Abstract
Stroke rehabilitation is expensive, and recent changes to Medicare reimbursement demand more efficient interventions. The use of cost-effectiveness analysis (CEA) can help occupational therapy practitioners, rehabilitation directors, and payers better understand the value of occupational therapy and decide whether or not to implement new treatments. The objective of this article is to illustrate the contribution of CEA to stroke rehabilitation using a hypothetical new intervention as an example.
What This Article Adds: This article facilitates an understanding of the importance of CEA to occupational therapy. It also explains how CEA improves consistency with reporting standards for cost-effectiveness studies.
This article illustrates the contribution of cost-effectiveness analysis in stroke rehabilitation using a hypothetical new intervention as an example.
The cost of poststroke care in the United States is the highest in the world (Godwin et al., 2011; Rajsic et al., 2019). The resources for medical care are finite, however. Cost-effectiveness analysis (CEA) can help us understand the long-term value of stroke rehabilitation, which may help inform the decision-making process for adopting new therapy approaches or rehabilitation devices. The objective of this article is to illustrate the contribution of CEA to stroke rehabilitation using a hypothetical new intervention as an example.
Arguments to Support Cost-Effectiveness Analysis
With changes to Medicare in 2020 (Unruh et al., 2020), CEAs are essential to the delivery of quality and value-based rehabilitation interventions. As a result, there is a need for research that examines interventions that can be used to maximize gains and minimize cost in outpatient settings. CEA added to clinical trials is needed to generate data documenting the intervention’s effect on patient outcomes, quality of life, cost, and cost-effectiveness as part of the implementation strategy. This point is recognized by the National Institutes of Health, which recommended economic evaluations for rehabilitation (Frontera et al., 2017).
Economic evaluations of new treatments during clinical trials are common in health care for pharmaceutical products (Jönsson, 2003) and for prevention interventions, such as vaccinations (Simpson et al., 1995) and screening (Marshall et al., 2001), which are relatively low-cost but high-volume services for which costs are immediate but benefits are delayed. The use of economic evaluation in rehabilitation, however, is limited. Although some economic evaluations have been reported for rehabilitation (Bürge et al., 2016; Chiatti & Iwarsson, 2014; Clarke et al., 2016; Fernández-de-Las-Peñas et al., 2019; Rahja et al., 2018; Schene et al., 2007), a recent assessment of reporting quality for economic evaluations in rehabilitation indicated that many authors fail to adhere to the accepted standards for describing how their study was performed (Flemming et al., 2020).
Access to occupational therapy for clients and efficiency in occupational therapy interventions can benefit from CEA. Occupational therapy practitioners take a client-centered approach to treatment to improve the client’s functional status specific to that client’s needs and wants (American Occupational Therapy Association, 2020; Pollock, 1993). Although occupational therapy practitioners traditionally do not consider information about cost, comparative effectiveness research related to interventions and rehabilitation devices will improve practitioners’ ability to make informed decisions about which treatments improve clients’ quality of life.
Process for Cost-Effectiveness Analysis
Well-conducted CEAs use standardized methods and allow for comparisons across different types of medical interventions. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS; Husereau et al., 2013) are a useful quality check (Cheng et al., 2018) for economic analyses. The use of CHEERS can standardize CEA and enable us to compare findings to ascertain their value in relation to other medical interventions.
In building a valid CEA model, clinician input is critical, because a model is a simplification of current practice conditions and must capture usual practice and treatment realities. First, economic researchers conduct preliminary qualitative interviews and analysis to provide a sufficient amount of data to determine clinically relevant variables within a model. After model completion, researchers contact therapists for validation of the model, to ensure accuracy and clarity. Choosing the right type of model also is important (Brennan et al., 2006). CEA uses two common models—(1) decision trees and (2) Markov models (Simpson et al., 2009)—both of which can be used to structure interventions under any reimbursement configuration for many different diseases from different data sources. Researchers must choose a model time horizon that is adequate for capturing major cost and effect differences between treatments, select a unit of analysis, and account for interactions among factors in the model (Neumann et al., 2017). Identifying the model’s costing perspective is also essential, and models are created to be country specific (Rascati, 2014).
Decision trees provide a visual example that includes pathways for different treatment approaches and associated probabilities. They make it easy to understand the major differences in treatments and their impact on outcomes. Clients are grouped on branches by different possible processes and outcomes (Neumann et al., 2017).
