Abstract
Background
Premature birth affects millions of neonates each year, placing them at risk for respiratory disease due to prematurity. Bronchopulmonary dysplasia is the most common chronic lung disease of infancy, but recent data suggest that even premature infants who do not meet the strict definition of bronchopulmonary dysplasia can develop adverse pulmonary outcomes later in life. This post-prematurity respiratory disease (PPRD) manifests as chronic respiratory symptoms, including cough, recurrent wheezing, exercise limitation, and reduced pulmonary function. This document provides an evidence-based clinical practice guideline on the outpatient management of infants, children, and adolescents with PPRD.
Methods
A multidisciplinary panel of experts posed questions regarding the outpatient management of PPRD. We conducted a systematic review of the relevant literature. The Grading of Recommendations, Assessment, Development, and Evaluation approach was used to rate the quality of evidence and the strength of the clinical recommendations.
Results
The panel members considered the strength of each recommendation and evaluated the benefits and risks of applying the intervention. In formulating the recommendations, the panel considered patient and caregiver values, the cost of care, and feasibility. Recommendations were developed for or against three common medical therapies and four diagnostic evaluations in the context of the outpatient management of PPRD.
Conclusions
The panel developed recommendations for the outpatient management of patients with PPRD on the basis of limited evidence and expert opinion. Important areas for future research were identified.
Keywords: prematurity, bronchopulmonary dysplasia, chronic lung disease
Contents
- Summary of Recommendations
- Recommendation 1a
- Recommendation 1b
- Recommendation 2a
- Recommendation 2b
- Recommendation 3a
- Recommendation 3b
- Recommendation 4a
- Recommendation 4b
- Recommendation 4c
- Recommendation 5
- Recommendation 6
- Recommendation 7a
- Recommendation 7b
Introduction
Use of These Guidelines
Definition of PPRD
Methods
- Results
- Question 1: Should infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD and who have respiratory symptoms (wheezing, cough, tachypnea) receive short- acting, inhaled bronchodilators?
- Question 2: Should infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD and who have respiratory symptoms (wheezing, cough, tachypnea) routinely receive inhaled corticosteroids?
- Question 3: Should infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD and who have respiratory symptoms (wheezing, cough, tachypnea) routinely receive diuretic therapy?
- Question 4: Should all infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD undergo baseline diagnostic PSG?
- Question 5: Should all infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD undergo a swallow evaluation?
- Question 6: Should all infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD undergo airway endoscopy?
- Question 7: Should all infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD undergo diagnostic imaging for TBM?
Limitations and Future Research
Conclusions
Summary of Recommendations
Recommendation 1a
For infants, children, and adolescents with post-prematurity respiratory disease (PPRD) who do not have recurrent respiratory symptoms, we suggest that short-acting inhaled bronchodilator therapy not be routinely prescribed (conditional recommendation, very-low-certainty evidence).
Recommendation 1b
For infants, children, and adolescents with PPRD who have recurrent respiratory symptoms (such as cough or wheeze), we suggest a trial of a short-acting inhaled bronchodilator with monitoring to assess for clinical improvement in symptoms (conditional recommendation, very-low-certainty evidence).
Recommendation 2a
For infants, children, and adolescents with PPRD who do not have chronic cough and recurrent wheezing, we suggest that inhaled corticosteroids not be routinely prescribed (conditional recommendation, very-low-certainty evidence).
Recommendation 2b
For infants, children, and adolescents with PPRD who have chronic cough or recurrent wheezing, we suggest a trial of inhaled corticosteroids with monitoring to assess for clinical improvement in symptoms (conditional recommendation, very-low-certainty evidence).
Recommendation 3a
For infants, children, and adolescents with PPRD, we suggest against the routine use of diuretics (conditional recommendation, very-low-certainty evidence).
Recommendation 3b
For infants with PPRD who are discharged from the Neonatal Intensive Care Unit (NICU) on chronic diuretic therapy, we suggest discontinuation in a judicious manner (conditional recommendation, very-low-certainty evidence).
Recommendation 4a
For infants with PPRD who are otherwise ready to be discharged from the NICU, we suggest the use of polysomnography (PSG) for patients with persistent apnea, intermittent desaturation, or bradycardia at greater than 40 weeks’ postmenstrual age (PMA) (conditional recommendation, very-low-certainty evidence).
Recommendation 4b
For infants, children, and adolescents with PPRD, we suggest the use of PSG and/or a sleep medicine referral for those with symptoms of sleep-disordered breathing (SDB), including persistent snoring, failure to thrive, or a persistent need for supplemental oxygen at 2 years of age (conditional recommendation, very-low-certainty evidence).
Recommendation 4c
When PSG is indicated but not available, we recommend that overnight or 24-hour oximetry be performed to screen for SDB, followed by PSG and/or a sleep medicine referral for further evaluation if needed (conditional recommendation, very-low-certainty evidence).
Recommendation 5
For infants, children, and adolescents with PPRD, we suggest a swallow evaluation (videofluoroscopic swallow study [VFSS]) for those who are eating by mouth and have cough or persistent oxygen desaturation during feeding, suspected or confirmed vocal cord paralysis or other airway anomalies, failure to wean from oxygen therapy or ventilatory support as expected, persistent or worsening pulmonary hypertension, failure to thrive, or chronic pulmonary symptoms out of proportion to viral respiratory infections (conditional recommendation, very-low-certainty evidence).
Recommendation 6
For infants, children, and adolescents with PPRD, we suggest airway endoscopy for those with unexplained symptoms such as chronic cough, wheezing, ventilator dependence, persistent hypoxemia, or a history of patent ductus arteriosus (PDA) ligation with stridor and weak cry (conditional recommendation, very-low-certainty evidence).
Recommendation 7a
For infants, children, and adolescents with PPRD who do not have symptoms suggestive of airway malacia, we suggest that dynamic airway imaging (computed tomography [CT] or magnetic resonance imaging [MRI]) not be used as a screening test for the routine diagnosis of tracheobronchomalacia (TBM) (conditional recommendation, very-low-certainty evidence).
Recommendation 7b
We suggest that unsedated, dynamic airway imaging (CT or MRI) be used for the diagnosis or reevaluation of TBM in patients with PPRD who have recurrent symptoms suggestive of airway malacia as an alternative to bronchoscopy when the risks from anesthesia for bronchoscopy are judged to be greater than the risks from radiation or when bronchoscopy is not feasible or available (see Figure 1) (conditional recommendation, very-low-certainty evidence).
Figure 1.
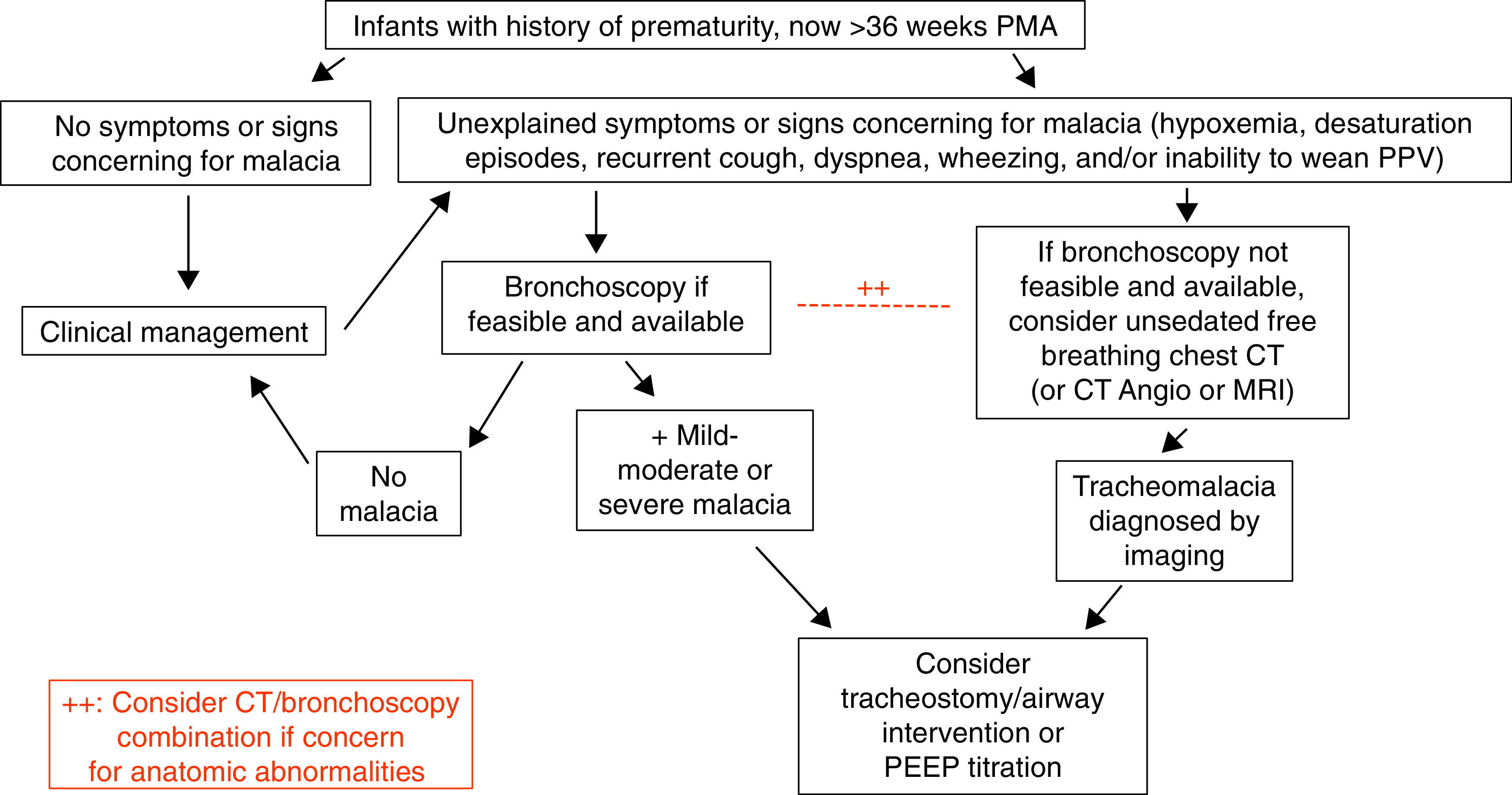
Diagnostic algorithm for patients with post-prematurity respiratory disease and concern for tracheobronchomalacia. Angio = angiography; CT = computed tomography; MRI = magnetic resonance imaging; PEEP = positive end-expiratory pressure; PMA = postmenstrual age; PPV = positive-pressure ventilation.
Introduction
Worldwide, approximately 12 million neonates (10% of live births) are born prematurely and are at risk for respiratory disease, which leads to increased morbidity and mortality (1–3). The most common chronic lung disease of infancy (CLDI) associated with preterm birth is bronchopulmonary dysplasia (BPD), with approximately 12–15,000 cases of BPD occurring annually in the United States alone (4–6).
Premature infants who do not meet the definition of BPD may develop adverse pulmonary outcomes later in life, including cough, recurrent wheezing, exercise intolerance, hypoxemia, and reduced pulmonary function (7–12). Disrupted lung development due to prematurity is associated with lifelong respiratory sequelae, including chronic obstructive pulmonary disease (13–20). Children born preterm require frequent clinic visits, increased use of respiratory medications, and increased hospitalizations (21–25).
In 2003, the American Thoracic Society (ATS) published the “Statement on the Care of the Child with Chronic Lung Disease of Infancy and Childhood,” which addressed the epidemiology, pathophysiology, and treatment of CLDI and childhood (26). This ATS statement has been a highly used clinical tool for the management of patients with CLDI. Advancements such as antenatal steroids, postnatal surfactants, and protective ventilation strategies have led to a marked increase in the survival of premature infants born much earlier in gestation. However, the increased survival of extremely preterm infants has led to a developmental arrest of alveolar and pulmonary vascular growth termed “new BPD” (27). Thus, there was a pressing need for updated guidance to assist clinicians who care for patients born preterm in the modern era.
Use of These Guidelines
These recommendations are intended to aid clinicians in the outpatient management of infants, children, and adolescents with PPRD, regardless of the degree of prematurity, the severity of disease, the disease phenotype (28), or the age of the patient at the time of presentation. Whether or not to begin a therapy or order a diagnostic study should be made on an individual basis, considering the patient’s symptoms and clinical presentation.
Definition of PPRD
We have used the term “PPRD” for defining the patient population with respiratory disease that is directly associated with premature birth (less than 37 weeks’ PMA), including those who were born prematurely but did not meet the definition for BPD (29). This is in contrast with the previous ATS statement, in which the term “CLDI” was used. CLDI consists of a heterogeneous group of respiratory diseases in preterm and full-term infants (26). CLDI includes BPD, other chronic respiratory diseases of prematurity not meeting the strict criteria for BPD, and chronic lung diseases in full-term infants, such as those associated with congenital diaphragmatic hernia and other forms of pulmonary hypoplasia. Although these conditions in full-term infants may be clinically similar to BPD, their underlying pathophysiology is strikingly different. For this reason, these recommendations focus on the outpatient management of patients with PPRD (29).
Methods
This clinical practice guideline was developed in accordance with ATS policies and procedures. We used the Grading of Recommendations, Assessment, Development, and Evaluation approach (30, 31) to formulate clinical questions, identify and summarize relevant evidence, and develop recommendations for clinical practice.
The co-chairs (A.I.C. and C.D.B.) submitted a proposal that was reviewed and approved by the ATS Assembly of Pediatrics, Program Review Subcommittee, and Board of Directors. A multidisciplinary panel of international specialists with expertise in PPRD and guideline development methodology was formed. Represented disciplines included pediatric pulmonology, neonatology, otolaryngology, sleep medicine, radiology, and nursing, and families of patients with PPRD were also included. Conflicts of interest were disclosed and managed appropriately. The committee identified seven specific questions, with three addressing the clinical management of patients with active symptoms and four addressing diagnostic methods. The patient/intervention/comparator/outcome (PICO) format was used to formulate each question. A formal search strategy of EMBASE, Medline, the Cochrane Library, the Cumulative Index to Nursing and Allied Health Literature, and the Web of Science was performed (see the online supplement). We included studies of infants, children, and adolescents who were born preterm (less than 37 weeks’ PMA) who had already been discharged from the hospital. Because of the lack of outpatient-based evidence for several questions, studies of preterm infants before NICU discharge (age greater than 36 weeks’ PMA at the time of study) were included. Detailed methods are included in the online supplement.
Results
Question 1: Should infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD and who have respiratory symptoms (wheezing, cough, tachypnea) receive short-acting, inhaled bronchodilators?
Background
Patients with PPRD are at increased risk of respiratory morbidity, cough and wheeze, and respiratory hospitalizations, and asthma is diagnosed in up to 25% (25, 32–35). Morbidity increases with decreasing gestational age but remains significant among children born mid-to-late preterm (36, 37). Cohort studies demonstrate a high incidence of bronchodilator use in this population (38–40). However, the overlap between respiratory disease in children born preterm and asthma is not clear (41). Although many children born preterm develop asthma-like symptoms, they are less likely to demonstrate airway hyperresponsiveness and may be less responsive to bronchodilators (42–44). They may have fixed airway obstruction or large airway diseases like tracheomalacia (45).
