Abstract
Rationale
Studies have suggested some patients with asthma are at risk of severe coronavirus disease (COVID-19), but they have had limited data on asthma phenotype and have not considered if risks are specific to COVID-19.
Objectives
To determine the effect of asthma phenotype on three levels of COVID-19 outcomes. Compare hospitalization rates with influenza and pneumonia.
Methods
Electronic medical records were used to identify patients with asthma and match them to the general population. Patient-level data were linked to Public Health England severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test data, hospital, and mortality data. Asthma was phenotyped by medication, exacerbation history, and type 2 inflammation. The risk of each outcome, adjusted for major risk factors, was measured using Cox regression.
Measurements and Main Results
A total of 434,348 patients with asthma and 748,327 matched patients were included. All patients with asthma had a significantly increased risk of a General Practice diagnosis of COVID-19. Asthma with regular inhaled corticosteroid (ICS) use (hazard ratio [HR], 1.27; 95% confidence interval [CI], 1.01–1.61), intermittent ICS plus add-on asthma medication use (HR, 2.00; 95% CI, 1.43–2.79), regular ICS plus add-on use (HR, 1.63; 95% CI, 1.37–1.94), or with frequent exacerbations (HR, 1.82; 95% CI, 1.34–2.47) was significantly associated with hospitalization. These phenotypes were significantly associated with influenza and pneumonia hospitalizations. Only patients with regular ICS plus add-on asthma therapy (HR, 1.70; 95% CI, 1.27–2.26) or frequent exacerbations (HR, 1.66; 95% CI, 1.03–2.68) had a significantly higher risk of ICU admission or death. Atopy and blood eosinophil count were not associated with severe COVID-19 outcomes.
Conclusions
More severe asthma was associated with more severe COVID-19 outcomes, but type 2 inflammation was not. The risk of COVID-19 hospitalization appeared to be similar to the risk with influenza or pneumonia.
Keywords: asthma, COVID-19, disease severity, allergic rhinitis, influenza
At a Glance Commentary
Current Scientific Knowledge on the Subject
Some coronavirus disease (COVID-19) studies have suggested patients with more severe asthma are at higher risk of adverse outcomes, but whether this is specific to COVID-19 has not been addressed, and the ability to phenotype patients with asthma has been limited owing to a lack of data. Furthermore, there have been conflicting findings regarding the role of type 2 inflammation.
What This Study Adds to the Field
This large longitudinal study, using individually linked community, hospital, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test data, has found that patients with asthma with higher use of asthma maintenance medication and more frequent exacerbations were significantly associated with severe COVID-19 outcomes, including hospital admission, ICU admission, and death; the same patients were also associated with a higher risk of hospitalization with influenza and pneumonia. Patients with markers suggestive of having type 2 inflammation were not found to be associated with more severe COVID-19 outcomes.
The effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in asthma continues to be unclear, one and a half years after the start of the coronavirus disease (COVID-19) pandemic. Health organizations initially declared patients with asthma to be highly vulnerable, but epidemiological studies from the earliest outbreak centers indicated they had reduced susceptibility (1–3). Subsequently, several preclinical studies provided some biological plausibility to a protective effect, indicating type 2 inflammation and corticosteroid use could downregulate key SARS-CoV-2 entry genes (4–7).
Two large UK consortia were established to determine risk factors for COVID-19–related death. Using primary care electronic medical records, OpenSAFELY reported no associated risk or protective effect with asthma, except for a slight increase in risk for patients with more severe asthma, defined by a prescription for a short course of oral corticosteroids in the year before study entry (8). ISARIC (International Severe Acute Respiratory and emerging Infection Consortium), addressed the risk of in-hospital death from COVID-19; only patients with asthma using three maintenance asthma medications were at significant risk (9, 10).
The OpenSAFELY and ISARIC cohorts were also used to investigate the relationship between mortality and inhaled corticosteroid (ICS) use. Whereas ISARIC found a reduced risk of in-hospital death associated with recent ICS use, OpenSAFELY found an increased mortality with regular high-dose ICS as compared with short-acting β-agonist (SABA) use alone (10, 11). To examine the influence of allergic asthma, two other large cohorts have been used; the population-based UK Biobank database and the South Korean national health insurance claims–based database. Both studies found that nonallergic asthma was significantly associated with severe outcomes but differed on their findings for allergic asthma (12, 13).
These studies have been limited by access to either only primary care or secondary care patient information. Here, we build on and improve our current knowledge through individual-level linkage of primary care data to data on SARS-CoV-2 test results, hospital and ICU admissions, and mortality across a longer period than previous studies. This has allowed more in-depth asthma phenotyping and measurement of outcomes. We have also more comprehensively addressed the effect of type 2 inflammation, assessing the effect of atopy and blood eosinophil count in asthma, and patients with another type 2 inflammatory condition, allergic rhinitis. Finally, we have compared the effect of SARS-CoV-2 infection with that of two other respiratory infections, pneumonia and influenza.
Methods
Data Sources
We used the Clinical Practice Research Datalink (CPRD) Aurum, a database of deidentified UK primary care electronic medical records. CPRD covers ∼19% of the population, is one of the largest longitudinal healthcare databases worldwide, and has been validated extensively (14). CPRD data were individually linked to Hospital Episode Statistics (HES) data (hospital admissions for England), socioeconomic data (Index of Multiple Deprivation), mortality data (Office of National Statistics [ONS]), and two Public Health England databases: the Second Generation Surveillance System data (laboratory test results for SARS-CoV-2 in hospital patients and National Health Service key workers) and the COVID-19 Hospitalisations in England Surveillance System (CHESS) data (outcome information including ICU admission, discharge, or death for patients admitted to hospital with confirmed COVID-19) (15).
Study Population and Design
Adults (⩾18 yr) contributing to CPRD Aurum from February 1, 2020, with linked HES-ONS-Public Health England data, and at least 12 months of data before study entry, were eligible for inclusion. Follow-up ended at the earliest of the following: study end date (June 26, 2020), transfer out of practice, last General Practice (GP) data collection date, or death. The first wave of the pandemic in the United Kingdom occurred between March 2020 and May 2020 inclusive (16); the study follow-up period is inclusive of these dates.
