LETTER
Innate immune activation by central nervous system (CNS)-resident cells is critical for controlling herpes simplex virus 1 (HSV-1) replication. In particular, the type I interferon (IFN) response has been shown to limit early replication and reduce mortality in mouse models of herpes simplex encephalitis (HSE) (1, 2). However, the relative importance of different CNS cell populations in eliciting a complete type I IFN response during HSE remains largely understudied. Microglia are well known to have an important role in the innate response to neurotropic pathogens, but recent work in our laboratory and others demonstrated that cross talk between microglia and astrocytes also plays an important role in the immune response to HSV-1 (3, 4). Astrocyte-specific production of type I IFNs has also been shown to be protective in an ocular model of HSV-1 infection (5).
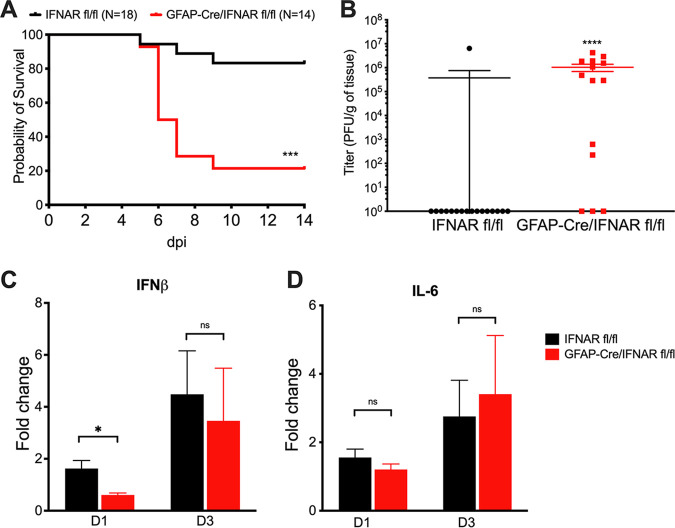
To assess the effect that type I IFN signaling in astrocytes has on HSE pathogenesis, we generated astrocyte-specific IFN-α receptor (IFNAR) knockout (KO) mice by crossing a mouse line expressing Cre recombinase driven by the glial fibrillary acidic protein (GFAP) promoter with a mouse line containing floxed IFNAR1 loci (IFNARfl/fl) (6, 7). The specificity of Cre expression in astrocytes has previously been demonstrated (8). GFAP-Cre+/IFNARfl/fl mice had significantly worse survival than littermate controls when intracranially (i.c.) infected with the low-virulence strain KOS in a model of HSE previously used in our laboratory (Fig. 1A) (9). Importantly, HSV-1 replicated to high levels in the brains of all GFAP-Cre+/IFNARfl/fl mice that succumbed to infection, indicating that type I IFN signaling in astrocytes is important for the clearance of viral replication in HSE (Fig. 1B). GFAP-Cre+/IFNARfl/fl mice had significantly lower expression levels of IFN-β in their brains at 24 h postinfection (hpi) than IFNARfl/fl littermate controls as measured by quantitative PCR (qPCR) (Fig. 1C). Levels of interleukin-6 (IL-6), a proinflammatory cytokine produced independently of type I IFN signaling, did not significantly differ between GFAP-Cre+/IFNARfl/fl mice and littermate controls at either time point (Fig. 1D).
FIG 1.
Astrocyte IFNAR signaling is required for survival and contributes to early IFN production following HSV-1 infection. (A and B) Survival (A) and viral titers in brains at mortality or the experimental endpoint at day 14 (B) of IFNARfl/fl or GFAP-Cre/IFNARfl/fl mice inoculated i.c. with 103 PFU of HSV-1 KOS at 8 to 10 weeks of age. dpi, days postinfection. (C and D) IFN-β (C) and IL-6 (D) mRNA levels quantified by qPCR following i.c. inoculation with 103 PFU HSV-1 (KOS) in IFNARfl/fl or GFAP-Cre/IFNARfl/fl mice at different time points (day 1 [D1] and day 3 [D3]). Samples were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression for each group (n = 3 to 4 for qPCR experiments). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001; ns, not significant. Values are expressed as means ± standard errors of the means (SEM). Kaplan-Meier survival curves were compared using log rank analysis. Viral titers were compared using Student’s t test. Gene expression was analyzed using multiple t tests with the Holm-Sidak correction for multiple comparisons.