Children with atopy or asthma (or a family history of these conditions) may benefit from bronchodilators (46). Those born at less than 32 weeks’ PMA or those with BPD may benefit differently from children without PPRD. Children with TBM can have a paradoxical response to bronchodilator therapy (47). Long-acting bronchodilators and anticholinergic agents, although components of asthma therapy, are infrequently used in patients with PPRD. This question focuses specifically on the use of short-acting β2-agonists (such as albuterol) as needed, given that this is the most common type and indication for bronchodilators in this population.
Evidence base
The outcomes considered critical or important for this review were wheezing, dyspnea, quality of life, missed school, hospital length of stay (LOS), improvement in pulmonary function test results (forced expiratory flows, airway resistance, lung volumes), tachycardia, attention deficit, airway obstruction, and hypoxemia.
The relevant medical literature described the effect of single or multiple doses of inhaled bronchodilators (online supplement). Two studies compared chronic (at least 2 wk) daily inhaled bronchodilators in children born preterm. We excluded one study whose primary outcome was peak expiratory flow (PEF), as PEF typically defines an exacerbation of respiratory disease and is not used for routine management (48). A nonrandomized, crossover, placebo-controlled trial examined 2 weeks of twice-daily bronchodilator use (500 μg of inhaled terbutaline or placebo) in 10 preterm infants with recurrent respiratory symptoms (49). All infants (postnatal age, 12.5 mo) had experienced cough or wheezing at least 4 d/wk for the prior month. The symptom score was significantly reduced during the treatment period. The greatest improvements were noted in wheeze and cough. The FRC increased by 32%. Although this was reported as improvement, the panel noted that this could also be due to worsening air trapping.
Supplemental evidence from studies of a single bronchodilator dose were examined. A systematic review of 21 studies of children and adolescents born preterm demonstrated a mean improvement in the FEV1 of 4–13% after albuterol treatment (50). A study of 17 infants with BPD (68 weeks’ PMA) demonstrated a response to albuterol in 35% of subjects (51). Specifically, 55% of those with recurrent wheeze responded, as compared with only 12.5% of those without wheezing. Another study of 52 preterm infants who were mechanically ventilated at birth (22 with BPD and 30 control subjects) demonstrated increased inspiratory or expiratory resistance in patients with BPD at 1 year of age and decreased resistance after salbutamol treatment in half of these patients (52). Sixty-five percent of hospitalized infants with severe BPD were responsive to bronchodilator treatment as determined by using infant pulmonary function testing at 52 weeks’ PMA (53). Finally, 75% of young children with BPD and tracheostomy were responsive to bronchodilator treatment at the ages of 6, 12, and 18 months (54).
Certainty of evidence
The panel’s confidence in the accuracy of these estimated effects of bronchodilator administration for critical outcomes was very low. Only one small study evaluated repeated bronchodilator use, but this study was not randomized and used a nonvalidated symptom score. Evidence evaluating the effect of single doses of a bronchodilator on pulmonary function was heterogeneous. Critical outcomes such as quality of life, hospitalizations, and school absences were not described. Questions remain regarding which subpopulations with PPRD are most likely to benefit from bronchodilators.
Benefits
In preterm infants with cough and wheeze, inhaled short-acting bronchodilators improved symptoms. In addition, 27–94% of school-aged children born preterm demonstrated an improved FEV1 after bronchodilator treatment.
Harms
A small proportion of infants may have increased airway resistance after short-acting bronchodilator administration because of underlying TBM (47). Patients with PPRD can also experience common adverse effects of bronchodilators, such as tachycardia, transient oxygen desaturation, and tremors (55–57).
Costs
Panel members did not consider the cost of medication to be a significant hinderance to their use but acknowledged that reducing their use when not indicated could result in significant cost savings (58).
Conclusions
The panel concluded that the benefits of bronchodilator administration, specifically short-acting β2-agonists, likely exceed the harms for patients born preterm with recurrent respiratory symptoms including cough and wheeze. Although the evidence is largely indirect, there is consistent evidence that inhaled β2-agonists can significantly improve airflow in patients with PPRD. Given the fact that even late-preterm infants demonstrate diminished lung function, the potential benefit of inhaled bronchodilators in the context of acute viral illness and bronchiolitis may differ from that of full-term infants, for whom routine bronchodilator administration is not recommended (55, 59). Forced expiratory flows in children and adolescents born preterm are abnormal and may improve with inhaled bronchodilator treatment (50). In addition, the harm of the intervention is likely very low, further supporting a trial of therapy. Data supporting chronic use of bronchodilators, including for airway clearance, in this population are limited. The panel also recognized that there may be individual factors, such as the presence of underlying TBM, a family history of asthma, and intercurrent lower respiratory tract bacterial infection that may impact the decision to trial bronchodilators in this patient population.
What others are saying
The 2020 European Respiratory Society (ERS) guidelines on long-term management of children with BPD made a conditional recommendation (based on consensus) for bronchodilators in certain subgroups with asthma-like symptoms, recurrent respiratory hospitalizations, exercise intolerance, or reversibility in lung function (60).
ATS recommendations
Recommendation 1a.
For infants, children, and adolescents with PPRD who do not have recurrent respiratory symptoms, we suggest that short-acting inhaled bronchodilator therapy not be routinely prescribed (conditional recommendation, very-low-certainty evidence).
Recommendation 1b.
For a subgroup of patients with PPRD who have recurrent respiratory symptoms (such as cough or wheeze), we suggest a trial of short-acting inhaled bronchodilator with monitoring to assess for clinical improvement in symptoms (conditional recommendation, very-low-certainty evidence).
Implementation
We suggest a short trial (1–2 doses) of a short-acting β2-agonist with continued use if there is demonstrated improvement in respiratory symptoms or pulmonary function on at least one occasion. Parental education around indications for use and training for proper administration, especially in infants and in children who have tracheostomies, should also be considered. In addition, children born preterm in whom asthma is later diagnosed may benefit from both short-acting and long-acting bronchodilators or daily controller medications as per international asthma guidelines (61).
Justification
This recommendation places a high value on the potential improvement of patient-important outcomes, such as quality of life, respiratory symptoms such as wheeze and dyspnea, respiratory exacerbations, and objective measures such as improvement in pulmonary function test results. In addition to the evidence above, our decision was also based on the consensus that most families would want a trial of bronchodilator therapy in their child born preterm with recurrent respiratory symptoms.
Question 2: Should Infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD and who have respiratory symptoms (wheezing, cough, tachypnea) routinely receive inhaled corticosteroids?
Background
A trial of inhaled corticosteroids is often attempted in these patients, as airway obstruction is a key feature of PPRD (34, 62). Up to one-third of the general preschool population has experienced wheezing in the last 6 months (63). A meta-analysis found that inhaled corticosteroids initiated at the onset of wheezing reduced the need for oral corticosteroids by 50% (64). Asthma is diagnosed in nearly 60% of preterm infants without BPD, whereas 30% require hospitalization for respiratory complications during the first year of life (65). The use of inhaled corticosteroids in full-term children with intermittent wheezing reduces the need for oral corticosteroids, hospitalization rates, emergency department visits, and overall healthcare costs (66), but it is unclear whether children born preterm benefit similarly to those born full-term.
Evidence base
We identified two randomized controlled trials (RCTs), two crossover RCTs, and one prospective observational trial that compared inhaled corticosteroids with placebo in children with PPRD (67–71) (online supplement).
In an RCT, 30 infants born preterm who required supplemental oxygen at 36 weeks’ PMA received fluticasone propionate or placebo for 1 year (67). There was no significant difference between study groups in the primary outcome (symptom-free days) or secondary outcomes (length of supplemental oxygen use, weight, bronchodilator use, number of hospital admissions, and LOS).
Another RCT compared inhaled beclomethasone dipropionate with placebo in infants with BPD from 36 weeks’ PMA until 3 months after discharge (68). There was no difference in the reported outcomes, including the length of supplemental oxygen use, bronchodilator use, weight, height, the number of hospital admissions, the LOS, oral corticosteroid use, additive inhaled or systemic corticosteroid administration, pediatric ICU days, or death before NICU discharge.
A prospective study evaluated 21 children born preterm (6 with BPD) with airway obstruction (FEV1 < 80%, PEF < 75%, or forced expiratory flow at 50% of FVC < 62% of predicted values), increased airway hyperreactivity with responsiveness to a β-agonist, or abnormal diurnal PEF variation >20% (69). Children (10 yr of age) received budesonide for 4 months. Eighteen children completed the study, with improvement in symptom scores but no differences in spirometric measurements being shown.
A crossover RCT compared beclomethasone dipropionate with placebo in 25 infants born preterm (3–24 mo of age) with wheeze or cough on ⩾4 d/wk that did not improve after 2 weeks of daily bronchodilator therapy (70). Seven infants did not complete the study; in four, this was due to worsening symptoms during placebo administration. The intervention group had significantly improved symptom scores, a significantly improved FRC, and fewer days of bronchodilator use.
Another crossover RCT compared 4 weeks of inhaled beclomethasone dipropionate with placebo in 15 school-aged children born preterm with wheezing or a troublesome cough and with a positive hyperreactive airway response to histamine (71). There was no difference in the respiratory symptom score, baseline airway function, or airway hyperreactivity between the treatment periods. There was a small but statistically insignificant increase in the FEV1 after treatment with beclomethasone compared with placebo.
Certainty of evidence
The panel’s confidence in the accuracy of the estimated effects of inhaled corticosteroid administration for critical outcomes was very low. Available studies evaluating the use of inhaled corticosteroids in children with PPRD have small cohorts (15–38 patients) and have not been adequately powered to show significant differences in the desired outcomes. Improvements in short-term symptom scores in full-term symptomatic children or children with documented airway hyperresponsiveness provide indirect evidence of a benefit.
Benefits
The two RCTs evaluating patients with BPD did not show statistically significant changes in reported outcomes, and hence did not show any benefit for the use of inhaled corticosteroids (67, 68). The three studies that included those with wheezing, cough, or changes in pulmonary function showed mixed results (69–71). However, data from full-term children with wheezing showed an improvement in symptom scores and decreased hospitalizations when subjects were treated with inhaled corticosteroids (63, 64, 66). Therefore, select patients with PPRD may benefit from inhaled corticosteroids.
Harms
Studies included in this review did not show evidence of harm with the use of inhaled corticosteroids. Evidence from full-term children is mixed regarding the effect of inhaled corticosteroids on growth (72, 73). This effect may be decreased with intermittent dosing (74). Inhaled corticosteroids increase the likelihood of sore throat and oral candidiasis, with one study finding one case of oral candidiasis for every 21 patients treated (75).
Parental preference
In our clinical experience, given the minimal available evidence regarding the potential benefit and toxicities, parents defer to the medical team concerning whether or not to trial inhaled corticosteroids.
Costs
The cost of daily therapy can be substantial; reducing inhaled corticosteroid use when not indicated could result in significant cost savings.
Conclusions
Given the low quality of evidence available and the lack of a significant benefit, routine use of inhaled corticosteroids has not been shown definitively to improve outcomes in patients with PPRD. However, when using data extrapolated from other populations, there may be some benefit for a trial of inhaled corticosteroids in selected patients with PPRD and chronic cough or wheezing.
What others are saying
The 2020 ERS guidelines on long-term management of children with BPD recommend against treating with inhaled corticosteroids. However, these guidelines made a conditional recommendation for a carefully monitored trial period of inhaled or systemic corticosteroids in some patients with severe BPD, severe respiratory symptoms, and/or recurrent hospitalizations and in those whose BPD was not controlled with regular bronchodilator use (60).
ATS recommendations
Recommendation 2a.
For infants, children, and adolescents with PPRD who do not have chronic cough and recurrent wheezing, we suggest that inhaled corticosteroids not be routinely prescribed (conditional recommendation, very-low-certainty evidence).
Recommendation 2b.
For infants, children, and adolescents with PPRD who have chronic cough or recurrent wheezing, we suggest a trial of inhaled corticosteroids with monitoring to assess for clinical improvement in symptoms (conditional recommendation, very-low-certainty evidence).
Implementation
Before a trial of inhaled corticosteroids, the baseline status (severity of symptoms, spirometric results) should be assessed and documented. Without evidence regarding an appropriate length of time for this trial, we suggest a duration of 3 months on the basis of extrapolation from other populations and full consensus of the panel. After this, patients should be reassessed, with pulmonary function testing being conducted if possible.
Justification
This recommendation places a high value on the potential improvement of patient-important outcomes, such as quality of life, respiratory symptoms, respiratory exacerbations, and objective measures such as improvement in pulmonary function test results. Potential side effects and adverse events were taken into consideration and compared with the possible benefits of therapy.
Question 3: Should infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD and who have respiratory symptoms (wheezing, cough, tachypnea) routinely receive diuretic therapy?
Background
Most studies examining the beneficial pulmonary effects of diuretics are limited by a short length of treatment and were conducted before the widespread use of surfactants (76–79). To date, no study has demonstrated an association between diuretic use and reductions in the incidence of BPD, the duration of mechanical ventilation, or NICU LOS. Diuretics, especially furosemide, are associated with comorbidities such as nephrolithiasis and metabolic bone disease (80–87). Although many infants are discharged from the NICU receiving diuretic therapy, their utility in post-NICU care remains uncertain.
Evidence base
The outcomes considered important for the literature review were mortality, the burden of disease, improvement in pulmonary function tests, the duration of supplemental oxygen being required, pulmonary edema, metabolic alkalosis, dehydration, osteopenia, ototoxicity, nephrolithiasis, and electrolyte abnormalities. Included were studies that described chronic diuretic use, defined as a minimum of 1 consecutive week of scheduled therapy (online supplement).
We identified four RCTs (88–91) that were conducted between 1983 and 1992, before the widespread use of surfactants (92). As such, the infants included in these studies had “old BPD,” characterized by airway injury, parenchymal fibrosis, and areas of severe hyperinflation due to oxygen toxicity and ventilator-induced lung injury (93). Only one study describes outcomes after NICU discharge (89). These studies enlisted few patients, with the largest including 43 infants (89).
Two RCTs evaluated the impact of furosemide therapy on pulmonary function, supplemental oxygen requirements, weight gain, electrolyte derangements, and hearing deficits. The first study included 11 infants born preterm, who were older than 28 days and who required continuous oxygen therapy (90). Subjects received alternating furosemide or placebo. On Day 8, furosemide therapy was significantly associated with improved dynamic pulmonary compliance and airway resistance, a decreased FiO2 requirement, and decreased average weight gain, with no significant difference in serum electrolytes concentrations being demonstrated when compared with placebo. In the second study of 17 mechanically ventilated infants born preterm, furosemide was administered for 7 consecutive days at 36–72 days of life (91). Furosemide therapy was significantly associated with improved lung compliance and a decreased serum chloride concentration. Weight gain was greater in the placebo group (P = 0.04). At 1 year of age, all infants had normal hearing. No respiratory outcomes were evaluated at 1 year.
