INTRODUCTION
Tumors characterized by homologous recombination deficiency, including BRCA1/2-mutated cancers, are sensitive to inhibition of poly(ADP-ribose) polymerases (PARPs), enzymes that regulate DNA repair.1,2 In tumor cells with mutated homologous recombination repair (HRR) genes, PARP inhibition synergizes with homologous recombination deficiency leading to synthetic lethality because of accumulated DNA damage.2,3
Rational combinations designed to increase DNA damage and reliance on HRR are promising strategies for increasing sensitivity to PARP inhibitors, although overlapping toxicities, such as myelosuppression, suggest a need for more selective and rational targeted agents.2,4-6 In human tumor cell lines, topoisomerase 1 (Topo1) inhibitors, including irinotecan and topotecan, have demonstrated synergy with PARP inhibitors.4,5 Since PARP1 is required for the clearance of Topo1-DNA cleavable complexes, PARP inhibition may augment Topo1-mediated DNA damage or delay repair.7,8 PARP inhibition has been shown to potentiate the cytotoxicity of SN-38, the active metabolite in irinotecan and topotecan, in mismatch repair-deficient and repair-proficient cell lines.9 Furthermore, combination of a PARP inhibitor with topotecan or irinotecan in early clinical studies delayed repair of Topo1-mediated DNA damage, but also demonstrated challenges with overlapping hematologic and/or gastrointestinal toxicities.10,11
The phase Ib SEASTAR study (ClinicalTrials.gov identifier: NCT03992131) was designed to evaluate the safety, tolerability, and preliminary efficacy of the PARP inhibitor rucaparib in combination with other anticancer agents. Rucaparib is approved in the United States and European Union for treatment or maintenance treatment of patients with recurrent ovarian cancer (OC),12,13 and in the United States for patients with metastatic castration-resistant prostate cancer,12 and is under investigation in patients with solid tumors harboring mutations in HRR genes.14 Arm B of the SEASTAR study investigated the combination of rucaparib with sacituzumab govitecan (SG), a conjugate of SN-38 with a humanized antibody targeting Trop-2 (trophoblast cell-surface antigen-2), a cell surface antigen overexpressed in epithelial cancers that has been linked to aggressive disease and poor prognosis. Targeted delivery of SN-38 to cancer cells through an antibody-drug conjugate (ADC) is a rational and effective strategy for combination therapy with a PARP inhibitor by potentially reducing off-target and additive toxicity.15,16 SG is approved in the United States for the treatment of patients with metastatic triple-negative breast cancer (TNBC) and urothelial cancer (UC),17 and has shown preliminary antitumor activity in other cancer types.18 Here, we report the results for six patients who received the combination of rucaparib and SG in arm B of the SEASTAR study.
METHODS
Study Design and Patients
The phase Ib open-label, parallel-arm SEASTAR study was approved by local and/or national institutional review boards and performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Council for Harmonisation. All patients provided written informed consent for participation. The primary aim of the study was to determine the maximum tolerated dose and recommended phase II dose; investigator-assessed objective response rate was a key secondary end point.
Arm B enrolled adult patients (≥ 18 years) with previously treated, locally advanced or metastatic TNBC or UC; or relapsed, platinum-resistant OC. Patients with advanced, recurrent, or metastatic solid tumors with documented evidence of a deleterious alteration in BRCA1, BRCA2, PALB2, RAD51C, and/or RAD51D were also eligible. Genomic alterations were identified by local testing or through central next-generation sequencing of tumor tissue or baseline plasma by Foundation Medicine (Cambridge, MA). Prior PARP inhibitor treatment was allowed, but patients previously treated with irinotecan, topotecan, or any derivative were excluded. Additional inclusion and exclusion criteria are included in the Protocol.
Study Treatment and Assessments
This study used a standard 3 + 3 dose escalation design, with a starting dose of 300 mg rucaparib twice a day (cohort 1) or 300 mg rucaparib once daily (cohort 2) in combination with 6 mg/kg SG administered intravenously (IV), on days 1 and 8 of a 21-day cycle. Dose-limiting toxicities (DLTs) were evaluated in cycle 1. Treatment interruptions and/or dose reductions were permitted for rucaparib (in 100-mg decrements) or SG (by 25% dose reduction) in the event of toxicity. Growth factor administration was permitted for treatment of toxicity when clinically indicated. Prophylactic administration of growth factors was allowed after the DLT evaluation period. Response was assessed per RECIST, version 1.1 (v1.1). Detailed descriptions of predefined DLTs, management of adverse events (AEs), and safety and efficacy assessments are included in the Protocol.
RESULTS
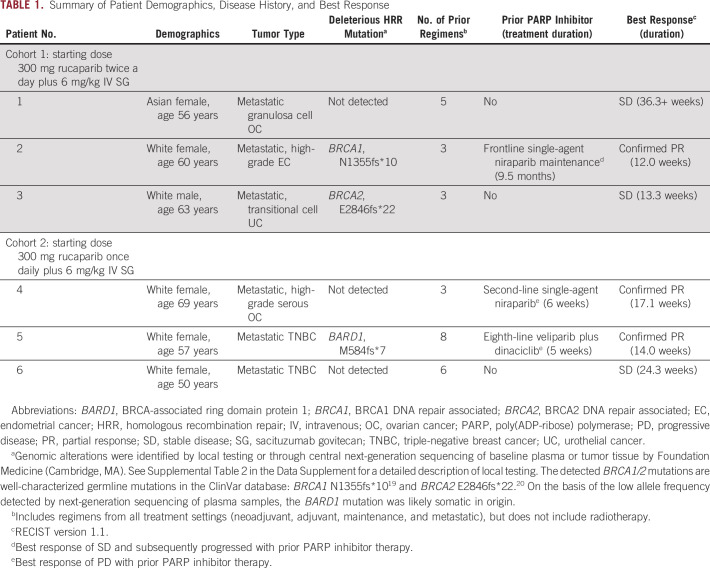
Six patients were enrolled in two dose cohorts (n = 3 each). Patients in cohort 1 received a starting dose of 300 mg rucaparib twice a day plus 6 mg/kg IV SG on days 1 and 8 of each cycle; cohort 2 received 300 mg rucaparib once daily plus 6 mg/kg IV SG on days 1 and 8 of each cycle (Table 1). All patients had metastatic solid tumors, including TNBC (n = 2), OC (n = 2), endometrial (n = 1), and UC (n = 1). Two patients had a known deleterious BRCA1 or BRCA2 gene mutation at enrollment, and one patient had a deleterious BARD1 mutation detected in circulating tumor DNA at baseline using central plasma testing. Patients received a median of 4 prior regimens (range, 3-8), with three patients previously receiving a PARP inhibitor (Fig 1).
