Abstract
Rationale
Pulmonary sarcoidosis is generally presumed to be a T-helper cell type 1– and macrophage-driven disease. However, mouse models have recently revealed that chronically inflamed lung tissue can also comprise T follicular helper (Tfh)-like cells and represents a site of active T-cell/B-cell cooperation.
Objectives
To assess the role of pulmonary Tfh- and germinal center–like lymphocytes in sarcoidosis.
Methods
BAL fluid, lung tissue, and peripheral blood samples from patients with sarcoidosis were analyzed by flow cytometry, immunohistology, RNA sequencing, and in vitro T-cell/B-cell cooperation assays for phenotypic and functional characterization of germinal center–like reactions in inflamed tissue.
Measurements and Main Results
We identified a novel population of Tfh-like cells characterized by high expression of the B helper molecules CD40L and IL-21 in BAL of patients with sarcoidosis. Transcriptome analysis further confirmed a phenotype that was both Tfh-like and tissue resident. BAL T cells provided potent help for B cells to differentiate into antibody-producing cells. In lung tissue, we observed large peribronchial infiltrates with T and B cells in close contact, and many IgA+ plasmablasts. Most clusters were nonectopic; that is, they did not contain follicular dendritic cells. Patients with sarcoidosis also showed elevated levels of PD-1high CXCR5− CD40Lhigh ICOShigh Tfh-like cells, but not classical CXCR5+ Tfh cells, in the blood.
Conclusions
Active T-cell/B-cell cooperation and local production of potentially pathogenic antibodies in the inflamed lung represents a novel pathomechanism in sarcoidosis and should be considered from both diagnostic and therapeutic perspectives.
Keywords: pulmonary sarcoidosis, T-cell/B-cell cooperation, follicular helper–like T cells, lymphocytic lung infiltrates, peripheral T-helper cells (Tph)
At a Glance Commentary
Scientific Knowledge on the Subject
Pulmonary sarcoidosis has previously been considered to be mainly a T-helper type 1– and macrophage-driven disease. Recently, a novel population of T follicular helper (Tfh)-like (also known as T peripheral helper) cells that promotes B-cell responses in inflamed nonlymphoid tissues has been described. These cells lack the classical Tfh markers BCL-6 and CXCR5, but they express high levels of the two key B-cell helper molecules, CD40L and IL-21.
What This Study Adds to the Field
We demonstrate for the first time the presence of Tfh-like cells in the lungs of patients with pulmonary sarcoidosis. They coproduce high levels of IFN-γ and IL-21 and provide potent B-cell help in vitro. In the lung tissue, they are found in large lymphocytic infiltrates in close contact with B cells, indicating that they drive the local generation of plasmablasts. Consistent with this, BAL fluid from patients with sarcoidosis contains high concentrations of immunoglobulins. These findings highlight an underappreciated role of T-cell/B-cell collaboration in sarcoidosis. Moreover, elevated frequencies of circulating Tfh-like cells in peripheral blood may be a useful marker for monitoring sarcoidosis disease activity.
Pulmonary sarcoidosis is an interstitial lung disease (ILD) of unknown origin, characterized by a high frequency of lung-infiltrating CD4+ T cells, conversely to the predominance of CD8+ T cells found in most other ILDs (1, 2). Sarcoidosis-associated CD4+ T cells are strongly polarized toward a T-helper cell type 1 (Th1) phenotype (3–5) and are clonally expanded, although disease-driving antigens have not been identified yet. Th1 cell–derived IFN-γ leads to macrophage activation and granuloma formation and is generally assumed the major disease-contributing factor (1, 2). Interestingly, reports from the 1980s and early 1990s described the presence of B cells in the lung tissue in sarcoidosis (6, 7). However, the role of B cells in sarcoidosis and other granulomatous lung diseases remains ill-defined. It is unknown whether T-helper cells interact with B cells in sarcoidosis. Cognate T-cell/B-cell cooperation normally takes place in secondary lymphoid organs during the germinal center (GC) reaction. The GC reaction involves a specialized population of T-helper cells, called T follicular helper (Tfh) cells, which interact with antigen-specific B cells to support their expansion, antibody affinity maturation, and differentiation into antibody-producing plasma cells or memory B cells (8, 9). Tfh cells are characterized by the lineage-defining transcription factor BCL-6, high expression of PD-1, the chemokine receptor CXCR5, which enables them to enter the B-cell zone in secondary lymphoid organs, and high levels of IL-21 (8, 9). However, under chronic inflammatory conditions, activated T and B cells are also frequently found in nonlymphoid tissues, substantially contributing to tissue damage through the production of proinflammatory cytokines and pathogenic antibodies. Still, a frequently underestimated aspect is their ability to actively interact and propagate the pathological inflammatory response in the inflamed tissue (10). In a mouse model of lung inflammation, we previously discovered a so far unknown population of Tfh-like cells that drive the local generation of GC-like B cells and plasmablasts producing large amounts of antibodies directly in the inflamed tissue (11). Even though these Tfh-like cells lack the classical Tfh cell markers BCL-6 and CXCR5, they have potent B helper capabilities owing to high production of IL-21 and CD40L, two major factors for B-cell activation and differentiation. Tfh-like cells are found in close contact with antigen-specific B cells within unstructured lymphocytic infiltrates in the lung, which lack follicular dendritic cells (FDCs) and other signs of ectopic lymphoid structures (10, 11).
Here, we asked whether local T-cell/B-cell cooperation in the lung might play a role for the pathogenesis of sarcoidosis and whether lung-infiltrating T cells exhibit Tfh-like features. Some results of these studies have been previously reported in the form of an abstract (12).
Methods
Patients
A total number of 33 patients with sarcoidosis were included in this study. A diagnosis of pulmonary sarcoidosis was made by a board-certified pulmonologist according to American Thoracic Society, European Respiratory Society, and World Association for Sarcoidosis and Other Granulomatous Disorders guidelines (13, 14). Criteria specifically included compatible clinicoradiological findings, the exclusion of relevant differential diagnoses, especially pulmonary tuberculosis, and the histopathological finding of granulomatous inflammation in all patients. From these patients, different types of samples were analyzed as outlined in Table 1. BAL samples were obtained during routine bronchoscopy for diagnostic purposes by sequential instillation of a total volume of 150 ml NaCl solution using a flexible fiberoptic bronchoscope (15). Peripheral blood mononuclear cells (PBMCs) were prepared from blood by Ficoll density centrifugation. Frozen lung tissue samples were provided by the Universities of Giessen and Marburg Lung Center Giessen Biobank, member of the German Center for Lung Research Platform Biobanking. As a control group for flow cytometric analyses of T and B cells from peripheral blood, 16 healthy volunteers were recruited to match the patient group in average age and sex distribution. As a control group for the immunoglobulin analysis from BAL supernatants, seven patients with non-ILDs (idiopathic cough, lung cancer, pulmonary metastases, or patients without apparent lung disease receiving BAL for exclusion of pulmonary tuberculosis) were enrolled. As a positive control for T and B cells from a secondary lymphoid organ, palatine tonsils were obtained from four patients undergoing routine tonsillectomy. For flow cytometry, cells were prepared from the tissue by mechanical disruption and Ficoll density centrifugation. For histology, tissue blocks were snap-frozen in liquid nitrogen and stored at −80°C. All human samples were obtained after informed consent and in accordance with the local ethics committee (IRB, EA2/086/16).
Table 1.
Sarcoidosis Patient and Control Cohorts: Demographics and Clinical Characteristics
| Patients with Sarcoidosis Cohort 1 | Patients with Sarcoidosis Cohort 2 | Patients with Sarcoidosis Cohort 3 | Patients with Sarcoidosis Cohort 4 | Control Cohort 1 | Control Cohort 2 | |
|---|---|---|---|---|---|---|
| Tissues analyzed | BAL cells, BAL supernatant, blood | BAL cells, BAL supernatant | BAL supernatant | Frozen lung biopsies | Blood | BAL supernatant |
| Total number | 12 | 6 | 7 | 8 | 16 | 7 |
| Age, yr, mean ± SD | 41.1 ± 10.4 | 45.3 ± 13.4 | 57.9 ± 19.1 | 49.6 ± 9.6 | 39.4 ± 12.3 | 58.1 ± 15.8 |
| Sex, M/F | 6/6 | 4/2 | 4/3 | 5/3 | 8/8 | 5/2 |
| CD4+/CD8+ ratio, mean ± SD | 9.2 ± 7.7 | 4.7 ± 1.6 | 5.3 ± 5.7 | n/a | n/a | n/a |
| Lymphocytic alveolitis, mean ± SD | 26.4 ± 14.7 | 31.8 ± 18.6 | 26.5% ± 15.5 | n/a | n/a | n/a |
Definition of abbreviation: n/a =not available.
Flow Cytometry
For analysis of cytokine production, cells were restimulated for 4 hours with phorbol 12-myristate 13-acetate and ionomycin, with brefeldin A added for the last 3 hours. To assess CXCL13 production, cells were activated for 24 hours with plate-bound anti-CD3 and anti-CD28 (10 μg/ml each), with 5 μg/ml brefeldin A added for the last 5 hours. Single-cell suspensions were stained on ice with the antibodies listed in Table E1 in the online supplement. Fc receptors were blocked with 1 mg/ml human IgG. Live/dead discrimination was performed by staining with succinimidyl esters as described previously (16). Cells were fixed with either 2% paraformaldehyde or the eBioscience Transcription Factor Staining Kit. For intracellular staining of cytokines or chemokines, cells were permeabilized with 0.5% saponin. Cells were acquired on an LSRFortessa or Symphony Flow Cytometer (Becton Dickinson) and further analyzed using FlowJo Version 9.9 (Tree Star).
Immunohistology
Frozen lung sections of 8 μm were fixed with acetone or 2% paraformaldehyde (for staining of nuclear factors) and stained with the antibodies listed in Table E2. Nuclei were counterstained with DAPI and slides mounted with Fluoromount (Sigma-Aldrich). Specimens were analyzed on a Carl Zeiss LSM 880 with ZEN 2.3 software.
Transcriptome Analysis
T cells from BAL, palatine tonsil, and PBMCs were sorted on an ARIA II flow sorter (Becton Dickinson) as DAPI− CD14− CD16− CD19− CD3+ CD4+ CD45RA− CD45RO+. Tonsillar T cells were further sorted for CXCR5/PD-1 double-positive or -negative to discriminate Tfh and non-Tfh cells. RNA was isolated using the Qiagen RNeasy Micro Kit. Libraries were prepared as described recently (16) and sequencing performed on a NextSeq500 (Illumina). Raw sequences were mapped to the GRCh37/hg19 genome. Differential gene expression was analyzed using DESeq2 (17) and was considered significant when the adjusted P value was less than 0.05 and the log2 fold change at least 1.3. These differences (∼30%) can already result in significant biological differences in overall biology, especially for transcription factors (16).
Data can be accessed in the NCBI Gene Expression Omnibus repository, accession number GSE162712.