The remaining two RCTs evaluated the efficacy of combined thiazide and spironolactone therapy. One RCT evaluated 43 infants born preterm who were receiving spironolactone and chlorothiazide or placebo until they were weaned off oxygen therapy (within 10 wk of enrollment) (89). Four weeks after the initiation of diuretics, the therapy group had significantly improved pulmonary compliance and airway resistance compared with the placebo group. However, these measures returned to prediuretic concentrations within 1 week after the completion of diuretic therapy. By 1 year of age, there was no difference in airway resistance or pulmonary compliance, the duration of supplemental oxygen being required, or the number of rehospitalizations between the two groups. The final RCT assessed 34 ventilator-dependent infants born preterm between Days of Life 31 and 40 who received both spironolactone and hydrochlorothiazide twice daily or placebo for 8 weeks (88). More infants were alive at NICU discharge in the diuretic group (P < 0.001). Respiratory system compliance was improved in the treatment group at 4 weeks (P = 0.016), but this difference was not significant by the end of the study. The NICU LOS, number of ventilator days, respiratory system compliance and resistance, and serum electrolytes were not significantly different.
Meta-analysis revealed a significant increase in pulmonary compliance in patients receiving diuretics at 1 and 4 weeks compared with at baseline. Compliance at 4 weeks compared with compliance at 1 week was also increased. Resistance at 4 weeks was decreased compared with resistance at 1 week. Mortality before NICU discharge was reduced, whereas rehospitalization within the first year and electrolyte supplementation were both increased in the diuretic group. No significant change was noted in the duration of supplemental oxygen use, the NICU LOS, the weight and length at 1 year, nephrolithiasis, sensorineural hearing deficits, or severe electrolyte abnormalities.
We identified 15 observational studies describing the potential harmful effects of diuretic use, including sensorineural hearing deficits or losses, nephrolithiasis, and metabolic bone disease. Six studies evaluated the rates of sensorineural hearing deficits associated with furosemide use. Of these, three studies showed that furosemide use was significantly associated with hearing impairment (94–96). However, the remaining studies showed no significant difference in hearing between those who received furosemide and those who did not, even in cases of furosemide exposure for greater than 28 days (97–99). Meta-analysis revealed a significantly increased risk of sensorineural hearing deficits in those receiving furosemide.
Eight studies assessed the risk of nephrolithiasis. Furosemide was the only diuretic used. Seven studies showed an increased incidence of nephrolithiasis with furosemide treatment (81–87). The remaining study showed no difference in the detection of nephrolithiasis in infants who received furosemide (100). Several authors noted that a lower gestational age at birth and lower birth weight were risk factors for renal stones. Meta-analysis revealed a significant increased risk of nephrolithiasis in those receiving furosemide.
We identified only one study that evaluated severe metabolic bone disease in neonates receiving furosemide (80). For every 2 weeks of furosemide therapy, the probability of developing severe metabolic bone disease increased by 1.4% (P < 0.001).
We did not identify any studies describing the effect of early diuretic therapy on late respiratory outcomes.
Certainty of evidence
The panel’s confidence in the accuracy of the estimated effects of diuretic administration for critical outcomes was very low. Evidence was considered indirect, as most studies were performed during the NICU stay and were performed before the routine use of surfactants. Several studies had incomplete outcome data. Other limitations included the lack of intention-to-treat analysis, varying chronological age at the time of testing, wide confidence intervals, potential confounders from co-interventions, bias due to missing data, and the inclusion of only infants with severe BPD in some of the studies. In addition, most studies were restricted to infants with limited comorbidities relevant to diuretic use, such as atrial septal defects and other intracardiac shunts.
Benefits
We identified no studies describing the potential beneficial effects of diuretics on important outcomes in children with PPRD after discharge from the hospital.
Harms
The use of furosemide was associated with an increased risk of nephrolithiasis and sensorineural hearing deficits. Electrolyte imbalances were also described.
Costs
Financial costs associated with diuretic use were considered to be minimal.
Conclusions
Although diuretics can improve pulmonary mechanics in the short term, studies investigating long-term diuretic use after NICU discharge are lacking. Currently available data showed that long-term diuretic use was not associated with improvements in patient-centered outcomes; however, it was associated with an increased likelihood of harm (sensorineural hearing deficits and nephrolithiasis), especially with furosemide.
What others are saying
A recent position statement from the Thoracic Society of Australia and New Zealand reviewed the literature and concluded that no formal recommendation could be made regarding long-term diuretic therapy in infants with BPD (101). The ERS guidelines on long-term management of children with BPD provided a conditional recommendation that infants who were treated with diuretics at the time of NICU discharge should be naturally weaned by allowing the dose to decrease slowly relative to the child’s weight (60).
ATS recommendations
Recommendation 3a.
For infants, children, and adolescents with PPRD, we suggest against the routine use of diuretics (conditional recommendation, very-low-certainty evidence).
Recommendation 3b.
For infants with PPRD who are discharged from the NICU on chronic diuretic therapy, we suggest discontinuation in a judicious manner (conditional recommendation, very-low-certainty evidence).
Implementation
-
1.
Given the short-term improvement in pulmonary mechanics, a trial of diuretics may be justified in situations in which lung compliance is believed to have acutely worsened. While administering diuretics, ongoing monitoring for electrolyte abnormalities, nephrolithiasis, and sensorineural hearing loss is recommended.
-
2.
The timing of discontinuation should be discussed with the family and made on an individual basis. Several panelists practice discontinuation without weaning. Others decrease the diuretic dose or lengthen the time interval of administration before stopping. Few panelists described allowing children to “outgrow” the dose.
Justification
This recommendation acknowledges the potential for improvement in patient-related outcomes, while emphasizing that the long-term use of diuretics may have deleterious side effects and that adverse events may outweigh the potential, yet unlikely, benefits.
Question 4: Should all infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD undergo baseline diagnostic PSG?
Background
Infants born preterm have an increased risk of SDB (102–105). Sleep disruption, hypoxemia, and hypercarbia can have lasting neurologic, cardiac, and behavioral effects that persist into adulthood (106–108).
Supplemental oxygen therapy improves hypoxemia, central apnea, and periodic breathing (104, 105). The increased risk of SDB and potential benefits of supplemental oxygen raise the question of the necessity of undergoing PSG (106, 108). However, costs, a lack of normative data, and the insufficient access to and limited capacity of pediatric sleep laboratories limit the ability to conduct PSG for all patients born preterm. Therefore, clinicians would benefit from guidelines regarding which infants require PSG and what alternatives may be useful when PSG is not indicated or unavailable.
Evidence base
The panel chose to focus on three clinical contexts: preterm infants before hospital discharge, preterm infants after hospital discharge, and the benefits of PSG versus overnight pulse oximetry (online supplement). We identified 32 articles relevant to the population of interest.
Preterm infants before hospital discharge.
Nine studies evaluated the role of PSG before discharge for infants with BPD. Elder and colleagues (109) compared infants with apnea born preterm with asymptomatic neonates by using nap PSG after 35 weeks’ PMA, and they observed no differences in the mean duration, type, or number of apnea events. However, the duration of the longest apnea and the degree of desaturation were greater in symptomatic cases versus controls, during both active and quiet sleep. Fajardo and colleagues (110) studied apneas in infants born preterm with and without BPD at 33 weeks’ PMA. All studied infants had central events, but obstructive apnea was more frequent in the BPD group (P = 0.004). Another study evaluated the impact of a supine versus prone sleep position on respiratory events in infants born preterm with and without BPD by using nap PSG at 37 weeks’ PMA (111). Obstructive apnea was significantly improved, whereas central apnea was significantly increased when going from the supine to the prone position. In a study describing 76 premature infants with clinical apnea, bradycardia, or cyanosis studied by using PSG before NICU discharge, the majority of infants had some degree of obstructive apnea, but patients with bradycardia (with or without clinically witnessed apnea) were noted to have significant obstructive events at PSG (102).
Infants with significant desaturations were noted to have improved PSG findings when treated with supplemental oxygen (104, 105) that persisted after discharge home (112). One study compared infants with BPD who had been weaned from supplemental oxygen before PSG with age-matched premature infants (104). Obstructive apnea or periodic breathing indices did not differ between the groups; however, infants with BPD had higher central apnea indices and lower oxygen saturation, which improved with supplemental oxygen. In another study of preterm infants who underwent PSG at 38 weeks’ PMA, supplemental oxygen significantly improved the apnea index, periodic breathing, and bradycardic events (105). Kulkarni and colleagues (112) identified that infants with BPD had elevated central apnea indices and oxygen desaturation that improved with supplemental oxygen administration to degrees similar to those of healthy infants. Over sequential PSG studies, patients with BPD were noted to have consistent improvement in the apnea–hypopnea index and respiratory disturbance index without complete normalization (113, 114).
Preterm infants after hospital discharge.
Four studies identified an increased risk of SDB in preterm patients as they age. In a prospective study of 850 children (46% premature) undergoing outpatient PSG, the prevalence of SDB was 2.2% overall and was three to five times more likely in preterm subjects (108). A reanalysis showed SDB in 7.3% of the preterm cohort (115). A cohort study of over 4 million Swedish children born over 40 years revealed SDB in 4.1% of subjects; those with preterm and extremely preterm birth had a 1.4- and 2.6-fold increased risk of SDB, respectively, from birth up to age 43 years (106). In addition to the lasting increased risk of SDB, chronic intermittent hypoxemia has also been associated with negative cognitive and behavioral effects (106, 107). Another retrospective review of 387 infants with BPD showed a 3% incidence of obstructive sleep apnea (107).
PSG versus overnight oximetry monitoring.
Overnight monitoring with pulse oximetry can be used as a screening test or in lieu of formal PSG when PSG is not available. Three studies were identified that examined the diagnostic accuracy of oximetry monitoring. Roberts and colleagues (116) studied the correlation between the intermittent hypoxemia measures derived from 24-hour oximetry and the measures of apnea derived from nap PSG for preterm infants after 35 weeks’ PMA but before NICU discharge. The desaturation index from the 24-hour oximetry correlated with the obstructive and central indices from the nap PSG. The desaturation index was 100% sensitive and 83% specific for predicting an apnea–hypopnea index greater than 10 events/h at nap PSG. Two studies evaluated daytime oximetry as a surrogate for overnight oximetry and showed conflicting results (117, 118). In a cohort of 63 infants with BPD and hypoxemia, there was no correlation between the 20-minute daytime and overnight oximetry studies (117). However, in 22 infants with BPD and hypoxemia, a longer 60-minute daytime oximetry study was more accurate, with a sensitivity of 100% for predicting overnight oximetry (by PSG) and a specificity of 65% being shown (118).
Certainty of evidence
With no RCT evaluating the use of PSG for this patient population, the panel’s confidence in the accuracy of these estimated effects on the utility of PSG for critical outcomes was very low. In addition, the studies were mostly retrospective and published over 4 decades. During this time, there have been significant clinical advancements in pediatric sleep medicine and the care of premature infants that are not represented in the reviewed studies.
Benefits
PSG provides detailed information regarding SDB and evaluating respiratory effort, oxygenation, ventilation, and obstructive and central events. It also provides limited information about other organ systems, including cardiopulmonary reserve and the central nervous system with respect to the control of breathing.
Harms
PSG is a prolonged and resource-intensive diagnostic test that is not indicated or feasible for all patients born preterm. This review has shown that even asymptomatic infants can have abnormal PSG findings. Their effect on long-term development is not clear, particularly in the context of events without desaturation. There is no consensus on “normal central apnea indices” in infancy because of a lack of normative data. Therefore, PSG may lead to unnecessary interventions, increased costs, and caregiver stress when the results will not change medical management. Finally, pediatric and neonatal PSG are not available in all locations. Facility transfers or prolonged stays may be required to complete these studies.
Parental preference
PSG is performed in a sleep medicine laboratory, and the child is monitored overnight. Many parents would prefer a home sleep apnea test as compared with formal PSG. However, home sleep apnea tests are not validated or recommended for children (119).
Costs
Although it is the gold standard to diagnose SDB, PSG is a resource-intensive diagnostic test (120). SDB itself is known to be associated with significant healthcare costs when diagnosis is delayed (121). This emphasizes the need for timely recognition of SDB symptoms and prompt diagnosis. Clinicians may consider overnight oximetry, a less expensive alternative, as a screening tool in certain situations.
Conclusions
PSG can be a useful tool in evaluating preterm patients. However, PSG may not change clinical management. Therefore, the panel considered the utility of PSG in distinct clinical contexts. The panel determined that before NICU discharge, PSG testing should be limited to patients whose care will be affected by the study results, such as those with persistent apnea, intermittent desaturations, or bradycardia. The panel observed a higher rate of SDB after NICU discharge across early and middle childhood and into midadulthood after preterm birth. PSG should be performed in patients born preterm with snoring, hypoxemia, or secondary effects of hypoxemia, such as failure to thrive and cognitive or behavioral impairment. Finally, as PSG is costly and not universally accessible, the panel recognizes that overnight or 24-hour oximetry studies may be an appropriate alternative, with sleep medicine referral being used when indicated.
What others are saying
Guidelines from the ATS, ERS, and British Thoracic Society support treating preterm infants with supplemental oxygen to maintain normal oxygen saturation (60, 122, 123). However, the role of diagnostic PSG was not addressed by these panels. A position statement from the Thoracic Society of Australia and New Zealand suggested that PSG be considered when obstructive or central apnea is suspected (101).
ATS recommendations
Recommendation 4a.
For infants with PPRD who are otherwise ready to be discharged from the NICU, we suggest the use of PSG for patients with persistent apnea, intermittent desaturation, or bradycardia at greater than 40 weeks’ PMA (conditional recommendation, very-low-certainty evidence).
Recommendation 4b.
For infants, children, and adolescents with PPRD, we suggest the use of PSG and/or sleep medicine referral for those with symptoms of SDB, including persistent snoring, failure to thrive, or a persistent need for supplemental oxygen at 2 years of age (conditional recommendation, very-low-certainty evidence).
Recommendation 4c.
When a PSG is indicated but not available, we recommend that an overnight or 24-hour oximetry be performed to screen for SDB, followed by PSG and/or a sleep medicine referral for further evaluation if needed (conditional recommendation, very-low-certainty evidence).
Implementation
When a PSG is indicated but not readily available, clinicians must consider whether oximetry monitoring is sufficient or whether referral to a sleep medicine program is needed for clinical management.
Justification
This recommendation places a high value on the potential improvement of quality of life and other clinical outcomes as well as respiratory symptoms such as apnea or hypoxemia. Limitations such as costs, access to PSG, and the lack of normative data in these patients are also taken in consideration.
Question 5: Should all infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD undergo a swallow evaluation?
