The primary life-limiting pulmonary morbidity of severe coronavirus disease (COVID-19) is characterized by pulmonary endothelialitis, microangiopathy, and aberrant angiogenesis (1). Although numerous studies have highlighted the pronounced microangiopathy in pulmonary circulation, the impact of the bronchial vascular system has not been fully elucidated. Therefore, we comprehensively analyzed complete lung lobes from three male patients (age, 63.7 ± 14.2 years; hospitalization time, 22 ± 1 days, mechanically ventilated) who succumbed to severe COVID-19 using conventional computed tomography, histology, microvascular corrosion casting, and hierarchical phase-contrast tomography (2). We used three control lungs from body donors (age, 78.3 ± 13.6 yr; nonventilated, two females and one male died from cerebral stroke or uterine carcinoma).
In pulmonary computed tomography angiography, we found the previously reported pulmonary sequelae of COVID-19 lung injury in the form of bilateral peripheral ground-glass opacities, peribronchial consolidations, and peripheral macrovascular congestion (3) (Figures 1A and 1B). Peribronchial and perivascular microvessels (vasa vasorum) were distinctly dilated (Figures 1C–1F). This intrapulmonary shunting by the bronchial circulation (Figure 2A) accounts for the continued perfusion in a variety of airway conditions, such as inflammation, acute respiratory distress syndrome, and chronic thromboembolism (3). In severe COVID-19 pneumonia, the microvascular architecture of the peribronchial vessels showed a microvascular architecture with densely packed aberrant bundles of blood vessels (Figures 2B–2E). The expansion of the peribronchial plexus is mainly driven by intussusceptive angiogenesis as evidenced by the appearance of transluminal endothelial tissue pillars (Figures 2B–2D) (1, 4, 5).
Figure 1.
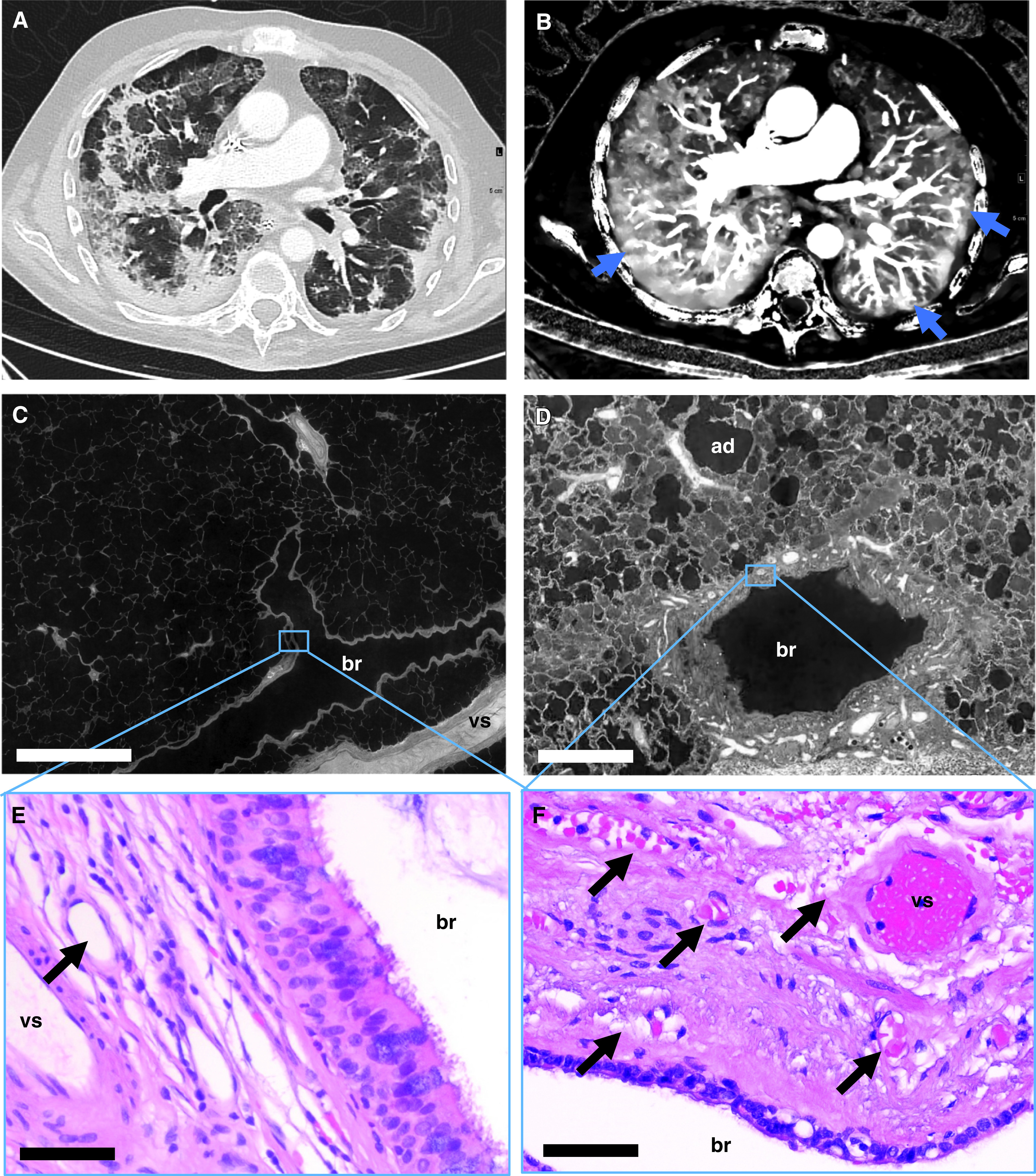
