To the Editor:
After acute infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), many individuals experience a range of symptoms including dyspnea, exercise intolerance, and chest pain commonly referred to as “post–COVID-19 syndrome” or as post-acute sequelae of SARS-CoV-2 infection (PASC) (1). Exertional dyspnea and physical activity intolerance in PASC can be debilitating despite mild acute coronavirus disease (COVID-19) and normal resting pulmonary physiology and cardiac function (2). There is an urgent need to understand the pathogenesis of PASC and find effective treatments. The cardiopulmonary exercise test (CPET) is commonly used to investigate unexplained exertional dyspnea; as such, it could provide insight into mechanisms of PASC. CPET data can be used to calculate rates of β-oxidation of fatty acids (FATox) and of lactate clearance, providing insight into mitochondrial function (3). Fit individuals have better mitochondrial function and a higher rate of FATox during exercise than less fit individuals (4). Our results suggest that patients with PASC have significant impairment in fat β-oxidation and increased blood lactate accumulation during exercise, regardless of previous comorbidities.
In this study, we investigated whether patients with PASC had compromised mitochondrial function during graded exercise. Data were obtained via retrospective review of the electronic medical record from a cohort of 50 subjects with PASC (n = 50, age = 50 ± 15 yr, 35 female) that were consecutively referred to and willing to complete CPET in the Pulmonary Physiology Laboratory at National Jewish Health between June 2020 and April 2021 (Table 1). No subject was excluded. Patients were assessed on a cycle ergometer (Vmax 29; SensorMedics Corp) using a continuous ramp protocol to exhaustion. Cardiovascular, ventilatory, metabolic, and gas exchange data were collected using a metabolic cart (Ultima Cardio2 System; Med graphics) per standard protocol (5). For calculations of total FATox and carbohydrate oxidation (CHOox), we used the stoichiometric equations by Frayn and colleagues: FATox [g/min] = 1.67 × o2 [L/min] – 1.67 × co2 [L/min] and CHOox [g/min] = 4.55 × o2 [L/min] – 3.21 × co2 [L/min] (6). Patient data were compared with results from two published cohorts that included subjects tested with CPET in Denver, Colorado (i.e., the same altitude as our cohort). The characteristics of these cohorts have been previously described (3). Statistical analyses were performed in SPSS Statistics V.27 (IBM) and graphed with Prism V.9.1.0(221) (GraphPad Software). Our study was approved by The Biomedical Research Alliance of New York (BRANY) Institutional Review Board (IRB) study # 20-12-582-528.
Table 1.
Patient Characteristics and Data at the Time of CPET
| Value | |
|---|---|
| Demographics | |
| Age, yr, mean ± SD | 50 ± 15 |
| Sex, F, n (%) | 35 (70) |
| BMI, kg m−1, mean ± SD | 26 ± 5 |
| Comorbidities, n (%) | |
| Asthma | 12 (24) |
| CAD | 4 (8) |
| COPD | 2 (4) |
| Diabetes mellitus | 2 (4) |
| Hypertension | 14 (28) |
| ILD | 2 (4) |
| Smoking status, n (%) | |
| Former | 10 (2) |
| Never | 40 (80) |
| Medications, n (%) | |
| ACE inhibitor/ARB | 8 (16) |
| BB | 5 (10) |
| CCB | 1 (2) |
| COVID-19 clinical characteristics | |
| Time from diagnosis to CPET, mean ± SD, mo | 6 ± 4 |
| Hospital admission, n (%) | 5 (10) |
| ICU admission, n (%) | 3 (6) |