Background
Acute and chronic pulmonary aspiration in patients with PPRD carries significant morbidity (124). Premature infants with pulmonary disease may be more susceptible to injury from aspiration. Faster respiratory rates in premature infants can contribute to swallow dysfunction when the time between breaths is less than the time needed to coordinate an effective swallow (125). Premature infants with airway anomalies, neurologic comorbidities, or recent viral infections may have a higher risk for aspiration (126, 127). Oral secretions, ingested liquids or solids, and gastric contents can be aspirated, leading to frequent respiratory exacerbations or infections, chronic symptoms, the development of bronchiectasis, or pulmonary hypertension (128, 129). Clinical signs of aspiration can include cough, stridor, wheeze, tachypnea, increased secretions, and oxygen desaturation. A subset has silent aspiration, which is identified through diagnostic evaluation without clinical symptoms while feeding. Diagnosing aspiration is complex. In addition to clinical feeding evaluation, evaluating aspiration can occur via a VFSS, fiberoptic endoscopic evaluation of swallowing (FEES), upper gastrointestinal series, bronchoscopy, or chest imaging. Because upper gastrointestinal series serve primarily to evaluate esophageal anatomy and gastrointestinal function, we focused on VFSS and FEES. Bronchoscopy and chest imaging are addressed in the PICO questions that follow.
Evidence base
We identified five retrospective studies of patients with PPRD who demonstrated aspiration during a VFSS. The reported prevalence of aspiration in premature infants who underwent a VFSS ranged from 29–100% (130–134) (online supplement).
Silent aspiration was described in several studies of preterm infants (130–132, 134). Those with vocal cord paralysis are at high risk for silent aspiration. One retrospective review revealed that 57% of 28 children with vocal cord paralysis referred for a VFSS had aspiration, including nine premature infants, all of whom had silent aspiration (132).
A retrospective review compared clinical feeding evaluations with VFSSs in 412 children under 2 years of age (131). Among those with normal clinical feeding evaluation results, approximately one-third demonstrated silent aspiration. In the subgroup of 130 preterm infants, 77% had aspiration or laryngeal penetration.
Among premature infants, an abnormal VFSS result in the first year of life is not associated with subsequent acute respiratory infections before the age of 3 years (130). However, infants with silent aspiration who are treated with thickened feeds are at decreased risk for subsequent acute respiratory infection.
Studies differ on whether premature infants are at an increased risk for swallowing dysfunction. Two retrospective studies noted a higher incidence of feeding problems after premature birth (133, 135). Yet other retrospective reviews noted similar rates of swallowing dysfunction in both preterm and term infants, although preterm infants more often experienced oxygen desaturation (134, 136).
We did not find any substantive literature describing the outcomes of using FEESs in patients with PPRD.
Certainty of evidence
The panel’s confidence in the accuracy of these estimated effects of the utility of swallow evaluation for critical outcomes was very low. Studies were primarily retrospective reviews of infants referred for feeding difficulties. Symptoms of aspiration and indications for testing with a VFSS are not standardized between centers.
Benefits
Swallow studies can be used to diagnose aspiration in preterm patients. Diagnosing aspiration can make feeding safer and decrease the risk for subsequent lung disease, including obliterative bronchiolitis and bronchiectasis.
Harms
Clinical symptoms lack sensitivity and specificity for diagnosing aspiration. The degree of radiation exposure during a VFSS differs between institutions. Advanced imaging of young children and its interpretation requires specialty expertise that may not be available at all centers. For patients with PPRD, aspirating contrast can carry clinical morbidity.
Parental preference
Families may experience anxiety related to understanding swallow study results and implementing changes to feeding after swallowing dysfunction is identified. Some parents may choose to have their children followed clinically, particularly if they have concerns regarding radiation exposure.
Costs
The cost of fluoroscopic studies should be considered. When swallowing dysfunction is identified, additional procedures may also be indicated.
Conclusions
The panel concluded that the benefit of using a VFSS to identify aspiration exceeded the burden and costs associated with the procedure for symptomatic patients with PPRD.
What others are saying
The utility of swallow evaluations was not addressed in other outpatient guidelines for patients with PPRD. However, for infants without neurologic pathology with persistent wheezing not relieved by bronchodilators or corticosteroids, the 2016 ATS guidelines for infants with recurrent or persistent wheezing recommend a swallow study to evaluate for aspiration (137).
ATS recommendation
Recommendation 5.
For infants, children, and adolescents with PPRD, we suggest a swallow evaluation (VFSS) for those who are eating by mouth and have cough or persistent oxygen desaturation during feeding, suspected or confirmed vocal cord paralysis or other airway anomalies, failure to wean from oxygen therapy or ventilatory support as expected, persistent or worsening pulmonary hypertension, failure to thrive, or chronic pulmonary symptoms out of proportion to viral respiratory infections (conditional recommendation, very-low-certainty evidence).
Implementation
An organized, multidisciplinary team is necessary to perform quality swallowing evaluations in preterm patients. When deciding to order a swallow evaluation, clinicians should consider how the results will impact care as well as the risks and benefits. Access to appropriate radiologic and technical expertise as well as the inclusion of experienced speech and occupational therapists are critical to interpreting study results.
Justification
This recommendation places a high value on the potential quality of life gained through the enjoyment of oral feeding while balancing the potential risks of both feeding and the evaluations themselves.
Question 6: Should all infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD undergo airway endoscopy?
Background
Infants with PPRD may require prolonged mechanical ventilation, undergo multiple intubations and extubation trials, and are at high risk for airway abnormalities, including subglottic or tracheal stenosis, subglottic cysts, TBM, and vocal cord immobility after PDA ligation. Such abnormalities may prolong mechanical ventilation, impact feeding, and can be life-threatening. Up to 50% of infants with PPRD demonstrate TBM at airway endoscopy (138, 139). Identifying airway abnormalities can guide management, including surgical correction of airway abnormalities. In the absence of a gold standard, airway endoscopy is considered the best available test to identify airway pathology. Endoscopic airway techniques consist of flexible laryngoscopy, flexible bronchoscopy, and rigid direct laryngobronchoscopy (DLB).
Evidence base
In the absence of RCTs, we identified 11 observational studies assessing airway endoscopy (flexible bronchoscopy, laryngoscopy, and DLB) in children with PPRD. Six indirectly addressed diagnostic yield and prognostic data from flexible bronchoscopy. Four studies addressed flexible laryngoscopy to identify left vocal cord paralysis (LVCP) after PDA ligation in preterm infants with respiratory or feeding symptoms. One study reported findings on DLB versus flexible laryngoscopy in children with aspiration (online supplement).
Two retrospective studies addressed the prognostic value of flexible bronchoscopy in preterm infants with BPD and TBM (45, 140). Three hundred fifty-three of 974 infants with BPD (36.2%) undergoing flexible bronchoscopies demonstrated malacia (140). Those with TBM more commonly underwent tracheostomy and gastrostomy, had longer mechanical ventilation and hospital LOSs, and were more likely to be mechanically ventilated at discharge. In 20 of 27 infants with severe BPD undergoing flexible bronchoscopy, airway pathologies were identified, including tracheomalacia (48%), bronchomalacia (40.7%), and airway edema (48%), often leading to changes in management (63%) (45).
Three prospective studies reported flexible bronchoscopy findings in preterm infants (141–143). Among 174 preterm infants undergoing flexible bronchoscopy, 7.5% had subglottic cysts (141). Another study of 117 preterm infants identified TBM or subglottic stenosis (SGS) in 48 patients (15 with moderate/severe SGS; 19 with moderate/severe malacia, and two with both) (142). Flexible bronchoscopy of 13 mechanically ventilated preterm infants revealed vocal cord nodules (62%) and airway malacia (31%) (143).
Four studies focused on flexible laryngoscopy used to identify LVCP in preterm infants after PDA ligation (144–147). A meta-analysis (33 studies; n = 4,887) showed an 11% incidence of LVCP in preterm infants, which was associated with an increased risk of BPD, gastrostomy, and mechanical ventilation days (144). A retrospective study showed that 22 of 55 extremely preterm infants had LVCP after PDA ligation (145). Those with LVCP were more likely to develop BPD, reactive airway disease, and gastrostomy dependence, suggesting that flexible laryngoscopy be considered for patients with respiratory or feeding difficulties after PDA ligation. A study of 23 preterm infants after PDA ligation and 12 matched preterm control subjects reported that 12 of 23 had LVCP after PDA ligation, with seven showing signs of aspiration (146). Finally, a study examining long-term morbidities in preterm infants after PDA ligation noted that 44% had LVCP, with most requiring gastrostomies (147).
One study examined the benefit of DLB in addition to flexible laryngoscopy for 532 patients with aspiration (mean age at enrollment, 2.2 yr; 131 preterm) (127). Flexible laryngoscopy enabled identification of 93 patients with airway lesions, whereas DLB enabled identification of 173 additional lesions (136 laryngeal clefts, 40 laryngomalacia cases, 25 vocal fold immobility cases, 22 tracheomalacia cases [with 6 also showing laryngomalacia], 12 SGS cases, three H-type tracheoesophageal fistulae, and 2 glottic webs), with 66.3% requiring surgical intervention.
Certainty of evidence
The panel’s confidence in the accuracy of these estimated effects for the utility of airway endoscopy for critical outcomes was very low. Small, observational studies often lacked a comparison group. The mode of endoscopy (flexible laryngoscopy, flexible bronchoscopy, DLB) differed between studies, limiting generalizability.
Benefits
In patients with PPRD and unexplained prolonged ventilation or chronic respiratory symptoms, airway endoscopy can be used to identify treatable anatomic abnormalities and can provide prognostic information. When possible, although not essential, airway endoscopy may be best performed by a multidisciplinary aerodigestive team, assessing all possible upper and lower airway pathologies at once without repeat anesthesia.
Harms
One study reported mild hypoxemia (an SpO2 less than 90% but greater than 80%) during bronchoscopy in 5 of 27 patients (18.5%), with one patient requiring bronchoscopy for severe hypoxemic events developing severe hypoxemia (SpO2 less than 50%) with bradycardia (45). Patients with PPRD have impaired lung function and an increased risk of cardiopulmonary sequelae (pulmonary hypertension, respiratory decompensation, atelectasis, pneumonia) after airway endoscopy (148).
Coordination of endoscopy with other procedures can increase the anesthesia time; thus, unnecessary procedures should be avoided. Complications after flexible bronchoscopy occur more frequently in children undergoing multiple same-day procedures. In a study of 1,297 bronchoscopic procedures, 27 (2.1%) unplanned outcomes occurred, with 21 of 27 patients undergoing multiple procedures (148). However, unplanned outcomes were not different from those of flexible bronchoscopy alone in the subset with multiple procedures coordinated through a multidisciplinary aerodigestive clinic. This supports the management of such children through a collaborative aerodigestive team, when available.
The neurodevelopmental risks of general anesthesia must also be considered. In cases in which general anesthesia poses a high risk, less invasive modes of airway evaluation such as airway imaging (dynamic CT or MRI) can be considered, although the panel recognizes that imaging is not as sensitive as endoscopy for the identification of tracheomalacia [see Question 7: Should all infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD undergo diagnostic imaging for TBM?].
Parental preference
Expert opinion from the panel reported that the majority of parents prefer to proceed with coordination of all necessary airway endoscopies (flexible and rigid) to identify treatable airway abnormalities and inform future management. When parental concern regarding general anesthesia is high, nonsedated airway imaging may be preferred.
Costs
Endoscopic airway evaluations, especially those done by multiple services, are expensive. Costs need to be weighed against the costs of failing to treat airway abnormalities while accounting for the child’s quality of life.
Conclusions
The panel concluded that airway endoscopy is the best available test for the identification of large airway disease. When indicated, the benefits of airway endoscopy exceed its risks. Airway endoscopy can be considered when less invasive diagnostic methods have been exhausted. Adverse outcomes are reduced when airway evaluations are performed by a multidisciplinary aerodigestive team.
What others are saying
Other guidelines have not addressed the role of airway endoscopy in the outpatient management of preterm patients. However, the ATS guidelines for infants with recurrent or persistent wheezing recommend bronchoscopy with BAL when wheezing persists despite medical management (137).
ATS recommendation
Recommendation 6.
For infants, children, and adolescents with PPRD, we suggest airway endoscopy for those with unexplained symptoms such as chronic cough, wheezing, ventilator dependence, persistent hypoxemia, or a history of PDA ligation with stridor and weak cry (conditional recommendation, very-low-certainty evidence).
Implementation
The optimal mode of airway endoscopy (flexible bronchoscopy, flexible laryngoscopy, DLB) is not clear in the studies reviewed. The panel suggests a collaborative approach to airway endoscopy by a multidisciplinary team. Endoscopic techniques complement each other and facilitate identification of different airway pathologies. Flexible laryngoscopy should be performed in an awake child to identify upper airway pathology (laryngomalacia, vocal cord immobility, glottic webs, stenosis). Direct or rigid laryngobronchoscopy performed with the child spontaneously breathing under anesthesia is appropriate for the identification of glottic/subglottic pathology (subglottic cysts, stenosis, laryngeal clefts) and tracheal/bronchial pathology (tracheal stenosis, TBM). Flexible bronchoscopy and sampling through BAL can be used to identify large airway disease (tracheal stenosis and TBM, as well as distal bronchial anatomic abnormalities).
Justification
This recommendation places a high priority on patient safety and the need to identify airway anomalies that could significantly impact patient-centered outcomes.
Question 7: Should all infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD undergo diagnostic imaging for TBM?
Background
Premature infants are at risk for developing large airway disease, especially those who require prolonged positive-pressure ventilation (28, 140). Positive pressure can distend the abnormally compliant and structurally immature airways of preterm infants, resulting in acquired or secondary TBM (149). The adverse consequences of TBM include decreased intrathoracic airflow, cyanotic episodes, difficulty weaning from positive-pressure ventilation, and higher mortality (140). The diagnosis of TBM is important for prognostication and individualized management of patients with PPRD (28, 150). The best available test for diagnosing tracheomalacia, bronchomalacia, or TBM is airway endoscopy. However, this technique is invasive, requires sedation or anesthesia, and may not be safe for critically ill neonates with small endotracheal or tracheostomy tubes. An alternative to bronchoscopy is dynamic imaging of the large airways.
Evidence base
Ten studies of preterm infants at risk for TBM were evaluated to determine the utility of airway imaging to complement or replace airway endoscopy in the management of PPRD. Although these guidelines focus on outpatients with PPRD, studies of preterm infants before NICU discharge (age greater than 36 weeks’ PMA at time of study) were included (online supplement).
Studies evaluating the use of imaging were compared with those evaluating the use of bronchoscopy, recognizing that the bronchoscopic diagnosis of airway malacia is subjective and lacks a standardized definition. Some define airway collapse of more than 50% as TBM (149). Others have graded TBM as none (0–25% collapse), mild to moderate (26–75% collapse), or severe (greater than 75% collapse) (151).
We identified a single prospective study comparing using a tracheobronchogram with using a dynamic spiral CT scan to diagnose TBM in 16 infants (15 preterm) (152). Compared with bronchography, CT was less sensitive, enabling correct identification of 10 of 16 infants with tracheomalacia and 14 of 28 infants with bronchomalacia. The dynamic CT scans resulted in an underestimation of opening pressure and required a larger radiation dose than bronchograms.
In a study of 24 infants and children, two of whom had BPD, undergoing large airway evaluation through dynamic CT with three-dimensional reconstruction, 5 had TBM, 5 had right bronchomalacia, 8 had left bronchomalacia, and 8 had lobar bronchomalacia (153). An evaluation of the correlation with bronchoscopy results was not performed.