In Vitro T-Cell/B-Cell Cooperation Assay
Naive T cells from PBMCs (CD19− CD4+ CD45RA+), memory T cells from BAL (CD19− CD16− CD14− CD4+ CD45RA−), and non-Tfh memory cells (CD19− CD4+ CD45RA− CXCR5−) or Tfh cells (CD19− CD4+ CD45RA− CXCR5+) from tonsils were sorted on an ARIA II flow sorter. Sorted T cells were cocultured for 7 days with heterologous tonsillar memory B cells (CD19+ CD4− IgD− CD38−) at a 1:1 ratio in the presence of 4 ng/ml staphylococcal enterotoxin B (Toxin Technology) as described previously (18). To block T-cell help, an antibody against CD40L (clone TRAP1; 20 μg/ml) and/or recombinant soluble IL-21 receptor (R&D Systems; 10 μg/ml) were added.
Statistical Analysis
Data were analyzed using GraphPad Prism 8. After checking for normal distribution (Shapiro-Wilk test), significant differences were determined by either ordinary one-way ANOVA, Kruskal-Wallis, unpaired t, or Mann-Whitney U test. For comparisons with eight or fewer data points, nonparametric tests were used. For transcriptome data, the Wald test from the DESeq2 package (17) was applied. Cytokine coexpression was analyzed by comparing the observed percentage of double-positive cells with the expected value calculated for random coincidence of two independent variables. For evaluation of coordinate or stochastic expression, the φ-correlation coefficient was calculated. As for cytokine coexpression, this value rarely exceeds 0.4, coefficients of φ ⩽ −0.1 or φ ⩾ 0.1 were considered significant in this analysis (19). For all tests, no data were excluded.
Results
Lung-Infiltrating T Cells from Patients with Sarcoidosis Exhibit a Tfh-like Phenotype
Lung-infiltrating T cells were analyzed from the BAL fluid of 18 patients with sarcoidosis with pulmonary involvement (Table 1). To compare their phenotype to T cells from a secondary lymphoid organ and circulating peripheral T cells, tonsillar cells from otherwise healthy individuals undergoing routine tonsillectomy and blood samples from healthy volunteers were obtained. Multicolor flow cytometry was used for detailed characterization of CD4+ T cells from these three sites. As expected, the frequency of T cells with an antigen-experienced phenotype (CD45RO+) varied substantially from 42.7% (±10.8) in blood, 61.5% (±3.7) in tonsils, to 98.7% (±1.1) in BAL. Thus, we directly compared CD45RO+ CD3+ CD4+ T cells and additionally excluded FoxP3+ regulatory T cells from analysis (Figure E1).
As expected, tonsillar samples contained a large population of classical CXCR5+ PD- 1+ Tfh as well as a unique subpopulation of CXCR5++ PD-1++ GC Tfh cells, whereas in the blood only a small population of memory Tfh cells was present. In contrast, bona fide CXCR5+ PD-1+ Tfh cells were absent in sarcoidosis-affected BAL (Figures 1A and 1C). However, when we analyzed expression of the two functionally important Tfh molecules, CD40L and IL-21, their expression in sarcoidosis-affected BAL T cells was more than twice as high as in tonsillar CD45RO+ T cells (Figures 1B and 1D). In both sarcoidosis-affected BAL and tonsillar T cells, IL-21 production and CD40L expression was clearly associated with high expression of PD-1, one of the prototypical Tfh cell markers, which, in CD4+ T cells, is not a marker of functional “exhaustion” but marks T cells with high B-cell helper capacity (8).
Figure 1.
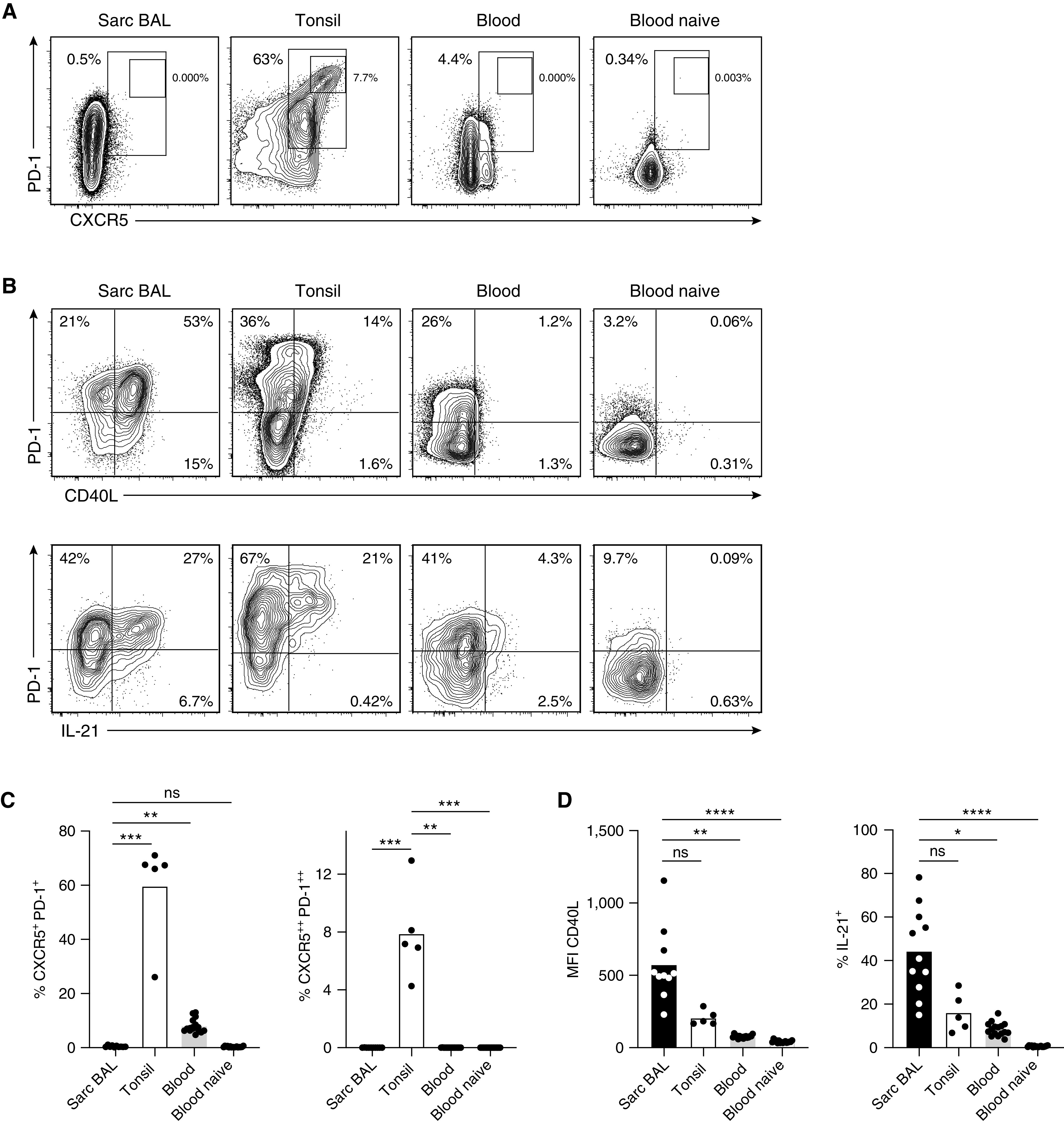
Sarcoidosis-affected BAL T cells lack classical T follicular helper (Tfh) cell markers but express high levels of CD40L and IL-21. Antigen-experienced CD4+ T cells (CD45RO+; for complete gating, see Figure E1 in the online supplement) from BAL of a patient with sarcoidosis, tonsillar tissue, and peripheral blood of healthy donors (CD45RO-negative [naive] cells are shown as additional control) were analyzed by flow cytometry. (A and C) Frequency of T cells with a CXCR5+ PD-1+ Tfh and CXCR5++ PD-1++ germinal center Tfh cell phenotype. (B and D) Analysis of CD40L expression (no restimulation; geometric mean fluorescence intensity [MFI]) and IL-21 (after short-term restimulation). Representative flow cytometry data and statistical analysis are shown. Individual samples are depicted by symbols with bars showing the mean. Data are from 10 sarcoidosis-affected BAL (11 for IL-21), 5 tonsil, and 16 healthy donor blood samples. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 (Kruskal-Wallis test). ns = not significant (P ⩾ 0.05, Kruskal-Wallis test); Sarc = sarcoidosis.
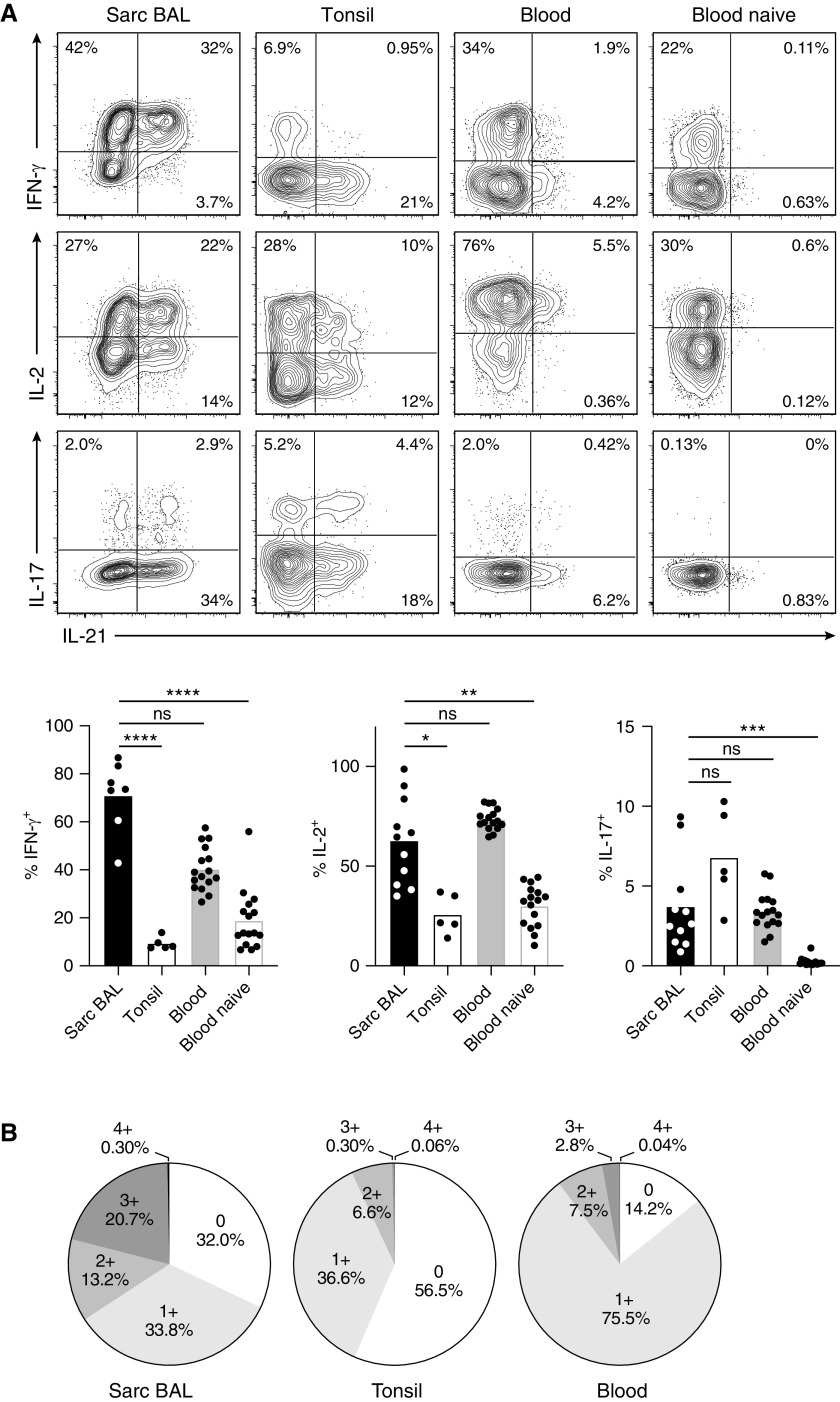
As reported (3–5), around 70% of sarcoidosis-affected BAL T cells produced IFN-γ upon restimulation, which is in stark contrast to tonsillar T cells with only around 10% IFN-γ producers (Figure 2A). Moreover, IFN-γ production in sarcoidosis-affected BAL T cells was associated with IL-21 (correlation coefficient φ = 0.22 ± 0.06), whereas tonsil and healthy donor blood were dominated by T cells producing IFN-γ alone (φ = 0.00 ± 0.03 and φ = −0.05 ± 0.04, respectively). Sarcoidosis-affected BAL T cells also expressed high levels of IL-2 but reduced levels of IL-17 in comparison to tonsillar T cells. Again, expression of IL-2 in sarcoidosis-affected BAL was associated with the Tfh cytokine IL-21 (φ = 0.22 ± 0.06). A detailed analysis of the percentage of CD4+ CD45RO+ T cells producing IL-21, IL-2, IL-17, or IFN-γ alone (1+), IL-21 in combination with one of the other cytokines (2+), IL-21 in combination with two other cytokines (3+), all four cytokines together (4+), or none of these cytokines (0), revealed that peripheral blood of healthy donors was dominated by cells producing IL-2 alone and tonsils by nonproducers (Figure 2B). In contrast, sarcoidosis-affected BAL contained a high proportion of polyfunctional T cells, that is, cells producing IL-21 in combination with a second or even third proinflammatory cytokine.
