Abstract
Introduction
Blood metabolomics‐based biomarkers may be useful to predict measures of neurocognitive aging.
Methods
We tested the association between 707 blood metabolites measured in 1451 participants from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), with mild cognitive impairment (MCI) and global cognitive change assessed 7 years later. We further used Lasso penalized regression to construct a metabolomics risk score (MRS) that predicts MCI, potentially identifying a different set of metabolites than those discovered in individual‐metabolite analysis.
Results
We identified 20 metabolites predicting prevalent MCI and/or global cognitive change. Six of them were novel and 14 were previously reported as associated with neurocognitive aging outcomes. The MCI MRS comprised 61 metabolites and improved prediction accuracy from 84% (minimally adjusted model) to 89% in the entire dataset and from 75% to 87% among apolipoprotein E ε4 carriers.
Discussion
Blood metabolites may serve as biomarkers identifying individuals at risk for MCI among US Hispanics/Latinos.
Keywords: global cognitive change, Hispanics/Latinos, metabolite biomarkers, metabolomic risk score, mild cognitive impairment, risk prediction
1. INTRODUCTION
Hispanics/Latinos in the United States are at higher risk for mild cognitive impairment (MCI) and Alzheimer's disease and related dementias (ADRD), compared to non‐Hispanic Whites, and are a rapidly growing ethnic population in the United States. 1 Various risk factors may explain the rate differences between populations, including genetic susceptibility, health conditions, lifestyle, and environmental factors. 2 For example, cardiovascular disease and diabetes are more prevalent in Hispanics/Latinos compared to non‐Hispanic Whites and are known to increase the risk for ADRD. On the other hand, the strongest known genetic risk factor for ADRD, apolipoprotein E (APOE) ε4, demonstrates a weaker association in Hispanics/Latinos, compared to other populations. 3
MCI is the stage between the expected cognitive decline of normal aging and the development of dementia, 4 although some individuals with MCI may revert to normal. 5 Individuals diagnosed with MCI experience mild changes in thinking, memory, language, and/or judgment abilities greater than age‐related expected changes. MCI can result from a variety of etiologies, some of which are reversible, and are affected by medications, injuries, sleep, exercise, education, and diet. 6 These MCI risk factors may lead to metabolic dysregulation. In the last decade, metabolome assessment has emerged as a new approach for biomarker discovery, and for evaluating the progress of disease and its underlying pathophysiology. 7 Recent studies have demonstrated risk prediction of MCI and ADRD based on blood metabolite biomarkers in prospective studies. 8 , 9 For example, higher plasma anthranilic acid levels were associated with a greater risk of dementia in the Framingham Offspring Study. 10 Thus, metabolome screening in middle‐aged adults can detect plausible biomarkers that may improve risk prediction for MCI and can facilitate modifiable interventions at earlier stages of the disease. 11 , 12 However, most studies were conducted in non‐Hispanic US populations, 8 and metabolite associations may not generalize between populations. In one study, 10 prospectively predictive phospholipids metabolites for MCI or dementia found in non‐Hispanic Whites failed replication in a Black cohort. 13 Another study in European Americans 12 replicated only one (out of seven) prospective predictive metabolite for dementia previously found in Blacks. Given the differences in risk factors and rates of cognitive decline and MCI across populations, we hypothesized that optimal metabolomics biomarkers in Hispanics/Latinos may differ from those in other populations.
We examined the associations of blood metabolites with MCI and global cognitive change over an average 7‐year follow‐up period among middle‐aged and older adults in the Study of Latinos‐Investigation of Neurocognitive Aging (SOL‐INCA). 14 SOL‐INCA is an ancillary study to the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). The analysis steps are described in Figure 1. We hypothesized that there are metabolites associated with MCI and cognitive decline in Hispanics/Latinos. We first tested the association of 707 metabolites with MCI and global cognitive change (Step A). We assessed the single‐metabolite associations based on previous literature (Step B) and generalization to Europeans and Blacks from the Atherosclerosis Risk in Communities (ARIC) study using a proxy‐trait approach by testing metabolite associations with change in cognitive tests (Step C). Next, we used a Lasso regression to construct a metabolite risk score (MRS, Step D). An MRS is a combined metabolomics measure, integrating information across multiple metabolites at once. Therefore, an MRS can potentially serve as a better biomarker of the metabolic environment related to a specific outcome compared to individual metabolites. Finally, we also explored whether previously reported metabolite associations with cognitive function generalize to the Hispanics/Latinos in the SOL‐INCA target population (Step E).
FIGURE 1.
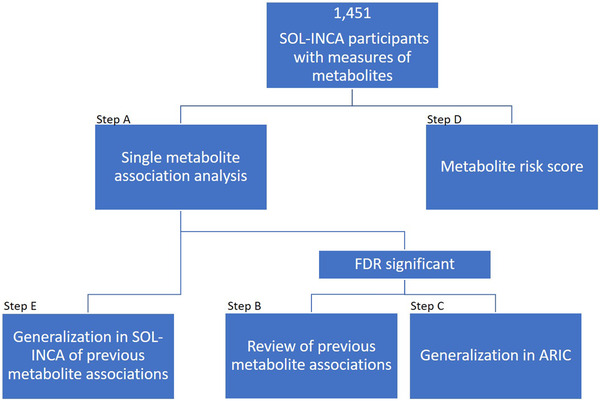
Metabolite—neurocognitive outcomes analyses flowchart in the Study of Latinos‐Investigation of Neurocognitive Aging (SOL‐INCA) analytic dataset. Step (A), association analysis between single metabolites and neurocognitive outcomes. Step (B), review of previous literature reporting the metabolites with false discovery rate (FDR)‐significant associations. Step (C), generalization of the FDR‐significant metabolite associations in the Atherosclerosis Risk in Communities (ARIC) dataset. Step (D), construction of a metabolite risk score (MRS) for neurocognitive outcomes. Step (E), generalization of previously reported metabolite‐neurocognitive associations in SOL‐INCA
2. METHODS
2.1. Study population
The HCHS/SOL is a US population‐based longitudinal cohort study with 16,415 Hispanic/Latino participants recruited from four field centers (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA, USA) by a sampling design previously described. 15 , 16 Adults ages 18 to 74 years old were recruited during the first visit, between 2008 and 2011, and various biospecimen and health measures were collected. At baseline, cognitive function was assessed in 9714 individuals aged 45 years or older. The baseline cognitive battery included the Six‐Item Screener (SIS; mental status), 17 Brief‐Spanish English Verbal Learning Test (B‐SEVLT; verbal episodic learning and memory), 18 Word Fluency (WF; verbal functioning), 19 and Digit Symbol Substitution test (DSS; processing speed, executive function). 20 SOL‐INCA is an ancillary study of HCHS/SOL, focusing on the middle‐aged and older adult group who underwent cognitive assessment at Visit 1. Overall, 6377 individuals 50 years or older with baseline cognitive testing participated in the SOL‐INCA examination, taking place at or after HCHS/SOL Visit 2, with an average of 7 years since Visit 1. Metabolites were measured in serum, after fasting, on a random subset of 3978 from HCHS/SOL participants from Visit 1. The current study population consists of 1451 individuals who participated in SOL‐INCA and additionally have measures of metabolites.
All study participants provided written informed consent in their preferred language (Spanish/English) to use their genetic and non‐genetic data. The study was reviewed and approved by the institutional review boards at all collaborating institutions.
2.2. Neurocognitive outcomes
We studied two primary neurocognitive outcomes: MCI at the SOL‐INCA visit, and global cognitive change in the 7‐year follow‐up between the HCHS/SOL Visit 1 and the SOL‐INCA visit. Individuals were classified with MCI according to National Institute on Aging–Alzheimer's Association criteria. 21 Details about the SOL‐INCA MCI diagnostic operational procedures have been previously published. 14 , 22 The global cognitive change is a continuous measure constructed using a confirmatory latent factor model based on the HCHS/SOL baseline and SOL‐INCA cognitive function tests, as previously described. 14 We performed secondary analyses in which we studied the association between the top identified metabolites and change in cognitive function measured by three cognitive tests. These cognitive tests correspond to different domains: B‐SEVLT for new learning and verbal memory, WF for verbal fluency, and DSS for psychomotor speed and sustained attention. 21 The changes were computed as the difference between the test scores in the SOL‐INCA and Visit 1. These associations were used for the analysis in Figure 1, Step C, as described later.