Two different matched cohorts were derived, one each for asthma and allergic rhinitis. Each exposed patient was matched to at least one (maximum three) unexposed patient(s) drawn from the rest of the CPRD population with the same year of birth, sex, and GP. These unexposed patients are termed “general population.” Patients with asthma were identified using a validated algorithm that identified patients using specific asthma codes. The three most commonly used codes were “asthma,” “asthma annual review,” and “asthma monitoring” (17), whereas those with allergic rhinitis were identified using specific Read codes (see below), recorded within 3 years of February 1, 2020. Patients with asthma had to have at least one prescription for a relevant medication (inhaler or oral asthma medication) in the year before the start of the study (baseline year). Patients with allergic rhinitis had to have been prescribed an antihistamine and a nasal corticosteroid in the baseline year. In the asthma matched cohort, patients from both populations were excluded if they had a chronic obstructive pulmonary disease (COPD) codiagnosis. In the allergic rhinitis matched cohort, patients were excluded if they had a COPD or asthma codiagnosis.
Outcomes
Three main COVID-19 outcomes were assessed: 1) GP diagnosis (suspected or confirmed COVID-19; CPRD and Second Generation Surveillance System), 2) hospital admission for COVID-19 (International Classification of Disease [ICD]-10 codes U07.1 or U07.2, HES, and CHESS), and 3) ICU admission or death (HES, CHESS, and ONS).
Two additional outcomes were assessed in the asthma cohort to better understand the association with a GP diagnosis: 1) GP consultation for COVID-19 advice or being a COVID-19 contact and 2) receiving a confirmed COVID-19 diagnosis in those that initially received a suspected COVID-19 diagnosis.
To compare the magnitude of association between asthma phenotype and COVID-19 with other respiratory infections, we measured hospitalization for pneumonia (J18.9) and influenza (J09-J11).
We also undertook three negative control analyses, using the same model in the same cohort but measuring the effect estimates for negative control outcomes, type 2 diabetes (E11-E14), myocardial infarction (I21-I23), and fracture of the forearm (S52). These outcomes are common in adults of all ages and not believed to be associated with asthma phenotype; if a similar association with asthma phenotype was found, this would suggest residual confounding. The control outcomes were measured between February 1, 2019, and June 26, 2019. As a sensitivity analysis, influenza was also measured during the seasonal influenza season, December 1, 2018, to April 26, 2019.
Exposures
Asthma was phenotyped by medication use (type and frequency), exacerbation history, and type 2 inflammation (atopy history and blood eosinophil count). For medication use, asthma was categorized by prescriptions in the baseline year: SABA only, ICS ± SABA, ICS plus an additional (“add-on”) asthma maintenance medication (inhaled long-acting β-agonist, oral leukotriene receptor antagonist, oral theophylline). ICS users were further categorized by the number of prescriptions in the baseline year, using an arbitrary cutoff based on clinical experience of 1–3 prescriptions (“intermittent” ICS users) or ⩾4 prescriptions (“regular” ICS users). This dichotomization was intended as a further proxy for asthma severity, alongside the type of medication used. Patients with asthma were additionally categorized according to exacerbations in the baseline year: 1) 1 GP-managed, or 2) >1 GP-managed or ⩾1 hospital admission for asthma in the past 5 years; the latter stratum was labeled as “frequent exacerbations.” Our previous work found hospital admissions up to 5 years prior were associated with a significant risk of a future exacerbation (18). GP-managed exacerbations were defined as a prescription of a short course of oral corticosteroids (19). Finally, asthma was categorized as atopic or not; atopy included a diagnosis of allergic rhinitis, allergic dermatitis, eczema, house dust mite allergy, animal allergy, or food allergy. Blood eosinophil counts were taken as the maximum absolute value from all values within 24 months before the study start date.
Covariates
Confounders were considered based on previous studies and included as covariates in all models (8, 9). Code lists were used to identify covariates (https://github.com/chloebloom/CovidAsthma.git); ethnicity was obtained from HES data variable. Obesity was defined as a body mass index (BMI) ⩾30 kg/m2.
Statistical Analysis
Patients’ characteristics were reported using descriptive statistics, and Kaplan-Meier plots were used to show time to outcome. We used a cause-specific competing risk analysis, with patients dying of non–COVID-19 causes censored on their date of death. We used multivariable Cox’s proportional hazard models, stratified by matched set (matched on age, sex, and GP practice), with time in study as the time scale, to estimate hazard ratios (HRs) for the association between each respiratory condition and time to first event for each outcome. The models were adjusted for ethnicity, socioeconomic status, obesity, cardiac disease, diabetes, cerebrovascular accident (CVA), dementia, cancer, and chronic renal failure (CRF). Asthma models were additionally adjusted for atopy, respiratory disease severity, and exacerbation history. We also undertook an interaction analysis between atopy and each outcome.
Four sensitivity analyses were conducted: 1) to circumvent the issue of misclassification of asthma, the asthma cohort was stratified by age, using a cutoff of 55 years; 2) only confirmed GP codes were included as a GP diagnosis of COVID-19; 3) only deaths were included in the last COVID-19 outcome, that is, ICU admissions were removed; and 4) for the influenza model, instead of applying the equivalent dates of the COVID-19 model to the year 2019, the same length of time was used but during the peak influenza season (December 1 to April 26). In all models, patients with missing ethnicity data were included and recorded as “unknown,” patients with missing BMI were presumed nonobese, (missing BMI data in primary care is expected to be missing not at random (20)), and patients with missing Index of Multiple Deprivation data (0.1%) were excluded. We assessed the proportional hazards assumption for all models by testing that the Schöenfeld residuals were independent of time, through inspecting plots against time and statistical tests.
A mixed-effects logistic regression model (GP practice as a random effect) was used to measure the association with receiving a confirmed COVID-19 diagnosis in the subgroup of the asthma cohort that received a suspected COVID-19 GP diagnosis; the sample size was insufficient for a matched Cox model. The model was adjusted for sex, age (as a transformed continuous variable), ethnicity, socioeconomic deprivation, obesity, atopy, cardiac disease, diabetes, CVA, CRF, dementia, and cancer. We used a restricted cubic spline with three knots (0.10, 0.29, 0.60 × 109/L) to evaluate the nonlinear association between blood eosinophil count and risk of ICU or death in patients with asthma only, using complete case analysis; the model was adjusted for sex, age, ethnicity, socioeconomic deprivation, obesity, cardiac disease, diabetes, CVA, CRF, dementia, and cancer. All statistical analyses were performed using STATA 16 (StataCorp).