Figure 2.
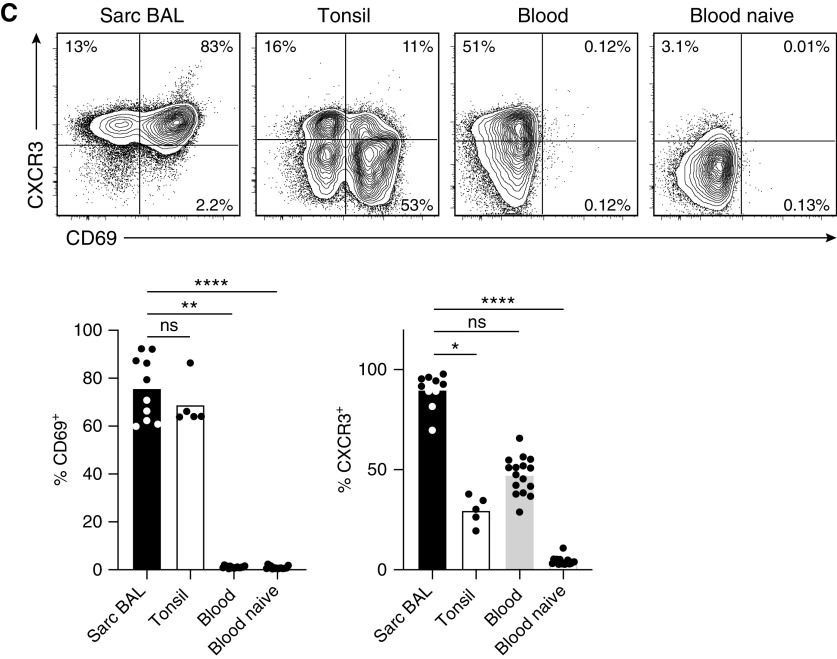
Lung-infiltrating T cells are polyfunctional cytokine producers and have a tissue-resident phenotype. T cells from BAL of a patient with sarcoidosis, tonsillar tissue, and peripheral blood of healthy donors were analyzed by flow cytometry. Plots are gated on antigen-experienced (CD45RO+) CD4+ T cells except for the “naive” panel, which was gated on CD45RO− cells. (A) Analysis of IL-21, IFN-γ, IL-2, and IL-17 production by intracellular staining after short-term restimulation in vitro. (B) Cytokine coexpression analysis indicating the percentage of cells producing neither IL-21, IFN-γ, IL-2, nor IL-17 (0), a single one of these four cytokines (1+), IL-21 in combination with one of the other cytokines (2+), IL-21 together with two of the other cytokines (3+), and all four cytokines together (4+). (C) Analysis of CD69 and CXCR3 expression. Representative flow cytometry data and statistical analysis are shown. Individual samples are depicted by symbols with bars showing the mean. Data are from 11 sarcoidosis-affected BAL (7 for IFN-γ and 10 for CD69 and CXCR3), 5 tonsil, and 16 healthy donor blood samples. *P < 0.05, **P < 0.005, ***P < 0.001, and ****P < 0.0001 (Kruskal-Wallis test). ns = not significant (P ⩾ 0.05, Kruskal-Wallis test); Sarc = sarcoidosis.
In accordance with their presence in the lung, sarcoidosis-affected BAL T cells expressed high levels of the tissue-homing receptor CXCR3 and CD69, which is inversely regulated to sphingosine-1-phosphate receptor and thereby considered a tissue-residence marker (20) (Figure 2C). Taken together, pulmonary CD4+ T cells in sarcoidosis lack classical Tfh cell markers such as CXCR5 but express high levels of functionally important molecules for B-cell help. Additionally, they are characterized by exceptionally high production of IFN-γ and IL-2 in combination with IL-21 and markers of tissue residency. Thus, they closely resemble Tfh-like cells from murine lung tissue (11, 21).
Transcriptome Analysis Reveals Similarities and Differentiating Features of Pulmonary Tfh-like Cells and Classical Tonsillar Tfh Cells
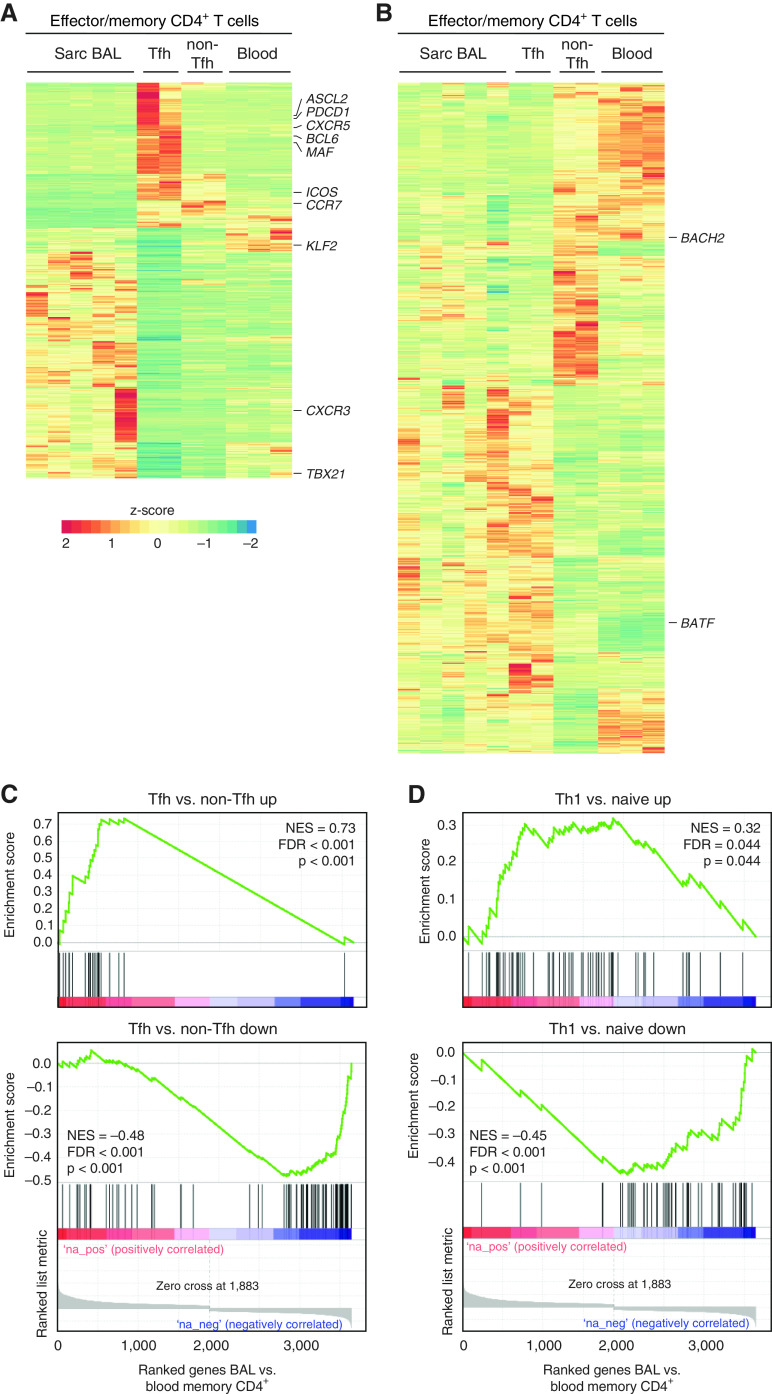
To obtain more comprehensive insights into similarities and differences between lung-infiltrating T cells and classical tonsillar Tfh cells, we assessed the transcriptome of BAL T cells from five patients with sarcoidosis through RNA sequencing. Tfh and non-Tfh effector cells from two tonsils and memory CD4+ T cells from peripheral blood of three healthy donors were sorted for comparison. This sample size resulted in clearly separated clusters for each group in a principal component analysis of the transcriptome data (data not shown). Of the 12,660 significantly expressed genes in this analysis (>50 reads for at least one sample), we found 1,068 genes (8.4%) that were differentially expressed (log2 fold change ⩾ 1.3 and adjusted P value < 0.05) between sarcoidosis-affected BAL T cells and tonsillar Tfh cells (Figure 3A). To examine the remaining 11,592 genes in more detail, we focused on Tfh signature genes, that is, genes that were differentially expressed between tonsillar Tfh and non-Tfh cells. Within these Tfh signature genes, the expression pattern in sarcoidosis-affected BAL T cells showed striking similarity to tonsillar Tfh cells (Figure 3B). For further in-depth comparison, we selected the 200 most differentially regulated genes between Tfh and non-Tfh cells to generate a Tfh cell gene signature list (Table E4). Gene set enrichment analysis revealed a strong enrichment of the Tfh cell signature in pulmonary T cells compared with peripheral blood memory T cells (Figure 3C). Given the strong Th1 polarization of sarcoidosis-affected BAL T cells (3–5), we performed a similar analysis with a published Th1 gene signature (22) and found a similar, albeit less pronounced, enrichment (Figure 3D).
