Abstract
Enhanced surveillance of patients with upper respiratory symptoms in a Northern Plains community revealed that approximately 4% of them were infected by toxigenic Corynebacterium diphtheriae of both mitis and gravis biotypes, showing that the organism is still circulating in the United States. Toxigenic C. diphtheriae was isolated from five members of four households. Four molecular subtyping methods—ribotyping, multilocus enzyme electrophoresis (MEE), random amplified polymorphic DNA (RAPD), and single-strand conformation polymorphism—were used to molecularly characterize these strains and compare them to 17 archival South Dakota strains dating back to 1973 through 1983 and to 5 isolates collected from residents of diverse regions of the United States. Ribotyping and RAPD clearly demonstrated the household transmission of isolates and provided precise information on the circulation of several distinct strains within three households. By MEE, most recent and archival South Dakota strains were identified as closely related and clustered within the newly identified ET (electrophoretic type) 215 complex. Furthermore, three recent South Dakota isolates and eight archival South Dakota isolates were indistinguishable by both ribotyping and RAPD. All of these molecular methods showed that recent South Dakota isolates and archival South Dakota isolates were more closely related to each other than to the C. diphtheriae strains isolated in other parts of the United States or worldwide. The data also supported the improbability of importation of C. diphtheriae into this area and rather strongly suggest the long-term persistence of the organism in this region.
With the inclusion of the diphtheria vaccine in routine infant immunization programs, the number of reported cases of diphtheria dramatically declined worldwide. In 1921, more than 200,000 cases of diphtheria were reported in the United States, but following the introduction of the diphtheria vaccine in the mid-1920s, the number of cases was greatly reduced. By 1945, the case count had dropped to 19,000 (1). This decline continued until the mid-1960s, when there was a small resurgence in diphtheria incidence. From 1971 to 1981, close to 1,300 cases were reported, including seven outbreaks of more than 15 cases each (4). The total number of cases reported between 1988 and June 1998 declined to less than 50 (1, 3, 4); 8 of these were linked to importation of Corynebacterium diphtheriae into the United States. However, while the number of cases reported in the United States has significantly declined, studies of diphtheria immunity levels among adults in the United States have shown that many adults (from 20 to 90%) do not possess adequate immunity against this disease. This, in combination with circulation of toxigenic strains, could result in a resurgence of the disease (1). In the summer of 1996, a 61-year-old American Indian woman in the Northern Plains region of the United States was hospitalized for alcohol intoxication and severe necrotizing ulcers on both legs. Even though no cultures were obtained from the skin lesions during her hospitalization, toxigenic C. diphtheriae was isolated from her blood culture, which had been drawn because she was unresponsive and her general condition was described as severe (3).
The discovery of this organism prompted public health officials to conduct enhanced diphtheria surveillance in the patient’s community; five C. diphtheriae isolates were recovered from the throat cultures of patients with acute pharyngitis, and a single strain was recovered from the ear drainage of a patient with otitis media. Four additional isolates were cultured from the throat cultures of four healthy household contacts in three households. These isolates were collected by the local public health nurses and the South Dakota Department of Health staff who visited each household where culture-positive patients were identified.
Given that diphtheria remained endemic in South Dakota through the 1970s, with reported incidence rates of >1.0 per million population, we wanted to characterize these 11 isolates collected in 1996 molecularly and to compare them to isolates selected from the same geographic region approximately 20 years earlier. Our hypothesis was that the 1996 isolates were not the result of the importation and that C. diphtheriae has endemically persisted in this region for more than 20 years. Single-strand conformation polymorphism (SSCP), random amplified polymorphic DNA (RAPD), multilocus enzyme electrophoresis (MEE), and ribotyping were chosen because these methods have been shown previously to provide useful molecular subtyping data in epidemiologic studies of C. diphtheriae and have recently allowed us to identify the epidemic clone associated with the current diphtheria epidemic in Russia and the New Independent States (NIS) of the former Soviet Union (12, 15, 16).
MATERIALS AND METHODS
Isolates. (i) Clinical isolates.
A total of 11 clinical isolates were included in this study; a single isolate was obtained from the routine blood culture of a 61-year-old American Indian woman hospitalized for alcohol intoxication and infected leg ulcers, and 10 isolates were recovered as a result of the subsequent enhanced diphtheria surveillance in her American Indian community in South Dakota. The surveillance was carried out by the Public Health Service Indian Hospital. This hospital, including its satellite clinics, is the only health care facility in this community. Clinical specimens (throat, skin, and swabs of ear drainage) from all patients presenting with pharyngitis, draining middle-ear infections, and skin lesions between 1 August 1996 to 7 October 1996 were collected. The condition of only one patient met the clinical definition of diphtheria (isolate PR101 was isolated from this patient) (2). All swabs were streaked onto a Tinsdale medium and a blood agar plate (tryptic soy agar with 5% sheep blood; Becton Dickinson Microbiology Systems, Cockeysville, Md.). Each swab was also inoculated in heart infusion broth. All media were incubated for 24 to 48 h at 37°C. C. diphtheriae was identified according to current World Health Organization recommendations (8).
(ii) Archival isolates.
Seventeen archival isolates collected from diphtheria patients and carriers from South Dakota between 1973 and 1983 were assayed. For comparative purposes, five archival isolates obtained from four other states were also included: Alaska (two isolates, isolated in 1978), California (one isolate, isolated in 1979), Colorado (one isolate, isolated in 1978), and New Mexico (one isolate, isolated in 1977). All strains were stored in sterile defibrinated sheep blood at −70°C until needed.