HIGHLIGHTS
Blood metabolomic biomarkers may predict measures of neurocognitive aging.
Metabolite levels are affected by both genetics and lifestyle.
Optimal metabolomics biomarkers may differ among population groups.
We identified metabolites associated with neurocognitive aging in US Hispanics/Latinos.
Metabolite risk score improved the prediction of mild cognitive impairment in this population.
RESEARCH IN CONTEXT
Systematic Review: The authors reviewed the literature using traditional sources. In the last decade, several studies have demonstrated risk prediction of early neurocognitive aging outcomes, such as mild cognitive impairment (MCI), by testing blood metabolites. However, these predictions may not generalize between populations, and only a few studies were conducted in the US Hispanic/Latino population. Publications are appropriately cited.
Interpretation: We identified novel metabolite associations and replicated several previously published metabolite associations. We also constructed a metabolomic risk score, improving the accuracy of MCI prediction models in Hispanics/Latinos.
Future Directions: Future studies are needed to confirm the effectiveness of metabolomic biomarkers in the prediction of MCI, and compare the metabolite biomarkers across diverse populations.
2.3. Metabolomics measurement and processing
Metabolites were measured in serum of blood drawn after at least 8 hours of overnight fasting. Profiling was done using untargeted liquid chromatography‐mass spectrometry (LC‐MS) using the discovery HD4 platform in 2017 at Metabolon Inc. (Durham, NC). Of 1136 metabolites quantified, 784 were identified as known compounds, and 352 were unidentified. 23 Further details on metabolites’ quality control are provided in Figure S1 in supporting information. Overall, we tested 707 metabolites in this study.
2.4. Statistical analysis
We characterized the target population that the sample represents using weighted analysis, stratified by MCI. The weights are used to obtain estimates of characteristics generalizable to the target population of SOL‐INCA. A detailed discussion of the sampling design, including the generation and use of weights for the HCHS/SOL, was previously published. 15 , 16
2.5. Single metabolite association analyses
We tested the associations of each metabolite with each of the neurocognitive outcomes accounting for the complex survey design of the data using the R “survey” package, 24 with a “quasi‐Poisson” family for a binary outcome (Figure 1, Step A). We used three nested regression models, the first with basic adjustment for sex, age, study center, self‐reported background (Dominican, Central American, Cuban, Mexican, Puerto Rican, South American, or more than one/other heritage), education, and years between HCHS/SOL Visit 1 and the SOL‐INCA visit. The second model with further adjustment for health measures including body mass index (BMI), estimated glomerular filtration rate (eGFR, estimated from serum creatinine using the CKD‐EPI equation), type II diabetes (T2D), and hypertension. The third model with further adjustment for APOE ɛ4 carrier status (dominant mode), and lifestyle variables including alcohol consumption, and smoking status. We also stratified models 1 and 2 by APOE ɛ4 allele carrier status. The significance threshold was false discovery rate (FDR) adjusted P‐value < .05 (q‐value) computed in each analysis separately. Biological pathways for metabolites were provided by Metabolon's annotation file.
2.6. Evidence from previous publications for single‐metabolite–identified associations
For each metabolite significantly associated with MCI or global cognitive change after multiple‐testing correction, we searched for relevant published associations in Google Scholar, PubMed, PubChem, and The Human Metabolome Database (HMDB). Keywords included in the search: the name/identification (eg., HMDB ID) of the metabolite, “cognitive function,” “dementia,” “Alzheimer's disease (AD)” (Figure 1, Step B).
2.7. Generalization analysis of metabolite associations in the Atherosclerosis Risk in Communities (ARIC) study
We evaluated the generalizability of the discovered metabolite associations in the ARIC study (Figure 1, Step C). ARIC is a longitudinal cohort study with cognitive measures and metabolite profiling based on a similar Metabolon platform. ARIC did not have equivalent measures of MCI and global cognitive change; therefore, we used a proxy‐generalization approach testing the metabolites’ associations with change in cognitive test results. Further details are provided in the supporting information.
2.8. Metabolic risk score for MCI
We performed Lasso‐penalized regression to optimally select and estimate the joint effect of multiple metabolites that together predict MCI in the HCHS/SOL analytic sample (Step D in Figure 1). 25 Based on the selected metabolites and their estimated effects, we constructed an MRS for MCI. Further details are provided in the supporting information.
2.9. Generalization of previously reported metabolite associations with neurocognitive outcomes in SOL‐INCA
We identified two manuscripts reporting associations of metabolites with MCI and AD, using a similar Metabolon platform. 12 , 26 To study whether their reported associations generalize to associations with MCI and global cognitive change in Hispanics/Latinos, we inspected the metabolites they reported in our single‐metabolite association analyses (Figure 1, Step E).
2.10. Data availability
HCHS/SOL and SOL‐INCA data can be obtained from dbGaP under accession number phs000810.v1.p1. Summary statistics from association analyses of all metabolites and all studied phenotypes are available in a GitHub repository. In addition, code for constructing the MCI MRS based on the Lasso‐selected metabolites is provided in the same GitHub repository.
3. RESULTS
Table 1 characterizes demographic, health, and lifestyle characteristics of the study population, stratified by MCI status. Overall, our dataset included 1451 individuals (558 men, 893 women), with a weighted mean age of 56 years at Visit 1.
TABLE 1.
Demographics, health, and lifestyle characteristics of the study population by MCI status
| Without MCI | With MCI | Total | |
|---|---|---|---|
| Sample size | 1,286 | 165 | 1,451 |
| Age at visit 1 (mean [SD]) | 54.33 (7.05) | 58.12 (8.01) | 56.03 (8.18) |
| Sex = M (%) | 506 (49.1) | 52 (40.6) | 558 (48.1) |
| Study center (%) | |||
| Bronx | 330 (30.0) | 40 (25.8) | 370 (29.5) |
| Chicago | 266 (10.1) | 38 (12.2) | 304 (10.3) |
| Miami | 386 (37.0) | 45 (41.4) | 431 (37.5) |
| San Diego | 304 (23.0) | 42 (20.6) | 346 (22.7) |
| Self‐reported background (%) | |||
| Dominican | 139 (11.6) | 16 (9.0) | 155 (11.3) |
| Central American | 134 (7.5) | 16 (9.0) | 150 (7.6) |
| Cuban | 239 (26.1) | 27 (27.4) | 266 (26.3) |
| Mexican | 461 (31.3) | 57 (24.8) | 518 (30.6) |
| Puerto Rican | 216 (16.0) | 35 (17.2) | 251 (16.1) |
| South American | 76 (4.4) | 9 (3.9) | 85 (4.3) |
| More than one/other heritage | 20 (3.2) | 5 (8.7) | 25 (3.8) |
| Education years (%) | |||
| <12 | 468 (32.7) | 85 (50.3) | 553 (34.8) |
| 12 | 294 (22.9) | 30 (12.9) | 324 (21.7) |
| >12 | 524 (44.4) | 50 (36.7) | 574 (43.4) |
| BMI (mean [SD]) | 29.91 (5.35) | 30.37 (5.50) | 29.50 (5.13) |
| eGFR (mean [SD]) | 90.64 (15.75) | 87.25 (15.96) | 88.72 (16.41) |
| Type II diabetes (%) | 308 (25.4) | 68 (45.4) | 376 (27.8) |
| Hypertension (%) | 511 (42.0) | 91 (63.3) | 602 (44.5) |
| APOE Ɛ4 carriers (%) | 288 (22.0) | 41 (20.3) | 329 (21.8) |
| Alcohol consumption (n [%]) | |||
| Never | 249 (18.9) | 45 (27.9) | 294 (20.0) |
| Former | 403 (27.8) | 55 (28.6) | 458 (27.9) |
| Current | 634 (53.3) | 65 (43.5) | 699 (52.1) |
| Smoking status (n [%]) | |||
| Never | 726 (55.6) | 97 (51.1) | 823 (55.1) |
| Former | 324 (25.1) | 35 (24.5) | 359 (25.0) |
| Current | 236 (19.3) | 33 (24.4) | 269 (19.9) |
| Years between visits (mean [SD]) | 7.05 (1.16) | 7.17 (1.16) | 7.06 (1.16) |
Abbreviation: APOE, apolipoprotein E; BMI, body mass index; eGFR, estimated glomerular filtration rate; MCI, mild cognitive impairment; SD, standard deviation; SOL‐INCA, Study of Latinos‐Investigation of Neurocognitive Aging.