Figure 3.
Lung-infiltrating T cells are a distinct cell population but share many similarities with T follicular helper (Tfh) cells. Antigen-experienced CD4+ T cells (CD45RO+ CD45RA−) were sorted from sarcoidosis-affected BAL (5 samples), healthy donor blood (3 samples), and tonsils (2 samples). Tonsillar T cells were further subdivided into non-Tfh and germinal center Tfh cells according to their expression of PD-1 and CXCR5 (for gating, see Figure 1A). Transcriptome analysis was performed by RNA-sequencing. (A) Heat map (z-scores rescaled to −2 to 2 as rainbow scale) showing all genes differentially regulated (log2 fold change ⩾ 1.3 and adjusted P value < 0.05) between BAL and Tfh cells (677 genes with higher expression in BAL vs. Tfh and 391 genes with lower expression). (B) Display of genes, which are specific for Tfh cells (differentially regulated between Tfh and non-Tfh cells; 994 genes with higher and 822 genes with lower expression), and comparison to healthy donor peripheral blood and sarcoidosis-affected BAL T cells. (C) Gene set enrichment analysis using the Broad Institute software package (http://www.gsea-msigdb.org/gsea/index.jsp) and a signature of the top 200 genes up- or downregulated between tonsillar Tfh and non-Tfh cells. (D) Gene set enrichment analysis using a published Th1 signature of genes up- or downregulated between naive CD4+ T cells and CD4+ T cells cultured in vitro for 48 hours under Th1-polarizing conditions (22). FDR = false discovery rate; NES = normalized enrichment score; Sarc = sarcoidosis; Th1 = T-helper cell type 1.
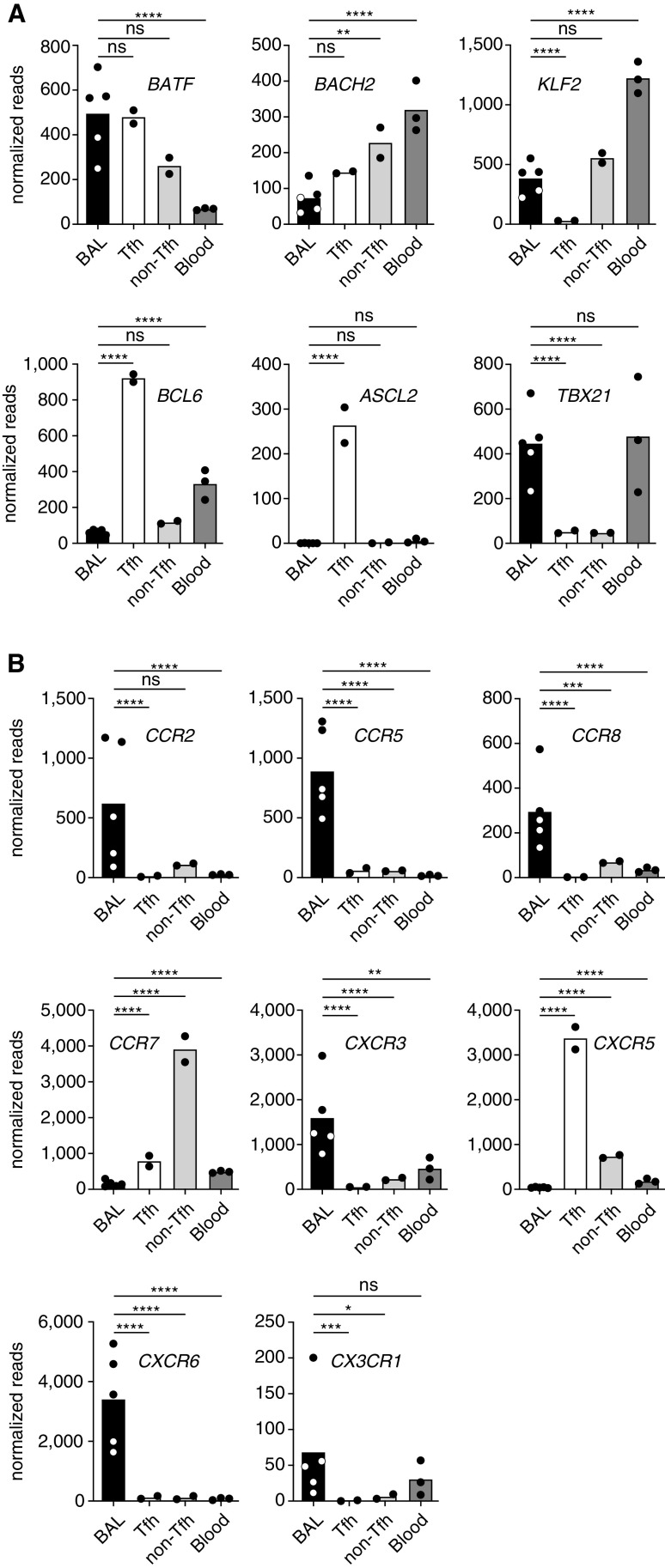
To gain further insights into the relationship between classical Tfh cells and sarcoidosis-affected BAL T cells, we analyzed expression of transcription factors known to be important for Tfh cells (8). Sarcoidosis-affected BAL T cells and classical tonsillar Tfh cells showed a similar expression pattern of BATF, a factor with higher expression in Tfh than non-Tfh cells, and KLF2 and BACH2, which need to be kept at low levels to maintain the Tfh cell phenotype (Figure 4A). However, other hallmark transcription factors of classical Tfh cells, like BCL6 and ASCL2, were not detectable in sarcoidosis-affected BAL T cells. In line with their high IFN-γ production, expression of TBX21 (encoding T-bet) was substantially higher in BAL compared with tonsillar T cells. We also analyzed several chemokine receptors important for T-cell homing (23) (Figure 4B). Interestingly, sarcoidosis-affected BAL T cells showed a marked upregulation of chemokine receptors known to guide T cells into inflamed tissues with a more than 15-fold higher expression of CCR2, CCR5, CCR8, CXCR3, CXCR6, and CX3CR1 compared with tonsillar Tfh cells. Expression of these chemokine receptors is in line with published studies showing enhanced production of the corresponding chemokine ligands, CCL16, CCL5, CCL1, CCL8, CXCL10, CXCL16, and CX3CL1, in the lung of patients with sarcoidosis (24–28). Overall, global transcriptome analysis confirmed that T cells from sarcoidosis-affected lungs share a number of Tfh features but express unique factors for tissue homing, which is in line with their presence in inflamed, nonlymphoid lung tissue.
Figure 4.
Expression pattern of key T follicular helper (Tfh) transcription factors and chemokine receptors identifies sarcoidosis-affected BAL T cells as a unique but Tfh-like population with a homing profile for inflamed tissues. Graphical display of selected genes from the transcriptome shown in Figure 3. (A) Display of key transcription factors important for the differentiation of Tfh cells. (B) Analysis of chemokine receptors important for tissue homing. Individual samples are depicted by symbols with bars showing the mean. Sarcoidosis-affected BAL T cells (5 patients) were compared with Tfh and non-Tfh cells from tonsils (2 donors) and memory T cells from peripheral blood (3 healthy donors). Significance was determined according to Wald. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. ns = not significant (P ⩾ 0.05).
Lung-Infiltrating T Cells Provide Potent B-Cell Help
To confirm that T cells from sarcoidosis-affected lungs not only express molecules important for B-cell help but can actually drive B-cell differentiation, we used an in vitro T-cell/B-cell cooperation assay, based on the coculture of T cells with heterologous B cells (isolated from tonsils) in the presence of superantigen (Staphylococcus enterotoxin B) at low concentration (18). As a reference, we used naive T cells from healthy donor blood and GC Tfh and non-Tfh effector cells isolated from tonsils. Sarcoidosis-affected BAL T cells were almost equally potent as classical Tfh cells in inducing B-cell differentiation into CD27high CD38high plasmablasts (Figure 5A). This is an impressive finding, considering that sorted GC Tfh cells with a very high expression of IL-21 (>80% producers) and CD40L were compared with total sarcoidosis-affected BAL T cells. T-cell help was almost completely abrogated by neutralization of IL-21 but not CD40L blockade. These findings were corroborated by determination of immunoglobulins in the culture supernatant by ELISA, demonstrating robust production of IgM, IgG, and IgA upon coculture with sarcoidosis-affected BAL Tfh-like cells (Figure 5B).
Figure 5.
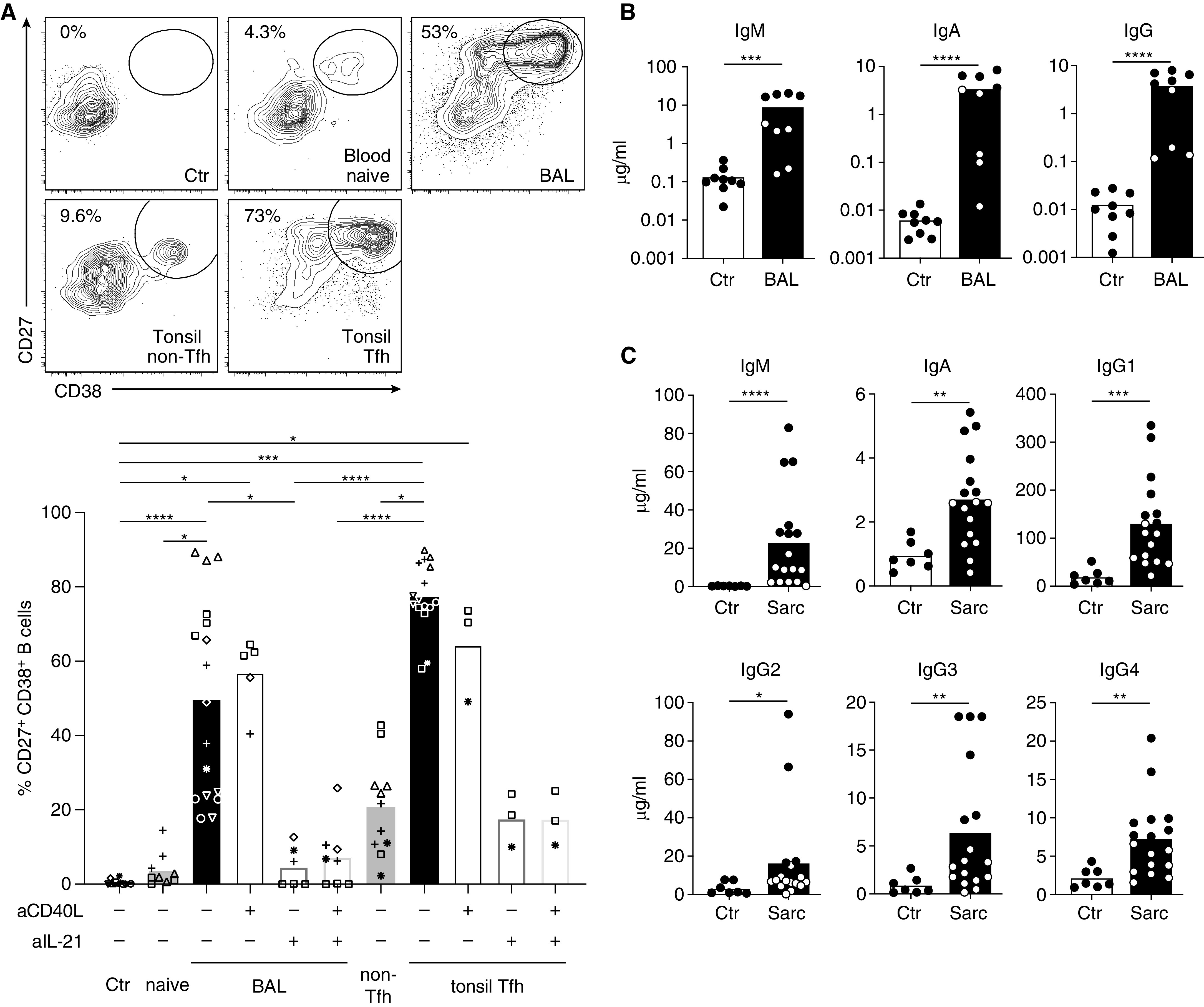
BAL T cells provide potent help for the differentiation of B cells into plasmablasts. (A) Heterologous memory B cells were cocultured with no T cells (Ctr), naive CD4+ T cells from blood, sarcoidosis-affected BAL CD4+ T cells, non–T follicular helper and germinal center T follicular helper cells from tonsils for 7 days in the presence of superantigen (staphylococcal enterotoxin B). T-cell help was blocked by addition of an antibody against CD40L and neutralization of IL-21 with recombinant soluble IL-21 receptor. Percentage of B cells with a plasmablast phenotype (CD27high CD38+) was determined by flow cytometry. Representative flow cytometry data and statistical analysis of two to seven independent experiments are shown. Each experiment is depicted by a different symbol and consisted of one to three individual cell culture wells. Only statistically significant differences are indicated. (B) Analysis of the culture supernatants from the experiment above for IgM, IgA, and IgG by ELISA. (C) BAL supernatants from 17 patients with sarcoidosis (10 patients from the study cohort and seven additional samples) (Table 1) and 7 control subjects (idiopathic cough, lung cancer, pulmonary metastases, or patients without apparent lung disease receiving BAL for exclusion of pulmonary tuberculosis) were analyzed for immunoglobulin subclasses by ELISA. Capture and biotinylated detection antibodies for ELISA are listed in Table E3. Human Immunoglobulin Calibrator (The Binding Site) was used as standard. Bars in all panels display mean with SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Statistical tests: Kruskal-Wallis test (A) and Mann-Whitney U test (B and C). Sarc = sarcoidosis; Tfh = T follicular helper cell.