Toxigenicity status.
The Elek test detects toxin production by the organism in an immunoprecipitation format so that lines of precipitation form between the diphtheria toxin antibodies and the toxin secreted by the test organism. This assay was performed for all strains as previously described (8).
PCR for detection of the diphtheria toxin gene, tox.
The PCR assay used detects a 249-bp fragment of the A subunit and a 297-bp fragment of the B subunit of the diphtheria toxin gene, tox. All isolates were assayed for the presence of both A and B subunits of the tox gene (14).
Molecular subtyping. (i) Ribotyping.
DNA was extracted from all isolates by the universal DNA isolation procedure (9). Briefly, overnight growth of isolates was collected from blood agar plates, transferred to polypropylene tubes containing 1× SSC (1× SSC is 0.15 M NaCl, plus 0.015 M sodium citrate), and spun down at 5,000 × g for 15 min at 4°C. Cells were lysed with 0.75 mg of lysozyme incubated at 37°C for 75 min and incubated for an additional hour at 37°C after the addition of 20 μl of proteinase K (20 mg/ml). DNA was extracted by phenol/chloroform purification and precipitated with ethanol. Subsequent steps were performed as previously described (16) with the following modifications. Electrophoresis of fragments restricted with BstEII was conducted on a 1% (wt/vol) agarose gel in 1× Tris-acetate-EDTA (TAE) at 75 V for 18 h. Hybridization by using five oligonucleotide probes at 37°C for 4 h and posthybridization washes were performed as recently described by Regnault et al. (18). Colorimetric detection was done by using the DIG Wash and Block Buffer Set (Boehringer Mannheim Biochemicals, Indianapolis, Ind.), sheep anti-digoxigenin antibody conjugated with alkaline phosphatase to detect digoxigenin-labeled nucleic acid fragments, and 4-nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (BCIP) to produce a blue precipitate to visualize fragments.
Ribotyping designations were based on the ribotyping scheme proposed earlier by Popovic et al. (16). A difference in one band was defined as an individual ribotype. When pattern resemblances between test strains and established type strains were observed, the ribotype designation of the test strain was given as a variant of the type strain pattern that a test strain resembled (e.g., the designation M13a signifies similarity to M13, and the designations M11a and M11b denote similarities to M11; the use of the designations a and b indicates that a minor difference in the ribotyping pattern between the test strains exists). Strains were given new designations if the obtained patterns did not resemble previously established type strain ribotypes.
(ii) RAPD.
All isolates were assayed by RAPD. One loopful of overnight growth was suspended in 200 μl of Tris buffer and boiled at 95°C for 20 min. One microliter (5 to 50 ng) of supernatant was used in the reaction. For each sample, in addition to DNA, 0.5 μl of Clontech Advantage cDNA polymerase (Clontech, Palo Alto, Calif.), 25 pmol of primer 3 from Ready-to-Go RAPD kit (Pharmacia Biotech, Piscataway, N.J.), 0.5 μl of 50× deoxynucleoside triphosphate (0.2 mM final concentration for each deoxynucleoside triphosphate), 2.5 μl of 10× Clontech Advantage cDNA reaction buffer (Clontech), and 19.5 μl of milli-Q water were used. Each sample was initially denatured at 95°C for 1 min and subjected to 35 amplification cycles at 94°C for 15 s, 36°C for 30 s, and 72°C for 3 min. Amplified products were electrophoresed on 0.70% SeaKem GTG agarose gel (FMC Corp., Philadelphia, Pa.) with 0.65% Synergel (Diversified Biotech, Boston, Mass.), stained with ethidium bromide, and visualized under UV light.
(iii) MEE.
Twenty-six of the 33 isolates were assayed by MEE, as previously described (16). MEE detects amino acid substitutions affecting charge and conformation in cellular housekeeping enzymes. Such mobility variants, or electromorphs, of the same enzyme are visualized in a starch gel matrix as bands with different migration rates. Each electromorph was considered to represent a distinct allele of that enzyme. By testing 27 different enzymes, a profile of electromorphs, which defined the electrophoretic type (ET) of each strain, was obtained. The genetic relatedness of the ETs was illustrated as a dendrogram, which was generated by the average-linkage method of clustering the ETs, as described by Selander et al. (19), using an SAS macroprogram described by Jacobs (13).
(iv) SSCP.
SSCP was performed on 22 of the 33 strains, as described earlier (15). In this study, we focused on two regions, designated regions 6 and 8, within the B subunit of the tox gene. All SSCP regions and patterns were previously described by Nakao et al. (15).
RESULTS
Of the 11 clinical isolates included in this study, 10 strains were isolated as a part of the diphtheria surveillance conducted in 1996, and 5 of these were biotype mitis. Two of these strains were toxigenic. The single toxigenic C. diphtheriae isolate obtained from the blood culture of the index patient was also biotype mitis. The remaining five isolates were of gravis biotype; three of these were toxigenic (Table 1).
TABLE 1.