| Mechanical ventilation, n (%) | 3 (6) |
| Post–COVID-19 symptoms, n (%) | |
| Two or more of the following | 6 (12) |
| Dyspnea on exertion | 28 (56) |
| Decreased endurance | 13 (26) |
| Chest pain | 3 (6) |
| Testing results | |
| CT chest, n (%) | 41 (82) |
| Time from CT chest to CPET, mean ± SD, mo | 1 ± 2 |
| Normal findings, n (%) | 14 (34) |
| Minimal GGO, n (%) | 10 (24) |
| Bronchial wall thickening, n (%) | 13 (32) |
| Peripheral reticulation, n (%) | 1 (2) |
| Pulmonary function test, n (%) | 50 (100) |
| Time from PFT to CPET, mean ± SD, mo | 1 ± 2 |
| FVC, L; FVC % predicted, mean ± SD | 3.8 ± 1; 96 ± 14 |
| FEV1, L; FEV1% predicted, mean ± SD | 3.1 ± 0.9; 98 ± 13 |
| FEV1/FVC, %, mean ± SD | 95 ± 12 |
| TLC, L; TLC % predicted, mean ± SD | 5.9 ± 1.3; 103 ± 13* |
| RVol, L; RVol % predicted, mean ± SD | 2.2 ± 0.6; 108 ± 21* |
| DlCOcor mL min-1 mm Hg; DlCOcor % predicted, mean ± SD | 26 ± 8; 109 ± 22† |
| Echocardiography at rest, n (%) | 39 (78) |
| Time from echocardiogram to CPET, mean ± SD, mo | 2 ± 3 |
| Normal LV ejection fraction, n (%) | 39 (100) |
| Grade 1 RV diastolic dysfunction, n (%) | 2 (5) |
| RV systolic dysfunction, n (%) | 8 (21) |
| RVSP, mean ± SD, mm Hg | 38 ± 3 |
| MPS at rest, n (%) | 8 (16) |
| Time from CPET to MPS, mean ± SD, d | 10 ± 90 |
| Normal, n (%) | 8 (100) |
| C-MRI at rest, n (%) | 9 (18) |
| Time from CPET to C-MRI, mean ± SD, mo | 1 ± 3 |
| CPET | |
| Reason for discontinuation of CPET, n (%) | |
| Dyspnea | 20 (40) |
| Overall fatigue | 15 (30) |
| Leg fatigue | 11 (22) |
| Lightheadedness | 2 (4) |
| Chest pain | 2 (4) |
| Dyspnea, Borg score, mean ± SD | 8 ± 1 |
| Leg fatigue, Borg score, mean ± SD | 8 ± 2 |
| WR max, Watts; % predicted, mean ± SD | 144 ± 49; 95 ± 22 |
| o2 max, L min−1; % predicted, mean ± SD | 1.6 ± 0.4; 95 ± 28 |
| o2 max/BW, ml kg min−1; % predicted, mean ± SD | 22.2 ± 6.0; 94 ± 29 |
| co2 max, L min−1; % predicted, mean ± SD | 2.0 ± 0.5; 95 ± 26 |
| RER, mean ± SD | 1.2 ± 0.1 |
| e max, L min−1, mean ± SD | 77 ± 19 |
| Vd/Vt, mean ± SD | 0.2 ± 0.1 |
| HR max, beats min−1, mean ± SD | 155 ± 22 |
| HRR, beats min−1, mean ± SD | 16 ± 14 |
| SpO2, % mean ± SD | 94 ± 4 |
| Lactate at peak, mmol L−1, mean ± SD | 10 ± 3‡ |
| Anaerobic threshold, %, mean ± SD | 51 ± 16 |
| Oxygen pulse, ml beat; % predicted, mean ± SD | 11 ± 3.8; 99 ± 33 |
| SBP; DBP, mm Hg, mean ± SD | 169 ± 25; 81 ± 13 |
Definition of abbreviations: ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BB = β-adrenergic receptor antagonist; BMI = body mass index; CAD = coronary artery disease; COVID-19 = coronavirus disease; CCB = calcium channel blocker; C-MRI = cardiac magnetic resonance imaging; COPD = chronic obstructive pulmonary disease; CPET = cardiopulmonary exercise testing; CT = computed tomography; DBP = diastolic blood pressure; DlCOcor = DlCO corrected for altitude; GGO = ground glass opacifications; HR max = maximum heart rate; HRR = heart rate reserve; ILD = interstitial lung disease; LV = left ventricle; MPS = myocardial perfusion scan; PFT = pulmonary function test; RER = respiratory exchange ratio; RVol = residual volume; RV = right ventricle; RVSP = right ventricular systolic pressure; SBP = systolic blood pressure; SpO2 = oxygen saturation as measured by pulse oximetry; Vco2 max = exhaled maximum co2; o2 max = maximum o2; o2 max/BW = maximum Vo2 per kilogram body weight; WR max = maximum work rate.