Moreover, there is strong evidence that sarcoidosis-affected BAL T cells not only provide B-cell help in vitro, but that similar reactions take place in the inflamed lung in vivo. When we analyzed BAL supernatant from 17 patients with sarcoidosis compared with patients with non-ILDs, we observed markedly elevated levels of all immunoglobulin subclasses in sarcoidosis-affected BAL samples (Figure 5C). Taken together, these data demonstrate that T cells from patients with sarcoidosis possess a B-cell helping capacity and may contribute to lung pathology through the production of proinflammatory cytokines and through their ability to provide help for plasma cell generation and antibody production within inflamed lung tissue.
Sarcoidosis-affected BAL Is Dominated by Antigen-experienced B Cells with Characteristics of Autoimmune-associated and Tissue-Resident Populations
The presence of Tfh-like cells in the lungs of patients with sarcoidosis raised the obvious question of the phenotype of lung-infiltrating B cells. Compared with secondary lymphoid organs, the frequency of B cells in sarcoidosis-affected BAL was generally low (0.7% ± 0.4%), but B cells were regularly found in all samples analyzed. Except for one patient, CD27high CD38high plasmablasts were not present in the BAL. The vast majority of B cells in the lungs exhibited a CD27+ antigen-experienced phenotype, with less than 20% of B cells being naive (CD27− IgD+), which contrasts with 50% and 70% naive cells in tonsils and blood, respectively (Figure 6). Moreover, the high frequency of class-switched (IgD−) B cells suggests their origin from a T cell–dependent immune response. Although not as pronounced as in tonsils, a subset of sarcoidosis-affected BAL B cells exhibited a proliferative (Ki-67+) CD38+ GC-like phenotype. A high proportion of sarcoidosis-affected BAL B cells expressed CD69, CXCR3 and Fc receptor–like 4 (Figure 6), which are typical markers of tissue-resident memory B cells (29). These cells also coexpressed CD11c, had lower levels of CD21 compared with tonsillar and healthy donor blood B cells (Figure 6), and were negative for IgD (Figure E2). With this expression pattern, they at least partially resembled the phenotype of CD11c+ CD21low CD27−/IgD− (“double-negative”) T-bet+ “autoimmunity-associated” or “inflammatory” B cells (30–34). Contrary to this, however, they expressed high levels of CD27 and CXCR5 (Figure E2).
Figure 6.
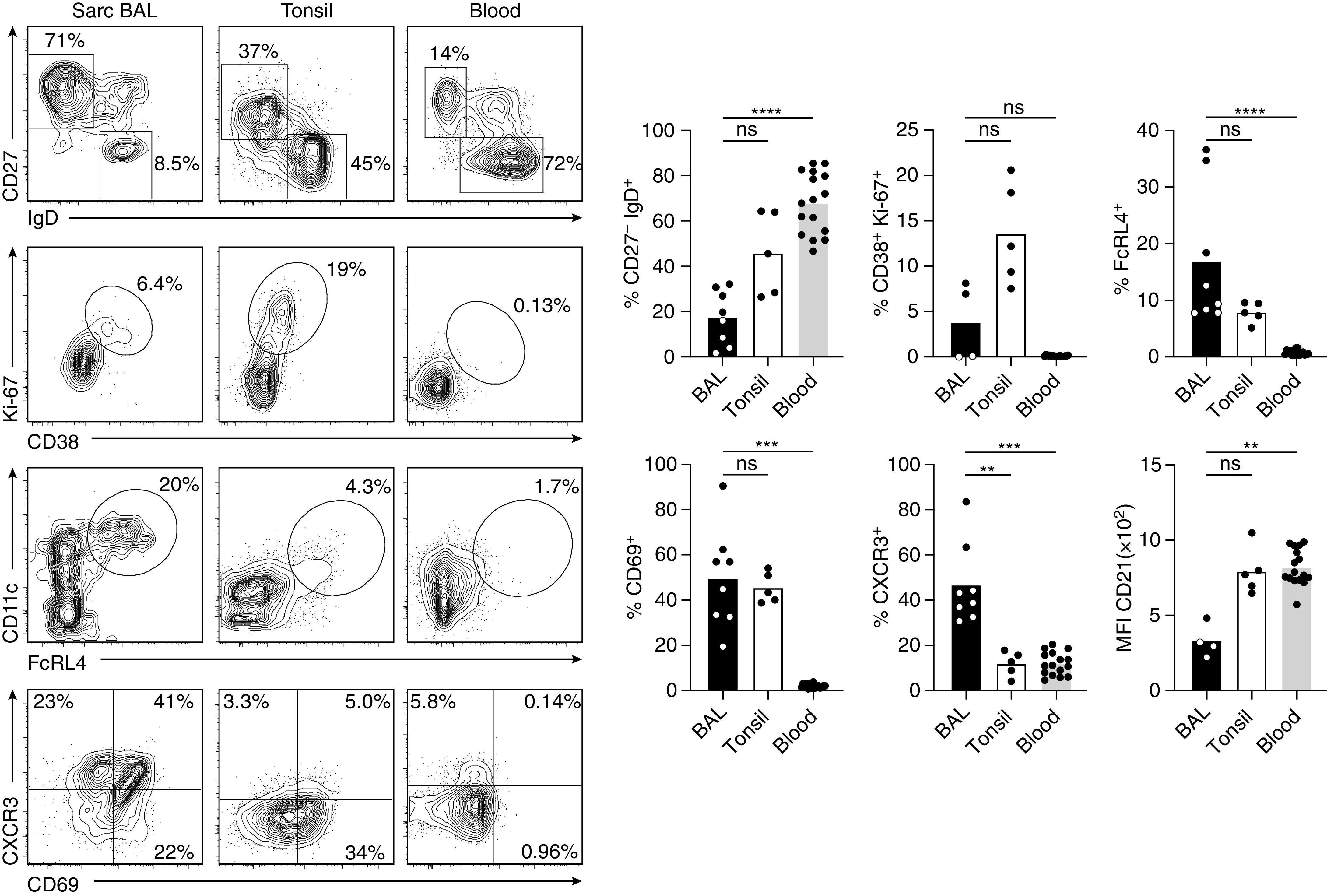
Sarcoidosis-affected BAL is strongly enriched in antigen-experienced B cells with an effector-memory phenotype. Flow cytometry analysis of B cells from sarcoidosis-affected BAL, tonsil, and healthy donor peripheral blood. For full gating, see Figure E1 in the online supplement. Representative flow cytometry data and statistical analysis for B cells with a naive or switched effector-memory phenotype (CD27− IgD+ vs. CD27+ IgD−), germinal center–like phenotype (CD38+ Ki-67+), “autoimmune B-cell” phenotype (FcRL4+ CD11c+ and CD21low), tissue-resident (CD69+), or CXCR3+ phenotype. Data are from 4–8 sarcoidosis-affected BAL, 5 tonsil, and 16 healthy donor blood samples. Individual samples are depicted by symbols with bars showing the mean. **P < 0.01, ***P < 0.001, and ****P < 0.0001 (Kruskal-Wallis test). MFI = mean fluorescence intensity; ns = not significant (P ⩾ 0.05, Kruskal-Wallis test); Sarc = sarcoidosis.
