Abstract
Introduction:
Previous studies about osteosarcoma patient characteristics, management, and outcomes have limited patient numbers, combine varied tumor types, and/or are older studies.
Methods:
Patients with osteosarcoma from the 2004 to 2015 National Cancer Database data sets were separated into axial, appendicular, and other. Demographic and treatment data as well as 1-, 5-, and 10-year survival were determined for each group. A multivariate Cox analysis of patient variables with the likelihood of death was performed, and the Kaplan Meier survival curves were generated.
Results:
Four thousand four hundred thirty patients with osteosarcoma (3,435 appendicular, 810 axial, and 185 other) showed survival at 1-year, 5-year, and 10-year and was highest among the appendicular cohort (91.17%, 64.43%, and 58.58%, respectively). No change in survival was seen over the periods studied. The likelihood of death was greater with increasing age category, distant metastases, and treatment with radiation alone but less with appendicular primary site, treatment with surgery alone, or surgery plus chemotherapy.
Discussion:
Despite advances in tumor management, surgical excision remains the best predictor of survival for osteosarcomas. No difference was observed in patient survival from 2004 to 2015 and, as would be expected, distant metastases were a poor prognostic sign, as was increasing age, male sex, and axial location.
Osteosarcoma is the most common primary osseous sarcoma.1,2 The incidence has been estimated to be approximately 5.0 per million patients aged zero to 191 and 3.5 per million patients for patients aged older than 60.3 Osteosarcoma has a predilection for invading the metaphysis of the long bones of the appendicular skeleton, chiefly the distal femur and proximal tibia.4 Osteosarcoma can also affect the axial skeleton/other locations, although this is less common.4
Owing to the relatively rare nature of osteosarcoma, previous studies have been limited in sample size (ranging from 34 to 648).5,6,7,8,9 These have tended to show positive prognostic factors to include but are not limited to younger age at the time of diagnosis,1 lack of metastases at the time of diagnosis,5 time to treatment initiation,10 parosteal subtype, smaller tumor size, and greater than 90% necrosis with chemotherapy.11 Depending on the combination of these prognostic factors, 5-year survival rates for osteosarcoma have been reported to vary from 70% in patients with local disease at the time of diagnosis to 20% in patients with metastatic or recurrent disease.12
The mainstay of treatment for osteosarcoma consists of surgical resection and reconstruction along with neoadjuvant chemotherapy (before surgery) and adjuvant chemotherapy (after surgery). Despite advances and numerous attempts to refine treatment regimens, the survival rates of patients with osteosarcoma have largely remained unchanged over the last 3 decades.13 Radiation therapy is not a mainstay in the treatment of osteosarcoma and is largely reserved for cases without negative resection margins.14,15 No treatment is rarely considered except in cases of exceedingly poor prognosis/patient choice.
Previous studies investigating treatment management of osteosarcoma have largely been limited by patient numbers. The National Cancer Database (NCDB) contains patient information from multiple centers across the United States with more than 30 million patient records representing a unique opportunity to learn additional insights concerning this patient population.16,17 To our knowledge, no previous studies assessing the outcomes in patients with primary osteosarcoma using the NCDB database exist. Thus, this study sought to evaluate the incidence, treatment, and outcomes of patients with osteosarcoma using the large, robust, and multicenter national patient population found within the NCDB database to further characterize the osteosarcoma population, treatments, and survivals.
Methods
Data Source and Study Population
The NCDB was used to conduct a retrospective database cohort study. This database represents an initiative of the American College of Surgeons and American Cancer Society to track and improve outcomes in cancer.17 The data contain information gathered from multiple centers, with demographic variables and outcome variables on more than 70% of new cancer diagnoses in the United States.16-18 Data are collected until either death of the patient or the patient is lost to follow-up.
Patients diagnosed with osteosarcoma were identified from the 2004 to 2015 NCDB databases by International Classification of Diseases for Oncology, third edition histology codes 9180, 9183, 9185, 9187, 9192, 9193, and 9194. Osteosarcoma cases were then divided into three cohorts by the site of the primary tumor: axial (International Classification of Diseases for Oncology, third edition codes C41.0, C41.1, C41.2, and C41.4), appendicular (C40.0, C40.1, C40.2, C40.3, and C40.9), or other location (C40.8, C41.3, C41.8, and C41.9). Patients were excluded if treatment was performed at another location than the reporting facility (Class of Case = 00), along with those missing data related to the presence of metastases at diagnosis.
Population Characteristics
Demographic variables extracted from the database include age, sex, Charles-Deyo score, and metastasis at presentation. Patients were also split into 3 era groups based on year of diagnosis: 2004 to 2007, 2008 to 2011, and 2012 to 2015.