TABLE 1.
Summary of Patient Demographics, Disease History, and Best Response
FIG 1.
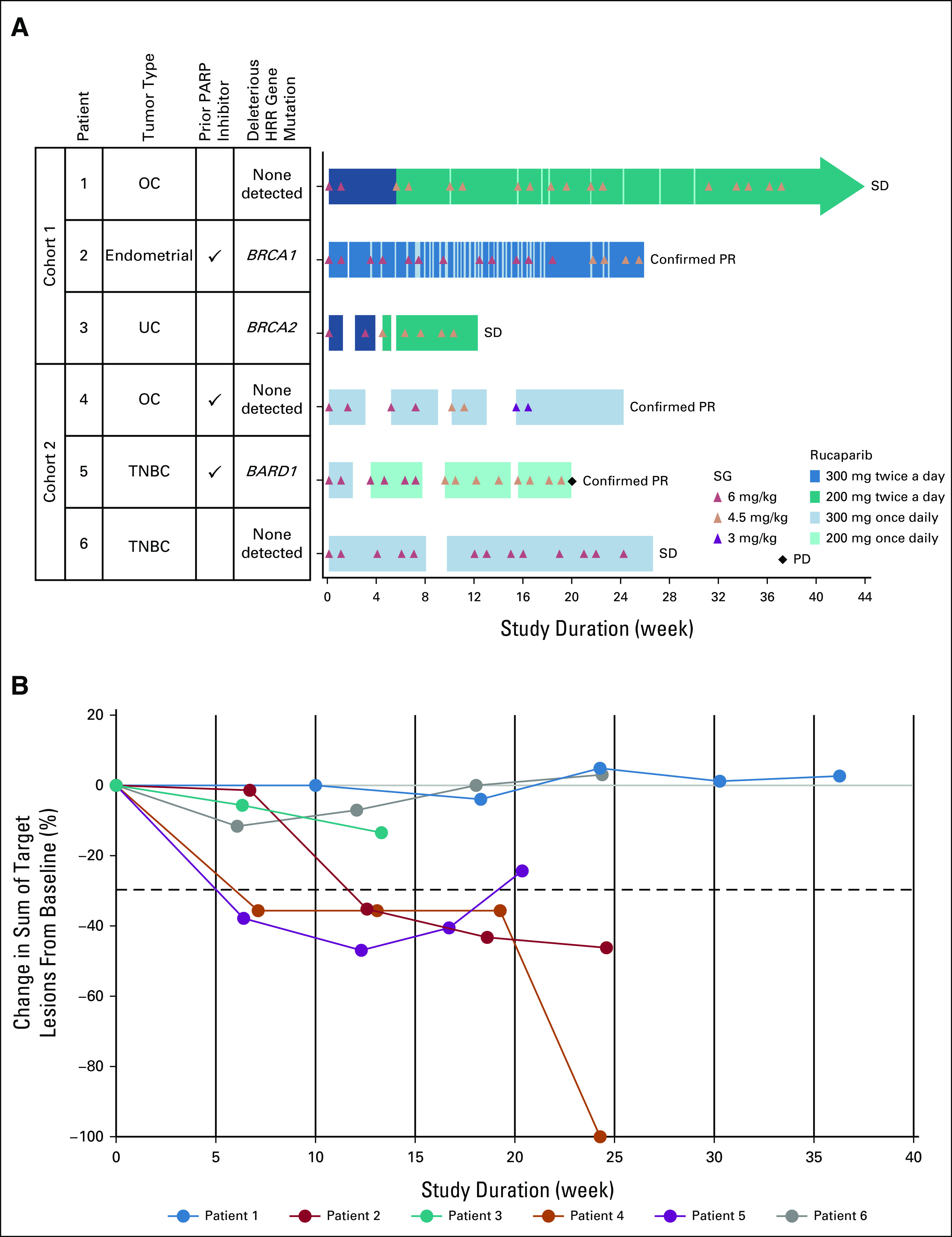
Overview of efficacy and treatment with rucaparib plus SG. (A) Duration of treatment and best overall response. Arrowhead denotes ongoing treatment as of the August 11, 2020, data cutoff date. (B) Change in tumor volume over time for each patient. Dotted line indicates the threshold for partial response (30% decrease from baseline). Because of the COVID-19 pandemic, SG was withheld after cycle 6 for patient 1. SG was then discontinued during cycle 11 at the patient's request. SG was withheld after cycle 4 for patient 4 because of the pandemic. BARD1, BRCA-associated ring domain protein 1; BRCA1, BRCA1 DNA repair associated; BRCA2, BRCA2 DNA repair associated; HRR, homologous recombination repair; OC, ovarian cancer; PARP, poly(ADP-ribose) polymerase; PD, progressive disease; PR, partial response; SD, stable disease; SG, sacituzumab govitecan; TNBC, triple-negative breast cancer; UC, urothelial cancer.
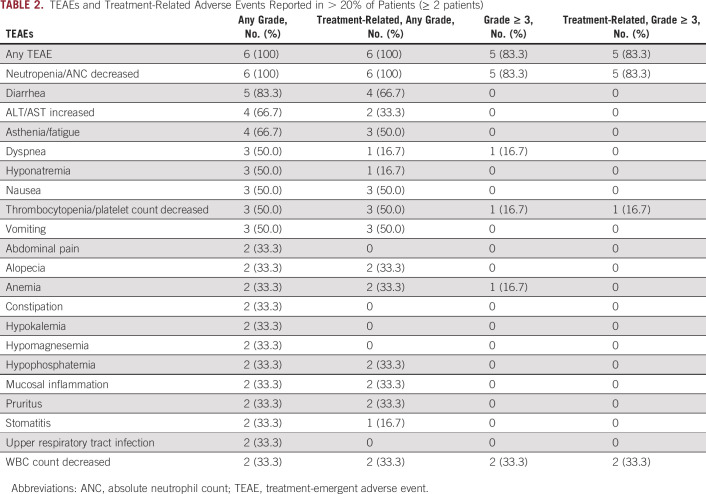
Two of three patients in cohort 1 experienced DLTs of grade 4 neutropenia. No DLTs were observed in cohort 2, although grade 3/4 neutropenia led to 1- to 2-week delays in starting cycle 2 in all three patients. All patients experienced at least one treatment-emergent AE (TEAE) (Table 2). The most common TEAEs were neutropenia/decreased absolute neutrophil count (ANC) (n = 6), diarrhea (n = 5), increased ALT/AST (n = 4), and asthenia/fatigue (n = 4). Grade ≥ 3 TEAEs were reported in five patients; those reported in ≥ 2 patients were neutropenia/decreased ANC (n = 5) and decreased WBC count (n = 2), all considered treatment-related. Genotypic analysis of ABCC2, SLCO1B1, and UGT1A121,22 showed no clear trends relating patient genotype and toxicity (Data Supplement, Supplemental Table 1). With management of TEAEs via treatment interruption, dose reduction, and/or granulocyte colony-stimulating factor support, all patients continued treatment for ≥ 12 weeks, with a mean (±standard deviation) exposure of 25.7 ± 10.5 weeks for rucaparib and 22.1 ± 9.3 weeks (7.3 ± 2.9 cycles) for SG (Fig 2). As of the cutoff date of August 11, 2020, one patient with OC in cohort 1 (patient 1) remained on rucaparib for 44+ weeks (having discontinued SG after week 37 [cycle 11]).