If decision trees become too “bushy” and complicated, the approach shifts to Markov modeling, which allows for data from groups with similar clinical statuses to be aggregated for specific time periods depending on the health state. Markov modeling is more complex, given that it represents more variables in the intervention process, but it still helps decision makers organize risks, forecast clinical outcomes, and estimate economic effects over a long time period. Once developed, the Markov model can be estimated using a probabilistic sensitivity analysis, cohort simulation, or algebraic matrix (Sonnenberg & Beck, 1993).
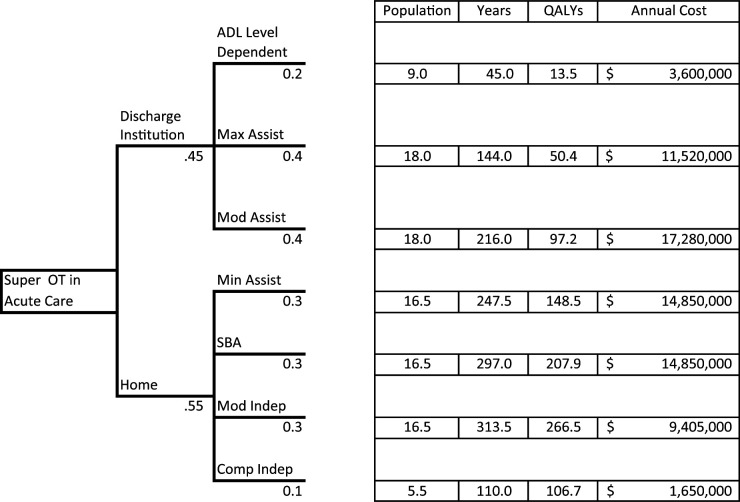
Hypothetical Decision Tree: Super OT Versus Treatment as Usual
The best way to show the powerful contributions of a cost-effectiveness model may be to create a simple decision tree from “real” practice: What if we implemented an intensive in-hospital occupational therapy program to prepare stroke patients for discharge to home? Would it be cost-effective if this “Super OT” program improved discharge to home by 5%, compared with treatment as usual (TAU), and required 10 additional hr of occupational therapy for all eligible patients? We designed a decision tree to capture this question (the full decision tree is available from the first author on request). Figure 1 depicts a hypothetical model that compares the new Super OT with TAU. For this model, we estimated Super OT’s impact on years survived, quality-adjusted life years (QALYs) gained, and total annual cost for 100 patients receiving each treatment. Thus, this is really a synthetic estimate of 200 patients evenly randomized into either the Super OT condition or TAU group, where all factors are assumed to be equal except discharge destination and cost of occupational therapy care in the hospital.
Figure 1.
Example of a decision tree for a hypothetical Super OT intervention per 100 patients, based on discharge destination and ADL assistance level.
Note. All dollar values are USD. ADL = activity of daily living; Comp Indep = complete independence; Max Assist = maximal assistance; Min Assist = minimal assistance; Mod Assist = moderate assistance; Mod Indep = modified independence; OT = occupational therapy; QALYs = quality-adjusted life years; SBA = standby assistance (supervision).
Several parameters need to be set to create the working model (Table 1). We categorized all stroke survivors by the level of assistance needed using FIM® scores1 (Davidoff et al., 1990; Keith et al., 1987). Next, we searched the literature for the average years survived for each level of assistance (hypothetical in this example). We also found the estimated annual cost of care for each patient at each level of assistance defined by the FIM. The Utility measure is an estimate of the ratio of quality of life per year a stroke survivor experiences at each FIM level. For example, a dependent patient may live for 5 yr after a stroke but, when adjusted for quality, experience only 1.5 yr of quality. The Utility measure is made by averaging scores of a quality-of-life assessment for a population of stroke survivors at each functional level. In our example, we hypothesized that each year of life for a dependent patient is worth about 30% of a year when adjusted for quality. In addition, occupational therapy treatment is estimated to cost $90/hr; 10 hr of service per patient equates to $900 per patient.
Table 1.
Model Inputs: ADL Scores, Utility Measures, Expected Survival, and Annual Cost Used for Estimations
| ADL FIM® Score | Utility (in QALYs) | Survival, Yr | Annual Cost (USD) |
|---|---|---|---|
| 1 | .30 | 5 | 80,000 |
| 2 | .35 | 8 | 80,000 |
| 3 | .45 | 12 | 80,000 |
| 4 | .60 | 15 | 60,000 |
| 5 | .70 | 18 | 50,000 |
| 6 | .85 | 19 | 30,000 |
| 7 | .97 | 20 | 15,000 |
| Additional Super OT Treatment | ||
|---|---|---|
| Hr | Cost/Hr | Cost/Patient |
| 10 | $90 | 900 |
Note. ADL = activity of daily living; OT = occupational therapy; QALYs = quality-adjusted life years.