Notes: (%) were computed using sampling weights and therefore characterize the SOL‐INCA target population.
All measures are provided from visit 1.
3.1. Single metabolite association analyses (Figure 1, Step A)
We performed metabolite association analysis across the total analytic sample and stratified by APOE ε4 carrier status. Table 2 summarizes the associations that passed the FDR‐adjusted threshold in any one of the three nested regression models. Table S1 in supporting information provides annotations for these metabolites. Across the three regression models, we identified 13 metabolites associated with MCI and 8 metabolites associated with global cognitive change. Effect directions persisted across the three models for all associations (data not shown). One metabolite, quinolinate, was associated with both MCI and global cognitive change.
TABLE 2.
Single‐metabolite associations with MCI or global cognitive decline in our analytic sample
| All participants | APOE Ɛ4 carriers | APOE Ɛ4 non‐carriers | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites | Super pathway | Effect | SE | Unadj‐P ‐value | FDR adj. P‐value | Effect | SE | Unadj P‐value | FDR adj. P‐value | Effect | SE | Unadj. P‐value | FDR adj. P‐value |
| Outcome: MCI | |||||||||||||
| MODEL 1 | |||||||||||||
| 3‐aminoisobutyrate | Nucleotide | –0.04 | 0.01 | 1.26E‐02 | 1.00E + 00 | –0.69 | 0.17 | 3.00E‐05 | 1.76E‐02 | –0.21 | 0.10 | 4.19E‐02 | 1.00E+00 |
| 1‐arachidonoyl‐GPE (20:4n6) | Lipid | 0.03 | 0.01 | 1.44E‐02 | 1.00E + 00 | –0.20 | 0.15 | 1.80E‐01 | 1.00E + 00 | 0.40 | 0.10 | 4.00E‐05 | 1.71E‐02 |
| Glycolithocholic acid 3‐sulfate | Lipid | 0.04 | 0.01 | 1.12E‐03 | 1.00E + 00 | –0.29 | 0.20 | 1.38E‐01 | 1.00E + 00 | 0.42 | 0.11 | 5.00E‐05 | 1.71E‐02 |
| 1‐palmitoyl‐2‐arachidonoyl‐gpe (16:0/20:4) | Lipid | 0.03 | 0.01 | 6.63E‐03 | 1.00E + 00 | –0.04 | 0.16 | 7.82E‐01 | 1.00E + 00 | 0.40 | 0.11 | 2.20E‐04 | 3.52E‐02 |
| Quinolinate | Cofactors and vitamins | –0.03 | 0.01 | 6.66E‐04 | 5.99E‐01 | –0.13 | 0.17 | 4.37E‐01 | 1.00E + 00 | –0.33 | 0.09 | 4.30E‐04 | 4.62E‐02 |
| 9,10‐DiHOME | Lipid | –0.04 | 0.01 | 4.16E‐04 | 3.74E‐01 | –0.13 | 0.20 | 5.23E‐01 | 1.00E + 00 | –0.38 | 0.11 | 5.10E‐04 | 4.66E‐02 |
| Glycodeoxycholatesulfate | Lipid | 0.04 | 0.01 | 8.64E‐04 | 7.77E‐01 | 0.02 | 0.20 | 9.23E‐01 | 1.00E + 00 | 0.34 | 0.09 | 1.70E‐04 | 3.52E‐02 |
| 2‐hydroxyoctanoate | Lipid | –0.04 | 0.01 | 2.06E‐04 | 1.86E‐01 | –0.08 | 0.14 | 5.64E‐01 | 1.00E + 00 | –0.36 | 0.10 | 3.10E‐04 | 3.93E‐02 |
| MODEL 2 | |||||||||||||
| 3‐aminoisobutyrate | Nucleotide | –0.04 | 0.02 | 1.93E‐02 | 1.00E + 00 | –0.66 | 0.14 | 1.90E‐01 | 8.50E‐04 | –0.17 | 0.11 | 1.11E‐01 | 1.00E + 00 |
| 3‐hydroxy‐3‐methylglutarate | Lipid | –0.02 | 0.01 | 9.42E‐02 | 1.00E + 00 | –0.68 | 0.16 | 1.00E‐05 | 4.27E‐03 | –0.06 | 0.11 | 6.16E‐01 | 1.00E + 00 |
| 1‐arachidonoyl‐GPE (20:4n6) | Lipid | 0.03 | 0.01 | 3.92E‐02 | 1.00E + 00 | –0.19 | 0.16 | 2.16E‐01 | 1.00E + 00 | 0.42 | 0.10 | 4.00E‐05 | 2.69E‐02 |
| Glycolithocholic acid 3‐sulfate | Lipid | 0.04 | 0.01 | 2.60E‐03 | 1.00E + 00 | –0.27 | 0.18 | 1.46E‐01 | 1.00E + 00 | 0.39 | 0.11 | 2.20E‐04 | 4.78E‐02 |
| 1‐palmitoleoyl‐GPC (16:1) | Lipid | 0.03 | 0.02 | 4.40E‐02 | 1.00E + 00 | –0.20 | 0.13 | 1.14E‐01 | 1.00E + 00 | 0.42 | 0.11 | 1.60E‐04 | 4.78E‐02 |
| MODEL 3 | |||||||||||||
| 3‐aminoisobutyrate | Nucleotide | –0.04 | 0.02 | 1.38E‐02 | 1.00E + 00 | –0.68 | 0.16 | 1.00E‐05 | 4.45E‐03 | –0.19 | 0.11 | 1.00E‐01 | 1.00E + 00 |
| 3‐hydroxy‐3‐methylglutarate | Lipid | –0.02 | 0.01 | 9.53E‐02 | 1.00E + 00 | –0.73 | 0.17 | 1.00E‐05 | 4.45E‐03 | –0.05 | 0.11 | 6.78E‐01 | 1.00E + 00 |
| Isocitrate | Energy | 0.01 | 0.01 | 5.44E‐01 | 1.00E + 00 | 0.57 | 0.16 | 2.60E‐04 | 3.38E‐02 | 0.04 | 0.12 | 7.49E‐01 | 1.00E + 00 |
| Sulfate | Xenobiotics | –0.01 | 0.01 | 4.19E‐01 | 1.00E + 00 | –0.52 | 0.14 | 1.70E‐04 | 2.72E‐02 | 0.08 | 0.12 | 4.82E‐01 | 1.00E + 00 |
| Tartarate | Xenobiotics | 0.01 | 0.01 | 7.72E‐01 | 1.00E + 00 | 0.52 | 0.14 | 1.70E‐04 | 2.72E‐02 | –0.14 | 0.10 | 1.64E‐01 | 1.00E + 00 |