Treatment data including surgery, radiation, chemotherapy, and combination treatments were also extracted from the database. Finally, 1-, 5-, and 10-year survival was calculated for each primary site cohort.
Comparison of Populations
Bivariate analyses were used to compare preoperative and surgical variables between the axial, appendicular, and other cohorts. Pearson chi-squared test was used for categorical variables, one-way Analysis of Variance (ANOVA) for continuous variables, and the Kruskal-Wallis test for ordinal variables (American Society of Anesthesiologists (ASA) class).
To study the influence of preoperative variables and treatment choice on the likelihood of death, a multivariate Cox analysis was conducted for the axial and appendicular cohorts. Incidence rate ratio (IRR) was calculated, which is a more accurate measure of the relative effect of a given exposure on the risk of the occurrence of an event between the exposed and unexposed populations. It takes the incidence rate (defined as the number of events per person time–person-years in this study) of the exposed population and divides this by all the people at risk for an outcome (in this instance death) at any one point in time. IRR is useful when calculating relative risk over a period when the population at risk is constantly changing (because of deaths or remission).19
Finally, the Kaplan Meier survival curves were generated showing long-term survival of various cohorts. First, long-term survival was shown for patients with and without distant metastases at presentation. Then, long-term survival was shown for the appendicular, axial, and other cohorts. Finally, long-term survival was shown for each of the 3 era groups.
All statistical analyses were conducted using Stata version 13.0 (StataCorp, LP). Significance was set at P < 0.05.
Results
Population and Treatment Characteristics
Of the 4,430 patients identified with osteosarcoma, 3,435 were classified as appendicular (77.54%), 810 cases were classified as axial (18.28%), and 185 were other (4.18%). These cohorts are defined in Table 1. The appendicular group was younger and healthier (lower Charles-Deyo score) than the axial and other groups. Metastases were present for a similar percent of cases for the appendicular (18.05%) and axial (18.40%) groups (but lower than the other group, 40.0%). No statistically significant difference existed is the distribution between the appendicular, axial, and other between the years of diagnosis.
Table 1.
Comparison of Demographics Between the Appendicular, Axial, and Other Osteosarcoma Cohorts
| Patient Characteristic | Total | Appendicular | Axial | Other | P |
| No. of patients | 4,430 | 3,435 | 810 | 185 | |
| Mean age in yrs (±SD) | 29.53 ± 20.32 | 25.52 ± 17.99 | 43.08 ± 21.11 | 44.57 ± 24.51 | <0.001 |
| Women | 1,963 (44.31) | 1,495 (43.52) | 383 (47.28) | 85 (45.95) | 0.138 |
| Charles-Deyo score | <0.001 | ||||
| 0 | 4,021 (90.77) | 3,157 (91.91) | 699 (86.30) | 165 (89.19) | |
| 1 | 348 (7.86) | 242 (7.05) | 92 (11.36) | 14 (7.57) | |
| 2 | 50 (1.13) | 28 (0.82) | 16 (1.98) | 6 (3.24) | |
| 3 | 11 (0.25) | 8 (0.23) | 3 (0.37) | 0 (0.00) | |
| Metastasis at presentation | < 0.001 | ||||
| No | 3,587 (80.97) | 2,815 (81.95) | 661 (81.60) | 111 (60.00) | |
| Yes | 843 (19.03) | 620 (18.05) | 149 (18.40) | 74 (40.00) | |
| Year of diagnosisa | 0.167 | ||||
| 2004-2007 | 1,574 (35.53) | 1,233 (35.90) | 288 (35.56) | 53 (28.65) | |
| 2008-2011 | 1,607 (36.28) | 1,227 (35.72) | 298 (36.79) | 82 (44.32) | |
| 2012-2015 | 1,249 (28.19) | 975 (28.38) | 224 (27.65) | 50 (27.03) |
No statistically significant difference exists between age, sex, Charles-Deyo score, and the prevalence of distant metastases at presentation between the 3 era groups in the appendicular or axial cohorts.
Significance set at <0.05.
Treatment data for the axial, appendicular, and other cohorts are shown in Table 2. The appendicular group was more likely to receive surgery and radiation but less likely to receive isolated radiation than the axial or other groups. For both the axial and other cohorts, radiation therapy was more likely to be given postoperatively (axial: 13.58%; other: 9.73%) than preoperatively (axial: 1.60%; other: 2.16%) or intraoperatively (axial: 0.12%; other: 0.00%). Regarding chemotherapy, the appendicular cohort was more likely to receive chemotherapy (83.00%) than the axial (64.81%) and other (67.03%) cohorts. In treatment combinations, surgery and chemotherapy were the most common for all scenarios but more common in appendicular cases (69.43%) than axial cases (34.57%) or other cases (41.08%).