Ethical Approval
A protocol for this research was approved by the Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agenc Database Research (protocol number 20_096). Generic ethical approval for observational research using the CPRD with approval from the Independent Scientific Advisory Committee has been granted by a Health Research Authority Research Ethics Committee (East Midlands – Derby, Research Ethics Committee reference number 05/MRE04/87).
Results
Patient Characteristics
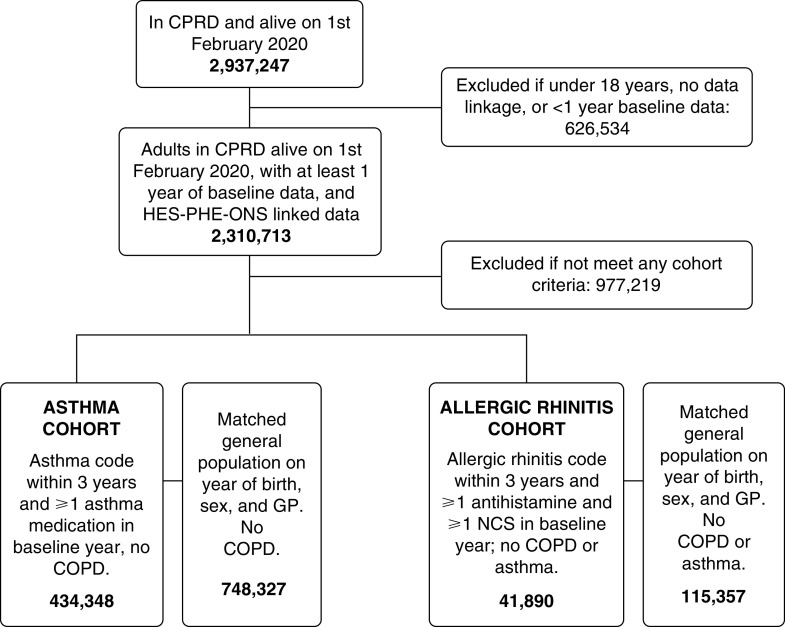
There were 1,182,675 patients in the asthma cohort (434,348 asthma and 748,327 matched general population) and 157,247 patients in the allergic rhinitis cohort (41,890 allergic rhinitis and 115,357 matched general population) (Figure 1). Demographic and clinical characteristics of each cohort are shown in Table 1 and Table E1 in the online supplement. The median age of the asthma cohort was 48.5 years and 42.5% were male, 59.2% of the patients with asthma were using ICS, and 19.2% had an exacerbation in the year prior.
Figure 1.
Flow diagram of inclusion and exclusion criteria. COPD = chronic obstructive pulmonary disease; CPRD = Clinical Practice Research Datalink; GP = General Practice; HES = Hospital Episode Statistics; NCS = nasal corticosteroid; ONS = Office of National Statistics; PHE = Public Health England.
Table 1.
Characteristics of the Asthma Cohort
| General Population | Asthma | Total | |
|---|---|---|---|
| Total, N | 748,327 | 434,348 | 1,182,675 |
| GP diagnosis (COVID-19) | |||
| No | 741,545 (99.1%) | 426,292 (98.1%) | 1,167,837 (98.7%) |
| Yes | 6,782 (0.9%) | 8,056 (1.9%) | 14,838 (1.3%) |
| Hospital admissions (COVID-19) | |||
| No | 747,348 (99.9) | 433,358 (99.8) | 1,180,706 (99.8) |
| Yes | 979 (0.1) | 990 (0.2) | 1,969 (0.2) |
| Death or ICU (COVID-19) | |||
| No | 747,936 (99.9) | 433,990 (99.9) | 1,181,926 (99.9) |
| Yes | 391 (0.1) | 358 (0.1) | 749 (0.1) |
| Age, yr, median (IQR) | 48.5 (34.5–61.5) | 49.5 (35.5–62.5) | 48.5 (35.5–61.5) |
| Sex | |||
| M | 319,577 (42.7) | 182,476 (42.0) | 502,053 (42.5) |
| F | 428,750 (57.3) | 251,872 (58.0) | 680,622 (57.5) |
| Ethnicity | |||
| White | 423,960 (56.7) | 289,872 (66.7) | 713,832 (60.4) |
| Asian | 39,688 (5.3) | 28,078 (6.5) | 67,766 (5.7) |
| Black | 16,776 (2.2) | 11,237 (2.6) | 28,013 (2.4) |
| Unknown | 267,903 (35.8) | 105,161 (24.2) | 373,064 (31.5) |
| Socioeconomic deprivation (IMD) | |||
| 1 | 175,451 (23.4) | 97,810 (22.5) | 273,261 (23.1) |
| 2 | 154,484 (20.6) | 88,318 (20.3) | 242,802 (20.5) |
| 3 | 144,689 (19.3) | 83,284 (19.2) | 227,973 (19.3) |
| 4 | 143,182 (19.1) | 84,305 (19.4) | 227,487 (19.2) |
| 5 (most deprived) | 129,909 (17.4) | 80,150 (18.5) | 210,059 (17.8) |
| Missing | 612 (0.1) | 481 (0.1) | 1,093 (0.1) |
| Asthma severity | |||
| Asthma | |||
| No asthma | 748,327 (100.0) | 0 (0.0) | 748,327 (63.3) |
| SABA only | N/A | 133,612 (30.8) | 133,612 (11.3) |
| Intermittent ICS (1–3 in baseline year) | N/A | 62,172 (14.3) | 62,172 (5.3) |
| Regular ICS (⩾4 in baseline year) | N/A | 57,061 (13.1) | 57,061 (4.9) |
| Intermittent (ICS plus add-on 1–3 in baseline year) | N/A | 44,118 (10.2) | 44,118 (3.8) |
| Regular ICS plus add-on (⩾4 in baseline year) | N/A | 137,385 (31.6) | 137,385 (11.7) |