| 1‐arachidonoyl‐GPE (20:4n6) | Lipid | 0.03 | 0.01 | 4.29E‐02 | 1.00E + 00 | –0.22 | 0.16 | 1.65E‐01 | 1.00E + 00 | 0.42 | 0.10 | 5.00E‐05 | 2.01E‐02 |
| Glycolithocholatesulfate | Lipid | 0.04 | 0.01 | 3.01E‐03 | 1.00E + 00 | –0.29 | 0.18 | 1.09E‐01 | 1.00E + 00 | 0.39 | 0.10 | 1.50E‐04 | 3.15E‐02 |
| 1‐palmitoleoyl‐GPC (16:1) | Lipid | 0.03 | 0.02 | 4.29E‐02 | 1.00E + 00 | –0.18 | 0.13 | 1.63E‐01 | 1.00E + 00 | 0.42 | 0.10 | 6.00E‐05 | 2.01E‐02 |
| Outcome: Global cognitive change | |||||||||||||
| MODEL 1 | |||||||||||||
| Kynurenine | Amino acid | 0.14 | 0.03 | 4.00E‐05 | 2.43E‐02 | 0.04 | 0.06 | 5.24E‐01 | 1.00E + 00 | 0.16 | 0.04 | 6.00E‐05 | 1.22E‐02 |
| 7‐Methylguanine | Nucleotide | 0.12 | 0.03 | 2.10E‐04 | 4.45E‐02 | 0.03 | 0.06 | 6.35E‐01 | 1.00E + 00 | 0.15 | 0.04 | 3.00E‐05 | 1.22E‐02 |
| Quinolinate | Cofactors and vitamins | 0.13 | 0.03 | 8.00E‐05 | 2.43E‐02 | 0.06 | 0.06 | 3.17E‐01 | 1.00E + 00 | 0.15 | 0.04 | 1.20E‐04 | 1.86E‐02 |
| Creatinine | Amino acid | 0.12 | 0.04 | 3.46E‐03 | 1.00E + 00 | 0.02 | 0.07 | 7.99E‐01 | 1.00E + 00 | 0.16 | 0.05 | 4.20E‐04 | 4.45E‐02 |
| Gamma‐glutamylleucine | Peptide | 0.11 | 0.03 | 6.60E‐04 | 5.93E‐01 | –0.02 | 0.07 | 7.51E‐01 | 1.00E + 00 | 0.16 | 0.04 | 4.00E‐05 | 1.22E‐02 |
| Gamma‐glutamylvaline | Peptide | 0.13 | 0.04 | 3.53E‐04 | 3.17E‐01 | 0.06 | 0.06 | 3.71E‐01 | 1.00E + 00 | 0.15 | 0.04 | 4.90E‐04 | 4.45E‐02 |
| N‐acetylcarnosine | Peptide | 0.16 | 0.05 | 4.95E‐04 | 4.45E‐01 | 0.09 | 0.08 | 2.60E‐01 | 1.00E + 00 | 0.19 | 0.05 | 2.90E‐04 | 3.68E‐02 |
| Thymol sulfate | Xenobiotics | 0.08 | 0.03 | 6.06E‐03 | 1.00E + 00 | –0.06 | 0.06 | 3.18E‐01 | 1.00E + 00 | 0.11 | 0.03 | 5.90E‐04 | 4.72E‐02 |
Abbreviation: APOE, apolipoprotein E; BMI, body mass index; eGFR, estimated glomerular filtration rate; FDR, false discovery rate; MCI, mild cognitive impairment; SE, standard error; T2D, type 2 diabetes.
Notes: The positive effect for MCI is risk increasing, and the positive effect for global cognitive change is risk decreasing.
MCI effect is log (odds ratio).
Model 1: Adjusted for sex, age, study center, self‐reported background, education, and duration in years between the visits.
Model 2: Model 1 with further adjustment for BMI, eGFR, T2D, and hypertension.
Model 3: Model 2 with further adjustment for APOE ɛ4 carriers, alcohol consumption, and smoking status.
Results from complete‐cases metabolite analyses were similar to the analyses of the imputed metabolites. All but three associations were identified in one of the APOE ε4 stratification groups (carriers or non‐carriers), with most lipid metabolites identified in the APOE ε4 non‐carrier group. Figure 2 visualizes the correlations between the metabolites predicting MCI and/or global cognitive change as outlined in Table 2.
FIGURE 2.
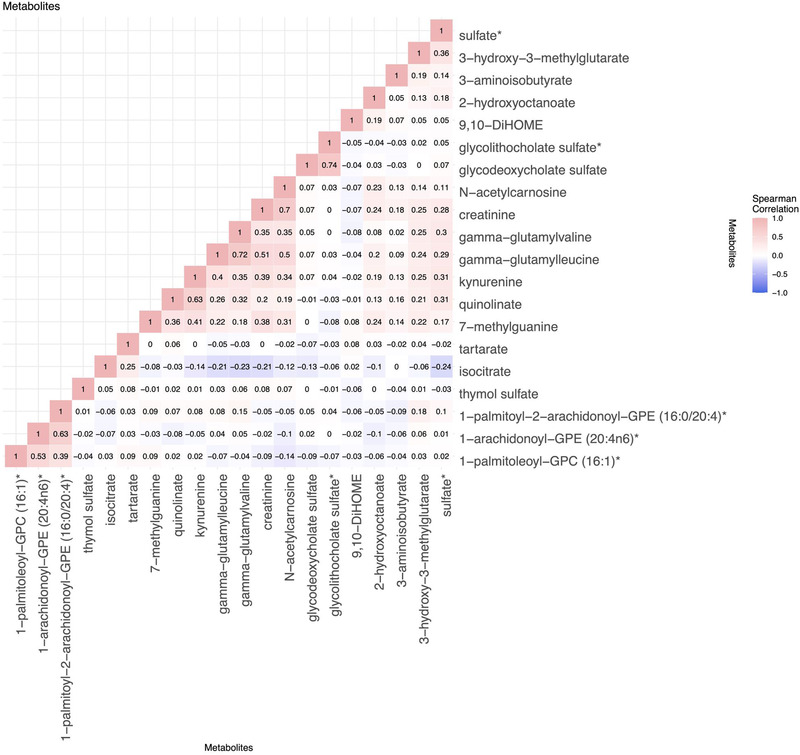
Correlations between significant false discovery rate (FDR)‐adjusted metabolites associated with mild cognitive impairment (MCI) or global cognitive decline. The metabolite correlations were computed within the total study population (not stratified by apolipoprotein E ɛ4 carrier status)
Table 3 summarizes previously reported metabolite associations for the metabolites from Table 2 (Figure 1, Step B). Six metabolites associated with MCI in our study had no previously reported associations with neurocognitive phenotypes. Of these, three lipids were associated with MCI in APOE ε4 non‐carriers: 1‐arachidonoyl‐GPE (20:4n6), 2‐hydroxyoctanoate, and 1‐Palmitoyl‐2‐arachidonoyl‐gpe (16:0/20:4). Two xenobiotics, tartarate and sulfate, and one nucleotide, aminoisobutyrate, were associated with MCI in APOE ε4 carriers. All other 14 metabolites were previously reported as associated with relevant neurocognitive phenotypes in primarily non‐Latino White and Black populations. Eight metabolites had a consistent direction of effects with the published literature, and six metabolites had opposite direction of effects.
TABLE 3.