N = 50.
n = 38.
n = 41.
n = 39.
Pulmonary function testing (PFT) showed mostly normal resting airflow and gas transfer capacity. All six patients (12%) with PFT abnormalities had preexisting illnesses, including asthma (n = 3) or interstitial lung disease (n = 1). Resting transthoracic echocardiogram was obtained in 39 patients (78%) within 2 ± 3 months of CPET. Left ventricular systolic function was normal (left ventricular ejection fraction 50–70%) in all patients. Among the 50 patients who underwent CPET, the mean time from COVID-19 diagnosis to the CPET was 6 ± 4 months. o2max was normal (>84% predicted) in 34 (68%; mean peak o2 107 ± 22% predicted) and reduced in 16 patients (32%; mean peak o2 67 ± 13% predicted). Among these 16, none had ventilatory limitation, but 9 (56%) had cardiovascular limitation (heart rate reserve <15 beats per minute at peak exercise), and 10 (63%) had a low O2 pulse value at peak exercise.
Regardless of the presence of comorbidities, among the 39 patients with PASC who had arterial catheters in place, mean lactate was significantly higher (Figures 1A and 1B); and in all 50 patients with PASC, calculated levels of FATox were significantly lower (Figures 1C and 1D) during exercise when compared with historical cohorts of subjects who are moderately active or with metabolic syndrome (3). Calculated levels of CHOox in patients with PASC were not significantly different from these published cohorts (data not shown). Average power output, oxygen consumption, and blood lactate did not differ between patients with PASC with or without comorbidities (data not shown). Correlations between post–COVID-19 symptoms, Borg score for dyspnea and leg fatigue, and time lapse from COVID-19 diagnosis to CPET with the anaerobic threshold and mitochondrial dysfunction (i.e., FATox) did not yield statistically significant relationships.
Figure 1.
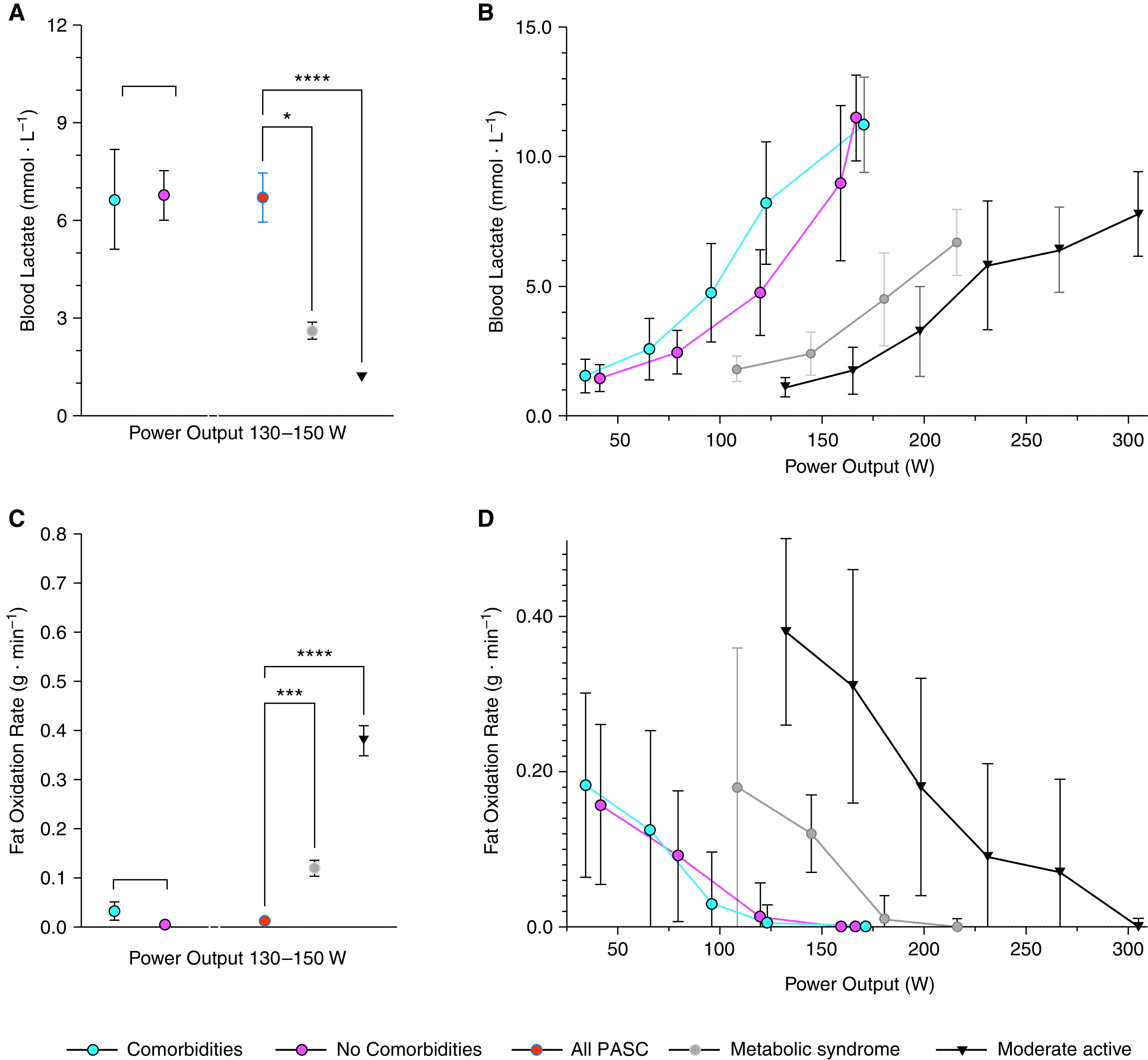