Several studies compared bronchoscopy and CT imaging. Ten of 17 infants less than 12 months old had airway malacia at bronchoscopy, and 50% of those infants with malacia were premature (154). The sensitivity of using CT to diagnose tracheomalacia was 37.5%, and the sensitivity for using CT to diagnose bronchomalacia was 75%. Among eight infants (50% preterm) evaluated by using dynamic volumetric CT without sedation to diagnose large airway disease, four patients had TBM at CT imaging, and there was 75% concordance with bronchoscopy results (155). Another study of 34 pediatric patients (35% preterm) who underwent a cine multidetector CT scan for clinical indications identified isolated tracheomalacia in four patients and right or left bronchomalacia in 10 patients (80% with a history of cardiac surgery) (156). In the 29 patients who underwent bronchoscopy, there was complete concordance between bronchoscopy and CT results in 23 patients and partial concordance in four patients. Importantly, in two patients, the CT result was positive, whereas the bronchoscopy result was negative. Dynamic CT was calculated to be 100% sensitive and 81% specific, with a positive predictive value of 90% and a negative predictive value of 100% being demonstrated. Another retrospective study included 28 patients who underwent bronchoscopy for suspected tracheomalacia (157). Paired static end-inspiratory and end-expiratory CT was performed on 26 of 28 patients with tracheomalacia and five “control subjects” with dry cough. The CT-enabled diagnosis of tracheomalacia was determined by using the change in the cross-sectional area (CSA) at the level of maximum collapse, resulting in a sensitivity of 85% and specificity of 100%. Finally, 27 children with bronchoscopic evidence of tracheomalacia and 320 control subjects were evaluated to determine the accuracy of using free-breathing cine CT for diagnosing tracheomalacia, as defined by the change in the tracheal CSA (158). Fifty-seven percent of patients with malacia diagnosed through bronchoscopy had a significant change in the CSA, compared with 11% of control subjects, with 96% sensitivity, 97% specificity, and 97% accuracy being demonstrated.
Wu and colleagues (28) described 76 premature infants with severe BPD between 40–50 weeks’ PMA by using a combination of CT with angiography and bronchoscopy/tracheoscopy to diagnose large airway disease (defined as a >50% decrease in the tracheal or bronchial CSA from inspiration to expiration) and determined its effect on BPD-related morbidity. Large airway malacia was associated with increased risks of death before hospital discharge, tracheostomy, and home pulmonary vasodilator therapy.
An emerging area for the diagnosis of large airway disease is respiratory-gated MRI. Bates and colleagues (159) performed a retrospective study of 27 neonates by using a specialized MRI system within their NICU to evaluate for tracheomalacia. The change in the luminal CSA as determined through bronchoscopy was used to identify those with tracheomalacia. A recent study by Hysinger and colleagues (160) validated the use of ultrashort echo-time MRI for diagnosing tracheomalacia as compared with bronchoscopy. The use of MRI to diagnose airway malacia had a moderate correlation with the use of bronchoscopy.
Certainty of evidence
Overall, the quality of evidence is very low. No direct evidence answers this PICO question for patients with PPRD. With one exception, studies were retrospective, single-center studies that included small sample sizes. The available data demonstrate that using CT to diagnose large airway disease results in variable sensitivity, specificity, and concordance with using bronchoscopy. This may be due to the state dependence of imaging findings that vary according to whether the infant is calm, agitated, or in respiratory distress.
Benefits
In patients with PPRD, large airway imaging can lead to the diagnosis and treatment of surgically correctable findings. Without treatment, TBM is associated with significant morbidity (140). Large airway imaging can be used to avoid the sedation associated with bronchoscopy. Dynamic CT can provide objective data to complement bronchoscopy data. CT imaging can be used to noninvasively evaluate parenchymal and vascular pathologies that contribute to airway disease when sedated bronchoscopy is not feasible (lack of subspecialist availability, clinical instability, or prohibitively small artificial airway).
Harms
Advanced imaging of young patients and its interpretation requires specialty expertise that may not be available at all centers. CT imaging requires radiation exposure, albeit at a relatively small dose designed for pediatric imaging studies (161–166). The amount of radiation is variable (0.75–1.96 mSv in the studies above). There are center-specific differences in imaging protocols, including positive end-expiratory pressure (PEEP) titration and degree of patient sedation, which can lead to decreased generalizability of the findings. Finally, in the studies referenced here and in the panelists’ practices, CT and MRI are not as sensitive as bronchoscopy for diagnosing TBM and thus may not provide a definitive diagnosis.
Parental preference
Parents are understandably concerned about the radiation exposure associated with CT. Should noninvasive dynamic CT be offered without sedation and ventilation, it may be preferred to bronchoscopy. Alternatively, if reliable airway imaging requires a combination of sedation, mechanical ventilation, and radiation exposure, bronchoscopy may be preferred. Dynamic MRI of the airways, when available, is an alternative that can be performed without radiation or sedation (159).
Costs
There is a cost associated with airway imaging, which includes radiologist expertise for protocol design, implementation, and interpretation of the studies. We recommend unsedated CT because anesthesia is associated with increased risk and cost and affects dynamic airway collapse. Alternatively, earlier identification of large airway disease can reduce costs by allowing for PEEP titration to decrease hospital LOSs and readmissions.
Conclusions
The use of bronchoscopy is most likely to result in an accurate identification of large airway disease [see Question 6: Should all infants, children, and adolescents born preterm (gestational age less than 37 wk) with PPRD undergo airway endoscopy?]. Large airway CT imaging is noninvasive but requires radiation exposure and is less sensitive and specific than bronchoscopy. However, dynamic airway imaging may be useful when bronchoscopy is not possible or in cases in which imaging would identify extrinsic compression or other anatomic features causing central airway collapse. A subset of patients may require a combination of bronchoscopy and imaging (Figure 1).
What others are saying
The 2020 ERS guidelines for the long-term management of children with BPD recommended consideration of lung imaging for children with more severe BPD.
ATS recommendations
Recommendation 7a.
For infants, children, and adolescents with PPRD who do not have symptoms suggestive of airway malacia, we suggest that dynamic airway imaging (CT or MRI) not be used as a screening test for the routine diagnosis of TBM (conditional recommendation, very-low-certainty evidence).
Recommendation 7b.
We suggest that unsedated, dynamic airway imaging (CT or MRI) be used for the diagnosis or reevaluation of TBM in patients with PPRD who have recurrent symptoms suggestive of airway malacia as an alternative to bronchoscopy when the risks from anesthesia for bronchoscopy are judged to be greater than the risks from radiation or when bronchoscopy is not feasible or available (see Figure 1) (conditional recommendation, very-low-certainty evidence).
Implementation
TBM is diagnosed by measuring the CSA ratio. This recommendation was informed by available literature, pediatric radiology guidelines, and expert opinion. Although technical image-acquisition details will differ by institution and device manufacturer, CT should be performed by using appropriate dose-reduction techniques (161), which can be as low as 0.5 mSv. Airway MRI can be performed by using dynamic cine or gating techniques (active or retrospective), with time resolutions as low as one-eighth of a respiratory cycle for retrospectively gated ultrashort echo-time sequences at high resolution (159). Although dynamic airway MRI is currently limited to centers with this unique resource, this technique may represent the future of TBM diagnosis, as it does not require radiation or sedation. Sedated imaging studies are less likely to capture dynamic airway collapse and are therefore not recommended.
Justification
This recommendation accounts for the fact that dynamic airway imaging may not be available at all centers. It places a high value on properly diagnosing conditions that significantly impact clinical care. The decision to perform airway imaging in addition to or in lieu of bronchoscopy requires careful consideration by the clinician.
Limitations and Future Research
Despite the frequency of PPRD in preterm infants, children, and adolescents, we were unable to make strong recommendations because all of the evidence was of very low certainty. Most studies focused on infants, which limits their applicability and suggests that much could be learned from evaluating these therapies and diagnostic studies in older children and adolescents with PPRD. Given the frequently unrecognized risks that late-preterm infants experience, we elected to include them in the systematic review. However, differences between extremely preterm and late-preterm infants may affect clinical management.
In some cases, multicenter studies are warranted to investigate our specific recommendations more comprehensively. Studies examining the duration and weaning or discontinuation of inhaled bronchodilator and corticosteroid therapy in patients with PPRD who have respiratory symptoms (wheezing, cough, tachypnea) will be highly beneficial. However, further RCTs of diuretics in children with PPRD require careful consideration of the risks of treatment before examining the short- and long-term outcomes of this common but potentially unnecessary intervention. Further studies are needed to determine the role of diagnostic PSG in patients with PPRD. The utility of an interdisciplinary aerodigestive team approach to conducting formal evaluations of swallowing (including both VFSS and FEES), airway endoscopy, and airway imaging should be studied to determine its clinical benefit and feasibility for patients with PPRD.
Studies in areas not specifically addressed by these clinical practice guidelines may also warrant further investigation. Such topics include longitudinal assessments of pulmonary function, potentially using infant pulmonary function testing, forced oscillation techniques, spirometry, plethysmography, and other novel modalities for measuring respiratory physiology in patients with PPRD across the lifespan. The management of both acute respiratory exacerbations and chronic care warrants further study. The impact of environmental exposures, including but not limited to inhaled tobacco smoke and air pollution, on those with PPRD is likewise an important area for future research. How PPRD affects the risk of chronic obstructive pulmonary disease, emphysema, and other chronic respiratory conditions in adults remains to be determined as populations of extremely preterm neonates reach this age in the coming decades. Approaches to the prevention of these severe, life-limiting conditions should also be studied in adult cohorts with PPRD.
Conclusions
PPRD manifests as a spectrum of disease severity. Even those with milder disease are at risk for long-term respiratory impairment (167). Given the lack of clinical studies in infants, children, and adolescents with PPRD, this clinical practice guideline will aid primary care providers and specialists in diagnosing and treating respiratory symptoms commonly seen in this population. There are currently no medications or diagnostic testing that are universally indicated for those with PPRD. However, future studies will elucidate which subgroups of patients will benefit from targeted therapies for the prevention and treatment of PPRD in a personalized approach. As patients with PPRD enter into adulthood, additional clinical and translational research will be critical for determining the risk of cardiopulmonary disease later in life. The recommendations in this guideline were reviewed by the ATS Quality Improvement and Implementation Committee, and none are considered suitable for performance measure development.
Acknowledgments
This official clinical practice guideline was prepared by an ad hoc subcommittee of the ATS Assembly on Pediatrics.
Members of the subcommittee are as follows:
A. Ioana Cristea, M.D., M.S. (Co-Chair)1
Christopher D. Baker, M.D. (Co-Chair)2
Julian Allen, M.D.3,4*
Reshma Amin, M.D.5*
Eric D. Austin, M.D.6‡
Mary E. Cataletto, M.D.7§
Joseph M. Collaco, M.D., Ph.D.8ǁ
Laurie C. Eldredge, M.D., Ph.D.9ǁ
Robert J. Fleck, M.D.10ǁ
Andrew Gelfand, M.D.11‡
Don Hayes, Jr., M.D.12,13¶
Narayan Iyer, M.D.14,15**
Marcus H. Jones, M.D.16‡‡
Sheila S. Kun, R.N.17*
Jonathan C. Levin, M.D.18‡‡
Parevi P. Majmudar, D.O.19‡
Erica W. Mandell, M.D.20‡
Anne E. May, M.D.21,22,23*
Sharon A. McGrath-Morrow, M.D.3,4ǁ
Howard B. Panitch, M.D.3,4‡‡
Rizwana Popatia, M.D.24¶
Clement L. Ren, M.D.3,4‡‡
Lawrence M. Rhein, M.D.25¶
Rebecca S. Rose, M.D.26¶
Alejandro Teper, M.D.27‡‡
Michael C. Tracy, M.D.28ǁ
Karen F. Watters, M.D., M.P.H.29ǁ
Jason C. Woods, Ph.D.10,12,13ǁ
*Polysomnogram patient/intervention/comparator/outcome subgroup.
‡Diuretics patient/intervention/comparator/outcome subgroup.
§Methodologist.
ǁAerodigestive patient/intervention/comparator/outcome subgroup.
¶Inhaled corticosteroid patient/intervention/comparator/outcome subgroup.
**Lead methodologist.
‡‡Short-acting inhaled bronchodilator patient/intervention/comparator/outcome subgroup.
1Division of Pediatric Pulmonology, Allergy and Sleep Medicine and 26Division of Neonatal–Perinatal Medicine, Department of Pediatrics, Riley Hospital for Children and Indiana University, Indianapolis, Indiana; 2Division of Pediatric Pulmonary and Sleep Medicine and 20Division of Neonatology, Department of Pediatrics, School of Medicine, University of Colorado Denver, Denver, Colorado; 3Division of Pulmonary and Sleep Medicine, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; 4Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; 5Division of Respiratory Medicine, The Hospital for Sick Children and University of Toronto, Toronto, Ontario, Canada; 6Department of Pediatric Pulmonary Medicine, Vanderbilt University Medical Center, Nashville, Tennessee; 7Division of Pediatric Pulmonology, New York University Health, School of Medicine, New York University Long Island, Mineola, New York; 8Eudowood Division of Pediatric Respiratory Sciences, Johns Hopkins University, Baltimore, Maryland; 9Division of Pediatric Pulmonary and Sleep Medicine, Department of Pediatrics, Seattle Children’s Hospital and School of Medicine, University of Washington, Seattle, Washington; 10Department of Radiology and 12Division of Pulmonary Medicine, Cincinnati Children’s Hospital Medical Center and College of Medicine, University of Cincinnati, Cincinnati, Ohio; 11Division of Respiratory Medicine, Department of Pediatrics, Children’s Medical Center of Dallas, University of Texas Southwestern Medical Center, Dallas, Texas; 13Department of Pediatrics, College of Medicine, University of Cincinnati, Cincinnati, Ohio; 14Fetal and Neonatal Institute, Division of Neonatology and 17Division of Pediatric Pulmonology, Children’s Hospital Los Angeles, Los Angeles, California; 15Department of Pediatrics, Keck School of Medicine, University of Southern California, Los Angeles, California; 16Department of Pediatrics, School of Medicine, Pontifical Catholic University of Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil; 18Division of Newborn Medicine, Division of Pulmonary Medicine, Boston Children’s Hospital, Boston, Massachusetts; 19Division of Pediatric Pulmonary and Sleep Medicine, Department of Pediatrics, Cleveland Clinic Children’s Center for Pulmonary Medicine, Cleveland, Ohio; 21Section of Pulmonary Medicine and 22Sleep Disorders Center, Nationwide Children’s Hospital, Columbus, Ohio; 23Department of Pediatrics, School of Medicine, The Ohio State University, Columbus, Ohio; 24College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, United Arab Emirates; 25Neonatal–Perinatal Medicine Fellowship Program, Division of Pediatric Pulmonary/Allergy, Chan Medical School, University of Massachusetts, Worcester, Massachusetts; 27Hospital de Niños R. Gutiérrez of Buenos Aires, Buenos Aires, Argentina; 28Division of Pediatric Pulmonary, Asthma and Sleep Medicine, Department of Pediatrics, School of Medicine, Stanford University, Stanford, California; and 29Department of Otolaryngology and Communication Enhancement, Boston Children’s Hospital and Harvard Medical School, Harvard University, Boston, Massachusetts
Acknowledgment
The authors thank Kimberly Lawrence for her excellent work organizing the panel, coordinating both in-person and virtual meetings, and performing administrative responsibilities related to this document. They thank Ms. Lynn Kysh for performing a thorough literature search and procuring full-text articles. Finally, they thank the patients with post-prematurity respiratory disease and their families, who provided critical feedback and insight that strengthened these guidelines.