| Exacerbations | |||
| None | 748327 (100.0) | 350,766 (80.8) | 1,099,093 (92.9) |
| 1 GP managed in past year | N/A | 50,951 (11.7) | 50,951 (4.3) |
| >1 GP managed or ⩾1 hospital admission in 5 yr | N/A | 32,631 (7.5) | 32,631 (2.8) |
| Comorbidities | |||
| Atopy | |||
| No | 532,142 (71.1) | 182,046 (41.9) | 714,188 (60.4) |
| Yes | 216,185 (28.9) | 252,302 (58.1) | 468,487 (39.6) |
| Obesity | |||
| No | 607,562 (81.2) | 305,657 (70.4) | 913,219 (77.2) |
| Yes | 140,765 (18.8) | 128,691 (29.6) | 269,456 (22.8) |
| Cardiac disease | |||
| No | 580,109 (77.5) | 309,664 (71.3) | 889,773 (75.2) |
| Yes | 168,218 (22.5) | 124,684 (28.7) | 292,902 (24.8) |
| Diabetes | |||
| No | 502,529 (67.2) | 262,321 (60.4) | 764,850 (64.7) |
| Yes | 245,798 (32.8) | 172,027 (39.6) | 417,825 (35.3) |
| Dementia | |||
| No | 708,817 (94.7) | 410,548 (94.5) | 1,119,365 (94.6) |
| Yes | 39,510 (5.3) | 23,800 (5.5) | 63,310 (5.4) |
| Cerebrovascular disease | |||
| No | 741,071 (99.0) | 429,049 (98.8) | 1,170,120 (98.9) |
| Yes | 7,256 (1.0) | 5,299 (1.2) | 12,555 (1.1) |
| Chronic renal failure | |||
| No | 728,907 (97.4) | 420,029 (96.7) | 1,148,936 (97.1) |
| Yes | 19,420 (2.6) | 14,319 (3.3) | 33,739 (2.9) |
| Cancer | |||
| No | 705,792 (94.3) | 407,426 (93.8) | 1,113,218 (94.1) |
| Yes | 42,535 (5.7) | 26,922 (6.2) | 69,457 (5.9) |
Definition of abbreviations: COVID-19 = coronavirus disease; GP = General Practice; ICS = inhaled corticosteroid; IMD = Index of Multiple Deprivation; IQR = interquartile range; N/A = not applicable; SABA = short-acting β-agonist.
Data are shown as n (%) unless otherwise noted.
Temporal Distribution and Frequency of COVID-19 Outcomes
The distribution of all three outcomes followed a similar pattern, although deaths lagged hospital admissions; peaks occurred in April and March 2020 (Figures E1–E3). There were more COVID-19 GP diagnoses (1.85% of patients with asthma and 1.94% of patients with allergic rhinitis) than hospital admissions (0.23% of patients with asthma and 0.15% of patients with allergic rhinitis) or ICU admission or death (0.08% of patients with asthma and 0.03% of patients with allergic rhinitis) (Tables 1 and E1). Time to each of the COVID-19 outcomes for the asthma cohort is shown in Figure E4. Compared with patients who died during or after a hospital admission, those who died without a prior hospital admission were older, with a higher proportion with dementia and CVA (Table E2).
Association between Asthma and GP COVID-19 Diagnosis
All types of asthma, regardless of medication phenotype, were significantly associated with receiving a GP diagnosis of COVID-19 (adjusted HR [95% CI]: SABA only = 1.63 [1.53–1.74]; intermittent ICS = 1.42 [1.29–1.56]; regular ICS = 1.41 [1.28–1.55]; intermittent ICS plus add-on = 1.79 [1.61–1.99]; regular ICS plus add-on = 1.73 [1.62–1.85]; 1 exacerbation = 1.42 [1.29–1.58]; more frequent exacerbations = 1.76 [1.57–1.97]; Figures 2 and E5 and Table E3) and with consulting their GP for COVID-19 advice or contact (Figure E6). All patients with asthma who received a diagnosis of suspected COVID-19, regardless of medication phenotype, had a significantly lower odds of receiving a confirmed COVID-19 diagnosis (Figure E7). Owing to the low availability of testing within the community during the first wave of the pandemic in the United Kingdom, only 9.4% of GP codes were confirmed COVID-19. A sensitivity analysis only including confirmed codes as a GP diagnosis had little impact on the effect estimates (Figure E8).