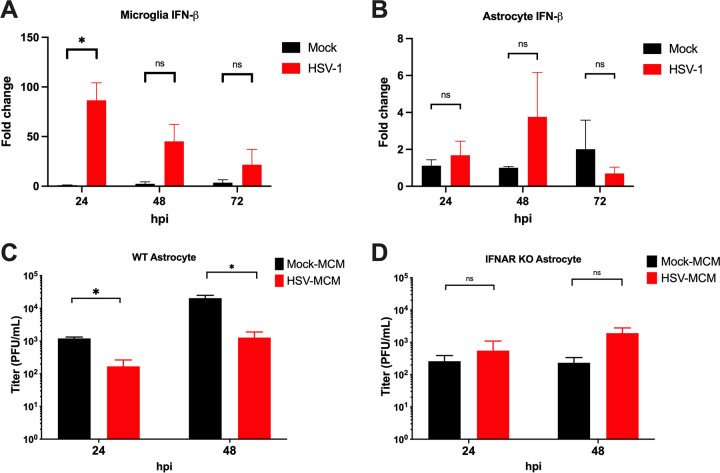
To assess the contribution of microglia to the astrocyte type I IFN response during infection, primary microglia and astrocytes were isolated from mice and infected with HSV-1 at a multiplicity of infection (MOI) of 0.1 as previously reported (4). Microglia robustly upregulated IFN-β at 24 hpi as measured by qPCR (Fig. 2A), whereas astrocytes were poor producers of IFN-β, with no significant upregulation at any time point (Fig. 2B). These results suggest that microglia, not astrocytes, are the primary drivers of IFN-β expression during infection among these cell types. We next collected UV-inactivated microglia-conditioned medium (MCM) from mock- and HSV-1-infected primary microglia and pretreated astrocytes with MCM before infection. Wild-type (WT) astrocytes pretreated with MCM from HSV-1-infected microglia had significantly lower HSV-1 replication than astrocytes treated with MCM from mock-infected microglia as measured by a plaque assay (Fig. 2C). However, there was no significant difference in HSV-1 replication in IFNAR−/− astrocytes pretreated with MCM from infected or mock-infected microglia (Fig. 2D). Interestingly, viral titers in IFNAR−/− astrocytes may be lower at baseline than those in WT astrocytes, which suggests a reduced ability of IFNAR−/− astrocytes to support HSV-1 replication (Fig. 2C and D). These results suggest that functional IFNAR signaling in astrocytes is critical for controlling viral replication and that soluble factors produced by microglia act on astrocytes through IFNAR to suppress viral replication. While microglia have been studied extensively in the context of the immune response to HSV-1, our work shows that astrocytes and their immune interactions with other cell types are deserving of further study.
FIG 2.
IFN signaling from microglia contributes to viral control in astrocytes. WT astrocytes and microglia were isolated from 7-day-old mice and cultured for 7 days prior to infection with HSV-1 KOS at an MOI of 0.1. (A and B) IFN-β mRNA levels from microglia (A) and astrocytes (B) were quantified by qPCR following HSV-1 infection at 24, 48, and 72 h postinfection (hpi). Samples were normalized to GAPDH gene expression in mock-treated cells at each time point (n = 3 per group). Microglia-conditioned medium (MCM) was collected from microglia 24 h after infection with HSV-1 KOS at an MOI of 1 or mock. (C and D) WT (C) and IFNAR−/− (D) astrocytes were pretreated with 20% MCM for 24 h before infection with HSV-1 KOS at an MOI of 0.1. Supernatants were collected from HSV- or mock-infected astrocytes, and HSV-1 was quantified by a plaque assay (n = 3 to 5 per group). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001. Values are expressed as means ± SEM. For viral titers and gene expression analysis, multiple t tests were performed with the Holm-Sidak correction for multiple comparisons.
This study was carried out in adherence to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (10). The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Northwestern University (protocol IS00003204).
ACKNOWLEDGMENTS
We thank Nan Susmarski for her expertise in cell culture.
This work, including the efforts of R.L., was funded by HHS, National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS) (R01NS110631). This work, including the efforts of C.K.H., was funded by HHS, NIH, National Institute of Allergy and Infectious Diseases (NIAID) (F30AI150049), and by HHS, NIH (T32GM008152). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Richard Longnecker, Email: r-longnecker@northwestern.edu.
Jae U. Jung, Lerner Research Institute, Cleveland Clinic
REFERENCES
- 1.Wilcox DR, Folmsbee SS, Muller WJ, Longnecker R. 2016. The type I interferon response determines differences in choroid plexus susceptibility between newborns and adults in herpes simplex virus encephalitis. mBio 7:e00437-16. 10.1128/mBio.00437-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giraldo D, Wilcox DR, Longnecker R. 2020. The innate immune response to herpes simplex virus 1 infection is dampened in the newborn brain and can be modulated by exogenous interferon beta to improve survival. mBio 11:e00921-20. 10.1128/mBio.00921-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinert LS, Lopušná K, Winther H, Sun C, Thomsen MK, Nandakumar R, Mogensen TH, Meyer M, Vægter C, Nyengaard JR, Fitzgerald KA, Paludan SR. 2016. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat Commun 7:13348. 10.1038/ncomms13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes CK, Wilcox DR, Yang Y, Coleman GK, Brown MA, Longnecker R. 2021. ASC-dependent inflammasomes contribute to immunopathology and mortality in herpes simplex encephalitis. PLoS Pathog 17:e1009285. 10.1371/journal.ppat.1009285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr DJJ, Veress LA, Noisakran S, Campbell IL. 1998. Astrocyte-targeted expression of IFN-α1 protects mice from acute ocular herpes simplex virus type 1 infection. J Immunol 161:4859–4865. [PubMed] [Google Scholar]
- 6.Prigge JR, Hoyt TR, Dobrinen E, Capecchi MR, Schmidt EE, Meissner N. 2015. Type-I-IFNs act upon hematopoietic progenitors to protect and maintain hematopoiesis during Pneumocystis lung infection in mice. J Immunol 195:5347–5357. 10.4049/jimmunol.1501553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, Smith KB, Groszer M, Garcia AD, Sofroniew MV, Carmichael ST, Kornblum HI, Liu X, Wu H. 2009. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci 29:1874–1886. 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels BP, Jujjavarapu H, Durrant DM, Williams JL, Green RR, White JP, Lazear HM, Gale M, Diamond MS, Klein RS. 2017. Regional astrocyte IFN signaling restricts pathogenesis during neurotropic viral infection. J Clin Invest 127:843–856. 10.1172/JCI88720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp SJ, Banisadr G, Glajch K, Maurer UE, Grünewald K, Miller RJ, Osten P, Spear PG. 2009. Infection of neurons and encephalitis after intracranial inoculation of herpes simplex virus requires the entry receptor nectin-1. Proc Natl Acad Sci USA 106:17916–17920. 10.1073/pnas.0908892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]