515666151016123923. [DOI] [PubMed] [Google Scholar]
- Tohge T. Watanabe M. Hoefgen R. Fernie A. R. Front. Plant Sci. 2013;4:62. doi: 10.3389/fpls.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanta T. K. J. Funct. Foods. 2020;68:103910. doi: 10.1016/j.jff.2020.103910. [DOI] [Google Scholar]
- Eko Kuswanto I. A. Putra R. E. Harahap I. S. J. Entomol. 2015;12:87–94. doi: 10.3923/je.2015.87.94. [DOI] [Google Scholar]
- Yang G. Ahmad F. Liang S. Fouad H. Guo M. Gaal H. A. Mo J. Appl. Biochem. Biotechnol. 2020;192:1270–1283. doi: 10.1007/s12010-020-03376-w. [DOI] [PubMed] [Google Scholar]
- Nyegue M. Zollo P.-H. A. Bessière J.-M. Rapior s. J. Essent. Oil-Bear. Plants. 2003;6:153–160. doi: 10.1080/0972-060X.2003.10643344. [DOI] [Google Scholar]
- Reyes B. A. S. Dufourt E. C. Ross J. Warner M. J. Tanquilut N. C. Leung A. B. Stud. Nat. Prod. Chem. 2018;55:111–143. [Google Scholar]
- Guimaraes A. C. Meireles L. M. Lemos M. F. Guimaraes M. C. C. Endringer D. C. Fronza M. Scherer R. Molecules. 2019;24:2471. doi: 10.3390/molecules24132471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt I. Kreuzenbeck N. B. Beemelmanns C. Dickschat J. S. Org. Biomol. Chem. 2019;17:3348–3355. doi: 10.1039/C8OB02744G. [DOI] [PubMed] [Google Scholar]
- Bonikowski R. Switakowska P. Sienkiewicz M. Zaklos-Szyda M. Molecules. 2015;20:11272–11296. doi: 10.3390/molecules200611272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva A. C. R. Lopes P. M. de Azevedo M. M. B. Costa D. C. M. Alviano C. S. Alviano D. S. Molecules. 2012;17:6305–6316. doi: 10.3390/molecules17066305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M. Ogura T. Kusama Y. Iwabuchi N. Imawaka T. Araki A. Sasaki T. Hirose E. Sunairi M. Water Res. 1996;30:2508–2511. doi: 10.1016/0043-1354(96)00104-2. [DOI] [Google Scholar]
- Kovac J. Simunovic K. Wu Z. W. Klancnik A. Bucar F. Zhang Q. J. Mozina S. S. Plos One. 2015;10:e0122871. doi: 10.1371/journal.pone.0122871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajini K. S. Aparna P. Sasikala C. Ramana Ch V. Crit. Rev. Microbiol. 2011;37:99–112. doi: 10.3109/1040841X.2010.512267. [DOI] [PubMed] [Google Scholar]
- Woolfson A. Rothschild M. Proc. Royal Soc. B. 1990;242:113–119. doi: 10.1098/rspb.1990.0113. [DOI] [PubMed] [Google Scholar]
- Mortzfeld F. B. Hashem C. Vrankova K. Winkler M. Rudroff F. Biotechnol. J. 2020:15. [Google Scholar]
- Schulz-Bohm K. Martin-Sanchez L. Garbeva P. Front. Microbiol. 2017;8:2484. doi: 10.3389/fmicb.2017.02484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R. Rappert S. Appl. Microbiol. Biotechnol. 2010;85:1315–1320. doi: 10.1007/s00253-009-2362-4. [DOI] [PubMed] [Google Scholar]
- Rappert S. Botsch K. C. Nagorny S. Francke W. Muller R. Appl. Environ. Microbiol. 2006;72:1437–1444. doi: 10.1128/AEM.72.2.1437-1444.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meer R. K. V. Preston C. A. Choi M. Y. J. Chem. Ecol. 2010;36:163–170. doi: 10.1007/s10886-010-9743-0. [DOI] [PubMed] [Google Scholar]
- Bi S. F. Guo Z. K. Jiang N. Jiao R. H. Ge H. M. Tan R. X. J. Asian Nat. Prod. Res. 2013;15:422–425. doi: 10.1080/10286020.2013.767246. [DOI] [PubMed] [Google Scholar]