Characteristics of 33 C. diphtheriae strains from five states isolated between 1973 and 1996
| Ribotype | RAPD pattern | Strain designation | Yr of isolation | State of isolation | Biotypea | Toxigenicity
|
SSCP type
|
ET | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Elek assay | PCR | Region 6 | Region 8 | |||||||
| M7a | 1 | C5214 | 1973 | S.Dak. | M | − | − | 216 | ||
| 2 | G4219 | 1978 | Alaska | M | + | + | 1 | 2 | 35 | |
| 2 | G4220 | 1978 | Alaska | M | + | + | 1 | 2 | 35 | |
| M11a | 3 | E8277 | 1980 | S.Dak. | G | + | + | 3 | 2 | 215 |
| 4 | D7643 | 1979 | S.Dak. | G | + | + | 3 | 2 | 215 | |
| 5 | C4943 | 1973 | S.Dak. | G | + | + | 3 | 2 | 215 | |
| 3 | E5276 | 1973 | S.Dak. | G | + | + | 3 | 2 | 218 | |
| 5 | E5449 | 1979 | S.Dak. | G | + | + | 3 | 2 | 40 | |
| 5 | G4218 | 1978 | Colo. | G | + | + | 3 | 2 | 34 | |
| M11b | 6 | PR110 | 1996 | S.Dak. | M | + | + | 1 | 1 | 228 |
| 6 | PR115 | 1996 | S.Dak. | M | + | + | 1 | 1 | 228 | |
| 6 | UT6-96 | 1996 | S.Dak. | M | + | + | 1 | 2 | 226 | |
| M11c | 7 | C5276 | 1973 | S.Dak. | M | + | + | 1 | 1 | 217 |
| M11d | 8 | PR79 | 1996 | S.Dak. | G | + | + | 3 | 2 | 225 |
| 8 | PR109 | 1996 | S.Dak. | G | + | + | 3 | 2 | 221 | |
| M1a | 9 | PR20 | 1996 | S.Dak. | M | − | + | 1 | 1 | 222 |
| 9 | PR101 | 1996 | S.Dak. | M | − | + | 1 | 1 | 221 | |
| 9 | PR130 | 1996 | S.Dak. | M | − | + | 1 | 1 | 229 | |
| 9 | E5340 | 1979 | S.Dak. | M | − | + | 1 | 1 | 219 | |
| 9 | E7798 | 1980 | S.Dak. | M | − | + | 1 | 1 | 220 | |
| 9 | E8392 | 1980 | S.Dak. | M | − | + | NDb | ND | ND | |
| 9 | E9026 | 1980 | S.Dak. | M | − | + | ND | ND | ND | |
| 9 | F1788 | 1981 | S.Dak. | M | − | + | ND | ND | ND | |
| 9 | F1803 | 1981 | S.Dak. | M | − | + | ND | ND | ND | |
| 9 | F2726 | 1982 | S.Dak. | M | − | + | ND | ND | ND | |
| 9 | F4306 | 1983 | S.Dak. | M | − | + | ND | ND | ND | |
| 10 | E9134 | 1980 | S.Dak. | M | − | + | ND | ND | ND | |
| M15 | 11 | D7920 | 1979 | S.Dak. | I | + | + | ND | 38 | |
| G4221 | 1977 | N.Mex. | I | + | + | 1 | 2 | 36 | ||
| M16 | 12 | G4217 | 1979 | Calif. | M | + | + | 3 | 2 | 33 |
| G3a | 13 | PR26 | 1996 | S.Dak. | G | − | − | ND | ND | 223 |
| G3b | 13 | PR120 | 1996 | S.Dak. | G | − | − | ND | ND | 230 |
| G5 | 3 | PR75 | 1996 | S.Dak. | G | + | + | 3 | 2 | 224 |
M, mitis; I, intermedius; G, gravis.
ND, not done.
Among the archival isolates, 14 of the 22 strains assayed were biotype mitis (5 were toxigenic, and 9 were nontoxigenic). Six strains were biotype gravis (all were toxigenic), and the remaining 2 strains were of intermedius biotype (both were toxigenic) (Table 1).
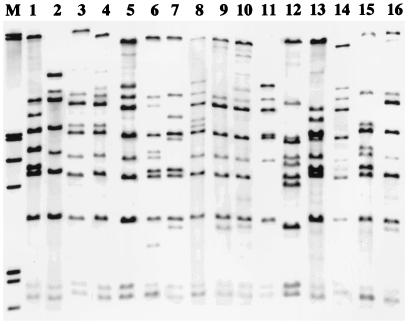
Ribotyping of all 33 strains resulted in 11 different ribotypes (Fig. 1). No ribotypes were identical to those described in the ribotyping scheme proposed earlier by Popovic et al. (16). Eight ribotypes observed among the test strains resembled established ribotypes and were designated accordingly. Twelve strains, all originating from South Dakota, were ribotype M1a. Three of these 12 strains were recent isolates, and the remaining 9 were archival isolates isolated in the 1970s and 1980s. Four distinct ribotypes similar to M11 were observed. Six strains were ribotype M11a, and five of these had also been isolated from residents of South Dakota between 1973 and 1980; the single exception was a strain isolated from a resident of Colorado in 1978. Three recent strains from South Dakota were ribotype M11b. In addition, two recent strains were ribotype M11d, and a single archival isolate was identified as M11c. Finally, three isolates isolated in the 1970s resembled M7, while two isolates (identified as G3a and G3b) were observed to have similarities to ribotype G3. The four remaining strains produced three new ribotypes.
FIG. 1.