Table 2.
Comparison of Surgical Data and Long-Term Survival for the Appendicular and Axial Cohorts
| Patient Characteristic | Total | Appendicular | Axial | Other | P |
| No. of patients | 4,430 | 3,435 | 810 | 185 | |
| Surgical treatment | <0.001 | ||||
| No surgery | 755 (17.04) | 470 (13.68) | 225 (27.78) | 60 (32.43) | |
| Surgery | 3,669 (82.82) | 2,959 (86.14) | 585 (72.22) | 125 (67.57) | |
| Unknown | 6 (0.14) | 6 (0.17) | 0 (0.00) | 0 (0.00) | |
| Radiation therapy | <0.001 | ||||
| None | 4,147 (93.61) | 3,321 (96.69) | 667 (82.35) | 159 (85.95) | |
| Preoperative | 31 (0.70) | 14 (0.41) | 13 (1.60) | 4 (2.16) | |
| Postoperative | 182 (4.11) | 454 (1.57) | 110 (13.58) | 18 (9.73) | |
| Both or intraoperative | 8 (0.18) | 4 (0.12) | 4 (0.49) | 0 (0.00) | |
| Unknown | 62 (1.40) | 42 (1.22) | 16 (1.98) | 4 (2.16) | |
| Chemotherapy | <0.001 | ||||
| No (0) | 862 (19.46) | 542 (15.78) | 261 (32.22) | 59 (31.89) | |
| Yes (1) | 3,500 (79.01) | 2,851 (83.00) | 525 (64.81) | 124 (67.03) | |
| Unknown (2) | 68 (1.53) | 42 (1.22) | 24 (2.96) | 2 (1.08) | |
| Treatment combination | <0.001 | ||||
| No treatment | 274 (6.19) | 152 (4.43) | 96 (11.85) | 26 (14.05) | |
| Isolated surgery | 625 (14.11) | 445 (12.95) | 157 (19.38) | 23 (12.43) | |
| Isolated radiation | 6 (0.14) | 1 (0.03) | 3 (0.37) | 2 (1.08) | |
| Isolated chemotherapy | 570 (12.87) | 381 (11.09) | 152 (18.77) | 37 (20.00) | |
| Surgery and radiation | 69 (1.56) | 16 (0.47) | 40 (4.94) | 13 (7.03) | |
| Surgery and chemotherapy | 2,741 (61.87) | 2,385 (69.43) | 280 (34.57) | 76 (41.08) | |
| Chemotherapy and radiation | 2 (0.05) | 1 (0.03) | 1 (0.12) | 0 (0.00) | |
| Surgery, radiation, and chemotherapy | 143 (3.23) | 54 (1.57) | 81 (10.00) | 8 (4.32) | |
| Survival | <0.001 | ||||
| 1-yr survival (%) | 87.1 | 91.17 | 74.22 | 67.33 | |
| 5-yr survival (%) | 60.17 | 64.43 | 45.97 | 42.57 | |
| 10-yr survival (%) | 53.92 | 58.58 | 38.27 | NA |
No statistically significant difference exists between the era groups for the above variables, within the appendicular or axial cohorts.
Significance set at <0.05.
Survival Data and Kaplan Meier Curves
Survival is also shown in Table 2. The appendicular cohort demonstrated a statistically higher 1-year survival rate (91.17%), 5-year survival rate (64.43%), and 10-year survival rate (58.58%) than the other two cohorts (axial: 1-year [74.22%], 5-year [45.97%], and 10-year [38.27%]; other: 1-year [67.33%], 5-year [45.57%], and 10-year [N/A]).