TABLE 2.
TEAEs and Treatment-Related Adverse Events Reported in > 20% of Patients (≥ 2 patients)
FIG 2.
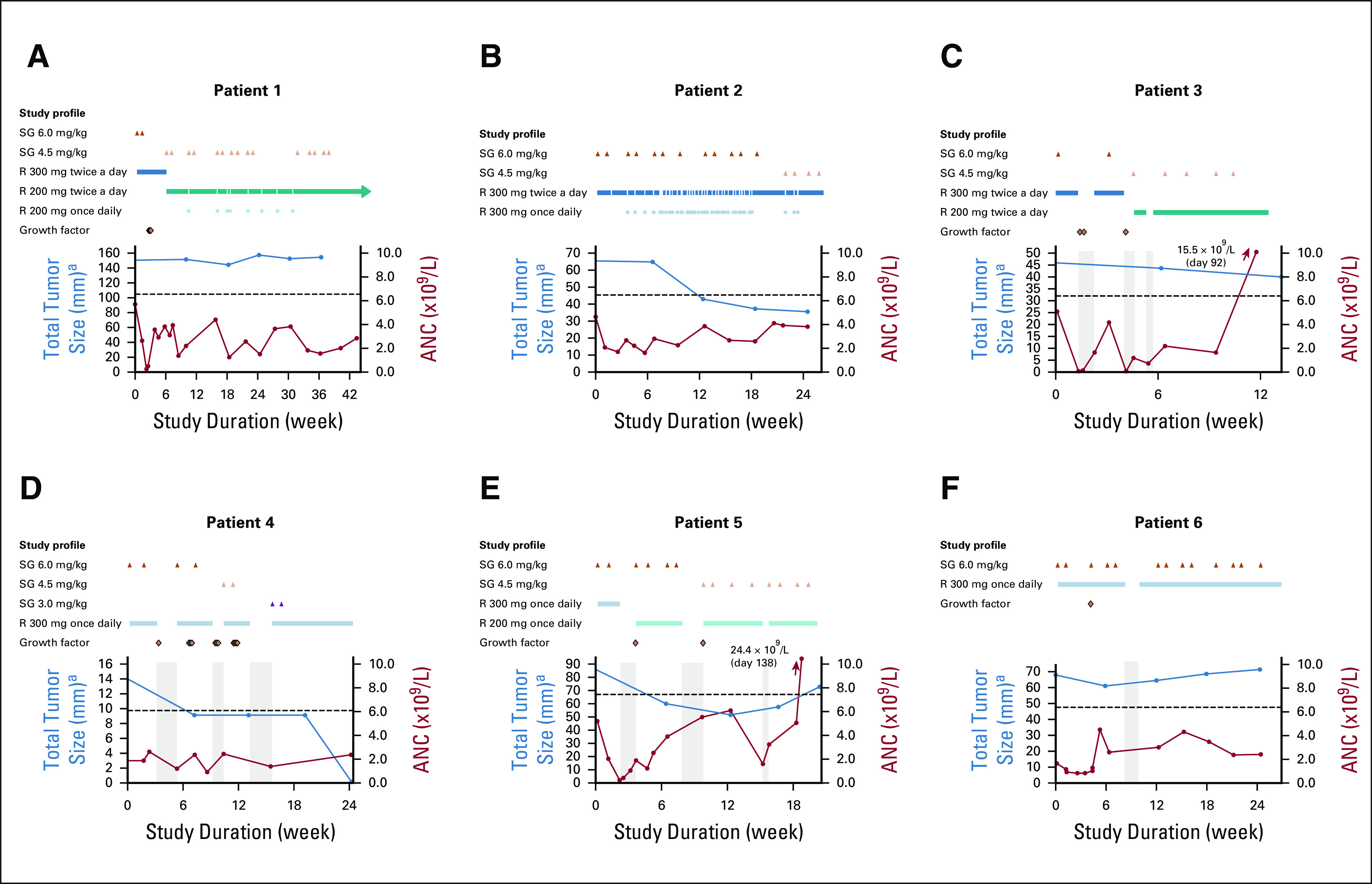
Investigator-assessed tumor response and ANC over the course of treatment. A timeline is included for each patient showing treatment interruptions (gray bars) and dose reductions of oral rucaparib (twice a day or once daily) and SG (administered intravenously on days 1 and 8 of a 21-day cycle), as well as administration of growth factors. Dotted line indicates the threshold for partial response (30% decrease from baseline). ANC, absolute neutrophil count; R, rucaparib; SG, sacituzumab govitecan. aEvaluated per RECIST v1.1.
All patients had an investigator-assessed best response of RECIST v1.1 stable disease or better (Fig 1). Three patients had a confirmed RECIST v1.1 partial response (Fig 3); all three patients were previously treated with a PARP inhibitor until disease progression (two with niraparib monotherapy, and one with veliparib plus dinaciclib), including one patient with no known deleterious HRR gene mutation (Table 1). No reversion mutations in HRR genes were identified in these three patients by central testing.