Eleven BstEII ribotypes identified among 33 C. diphtheriae strains isolated in the United States from 1973 to 1996. Lane M, molecular weight marker; lane 1, ribotype M7a; lane 2, M11a; lane 3, M11b; lane 4, M11c; lane 5, M11d; lane 6, M1a; lane 7, M15; lane 8, M16; lane 9, G3a; lane 10, G3b; and lane 11, G5. Lanes 12 to 16 contain previously established ribotypes which the new ribotypes resemble. Lane 12, ribotype M1; lane 13, M7; lane 14, M11; lane 15, M13; and lane 16, G3.
Ribotyping correlated well with biotyping and with the toxigenicity status of the isolates, and it provided useful information about the circulation of C. diphtheriae in several households (Table 2; Fig. 2). The two nontoxigenic strains of the same biotype isolated from one household (household 1) were also of the same ribotype (M1a). In another household (household 2), where three strains were isolated, the two mitis toxigenic strains had the same ribotype (M11b) and, as anticipated, the third gravis toxigenic strain produced a different ribotype (M11d). The strains isolated from the third household, in which one gravis toxigenic strain and one mitis nontoxigenic strain were isolated, produced two different ribotypes, G5 and M1a, respectively. However, the mitis strain isolated from this household (household 3) had a ribotype (M1a) identical to that of the mitis strain isolated from the first household. In addition, the two mitis strains isolated from household 2 had ribotyping patterns identical to that of the strain isolated from the index patient.
TABLE 2.
Seven C. diphtheriae strains isolated within three different surveyed households
| House-hold | Strain designation | Bio-typea | Toxi-genicity | Relationship within household | Ribo-type | ET |
|---|---|---|---|---|---|---|
| 1 | PR101 | M | − | Sibling | M1a | 221 |
| PR130 | M | − | Sibling | M1a | 229 | |
| 2 | PR79 | G | + | Sibling | M11d | 225 |
| PR110 | M | + | Sibling | M11b | 228 | |
| PR115 | M | + | Sibling | M11b | 228 | |
| 3 | PR20 | M | − | Daughter | M1a | 222 |
| PR75 | G | + | Mother | G5 | 224 |
M, mitis; G, gravis.
FIG. 2.
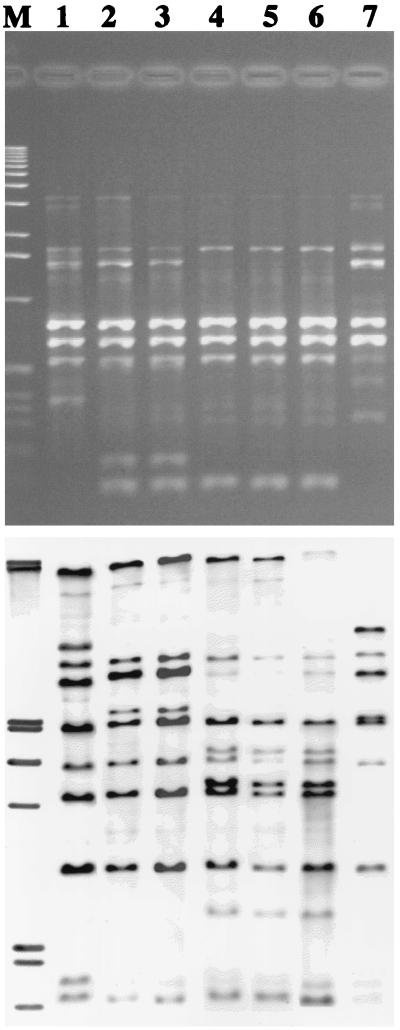
RAPD and ribotyping patterns of seven C. diphtheriae strains isolated from members of three households. Lane M contains molecular weight marker. Lanes 1 to 3 contain strains isolated from members of household 2. Lane 1, PR79 (biotype gravis, toxigenic); lane 2, PR110 (biotype mitis, toxigenic); and lane 3, PR115 (biotype mitis, toxigenic). Lanes 4 and 5 contain strains isolated from members of household 1. Lane 4, PR101 (biotype mitis, nontoxigenic); lane 5, PR130 (biotype mitis, toxigenic). Lanes 6 and 7 contain strains isolated from members of household 3. Lane 6, PR20 (biotype mitis, nontoxigenic); lane 7, PR75 (biotype gravis, toxigenic).
Isolates isolated in the 1970s and 1980s and in 1996 were ribotype M1a (Fig. 3). All of these strains were identified by the Elek test as nontoxigenic. However, these strains were positive by the PCR assay which amplified fragments of both A and B subunits. In addition, strains isolated more than 20 years apart had four closely related ribotypes resembling ribotype M11. With the exception of one strain, strains of this ribotype had been isolated in South Dakota. Ribotypes M7a and M15 were identified in isolates collected in the 1970s in South Dakota, New Mexico, and Alaska.
FIG. 3.
Ribotyping and RAPD patterns of five C. diphtheriae strains isolated in 1996 and four C. diphtheriae strains isolated from 1973 to 1983. Lane 1, PR101 (biotype mitis, nontoxigenic; isolated in 1996); lane 2, PR130 (biotype mitis, nontoxigenic; isolated in 1996); lane 3, PR20 (biotype mitis, nontoxigenic; isolated in 1996); lane 4, F1803 (biotype mitis, nontoxigenic; isolated in 1981); lane 5, F2726 (biotype mitis, nontoxigenic; isolated in 1982); lane 6, F4306 (biotype mitis, nontoxigenic; isolated in 1983); lane 7, PR110 (biotype mitis, toxigenic; isolated in 1996); lane 8, PR115 (biotype mitis, toxigenic; isolated in 1996); and lane 9, C5276 (biotype mitis, toxigenic; isolated in 1973).