Previously reported metabolite associations for identified HCHS/SOL metabolite associations with cognitive functions
| Present study | Previously reported metabolite associations | ||||||
|---|---|---|---|---|---|---|---|
| Metabolite | APOE Ɛ4 stratum | Effect | Citation | Outcome | Effect | Study population | Note |
| MCI‐associated metabolites in SOL‐INCA | |||||||
| Glycodeoxycholatesulfate | Non‐carriers | Increased risk | Mahmoudian Dehkordi et al., 2019 36 | AD | Increased risk | ADNI cohort, N = 1464 | A serum metabolite, observed with increased levels in AD patients compared to cognitively normal elderlies and associated with worse cognitive function. |
| 9,10‐DiHOME | Non‐carriers | Decreased risk | Kim, et al., 2019 37 | Central Nervous System (CNS) Aβ | Decreased risk | Europeans with MCI, n = 236 | A plasma metabolite associated with lower central nervous system Aβ in MCI patients. |
| Glycolithocholic acid 3‐sulfate | Non‐carriers | Increased risk | Mahmoudian Dehkordi et al., 2019 36 | AD | Increased risk | ADNI cohort, N = 1464 | A serum metabolite, observed with increased levels in AD patients compared to cognitively normal elderlies and associated with worse cognitive function. |
| 3‐hydroxy‐3‐methylglutarate | carriers | Decreased risk | McFarlane et al., 2019 27 | AD | Decreased risk | Review | Retrospective studied show lower AD prevalence among patients on 3‐hydroxy‐3‐methylglutaryl coenzyme A (HMG‐CoA) reductase inhibitors, commonly known as statins. This metabolite inhibits the cholesterol biosynthesis activity of HMG‐CoA, thus acting as a statin. |
| 1‐palmitoleoyl‐GPC (16:1) | Non‐carriers | Increased risk | Shi et al., 2019; 38 Dong et al., 2021 39 | Processing speed test; CNS t‐tau | Increased risk | Bogalusa Heart Study, White and African Americans, n = 1177 | A serum metabolite associated with processing speed, and a cerebrospinal fluid metabolite associated with t‐tau measured in CNS. |
| Isocitrate | Carriers | Increased risk | González‐Domínguez et al., 2015 29 | AD | Increased risk | Europeans, N = 44 | A serum metabolite associated with AD. |
| Global cognitive change‐associated metabolites in SOL‐INCA | |||||||
| Kynurenine | Combined | Decreased risk | Tanaka et al., 2020 40 | AD, vascular cognitive dementia (VCD) | Increased risk | Review | Elevated levels of the metabolite have been observed in patients with AD and VCD, in serum, or brain tissue. |
| Methylguanine | Combined | Decreased risk | Shi et al., 2019 38 | Digit coding test | Increased risk | Bogalusa Heart Study, Whites and Blacks, n = 1177 | A serum metabolite negatively associated with digit coding test scores. |
| Quinolinate a | Combined | Decreased risk | Schwarcz et al., 2017 32 | Neurodegenerative diseases | Increased risk | Review | Interacts with the immune system contributing to neuronal damage. |
| Creatinine | Non‐carriers | Decreased risk | Tsuruoka et al., 2013 41 | Dementia | Increased risk | Japanese, n = 19 | A serum metabolite increased in dementia patients compared to controls. |
| Gamma‐glutamylleucine | Non‐carriers | Decreased risk | Jeitner et al., 2013 42 | Neurodegenerative diseases | Increased risk | Review | Gamma‐glutamylamines isoforms of this metabolite are found to have increased levels in major neurodegenerative disorders. |
| Gamma‐glutamylvaline | Non‐carriers | Decreased risk | Jeitner et al., 2013 42 | Neurodegenerative diseases | Increased risk | Review | Gamma‐glutamylamines isoforms of this metabolite, are found to have increased levels in major neurodegenerative disorders. |
| N‐acetylcarnosine | Non‐carriers | Decreased risk | Berezhnoy et al., 2019 43 | Neurodegenerative diseases | Decreased risk | Review | Carosine, an isoform of this metabolite, presents a therapeutic neuroprotective effect for neurodegenerative disorders. |
| Thymol sulfate | Non‐carriers | Decreased risk | Azizi et al., 2012 44 | Cognitive impairment | Decreased risk | Rats | Thymol treatment is associated with enhanced cognitive activity in rat models of dementia. |
Notes: In a case where an association was discovered in both the combined cohort and one of the strata (i.e., methylguanine, kynurenine, and quinolinate), we report the direction in the combined cohort.
Quinolinate was associated with both MCI and global cognitive change.
Abbreviations: Aβ, amyloid beta; AD, Alzheimer's disease; APOE, apolipoprotein E; CNS, central nervous system; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; MCI, mild cognitive impairment; SOL‐INCA, Study of Latinos‐Investigation of Neurocognitive Aging; t‐tau, total tau.
3.2. Generalization analysis of metabolite associations in ARIC (Figure 1, Step C)
Tables S2‐S5 in supporting information summarize proxy‐phenotype selection for the ARIC cohort generalization study as described in the supporting information. Out of the 20 associated metabolites with either MCI or global cognitive change in our analytic dataset, 11 were available for ARIC generalization tests. Table S6 in supporting information summarizes the results from the generalization study in ARIC stratified by race: European American and Black. One association was generalized; 7‐methylguanine was associated with improved cognitive change in the total analytic sample was associated with improved DSS change in Black ARIC APOE ε4 carriers and non‐carriers.
3.3. Metabolic risk score for MCI (Figure 1, Step D)
We performed Lasso regression to jointly select metabolites associated with MCI and estimate their effects. Sixty‐one metabolites were selected by the algorithm and formed an MRS. Figure 3 compares MCI classification accuracy in a model accounting for baseline covariates only (sex, age, study center, self‐reported Hispanic/Latino background, education, and time between visits), and a model further accounting for the MRS. Comparison of both models shows an increase in accuracy of 5% in the model that accounts for MRS within the total dataset, reaching an 89% accuracy. Within the APOE ε4 carriers, the increased accuracy due to the addition of the MRS to the model is 12%, reaching 87% accuracy, and in the APOE ε4 non‐carriers, the increased accuracy is 7%, reaching a total of 90% accuracy. The MRS had a P‐value < .0001 in all three models.
FIGURE 3.
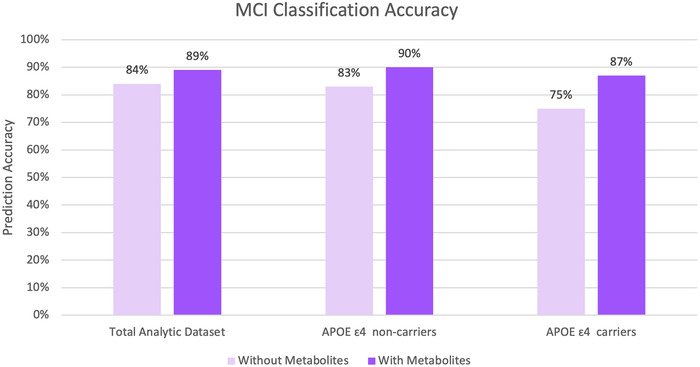
Mild cognitive impairment (MCI) prediction accuracy for the total analytic dataset, and stratified by apolipoprotein E (APOE) Ɛ4 status, for models adjusted for sex, age, study center, self‐reported Hispanic/Latino background, education, and time between visits, with and without the metabolite risk score
Of the 61 metabolites forming the MRS, four metabolites (aminoisobutyrate, quinolinate, 2‐hydroxyoctanoate, and 1‐palmitoleoyl‐GPC [16:1]) were associated with MCI in single‐metabolite analysis. Figure S2 in supporting information presents the MRS selected metabolites’ associations with SOL‐INCA cognitive outcomes.
3.4. Generalization of previously reported metabolite associations with neurocognitive outcomes to SOL‐INCA Hispanics/Latinos (Figure 1, Step E)
Figure S3 in supporting information provides generalization results of 14 previously reported associations of Metabolon‐measured metabolites available for SOL‐INCA, with all primary and secondary neurocognitive outcomes. The metabolites were reported either in the Black population or in a primarily White population. Five associations were generalized (one‐sided P‐value < .05) to MCI: androstenediol, docosapentaenoate, glycodeoxycholate, taurodeoxycholate were associated with increased risk of MCI (previously associated increased risk of dementia, AD, and reduced performance in DSS over time), and ursodeoxycholate was decreased risk for MCI (previously reported as decreasing risk for AD).
4. DISCUSSION
We studied the association of 707 serum metabolites with MCI and global cognitive change phenotypes in Hispanics/Latinos from SOL‐INCA. Overall, we identified 20 metabolite associations with either one or both of the phenotypes, mostly detected in APOE ε4 non‐carriers. To the best of our knowledge, 6 of the metabolites are novel associations and 14 had previously published evidence of association with neurocognitive phenotypes. The published evidence is from either diverse population‐based, clinical, animal, or in vitro studies. We constructed an MRS based on 61 metabolites to form a single score predictive of MCI, improving the accuracy of minimally adjusted MCI prediction models in our Hispanic/Latino population. Finally, we generalized to our Hispanic/Latino participants 5 out of 14 metabolite associations with neurocognitive phenotypes reported in previous publications that used the same metabolomics platform. All generalized associations were of lipid metabolites.