(A and B) Blood lactate and (C and D) fat oxidation rate as a function of power output, measured in Watts (W) during graded exercise in patients with PASC with and without comorbidities compared with a historic cohort (data obtained from Reference 3) of patients with metabolic syndrome and moderately active individuals. No comorbidities (n = 27); comorbidities (n = 23). Mean ± SEM; one-way ANOVA P < 0.0001 (Welch or Brown-Forsythe); Dunnett’s multiple comparison test: *P < 0.05, ***P < 0.001, and ****P < 0.0001. PASC = post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Our data suggest abnormally low FATox and altered lactate production by skeletal muscle as a putative cause of—or contributor to—the functional limitation of patients with PASC. Normally, as glycolysis increases with exercise intensity, lactate is oxidized for fuel in mitochondria, mainly in adjacent slow-twitch muscle fibers. Like FATox, lactate clearance capacity is a useful surrogate for mitochondrial function. In patients with PASC, even in those with normal pre–COVID-19 physical fitness and free of comorbidities, the metabolic disturbances of the skeletal muscle during exercise may be worse than those reported in moderately active individuals or in individuals with metabolic syndrome (3). Whereas rising blood lactate levels are expected during high exercise intensity (as glycolytic flux exceeds the rate of mitochondrial pyruvate oxidation), a high blood lactate at lower exercise levels indicates mitochondrial dysfunction (7). The inappropriately high arterial lactate levels at relatively low exercise intensity (e.g., >9 mM at 150 W) in patients with PASC indicate that the transition from FATox to CHOox occurs prematurely, suggesting metabolic reprogramming and dysfunctional mitochondria.
Dysregulated lipid oxidation and decreased mitochondrial biogenesis have been reported in acute critically ill patients admitted to the ICU (8). However, the long-term weakness in ICU survivors is associated with heterogeneous muscle pathophysiology with variable combinations of muscle atrophy and impaired contractile capacity and not solely by diminished mitochondrial content (9).