For this example, we divided patients into two categories: (1) discharged to some form of inpatient rehabilitation or (2) discharged to home (with home health, outpatient, or no therapy). First, we constructed the tree for the Super OT group (Figure 1). Rates of discharge from acute care to an inpatient rehabilitation institution (.45) versus home (.55) were provided from a hypothetical clinical trial for Super OT. We assumed that all patients who were discharged from acute care to inpatient rehabilitation were classified as dependent (20% of inpatient rehabilitation stroke survivors), maximal assistance (40%), or moderate assistance (40%) for ADLs. We also assumed that patients who were classified as minimal assistance (30%), standby assistance (supervision; 30%), modified independence (30%), or complete independence (10%) were discharged to home. If we have the corresponding QALYs Utility measure, we could just as easily divide the two discharge groups by Fugl-Meyer scores, modified Rankin scores, or any other organizational variable.
We computed the total number of survival years for each functional level by simply multiplying the subpopulation by the expected number of years survived (Figure 1). For example, the 9 Dependent stroke survivors are expected to live for a combined 45 yr. Similarly, we estimated the number of QALYs by multiplying the number of years by the Utility (in our Dependent example, this means 45 yr × .30 utility). Finally, we estimated the cost for each subpopulation by multiplying the years of survival by the average annual cost (e.g., for Dependents, 45 yr × $80,000). These steps are repeated for each functional level.
For the TAU group, we copied the Super OT spreadsheet and changed only the percentage discharged to each type of site. We then could reestimate the discharge disposition for the TAU group (50% discharged to inpatient rehabilitation institution vs. 50% discharged to home). Because all our formulas were carried over from the previous page, the outcome measures are easily computed with the rate change. We then found the difference between the Super OT and TAU groups (Table 2).
Table 2.
Economic Impact of Super OT Versus TAU per 100 Patients
| Metric | Total Impact | ||
|---|---|---|---|
| Super OT | TAU | Difference | |
| Survival, yr | 1,373 | 1,330 | 43 |
| QALYs | 890.7 | 878.3 | 12.4 |
| Follow-up cost, USD | 73,155,000 | 73,050,000 | 105,000 |
| Super OT cost, USD | 90,000 | — | 90,000 |
| Total cost, USD | 73,245,000 | 73,050,000 | 195,000 |
| ICER | |||
| $/patient to gain 1 QALY | 15,725 | ||
| $/patient to gain 1 yr survival | 4,535 | ||
Note. — = not applicable; ICER = incremental cost-effectiveness ratio; OT = occupational therapy; QALYs = quality-adjusted life years; TAU = treatment as usual.
Notice the change in outcomes, with just a 5% improvement in ADL performance attributable to the Super OT intervention. We calculated an estimated 43 additional years of survival per 100 patients, but those years are not in full health, so they resulted in only 12.4 QALYs. In addition, occupational therapy treatment cost was estimated at $90,000 per 100 patients. The total annual cost increases by $195,000 (or $1,950/person) because survival years increase. Overall, this result equates to an incremental cost-effectiveness ratio of $15,725 per QALY gained, or $4,535 per additional year of survival gained. Compared with standards in health care, this is a good cost value for an intervention, because the U.S. benchmark for good value for money for stroke care is at least $50,000 per QALY gained (Simpson et al., 2017), and our Super OT program is much cheaper than that benchmark (Simpson & Tilley, 2012).
One important strength of a decision tree is that, once programmed, the rates are easily modified and outcomes recomputed. This is true for all numbers within the decision tree. These outcome measures could help a rehabilitation director understand the impact of implementing a new treatment method.
Conclusion
CEA can be an effective tool to help rehabilitation decision makers make more informed choices. Although it has been explored in rehabilitation in the past, greater use and a more standardized approach will enable comparisons across studies to help decision makers determine which interventions are most practical and effective. However, these outcomes should not be the sole consideration for decision making (Pinkerton et al., 2002). Ethical, access, practical, and professional issues may not be captured in an economic model but need to be considered with any change. A model is a simplification of reality, and as such, its estimates may contribute objective information to discussions of the potential impact of a new approach on expected quality years gained and annual cost. However, it yields only one additional set of data for decision makers to consider. CEA is not yet common in the occupational therapy literature, but we believe this analytical approach holds great potential for contributing to occupational therapy research and practice.