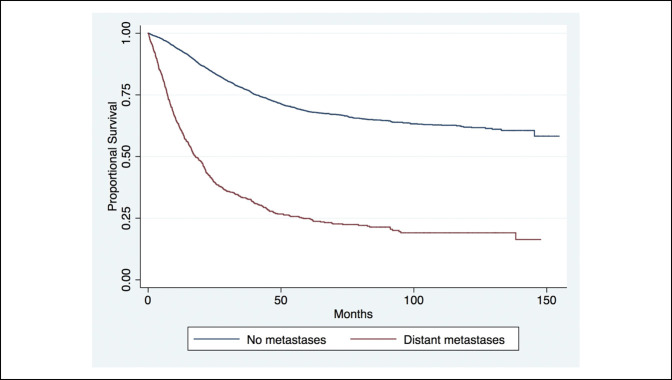
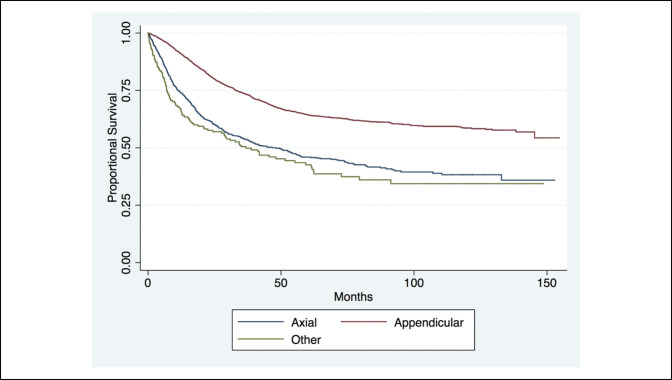
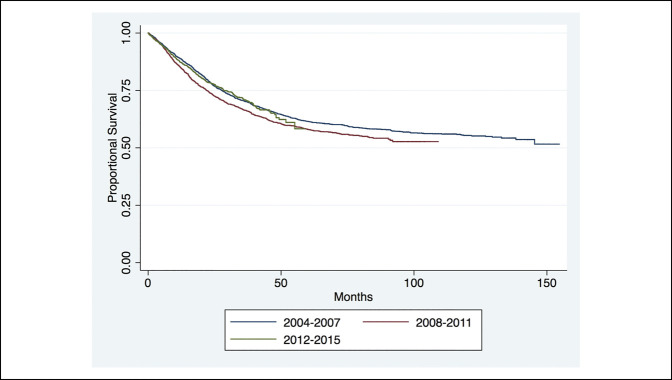
The Kaplan Meier curve analysis of proportional survival of patients with distant metastases on diagnosis revealed markedly decreased life expectancy in patients with distant metastases at 1, 5, and 10 years from time of diagnosis (Figure 1). The long-term survival of axial, appendicular, and other cohorts showed markedly a poorer long-term survival at 1, 5, and 10 years of those with tumors in the axial or other cohorts (Figure 2). Furthermore, no notable difference was observed in the long-term survival for patients diagnosed since the mid-2000s (Figure 3).
Figure 1.
Chart showing the long-term survival of all patients with and without distant metastases at the time of presentation (P < 0.001).
Figure 2.
Chart showing the long-term survival of patients in the axial, appendicular, and other cohorts (P < 0.001).
Figure 3.
Chart showing the long-term survival of patients in the 3 era groups (P = 0.220)
Multivariate Analyses
Looking at axial and appendicular cohorts individually, Table 3 demonstrates the multivariate Cox analysis of the likelihood of death at any given time for demographic and surgical variables for just the axial cohort. The likelihood of death increased in a sequential manner with increasing age category (23 to 45 age cohort: IRR = 1.325, P < 0.001; 46 to 62 age cohort: IRR = 2.107, P < 0.001; 62+ age cohort: IRR = 3.528, P < 0.001) and the presence of metastasis at the time of diagnosis demonstrated (IRR = 3.958, P < 0.001). The likelihood of death decreased with female sex (IRR = 0.798, P < 0.001). No significant change existed in the IRR with the year of diagnosis. The likelihood of death demonstrated an increase with the treatment choice of radiation alone (IRR = 16.575, P = 0.006), whereas the likelihood of death demonstrated a decrease with the treatment choice was surgery alone (IRR = 0.305, P < 0.001) and surgery with chemotherapy (IRR = 0.476, P < 0.001).
Table 3.
A Multivariate Cox Analysis of the Likelihood of Death at Any Given Time for Demographic and Surgical Variables for the Axial Cohort
| Likelihood of Death at Any Given Time | IRRa | 95% Confidence Interval | P | ||
| Age category | |||||
| <23 | Ref | Ref | Ref | Ref | |
| 23-45 | 1.325 | 1.137 | — | 1.545 | <0.001 |
| 46-62 | 2.107 | 1.734 | — | 2.561 | <0.001 |
| 62+ | 3.528 | 2.695 | — | 4.619 | <0.001 |
| Sex | |||||
| Men | Ref | Ref | Ref | Ref | |
| Women | 0.798 | 0.707 | — | 0.9 | <0.001 |
| Metastasis at time of diagnosis | |||||
| Yes | 3.958 | 3.478 | — | 4.505 | <0.001 |
| Year of diagnosis | |||||
| 2004-2007 | Ref | Ref | Ref | Ref | |
| 2008-2011 | 1.102 | 0.966 | — | 1.258 | 0.149 |
| 2012-2015 | 0.965 | 0.815 | — | 1.142 | 0.677 |
| Treatment choice | |||||
| None | Ref | Ref | Ref | Ref | |
| Surgery | 0.305 | 0.227 | — | 0.411 | <0.001 |
| Radiation | 16.575 | 2.263 | — | 121.416 | 0.006 |
| Chemotherapy | 0.919 | 0.707 | — | 1.193 | 0.525 |
| Surgery and radiation | 0.599 | 0.309 | — | 1.165 | 0.131 |
| Surgery and chemotherapy | 0.476 | 0.375 | — | 0.605 | <0.001 |
| Radiation and chemotherapy | 5.62 | 0.767 | — | 41.161 | 0.089 |
| Unknown | 0.363 | 0.217 | — | 0.606 | <0.001 |
IRR = Incidence Rate Ratio
Defined as the incidence rate of the exposed population divided by the total number of people at risk of death at any one point in time.