Large Numbers of B Cells Are Present in Sarcoidosis-affected Lung Tissue
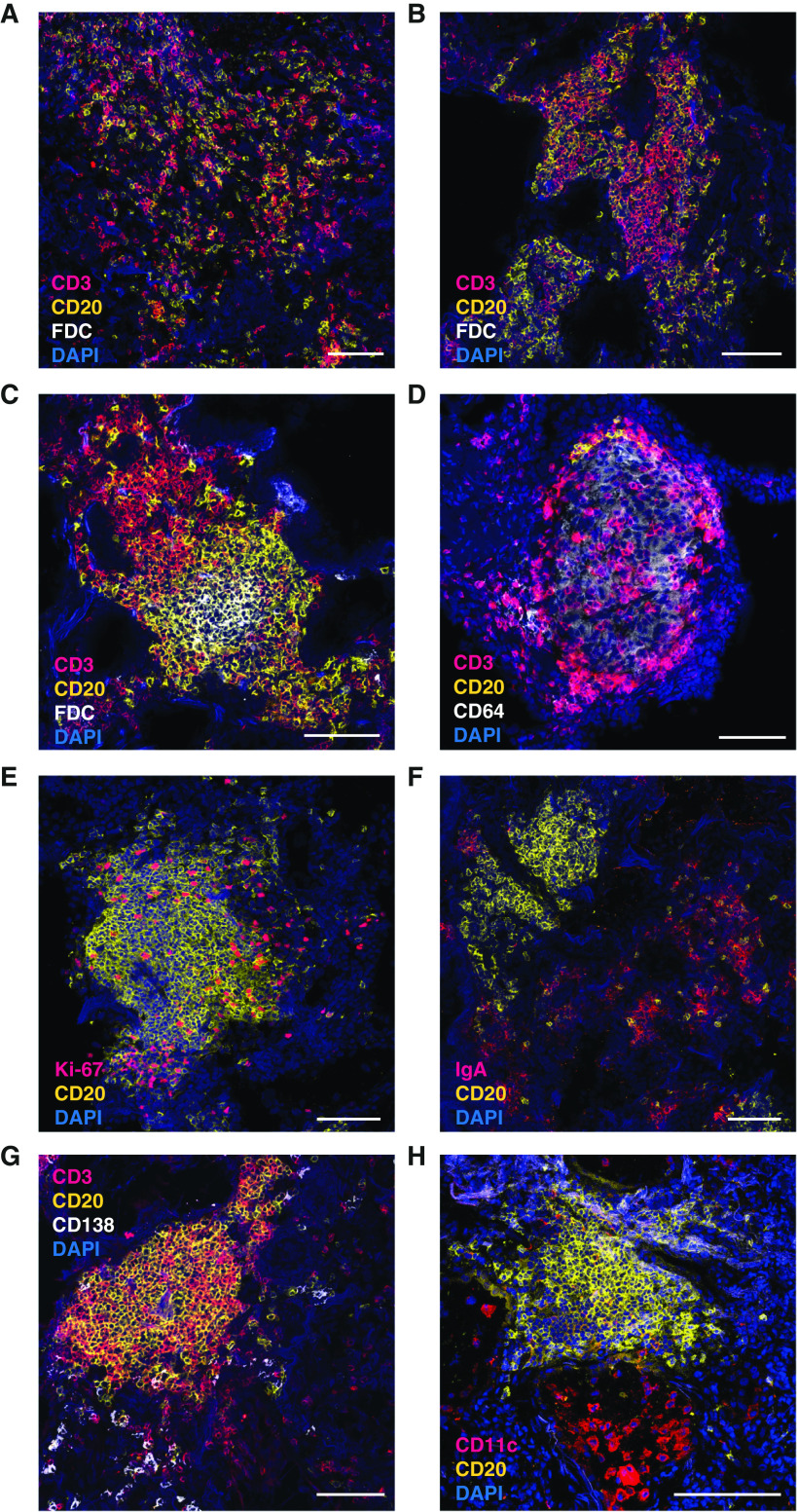
Data from a mouse lung inflammation model have demonstrated that GC-like B cells are trapped within the dense interstitial tissue infiltrates and are not present in the BAL (unpublished results). This necessitates the additional analysis of B cells not only from BAL but also directly in the lung tissue. In stark contrast to the findings in BAL, immunohistological analysis of lung tissue samples from eight patients with sarcoidosis revealed large numbers of B cells, which in some cases even outnumbered T cells. B cells were frequently in contact with T cells and typically found in large peribronchial lymphocytic infiltrates that exhibited varying sizes and degrees of organization (Figures 7A–7C and Figure E3). In contrast, T cells were also present as single cells scattered within the tissue. Whereas most infiltrates were FDC negative (Figures 7A and 7B), only a few infiltrates displayed typical signs of ectopic lymphoid tissue with separate T- and B-cell areas and an FDC network (Figure 7C). In contrast, there appeared no direct T/B contact within lung granulomas. These consisted mainly of CD64+ macrophages with scattered T cells, whereas B cells typically surrounded the granuloma (Figure 7D). Within T/B infiltrates, several B cells displayed a Ki-67+ proliferative phenotype (Figure 7E). In contrast to sarcoidosis-affected BAL, lung tissue also contained a large number of plasmablasts, mainly of IgA isotype, which were typically found near but not within the T/B clusters (Figures 7F and 7G). Few CD11c+ B cells were present within infiltrates (Figure 7H). Thus, the lymphocyte composition of the lung tissue markedly differed from BAL, resulting in an underestimation of B-cell frequencies. The close contact of T and B cells in GC-like but nonectopic infiltrates suggests sustained T/B cooperation leading to the local generation of plasmablasts.
Figure 7.
Sarcoidosis lung contains large T- and B-cell infiltrates. Lung sections from patients with sarcoidosis were stained with different combinations of fluorescently labeled antibodies (Table E2 in the online supplement) and analyzed by confocal laser-scanning microscopy. (A–C) Staining for T cells (CD3, magenta), B cells (CD20, yellow), and follicular dendritic cells (FDCs) (white). Representative examples for a loose infiltrate (A), an unstructured, FDC-negative infiltrate (B), and an FDC-positive infiltrate with clearly separated T- and B-cell zones (C) are shown. (D) Example for a large granuloma, containing CD64-positive macrophages (white), scattered T cells (magenta), and few B cells (yellow), which are exclusively located at the border of the granuloma. (E) Staining for B cells (yellow) and the proliferation marker Ki-67 (magenta). (F and G) Analysis of plasma cells with costaining of CD20 (yellow) and IgA (magenta) (F) and CD138 (white) in combination with CD3 (magenta) and CD20 (yellow) (G). (H) Costaining of CD20 (yellow) and CD11c (magenta). In addition to CD11c+ B cells, strong staining of alveolar macrophages is observed. Nuclei in all panels are stained with DAPI (blue). Scale bars, 100 μm.
Tfh-like Cells Are Enriched in the Peripheral Blood of Patients with Sarcoidosis
Tfh and Tfh-like memory cells can also be found as a circulating population in peripheral blood, which is easier to obtain for immunological analysis compared with BAL and lung tissue and thus accessible for diagnostic purposes (35). Costaining for CXCR5 and PD-1 discriminates three functionally distinct populations (Figure 8A): 1) CXCR5-negative PD-1 single-positive cells considered to be the circulating correlate of tissue-resident Tfh-like cells (36); 2) CXCR5/PD-1 double-positive cells, which functionally and transcriptionally resemble classical Tfh cells from secondary lymphoid organs (37, 38); and 3) a CXCR5 single-positive population, which also represents circulating Tfh cells but lacks the capacity to provide immediate B-cell help (38). Compared with age-matched healthy donor controls, the population of circulating PD-1+ CXCR5− Tfh-like cells was greatly increased in patients with sarcoidosis, whereas classical CXCR5+ PD-1+ Tfh cells were unchanged and the CXCR5 single-positive population even decreased (Figure 8A). Another hallmark of classical Tfh as well as Tfh-like cells is their ability to produce the chemokine, CXCL13 (36, 39–41). Intracellular staining for CXCL13 after restimulation in vitro revealed a small but elevated population within the PD-1 single-positive and PD-1/CXCR5 double-positive population compared with double-negative memory T cells (Figure 8B). In addition, the transcription factor c-MAF, which is functionally important for Tfh cell development (8), was increased in both Tfh and Tfh-like cells (Figure 8C).
Figure 8.
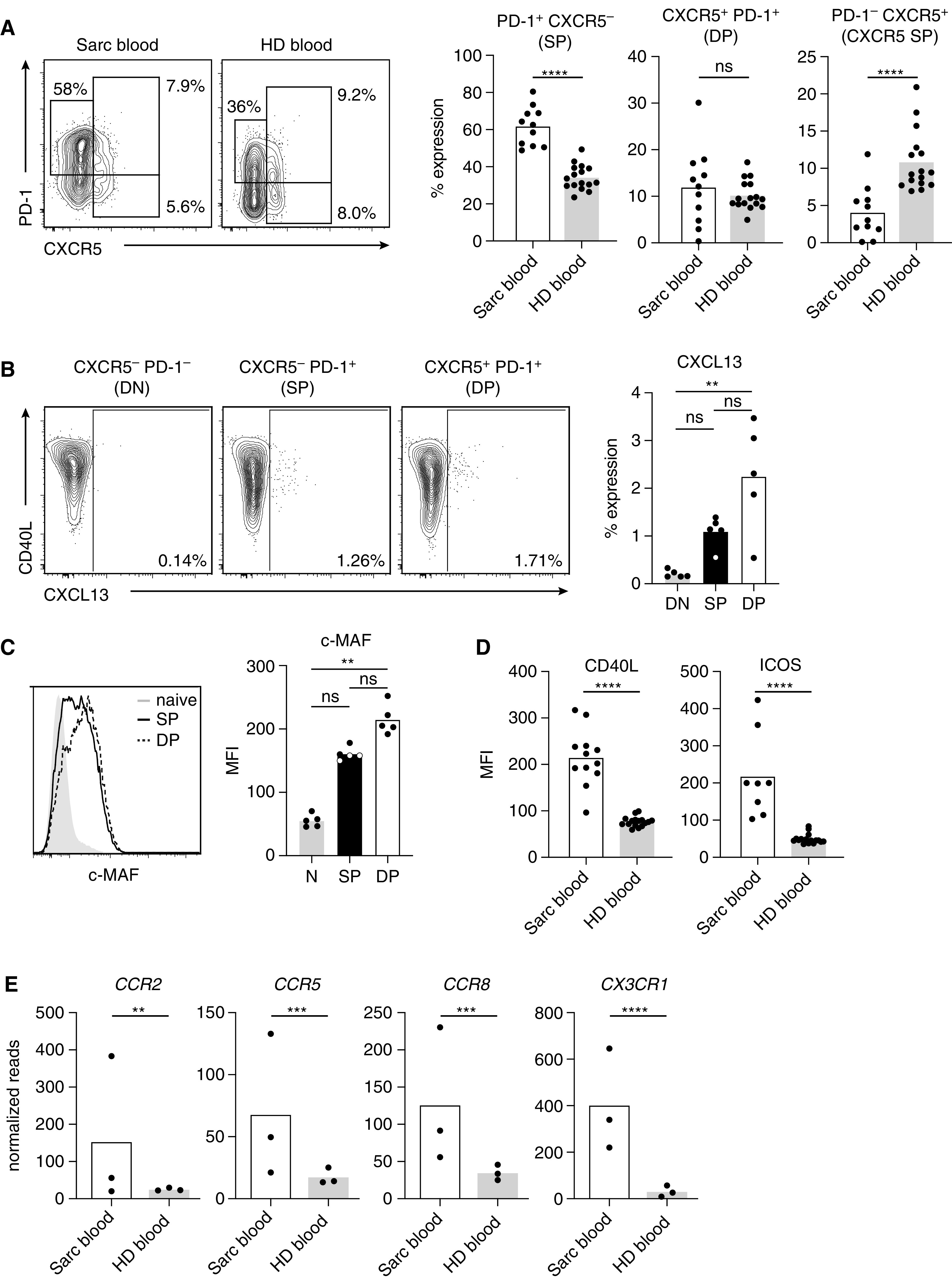
Peripheral blood T cells from patients with sarcoidosis have an activated T follicular helper (Tfh)-like phenotype and display signs of recent tissue egress. (A) Peripheral blood CD4+ CD45RO+ memory T cells from patients with sarcoidosis (Sarc blood) and age-matched healthy donors (HD blood) were analyzed by flow cytometry for coexpression of CXCR5 and PD-1. Representative staining and analysis of CXCR5− PD-1+ Tfh-like (SP) and PD-1+ CXCR5+ classical Tfh cells with further subdivision into double-positive (DP) (CXCR5+ PD-1+) versus CXCR5+ PD-1− single-positive subsets. (B) Blood memory CD4+ T cells from patients with sarcoidosis were sorted according to CXCR5 and PD-1 expression into double-negative (DN), PD-1 single-positive (SP) Tfh-like, and CXCR5/PD-1 double-positive (DP) classical Tfh cell subsets. Cells were stimulated in vitro with α-CD3/CD28 for 24 hours and stained intracellularly for CXCL13. (C) Intracellular staining for c-MAF in sarcoidosis-affected blood CD4+ T cells. The different expression levels between naive CD45RO−, single-positive (SP) and double-positive (DP) cells as in B are shown as histograms. (D) Analysis of CD40L and ICOS expression on peripheral blood memory T cells by flow cytometry (geometric mean fluorescence intensity [MFI]). (E) Expression levels of several chemokine receptors as analyzed by RNA sequencing. Individual samples are depicted by symbols with bars showing the mean. **P < 0.01, ***P < 0.001, and ****P < 0.0001. Data are obtained from 8–12 patients with sarcoidosis and 16 HD controls for the analysis in A and D, 5 patients for B and C, and 3 patients and healthy donors for E. The following statistical tests were used for data in the indicated panels: (A) unpaired t test (SP and DP), Mann-Whitney U test (CXCR5 SP); (B and C) Kruskal-Wallis test; (D) unpaired t test (CD40L) and Mann-Whitney U test (ICOS); and (E) Wald test. N = naive; ns = not significant (P ⩾ 0.05).