There were 13 metabolites associated with MCI after FDR control in our analysis. Of these, eight were lipids, and seven of them were identified in APOE ε4 non‐carriers (Table 2). As outlined in Table 3, five of the eight lipids were previously reported as associated with neurocognitive phenotypes with similar directions of associations (glycolithocholic acid 3‐sulfate, glycodeoxycholatesulfate, 1‐palmitoleoyl‐GPC [16:1], 3‐hydroxy‐3‐methylglutarate, and 9,10‐DiHOME). The single lipid association detected in APOE ε4 carriers was with 3‐hydroxy‐3‐methylglutarate previously reported as a risk‐decreasing factor for AD. 27 Lipids are known for their crucial function in cell signaling, physiological processes, and disease pathology, especially in the brain. 28 APOE ε4 genotype is known to disrupt lipid transport and metabolism in AD. Our results from the APOE ε4 non‐carriers emphasize the importance of lipids dysregulation in neurocognitive phenotypes, even in the absence of the APOE ε4 allele. 28 Isocitrate is another metabolite associated with increased MCI risk in APOE ε4 carriers. It was previously reported as having a higher concentration in serum of AD patients compared to cognitively normal older adults. 29 Isocitrate is a part of the citrate cycle pathway, responsible for the oxidation of carbohydrates and fatty acids.
We identified eight metabolites associated with global cognitive change; all were previously implicated with neurocognitive phenotypes (Table 3). The directions of six of the associations were inconsistent with the literature. The associations were risk‐decreasing in the present study whereas they were risk‐increasing in previous reports. All these six metabolites also were highly positively correlated in our dataset (Figure 2). The direction of association in our dataset remained consistent after adjusting for additional neurocognitive risk factors in regression models 2 and 3, though they became less statistically significant. One of these metabolites, 7‐methylguanine was also associated with improved cognitive function (change in DSS test) in ARIC Blacks (P‐values = .03 and .04 in APOE ε4 carriers and non‐carriers, respectively). The inconsistencies between our findings and prior literature could result from different neurocognitive phenotypes evaluated across studies, differences in population characteristics such as age and genetic architecture, or other lifestyle and environmental factors that could not be adequately accounted for in the models. 30 Such differences between populations may also explain the overall low generalization in the ARIC population (Table S6). Further investigation of these metabolites in additional data collected in Hispanics/Latinos, and across diverse populations, is needed to clarify the role of these metabolites in neurocognitive health.
A single metabolite, quinolinate, was associated with both MCI and cognitive change in our analysis. Quinolinate is part of the kynurenine pathway of tryptophan catabolism, involved in response to inflammation and infection. The kynurenine pathway is increasingly recognized as contributing to cognitive function. 31 , 32 Higher concentrations of both quinolinate and kynurenine were associated with improved cognitive change in our study, while previous studies primarily reported these two metabolites as associated with neuronal damage. 32 In generalization analysis in European Americans from ARIC, quinolinate was nominally associated (P‐value = .05) with worse 6‐year cognitive performance in the DSS test in APOE ε4 carriers. A possible explanation for these contrasting results could be the recognition that quinolinate is a double‐edged sword, acting as both an essential metabolite in the kynurenine pathway of tryptophan catabolism and a potent neurotoxin with a pro‐apoptotic effect on some cell types. 33 In fact, kynurenine pathway metabolites have recently been proposed as a potential treatment or therapeutic biomarker for neuropsychiatric disorders. 31 Interpretation of these contrasting results is further complicated because metabolites measured in peripheral blood, as done in this study, may show a different effect compared to other studies that measure the brain metabolites. 31 Future work is needed to elucidate their complex association and therapeutic potential for neurocognitive conditions.
We identified six novel associations of metabolites with MCI that were not previously implicated with neurocognition. Aminoisobutyrate, a risk decreasing factor, is a nucleotide in the pyrimidine metabolism pathway produced by skeletal muscle during physical activity. This metabolite was suggested to decrease the risk for metabolic syndrome and its cardiovascular complications, which are known risk factors for dementia. 34 Three other metabolites are lipids: risk‐increasing 1‐arachidonoyl‐GPE (20:4n6), risk‐decreasing 2‐hydroxyoctanoate, risk‐increasing 1‐palmitoyl‐2‐arachidonoyl‐gpe (16:0/20:4); and the two other metabolites are xenobiotics, chemicals that are not derived in humans and enter the body via food or other environmental exposure: risk‐increasing tartarate and risk‐decreasing sulfate. Of these, only the sulfate was available for generalization analysis in ARIC, with DSS as proxy‐phenotype, and there was no evidence of association (P‐value > .5 in both Whites and Blacks). Tartarate is an organic acid occurring in many fruits, thus it is regulated in the body by dietary consumption. Similarly, the quinolinate mentioned above is regulated by dietary consumption of vitamin B3, 35 thus supporting the investigation of diet as a risk factor for neurocognitive outcomes.
The MCI MRS created by Lasso comprised 61 metabolites, and MCI prediction models that included the MRS improved prediction accuracy compared to the minimally adjusted models for both the entire dataset and among the APOE ε4 carriers and non‐carriers. Future targeted data collection is needed to test the performance of the MRS in the prediction of MCI in an independent dataset with similar and/or different ancestries. Validation using existing datasets is challenging because measured metabolites often do not overlap between different studies, in addition to differences in measured phenotypes. Because MCI is often a transitional stage before the development of dementia, the MRS, if validated, could potentially be used as an early detection biomarker allowing for prevention strategies. The identified metabolites, individually and as an integrated measure represented by the MRS, are potential mediators of the effect of environmental exposures such as nutrition, on neurocognitive outcomes.
The present study adds to the growing body of research that uses fasting blood metabolites to predict neurocognitive aging outcomes. We provide an in‐depth analysis of the associations of a broad panel of metabolites with neurocognitive outcomes studied in a unique prospective cohort comprised solely of the US Hispanic/Latino understudied population. However, our study also has some limitations. First, despite the relatively large HCHS/SOL dataset, comprising 1451 participants, only 10% of them were classified as MCI, due to their relatively young age, thus limiting statistical power. Another limitation is that we used a prevalent, rather than the incident, MCI because MCI status is not available at baseline, so we could not verify that all individuals with MCI at the SOL‐INCA visit did not already have MCI at baseline. However, the majority of participants likely did not have MCI at baseline. Moreover, in association analysis within the stratum of APOE ɛ4 carriers, the sample size is quite small (n = 329), limiting the power and potential generalizability to other populations, and increasing the likelihood of overfitting. Second, despite accounting for several covariates in the nested models, these covariates may not fully capture the relationship of lifestyle and environmental confounders with both metabolites and cognitive outcomes, resulting in additional unobserved confounding. Finally, we were not able to fully test our novel metabolite in an independent study population. The ARIC generalization study was also conducted in young adults, used different phenotypes, and had only one of the six novel metabolites detected in the present study. Nevertheless, most of our findings validated previous metabolite associations with cognitive functions, offering external validity. Further analyses with other Hispanic/Latino data are needed to replicate our novel associations.
Overall, this study shows evidence those optimal metabolomics biomarkers predicting prevalent MCI and global cognitive change in Hispanics/Latinos are different than those in Whites and other populations. We found novel associated metabolites that may be specific to our population and replicated previously published metabolite associations, but some showed the opposite direction of associations. Further work should study the similarity and differences in metabolomics biomarkers predicting neurocognitive phenotypes across diverse populations.