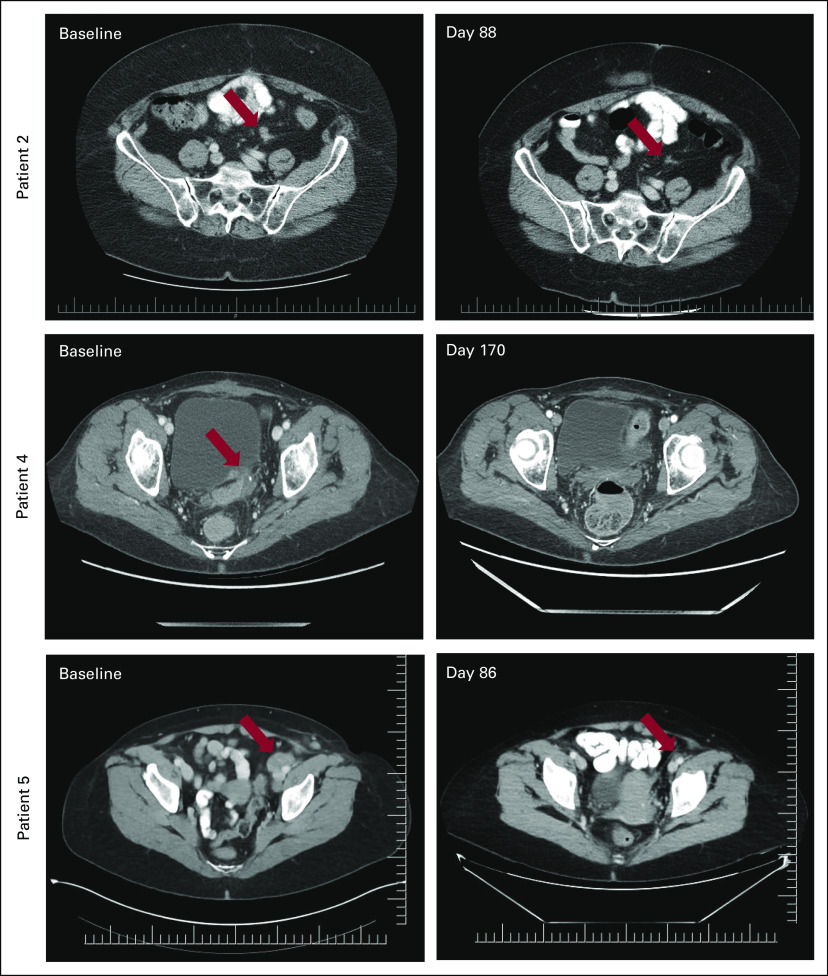
FIG 3.
Representative computed tomography scans showing confirmed RECIST version 1.1 partial responses. Patient 2 had an overall 46.2% reduction in endometrial cancer tumor burden at day 88, including a 41.2% decrease in diameter of the small, left pelvic mesenteric nodule shown. Patient 4 experienced complete regression of a 1.4-cm serosal ovarian cancer metastatic implant in the posterior bladder wall by day 170. Patient 5 had a 68.6% reduction in diameter of the left iliac lymph node lesion shown here at day 86 and an overall best response of 47.1% reduction in triple-negative breast cancer tumor burden.
DISCUSSION
The results from this case series suggest that rucaparib plus SG has promising antitumor activity in patients with advanced solid tumors, including PARP inhibitor–exposed patients with tumors with and without HRR gene mutations. Although submaximal doses of SG and rucaparib were combined, decreases in ANC levels were observed. DLTs because of neutropenia were not unexpected, given the known toxicity profiles of Topo1 and PARP inhibitors.12,15,23-25 In a previous study in advanced epithelial cancers, 33% of patients experienced grade ≥ 3 neutropenia with SG monotherapy.15 Combinations of topotecan or irinotecan with olaparib or irinotecan-based chemotherapy with veliparib resulted in hematologic DLTs or severe toxicities and are no longer in development.26-28 Although UGT1A1 genotype has been linked to elevated rates of neutropenia and diarrhea with irinotecan or SG15,22,29-31 and neutropenia with rucaparib plus irinotecan,32 the results from this study did not show any clear relationships with such toxicities. However, correlations may have been limited by the small number of patients in this series.
In patients treated with SG monotherapy, neutropenia is typically managed with a combination of treatment interruptions, dose reductions, or granulocyte colony-stimulating factor administration.17 By applying similar strategies in this study, all patients were able to continue therapy and had a best response of RECIST v1.1 stable disease or better. Antitumor activity in a patient with prior PARP inhibitor treatment without HRR mutation is notable, given the current unmet clinical need in identifying rational combinations capable of enhancing the efficacy of PARP inhibitor therapy in a broader range of patients beyond those harboring HRR-mutant tumors.2,6
In summary, the results from the SEASTAR study provide proof-of-concept clinical evidence supporting further development of PARP inhibitors in combination with ADCs carrying Topo1-inhibitor payloads. Importantly, recent data suggest that a pulse-dosing schedule of rucaparib plus irinotecan allows for long-term tolerability and has demonstrated encouraging efficacy in patients with tumors harboring ATM mutations.32 Combination of other Trop-2–directed ADCs, such as datopotamab deruxtecan,18 with more selective PARP inhibitors, such as the PARP1-targeted inhibitor AZD5305,33 may also improve tolerability. Although no optimal recommended phase II dose was established in the current study, these data suggest that combination trials are warranted to investigate intermittent dosing of PARP inhibitors together with SG or other ADCs to reduce myelosuppression and optimize antitumor efficacy; future research may also help clarify the relative contributions of each agent to the observed antitumor activity.