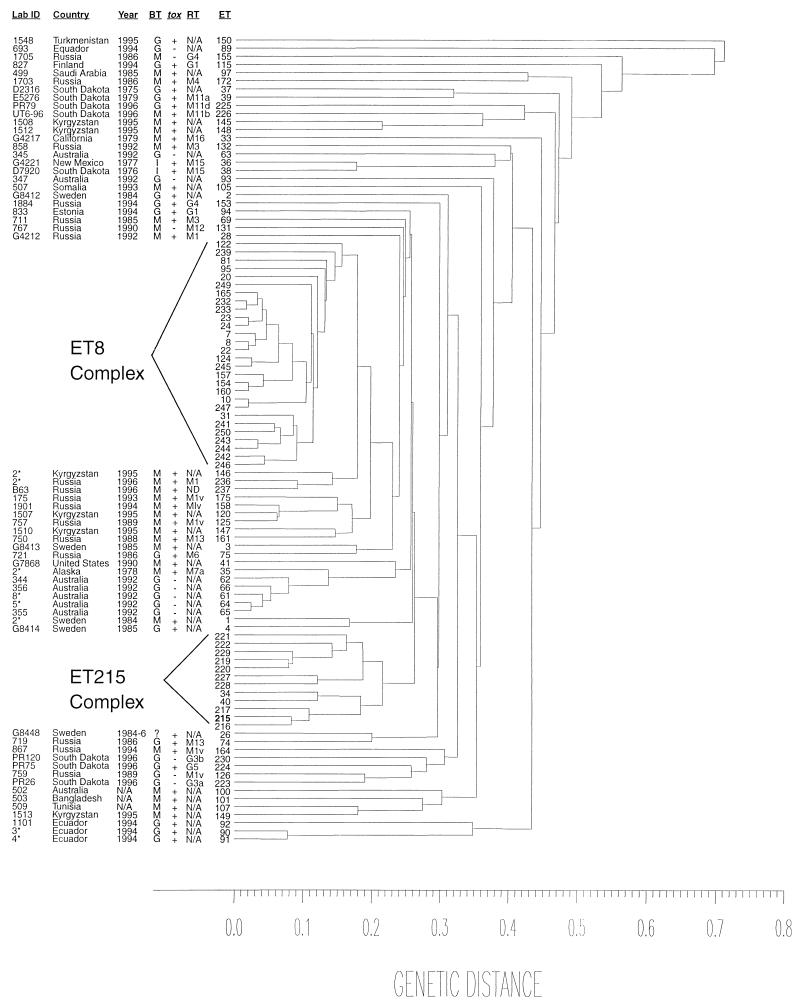
Significantly greater genetic diversity was observed among the strains studied by MEE. Among the 26 strains tested (15 archival strains and the 11 recently isolated strains), 21 different ETs were identified (Table 1). Seventeen strains had 17 different ETs; the nine other strains had a total of four ETs. Fifteen (57%) of the 26 strains tested were clustered within a genetic distance of <0.22, forming a distinct clonal group designated as the ET 215 complex (Fig. 4; Table 3). This complex contains 12 ETs; its designation was based on the observation that ET 215 was the most frequently identified ET in the complex. This complex is clearly distinct and only distantly related to the ET 8 complex previously verified to include strains associated with the diphtheria epidemic in Russia and the NIS (16). Nine of the 15 strains were archival strains, and 6 strains were recent isolates. By biotyping, nine of the strains within the ET 215 complex were of the biotype mitis (three were toxigenic, and six were nontoxigenic) and six were biotype gravis (all were toxigenic). By ribotyping, 5 of the 15 strains were ribotype M1a. With the exception of a single strain from Colorado, strains from states other than South Dakota were not identified within the ET 215 complex and were only distantly related to the complex. Of the 26 assayed strains 11 strains not included within the ET 215 complex were disbursed among nine different distantly related ETs. Only two of these strains (G4219 and G4220) shared the same ET (ET 35).
FIG. 4.
Dendrogram showing the genetic relatedness of 99 electrophoretic types of 216 C. diphtheriae isolates collected from various countries around the world between 1973 and 1996. ID, identifying designation; Year, year of isolation; BT, biotype; RT, ribotype; ET, electrophoretic type; tox, toxigenicity status; N/A, information not available; ND, not done. ET 8 complex includes 122 C. diphtheriae strains, clustered within 28 ETs, that were isolated in Russia between 1986 and 1997. All strains in the ET 8 complex, with one exception, are biotype gravis and toxigenic. Detailed information on strains within the ET 215 complex is provided in Table 3.
TABLE 3.
C. diphtheriae strains within the ET 215 complex isolated in the United States from 1973 to 1996
| Strain designationa | Yr | Biotypeb | Toxigenicity status | Ribotype | ET |
|---|---|---|---|---|---|
| PR101 | 1996 | M | + | M1a | 221 |
| PR20 | 1996 | M | + | M1a | 222 |
| PR130 | 1996 | M | + | M1a | 229 |
| E5340 | 1979 | M | − | M1a | 219 |
| E7798 | 1980 | M | − | M1a | 220 |
| PR109 | 1996 | G | + | M11d | 227 |
| PR110 | 1996 | M | + | M11b | 228 |
| PR115 | 1996 | M | + | M11b | 228 |
| G4218 | 1978 | G | + | M11a | 34 |
| E5449 | 1979 | G | + | M11a | 40 |
| C5276 | 1973 | M | + | M11c | 217 |
| C4943 | 1973 | G | + | M11a | 215 |
| D7643 | 1979 | G | + | M11a | 215 |
| E8277 | 1980 | G | + | M11a | 215 |
| C5214 | 1973 | M | − | M7a | 216 |
All strains except G4218, which was collected in Colorado, were collected in South Dakota.