Figure 2.
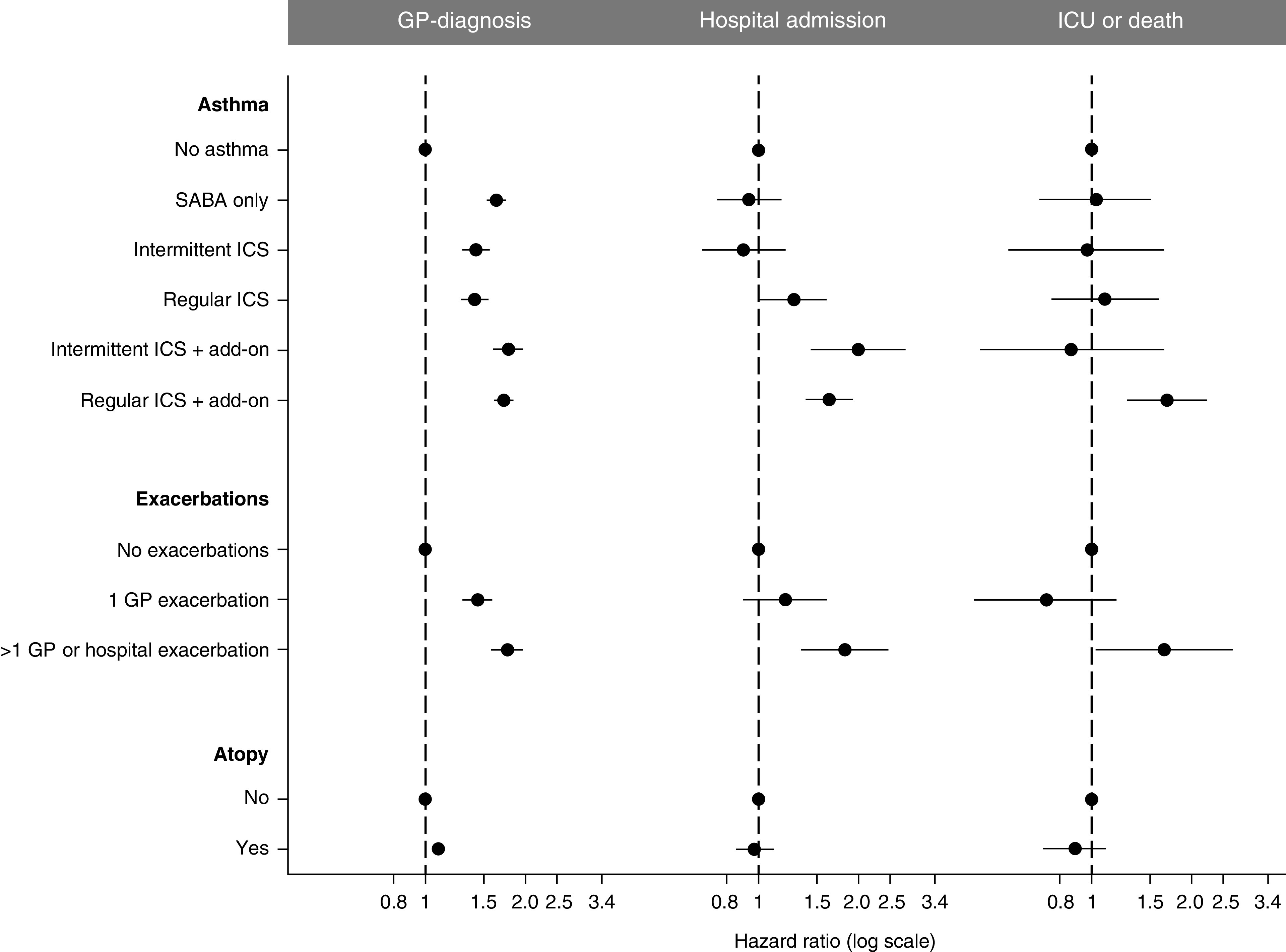
Comparing forest plots of the associations between asthma phenotype and each coronavirus disease (COVID-19) outcome, after adjusting for all other risk factors (see Figure E7 for forest plot including all variables and Tables E3, E5, and E6 for unadjusted and adjusted effect estimates). GP = General Practice; ICS = inhaled corticosteroid; SABA = short-acting β-agonist.
Association between Asthma and Hospital Admission, ICU Admission, or Death
Patients with asthma in whom there were significant associations with COVID-19 hospital admission were regular users of ICS (adjusted HR, 1.27; 95% CI, 1.01–1.61), intermittent users of ICS plus an add-on therapy (adjusted HR, 2.00; 95% CI, 1.43–2.79), regular uses of ICS plus add-on therapy (adjusted HR, 1.63; 95% CI, 1.37–1.94), or those with more frequent exacerbations (adjusted HR, 1.82; 95% CI, 1.34–2.47) (Figures 2 and E5 and Table E4). Patients with asthma in whom there were significant associations with COVID-19 ICU admission or death were regular users of ICS plus an add-on therapy (adjusted HR, 1.70; 95% CI, 1.27–2.26) or those with more frequent exacerbations (adjusted HR, 1.66; 95% CI, 1.03–2.68) (Figures 2 and E5 and Table E5).
Sensitivity analyses, only including death (excluding ICU admissions) (Figure E9) and stratifying the asthma cohort by age (Figures E10 and E11), found that associations with the same asthma phenotypes persisted, although the cumulative proportion of ICU admissions or death during the study period was considerably higher in patients aged ⩾55 years (<55 yr = 63 [0.06%] ICU admissions or deaths; ⩾55 yr or more = 683 [0.15%] ICU admissions or deaths).
Association between Asthma and Non–COVID-19 Hospital Admissions
The patients with asthma with a significantly increased risk of COVID-19 hospital admission had a significantly higher risk of pneumonia and influenza admissions, with similar levels of relative risk, but lower rates of events (adjusted HR [95% CI]: 1) influenza, SABA only = 1.02 [0.45–2.29]; intermittent ICS = 0.96 [0.26–3.48]; regular ICS = 1.17 [0.34–3.96]; intermittent ICS plus add-on = 2.72 [0.51–14.4]; regular ICS plus add-on = 3.82 [1.76–8.26]; 1 exacerbation = 5.18 [0.96–28.1]; more frequent exacerbations = 4.20 [1.16–15.22]; 2) pneumonia, SABA only = 1.34 [0.61–2.98]; intermittent ICS = 1.39 [0.40–4.82]; regular ICS = 4.10 [1.04–15.79]; intermittent ICS plus add-on = 8.43 [1.50–47.33]; regular ICS plus add-on = 7.53 [3.07–18.46]; 1 exacerbation = 2.80 [0.84–9.29]; more frequent exacerbations = 2.53 [0.61–10.38]) (Figure 3 and Table 2). As sensitivity analyses, using age cutoff of 55 years (Figure E12) or using peak seasonal influenza months (data not shown), there was little change to the effect estimates but a small increase in the rates of influenza cases during peak seasonal influenza months (Table 2).
Figure 3.