Significance set at <0.05.
Table 4 demonstrates the multivariate Cox analysis of the likelihood of death at any given time for demographic and surgical variables for the appendicular cohort. In similarity to the axial cohort, the likelihood of death increased in a sequential manner with increasing age category (23 to 45 age cohort: IRR = 1.496, P = 0.012; 46 to 62 age cohort: IRR = 2.243, P < 0.001; 62+ age cohort: IRR = 4.088, P < 0.001) and the presence of metastasis at time of diagnosis (IRR = 3.393, P < 0.001). Unlike the axial cohort, no statistically significant change existed in the IRR with female sex. No statistically significant change existed in the IRR with the year of diagnosis. The likelihood of death demonstrated a statistically significant increase with the treatment choice of chemotherapy alone (IRR = 1.682, P = 0.001), whereas the likelihood of death demonstrated a statistically significant decrease with the treatment choice was surgery alone (IRR = 0.443, P < 0.001).
Table 4.
A Multivariate Cox Analysis of the Likelihood of Death at Any Given Time for Demographic and Surgical Variables for the Appendicular Cohort
| Likelihood of Death at Any Given Time | IRRa | 95% Confidence Interval | P | ||
| Age category | |||||
| <23 | Ref | Ref | Ref | Ref | |
| 23-45 | 1.496 | 1.094 | — | 2.045 | 0.012 |
| 46-62 | 2.243 | 1.614 | — | 3.117 | <0.001 |
| 62+ | 4.088 | 2.776 | — | 6.020 | <0.001 |
| Sex | |||||
| Men | Ref | Ref | Ref | Ref | |
| Women | 0.782 | 0.641 | — | 0.955 | 0.016 |
| Metastasis at time of diagnosis | |||||
| Yes | 3.393 | 2.659 | — | 4.330 | <0.001 |
| Year of diagnosis | |||||
| 2004-2007 | Ref | Ref | Ref | Ref | |
| 2008-2011 | 1.008 | 0.804 | — | 1.263 | 0.947 |
| 2012-2015 | 0.844 | 0.644 | — | 1.106 | 0.218 |
| Treatment choice | |||||
| None | Ref | Ref | Ref | Ref | |
| Surgery | 0.443 | 0.309 | — | 0.637 | <0.001 |
| Radiation | 4.533 | 1.044 | — | 19.683 | 0.044 |
| Chemotherapy | 1.682 | 1.241 | — | 2.279 | 0.001 |
| Surgery and radiation | 0.840 | 0.514 | — | 1.372 | 0.486 |
| Surgery and chemotherapy | 0.788 | 0.592 | — | 1.049 | 0.103 |
| Radiation and chemotherapy | 8.244 | 1.046 | — | 64.997 | 0.045 |
| Unknown | 0.883 | 0.533 | — | 1.465 | 0.631 |
IRR = Incidence Rate Ratio
Defined as the incidence rate of the exposed population divided by the total number of people at risk of death at any one point in time.
Significance set at <0.05.
Discussion
Osteosarcoma remains a clinical challenge. This study evaluated 4,430 patients with osteosarcoma from the NCDB database, which is the largest osteosarcoma cohort presented to date in the literature. Despite advances in tumor management, long-term survival of patients with osteosarcoma was found to be similar over the last 15 years. As expected, metastasis at presentation heralded a worse prognosis and being a surgical candidate was correlated with increased life-expectancy.
Most cases identified were appendicular in nature. This is consistent with known epidemiology of osteosarcoma.20 In fact, 77.5% of the cases were appendicular. The second most common site was axial (18.3%). The least common site was other (eg, head, neck, or mandible, 4.2%). Again, these figures are consistent with previous studies and substantiate the representative nature of the National Trauma Data Bank (NTDB) population.