- Bi S. F. Li F. Song Y. C. Tan R. X. Ge H. M. Nat. Prod. Commun. 2011;6:353–355. doi: 10.1177/1934578X1100600310. [DOI] [PubMed] [Google Scholar]
- Yin C. Jin L. Li S. Xu X. Zhang Y. 3 Biotech. 2019;9:45. doi: 10.1007/s13205-019-1573-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew G. M. Ju Y. M. Lai C. Y. Mathew D. C. Huang C. C. FEMS Microbiol. Ecol. 2012;79:504–517. doi: 10.1111/j.1574-6941.2011.01232.x. [DOI] [PubMed] [Google Scholar]
- Aparicio J. F. Molnar I. Schwecke T. Konig A. Haydock S. F. Khaw L. E. Staunton J. Leadlay P. F. Gene. 1996;169:9–16. doi: 10.1016/0378-1119(95)00800-4. [DOI] [PubMed] [Google Scholar]
- Weng J. K. Noel J. P. Methods Enzymol. 2012;515:317–335. doi: 10.1016/B978-0-12-394290-6.00014-8. [DOI] [PubMed] [Google Scholar]
- Kim K. H. Ramadhar T. R. Beemelmanns C. Cao S. G. Poulsen M. Currie C. R. Clardy J. Chem. Sci. 2014;5:4333–4338. doi: 10.1039/C4SC01136H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen J. L. Lee S. R. Poulsen M. Beemelmanns C. Kim K. H. Front. Microbiol. 2019;10:1739. doi: 10.3389/fmicb.2019.01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi S. Rezaie S. Ghazvini R. D. Hashemi S. J. Badali H. Foroumadi A. Diba K. Chowdhary A. Meis J. F. Khodavaisy S. Mycopathologia. 2019;184:607–613. doi: 10.1007/s11046-019-00370-7. [DOI] [PubMed] [Google Scholar]
- Jia C. Zhang J. Zhuge Y. Z. Xu K. Liu J. H. Wang J. L. Li L. Chu M. P. Free Radical Res. 2019;53:618–628. doi: 10.1080/10715762.2019.1610563. [DOI] [PubMed] [Google Scholar]
- Fang A. Q. Wong G. K. Demain A. L. J. Antibiot. 2000;53:158–162. doi: 10.7164/antibiotics.53.158. [DOI] [PubMed] [Google Scholar]
- Ochel H. J. Eichhorn K. Gademann G. Cell Stress Chaperones. 2001;6:295. doi: 10.1379/1466-1268(2001)006<0295:>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova J. Dencheva-Zarkova M. J. Phys. Conf. 2017;794:012031. doi: 10.1088/1742-6596/794/1/012031. [DOI] [Google Scholar]
- Ohji G. Hidayat S. Nakashima A. Tokunaga C. Oshiro N. Yoshino K. Yokono K. Kikkawa U. Yonezawa K. J. Biochem. 2006;139:129–135. doi: 10.1093/jb/mvj008. [DOI] [PubMed] [Google Scholar]
- McLean T. C. Wilkinson B. Hutchings M. I. Devine R. Antibiotics. 2019;8:83. doi: 10.3390/antibiotics8020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M. Yin M. Wu S. Han X. Ji K. Wen M. Lu T. Sci. Rep. 2017;7:4803. doi: 10.1038/s41598-017-05073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H. Benndorf R. Leichnitz D. Klassen J. L. Vollmers J. Görls H. Steinacker M. Weigel C. Dahse H. M. Kaster A. K. de Beer Z. W. Poulsen M. Beemelmanns C. Chem. -Eur. J. 2017;23:9338–9345. doi: 10.1002/chem.201701005. [DOI] [PubMed] [Google Scholar]
- Yan Y. Ma Y. T. Yang J. Horsman G. P. Luo D. Ji X. Huang S. X. Org. Lett. 2016;18:1254–1257. doi: 10.1021/acs.orglett.6b00074. [DOI] [PubMed] [Google Scholar]
- Li L. L. Li S. F. Jiang B. Y. Zhang M. Q. Zhang J. P. Yang B. B. Li L. Yu L. Y. Liu H. Y. You X. F. Hu X. X. Wang Z. Li Y. H. Wu L. Z. J. Nat. Prod. 2019;82:1149–1154. doi: 10.1021/acs.jnatprod.8b00857. [DOI] [PubMed] [Google Scholar]
- Guo H. Schwitalla J. W. Benndorf R. Baunach M. Steinbeck C. Görls H. de Beer Z. W. Regestein L. Beemelmanns C. Org. Lett. 2020;22:2634–2638. doi: 10.1021/acs.orglett.0c00601. [DOI] [PubMed] [Google Scholar]