M, mitis; G, gravis.
Identical ETs were detected in two toxigenic biotype mitis strains collected from members of household 2. Conversely, two ETs were observed in two nontoxigenic biotype mitis strains, differing from each other at six loci, collected from members of household 1. Two distant ETs were identified in two strains collected from members of household 3, thus according with the biotype, toxigenicity status, and ribotyping data.
All 33 isolates (22 archival and 11 recent strains) were tested by RAPD, and 13 different patterns were identified. As with ribotyping, a difference of one band was recognized as an individual RAPD pattern. Eleven of the 33 isolates had the same RAPD pattern, and a single strain, E9134, very closely resembled this pattern. All 12 of these nontoxigenic strains were biotype mitis. Three of the 12 were newer isolates, and the remaining 9 were archival isolates. Five other identified RAPD patterns were also seen in more than one strain (2 to 4 strains), while seven strains had unique RAPD patterns.
All strains of ribotype M1a had identical RAPD patterns (Fig. 3) with the exception of one strain (E9134), for which the RAPD pattern exhibited minor differences. Strains of ribotype M11b and M11c also had the same RAPD patterns. In addition, the two intermedius biotype strains possessed matching RAPD patterns.
RAPD provided identical differentiation among the strains from the three households when compared to the ribotyping data (Fig. 2). For household 1, the two biotype mitis nontoxigenic strains isolated were ribotype M1a and possessed the same RAPD pattern. Conversely, for household 3, where two strains of differing biotypes and toxigenicities were isolated, the patterns identified for these two strains by both ribotyping and RAPD showed significant differences. Finally, for household 2, the two toxigenic strains of the same biotype isolated also had identical ribotypes and RAPD patterns. However, the patterns identified from the third strain (biotype gravis, toxigenic) differed by both ribotyping and RAPD from the other two biotype mitis strains.
Twenty-two (13 archival strains and 9 recent strains) of the 33 strains were assayed by SSCP. Three patterns (designated types 1, 2, and 3) were identified in each region. The types identified with the Park-Williams 8 (PW8) strain were designated type 1 for each region. SSCP types for eight assayed strains (five recent and three archival strains) were identical to those observed in the PW8 type strain (type 1 for both regions 6 and 8). Ten of the 22 strains (45%; 3 recent and 7 archival strains) were type 3 for tox region 6 and type 2 for tox region 8. Nine of these strains were biotype gravis; a single strain (G4217) was biotype mitis. The remaining four strains were type 1 for region 6 and type 2 for region 8.
DISCUSSION
Molecular subtyping of C. diphtheriae has recently been used successfully to identify a clonal group associated with the current diphtheria epidemic in Russia and the NIS of the former Soviet Union, where more than 150,000 cases have been reported since 1990 (12, 16, 17). MEE and ribotyping have proved to be an extremely sensitive and specific tool for the molecular subtyping of C. diphtheriae isolates. Numerous ETs and over 45 different ribotypes have been described to date. An excellent correlation with epidemiologic data has been demonstrated in studies by Popovic et al. (16) and De Zoysa et al. (6, 7). Furthermore, the WHO ribotyping database for C. diphtheriae has already been established at the Pasteur Institute in Paris, France (10). The presence of toxigenic C. diphtheriae in an American Indian community prompted us to use these molecular subtyping methods to evaluate the possibility that the recent isolates were not the result of importation but rather of a continuous focal persistence of C. diphtheriae in South Dakota. Based on the MEE data, a distinct clonal group, the ET 215 complex, containing both archival and new strains, was identified (Fig. 4; Table 3). This clonal group was distinctly different from the clonal group (ET 8 complex) associated with the current epidemic in Russia and the NIS. In addition, unlike the ET 8 complex, which includes only biotype gravis strains, the ET 215 complex includes strains of both mitis and gravis biotypes. This complex also includes a small group of nontoxigenic isolates that possess the tox gene. Although rare, the presence of the tox gene accompanied by the absence of toxin activity has been documented previously (5, 11). In 1983, Groman et al. reported that 11 nontoxigenic C. diphtheriae isolates collected from residents of South Dakota carried the tox gene (11). These isolates failed to produce a reaction when tested by intracutaneous inoculation of a rabbit. A representative isolate of that group, E8392, used in Groman’s study was also included in our study. Seven other isolates from that period (1979 to 1983) were also included. All eight strains had identical ribotypes and RAPD patterns. Two strains assayed by MEE belonged to the ET 215 complex. Interestingly, three recent South Dakota strains were indistinguishable from the eight older South Dakota strains by ribotyping and RAPD, and they also belonged to the ET 215 complex but formed a distinct cluster within this clonal group. The ET 215 complex appears to have persisted steadily over time, allowing subtle changes to evolve in the strains that belong within this complex, although the strains remain closely related to each other. Both ribotyping and RAPD data support the conclusions based on the MEE results, since they show that archival and recent strains had identical ribotypes and/or RAPD patterns. These data strongly support the hypothesis of endemic circulation of C. diphtheriae in this area. Interestingly, with the exception of a single strain (G4218, from Colorado), isolates collected in other states are only distantly related to those collected in South Dakota.