The overall 1-year osteosarcoma survival was 87.1%, the 5-year survival was 60.17%, and then 10-year survival was 53.92%, which is consistent with previous reports.3,21 These figures were slightly better for the appendicular cohort (1-year 91.17%, 5-year 64.43%, and 10-year 58.58%), but note that the overall numbers were largely driven by the appendicular numbers because of their overwhelming majority of the study population. Conversely, there was worse survival for the axial cohort (1-year 74.22%, 5-year 45.97%, 10-year 38.27%) presumably because of later stage diagnosis and more limited surgical options. The other group was more similar to axial group for presumed similar reasons.
Surgical excision of osteosarcoma is widely held as the best treatment option when possible. It is thus not surprising that when surgery is performed, outcomes are the highest. Surgical intervention addresses the source of the neoplasm. When examining the progress in overall survival over the past few decades, the increase in survival in the 1970s was attributed to the addition of chemotherapy to osteosarcoma treatment regimens.22 Although there have been advances in chemotherapy, a prospective study examined 31 consecutive patients with local osteosarcomas who were treated exclusively with chemotherapy found that only 3 of the 31 patients were cured exclusively with chemotherapy, suggesting that chemotherapy alone is insufficient for the management of osteosarcoma.23
It has also been shown that there has not been an increase in 5-year overall survival from the 1980s to the 2000s.22 The current investigation was able to evaluate for possible more recent changes in survival (time intervals evaluated were 2004 to 2007, 2008 to 2011, and 2012 to 2015). This study also found no increases were found in survival noted over these time intervals. Certainly, new treatment paradigms exist for osteosarcoma, including immune-based therapies—including inhaled aerosolized granulocyte-macrophage colony-stimulating factor and liposomal muramyl tripeptide phosphatidylethanolamine—to treat and prevent lung metastasis as well as using antitumor agents and antiangiogenesis agents24; however, the impact of such interventions may not be fully reflected in the time frame studied because of a lack of 10-year follow-up data for more recent years in the data set.
This investigation also demonstrated that axial disease and metastasis at the initial presentation were poor predictors of long-term survival, which is consistent with previous investigations.25,26 One may postulate that there is a multitude of surgical interventions to resect a tumor within the appendicular skeleton,27 whereas a tumor that originates in the axial skeleton may prove difficult and more complex for complete en bloc resection. Regarding the importance of primary metastasis, Ozaki et al25 examined the overall survival of 67 patients with high-grade osteosarcoma of the pelvis and found that primary metastasis represented a statistically significant poor prognostic factor. One may postulate that osteosarcomas with metastatic potential may represent a more advanced or aggressive lesion in comparison to those without metastasis, thereby conferring decreased overall survival.
Increased age was associated with worse survival. It should be noted that this may represent a confounding variable; however, an analysis of the demographic data between the cohorts demonstrated that a statistically significant difference existed in the age between the cohorts, with the appendicular cohort demonstrating a younger age in comparison to the axial cohort. One may propose that the decrease in the age of osteosarcoma within the appendicular skeleton is because the appendicular skeletal has the most areas with high epiphyseal plate proliferation,28 which would inevitably decrease the age range for the appendicular cohort. A previous investigation has demonstrated that patients older the age of 65 demonstrated worse prognosis because of more metastatic disease at initial presentation, fewer patients receiving chemotherapy, and more patients excluded from clinical trials.29 In a retrospective review of 438 patients with osteosarcoma, however, age was not a notable independent prognostic indicator of the overall survival or disease-free survival.30 Although age may represent a confounding variable, previous research has shown that tumor site and primary metastasis were statistically notable poor prognostic factors on a multivariate Cox regression analysis, which would have taken age into consideration.26
The fact that male sex represents a poor prognostic factor is in concordance with previous investigations that have demonstrated that male sex is associated with delayed presentation and worse outcomes for the same level of disease in comparison to the female sex.31,32 Bieleck et al31 examined 1,702 consecutive newly diagnosed patients with high-grade osteosarcoma and found that male sex was associated with poor response to chemotherapy on both univariate and multivariate analysis. However, it should be noted that on univariate analysis, male sex was not as statistically significant in comparison to tumor site, duration of symptoms before presentation, and treatment delay. In addition, male sex was not significantly associated with poor overall survival or event-free survival.31
There have also been advances regarding the surgical technique in performing surgical resections of osteosarcomas. Computer-assisted tumor surgery, using three-dimensional image-guided bone resection, may increase the accuracy of complex tumor resections,33-35 although there are issues with computer-assisted tumor surgery, including a learning curve to become comfortable with the technology and a mismatch between preoperative images and actual patient anatomy.33 More extensive research is needed to investigate whether technology-assisted tumor surgery helps to improve the overall survival and disease-free survival in patients with osteosarcoma.