- Benndorf R. Guo H. J. Sommerwerk E. Weigel C. Garcia-Altares M. Martin K. Hu H. F. Küfner M. de Beer Z. W. Poulsen M. Beemelmanns C. Antibiotics. 2018;7:83. doi: 10.3390/antibiotics7030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H. Feng P. X. Wan C. X. Nat. Prod. Res. 2019;33:1751–1755. doi: 10.1080/14786419.2018.1431630. [DOI] [PubMed] [Google Scholar]
- Boya C. A. Fernandez-Marin H. Mejia L. C. Spadafora C. Dorrestein P. C. Gutierrez M. Sci. Rep. 2017;7:5604. doi: 10.1038/s41598-017-05515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenian I. Spiteller M. Ghaste M. Wirth R. Herz H. Spiteller D. Proc. Natl. Acad. Sci. U. S. A. 2011;108:1955–1960. doi: 10.1073/pnas.1008441108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan C. N. Medoff G. Kobayashi G. S. Schlessinger D. Raskas H. J. Antimicrob. Agents Chemother. 1972;2:61–65. doi: 10.1128/AAC.2.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo K. Saito N. Otsuki K. Kikuchi M. Ishida N. J. Antibiot. 1971;24:907–910. doi: 10.7164/antibiotics.24.907. [DOI] [PubMed] [Google Scholar]
- Lee S. R. Lee D. Yu J. S. Benndorf R. Lee S. Lee D. S. Huh J. de Beer Z. W. Kim Y. H. Beemelmanns C. Kang K. S. Kim K. H. Molecules. 2018;23:3003. doi: 10.3390/molecules23113003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich E. J. N. Piel J. Nat. Prod. Rep. 2016;33:231–316. doi: 10.1039/C5NP00125K. [DOI] [PubMed] [Google Scholar]
- Moldenhauer J. Chen X. H. Borriss R. Piel J. Angew. Chem. Int. Ed. 2007;46:8195–8197. doi: 10.1002/anie.200703386. [DOI] [PubMed] [Google Scholar]
- Patel P. S. Huang S. Fisher S. Pirnik D. Aklonis C. Dean L. Meyers E. Fernandes P. Mayerl F. J. Antibiot. 1995;48:997–1003. doi: 10.7164/antibiotics.48.997. [DOI] [PubMed] [Google Scholar]
- Nonejuie P. Trial R. M. Newton G. L. Lamsa A. Perera V. R. Aguilar J. Liu W. T. Dorrestein P. C. Pogliano J. Pogliano K. J. Antibiot. 2016;69:353–361. doi: 10.1038/ja.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyche T. P. Ruzzini A. C. Beemelmanns C. Kim K. H. Klassen J. L. Cao S. G. Poulsen M. Bugni T. S. Currie C. R. Clardy J. Org. Lett. 2017;19:1772–1775. doi: 10.1021/acs.orglett.7b00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D. C. Poulsen M. Currie C. R. Clardy J. Nat. Chem. Biol. 2009;5:391–393. doi: 10.1038/nchembio.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D. Byun W. S. Moon K. Kwon Y. Bae M. Um S. Lee S. K. Oh D. C. Front. Chem. 2018;6:498. doi: 10.3389/fchem.2018.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S. Li S. F. Wu W. Zhao F. Bao L. Ding R. Gao H. Wen H. A. Song F. H. Liu H. W. Chem. Biodivers. 2011;8:1689–1700. doi: 10.1002/cbdv.201100026. [DOI] [PubMed] [Google Scholar]
- Guo H. Kreuzenbeck N. B. Otani S. Garcia-Altares M. Dahse H. M. Weigel C. Aanen D. K. Hertweck C. Poulsen M. Beemelmanns C. Org. Lett. 2016;18:3338–3341. doi: 10.1021/acs.orglett.6b01437. [DOI] [PubMed] [Google Scholar]
- Schalk F. Um S. Guo H. Kreuzenbeck N. B. Gorls H. de Beer Z. W. Beemelmanns C. Chembiochem. 2020;21:2991–2996. doi: 10.1002/cbic.202000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. Nong X. H. Huang Z. H. Qi S. H. J. Agr. Food. Chem. 2017;65:5114–5121. doi: 10.1021/acs.jafc.7b01238. [DOI] [PubMed] [Google Scholar]