Bronchial artery and vasa vasorum dilatation and expansion in coronavirus disease (COVID-19) pneumonia. (A) Dual energy computed tomography revealed the characteristic features of severe COVID-19 pneumonia as bilateral ground-glass opacities, peribronchial consolidations, and a diffuse crazy-paving pattern. (B) Iodine map showed dilatation of macrovessels in or leading to opacified air spaces and frequently evident vascular “tree-in-bud” (blue arrows). (C and D) Hierarchical phase-contrast tomography (high-resolution tomographic images analyzed by Synchrotron radiation X-ray tomographic microscopy) demonstrated the regular architecture in healthy control lungs (C), whereas in COVID-19 pneumonia, a dilatation and an expansion of vasa vasorum and peribronchial vessels was observed (D). Scale bars: C, 1,000 μm; D, 400 μm. (E and F) Representative hematoxylin and eosin–stained sections show sporadic peribronchial vessels (black arrow) in the control tissue (E), whereas the peribronchial plexus in COVID-19 lungs was characterized by an expansion and the formation of angiomatoid- and plexiform-like vessels (black arrows) (F). Scale bars: 20 μm. ad = alveolar duct; br = bronchus; vs = pulmonary vessel.
Figure 2.
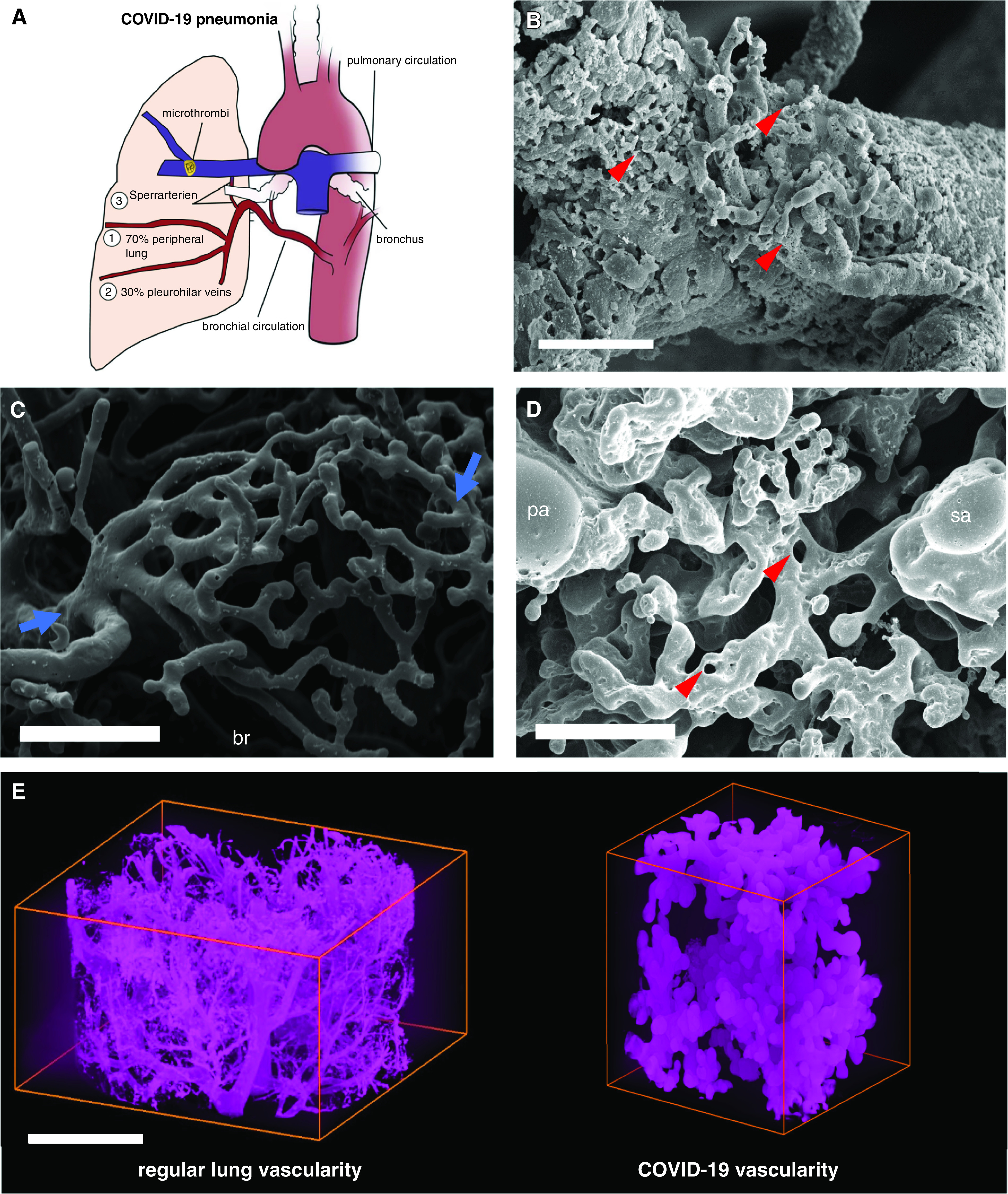
Intrapulmonary shunting via bronchial circulation in coronavirus disease (COVID-19) pneumonia. (A) The schematic illustrates intrapulmonary cross-circulation and draining by bronchial arteries to the peripheral lung, pleurohilar veins, and “Sperrarterien,” specialized, spiral-like intrapulmonary arteriovenous anastomoses recruited by hypoxia and flow irregularities. The bronchial circulation arises primarily direct from the thoracic aorta and intercostal arteries and, under normal conditions, constitutes less than 2% volume of the left cardiac output. However, the bronchial flow can significantly exceed the regular hemodynamics upon hypoxic and inflammatory stimulus. As part of a physiological right–left shunting, nearly two-thirds of the bronchial vessels are drained peribronchially to the peripheral lung and finally into the