Timothy A. Yap
Consulting or Advisory Role: Pfizer, EMD Serono, Clovis Oncology, Ignyta, AstraZeneca, Atrin Pharmaceuticals, Aduro Biotech, Merck, Almac Diagnostics, Bayer, Bristol Myers Squibb, Calithera Biosciences, Cybrexa Therapeutics, Janssen, Kyn Therapeutics, Roche, Seattle Genetics, Axiom Biotechnologies, F-Star, Guidepoint Global, I-Mab, Repare Therapeutics, Rubius Therapeutics, Schrodinger, Varian Medical Systems, Zai Lab
Research Funding: AstraZeneca (Inst), Vertex Pharmaceuticals (Inst), Pfizer (Inst), Bayer (Inst), Tesaro (Inst), Jounce Therapeutics (Inst), Seattle Genetics (Inst), Kyowa Hakko Kirin (Inst), Constellation Pharmaceuticals (Inst), Lilly (Inst), Artios (Inst), Clovis Oncology (Inst), Cyteir (Inst), EMD Serono (Inst), Forbius (Inst), F-Star (Inst), GlaxoSmithKline (Inst), Genentech (Inst), ImmuneSensor Therapeutics (Inst), Ipsen (Inst), Karyopharm Therapeutics (Inst), Merck (Inst), Novartis (Inst), Ribon Therapeutics (Inst), Regeneron (Inst), Repare Therapeutics (Inst), Sanofi (Inst), Scholar Rock (Inst)
Erika Hamilton
Consulting or Advisory Role: Pfizer (Inst), Genentech/Roche (Inst), Lilly (Inst), Puma Biotechnology (Inst), Daiichi Sankyo (Inst), Mersana (Inst), Boehringer Ingelheim (Inst), AstraZeneca (Inst), Novartis (Inst), Silverback Therapeutics (Inst), Black Diamond Therapeutics (Inst), Nanostring (Inst), CytomX Therapeutics (Inst), Dantari (Inst), H3 Biomedicine (Inst), Merck (Inst), Seattle Genetics (Inst), Eisai (Inst), Deciphera (Inst), Arvinas (Inst), Arcus Biosciences (Inst), ITeos Therapeutics (Inst), Janssen (Inst), Loxo (Inst), Relay Therapeutics (Inst)
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), OncoMed (Inst), MedImmune (Inst), Stem CentRx (Inst), Genentech/Roche (Inst), Curis (Inst), Verastem (Inst), Zymeworks (Inst), Syndax (Inst), Lycera (Inst), Rgenix (Inst), Novartis (Inst), Mersana (Inst), Millennium (Inst), TapImmune Inc (Inst), Lilly (Inst), Pfizer (Inst), Tesaro (Inst), Boehringer Ingelheim (Inst), H3 Biomedicine (Inst), Radius Health (Inst), Acerta Pharma (Inst), Macrogenics (Inst), AbbVie (Inst), Immunomedics (Inst), Fujifilm (Inst), eFFECTOR Therapeutics (Inst), Merus (Inst), Nucana (Inst), Regeneron (Inst), Leap Therapeutics (Inst), Taiho Pharmaceutical (Inst), EMD Serono (Inst), Daiichi Sankyo (Inst), ArQule (Inst), Syros Pharmaceuticals (Inst), Clovis Oncology (Inst), CytomX Therapeutics (Inst), InventisBio (Inst), Deciphera (Inst), Sermonix Pharmaceuticals (Inst), Sutro Biopharma (Inst), Zenith Epigenetics (Inst), Arvinas (Inst), Harpoon (Inst), Fochon Pharmaceuticals (Inst), Black Diamond Therapeutics (Inst), Orinove (Inst), Molecular Templates (Inst), Silverback Therapeutics (Inst), Seattle Genetics (Inst), Compugen (Inst), G1 Therapeutics (Inst), Puma Biotechnology (Inst), BerGenBio (Inst), Medivation (Inst), Eisai (Inst), Takeda (Inst), Aravive (Inst), Unum Therapeutics (Inst), Torque Therapeutics (Inst), Karyopharm Therapeutics (Inst), Dana Farber Cancer Hospital (Inst), Onconova Therapeutics (Inst), Shattuck Labs (Inst), Merck (Inst), PharmaMar (Inst), Olema Pharmaceuticals (Inst), Immunogen (Inst), Plexxikon (Inst), Amgen (Inst), Akesobio (Inst), ADC Therapeutics (Inst), AtlasMedx (Inst), Aravive (Inst), Ellipses Pharma (Inst), Incyte (Inst), MabSpace Biosciences (Inst), Myriad Genetics (Inst), ORIC Pharmaceuticals (Inst), Pieris Pharmaceuticals (Inst), Pionyr (Inst), Repertoire Immune Medicines (Inst), Treadwell Therapeutics (Inst), Vincerx Pharma (Inst)
Todd Bauer
Employment: Tennessee Oncology
Consulting or Advisory Role: Ignyta (Inst), Guardant Health, Loxo, Moderna Therapeutics (Inst), Pfizer (Inst), Exelixis, Blueprint Medicines, Foundation Medicine, Bayer, AstraZeneca
Speakers' Bureau: Bayer, Bristol Myers Squibb, Lilly
Research Funding: Daiichi Sankyo (Inst), Medpacto (Inst), Incyte (Inst), Mirati Therapeutics (Inst), MedImmune (Inst), AbbVie (Inst), AstraZeneca (Inst), Leap Therapeutics (Inst), MabVax (Inst), Stemline Therapeutics (Inst), Merck (Inst), Lilly (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Pfizer (Inst), Genentech/Roche (Inst), Deciphera (Inst), Merrimack (Inst), Immunogen (Inst), Millennium (Inst), Ignyta (Inst), Calithera Biosciences (Inst), Kolltan Pharmaceuticals (Inst), Principa Biopharma (Inst), Peleton (Inst), Immunocore (Inst), Roche (Inst), Aileron Therapeutics (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Moderna Therapeutics (Inst), Sanofi (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), Five Prime Therapeutics (Inst), Jacobio (Inst), Top Alliance BioScience (Inst), Loxo (Inst), Janssen (Inst), Clovis Oncology (Inst), Takeda (Inst), Karyopharm Therapeutics (Inst), Onyx (Inst), Phosplatin Therapeutics (Inst), Foundation Medicine (Inst), ARMO BioSciences (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, AstraZeneca, Celgene, Clovis Oncology, EMD Serono, Genentech, Lilly, Merck, Novartis, Pharmacyclics, Sysmex, Pfizer
Ecaterina E. Dumbrava
Consulting or Advisory Role: Bolt Biotherapeutics, Catamaran Bio
Research Funding: Bayer (Inst), Immunocore (Inst), Amgen (Inst), NCI (Inst), Aileron Therapeutics (Inst), Compugen (Inst), TRACON Pharma (Inst), Unum Therapeutics (Inst), Immunomedics (Inst), Bolt Biotherapeutics (Inst), Aprea Therapeutics (Inst), Bellicum Pharmaceuticals (Inst), PMV Pharma (Inst), Triumvira Immunologics Inc (Inst), Seattle Genetics (Inst), Mereo BioPharma 5 Inc (Inst), Sanofi (Inst), Astex Therapetics (Inst)
Rinath Jeseslsohn
Consulting or Advisory Role: Luminex, Carrick Therapeutics
Research Funding: Lilly, Pfizer
Aaron Enke
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Sabrina Hurley
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology, Pfizer
Kevin K. Lin
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Travel, Accommodations, Expenses: Clovis Oncology
Jenn Habeck
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Heidi Giordano
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Geoffrey I. Shapiro
Consulting or Advisory Role: G1 Therapeutics, Lilly, Pfizer, Roche, Merck Serono, Sierra Oncology, Cybrexa Therapeutics, Ipsen, Bayer, Fusion Pharmaceuticals, Bicycle Therapeutics, Almac Diagnostics, Astex Pharmaceuticals, Daiichi Sankyo, Angiex, Seattle Genetics, Artios, Boehringer Ingelheim, Concarlo, Atrin Pharmaceuticals, Syros Pharmaceuticals, Zentalis, CytomX Therapeutics, Blueprint Medicines, Kymera, ImmunoMet Therapeutics, Asana
Research Funding: Pfizer (Inst), Genentech (Inst), Bayer (Inst), Immune Design (Inst), Vertex (Inst), Millennium (Inst), Puma Biotechnology (Inst), Tensha Therapeutics (Inst), Covidien (Inst), Novartis (Inst), Cellceutix (Inst), Sanofi (Inst), Cyclacel (Inst), Mirati Therapeutics (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Lilly (Inst), Aileron Therapeutics (Inst), PharmaMar (Inst), PTC Therapeutics (Inst), Roche (Inst), CanBas (Inst), Tesaro (Inst), Merck Serono (Inst), Sierra Oncology (Inst), Syros Pharmaceuticals (Inst), Curis (Inst), Merck (Inst), Array BioPharma (Inst), Seattle Genetics (Inst), Clovis Oncology (Inst), Exelixis (Inst), Boehringer Ingelheim (Inst), Esperas Pharma (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Patent No.: 9872874, Title: Dosage regimen for sapacitabine and seliciclib Issue Date: January 23, 2018, Provisional Patent No.: 62/538,319, Title: Compositions and methods for predicting response and resistance to CDK4/6 inhibition, Filed: July 28, 2017
Travel, Accommodations, Expenses: Lilly, Pfizer, Bicycle Therapeutics, G1 Therapeutics, Sierra Oncology, Bayer
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the European Society for Medical Oncology Virtual Congress 2020, September 17, 2020.