Footnotes
This official clinical practice guideline of the American Thoracic Society was approved September 2021
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1164/rccm.202110-2269ST.
This document has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author Disclosures: E.D.A. served on an advisory committee for United Therapeutics. M.E.C. served as a speaker for the Asthma Coalition; and was an employee of Mary Ann Liebert Publishers. M.H.J. served on an advisory committee for Boehringer Ingelheim and GlaxoSmithKline; and served as a speaker for Abbvie, AstraZeneca, Boehringer Ingelheim, Chiesi, and Vertex. H.B.P. served on an advisory committee for Philips Respironics. C.L.R. served as a consultant for Vertex; and served on a data and safety monitoring board for Shire and Takeda. J.C.W. served as a consultant and received research support from Vertex. A.I.C., C.D.B., J.A., R.A., J.M.C., L.C.E., R.J.F., A.G., D.H., N.I., S.S.K., J.C.L., P.P.M., E.W.M., A.E.M., S.A.M.-M., R.P., L.M.R., R.S.R., A.T., M.C.T., and K.F.W. reported no commercial or relevant noncommercial interests.
References
- 1. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet . 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Fact sheet: preterm birth Geneva, Switzerland: World Health Organization; 2018. [created 2018 Feb 19; accessed 2020 Dec 22]. Available from: https://www.who.int/news-room/fact-sheets/detail/preterm-birth [Google Scholar]
- 3. Patel RM. Short- and long-term outcomes for extremely preterm infants. Am J Perinatol . 2016;33:318–328. doi: 10.1055/s-0035-1571202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol . 2013;37:124–131. doi: 10.1053/j.semperi.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol . 2014;100:145–157. doi: 10.1002/bdra.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bancalari E, Gerhardt T. Bronchopulmonary dysplasia. Pediatr Clin North Am . 1986;33:1–23. doi: 10.1016/s0031-3955(16)34967-7. [DOI] [PubMed] [Google Scholar]
- 7. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med . 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 8. Korhonen P, Laitinen J, Hyödynmaa E, Tammela O. Respiratory outcome in school-aged, very-low-birth-weight children in the surfactant era. Acta Paediatr . 2004;93:316–321. doi: 10.1080/08035250410023593. [DOI] [PubMed] [Google Scholar]
- 9. Anand D, Stevenson CJ, West CR, Pharoah PO. Lung function and respiratory health in adolescents of very low birth weight. Arch Dis Child . 2003;88:135–138. doi: 10.1136/adc.88.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farrell ET, Bates ML, Pegelow DF, Palta M, Eickhoff JC, O’Brien MJ, et al. Pulmonary gas exchange and exercise capacity in adults born preterm. Ann Am Thorac Soc . 2015;12:1130–1137. doi: 10.1513/AnnalsATS.201410-470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsopanoglou SP, Davidson J, Goulart AL, Barros MC, dos Santos AMN. Functional capacity during exercise in very-low-birth-weight premature children. Pediatr Pulmonol . 2014;49:91–98. doi: 10.1002/ppul.22754. [DOI] [PubMed] [Google Scholar]
- 12. Smith LJ, van Asperen PP, McKay KO, Selvadurai H, Fitzgerald DA. Reduced exercise capacity in children born very preterm. Pediatrics . 2008;122:e287–e293. doi: 10.1542/peds.2007-3657. [DOI] [PubMed] [Google Scholar]
- 13. Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, et al. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J . 2008;32:321–328. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]
- 14. Broström EB, Akre O, Katz-Salamon M, Jaraj D, Kaijser M. Obstructive pulmonary disease in old age among individuals born preterm. Eur J Epidemiol . 2013;28:79–85. doi: 10.1007/s10654-013-9761-7. [DOI] [PubMed] [Google Scholar]
- 15. Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med . 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 16. Baker CD, Alvira CM. Disrupted lung development and bronchopulmonary dysplasia: opportunities for lung repair and regeneration. Curr Opin Pediatr . 2014;26:306–314. doi: 10.1097/MOP.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol . 1998;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 18. Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr Opin Pediatr . 2013;25:329–337. doi: 10.1097/MOP.0b013e328360a3f6. [DOI] [PubMed] [Google Scholar]
- 19. Thibeault DW, Mabry SM, Ekekezie II, Truog WE. Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics . 2000;106:1452–1459. doi: 10.1542/peds.106.6.1452. [DOI] [PubMed] [Google Scholar]
- 20. Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet . 2006;367:1421–1431. doi: 10.1016/S0140-6736(06)68615-7. [DOI] [PubMed] [Google Scholar]
- 21. Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med . 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 22. Chye JK, Gray PH. Rehospitalization and growth of infants with bronchopulmonary dysplasia: a matched control study. J Paediatr Child Health . 1995;31:105–111. doi: 10.1111/j.1440-1754.1995.tb00756.x. [DOI] [PubMed] [Google Scholar]
- 23. Gross SJ, Iannuzzi DM, Kveselis DA, Anbar RD. Effect of preterm birth on pulmonary function at school age: a prospective controlled study. J Pediatr . 1998;133:188–192. doi: 10.1016/s0022-3476(98)70219-7. [DOI] [PubMed] [Google Scholar]
- 24. Keller RL, Feng R, DeMauro SB, Ferkol T, Hardie W, Rogers EE, et al. Prematurity and Respiratory Outcomes Program. Bronchopulmonary dysplasia and perinatal characteristics predict 1-year respiratory outcomes in newborns born at extremely low gestational age: a prospective cohort study. J Pediatr . 2017;187:89–97.e3. doi: 10.1016/j.jpeds.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr . 2004;144:799–803. doi: 10.1016/j.jpeds.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 26. Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A, et al. American Thoracic Society. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med . 2003;168:356–396. doi: 10.1164/rccm.168.3.356. [DOI] [PubMed] [Google Scholar]
- 27. Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr . 2011;23:167–172. doi: 10.1097/MOP.0b013e3283423e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu KY, Jensen EA, White AM, Wang Y, Biko DM, Nilan K, et al. Characterization of disease phenotype in very preterm infants with severe bronchopulmonary dysplasia. Am J Respir Crit Care Med . 2020;201:1398–1406. doi: 10.1164/rccm.201907-1342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pryhuber GS, Maitre NL, Ballard RA, Cifelli D, Davis SD, Ellenberg JH, et al. Prematurity and Respiratory Outcomes Program Investigators. Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr . 2015;15:37. doi: 10.1186/s12887-015-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction— GRADE evidence profiles and summary of findings tables. J Clin Epidemiol . 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Schünemann H, Brożek J, Guyatt G, Oxman Aeditors. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. The GRADE Working Group; 2013. [updated 2013 Oct; accessed 2019 Mar 20]. Available from: https://gdt.gradepro.org/app/handbook/handbook.html [Google Scholar]
- 32.Bhandari A, Panitch HB.Pulmonary outcomes in bronchopulmonary dysplasia Semin Perinatol 200630219–226.. [DOI] [PubMed] [Google Scholar]
- 33.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis PLoS Med 201411e1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pramana IA, Latzin P, Schlapbach LJ, Hafen G, Kuehni CE, Nelle M, et al. Respiratory symptoms in preterm infants: burden of disease in the first year of life. Eur J Med Res . 2011;16:223–230. doi: 10.1186/2047-783X-16-5-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med . 2010;182:237–245. doi: 10.1164/rccm.200912-1806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leps C, Carson C, Quigley MA. Gestational age at birth and wheezing trajectories at 3-11 years. Arch Dis Child . 2018;103:1138–1144. doi: 10.1136/archdischild-2017-314541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Natarajan G, Shankaran S. Short- and long-term outcomes of moderate and late preterm infants. Am J Perinatol . 2016;33:305–317. doi: 10.1055/s-0035-1571150. [DOI] [PubMed] [Google Scholar]
- 38. Collaco JM, Kole AJ, Riekert KA, Eakin MN, Okelo SO, McGrath-Morrow SA. Respiratory medication adherence in chronic lung disease of prematurity. Pediatr Pulmonol . 2012;47:283–291. doi: 10.1002/ppul.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Damgaard AL, Hansen BM, Mathiasen R, Buchvald F, Lange T, Greisen G. Prematurity and prescription asthma medication from childhood to young adulthood: a Danish national cohort study. PLoS One . 2015;10:e0117253. doi: 10.1371/journal.pone.0117253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ryan RM, Keller RL, Poindexter BB, D’Angio CT, Shaw PA, Bellamy SL, et al. Respiratory medications in infants< 29 weeks during the first year postdischarge: the Prematurity and Respiratory Outcomes Program (PROP) consortium. J Pediatr . 2019;208:148–155, e3. doi: 10.1016/j.jpeds.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Um-Bergström P, Hallberg J, Pourbazargan M, Berggren-Broström E, Ferrara G, Eriksson MJ, et al. Pulmonary outcomes in adults with a history of bronchopulmonary dysplasia differ from patients with asthma. Respir Res . 2019;20:102. doi: 10.1186/s12931-019-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Halvorsen T, Skadberg BT, Eide GE, Røksund O, Aksnes L, Øymar K. Characteristics of asthma and airway hyper-responsiveness after premature birth. Pediatr Allergy Immunol . 2005;16:487–494. doi: 10.1111/j.1399-3038.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 43. Narang I, Rosenthal M, Cremonesini D, Silverman M, Bush A. Longitudinal evaluation of airway function 21 years after preterm birth. Am J Respir Crit Care Med . 2008;178:74–80. doi: 10.1164/rccm.200705-701OC. [DOI] [PubMed] [Google Scholar]
- 44. Hamon I, Varechova S, Vieux R, Ioan I, Bonabel C, Schweitzer C, et al. Exercise-induced bronchoconstriction in school-age children born extremely preterm. Pediatr Res . 2013;73:464–468. doi: 10.1038/pr.2012.202. [DOI] [PubMed] [Google Scholar]
- 45. Hysinger E, Friedman N, Jensen E, Zhang H, Piccione J. Bronchoscopy in neonates with severe bronchopulmonary dysplasia in the NICU. J Perinatol . 2019;39:263–268. doi: 10.1038/s41372-018-0280-y. [DOI] [PubMed] [Google Scholar]
- 46. Edwards MO, Kotecha SJ, Lowe J, Richards L, Watkins WJ, Kotecha S. Management of prematurity-associated wheeze and its association with atopy. PLoS One . 2016;11:e0155695. doi: 10.1371/journal.pone.0155695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Panitch HB, Keklikian EN, Motley RA, Wolfson MR, Schidlow DV. Effect of altering smooth muscle tone on maximal expiratory flows in patients with tracheomalacia. Pediatr Pulmonol . 1990;9:170–176. doi: 10.1002/ppul.1950090309. [DOI] [PubMed] [Google Scholar]
- 48. Pelkonen AS, Hakulinen AL, Turpeinen M. Bronchial lability and responsiveness in school children born very preterm. Am J Respir Crit Care Med . 1997;156:1178–1184. doi: 10.1164/ajrccm.156.4.9610028. [DOI] [PubMed] [Google Scholar]
- 49. Yuksel B, Greenough A, Maconochie I. Effective bronchodilator treatment by a simple spacer device for wheezy premature infants. Arch Dis Child . 1990;65:782–785. doi: 10.1136/adc.65.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kotecha SJ, Edwards MO, Watkins WJ, Lowe J, Henderson AJ, Kotecha S. Effect of bronchodilators on forced expiratory volume in 1 s in preterm-born participants aged 5 and over: a systematic review. Neonatology . 2015;107:231–240. doi: 10.1159/000371539. [DOI] [PubMed] [Google Scholar]
- 51. Robin B, Kim YJ, Huth J, Klocksieben J, Torres M, Tepper RS, et al. Pulmonary function in bronchopulmonary dysplasia. Pediatr Pulmonol . 2004;37:236–242. doi: 10.1002/ppul.10424. [DOI] [PubMed] [Google Scholar]
- 52. De Boeck K, Smith J, Van Lierde S, Devlieger H. Response to bronchodilators in clinically stable 1-year-old patients with bronchopulmonary dysplasia. Eur J Pediatr . 1998;157:75–79. doi: 10.1007/s004310050771. [DOI] [PubMed] [Google Scholar]
- 53. Shepherd EG, Clouse BJ, Hasenstab KA, Sitaram S, Malleske DT, Nelin LD, et al. Infant pulmonary function testing and phenotypes in severe bronchopulmonary dysplasia. Pediatrics . 2018;141:e20173350. doi: 10.1542/peds.2017-3350. [DOI] [PubMed] [Google Scholar]
- 54. Mallory GB, Jr, Chaney H, Mutich RL, Motoyama EK. Longitudinal changes in lung function during the first three years of premature infants with moderate to severe bronchopulmonary dysplasia. Pediatr Pulmonol . 1991;11:8–14. doi: 10.1002/ppul.1950110103. [DOI] [PubMed] [Google Scholar]
- 55. Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, et al. American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics . 2014;134:e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 56. Gadomski AM, Bhasale AL. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev . 2006:CD001266. doi: 10.1002/14651858.CD001266.pub2. [DOI] [PubMed] [Google Scholar]
- 57. Levin DL, Garg A, Hall LJ, Slogic S, Jarvis JD, Leiter JC. A prospective randomized controlled blinded study of three bronchodilators in infants with respiratory syncytial virus bronchiolitis on mechanical ventilation. Pediatr Crit Care Med . 2008;9:598–604. doi: 10.1097/PCC.0b013e31818c82b4. [DOI] [PubMed] [Google Scholar]