Our study has several limitations, including a small cohort size, retrospective methodology, and lack of a contemporaneous control, relying on comparisons with historical cohorts that have distinct demographics. Therefore, our findings may not be generalizable and should be considered hypothesis-generating. They should provide impetus for further investigations into the molecular mechanisms linking COVID-19 to metabolic reprogramming and mitochondrial dysfunction. Our report is consistent with that by Pleguezuelos and colleagues, who also showed that patients with PACS that followed acute COVID-19 requiring admission to the ICU suffered from reduced exercise efficiency (10) and with that of Rinaldo and colleagues showing that disease severity does not impact exercise capacity in COVID-19 survivors; however, the study focused on peak values and cardiorespiratory peak values and not on metabolic/cellular adaptations as we did in our study (11).
To our knowledge, our study provides the first evidence of mitochondrial dysfunction that advances our understanding of the pathogenesis of PACS in patients with preserved pulmonary and cardiac function. Future studies into the mechanisms of mitochondrial dysfunction in individuals with PACS will help accelerate the development of therapies to improve their functional status.
Acknowledgments
Acknowledgment
The authors thank Janel L. Martinez, M.S., Valerie Clarkson Keever, and Bree Mabley, as well as the staff of the Pulmonary Physiology Laboratory and the Center for Post COVID Care at National Jewish Health, Denver, Colorado, for their contribution to this study.
Footnotes
Supported by Wollowick Chair in COPD Research (I.P.).
Originally Published in Press as DOI: 10.1164/rccm.202108-1903LE on October 19, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA . 2020;324:1723–1724. doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baratto C, Caravita S, Faini A, Perego GB, Senni M, Badano LP, et al. Impact of COVID-19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol (1985) . 2021;130:1470–1478. doi: 10.1152/japplphysiol.00710.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. San-Millán I, Brooks GA. Assessment of metabolic flexibility by means of measuring blood lactate, fat, and carbohydrate oxidation responses to exercise in professional endurance athletes and less-fit individuals. Sports Med . 2018;48:467–479. doi: 10.1007/s40279-017-0751-x. [DOI] [PubMed] [Google Scholar]
- 4. Wasserman DH, Halseth AE. An overview of muscle glucose uptake during exercise: sites of regulation. Adv Exp Med Biol . 1998;441:1–16. doi: 10.1007/978-1-4899-1928-1_1. [DOI] [PubMed] [Google Scholar]
- 5. American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med . 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 6. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol . 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 7. Parikh S, Goldstein A, Koenig MK, Scaglia F, Enns GM, Saneto R, et al. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet Med . 2015;17:689–701. doi: 10.1038/gim.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puthucheary ZA, Astin R, Mcphail MJW, Saeed S, Pasha Y, Bear DE, et al. Metabolic phenotype of skeletal muscle in early critical illness. Thorax . 2018;73:926–935. doi: 10.1136/thoraxjnl-2017-211073. [DOI] [PubMed] [Google Scholar]
- 9. Dos Santos C, Hussain SN, Mathur S, Picard M, Herridge M, Correa J, et al. MEND ICU Group RECOVER Program Investigators; Canadian Critical Care Translational Biology Group. Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay: a pilot study. Am J Respir Crit Care Med . 2016;194:821–830. doi: 10.1164/rccm.201512-2344OC. [DOI] [PubMed] [Google Scholar]
- 10. Pleguezuelos E, Del Carmen A, Llorensi G, Carcole J, Casarramona P, Moreno E, et al. Severe loss of mechanical efficiency in COVID-19 patients. J Cachexia Sarcopenia Muscle . 2021;12:1056–1063. doi: 10.1002/jcsm.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rinaldo RF, Mondoni M, Parazzini EM, Baccelli A, Pitari F, Brambilla E, et al. Severity does not impact on exercise capacity in COVID-19 survivors. Respir Med . 2021;187:106577. doi: 10.1016/j.rmed.2021.106577. [DOI] [PMC free article] [PubMed] [Google Scholar]