SUPPORT
Funded by Clovis Oncology Inc and was designed by the sponsor. Medical writing and editorial support were funded by Clovis Oncology and provided by Melanie Styers and Leslie Mitchell of Verascity Science. T. A. Y. is supported by MD Anderson Cancer Center Support grant (NIH/NCI P30 CA016672), the US Department of Defense Ovarian Cancer Research Program (OC200482), and the V Foundation Clinical Scholar Program (VC2020-001). T.A.Y. and E.E.D. acknowledge support from MD Anderson Cancer Center Support Grant No. NIH/NCI P30 CA016672, Clinical Translational Science Award No. 1UL1 TR003167, Cancer Prevention Research Institute of Texas Precision Oncology Decision Support Core RP150535, and Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy. G.I.S. is supported by the Dana-Farber/Harvard Cancer Center Support Grant No. (NIH/NCI P30 CA006516). T.A.Y. also acknowledges support from the US Department of Defense Ovarian Cancer Research Program (OC200482) and the V Foundation Clinical Scholar Program (VC2020-001).
AUTHOR CONTRIBUTIONS
Conception and design: Timothy A. Yap, Erika Hamilton, Aaron Enke, Heidi Giordano, Geoffrey I. Shapiro
Provision of study materials or patients: Timothy A. Yap, Erika Hamilton, Todd Bauer, Ecaterina E. Dumbrava, Rinath Jeselsohn, Geoffrey I. Shapiro
Collection and assembly of data: Timothy A. Yap, Erika Hamilton, Todd Bauer, Ecaterina E. Dumbrava, Rinath Jeselsohn, Geoffrey I. Shapiro
Data analysis and interpretation: Timothy A. Yap, Erika Hamilton, Ecaterina E. Dumbrava, Aaron Enke, Kevin K. Lin, Jenn Habeck, Geoffrey I. Shapiro
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Timothy A. Yap
Consulting or Advisory Role: Pfizer, EMD Serono, Clovis Oncology, Ignyta, AstraZeneca, Atrin Pharmaceuticals, Aduro Biotech, Merck, Almac Diagnostics, Bayer, Bristol Myers Squibb, Calithera Biosciences, Cybrexa Therapeutics, Janssen, Kyn Therapeutics, Roche, Seattle Genetics, Axiom Biotechnologies, F-Star, Guidepoint Global, I-Mab, Repare Therapeutics, Rubius Therapeutics, Schrodinger, Varian Medical Systems, Zai Lab
Research Funding: AstraZeneca (Inst), Vertex Pharmaceuticals (Inst), Pfizer (Inst), Bayer (Inst), Tesaro (Inst), Jounce Therapeutics (Inst), Seattle Genetics (Inst), Kyowa Hakko Kirin (Inst), Constellation Pharmaceuticals (Inst), Lilly (Inst), Artios (Inst), Clovis Oncology (Inst), Cyteir (Inst), EMD Serono (Inst), Forbius (Inst), F-Star (Inst), GlaxoSmithKline (Inst), Genentech (Inst), ImmuneSensor Therapeutics (Inst), Ipsen (Inst), Karyopharm Therapeutics (Inst), Merck (Inst), Novartis (Inst), Ribon Therapeutics (Inst), Regeneron (Inst), Repare Therapeutics (Inst), Sanofi (Inst), Scholar Rock (Inst)
Erika Hamilton
Consulting or Advisory Role: Pfizer (Inst), Genentech/Roche (Inst), Lilly (Inst), Puma Biotechnology (Inst), Daiichi Sankyo (Inst), Mersana (Inst), Boehringer Ingelheim (Inst), AstraZeneca (Inst), Novartis (Inst), Silverback Therapeutics (Inst), Black Diamond Therapeutics (Inst), Nanostring (Inst), CytomX Therapeutics (Inst), Dantari (Inst), H3 Biomedicine (Inst), Merck (Inst), Seattle Genetics (Inst), Eisai (Inst), Deciphera (Inst), Arvinas (Inst), Arcus Biosciences (Inst), ITeos Therapeutics (Inst), Janssen (Inst), Loxo (Inst), Relay Therapeutics (Inst)
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), OncoMed (Inst), MedImmune (Inst), Stem CentRx (Inst), Genentech/Roche (Inst), Curis (Inst), Verastem (Inst), Zymeworks (Inst), Syndax (Inst), Lycera (Inst), Rgenix (Inst), Novartis (Inst), Mersana (Inst), Millennium (Inst), TapImmune Inc (Inst), Lilly (Inst), Pfizer (Inst), Tesaro (Inst), Boehringer Ingelheim (Inst), H3 Biomedicine (Inst), Radius Health (Inst), Acerta Pharma (Inst), Macrogenics (Inst), AbbVie (Inst), Immunomedics (Inst), Fujifilm (Inst), eFFECTOR Therapeutics (Inst), Merus (Inst), Nucana (Inst), Regeneron (Inst), Leap Therapeutics (Inst), Taiho Pharmaceutical (Inst), EMD Serono (Inst), Daiichi Sankyo (Inst), ArQule (Inst), Syros Pharmaceuticals (Inst), Clovis Oncology (Inst), CytomX Therapeutics (Inst), InventisBio (Inst), Deciphera (Inst), Sermonix Pharmaceuticals (Inst), Sutro Biopharma (Inst), Zenith Epigenetics (Inst), Arvinas (Inst), Harpoon (Inst), Fochon Pharmaceuticals (Inst), Black Diamond Therapeutics (Inst), Orinove (Inst), Molecular Templates (Inst), Silverback Therapeutics (Inst), Seattle Genetics (Inst), Compugen (Inst), G1 Therapeutics (Inst), Puma Biotechnology (Inst), BerGenBio (Inst), Medivation (Inst), Eisai (Inst), Takeda (Inst), Aravive (Inst), Unum Therapeutics (Inst), Torque Therapeutics (Inst), Karyopharm Therapeutics (Inst), Dana Farber Cancer Hospital (Inst), Onconova Therapeutics (Inst), Shattuck Labs (Inst), Merck (Inst), PharmaMar (Inst), Olema Pharmaceuticals (Inst), Immunogen (Inst), Plexxikon (Inst), Amgen (Inst), Akesobio (Inst), ADC Therapeutics (Inst), AtlasMedx (Inst), Aravive (Inst), Ellipses Pharma (Inst), Incyte (Inst), MabSpace Biosciences (Inst), Myriad Genetics (Inst), ORIC Pharmaceuticals (Inst), Pieris Pharmaceuticals (Inst), Pionyr (Inst), Repertoire Immune Medicines (Inst), Treadwell Therapeutics (Inst), Vincerx Pharma (Inst)
Todd Bauer
Employment: Tennessee Oncology
Consulting or Advisory Role: Ignyta (Inst), Guardant Health, Loxo, Moderna Therapeutics (Inst), Pfizer (Inst), Exelixis, Blueprint Medicines, Foundation Medicine, Bayer, AstraZeneca