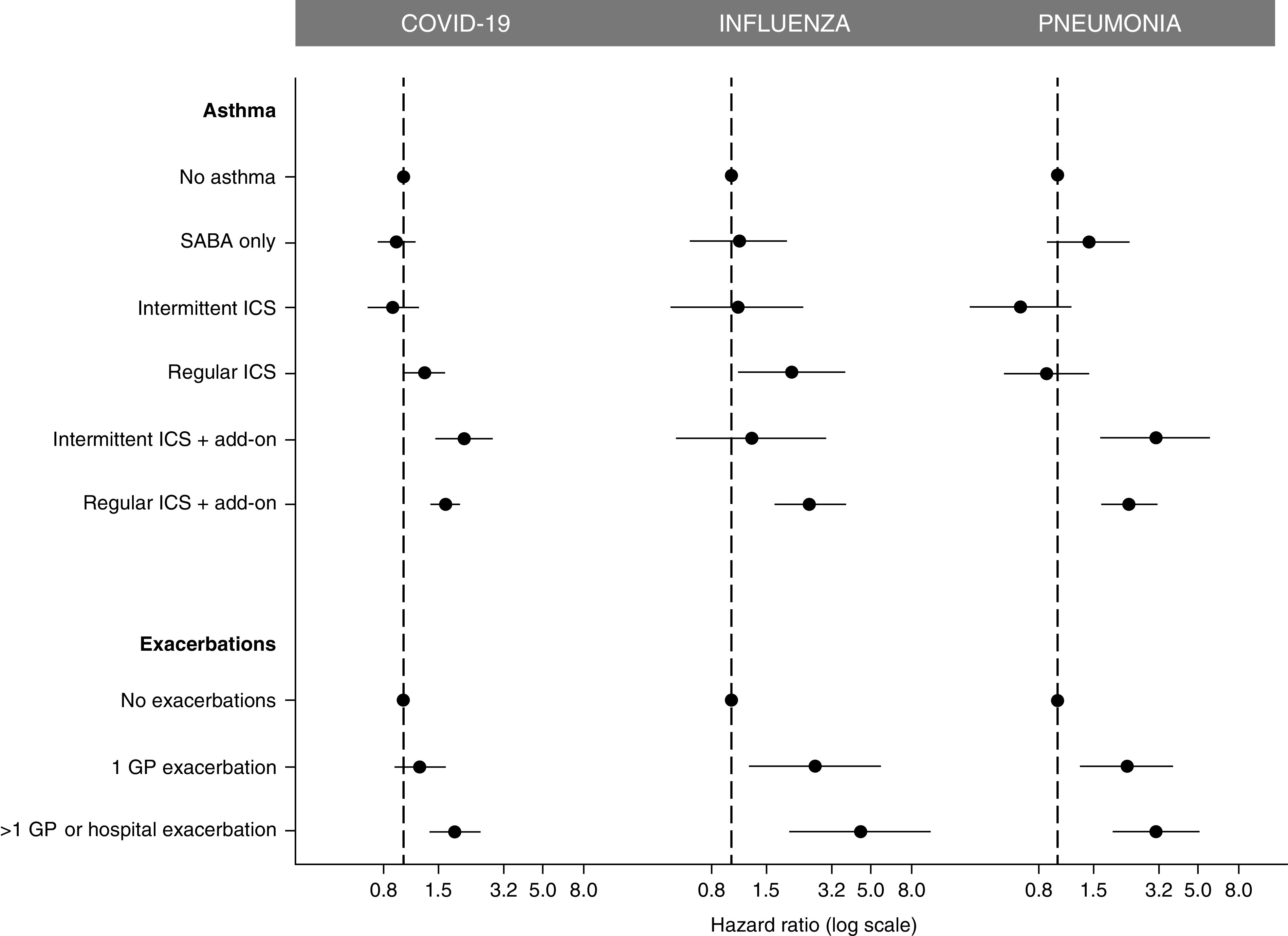
Comparing forest plots of the associations between asthma phenotype and hospitalization for the three different respiratory infections—COVID-19, influenza, and pneumonia—after adjusting for all other risk factors (ethnicity, socioeconomic deprivation, obesity, atopy, cardiac disease, diabetes, cerebrovascular accident, chronic renal failure, dementia, and cancer). COVID-19 = coronavirus disease; GP = General Practice; ICS = inhaled corticosteroid; SABA = short-acting β-agonist.
Table 2.
Rates of Hospitalization in the Asthma Cohort for Each Respiratory Infection, by Time Period
| Time Period | Admission | Number of Events |
Rates per 100,000 (95% CI) |
|||
|---|---|---|---|---|---|---|
| Asthma | General Population | Total | Asthma | General Population | ||
| February 1 to June 26, 2020 | COVID-19 | 990 | 979 | 1,969 | 19.33 (18.16–20.57) | 11.08 (10.41–11.80) |
| February 1 to June 26, 2019 | Influenza | 211 | 124 | 335 | 3.99 (3.49–4.57) | 1.37 (1.15–1.64) |
| February 1 to June 26, 2019 | Pneumonia | 373 | 245 | 618 | 7.06 (6.38–7.82) | 2.71 (2.40–3.08) |
| December 1, 2018, to April 26, 2019 | Influenza | 369 | 225 | 594 | 6.99 (6.31–7.74) | 2.49 (2.19–2.98) |
Definition of abbreviations: CI = confidence interval; COVID-19 = coronavirus disease.
No asthma patient groups had a significantly greater risk of the negative control outcomes of hospital admission for diabetes, myocardial infarction, or right arm fracture (Figure E13).
Association between Atopy and COVID-19 Outcomes
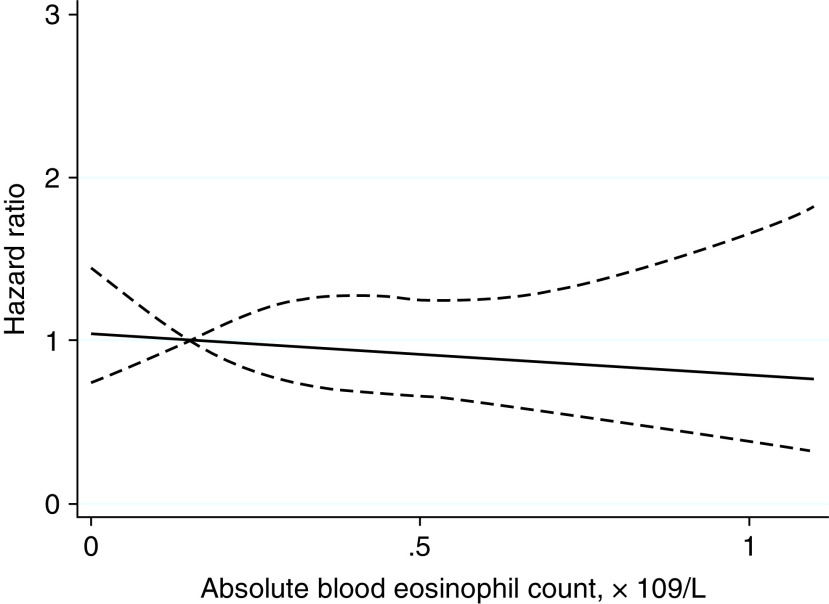
In the asthma cohort, atopy was significantly associated with a GP diagnosis but not with hospitalization or ICU admission or death (Figures 2 and E5 and Table E6). Atopy did not modify the association between asthma severity and outcomes (P < 0.05). There were 290,639 (66.9% of total asthma study population) patients included in the analysis of blood eosinophil count. Patients with an eosinophil count were broadly similar in characteristics to the total asthma study population but were slightly older, more often female, and more of White ethnicity and had more severe asthma, less obesity, less cardiac disease, and less diabetes (Table E6). Eosinophil count was not significantly associated with a COVID-19 hospital admission, ICU admission, or death (Figure 4).