- 58. Brandt SJ, Perez L, Künzli N, Lurmann F, McConnell R. Costs of childhood asthma due to traffic-related pollution in two California communities. Eur Respir J . 2012;40:363–370. doi: 10.1183/09031936.00157811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McEvoy C, Venigalla S, Schilling D, Clay N, Spitale P, Nguyen T. Respiratory function in healthy late preterm infants delivered at 33-36 weeks of gestation. J Pediatr . 2013;162:464–469. doi: 10.1016/j.jpeds.2012.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Duijts L, van Meel ER, Moschino L, Baraldi E, Barnhoorn M, Bramer WM, et al. European Respiratory Society guideline on long-term management of children with bronchopulmonary dysplasia. Eur Respir J . 2020;55:1900788. doi: 10.1183/13993003.00788-2019. [DOI] [PubMed] [Google Scholar]
- 61.Pocket guide for asthma management and prevention. Global Initiative for Asthma; Fontana, WI: 2019. [accessed 2020 Dec 22]. Available from: https://ginasthma.org/pocket-guide-for-asthma-management-and-prevention/ [Google Scholar]
- 62. Kotecha S, Clemm H, Halvorsen T, Kotecha SJ. Bronchial hyper-responsiveness in preterm-born subjects: a systematic review and meta-analysis. Pediatr Allergy Immunol . 2018;29:715–725. doi: 10.1111/pai.12957. [DOI] [PubMed] [Google Scholar]
- 63.Ducharme FM, Tse SM, Chauhan B.Diagnosis, management, and prognosis of preschool wheeze Lancet 20143831593–1604.. [DOI] [PubMed] [Google Scholar]
- 64.Chong J, Haran C, Chauhan BF, Asher I.Intermittent inhaled corticosteroid therapy versus placebo for persistent asthma in children and adults. Cochrane Database Syst Rev . 2015. p. CD011032. [DOI] [PMC free article] [PubMed]
- 65. Fierro JL, Passarella M, Lorch SA. Prematurity as an independent risk factor for the development of pulmonary disease. J Pediatr . 2019;213:110–114. doi: 10.1016/j.jpeds.2019.05.066. [DOI] [PubMed] [Google Scholar]
- 66. Colice G, Wu EQ, Birnbaum H, Daher M, Maryna M. Use of inhaled corticosteroids and healthcare costs in mild persistent asthma. J Asthma . 2007;44:479–483. doi: 10.1080/02770900701424124. [DOI] [PubMed] [Google Scholar]
- 67. Beresford MW, Primhak R, Subhedar NV, Shaw NJ. Randomised double blind placebo controlled trial of inhaled fluticasone propionate in infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed . 2002;87:F62–F63. doi: 10.1136/fn.87.1.F62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kugelman A, Peniakov M, Zangen S, Shiff Y, Riskin A, Iofe A, et al. Inhaled hydrofluoalkane-beclomethasone dipropionate in bronchopulmonary dysplasia: a double-blind, randomized, controlled pilot study. J Perinatol . 2017;37:197–202. doi: 10.1038/jp.2016.177. [DOI] [PubMed] [Google Scholar]
- 69. Pelkonen AS, Hakulinen AL, Hallman M, Turpeinen M. Effect of inhaled budesonide therapy on lung function in schoolchildren born preterm. Respir Med . 2001;95:565–570. doi: 10.1053/rmed.2001.1104. [DOI] [PubMed] [Google Scholar]
- 70. Yuksel B, Greenough A. Randomised trial of inhaled steroids in preterm infants with respiratory symptoms at follow up. Thorax . 1992;47:910–913. doi: 10.1136/thx.47.11.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chan KN, Silverman M. Increased airway responsiveness in children of low birth weight at school age: effect of topical corticosteroids. Arch Dis Child . 1993;69:120–124. doi: 10.1136/adc.69.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Axelsson I, Naumburg E, Prietsch SO, Zhang L.Inhaled corticosteroids in children with persistent asthma: effects of different drugs and delivery devices on growth. Cochrane Database Syst Rev . 2019. p. CD010126. [DOI] [PMC free article] [PubMed]
- 73. Loke YK, Blanco P, Thavarajah M, Wilson AM. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS One . 2015;10:e0133428. doi: 10.1371/journal.pone.0133428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fernandes RM, Wingert A, Vandermeer B, Featherstone R, Ali S, Plint AC, et al. Safety of corticosteroids in young children with acute respiratory conditions: a systematic review and meta-analysis. BMJ Open . 2019;9:e028511. doi: 10.1136/bmjopen-2018-028511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adams NP, Bestall JC, Lasserson TJ, Jones PW.Inhaled fluticasone versus placebo for chronic asthma in adults and children Cochrane Database Syst Rev 2005CD003135. [Published update appears in Cochrane Database Syst Rev 2005:CD003135.] [DOI] [PubMed]
- 76. Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet . 1980;1:55–59. doi: 10.1016/s0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- 77. Enhorning G, Shennan A, Possmayer F, Dunn M, Chen CP, Milligan J. Prevention of neonatal respiratory distress syndrome by tracheal instillation of surfactant: a randomized clinical trial. Pediatrics . 1985;76:145–153. [PubMed] [Google Scholar]
- 78. Hallman M, Merritt TA, Jarvenpaa A-L, Boynton B, Mannino F, Gluck L, et al. Exogenous human surfactant for treatment of severe respiratory distress syndrome: a randomized prospective clinical trial. J Pediatr . 1985;106:963–969. doi: 10.1016/s0022-3476(85)80253-5. [DOI] [PubMed] [Google Scholar]
- 79. Hamvas A, Wise PH, Yang RK, Wampler NS, Noguchi A, Maurer MM, et al. The influence of the wider use of surfactant therapy on neonatal mortality among blacks and whites. N Engl J Med . 1996;334:1635–1640. doi: 10.1056/NEJM199606203342504. [DOI] [PubMed] [Google Scholar]
- 80. Jensen EA, White AM, Liu P, Yee K, Waber B, Monk HM, et al. Determinants of severe metabolic bone disease in very low-birth-weight infants with severe bronchopulmonary dysplasia admitted to a tertiary referral center. Am J Perinatol . 2016;33:107–113. doi: 10.1055/s-0035-1560043. [DOI] [PubMed] [Google Scholar]
- 81. Chang H-Y, Hsu C-H, Tsai J-D, Li S-T, Hung H-Y, Kao H-A, et al. Renal calcification in very low birth weight infants. Pediatr Neonatol . 2011;52:145–149. doi: 10.1016/j.pedneo.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 82. Gimpel C, Krause A, Franck P, Krueger M, von Schnakenburg C. Exposure to furosemide as the strongest risk factor for nephrocalcinosis in preterm infants. Pediatr Int . 2010;52:51–56. doi: 10.1111/j.1442-200X.2009.02886.x. [DOI] [PubMed] [Google Scholar]
- 83. Jacinto JS, Modanlou HD, Crade M, Strauss AA, Bosu SK. Renal calcification incidence in very low birth weight infants. Pediatrics . 1988;81:31–35. [PubMed] [Google Scholar]
- 84. Mohamed GB, Ibrahiem MA, Abdel Hameed WM. Nephrocalcinosis in pre-term neonates: a study of incidence and risk factors. Saudi J Kidney Dis Transpl . 2014;25:326–332. doi: 10.4103/1319-2442.128524. [DOI] [PubMed] [Google Scholar]
- 85. Saarela T, Vaarala A, Lanning P, Koivisto M. Incidence, ultrasonic patterns and resolution of nephrocalcinosis in very low birthweight infants. Acta Paediatr . 1999;88:655–660. doi: 10.1080/08035259950169332. [DOI] [PubMed] [Google Scholar]
- 86. Schell-Feith EA, Kist-van Holthe JE, Conneman N, van Zwieten PH, Holscher HC, Zonderland HM, et al. Etiology of nephrocalcinosis in preterm neonates: association of nutritional intake and urinary parameters. Kidney Int . 2000;58:2102–2110. doi: 10.1111/j.1523-1755.2000.00383.x. [DOI] [PubMed] [Google Scholar]
- 87. Short A, Cooke R. The incidence of renal calcification in preterm infants. Arch Dis Child . 1991;66:412–417. doi: 10.1136/adc.66.4_spec_no.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Albersheim SG, Solimano AJ, Sharma AK, Smyth JA, Rotschild A, Wood BJ, et al. Randomized, double-blind, controlled trial of long-term diuretic therapy for bronchopulmonary dysplasia. J Pediatr . 1989;115:615–620. doi: 10.1016/s0022-3476(89)80297-5. [DOI] [PubMed] [Google Scholar]
- 89. Kao LC, Durand DJ, McCrea RC, Birch M, Powers RJ, Nickerson BG. Randomized trial of long-term diuretic therapy for infants with oxygen-dependent bronchopulmonary dysplasia. J Pediatr . 1994;124:772–781. doi: 10.1016/s0022-3476(05)81373-3. [DOI] [PubMed] [Google Scholar]
- 90. Rush MG, Engelhardt B, Parker RA, Hazinski TA. Double-blind, placebo-controlled trial of alternate-day furosemide therapy in infants with chronic bronchopulmonary dysplasia. J Pediatr . 1990;117:112–118. doi: 10.1016/s0022-3476(05)82458-8. [DOI] [PubMed] [Google Scholar]
- 91. McCann EM, Lewis K, Deming DD, Donovan MJ, Brady JP. Controlled trial of furosemide therapy in infants with chronic lung disease. J Pediatr . 1985;106:957–962. doi: 10.1016/s0022-3476(85)80252-3. [DOI] [PubMed] [Google Scholar]
- 92. Halliday HL. History of surfactant from 1980. Biol Neonate . 2005;87:317–322. doi: 10.1159/000084879. [DOI] [PubMed] [Google Scholar]
- 93. Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease: bronchopulmonary dysplasia. N Engl J Med . 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 94. Rastogi S, Mikhael M, Filipov P, Rastogi D. Effects of ventilation on hearing loss in preterm neonates: nasal continuous positive pressure does not increase the risk of hearing loss in ventilated neonates. Int J Pediatr Otorhinolaryngol . 2013;77:402–406. doi: 10.1016/j.ijporl.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 95. Ertl T, Hadzsiev K, Vincze O, Pytel J, Szabo I, Sulyok E. Hyponatremia and sensorineural hearing loss in preterm infants. Biol Neonate . 2001;79:109–112. doi: 10.1159/000047076. [DOI] [PubMed] [Google Scholar]
- 96. Borradori C, Fawer C-L, Buclin T, Calame A. Risk factors of sensorineural hearing loss in preterm infants. Biol Neonate . 1997;71:1–10. doi: 10.1159/000244391. [DOI] [PubMed] [Google Scholar]
- 97. Wang LA, Smith PB, Laughon M, Goldberg RN, Ku LC, Zimmerman KO, et al. Best Pharmaceuticals for Children Act—Pediatric Trials Network Steering Committee. Prolonged furosemide exposure and risk of abnormal newborn hearing screen in premature infants. Early Hum Dev . 2018;125:26–30. doi: 10.1016/j.earlhumdev.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rais-Bahrami K, Majd M, Veszelovszky E, Short BL. Use of furosemide and hearing loss in neonatal intensive care survivors. Am J Perinatol . 2004;21:329–332. doi: 10.1055/s-2004-831887. [DOI] [PubMed] [Google Scholar]
- 99. Coenraad S, Goedegebure A, van Goudoever JB, Hoeve LJ. Risk factors for auditory neuropathy spectrum disorder in NICU infants compared to normal-hearing NICU controls. Laryngoscope . 2011;121:852–855. doi: 10.1002/lary.21430. [DOI] [PubMed] [Google Scholar]
- 100. Narendra A, White MP, Rolton HA, Alloub ZI, Wilkinson G, McColl JH, et al. Nephrocalcinosis in preterm babies. Arch Dis Child Fetal Neonatal Ed . 2001;85:F207–F213. doi: 10.1136/fn.85.3.F207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kapur N, Nixon G, Robinson P, Massie J, Prentice B, Wilson A, et al. Respiratory management of infants with chronic neonatal lung disease beyond the NICU: a position statement from the Thoracic Society of Australia and New Zealand. Respirology . 2020;25:880–888. doi: 10.1111/resp.13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dransfield DA, Spitzer AR, Fox WW. Episodic airway obstruction in premature infants. Am J Dis Child . 1983;137:441–443. doi: 10.1001/archpedi.1983.02140310023005. [DOI] [PubMed] [Google Scholar]
- 103. Di Fiore JM, Arko MK, Miller MJ, Krauss A, Betkerur A, Zadell A, et al. Cardiorespiratory events in preterm infants referred for apnea monitoring studies. Pediatrics . 2001;108:1304–1308. doi: 10.1542/peds.108.6.1304. [DOI] [PubMed] [Google Scholar]
- 104. Sekar KC, Duke JC. Sleep apnea and hypoxemia in recently weaned premature infants with and without bronchopulmonary dysplasia. Pediatr Pulmonol . 1991;10:112–116. doi: 10.1002/ppul.1950100213. [DOI] [PubMed] [Google Scholar]
- 105. Simakajornboon N, Beckerman RC, Mack C, Sharon D, Gozal D. Effect of supplemental oxygen on sleep architecture and cardiorespiratory events in preterm infants. Pediatrics . 2002;110:884–888. doi: 10.1542/peds.110.5.884. [DOI] [PubMed] [Google Scholar]
- 106. Crump C, Friberg D, Li X, Sundquist J, Sundquist K. Preterm birth and risk of sleep-disordered breathing from childhood into mid-adulthood. Int J Epidemiol . 2019;48:2039–2049. doi: 10.1093/ije/dyz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sharma PB, Baroody F, Gozal D, Lester LA. Obstructive sleep apnea in the formerly preterm infant: an overlooked diagnosis. Front Neurol . 2011;2:73. doi: 10.3389/fneur.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr . 2003;142:383–389. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 109. Elder DE, Whale J, Galletly D, Campbell AJ. Respiratory events in preterm infants prior to discharge: with and without clinically concerning apnoea. Sleep Breath . 2011;15:867–873. doi: 10.1007/s11325-010-0457-x. [DOI] [PubMed] [Google Scholar]
- 110. Fajardo C, Alvarez J, Wong A, Kwiatkowski K, Rigatto H. The incidence of obstructive apneas in preterm infants with and without bronchopulmonary dysplasia. Early Hum Dev . 1993;32:197–206. doi: 10.1016/0378-3782(93)90012-j. [DOI] [PubMed] [Google Scholar]
- 111. Bhat RY, Hannam S, Pressler R, Rafferty GF, Peacock JL, Greenough A. Effect of prone and supine position on sleep, apneas, and arousal in preterm infants. Pediatrics . 2006;118:101–107. doi: 10.1542/peds.2005-1873. [DOI] [PubMed] [Google Scholar]
- 112. Kulkarni G, de Waal K, Grahame S, Collison A, Roddick L, Hilton J, et al. Polysomnography for the management of oxygen supplementation therapy in infants with chronic lung disease of prematurity. J Matern Fetal Neonatal Med . 2019;32:3640–3646. doi: 10.1080/14767058.2018.1470234. [DOI] [PubMed] [Google Scholar]
- 113. Ortiz LE, McGrath-Morrow SA, Sterni LM, Collaco JM. Sleep disordered breathing in bronchopulmonary dysplasia. Pediatr Pulmonol . 2017;52:1583–1591. doi: 10.1002/ppul.23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. McGrath-Morrow SA, Ryan T, McGinley BM, Okelo SO, Sterni LM, Collaco JM. Polysomnography in preterm infants and children with chronic lung disease. Pediatr Pulmonol . 2012;47:172–179. doi: 10.1002/ppul.21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hibbs AM, Johnson NL, Rosen CL, Kirchner HL, Martin R, Storfer-Isser A, et al. Prenatal and neonatal risk factors for sleep disordered breathing in school-aged children born preterm. J Pediatr . 2008;153:176–182. doi: 10.1016/j.jpeds.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Roberts T, Campbell A, Larsen P, Elder D. Preterm infants at discharge: nap polysomnography versus 24-hour oximetry. Acta Paediatr . 2017;106:1754–1759. doi: 10.1111/apa.13900. [DOI] [PubMed] [Google Scholar]