Speakers' Bureau: Bayer, Bristol Myers Squibb, Lilly
Research Funding: Daiichi Sankyo (Inst), Medpacto (Inst), Incyte (Inst), Mirati Therapeutics (Inst), MedImmune (Inst), AbbVie (Inst), AstraZeneca (Inst), Leap Therapeutics (Inst), MabVax (Inst), Stemline Therapeutics (Inst), Merck (Inst), Lilly (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Pfizer (Inst), Genentech/Roche (Inst), Deciphera (Inst), Merrimack (Inst), Immunogen (Inst), Millennium (Inst), Ignyta (Inst), Calithera Biosciences (Inst), Kolltan Pharmaceuticals (Inst), Principa Biopharma (Inst), Peleton (Inst), Immunocore (Inst), Roche (Inst), Aileron Therapeutics (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Moderna Therapeutics (Inst), Sanofi (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), Five Prime Therapeutics (Inst), Jacobio (Inst), Top Alliance BioScience (Inst), Loxo (Inst), Janssen (Inst), Clovis Oncology (Inst), Takeda (Inst), Karyopharm Therapeutics (Inst), Onyx (Inst), Phosplatin Therapeutics (Inst), Foundation Medicine (Inst), ARMO BioSciences (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, AstraZeneca, Celgene, Clovis Oncology, EMD Serono, Genentech, Lilly, Merck, Novartis, Pharmacyclics, Sysmex, Pfizer
Ecaterina E. Dumbrava
Consulting or Advisory Role: Bolt Biotherapeutics, Catamaran Bio
Research Funding: Bayer (Inst), Immunocore (Inst), Amgen (Inst), NCI (Inst), Aileron Therapeutics (Inst), Compugen (Inst), TRACON Pharma (Inst), Unum Therapeutics (Inst), Immunomedics (Inst), Bolt Biotherapeutics (Inst), Aprea Therapeutics (Inst), Bellicum Pharmaceuticals (Inst), PMV Pharma (Inst), Triumvira Immunologics Inc (Inst), Seattle Genetics (Inst), Mereo BioPharma 5 Inc (Inst), Sanofi (Inst), Astex Therapetics (Inst)
Rinath Jeseslsohn
Consulting or Advisory Role: Luminex, Carrick Therapeutics
Research Funding: Lilly, Pfizer
Aaron Enke
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Sabrina Hurley
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology, Pfizer
Kevin K. Lin
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Travel, Accommodations, Expenses: Clovis Oncology
Jenn Habeck
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Heidi Giordano
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Geoffrey I. Shapiro
Consulting or Advisory Role: G1 Therapeutics, Lilly, Pfizer, Roche, Merck Serono, Sierra Oncology, Cybrexa Therapeutics, Ipsen, Bayer, Fusion Pharmaceuticals, Bicycle Therapeutics, Almac Diagnostics, Astex Pharmaceuticals, Daiichi Sankyo, Angiex, Seattle Genetics, Artios, Boehringer Ingelheim, Concarlo, Atrin Pharmaceuticals, Syros Pharmaceuticals, Zentalis, CytomX Therapeutics, Blueprint Medicines, Kymera, ImmunoMet Therapeutics, Asana
Research Funding: Pfizer (Inst), Genentech (Inst), Bayer (Inst), Immune Design (Inst), Vertex (Inst), Millennium (Inst), Puma Biotechnology (Inst), Tensha Therapeutics (Inst), Covidien (Inst), Novartis (Inst), Cellceutix (Inst), Sanofi (Inst), Cyclacel (Inst), Mirati Therapeutics (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Lilly (Inst), Aileron Therapeutics (Inst), PharmaMar (Inst), PTC Therapeutics (Inst), Roche (Inst), CanBas (Inst), Tesaro (Inst), Merck Serono (Inst), Sierra Oncology (Inst), Syros Pharmaceuticals (Inst), Curis (Inst), Merck (Inst), Array BioPharma (Inst), Seattle Genetics (Inst), Clovis Oncology (Inst), Exelixis (Inst), Boehringer Ingelheim (Inst), Esperas Pharma (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Patent No.: 9872874, Title: Dosage regimen for sapacitabine and seliciclib Issue Date: January 23, 2018, Provisional Patent No.: 62/538,319, Title: Compositions and methods for predicting response and resistance to CDK4/6 inhibition, Filed: July 28, 2017
Travel, Accommodations, Expenses: Lilly, Pfizer, Bicycle Therapeutics, G1 Therapeutics, Sierra Oncology, Bayer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Amé JC, Spenlehauer C, De Murcia G.The PARP superfamily BioEssays 26882–8932004 [DOI] [PubMed] [Google Scholar]
- 2. Rose M, Burgess JT, O'Byrne K, et al. PARP inhibitors: Clinical relevance, mechanisms of action and tumor resistance. Front Cell Dev Biol. 2020;8:564601. doi: 10.3389/fcell.2020.564601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashworth A.A synthetic lethal therapeutic approach: Poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair J Clin Oncol 263785–37902008 [DOI] [PubMed] [Google Scholar]
- 4.Delaney CA, Wang LZ, Kyle S, et al. Potentiation of temozolomide and topotecan growth inhibition and cytotoxicity by novel poly(adenosine diphosphoribose) polymerase inhibitors in a panel of human tumor cell lines Clin Cancer Res 62860–28672000 [PubMed] [Google Scholar]
- 5.Murai J, Zhang Y, Morris J, et al. Rationale for poly(ADP-ribose) polymerase (PARP) inhibitors in combination therapy with camptothecins or temozolomide based on PARP trapping versus catalytic inhibition J Pharmacol Exp Ther 349408–4162014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilié PG, Gay CM, Byers LA, et al. PARP inhibitors: Extending benefit beyond BRCA-mutant cancers Clin Cancer Res 253759–37712019 [DOI] [PubMed] [Google Scholar]