CONFLICTS OF INTEREST
Jan Bressler reports receiving the following financial support, with all payments made to her institution: NIH/NHLBI HHSN268201700001I (University of North Carolina at Chapel Hill; this is support for the present manuscript), NIH/NHLBI HHSN268201700001I (University of North Carolina at Chapel Hill), NIH/NIA 1R01AG058921 01A1 (Wake Forest School of Medicine), NIH/NHLBI R01HL141292 (University of Michigan), NIH/NHLBI R01HL141291‐02 NIH/NIAMS R01AR073178‐03 (University of Mississippi Medical Center), NIH/NIA 1R03 AG065420‐02 NIH/NHLBI 2U01HL096812‐09 (Johns Hopkins University), NIH/OD 1OT2OD00275 (Baylor College of Medicine), NIH/NIDDK R01DK552556‐01 (Johns Hopkins University), NIH/NIEHS 5R01ES022165 NIH/NINDS R01NS087541 NIH/NCI 2P01CA138338 (University of Minnesota), NIH/NIDDK U01DK078616 (Massachusetts General Hospital), NIH/NHGRI UM1HG006542 (Johns Hopkins University). Tianyi Huang reports receiving grant support from NHLBI, to himself. Sylvia Wassertheil‐Smoller reports receiving an NIH grant, and support from the Fred Hutchinson Cancer Center, with payment made to her. She reports royalties for her textbook Biostatistics and Epidemiology from Springer Publishing and consulting fees from RxScholar for review of STEP 1 testing materials for medical students. Melissa Lamar reports receiving funding support from the NIH, with payments made to the institution; support from the International Neuropsychological Society, with payments made to her. She is a member of the Rush University Medical Center's Data Safety and Monitoring Board or Advisory Board, with no payment to her or her institute and receives a reimbursement in the form of an honorarium (later donated to student fellowship fund of INS) from the International Neuropsychological Society travel reimbursement, with payment made to her. Martha Daviglus reports receiving multiple NIH grants, with payments made to the University of Illinois at Chicago. Maria J. Marquine reports receiving support through multiple NIH grants: R01MD013502 and 3R01MD013502‐03S1 (as a PI, with payments made to her institution), as well as support from the Federal Defenders of San Diego, from the American Academy of Neuropsychology Annual Meeting, and the International Neuropsychological Society, with payments made to her. Jianwen Cai reports funding support from the NIH, 75N92019D00010, with payments made to her institution. She reports being a Chair of American Statistical Association Lifetime Data Science Section Chair of American Statistical Association Fellow Committee. Thomas Mosley reports receiving NIH grants, with payments made to the institution. He reports serving on the CARDIA Observational studies monitoring board with remuneration paid to him for time spent on meeting (<$200). Robert Kaplan reports receiving NIH grants and receiving consulting fees from the University of Texas. Eric Boerwinkle reports receiving multiple grants: ARIC Contract (support for the present manuscript), payments made to his institution; UM1HG008898 NIH/Baylor College of Medicine, R01HL131136 NIH/NHLBI, R01HL131136‐S1 NIH/NHLBI, HHSN268201700001I NIH/NHLBI/UNC at Chapel Hill, 1R01HL141824 NIH/NHLBI, 1OT2OD00275 NIH/Baylor College of Medicine, 1R01MD013349 NIH/UNC at Chapel Hill, 1U01AG058589‐01 NIH/Boston University, HHSN268201800002I NHLBI/U. of Michigan, R01AG059727 NIH/Boston University, R01AG061022 NIH/University of California‐San Diego, 1R01HL148050‐01 NIH/Baylor College of Medicine, U01 HL096812 NIH/Johns Hopkins University, HHS000866600001 Texas Department of State Health Services TOPMed Centralized Omics Resource (CORE) BCM/NIH/NHLBI, R01 HL148218 NIH/Brigham and Women's Hospital. He also reports participating in the PRIDE Programs to Increase Diversity‐Lecture at Washington University in St. Louis‐annual lecture, and payments were made to him and donated to the institution. He reports receiving reimbursement for travel expenses out of pocket for several NIH meetings. He reports serving as a member in numerous NIH Special Emphasis Panels (mostly NHLBI and NHGRI). Eric Boerwinkle further reports serving as a co‐cChair of the Alzheimer's Disease Sequencing Project Steering Committee; as a member of the Alzheimer's Disease Sequencing Project Executive Committee; a member of the NHLBI TOPMed Program Steering Committee; a member of the NHLBI TOPMed Program Executive Committee; a member of the All of Us Steering Committee; a member of the All of Us Science Committee; a member of the All of Us Reassessment Committee; and a member of the anchoring committee for the Greater Houston Coalition for the Social Determinants of Health. This group is working to bring together organizations and service providers to help those in need navigate available services and coordinate across service providers to reduce duplication and increase efficiency. He also reports serving as a chair of the Greater Houston Health Connect Research Committee, a group working to build health services research in the State's largest health information exchange (HIE), and as a board member for the Hackett Center for Mental Health Consulting member of the TMC CEO COVID‐19 Task Force Consulting member of the LHA (Local Health Authorities). Myriam Fornage reports receiving NIH grants. Charles DeCarli reports receiving NIH ADRC support with money paid to the institution. He reports receiving consulting fees from Novartis Novo Nordisk, and honoraria for teaching a Harvard Dementia Course. Bruce Kristal reports royalties from Metabolon, through Weill Medical of Cornell University, and consulting fees from Pfizer and Metabolon (both completed). He also reports holding Metabolon stock—both directly owned and through rights paid to Weill Medical College of Cornell University. He also reports an informatics patent‐pending, unrelated to the current study. His interests were reviewed by the Brigham and Women's Hospital and Mass General Brigham following their institutional policy. Accordingly, upon review, the institution determined that Kristal's financial interest in Metabolon does not create a significant financial conflict of interest with this research. Hector M. Gonzalez reports receiving NIA funding with payments made to his institution, and honoraria from USC and UTHSCSA, with payments made to him. Tamar Sofer reports funding through contracts from Brown University, the Broad Institute of MIT and Harvard, the Veterans Administration‐Boston, the University of California Davis, and the University of California San Diego, with payments made to the institution. She also reports funding from NHLBI, with payments made to the institution. She reports receiving honoraria after a presentation at the University of California San Diego, with payment made to her.
Supporting information
Supporting material
ACKNOWLEDGMENTS
The authors thank the staff and participants of HCHS/SOL for their important contributions. Investigators website–https://sites.cscc.unc.edu/hchs/. The Hispanic Community Health Study/Study of Latinos is a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (HHSN268201300001I/N01‐HC‐65233), University of Miami (HHSN268201300004I/N01‐HC‐65234), Albert Einstein College of Medicine (HHSN268201300002I/N01‐HC‐65235), University of Illinois at Chicago (HHSN268201300003I/N01‐HC‐65236 Northwestern University), and San Diego State University (HHSN268201300005I/N01‐HC‐65237). The following Institutes/Centers/Offices have contributed to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution‐Office of Dietary Supplements. This work was supported by the National Institute on Aging (R01AG048642, RF1AG054548, RF1AG061022, and R21AG056952). Dr. González also receives additional support from P30AG062429 and P30AG059299. Support for metabolomics data was graciously provided by the JLH Foundation (Houston, Texas). The Atherosclerosis Risk in Communities study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services (contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I). We thank the staff and participants of the ARIC study for their important contributions. The metabolomics research was sponsored by the National Human Genome Research Institute (3U01HG004402‐02S1).
He S, Granot‐Hershkovitz E, Zhang Y, et al. Blood metabolites predicting mild cognitive impairment in the study of Latinos‐investigation of neurocognitive aging (HCHS/SOL). Alzheimer's Dement. 2022;14:e12259. 10.1002/dad2.12259
Shan He and Einat Granot‐Hershkovitz equally contributed to this study.