left ventricle (up to 4% of left ventricular output), whereas the remaining third is perfused pleurohilar veins draining ultimately to the right heart. (B) Scanning electron micrograph of a microvascular corrosion cast demonstrates the expansion of vasa vasorum of an intralobular artery by intussusceptive angiogenesis (red arrowheads) in the lung tissue of a patient who succumbed to severe COVID-19 pneumonia. Scale bar, 50 μm. Intussusceptive angiogenesis is a well-characterized morphogenetic process observed during the capillary expansion in cancer, inflammation, and regeneration, but it has also been reported in the alveolar plexus of COVID-19 lungs. Scale bar, 100 μm. (C) Scanning electron micrograph of a microvascular corrosion cast demonstrates the peribronchial plexus in healthy control lungs (blue arrows). Scale bar, 200 μm. (D) Scanning electron micrograph of a microvascular corrosion cast reveals the cross-circulation in COVID-19 lung tissue between a Sperrarterie and a lobular artery in a secondary pulmonary lobule, which is characterized by plexiform-like vascular proliferations by intussusceptive angiogenesis (red arrowheads). Scale bar, 200 μm. (E) Three-dimensional evaluation of microvascular corrosion casts by Synchrotron radiation X-ray tomographic microscopy illustrating the irregular, hierarchical vascularity with dilated, blunt-like vessels in COVID-19 pneumonia. Scale bar, 100 μm. br = bronchiole, pa = lobular artery; sa = Sperrarterie.
A spatial analysis of peribronchial vessels in COVID-19 pneumonia by hierarchical phase-contrast tomography demonstrated the expansion of peribronchial and perivascular arteriovenous anastomoses and a recruitment of “Sperrarterien” (blockade arteries) across individual secondary pulmonary lobules in the third dimension for the first time (Figures 3A and 3B and Video E1 in the online supplement). This intralobular shunting is accompanied by different spots of glomerouid-like vascular expansion (Figures 3C and 3D).