- 7.Smith LM, Willmore E, Austin CA, et al. The novel poly(ADP-ribose) polymerase inhibitor, AG14361 sensitizes cells to topoisomerase I poisons by increasing the persistence of DNA strand breaks Clin Cancer Res 118449–84572005 [DOI] [PubMed] [Google Scholar]
- 8.Znojek P, Willmore E, Curtin NJ.Preferential potentiation of topoisomerase I poison cytotoxicity by PARP inhibition in S phase Br J Cancer 1111319–13262014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tahara M, Inoue T, Sato F, et al. The use of olaparib (AZD2281) potentiates SN-38 cytotoxicity in colon cancer cells by indirect inhibition of rad51-mediated repair of DNA double-strand breaks Mol Cancer Ther 131170–11802014 [DOI] [PubMed] [Google Scholar]
- 10.LoRusso PM, Li J, Burger A, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the poly(ADP-ribose) polymerase (PARP) inhibitor veliparib (ABT-888) in combination with irinotecan in patients with advanced solid tumors Clin Cancer Res 223227–32372016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kummar S, Chen A, Ji J, et al. Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas Cancer Res 715626–56342011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubraca (Rucaparib) Tablets [prescribing information] Boulder, CO: Clovis Oncology; 2020. https://clovisoncology.com/pdfs/RubracaUSPI.pdf [Google Scholar]
- 13.Rubraca (Rucaparib) Tablets [summary of product characteristics] Swords, Ireland: Clovis Oncology Ireland; 2019. https://www.ema.europa.eu/en/documents/product-information/rubraca-epar-product-information_en.pdf [Google Scholar]
- 14.ClinicalTrials.gov A study to evaluate rucaparib in patients with solid tumors and with deleterious mutations in HRR genes (LODESTAR) https://clinicaltrials.gov/ct2/show/NCT04171700
- 15.Ocean AJ, Starodub AN, Bardia A, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: Safety and pharmacokinetics Cancer 1233843–38542017 [DOI] [PubMed] [Google Scholar]
- 16.Stepan LP, Trueblood ES, Hale K, et al. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: Potential implications as a cancer therapeutic target J Histochem Cytochem 59701–7102011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trodelvy (Sacituzumab Govitecan-Hziy) Injection [prescribing information] Morris Plains, NJ: Immunomedics; 2021. https://www.gilead.com/-/media/files/pdfs/medicines/oncology/trodelvy/trodelvy_pi.pdf [Google Scholar]
- 18.Bardia A.Datopotamab deruxtecan (Dato-DXd), a TROP2-directed antibody-drug conjugate (ADC), for triple-negative breast cancer (TNBC): Preliminary results from an ongoing phase 1 trial Ann Oncol 32S60–S782021suppl 2 [Google Scholar]
- 19.ClinVar database: BRCA1 N1355fs*10. https://www.ncbi.nlm.nih.gov/clinvar/variation/17674/
- 20.ClinVar database: BRCA2 E2846fs*22. https://www.ncbi.nlm.nih.gov/clinvar/variation/52614/
- 21.Rhodes K, Zhang W, Yang D, et al. ABCB1, SLCO1B1 and UGT1A1 gene polymorphisms are associated with toxicity in metastatic colorectal cancer treated with first-line irinotecan Drug Metab Lett 123–302008 [DOI] [PubMed] [Google Scholar]
- 22.Innocenti F, Kroetz DL, Schuetz E, et al. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics J Clin Oncol 272604–26142009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seiter K.Toxicity of the topoisomerase I inhibitors Expert Opin Drug Saf 445–532005 [DOI] [PubMed] [Google Scholar]
- 24.Shitara K, Bang Y-J, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer N Engl J Med 3822419–24302020 [DOI] [PubMed] [Google Scholar]
- 25.Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer N Engl J Med 382610–6212020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samol J, Ranson M, Scott E, et al. Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: A phase I study Invest New Drugs 301493–15002012 [DOI] [PubMed] [Google Scholar]
- 27.Chen EX, Jonker DJ, Siu LL, et al. A phase I study of olaparib and irinotecan in patients with colorectal cancer: Canadian Cancer Trials Group IND 187 Invest New Drugs 34450–4572016 [DOI] [PubMed] [Google Scholar]
- 28.Chiorean EG, Guthrie KA, Philip PA, et al. Randomized phase II study of PARP inhibitor veliparib with modified FOLFIRI versus FOLFIRI as second line treatment of metastatic pancreatic cancer: SWOG S1513 Clin Cancer Res 276314–63222021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan J Clin Oncol 221382–13882004 [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Cheng D, Kuang Q, et al. Association of UGT1A1*28 polymorphisms with irinotecan-induced toxicities in colorectal cancer: A meta-analysis in Caucasians Pharmacogenomics J 14120–1292014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bardia A, Messersmith W, Kio E, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial Ann Oncol 32746–7562021 [DOI] [PubMed] [Google Scholar]
- 32. Dhawan MS, Rahimi R, Karipineni S, et al. Phase I study of rucaparib and irinotecan in advanced solid tumors with homologous recombination deficiency (HRD) mutations. J Clin Oncol. 2020;38(15 suppl) doi: 10.1200/PO.23.00494. abstr 3513. [DOI] [PubMed] [Google Scholar]
- 33.Leo E, Johannes J.Discovery and first structural disclosure of AZD5305: A next generation, highly selective PARP1 inhibitor and trapper. Presented at the Virtual American Association for Cancer Research annual meeting, April 10-15, 2021 (abstr ND05). https://www.abstractsonline.com/pp8/#!/9325/presentation/874.