The subtyping data also support the improbability of importation of C. diphtheriae into this region. When they were compared to Russian strains isolated during the current epidemic, the South Dakota strains bore little resemblance to the Russian strains. No South Dakota strain or other strain collected in the United States had the G1/G4 RAPD patterns predominant among the Russian strains. The RAPD patterns identified in the South Dakota isolates and in other isolates collected in the United States have not been previously observed in any Russian isolate tested by RAPD. Similarly, ribotyping results showed that none of the archival or recent South Dakota isolates had the same ribotype as the ribotypes (G1 and G4) currently prevailing among the epidemic Russian strains. In agreement with the RAPD results, the ribotypes identified among all of the South Dakota strains have not been previously seen in any epidemic Russian strain, although some resemblances between these two geographically distinct groups of strains were observed.
MEE, ribotyping, and RAPD data provided highly discriminative information about the strains circulating in several households. Strains with the same biotype and toxigenicity status isolated within the same household had identical ribotyping and RAPD patterns (households 1 and 2; Fig. 2 and Table 2). However, if strains found within a household were of different biotypes and/or toxigenicity status, their ribotypes and RAPD patterns also differed (households 2 and 3; Fig. 2 and Table 2). MEE data also showed that strains with identical biotypes and toxigenicity status were more closely genetically related to each other than to strains of differing biotypes and/or toxigenicity status. In household 2, the two toxigenic biotype mitis strains had identical ETs and were both within the ET 215 complex. The third toxigenic strain, of biotype gravis, from household 2 had a different ET and was very distantly related to the two other (biotype mitis) strains from this household. Both strains from household 1 were also included within the ET 215 complex (Fig. 4; Table 3). However, in household 3, one strain (mitis, nontoxigenic) was included within the ET 215 complex and the other isolated strain (gravis, toxigenic) was not. In addition, the isolation of two strains with differing biotypes from the same household suggests high rates of infection within this community (3). Isolation of the organism in the absence of reports of disease (with a single exception) may have several potential explanations. It could be an indication of a high prevalence of vaccine-induced or natural immunity within the population (3). Further studies are needed to determine the seroprevalence of diphtheria toxin antibodies among adults in this particular community. On the other hand, the incidence of disease may be underreported because of inadequate diagnosis, failure to report cases, and inadequate laboratory diagnosis.
Analysis of the tox gene provided information that could lead to a reevaluation of the components in the currently administered vaccine. In our earlier report regarding our study of the tox gene by the SSCP assay, we proposed that the type 1 designation for both regions 6 and 8 of the tox B subunit be the types identified in the PW8 reference strain, which is the strain used worldwide to produce the diphtheria toxoid; any variations from sequences seen in the PW8 strain were sequentially numbered. The SSCP assay showed that over 60% of the strains collected in the United States differed from the PW8 strain. The results from this assay showed that 45% of the archival and recent South Dakota strains and other isolates collected in the United States assayed were type 3 in region 6 of the toxin gene. In region 8 of the toxin gene, 64% of the strains were type 2. Only 36% of the strains were type 1 for both regions 6 and 8. While several base substitutions were detected within the 6 and 8 regions of the tox B subunit, none resulted in amino acid substitutions, suggesting that the tox gene remains highly conserved. In addition, differences between the strains isolated in the United States and those isolated during the Russian and NIS epidemic were noted. Russian epidemic isolates showed a predominance of type 3 in both region 6 and region 8 (15). However, among the South Dakota strains, type 3 prevailed in region 6 and type 2 prevailed in region 8. Thus, although nucleic acid differences in all strains tested have been noted and did not result in amino acid substitutions, leaving the produced toxin unchanged, the SSCP data suggest that the corynephage evolution seen here may be neutral or driven by selection at loci other than tox.
In 1997, four cases of respiratory diphtheria were reported in the United States. Recent enhanced surveillance in South Dakota has revealed that toxigenic C. diphtheriae is circulating among American Indian populations. This finding contradicts earlier, passive national surveillance data, which indicated that toxigenic strains had essentially disappeared from the United States. Routine surveillance is, however, limited by the lack of specialized laboratory expertise in the isolation of C. diphtheriae, including the necessary use of specialized culture techniques. Molecular analysis of these recent C. diphtheriae strains and strains from diphtheria cases reported in the United States in the late 1970s and early 1980s support persistent endemicity rather than importation from countries with current endemic or epidemic diphtheria. Isolation of C. diphtheriae from this American Indian community was the result of enhanced surveillance. To date, this is the only study of this nature. It is, therefore, difficult to provide generalized conclusions and recommendations before additional studies are carried out to determine if more such endemic foci persist in the United States. Although the diphtheria toxoid vaccine used in this country since the 1920s has proven to be effective in controlling the large-scale diphtheria epidemics, given that more than 50% of the adult population of the United States lacks protective levels of diphtheria toxin antibodies, the reemergence of diphtheria is a potentially significant, but as yet undetermined, risk to public health.
ACKNOWLEDGMENTS
We thank Mary Afraid of Bear and Tom Haase at Indian Health Service, Aberdeen Area Office, for their help in collecting the specimens for the enhanced surveillance. We also thank all local and state health officials, particularly Susan Lance at the South Dakota Department of Health, for their help in organizing the surveillance.