Expression of CD40L and ICOS, a marker of recently activated T cells, was highly significantly increased in sarcoidosis CD45RO+ T cells compared with healthy donor blood samples (Figure 8D). In addition, several chemokine receptors important for homing to inflamed tissue, such as CCR2, CCR5, CCR8, and CX3CR1, were highly increased, supporting the idea of recent egress from the inflamed tissue (Figure 8E). Thus, analysis of peripheral blood T cells to assess Tfh-like phenotypes should be further explored as a useful biomarker to monitor sarcoidosis disease activity.
Discussion
The pathogenesis of sarcoidosis remains enigmatic with controversial discussions about whether clonally expanded cells in the lung recognize an infectious agent or a self-antigen. In fact, sarcoidosis shares many characteristics with autoimmune diseases, including association with certain HLA genotypes and clonally expanded T-cell populations (42). For most autoimmune diseases, B cells have been recognized as key players in immunopathology. They are producers not only of potentially self-reactive antibodies but also proinflammatory cytokines and play a major role as antigen-presenting cells for T cells under conditions of limited antigen supply. It is therefore surprising that sarcoidosis research has focused almost exclusively on IFN-γ–producing T cells and macrophages and has overlooked T-cell/B-cell cooperation as an aspect of sarcoidosis immunopathology.
In this study, we demonstrate that lung-infiltrating T cells from patients with sarcoidosis not only produce large amounts of IFN-γ but also serve as a key source for the B-cell helper cytokine IL-21. With a CXCR5− BCL-6− phenotype but very potent B-cell helper qualities, they resemble Tfh-like cells originally identified in an antigen-driven murine lung inflammation model (11, 21). Tfh-like cells, also named peripheral T-helper cells, have now been identified in rheumatoid arthritis (36), systemic sclerosis (43, 44), celiac disease (44), lupus nephritis (40), and several other human autoimmune conditions (35). A common characteristic of these Tfh-like/peripheral T-helper cells is their preferential presence in inflamed nonlymphoid tissue, where they provide help for tissue-infiltrating B cells. Because of this “hidden” localization, this T-cell subset has only recently been investigated in more detail. Notably, studies from mouse models show that tissue-infiltrating T cells far outnumber their antigen-specific counterparts in secondary lymphoid organs (11). Furthermore, T-cell lymphopenia in peripheral blood typically observed in patients with sarcoidosis seems to be the result of enhanced recruitment into sarcoid lesions in nonlymphoid tissues (1). Therefore, tissue-resident T cells need to be investigated in more detail for their disease-driving potential and as a therapeutic target. In addition to extensive studies of human tissues from various stages of sarcoidosis and potentially other ILDs, this should be further explored in suitable animal models.
In the past, research on T/B cooperation in nonlymphoid tissues focused on ectopic lymphoid structures, which contain FDCs and separated T- and B-cell zones as a hallmark feature, thereby fully resembling GC in secondary lymphoid organs. Development of ectopic lymphoid structures, also called inducible bronchus-associated lymphoid tissue, in the lung requires strong stimuli like viral infections (10). Lung-infiltrating CD4+ T cells in such models express CXCR5 or at least depend on BCL-6 for their development and thereby differ substantially from lung-infiltrating T cells in patients with sarcoidosis (45–48). Furthermore, our study confirms the early finding of Fazel and colleagues (7) that sarcoidosis-affected lungs contain very few ectopic lymphoid structures, but at the same time shows that T/B cooperation can also occur in unstructured lymphocytic infiltrates.
We also demonstrate that Tfh-like responses in lung tissue are reflected by increased frequencies of circulating PD-1high CXCR5− CD40Lhigh ICOShigh Tfh-like cells in peripheral blood of patients with sarcoidosis, whereas classical CXCR5+ Tfh cells were unchanged in our study. These findings may have diagnostic potential and should be further explored in clinical studies with larger patient cohorts. Two recent studies of circulating CXCR5+ Tfh cells reported both increased (49) and decreased (50) frequencies in patients with sarcoidosis, suggesting that Tfh-like rather than classical Tfh cells may be useful for disease monitoring.
Although the presence of B cells in lung tissue in sarcoidosis has been known for almost four decades (6, 7), our study provides the first detailed phenotypic description of B cells from BAL and lung tissue. The antigen-experienced and class-switched B-cell phenotype strongly suggests that BAL B cells arise directly from T cell–dependent GC-like reactions in lung tissue. It will be important to determine whether these B cells represent a clonally expanded population. If so, this could be key to identifying the disease-driving antigen in sarcoidosis. The concept that B cells in sarcoidosis respond to autoantigens is further corroborated by enhanced levels of antinuclear antibodies in the serum (51, 52), a high degree of B-cell receptor hypermutation in blood (53), and a high percentage of B cells with the phenotype of age-associated (ABC-like) B cells, which are typical for multiple autoimmune diseases (31–34, 54). A pivotal role of B cells in sarcoidosis pathology is also supported by the finding that serum levels of BAFF, an important survival factor for B cells, directly correlate with disease activity (55–57). Finally, it has been demonstrated that patients with sarcoidosis can be successfully treated with B cell–depleting antibodies like Rituximab (58–60). Taken together, the identification of Tfh-like cells in the lung changes the current paradigm of sarcoidosis as a primarily Th1 cell–driven disease and warrants further investigation of Tfh-like cells and B cells as disease drivers in sarcoidosis.
Acknowledgments
Acknowledgment
The authors thank Ralf Uecker and Katrin Lehmann for excellent technical support and Klaus Warnatz, Anja Hauser, Ronja Mothes, Claudia Giesecke, and Pawel Durek for helpful discussions.
Footnotes
Supported by grants from Deutsche Forschungsgemeinschaft (HU 1294/8-1 TRR 130 P23 to A.H., and TRR 84 C8 and C10 to L.E.S.); the European Regional Development Fund (ERDF 2014–2020 and EFRE 1.8/11 to M.-F.M.); and the Jürgen Manchot Foundation to S.M.V.
Author Contributions: L.B. performed experiments, analyzed data, and prepared the manuscript. L.J.M. performed experiments and analyzed data. S.M.V. established the patient cohort. F.H and M.-F.M. performed transcriptome analyses. C.R. provided lung tissue biopsies. L.E.S. designed the study and provided patient material. A.H. designed and supervised the study, analyzed data, and prepared the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202012-4423OC on September 17, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Grunewald J, Grutters JC, Arkema EV, Saketkoo LA, Moller DR, Müller-Quernheim J. Sarcoidosis. Nat Rev Dis Primers . 2019;5:45. doi: 10.1038/s41572-019-0096-x. [DOI] [PubMed] [Google Scholar]
- 2. Greaves SA, Atif SM, Fontenot AP. Adaptive immunity in pulmonary sarcoidosis and chronic beryllium disease. Front Immunol . 2020;11:474. doi: 10.3389/fimmu.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prasse A, Georges CG, Biller H, Hamm H, Matthys H, Luttmann W, et al. Th1 cytokine pattern in sarcoidosis is expressed by bronchoalveolar CD4+ and CD8+ T cells. Clin Exp Immunol . 2000;122:241–248. doi: 10.1046/j.1365-2249.2000.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Möllers M, Aries SP, Drömann D, Mascher B, Braun J, Dalhoff K. Intracellular cytokine repertoire in different T cell subsets from patients with sarcoidosis. Thorax . 2001;56:487–493. doi: 10.1136/thorax.56.6.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wahlström J, Katchar K, Wigzell H, Olerup O, Eklund A, Grunewald J. Analysis of intracellular cytokines in CD4+ and CD8+ lung and blood T cells in sarcoidosis. Am J Respir Crit Care Med . 2001;163:115–121. doi: 10.1164/ajrccm.163.1.9906071. [DOI] [PubMed] [Google Scholar]
- 6. Hunninghake GW, Crystal RG. Mechanisms of hypergammaglobulinemia in pulmonary sarcoidosis. Site of increased antibody production and role of T lymphocytes. J Clin Invest . 1981;67:86–92. doi: 10.1172/JCI110036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fazel SB, Howie SE, Krajewski AS, Lamb D. B lymphocyte accumulations in human pulmonary sarcoidosis. Thorax . 1992;47:964–967. doi: 10.1136/thx.47.11.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T cells. Annu Rev Immunol . 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 9. Song W, Craft J. T follicular helper cell heterogeneity: Time, space, and function. Immunol Rev . 2019;288:85–96. doi: 10.1111/imr.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hutloff A. T follicular helper-like cells in inflamed non-lymphoid tissues. Front Immunol . 2018;9:1707. doi: 10.3389/fimmu.2018.01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vu Van D, Beier KC, Pietzke LJ, Al Baz MS, Feist RK, Gurka S, et al. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat Commun . 2016;7:10875. doi: 10.1038/ncomms10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutloff A.