Acknowledgments
This article was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Grants TL1 TR001451 and UL1 TR001450. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
FIM is a trademark of the Uniform Data System for Medical Rehabilitation, a division of UB Foundation Activities, Inc.
References
- American Occupational Therapy Association. (2020). Occupational therapy practice framework: Domain and process (4th ed.). American Journal of Occupational Therapy , 74(Suppl. 2), 7412410010. 10.5014/ajot.2020.74S2001 [DOI] [PubMed] [Google Scholar]
- Brennan, A., Chick, S. E., & Davies, R. (2006). A taxonomy of model structures for economic evaluation of health technologies. Health Economics , 15, 1295–1310. 10.1002/hec.1148 [DOI] [PubMed] [Google Scholar]
- Bürge, E., Monnin, D., Berchtold, A., & Allet, L. (2016). Cost-effectiveness of physical therapy only and of usual care for various health conditions: Systematic review. Physical Therapy , 96, 774–786. 10.2522/ptj.20140333 [DOI] [PubMed] [Google Scholar]
- Cheng, Q., Graves, N., & Pacella, R. E. (2018). Economic evaluations of guideline-based care for chronic wounds: A systematic review. Applied Health Economics and Health Policy , 16, 633–651. 10.1007/s40258-018-0403-9 [DOI] [PubMed] [Google Scholar]
- Chiatti, C., & Iwarsson, S. (2014). Evaluation of housing adaptation interventions: Integrating the economic perspective into occupational therapy practice. Scandinavian Journal of Occupational Therapy , 21, 323–333. 10.3109/11038128.2014.900109 [DOI] [PubMed] [Google Scholar]
- Clarke, C. E., Patel, S., Ives, N., Rick, C. E., Woolley, R., Wheatley, K., . . . Sackley, C. M. (2016). Clinical effectiveness and cost-effectiveness of physiotherapy and occupational therapy versus no therapy in mild to moderate Parkinson’s disease: A large pragmatic randomised controlled trial (PD REHAB). Health Technology Assessment , 20(63), 1–96. 10.3310/hta20630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff, G. N., Roth, E. J., Haughton, J. S., & Ardner, M. S. (1990). Cognitive dysfunction in spinal cord injury patients: Sensitivity of the Functional Independence Measure subscales vs neuropsychologic assessment. Archives of Physical Medicine and Rehabilitation , 71, 326–329. [PubMed] [Google Scholar]
- Fernández-de-Las-Peñas, C., Ortega-Santiago, R., Díaz, H. F., Salom-Moreno, J., Cleland, J. A., Pareja, J. A., & Arias-Buría, J. L. (2019). Cost-effectiveness evaluation of manual physical therapy versus surgery for carpal tunnel syndrome: Evidence from a randomized clinical trial. Journal of Orthopaedic and Sports Physical Therapy , 49, 55–63. 10.2519/jospt.2019.8483 [DOI] [PubMed] [Google Scholar]
- Flemming, J., Chojecki, D., Tjosvold, L., Paulden, M., & Armijo-Olivo, S. (2020). Quality of reporting of economic evaluations in rehabilitation research: A systematic review. Disability and Rehabilitation. Advance online publication. 10.1080/09638288.2020.1830441 [DOI] [PubMed] [Google Scholar]
- Frontera, W. R., Bean, J. F., Damiano, D., Ehrlich-Jones, L., Fried-Oken, M., Jette, A., . . . Thompson, A. (2017). Rehabilitation research at the National Institutes of Health: Moving the field forward (executive summary). American Journal of Occupational Therapy , 71, 7103320010. 10.5014/ajot.2017.713003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin, K. M., Wasserman, J., & Ostwald, S. K. (2011). Cost associated with stroke: Outpatient rehabilitative services and medication. Topics in Stroke Rehabilitation , 18(Suppl. 1), 676–684. 10.1310/tsr18s01-676 [DOI] [PubMed] [Google Scholar]
- Husereau, D., Drummond, M., Petrou, S., Carswell, C., Moher, D., Greenberg, D., . . . Loder, E.; ISPOR Health Economic Evaluation Publication Guidelines– CHEERS Good Reporting Practices Task Force. (2013). Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—Explanation and elaboration: A report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value in Health , 16, 231–250. 10.1016/j.jval.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Jönsson, L. (2003). Pharmacoeconomics of cholinesterase inhibitors in the treatment of Alzheimer’s disease. PharmacoEconomics , 21, 1025–1037. 10.2165/00019053-200321140-00003 [DOI] [PubMed] [Google Scholar]