REFERENCES
- 1.Bisgard K, Hardy I, Popovic T, Strebel P, Wharton M, Chen R, Hadler S. Respiratory diphtheria in the United States, 1980–1995. Am J Public Health. 1998;88:787–791. doi: 10.2105/ajph.88.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisgard K M, Vitek C, Golaz A, Popovic T. Diphtheria. In: Wharton M, Roush S, editors. Vaccine preventable diseases surveillance manual. Atlanta, Ga: Centers for Disease Control and Prevention; 1997. pp. 1-1–1-9. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Toxigenic Corynebacterium diphtheriae—Northern Plains Indian community, August–October 1996. Morbid Mortal Weekly Rpt. 1997;46:506–510. [PubMed] [Google Scholar]
- 4.Chen R T, Broome C V, Weinstein R A, Weaver R, Tsai T F. Diphtheria in the United States, 1971–1981. Am J Public Health. 1985;75:1393–1397. doi: 10.2105/ajph.75.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cianciotto N P, Groman N B. Characterization of bacteriophages from tox-containing, non-toxigenic isolates of Corynebacterium diphtheriae. Microb Pathog. 1997;22:343–351. doi: 10.1006/mpat.1996.0120. [DOI] [PubMed] [Google Scholar]
- 6.De Zoysa A, Efstratiou A, George R C, McNiff A, Vuopio-Varkila J, Mazurova I, Tseneva G, Thilo W, Andronescu C, Roure C. Program and abstracts of the 2nd International Meeting of the European Laboratory Working Group on Diphtheria. London, United Kingdom: Public Health Laboratory Services; 1995. Further studies on the molecular epidemiology of toxigenic and non-toxigenic Corynebacterium diphtheriae from U. K. and Europe using ribotyping and pulsed-field gel electrophoresis; p. 22. [Google Scholar]
- 7.De Zoysa A, Efstratiou A, et al. Molecular epidemiology of Corynebacterium diphtheriae from northwestern Russia and surrounding countries studied by using ribotyping and pulsed-field gel electrophoresis. J Clin Microbiol. 1995;33:1080–1083. doi: 10.1128/jcm.33.5.1080-1083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efstratiou A, Maple P A. WHO manual for the laboratory diagnosis of diphtheria. Reference ICP-EPI 038(C). Geneva, Switzerland: World Health Organization; 1994. [Google Scholar]
- 9.Graves L M, Swaminathan B. Universal bacterial DNA isolation procedure. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C: American Society for Microbiology; 1993. pp. 617–621. [Google Scholar]
- 10.Grimont P A D, Grimont F, Ruckly C. Program and abstracts of the 5th International Meeting of the European Laboratory Working Group on Diphtheria. London, United Kingdom: Public Health Laboratory Service; 1998. The Corynebacterium diphtheriae ribotype database; p. 31. [Google Scholar]
- 11.Groman N, Cianciotto N, Bjorn M, Rabin M. Detection and expression of DNA homologous to the tox gene in nontoxigenic isolates of Corynebacterium diphtheriae. Infect Immun. 1983;42:48–56. doi: 10.1128/iai.42.1.48-56.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy I R B, Dittman S, Sutter R W. Current situation and control strategies for resurgence of diphtheria in newly independent states of former Soviet Union. Lancet. 1996;347:1739–1744. doi: 10.1016/s0140-6736(96)90811-9. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs D. Proceedings of the 15th Annual Users Group International Conference. Cary, N.C: SAS Institute; 1990. SAS/GRAPH software and numerical taxonomy; pp. 1413–1418. [Google Scholar]
- 14.Mikhailovich V M, Melnikov V G, Mazurova I K, Wachsmuth I K, Wenger J D, Wharton M, Nakao H, Popovic T. Application of PCR for detection of toxigenic Corynebacterium diphtheriae strains isolated during the Russian diphtheria epidemic, 1990 through 1994. J Clin Microbiol. 1995;33:3061–3063. doi: 10.1128/jcm.33.11.3061-3063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakao H, Pruckler J M, Mazurova I K, Narvskaia O V, Glushkevich T, Marijevski V F, Kravetz A N, Fields B S, Wachsmuth I K, Popovic T. Heterogeneity of diphtheria toxin gene, tox, and its regulatory element, dtxR, in Corynebacterium diphtheriae strains causing epidemic diphtheria in Russia and Ukraine. J Clin Microbiol. 1996;34:1711–1716. doi: 10.1128/jcm.34.7.1711-1716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popovic T, Kombarova S, Reeves M, Nakao H, Mazurova I K, Wharton M, Wachsmuth I K, Wenger J D. Molecular epidemiology of diphtheria in Russia, 1985–1994. J Infect Dis. 1996;174:1064–1072. doi: 10.1093/infdis/174.5.1064. [DOI] [PubMed] [Google Scholar]
- 17.Popovic T, Wharton M, Wenger J D, McIntyre L, Wachsmuth I K. Are we ready for diphtheria? A report from the diphtheria diagnostic workshop, Atlanta, 11 and 12 July 1994. J Infect Dis. 1995;171:765–767. doi: 10.1093/infdis/171.4.765. [DOI] [PubMed] [Google Scholar]
- 18.Regnault B, Grimont R, Grimont P A D. Universal ribotyping method using a chemically-labeled oligonucleotide probe mixture. Res Microbiol. 1997;146:649–659. doi: 10.1016/S0923-2508(99)80064-3. [DOI] [PubMed] [Google Scholar]
- 19.Selander R K, Caugant D A, Ochman H, Musser J M, Gilmour M N, Whittam T S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986;51:873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]