Figure 4.
Association between maximum absolute blood eosinophil count and ICU admission or death in the patients with asthma. The model was adjusted for sex, age, ethnicity, socioeconomic deprivation, obesity, atopy, cardiac disease, diabetes, cerebrovascular accident, chronic renal failure, dementia, and cancer. The solid black line represents the adjusted hazard ratio for the outcome (ICU admission or death) for the continuous variable of maximum absolute blood eosinophil count. The dashed black lines represent the 95% confidence interval of the hazard ratio.
Allergic rhinitis was significantly associated with receiving a GP diagnosis but not with hospital admission, ICU admission, or death (GP diagnosis, adjusted HR, 2.17; 95% CI, 1.92–2.43; hospital admission, adjusted HR, 1.37; 95% CI, 0.91–2.06; ICU admission or death, 0.93; 95% CI, 0.34–2.56; Figure E14).
Discussion
In our asthma cohort, comprising more than 1 million patients with asthma and matched general population patients, there was an increased risk of GP-diagnosed COVID-19 for all asthma phenotypes, including those prescribed SABA alone and those with atopy. This could represent a greater risk of infection with SARS-CoV-2 for asthma, but there was also a significant association with GP consultation for advice on COVID-19 and reporting exposure to COVID-19. In addition, patients with asthma were significantly less likely to have their suspected COVID-19 diagnosis confirmed, indicating a higher risk of false-positive labeling of COVID-19 in asthma than the general population. Put together, these findings suggest that patients with asthma had increased healthcare-seeking behavior and GPs a lower threshold to diagnose COVID-19 in them. These healthcare behaviors are likely to reflect the initial global concern that asthma would be associated with severe COVID-19 outcomes.
Next, we examined whether patients with asthma were more likely to be admitted to the hospital with COVID-19, after accounting for all other major risk factors. We found those patients with regular ICS use, intermittent or regular use of ICS plus an add-on therapy, or those with frequent exacerbations were at a significantly higher risk of hospitalization. Lastly, we assessed the risk of ICU admission or death. Patients with regular use of ICS plus add-on therapy had a 70% increased risk and those with frequent exacerbations had a 66% increased risk, although the absolute risk was low. It is worth noting that patients with asthma did not change their pattern of inhaler prescriptions during this time period, except for a brief increase in inhaler prescriptions at the beginning of the first wave in those with mild asthma (21).
Our findings suggests that COVID-19 outcomes are related to asthma severity, as defined by use of maintenance inhaler medication and exacerbation history. Although a large systematic review did not find an association with asthma and COVID-19, this may have been because asthma was considered as a homogeneous condition without phenotyping (22). Analysis of the large OpenSAFELY and ISARIC data sets did apply phenotyping, and although limited by the data available on the patients’ asthma, they found that more severe asthma was linked to COVID-19–related mortality. The OpenSAFELY study, including community patients, only dichotomized asthma severity by prior prescriptions of oral corticosteroids from their GP (11). The ISARIC study, of a hospitalized cohort, was only able to stratify patients with asthma by their recent medication use (10). Importantly, both studies considered patients with asthma separately from patients with both asthma and COPD, as we have done here. But these earlier studies were not able to address the risk of hospitalization or diagnoses in primary care. To phenotype patients with asthma in the present study, we had access to all medications, frequency of prescriptions, and treatment of exacerbations within both primary and secondary care. This more granular phenotyping has further added to the evidence that milder asthma is neither protective nor associated with severe COVID-19 outcomes. Interestingly, age did not modify the relative risk between asthma phenotypes and ICU admission or death, but there were considerably more deaths in the older age group.
The pattern of hospitalization risk for the different asthma phenotypes was also apparent when examining the risk for pneumonia and influenza, but not for diabetes, myocardial infarction or fractures. This finding parallels and builds upon the results from another study from the OpenSAFELY consortium (23), in which the authors demonstrate that for many recognized COVID-19 risk factors (including age, male sex and obesity), the risk of death is a magnification of the risk that would be expected from non-COVID-19 death. In contrast, although asthma patients with recent oral-steroid use had a significantly raised risk of COVID-19 death, they had a much lower risk of non-COVID-19 death, suggesting the mortality risk was particular to COVID-19 (23).
Although the incident rate of hospitalization was considerably higher for COVID-19 than either pneumonia or influenza in the overall population, the elevated adjusted association between asthma and risk of hospitalization was similar between the three respiratory infections. This finding even held true for those younger patients, aged <55 years.
We found no association between severe COVID-19 outcomes and variables representing probable type 2 inflammation. There was no association in patients with asthma with atopy or a raised blood eosinophil count or in patients with allergic rhinitis. This is consistent with a large study using the UK Biobank data set that assessed the risk of allergic asthma (12). However, two studies published using the SARS-CoV-2–positive patients from the South Korean Health Insurance Review and Assessment Service found an elevated risk of severe COVID-19 outcomes with allergic asthma and chronic rhinosinusitis (13, 24). Neither study accounted for asthma severity, which may have confounded associations.
Limitations
The data in this study are only from the first wave of COVID-19 in the United Kingdom, before the availability of COVID-19 vaccination and before the routine use of corticosteroids and other treatments in hospitalized patients; associations with outcomes may have changed subsequently. This study may share the limitations of all observational research, including residual confounding and bias, and observational studies, such as this one, cannot be used to demonstrate causality. Patients may have inaccurate diagnoses of respiratory conditions or may not be using the medications prescribed by their GP. We have attempted to reduce misclassification in asthma by excluding patients with a codiagnosis of COPD and carrying out a sensitivity analysis using an age cutoff of 55 years (below this age, misclassification with COPD is rare). We did not have information on adherence to medication or subjective level of disease control. We did not include smoking as a variable owing to the large amount of missing data on current smoking history, particularly in the general population. Another limitation is misclassification of outcomes; mortality data were obtained from death certificates written by physicians, at a time when there was a shortage of PCR tests in the United Kingdom.