Limitations exist to this study. The data used in this study were acquired from the NCDB and therefore may demonstrate an overrepresentation of academic institutions with access to clinical trials. However, given the fact that the NCDB represents more than 70% of new cancer diagnoses in the United States and contains more than 30 million patient records,16,17 it is believed that this is a patient cohort that could be generalizable. Given the fact that the data within the NCDB are gathered from numerous institutions, the collection of these data may in fact be heterogenous. However, as multiple of the variables examined where objective in nature, it is believed this would not markedly affect the results of this investigation. Finally, tumor and treatment specifics (such as radiation-induced osteosarcomas) beyond general categories may affect outcomes and/or classifications and are not captured by the data set but may have an impact on survival.
Conclusion
In conclusion, this study evaluated more than 4,000 patients with osteosarcoma and defined modern survival rates as largely unchanged from more dated studies. Surgical excision remains the best predictor of survival for osteosarcomas. Furthermore, factors correlating with decreased survival include distant metastases, increasing age, male sex, and axial location. With survival having remained similar over the time frames studied, the need for further advances in this area are highlighted and leaves room for additional studies as cancer care progresses.
Footnotes
Dr. J. N. Grauer or an immediate family member has received or may receive payments or benefits, during the study period, in an amount of USD less than USD 10,000 from TIDI products (Neenah, WI) and in an amount of USD 10,000 to USD 100,000 from the North American Spine Society (Burr Ridge, IL), and is a member of the board of the Lumbar Spine Research Society (Oregon, WI). None of the following authors or any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: Dr. Ottesen, Shultz, Dr. Munger, Sibindi, Yurter, Dr. Varthi, and Dr. Grauer.
T. D. Ottesen, and B. S. Shultz are co-first authors.
IRB approval: This study received exemption by our institution's Human Investigations Committee.
Contributor Information
Taylor D. Ottesen, Email: taylor.ottesen@yale.edu.
Blake N. Shultz, Email: blake.shultz@yale.edu.
Alana M. Munger, Email: alana.munger@yale.edu.
Cosmas Sibindi, Email: cosmas.sibindi@yale.edu.
Alp Yurter, Email: alpyurter@gmail.com.
Arya G. Varthi, Email: arya.varthi@yale.edu.
References
- 1.Ottaviani G, Jaffe N: The epidemiology of osteosarcoma, in Jaffe N, Bruland OS, Bielack S, eds. Pediatric and Adolescent Osteosarcoma. Boston, MA, Springer US, 2010, pp 3-13. [Google Scholar]
- 2.U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on 2020 submission data (1999–2018): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; www.cdc.gov/cancer/dataviz, June 2021. [Google Scholar]
- 3.Mirabello L, Troisi RJ, Savage SA: International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer 2009;125:229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stitzlein RN, Wojcik J, Sebro RA, Balamuth NJ, Weber KL: Team approach: Osteosarcoma of the distal part of the femur in adolescents. JBJS Rev 2017;5:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC: Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res 2004;429:286-291. [DOI] [PubMed] [Google Scholar]
- 6.Kong CB, Kim MS, Lee SY, et al. : Prognostic effect of diaphyseal location in osteosarcoma: A cohort case-control study at a single institute. Ann Surg Oncol 2009;16:3094-3100. [DOI] [PubMed] [Google Scholar]
- 7.Lee JA, Kim MS, Kim DH, et al. : Risk stratification based on the clinical factors at diagnosis is closely related to the survival of localized osteosarcoma. Pediatr Blood Cancer 2009;52:340-345. [DOI] [PubMed] [Google Scholar]
- 8.Dahlin DC, Coventry MB: Osteogenic sarcoma. A study of six hundred cases. J Bone Joint Surg Am 1967;49:101-110. [PubMed] [Google Scholar]
- 9.Mukherjee D, Chaichana KL, Gokaslan ZL, Aaronson O, Cheng JS, McGirt MJ: Survival of patients with malignant primary osseous spinal neoplasms: Results from the surveillance, epidemiology, and end results (SEER) database from 1973 to 2003. J Neurosurg Spine 2011;14:143-150. [DOI] [PubMed] [Google Scholar]