- Keith, R. A., Granger, C. V., Hamilton, B. B., & Sherwin, F. S. (1987). The Functional Independence Measure: A new tool for rehabilitation. Advances in Clinical Rehabilitation , 1, 6–18. [PubMed] [Google Scholar]
- Marshall, D., Simpson, K. N., Earle, C. C., & Chu, C. (2001). Potential cost-effectiveness of one-time screening for lung cancer (LC) in a high risk cohort. Lung Cancer , 32, 227–236. 10.1016/S0169-5002(00)00239-7 [DOI] [PubMed] [Google Scholar]
- Neumann, P. J., Sanders, G. D., Russell, L. B., Siegel, J. E., & Ganiats, T. G. (2017). Cost-effectiveness in health and medicine. Oxford University Press. [Google Scholar]
- Pinkerton, S. D., Johnson-Masotti, A. P., Derse, A., & Layde, P. M. (2002). Ethical issues in cost-effectiveness analysis. Evaluation and Program Planning , 25, 71–83. 10.1016/S0149-7189(01)00050-7 [DOI] [Google Scholar]
- Pollock, N. (1993). Client-centered assessment. American Journal of Occupational Therapy , 47, 298–301. 10.5014/ajot.47.4.298 [DOI] [PubMed] [Google Scholar]
- Rahja, M., Comans, T., Clemson, L., Crotty, M., & Laver, K. (2018). Economic evaluations of occupational therapy approaches for people with cognitive and/or functional decline: A systematic review. Health and Social Care in the Community , 26, 635–653. 10.1111/hsc.12553 [DOI] [PubMed] [Google Scholar]
- Rajsic, S., Gothe, H., Borba, H. H., Sroczynski, G., Vujicic, J., Toell, T., & Siebert, U. (2019). Economic burden of stroke: A systematic review on post-stroke care. European Journal of Health Economics , 20, 107–134. 10.1007/s10198-018-0984-0 [DOI] [PubMed] [Google Scholar]
- Rascati, K. L. (2014). Essentials of pharmacoeconomics (2nd ed.). Lippincott Williams & Wilkins. [Google Scholar]
- Schene, A. H., Koeter, M. W., Kikkert, M. J., Swinkels, J. A., & McCrone, P. (2007). Adjuvant occupational therapy for work-related major depression works: Randomized trial including economic evaluation. Psychological Medicine , 37, 351–362. 10.1017/S0033291706009366 [DOI] [PubMed] [Google Scholar]
- Simpson, K. N., Biddle, A. K., & Rabinovich, N. R. (1995). A model for estimating the impact of changes in children’s vaccines. American Journal of Public Health , 85, 1666–1672. 10.2105/AJPH.85.12.1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, K. N., Simpson, A. N., Mauldin, P. D., Palesch, Y. Y., Yeatts, S. D., Kleindorfer, D., . . . Broderick, J. P.; Interventional Management of Stroke (IMS) III Investigators. (2017). Observed cost and variations in short term cost-effectiveness of therapy for ischemic stroke in Interventional Management of Stroke (IMS) III. Journal of the American Heart Association , 6, e004513. 10.1161/JAHA.116.004513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, K. N., Strassburger, A., Jones, W. J., Dietz, B., & Rajagopalan, R. (2009). Comparison of Markov model and discrete-event simulation techniques for HIV. PharmacoEconomics , 27, 159–165. 10.2165/00019053-200927020-00006 [DOI] [PubMed] [Google Scholar]
- Simpson, K. N., & Tilley, B. C. (2012). Economic analysis of secondary trial data. Progress in Cardiovascular Diseases , 54, 351–356. 10.1016/j.pcad.2011.08.004 [DOI] [PubMed] [Google Scholar]
- Sonnenberg, F. A., & Beck, J. R. (1993). Markov models in medical decision making: A practical guide. Medical Decision Making , 13, 322–338. 10.1177/0272989X9301300409 [DOI] [PubMed] [Google Scholar]
- Unruh, M. A., Khullar, D., & Jung, H. Y. (2020). The patient-driven payment model: Addressing perverse incentives, creating new ones. American Journal of Managed Care , 26, 150–152. 10.37765/ajmc.2020.42831 [DOI] [PubMed] [Google Scholar]