Figure 3.
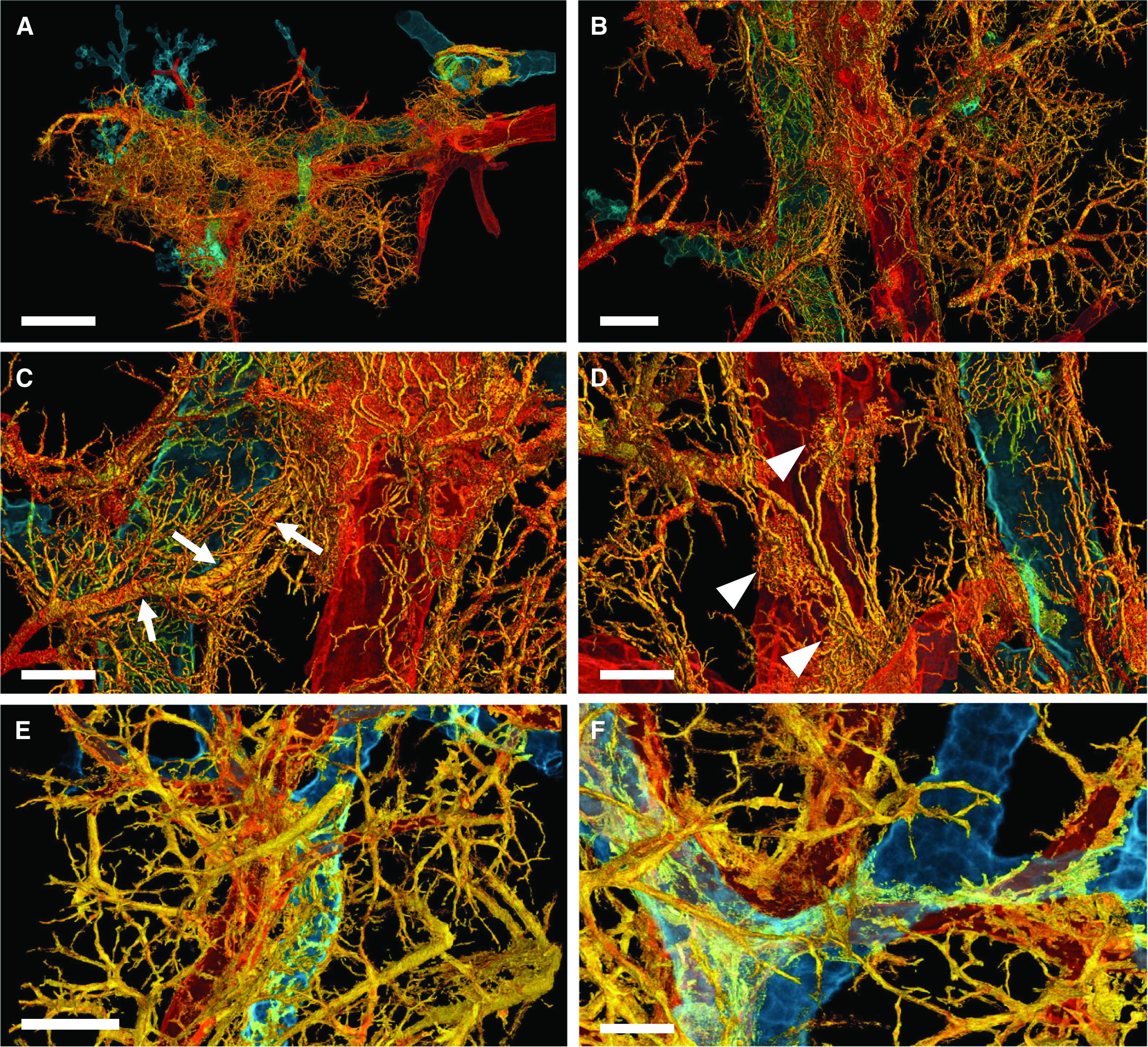
Three-dimensional complexity of vascular remodeling and bronchiopulmonary shunting in coronavirus disease (COVID-19) highlighted by hierarchical phase-contrast tomography. (A) The distal bronchiole (blue) is paralleled by a centrilobular pulmonary artery (red) and an expanded plexus of private vessels (vasa vasorum) (yellow) in a secondary pulmonary lobule in the periphery of the left lower lobe. Scale bar, 4 mm. (B) Dilatation and expansion of the peribronchiolar plexus and vasa vasorum (yellow) is observed around the distal terminal bronchiole (blue) and centrilobular pulmonary artery (red). Scale bar, 1.3 mm. (C) Numerous arteriovenous anastomoses (yellow) form between bronchial arteries and pulmonary artery drain into a pulmonary vein accompanied by a pronounced shunting of a Sperrarterie (white arrows). These specialized arteries are essential for intrapulmonary arteriovenous shunting and are located mainly at the septal margin of secondary pulmonary lobules, where up to 3 to 5 such “Sperrarterien” anastomoses can be found in each lobule. Scale bar, 0.7 mm. (D) The perivascular vasa vasorum and bronchial vascular plexus are characterized by complex nodular lesions with angiomatoid- and plexiform-like arrangements (white arrowheads). Scale bar, 0.7 mm. For high-resolution details, see COVID-19 Pneumonia in the online supplement . (E) In controls, the vascular architecture shows a well-organized hierarchy of peribronchial vessels. Scale bar, 1.3 mm. (F) Only few perivascular vasa vasorum are observed in healthy control tissue. Scale bar, 0.7 mm. For high-resolution details, see Control in the online supplement.