- 10.Lawrenz JM, Curtis GL, Styron JF, et al. : Adult primary bone sarcoma and time to treatment initiation: An analysis of the national cancer database. Sarcoma 2018;2018:1728302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson ME: Update on survival in osteosarcoma. Orthop Clin North Am 2016;47:283-292. [DOI] [PubMed] [Google Scholar]
- 12.Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R: Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther 2018;18:39-50. [DOI] [PubMed] [Google Scholar]
- 13.Bishop MW, Janeway KA, Gorlick R: Future directions in the treatment of osteosarcoma. Curr Opin Pediatr 2016;28:26-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson E, Brown HL: Understanding osteosarcomas. JAAPA 2018;31:15-19. [DOI] [PubMed] [Google Scholar]
- 15.DeLaney TF, Park L, Goldberg SI, et al. : Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol Biol Phys 2005;61:492-498. [DOI] [PubMed] [Google Scholar]
- 16.Merkow RP, Rademaker AW, Bilimoria KY: Practical guide to surgical data sets: National cancer database (NCDB). JAMA Surg 2018;153:850-851. [DOI] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Stewart AK, Winchester DP, Ko CY: The national cancer data base: A powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.About the National Cancer Database. 2019. https://www.facs.org/quality-programs/cancer/ncdb/about. Accessed December 23, 2019. [Google Scholar]
- 19.Sedgwick P: Incidence rate ratio. BMJ 2010;341:c4804. [DOI] [PubMed] [Google Scholar]
- 20.Fraumeni JF, Jr: Stature and malignant tumors of bone in childhood and adolescence. Cancer 1967;20:967-973. [DOI] [PubMed] [Google Scholar]
- 21.Misaghi A, Goldin A, Awad M, Kulidjian AA: Osteosarcoma: A comprehensive review. Sicot j 2018;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allison DC, Carney SC, Ahlmann ER, et al. : A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012;2012:704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaffe N, Carrasco H, Raymond K, Ayala A, Eftekhari F: Can cure in patients with osteosarcoma be achieved exclusively with chemotherapy and abrogation of surgery? Cancer 2002;95:2202-2210. [DOI] [PubMed] [Google Scholar]
- 24.Nagarajan R, Clohisy D, Weigel B: New paradigms for therapy for osteosarcoma. Curr Oncol Rep 2005;7:410-414. [DOI] [PubMed] [Google Scholar]
- 25.Ozaki T, Flege S, Kevric M, et al. : Osteosarcoma of the pelvis: Experience of the cooperative osteosarcoma study group. J Clin Oncol 2003;21:334-341. [DOI] [PubMed] [Google Scholar]
- 26.Lin L, Deng S, Zhang F, Liang Y, Huang Z: The extremity localized classic osteosarcomas have better survival than the axial non-classics. World J Surg Oncol 2018;16:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bus MP, Bramer JA, Schaap GR, et al. : Hemicortical resection and inlay allograft reconstruction for primary bone tumors: A retrospective evaluation in The Netherlands and review of the literature. J Bone Joint Surg Am 2015;97:738-750. [DOI] [PubMed] [Google Scholar]
- 28.Rogozhin DV, Bulycheva IV, Konovalov DM, et al. : Classical osteosarcoma in children and adolescent [in Russian]. Arkh Patol 2015;77:68-74. [DOI] [PubMed] [Google Scholar]
- 29.Longhi A, Errani C, Gonzales-Arabio D, Ferrari C, Mercuri M: Osteosarcoma in patients older than 65 years. J Clin Oncol 2008;26:5368-5373. [DOI] [PubMed] [Google Scholar]
- 30.Harting MT, Lally KP, Andrassy RJ, et al. : Age as a prognostic factor for patients with osteosarcoma: An analysis of 438 patients. J Cancer Res Clin Oncol 2010;136:561-570. [DOI] [PubMed] [Google Scholar]
- 31.Bielack SS, Kempf-Bielack B, Delling G, et al. : Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002;20:776-790. [DOI] [PubMed] [Google Scholar]
- 32.Saeter G, Elomaa I, Wahlqvist Y, et al. : Prognostic factors in bone sarcomas. Acta Orthop Scand Suppl 1997;273:156-160. [DOI] [PubMed] [Google Scholar]
- 33.Wong KC, Niu X, Xu H, Li Y, Kumta S: Computer navigation in orthopaedic tumour surgery. Adv Exp Med Biol 2018;1093:315-326. [DOI] [PubMed] [Google Scholar]
- 34.Hüfner T, Kfuri M, Jr, Galanski M, et al. : New indications for computer-assisted surgery: Tumor resection in the pelvis. Clin Orthop Relat Res 2004:219-225. [DOI] [PubMed] [Google Scholar]
- 35.Jeys L, Matharu GS, Nandra RS, Grimer RJ: Can computer navigation-assisted surgery reduce the risk of an intralesional margin and reduce the rate of local recurrence in patients with a tumour of the pelvis or sacrum? Bone Joint J 2013;95-b:1417-1424. [DOI] [PubMed] [Google Scholar]