- 117. Moyer-Mileur LJ, Nielson DW, Pfeffer KD, Witte MK, Chapman DL. Eliminating sleep-associated hypoxemia improves growth in infants with bronchopulmonary dysplasia. Pediatrics . 1996;98:779–783. [PubMed] [Google Scholar]
- 118. Vermeulen MJ, Weening FT, Battistutta D, Masters IB. Awake daytime oximetry measurements in the management of infants with chronic lung disease. J Paediatr Child Health . 1999;35:553–557. doi: 10.1046/j.1440-1754.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- 119. Kirk V, Baughn J, D’Andrea L, Friedman N, Galion A, Garetz S, et al. American Academy of Sleep Medicine position paper for the use of a home sleep apnea test for the diagnosis of OSA in children. J Clin Sleep Med . 2017;13:1199–1203. doi: 10.5664/jcsm.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics . 2012;130:e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 121. Walter RJ, Hagedorn SI, Lettieri CJ. Impact of diagnosing and treating obstructive sleep apnea on healthcare utilization. Sleep Med . 2017;38:73–77. doi: 10.1016/j.sleep.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 122. Hayes D, Jr, Wilson KC, Krivchenia K, Hawkins SMM, Balfour-Lynn IM, Gozal D, et al. Home oxygen therapy for children: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med . 2019;199:e5–e23. doi: 10.1164/rccm.201812-2276ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Balfour-Lynn IM, Field DJ, Gringras P, Hicks B, Jardine E, Jones RC, et al. Paediatric Section of the Home Oxygen Guideline Development Group of the BTS Standards of Care Committee. BTS guidelines for home oxygen in children. Thorax . 2009;64:ii1–ii26. doi: 10.1136/thx.2009.116020. [DOI] [PubMed] [Google Scholar]
- 124.Collaco JM, McGrath-Morrow SA.editors. Pediatric swallowing and feeding: assessment and management. In: Arvedson JC, Brodsky L, Lefton-Greif MA, editors. San Diego, CA: Plural Publishing; 2020. Pulmonary manifestations and management considerations for aspiration; pp. 453–478. [Google Scholar]
- 125.Newman LA, Cleveland RH, Blickman JG, Hillman RE, Jaramillo D.Videofluoroscopic analysis of the infant swallow Invest Radiol 199126870–873.. [DOI] [PubMed] [Google Scholar]
- 126. Khoshoo V, Edell D. Previously healthy infants may have increased risk of aspiration during respiratory syncytial viral bronchiolitis. Pediatrics . 1999;104:1389–1390. doi: 10.1542/peds.104.6.1389. [DOI] [PubMed] [Google Scholar]
- 127. Adil E, Gergin O, Kawai K, Rahbar R, Watters K. Usefulness of upper airway endoscopy in the evaluation of pediatric pulmonary aspiration. JAMA Otolaryngol Head Neck Surg . 2016;142:339–343. doi: 10.1001/jamaoto.2015.3923. [DOI] [PubMed] [Google Scholar]
- 128. Li AM, Sonnappa S, Lex C, Wong E, Zacharasiewicz A, Bush A, et al. Non-CF bronchiectasis: does knowing the aetiology lead to changes in management? Eur Respir J . 2005;26:8–14. doi: 10.1183/09031936.05.00127704. [DOI] [PubMed] [Google Scholar]
- 129. Krishnan U, Feinstein JA, Adatia I, Austin ED, Mullen MP, Hopper RK, et al. Pediatric Pulmonary Hypertension Network (PPHNet) Evaluation and management of pulmonary hypertension in children with bronchopulmonary dysplasia. J Pediatr . 2017;188:24–34, e1. doi: 10.1016/j.jpeds.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 130. Coon ER, Srivastava R, Stoddard GJ, Reilly S, Maloney CG, Bratton SL. Infant videofluoroscopic swallow study testing, swallowing interventions, and future acute respiratory illness. Hosp Pediatr . 2016;6:707–713. doi: 10.1542/hpeds.2016-0049. [DOI] [PubMed] [Google Scholar]
- 131. Duncan DR, Mitchell PD, Larson K, Rosen RL. Presenting signs and symptoms do not predict aspiration risk in children. J Pediatr . 2018;201:141–146. doi: 10.1016/j.jpeds.2018.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Irace AL, Dombrowski ND, Kawai K, Dodrill P, Perez J, Hernandez K, et al. Aspiration in children with unilateral vocal fold paralysis. Laryngoscope . 2019;129:569–573. doi: 10.1002/lary.27410. [DOI] [PubMed] [Google Scholar]
- 133. Mercado-Deane M-G, Burton EM, Harlow SA, Glover AS, Deane DA, Guill MF, et al. Swallowing dysfunction in infants less than 1 year of age. Pediatr Radiol . 2001;31:423–428. doi: 10.1007/s002470100456. [DOI] [PubMed] [Google Scholar]
- 134. Uhm KE, Yi SH, Chang HJ, Cheon HJ, Kwon JY. Videofluoroscopic swallowing study findings in full-term and preterm infants with dysphagia. Ann Rehabil Med . 2013;37:175–182. doi: 10.5535/arm.2013.37.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rommel N, De Meyer A-M, Feenstra L, Veereman-Wauters G. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J Pediatr Gastroenterol Nutr . 2003;37:75–84. doi: 10.1097/00005176-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 136. Silva-Munhoz LdeF, Bühler KE. Fluoroscopic findings of swallowing: comparison between preterm and full-term infants. J Soc Bras Fonoaudiol . 2011;23:206–213. doi: 10.1590/s2179-64912011000300005. [DOI] [PubMed] [Google Scholar]
- 137. Ren CL, Esther CR, Jr, Debley JS, Sockrider M, Yilmaz O, Amin N, et al. ATS Ad Hoc Committee on Infants with Recurrent or Persistent Wheezing. Official American Thoracic Society clinical practice guidelines: diagnostic evaluation of infants with recurrent or persistent wheezing. Am J Respir Crit Care Med . 2016;194:356–373. doi: 10.1164/rccm.201604-0694ST. [DOI] [PubMed] [Google Scholar]
- 138. Miller RW, Woo P, Kellman RK, Slagle TS. Tracheobronchial abnormalities in infants with bronchopulmonary dysplasia. J Pediatr . 1987;111:779–782. doi: 10.1016/s0022-3476(87)80267-6. [DOI] [PubMed] [Google Scholar]
- 139. Doull IJ, Mok Q, Tasker RC. Tracheobronchomalacia in preterm infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed . 1997;76:F203–F205. doi: 10.1136/fn.76.3.f203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hysinger EB, Friedman NL, Padula MA, Shinohara RT, Zhang H, Panitch HB, et al. Children’s Hospitals Neonatal Consortium. Tracheobronchomalacia is associated with increased morbidity in bronchopulmonary dysplasia. Ann Am Thorac Soc . 2017;14:1428–1435. doi: 10.1513/AnnalsATS.201702-178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Downing GJ, Hayen LK, Kilbride HW. Acquired subglottic cysts in the low-birth-weight infant: characteristics, treatment, and outcome. Am J Dis Child . 1993;147:971–974. doi: 10.1001/archpedi.1993.02160330061020. [DOI] [PubMed] [Google Scholar]
- 142. Downing GJ, Kilbride HW. Evaluation of airway complications in high-risk preterm infants: application of flexible fiberoptic airway endoscopy. Pediatrics . 1995;95:567–572. [PubMed] [Google Scholar]
- 143. Kercsmar CM, Martin RJ, Chatburn RL, Carlo WA. Bronchoscopic findings in infants treated with high-frequency jet ventilation versus conventional ventilation. Pediatrics . 1988;82:884–887. [PubMed] [Google Scholar]
- 144. Henry BM, Hsieh WC, Sanna B, Vikse J, Taterra D, Tomaszewski KA. Incidence, risk factors, and comorbidities of vocal cord paralysis after surgical closure of a patent ductus arteriosus: a meta-analysis. Pediatr Cardiol . 2019;40:116–125. doi: 10.1007/s00246-018-1967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Benjamin JR, Smith PB, Cotten CM, Jaggers J, Goldstein RF, Malcolm WF. Long-term morbidities associated with vocal cord paralysis after surgical closure of a patent ductus arteriosus in extremely low birth weight infants. J Perinatol . 2010;30:408–413. doi: 10.1038/jp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Clement WA, El-Hakim H, Phillipos EZ, Coté JJ. Unilateral vocal cord paralysis following patent ductus arteriosus ligation in extremely low-birth-weight infants. Arch Otolaryngol Head Neck Surg . 2008;134:28–33. doi: 10.1001/archoto.2007.2. [DOI] [PubMed] [Google Scholar]
- 147. Malcolm WF, Hornik C, Evans A, Smith PB, Cotten CM. Vocal fold paralysis following surgical ductal closure in extremely low birth weight infants: a case series of feeding and respiratory complications. J Perinatol . 2008;28:782–785. doi: 10.1038/jp.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. DeBoer EM, Prager JD, Kerby GS, Stillwell PC. Measuring pediatric bronchoscopy outcomes using an electronic medical record. Ann Am Thorac Soc . 2016;13:678–683. doi: 10.1513/AnnalsATS.201509-576OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Shaffer TH, Wolfson MR, Panitch HB. Airway structure, function and development in health and disease. Paediatr Anaesth . 2004;14:3–14. doi: 10.1046/j.1460-9592.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 150. Bamat NA, Zhang H, McKenna KJ, Morris H, Stoller JZ, Gibbs K. The clinical evaluation of severe bronchopulmonary dysplasia. Neoreviews . 2020;21:e442–e453. doi: 10.1542/neo.21-7-e442. [DOI] [PubMed] [Google Scholar]
- 151. Hysinger EB, Hart CK, Burg G, De Alarcon A, Benscoter D. Differences in flexible and rigid bronchoscopy for assessment of tracheomalacia. Laryngoscope . 2021;131:201–204. doi: 10.1002/lary.28656. [DOI] [PubMed] [Google Scholar]
- 152. Mok Q, Negus S, McLaren CA, Rajka T, Elliott MJ, Roebuck DJ, et al. Computed tomography versus bronchography in the diagnosis and management of tracheobronchomalacia in ventilator dependent infants. Arch Dis Child Fetal Neonatal Ed . 2005;90:F290–F293. doi: 10.1136/adc.2004.062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Greenberg SB. Dynamic pulmonary CT of children. AJR Am J Roentgenol . 2012;199:435–440. doi: 10.2214/AJR.11.8014. [DOI] [PubMed] [Google Scholar]
- 154. Lee S, Im SA, Yoon JS. Tracheobronchomalacia in infants: the use of non-breath held 3D CT bronchoscopy. Pediatr Pulmonol . 2014;49:1028–1035. doi: 10.1002/ppul.22931. [DOI] [PubMed] [Google Scholar]
- 155. Tan JZ, Crossett M, Ditchfield M. Dynamic volumetric computed tomographic assessment of the young paediatric airway: initial experience of rapid, non-invasive, four-dimensional technique. J Med Imaging Radiat Oncol . 2013;57:141–148. doi: 10.1111/1754-9485.12009. [DOI] [PubMed] [Google Scholar]
- 156. Ullmann N, Secinaro A, Menchini L, Caggiano S, Verrillo E, Santangelo TP, et al. Dynamic expiratory CT: an effective non-invasive diagnostic exam for fragile children with suspected tracheo-bronchomalacia. Pediatr Pulmonol . 2018;53:73–80. doi: 10.1002/ppul.23831. [DOI] [PubMed] [Google Scholar]
- 157. Douros K, Kremmydas G, Grammeniatis V, Papadopoulos M, Priftis KN, Alexopoulou E. Helical multi-detector CT scan as a tool for diagnosing tracheomalacia in children. Pediatr Pulmonol . 2019;54:47–52. doi: 10.1002/ppul.24188. [DOI] [PubMed] [Google Scholar]
- 158. Goo HW. Free-breathing cine CT for the diagnosis of tracheomalacia in young children. Pediatr Radiol . 2013;43:922–928. doi: 10.1007/s00247-013-2637-x. [DOI] [PubMed] [Google Scholar]
- 159. Bates AJ, Higano NS, Hysinger EB, Fleck RJ, Hahn AD, Fain SB, et al. Quantitative assessment of regional dynamic airway collapse in neonates via retrospectively respiratory-gated 1H ultrashort echo time MRI. J Magn Reson Imaging . 2019;49:659–667. doi: 10.1002/jmri.26296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hysinger EB, Bates AJ, Higano NS, Benscoter D, Fleck RJ, Hart CK, et al. Ultrashort echo-time MRI for the assessment of tracheomalacia in neonates. Chest . 2020;157:595–602. doi: 10.1016/j.chest.2019.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kim HJ, Yoo SY, Jeon TY, Kim JH. Model-based iterative reconstruction in ultra-low-dose pediatric chest CT: comparison with adaptive statistical iterative reconstruction. Clin Imaging . 2016;40:1018–1022. doi: 10.1016/j.clinimag.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 162. Kino A, Zucker EJ, Honkanen A, Kneebone J, Wang J, Chan F, et al. Ultrafast pediatric chest computed tomography: comparison of free-breathing vs. breath-hold imaging with and without anesthesia in young children. Pediatr Radiol . 2019;49:301–307. doi: 10.1007/s00247-018-4295-5. [DOI] [PubMed] [Google Scholar]
- 163. Shi JW, Xu DF, Dai HZ, Shen L, Ji YD. Evaluation of chest CT scan in low-weight children with ultralow tube voltage (70 kVp) combined with Flash scan technique. Br J Radiol . 2016;89:20150184. doi: 10.1259/bjr.20150184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Villanueva-Meyer JE, Naeger DM, Courtier JL, Hope MD, Lambert JW, MacKenzie JD, et al. Pediatric chest CT at chest radiograph doses: when is the ultralow-dose chest CT clinically appropriate? Emerg Radiol . 2017;24:369–376. doi: 10.1007/s10140-017-1487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Westra SJ. High-pitch CT, decreasing need for sedation and its potential side effects: some practical considerations and future directions. Pediatr Radiol . 2019;49:297–300. doi: 10.1007/s00247-018-4314-6. [DOI] [PubMed] [Google Scholar]
- 166. Zhu Y, Li Z, Ma J, Hong Y, Pi Z, Qu X, et al. Imaging the infant chest without sedation: feasibility of using single axial rotation with 16-cm wide-detector CT. Radiology . 2018;286:279–285. doi: 10.1148/radiol.2017170019. [DOI] [PubMed] [Google Scholar]
- 167. Doyle LW, Andersson S, Bush A, Cheong JLY, Clemm H, Evensen KAI, et al. Adults Born Preterm International Collaboration. Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with controls born at term or with normal birthweight: a meta-analysis of individual participant data. Lancet Respir Med . 2019;7:677–686. doi: 10.1016/S2213-2600(18)30530-7. [DOI] [PubMed] [Google Scholar]