Here, we show the first insight into the complex three-dimensional phenomenon of bronchiopulmonary shunting in COVID-19. However, the sequelae of this excessive neovascularization, the actual remodeling that may account for intralesional hyperperfusion and long-term implications, have so far not been understood.
Footnotes
Supported by NIH Grant HL94567 and NHLBI Grant HL134229 (M.A. and S.J.M.), an H2020 European Research Council Consolidator Grant XHale (771883, D.D.J.), Deutsche Forschungsgemeinschaft (Project Z2) Grant KFO311 (D.D.J.), Chan Zuckerberg Initiative Grant 2020-225394, an advised fund of Silicon Valley Community Foundation, the ESRF beamtime proposal md1252, Diamond Light Source (MG27025, MG27094), the Royal Academy of Engineering Grant CiET1819/10 (P.D.L.), and the MRC (MR/R025673/1). C.W. is supported by the MRC Skills Development Fellowship (MR/S007687/1). This work was also supported by the German Registry of COVID-19 Autopsies (DeRegCOVID), which is supported by the Federal Ministry of Health (ZMVI1-2520COR201), and the Federal Ministry of Education and Research as part of the Network of University Medicine (Bundesministerium für Bildung und Forschung DEFEAT PANDEMIcs, 01KX2021).
Author Contributions: M.A., W.L.W., C.W., S.J.M., and D.D.J. managed the patients. M.A., P.T., C.W., M.P.K., F.P.L., C.D., A.B., S.E.V., and D.D.J. worked up surgical specimens. M.A., P.T., W.L.W., C.L.W., C.W., M.P.K., C.D., A.J.B., A.B., S.E.V., P.D.L., S.J.M., and D.D.J. interpreted the data. M.A., S.E.V., S.J.M., and D.D.J. wrote the manuscript. M.A., P.T., C.W., M.P.K., C.D., A.B., S.E.V., and D.D.J. prepared the images. M.A., P.T., W.L.W., C.L.W., C.W., M.P.K., F.P.L., C.D., A.B., S.E.V., P.D.L., S.J.M., and D.D.J. revised the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202103-0594IM on August 31, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med . 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walsh C, Tafforeau P, Wagner WL, Jafree DJ, Bellier A, Werlein C, et al. Imaging intact human organs with local resolution of cellular structures using hierarchical phase-contrast tomography. Nat Methods . doi: 10.1038/s41592-021-01317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCullagh A, Rosenthal M, Wanner A, Hurtado A, Padley S, Bush A. The bronchial circulation--worth a closer look: a review of the relationship between the bronchial vasculature and airway inflammation. Pediatr Pulmonol . 2010;45:1–13. doi: 10.1002/ppul.21135. [DOI] [PubMed] [Google Scholar]
- 4. Eldridge L, Wagner EM. Angiogenesis in the lung. J Physiol . 2019;597:1023–1032. doi: 10.1113/JP275860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ackermann M, Mentzer SJ, Kolb M, Jonigk D. Inflammation and intussusceptive angiogenesis in COVID-19: everything in and out of flow. Eur Respir J . 2020;56:2003147. doi: 10.1183/13993003.03147-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