Conclusions
All asthma phenotypes were associated with an increased risk of receiving a GP diagnosis of COVID-19 during the first wave of the pandemic in the United Kingdom; this may have been related to behavioral factors. Higher use of asthma maintenance medication and a history of frequent exacerbations were significantly associated with severe COVID-19 outcomes, including hospital admission, ICU admission, and death. Measurements of type 2 inflammation were not significantly associated with severe COVID-19 outcomes. Those with higher medication use or frequent exacerbations were associated with a similar increased risk of hospitalization for influenza, pneumonia, and COVID-19, although the incidence was much higher for COVID-19. Patients with asthma who regularly use ICS and an add-on therapy or have frequent exacerbations are at higher risk of developing adverse consequences from COVID-19 and influenza than the general population, after accounting for other recognized risk factors; such patients should consider vaccination against both diseases.
Footnotes
Supported by the Imperial College COVID-19 Response Fund and the National Institute for Health Research Imperial Biomedical Research Centre.
Author Contributions: C.I.B. conceived the study and analyzed the data. C.I.B., P.C., and J.A.W. contributed substantially to study design, data interpretation, and writing.
Data sharing statement: Data are available on request from the Clinical Practice Research Datalink. Their provision requires the purchase of a license, and our license does not permit us to make them publicly available to all. We used data from the version collected in July 2020 and have clearly specified the data selected in our Methods section. To allow identical data to be obtained by others, via the purchase of a license, we have provided the code lists. Licenses are available from the Clinical Practice Research Datalink (http://www.cprd.com): The Clinical Practice Research Datalink Group, The Medicines and Healthcare products Regulatory Agency, 10 South Colonnade, Canary Wharf, London E14 4PU.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202107-1704OC on October 20, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. COVID-19 Lombardy ICU Network Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA . 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA . 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol . 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finney LJ, Glanville N, Farne H, Aniscenko J, Fenwick P, Kemp SV, et al. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon J Allergy Clin Immunol 2021147510–519.e5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peters MC, Sajuthi S, Deford P, Christenson S, Rios CL, Montgomery MT, et al. COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med . 2020;202:83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson DJ, Busse WW, Bacharier LB, Kattan M, O’Connor GT, Wood RA, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol . 2020;146:203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kimura H, Francisco D, Conway M, Martinez FD, Vercelli D, Polverino F, et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol . 2020;146:80–88.e8. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature . 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. ISARIC4C investigators Features of 20133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ . 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bloom CI, Drake TM, Docherty AB, Lipworth BJ, Johnston SL, Nguyen-Van-Tam JS, et al. ISARIC investigators Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med . 2021;9:699–711. doi: 10.1016/S2213-2600(21)00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schultze A, Walker AJ, MacKenna B, Morton CE, Bhaskaran K, Brown JP, et al. The OpenSAFELY Collaborative Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med . 2020;8:1106–1120. doi: 10.1016/S2213-2600(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA, Jr, Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol . 2020;146:327–329.e4. doi: 10.1016/j.jaci.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang JM, Koh HY, Moon SY, Yoo IK, Ha EK, You S, et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol . 2020;146:790–798. doi: 10.1016/j.jaci.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolf A, Dedman D, Campbell J, Booth H, Lunn D, Chapman J, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. Int J Epidemiol . 2019;48:1740–1740g. doi: 10.1093/ije/dyz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NHS Digital. SGSS and CHEST data 2021. [accessed 2021 Jul 1]. Available from: https://digital.nhs.uk/about-nhs-digital/corporate-information-and-documents/directions-and-data-provision-notices/data-provision-notices-dpns/sgss-and-chess-data.
- 16.Office of National Statistics. Coronavirus (COVID-19) Infection Survey technical article: waves and lags of COVID-19 in England, June 2021 2021. [accessed 2021 Jul 1]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsurveytechnicalarticle/wavesandlagsofcovid19inenglandjune2021
- 17. Nissen F, Morales DR, Mullerova H, Smeeth L, Douglas IJ, Quint JK. Validation of asthma recording in the Clinical Practice Research Datalink (CPRD) BMJ Open . 2017;7:e017474. doi: 10.1136/bmjopen-2017-017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bloom CI, Palmer T, Feary J, Quint JK, Cullinan P. Exacerbation patterns in adults with asthma in England. A population-based study. Am J Respir Crit Care Med . 2019;199:446–453. doi: 10.1164/rccm.201808-1516OC. [DOI] [PubMed] [Google Scholar]
- 19. Bloom CI, Nissen F, Douglas IJ, Smeeth L, Cullinan P, Quint JK. Exacerbation risk and characterisation of the UK’s asthma population from infants to old age. Thorax . 2018;73:313–320. doi: 10.1136/thoraxjnl-2017-210650. [DOI] [PubMed] [Google Scholar]
- 20. Bhaskaran K, Smeeth L. What is the difference between missing completely at random and missing at random? Int J Epidemiol . 2014;43:1336–1339. doi: 10.1093/ije/dyu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloom CI, Wong E, Hickman K, Elkin S.Influence of the first wave of COVID-19 on asthma inhaler prescriptions NPJ Prim Care Respir Med. [online ahead of print] 25 Nov 2021; DOI: 10.1038/s41533-021-00260-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Terry PD, Heidel RE, Dhand R. Asthma in adult patients with COVID-19: prevalence and risk of severe disease. Am J Respir Crit Care Med . 2021;203:893–905. doi: 10.1164/rccm.202008-3266OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhaskaran K, Bacon S, Evans SJ, Bates CJ, Rentsch CT, MacKenna B, et al. Factors associated with deaths due to COVID-19 versus other causes: population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet Reg Health Eur . 2021;6:100109. doi: 10.1016/j.lanepe.2021.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee SW, Kim SY, Moon SY, Yang JM, Ha EK, Jee HM, et al. Estimating COVID-19 infection and severity risks in patients with chronic rhinosinusitis: a Korean nationwide cohort study. J Allergy Clin Immunol Pract . 2021;9:2262–2271.e2. doi: 10.1016/j.jaip.2021.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]