- 13. The American Thoracic Society (ATS), the European Respiratory Society (ERS), and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) Statement on sarcoidosis. Am J Respir Crit Care Med . 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 14. Crouser ED, Maier LA, Wilson KC, Bonham CA, Morgenthau AS, Patterson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med . 2020;201:e26–e51. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, et al. American Thoracic Society Committee on BAL in ILD. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in ILD. Am J Respir Crit Care Med . 2012;185:1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 16. Lahmann A, Kuhrau J, Fuhrmann F, Heinrich F, Bauer L, Durek P, et al. Bach2 controls T follicular helper cells by direct repression of BCL-6. J Immunol . 2019;202:2229–2239. doi: 10.4049/jimmunol.1801400. [DOI] [PubMed] [Google Scholar]
- 17. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol . 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ugolini M, Gerhard J, Burkert S, Jensen KJ, Georg P, Ebner F, et al. Recognition of microbial viability via TLR8 drives TFH cell differentiation and vaccine responses. Nat Immunol . 2018;19:386–396. doi: 10.1038/s41590-018-0068-4. [DOI] [PubMed] [Google Scholar]
- 19. Löhning M, Grogan JL, Coyle AJ, Yazdanbakhsh M, Meisel C, Gutierrez-Ramos JC, et al. T1/ST2 expression is enhanced on CD4+ T cells from schistosome egg-induced granulomas: analysis of Th cell cytokine coexpression ex vivo. J Immunol . 1999;162:3882–3889. [PubMed] [Google Scholar]
- 20. Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol . 2015;194:2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 21. Van DV, Bauer L, Kroczek RA, Hutloff A. ICOS costimulation differentially affects T cells in secondary lymphoid organs and inflamed tissues. Am J Respir Cell Mol Biol . 2018;59:437–447. doi: 10.1165/rcmb.2017-0309OC. [DOI] [PubMed] [Google Scholar]
- 22. Abbas AR, Baldwin D, Ma Y, Ouyang W, Gurney A, Martin F, et al. Immune response in silico (IRIS): immune-specific genes identified from a compendium of microarray expression data. Genes Immun . 2005;6:319–331. doi: 10.1038/sj.gene.6364173. [DOI] [PubMed] [Google Scholar]
- 23. Lu E, Cyster JG. G-protein coupled receptors and ligands that organize humoral immune responses. Immunol Rev . 2019;289:158–172. doi: 10.1111/imr.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arakelyan A, Kriegova E, Kubistova Z, Mrazek F, Kverka M, du Bois RM, et al. Protein levels of CC chemokine ligand (CCL)15, CCL16 and macrophage stimulating protein in patients with sarcoidosis. Clin Exp Immunol . 2009;155:457–465. doi: 10.1111/j.1365-2249.2008.03832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iida K, Kadota J, Kawakami K, Matsubara Y, Shirai R, Kohno S. Analysis of T cell subsets and beta chemokines in patients with pulmonary sarcoidosis. Thorax . 1997;52:431–437. doi: 10.1136/thx.52.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamamoto H, Yasuo M, Komatsu M, Ushiki A, Hamano H, Hori A, et al. Comparison of the chemokine profiles in the bronchoalveolar lavage fluid between IgG4-related respiratory disease and sarcoidosis: CC-chemokine ligand 1 might be involved in the pathogenesis of sarcoidosis. Cytokine . 2019;120:125–129. doi: 10.1016/j.cyto.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 27. Agostini C, Cabrelle A, Calabrese F, Bortoli M, Scquizzato E, Carraro S, et al. Role for CXCR6 and its ligand CXCL16 in the pathogenesis of T-cell alveolitis in sarcoidosis. Am J Respir Crit Care Med . 2005;172:1290–1298. doi: 10.1164/rccm.200501-142OC. [DOI] [PubMed] [Google Scholar]
- 28. Stanton LA, Fenhalls G, Lucas A, Gough P, Greaves DR, Mahoney JA, et al. Immunophenotyping of macrophages in human pulmonary tuberculosis and sarcoidosis. Int J Exp Pathol . 2003;84:289–304. doi: 10.1111/j.0959-9673.2003.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ehrhardt GR, Hsu JT, Gartland L, Leu CM, Zhang S, Davis RS, et al. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med . 2005;202:783–791. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cancro MP. Age-associated B cells. Annu Rev Immunol . 2020;38:315–340. doi: 10.1146/annurev-immunol-092419-031130. [DOI] [PubMed] [Google Scholar]
- 31. Phalke S, Aviszus K, Rubtsova K, Rubtsov A, Barkes B, Powers L, et al. Age-associated B cells appear in patients with granulomatous lung diseases. Am J Respir Crit Care Med . 2020;202:1013–1023. doi: 10.1164/rccm.201911-2151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol . 2007;178:6624–6633. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 33. Yeo L, Lom H, Juarez M, Snow M, Buckley CD, Filer A, et al. Expression of FcRL4 defines a pro-inflammatory, RANKL-producing B cell subset in rheumatoid arthritis. Ann Rheum Dis . 2015;74:928–935. doi: 10.1136/annrheumdis-2013-204116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood . 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoshitomi H, Ueno H. Shared and distinct roles of T peripheral helper and T follicular helper cells in human diseases. Cell Mol Immunol . 2021;18:523–527. doi: 10.1038/s41423-020-00529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature . 2017;542:110–114. doi: 10.1038/nature20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity . 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 38. Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. International AIDS Vaccine Initiative Protocol C Principal Investigators. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity . 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manzo A, Vitolo B, Humby F, Caporali R, Jarrossay D, Dell’accio F, et al. Mature antigen-experienced T helper cells synthesize and secrete the B cell chemoattractant CXCL13 in the inflammatory environment of the rheumatoid joint. Arthritis Rheum . 2008;58:3377–3387. doi: 10.1002/art.23966. [DOI] [PubMed] [Google Scholar]
- 40. Bocharnikov AV, Keegan J, Wacleche VS, Cao Y, Fonseka CY, Wang G, et al. Accelerating Medicines Partnership (AMP) RA/SLE Network. PD-1hiCXCR5- T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight . 2019;4:e130062. doi: 10.1172/jci.insight.130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohée S, Garaud S, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight . 2017;2:e91487. doi: 10.1172/jci.insight.91487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaiser Y, Eklund A, Grunewald J. Moving target: shifting the focus to pulmonary sarcoidosis as an autoimmune spectrum disorder. Eur Respir J . 2019;54:1802153. doi: 10.1183/13993003.021532018. [DOI] [PubMed] [Google Scholar]
- 43. Taylor DK, Mittereder N, Kuta E, Delaney T, Burwell T, Dacosta K, et al. T follicular helper-like cells contribute to skin fibrosis. Sci Transl Med . 2018;10:eaaf5307. doi: 10.1126/scitranslmed.aaf5307. [DOI] [PubMed] [Google Scholar]
- 44. Christophersen A, Lund EG, Snir O, Solà E, Kanduri C, Dahal-Koirala S, et al. Distinct phenotype of CD4+ T cells driving celiac disease identified in multiple autoimmune conditions. Nat Med . 2019;25:734–737. doi: 10.1038/s41591-019-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strutt TM, McKinstry KK, Kuang Y, Bradley LM, Swain SL. Memory CD4+ T-cell-mediated protection depends on secondary effectors that are distinct from and superior to primary effectors. Proc Natl Acad Sci USA . 2012;109:E2551–E2560. doi: 10.1073/pnas.1205894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moguche AO, Shafiani S, Clemons C, Larson RP, Dinh C, Higdon LE, et al. ICOS and Bcl6-dependent pathways maintain a CD4 T cell population with memory-like properties during tuberculosis. J Exp Med . 2015;212:715–728. doi: 10.1084/jem.20141518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Swarnalekha N, Schreiner D, Litzler LC, Iftikhar S, Kirchmeier D, Künzli M, et al. T resident helper cells promote humoral responses in the lung. Sci Immunol . 2021;6:eabb6808. doi: 10.1126/sciimmunol.abb6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Son YM, Cheon IS, Wu Y, Li C, Wang Z, Gao X, et al. Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Sci Immunol . 2021;6:eabb6852. doi: 10.1126/sciimmunol.abb6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kudryavtsev I, Serebriakova M, Starshinova A, Zinchenko Y, Basantsova N, Malkova A, et al. Imbalance in B cell and T follicular helper cell subsets in pulmonary sarcoidosis. Sci Rep . 2020;10:1059. doi: 10.1038/s41598-020-57741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ly NTM, Ueda-Hayakawa I, Nguyen CTH, Okamoto H. Exploring the imbalance of circulating follicular helper CD4+ T cells in sarcoidosis patients. J Dermatol Sci . 2020;97:216–224. doi: 10.1016/j.jdermsci.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 51. Veien NK, Hardt F, Bendixen G, Genner J, Ringsted J, Wanstrup J, et al. Humoral and cellular immunity in sarcoidosis. Acta Med Scand . 1978;203:321–326. doi: 10.1111/j.0954-6820.1978.tb14881.x. [DOI] [PubMed] [Google Scholar]
- 52. Weinberg I, Vasiliev L, Gotsman I. Anti-dsDNA antibodies in sarcoidosis. Semin Arthritis Rheum . 2000;29:328–331. doi: 10.1016/s0049-0172(00)80019-0. [DOI] [PubMed] [Google Scholar]
- 53. Kamphuis LS, van Zelm MC, Lam KH, Rimmelzwaan GF, Baarsma GS, Dik WA, et al. Perigranuloma localization and abnormal maturation of B cells: emerging key players in sarcoidosis? Am J Respir Crit Care Med . 2013;187:406–416. doi: 10.1164/rccm.201206-1024OC. [DOI] [PubMed] [Google Scholar]
- 54. Claes N, Fraussen J, Vanheusden M, Hellings N, Stinissen P, Van Wijmeersch B, et al. Age-associated B Cells with proinflammatory characteristics are expanded in a proportion of multiple sclerosis patients. J Immunol . 2016;197:4576–4583. doi: 10.4049/jimmunol.1502448. [DOI] [PubMed] [Google Scholar]
- 55. Ueda-Hayakawa I, Tanimura H, Osawa M, Iwasaka H, Ohe S, Yamazaki F, et al. Elevated serum BAFF levels in patients with sarcoidosis: association with disease activity. Rheumatology (Oxford) . 2013;52:1658–1666. doi: 10.1093/rheumatology/ket186. [DOI] [PubMed] [Google Scholar]
- 56. Saussine A, Tazi A, Feuillet S, Rybojad M, Juillard C, Bergeron A, et al. Active chronic sarcoidosis is characterized by increased transitional blood B cells, increased IL-10-producing regulatory B cells and high BAFF levels. PLoS One . 2012;7:e43588. doi: 10.1371/journal.pone.0043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ando M, Goto A, Takeno Y, Yamasue M, Komiya K, Umeki K, et al. Significant elevation of the levels of B-cell activating factor (BAFF) in patients with sarcoidosis. Clin Rheumatol . 2018;37:2833–2838. doi: 10.1007/s10067-018-4183-2. [DOI] [PubMed] [Google Scholar]
- 58. Sweiss NJ, Lower EE, Mirsaeidi M, Dudek S, Garcia JG, Perkins D, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525–1528. doi: 10.1183/09031936.00224513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cinetto F, Compagno N, Scarpa R, Malipiero G, Agostini C. Rituximab in refractory sarcoidosis: a single centre experience. Clin Mol Allergy . 2015;13:19. doi: 10.1186/s12948-015-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Krause ML, Cooper LT, Chareonthaitawee P, Amin S. Successful use of rituximab in refractory cardiac sarcoidosis. Rheumatology (Oxford) . 2016;55:189–191. doi: 10.1093/rheumatology/kev309. [DOI] [PubMed] [Google Scholar]