- Kijima M. Yoshida M. Sugita K. Horinouchi S. Beppu T. J. Biol. Chem. 1993;268:22429–22435. doi: 10.1016/S0021-9258(18)41547-5. [DOI] [PubMed] [Google Scholar]
- Subekti N. Fibriana F. Widyaningrum P. Adfa M. Ukr. Biochem. J. 2017;89:77–82. doi: 10.15407/ubj89.04.077. [DOI] [Google Scholar]
- Ding K. Tan Y. Y. Ding Y. Fang Y. Yang X. Fang J. Xu D. C. Zhang H. Lu W. Li M. Huang S. C. Cai M. L. Song Y. Ding Y. J. Zhang S. M. J. Cell. Biochem. 2019;120:5687–5694. doi: 10.1002/jcb.27853. [DOI] [PubMed] [Google Scholar]
- Kiprono P. C. Kaberia F. Keriko J. M. Karanja J. N. Z. Naturforsch., C: J. Biosci. 2000;55:485–488. doi: 10.1515/znc-2000-5-629. [DOI] [PubMed] [Google Scholar]
- Brucker R. M. Harris R. N. Schwantes C. R. Gallaher T. N. Flaherty D. C. Lam B. A. Minbiole K. P. C. J. Chem. Ecol. 2008;34:1422–1429. doi: 10.1007/s10886-008-9555-7. [DOI] [PubMed] [Google Scholar]
- Eom S. H. Kang S. K. Lee D. S. Myeong J. I. Lee J. Kim H. W. Kim K. H. Je J. Y. Jung W. K. Kim Y. M. J. Microbiol. Biotechnol. 2016;26:784–789. doi: 10.4014/jmb.1511.11046. [DOI] [PubMed] [Google Scholar]
- Shin B. Park C. Imlay J. A. Park W. Appl. Microbiol. Biotechnol. 2018;102:2323–2335. doi: 10.1007/s00253-018-8791-1. [DOI] [PubMed] [Google Scholar]
- Kim J. H. Chan K. L. Mahoney N. Campbell B. C. Ann. Clin. Microbiol. Antimicrob. 2011;10:23. doi: 10.1186/1476-0711-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H. Mahoney N. Chan K. L. Molyneux R. J. May G. S. Campbell B. C. FEMS Microbiol. Lett. 2008;281:64–72. doi: 10.1111/j.1574-6968.2008.01072.x. [DOI] [PubMed] [Google Scholar]
- Himejima M. Kubo I. J. Agr. Food. Chem. 1993;41:1776–1779. doi: 10.1021/jf00034a048. [DOI] [Google Scholar]
- Cain C. C. Lee D. H. Waldo R. H. Henry A. T. Casida E. J. Wani M. C. Wall M. E. Oberlies N. H. Falkinham J. O. Antimicrob. Agents Chemother. 2003;47:2113–2117. doi: 10.1128/AAC.47.7.2113-2117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. Yeh P. J. J. Antibiot. 2017;70:1033–1042. doi: 10.1038/ja.2017.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. Benndorf R. de Beer Z. W. Vollmers J. Kaster A.-K. Beemelmanns C. Poulsen M. mSphere. 2021;6:e01233-20. doi: 10.1128/mSphere.01233-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y. Yu Z. Y. Zhao J. W. Zhuang X. X. Cao P. Guo X. W. Liu C. X. Xiang W. S. Front. Microbiol. 2020;11:201. doi: 10.3389/fmicb.2020.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. J. Sosa-Calvo J. Horn H. A. Pupo M. T. Clardy J. Rabeling C. Schultz T. R. Currie C. R. Proc. Natl. Acad. Sci. U. S. A. 2018;115:10720–10725. doi: 10.1073/pnas.1809332115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noknoy R. Sunantaraporn S. Phumee A. Siriyasatien P. Sanguansub S. Insects. 2020;11:318. doi: 10.3390/insects11050318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. Myrmecol. News. 2016;22:65–108. [Google Scholar]
- Liu G. C. Chen G. Zootaxa. 2019;4551:237–243. doi: 10.11646/zootaxa.4551.2.8. [DOI] [PubMed] [Google Scholar]
- Zran P. Yoro T. Dabonné S. N'Guessan K. Int. J. Approx. Reason. 2017;5:46–59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.