REFERENCES
- 1. Alzheimer's Association . 2018 Alzheimer's disease facts and figures. Alzheimer's Dement 2018;14:367‐429. [Google Scholar]
- 2. 2020 Alzheimer's disease facts and figures. Alzheimer's Dement. 2020;16:391‐460. [DOI] [PubMed] [Google Scholar]
- 3. Granot‐Hershkovitz E, Daviglus M, Tarraf W, et al. APOE alleles ’ association with cognitive function differs across Hispanic /Latino groups and genetic ancestry in the study of Latinos‐investigation of neurocognitive aging ( HCHS / SOL ). Alzheimer's Dement. 2020;17(3):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA. The natural history of cognitive decline in Alzheimer's disease. Psychol Aging. 2012;27:1008‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manly JJ, Tang M‐X, Schupf N, Stern Y, Vonsattel J‐PG, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duara R, Loewenstein DA, Wright C, Crocco E, Varon D. Mild cognitive impairment. Dementia. 2013:77‐95. [Google Scholar]
- 7. Trivedi DK, Hollywood KA, Goodacre R. Metabolomics for the masses: the future of metabolomics in a personalized world. New Horizons Transl Med. 2017;3:294‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang Y, Zhen Z, Jie S, Yanpeng A, et al. Metabolomics in the development and progression of dementia: a systematic review. Front Neurosci. 2019;1:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tynkkynen J, Chouraki V, van der Lee SJ, et al. Association of branched‐chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer's disease: a prospective study in eight cohorts. Alzheimer's Dement. 2018;14:723‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chouraki V, Preis SR, Yang Q, et al. Association of amine biomarkers with incident dementia and Alzheimer's disease in the Framingham Study. Alzheimer's Dement. 2017;13:1327‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trushina E, Dutta T, Persson X‐MT, Mielke MM, Petersen RC. Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer's disease using metabolomics. PLoS One. 2013;8:e63644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bressler J, Yu B, Mosley TH, et al. Metabolomics and cognition in African American adults in midlife: the atherosclerosis risk in communities study. Transl Psychiatry. 2017;7:e1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li D, Misialek JR, Boerwinkle E, et al. Prospective associations of plasma phospholipids and mild cognitive impairment/dementia among African Americans in the ARIC Neurocognitive Study. Alzheimer's Dement. 2017;6:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. González HM, Tarraf W, Fornage M, et al. A research framework for cognitive aging and Alzheimer's disease among diverse US Latinos: design and implementation of the Hispanic Community Health Study/Study of Latinos—Investigation of Neurocognitive Aging (SOL‐INCA). Alzheimer's Dement. 2019;15:1624‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lavange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorlie PD, Avilé S‐Santa LM, Wassertheil‐Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Callahan CM, Unverzagt FW, Hui SL, Anthony J. Six‐Item Screener to Identify Cognitive Impairment among Potential Subjects for Clinical Perkins and Hugh C. Hendrie: Lippincott Williams & Wilkins Stable; https://www.jstor.org/stable/3768143 [DOI] [PubMed] [Google Scholar]; Six‐item screener to identify cognitive im. Med Care. 2001;40:771‐781. [DOI] [PubMed] [Google Scholar]
- 18. González HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for English‐ and Spanish‐speaking older people. J Int Neuropsychol Soc. 2001;7:544‐555. [DOI] [PubMed] [Google Scholar]
- 19. Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4th ed.. New‐York: Oxford University Press; 2004. PsycNET. [Google Scholar]
- 20. HCHS/SOL Manual 9 Neurocognitive Function. 2008. Available from: https://sites.cscc.unc.edu/hchs/system/files/protocols‐manuals/UNLICOMMManual09Neurocognitivev1002182008.pdf
- 21. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. González HM, Tarraf W, Schneiderman N, et al. Prevalence and correlates of mild cognitive impairment among diverse Hispanics/Latinos: study of latinos‐investigation of neurocognitive aging results. Alzheimer's Dement. 2019:12:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feofanova EV, Chen H, Dai Y, et al. A genome‐wide association study discovers 46 loci of the human metabolome in the Hispanic Community Health Study/Study of Latinos. Am J Hum Genet. 2020;107:849‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lumley T, Scott A. Tests for regression models fitted to survey data. Aust New Zeal J Stat. 2014;56:1‐14. [Google Scholar]
- 25. Tibshirani R. Regression shrinkage and selection via the lasso. JR Stat Soc. 1996;58:267‐288. [Google Scholar]
- 26. Nho K, Kueider‐Paisley A, MahmoudianDehkordi S, et al. Altered bile acid profile in mild cognitive impairment and Alzheimer's disease: relationship to neuroimaging and CSF biomarkers. Alzheimer's Dement. 2019;15:232‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McFarlane O, Kędziora‐Kornatowska K. Cholesterol and dementia: a long and complicated relationship. Curr Aging Sci. 2019;13:42‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chew H, Solomon VA, Fonteh AN. Involvement of lipids in Alzheimer's disease pathology and potential therapies. Front Physiol. 2020;11:1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. González‐Domínguez R, García‐Barrera T, Gómez‐Ariza JL. Metabolite profiling for the identification of altered metabolic pathways in Alzheimer's disease. J Pharm Biomed Anal. 2015;107:75‐81. [DOI] [PubMed] [Google Scholar]
- 30. Liu J, Lahousse L, Nivard MG, et al. Integration of epidemiologic, pharmacologic, genetic and gut microbiome data in a drug–metabolite atlas. Nat Med. 2020;26:110‐117. [DOI] [PubMed] [Google Scholar]
- 31. Savitz J. The kynurenine pathway: a finger in every pie. Mol Psychiatry. 2020;25:131‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schwarcz R, Stone TW. The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology. 2017;112:237‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moffett JR, Arun P, Puthillathu N, et al. Quinolinate as a marker for kynurenine metabolite formation and the unresolved question of NAD+ synthesis during inflammation and infection. Front Immunol. 2020;11:1‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanianskii DA, Jarzebska N, Birkenfeld AL, O'sullivan JF, Rodionov RN. Beta‐aminoisobutyric acid as a novel regulator of carbohydrate and lipid metabolism. Nutrients. 2019;11:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gasperi V, Sibilano M, Savini I, Catani MV. Niacin in the central nervous system: an update of biological aspects and clinical applications. Int J Mol Sci. 2019;20:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. MahmoudianDehkordi S, Arnold M, Nho K, et al. Altered bile acid profile associates with cognitive impairment in Alzheimer's disease—An emerging role for gut microbiome. Alzheimer's Dement. 2019;15:76‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim M, Snowden S, Suvitaival T, et al. Primary fatty amides in plasma associated with brain amyloid burden, hippocampal volume, and memory in the European Medical Information Framework for Alzheimer's disease biomarker discovery cohort. Alzheimer's Dement. 2019;15:817‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi M, Bazzano LA, He J, et al. Novel serum metabolites associate with cognition phenotypes among Bogalusa Heart Study participants. Aging (Albany NY). 2019;11:5124‐5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dong R, Darst BF, Deming Y, et al. CSF metabolites associate with CSF tau and improve prediction of Alzheimer's disease status. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2021;13(1). 10.1002/dad2.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tanaka M, Bohár Z, Vécsei L. Are kynurenines accomplices or principal villains in dementia? Maintenance of kynurenine metabolism. Molecules. 2020;25:1‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsuruoka M, Hara J, Hirayama A, et al. Capillary electrophoresis‐mass spectrometry‐based metabolome analysis of serum and saliva from neurodegenerative dementia patients. Electrophoresis. 2013;34:2865‐2872. [DOI] [PubMed] [Google Scholar]
- 42. Jeitner TM, Battaile K, Cooper AJL. γ‐Glutamylamines and neurodegenerative diseases. Amino Acids. 2013;44:129‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berezhnoy DS, Stvolinsky SL, Lopachev AV, et al. Carnosine as an effective neuroprotector in brain pathology and potential neuromodulator in normal conditions. Amino Acids. 2019;51:139‐150. [DOI] [PubMed] [Google Scholar]
- 44. Azizi Z, Ebrahimi S, Saadatfar E, Kamalinejad M, Majlessi N. Cognitive‐enhancing activity of thymol and carvacrol in two rat models of dementia. Behav Pharmacol. 2012;23:241‐249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting material
Data Availability Statement
HCHS/SOL and SOL‐INCA data can be obtained from dbGaP under accession number phs000810.v1.p1. Summary statistics from association analyses of all metabolites and all studied phenotypes are available in a GitHub repository. In addition, code for constructing the MCI MRS based on the Lasso‐selected metabolites is provided in the same GitHub repository.


