Abstract
Background
Sodium–glucose cotransporter 2 (SGLT2) inhibitors are effective for glycemic control and have demonstrated cardiorenal benefits. The U.S. Food and Drug Administration (FDA) released a boxed warning in 2018 regarding the potential development of Fournier’s gangrene (FG) with the use of SGLT2 inhibitors. FG is a serious perineal infection with a mortality rate of up to 88% in some cases.
Objectives
To report spontaneous post-marketing cases from the FDA Adverse Event Reporting System (FAERS) database and case reports from the literature of FG associated with the use of SGLT2 inhibitors and to determine whether correlations exist with specific agents.
Methods
A search of the FAERS database was conducted to identify reported cases of FG associated with the use of any FDA-approved SGLT2 inhibitor between 1 March 2013 and 30 June 2020. Additionally, a literature search was conducted of PubMed, Embase, and the Cochrane library using PRISMA guidelines to identify case reports of FG with the use of SGLT2 inhibitors up to 9 October 2020.
Results
A total of 491 cases from the FAERS database were included for review. Descriptive analysis depicted more cases in the empagliflozin, canagliflozin, and dapagliflozin groups than in the ertugliflozin group. Nine case reports were included from the literature review; four attributed to dapagliflozin, three to empagliflozin, and two to canagliflozin. The median ages from cases reported in the FAERS database and from the literature review were 54 and 52 years, respectively. In both datasets, males had a higher incidence of FG than females. Additional data reported include clinical outcomes and concomitant antihyperglycemic medications.
Conclusion
Consistent findings are noted in this systematic review and warrant further investigation to elucidate the association between SGLT2 inhibitor use and the development of FG. These results may drive enhanced prescribing patterns to consider patient-specific risk factors and timely monitoring, especially as more indications are approved related to these medications’ cardiorenal protective properties.
Sodium–glucose cotransporter 2 (SGLT2) inhibitors are a fairly new class of medications that includes canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. The first SGLT2 inhibitor was approved by the U.S. Food and Drug Administration (FDA) in 2013 for the treatment of type 2 diabetes. SGLT2 inhibitors are effective for improving glycemic control, and two (empagliflozin and canagliflozin) have also been approved to reduce the risk for major adverse cardiovascular events in adults with type 2 diabetes and established cardiovascular disease (1–4). In addition, they have been found to be beneficial for reducing hospitalization for heart failure and kidney disease progression (4,5).
SGLT2 inhibitors lower blood glucose by increasing excretion of excess glucose through the kidneys and urine. Because of this unique mechanism, the most commonly associated adverse effects include increased risks of genital infections such as yeast infections and urinary tract infections (6). Other common adverse effects include diabetic ketoacidosis, cancer, bone fractures, and foot and leg amputations (7–11). In August 2018, the FDA issued a warning that cases of a rare but serious infection called Fournier’s gangrene (FG) have been reported with SGLT2 inhibitor use, with 12 cases identified between March 2013 (when the first agent was approved) and May 2018 (12).
FG is a bacterial infection of the tissue under the skin that surrounds the muscles, nerves, fat, and blood vessels of the perineum. It has an acute onset with rapid progression and is considered a urologic emergency. FG most commonly affects men but can also occur in women. The mainstay of therapy for FG includes treatment with broad-spectrum antibiotics and invasive surgical debridement (13). Early diagnosis and initiation of treatment is crucial, as mortality rates average 20–40% and have been as high as 88% in some instances, despite advanced management (13,14). Comorbidities are an important factor in determining mortality in patients with FG. A systematic review and meta-analysis by El-Qushayri et al. (15) indicated that higher mortality rates were detected in people with diabetes, heart disease, renal failure, and kidney disease. Within this meta-analysis, sepsis was the most common cause of death, followed by multiple organ failure.
It has been determined that FG may be a class effect of SGLT2 inhibitors. Fadini et al. (16) published such findings shortly after the FDA warning was issued. Interestingly, they found 47 cases of FG reported during the same time frame as the FDA (March 2013 through May 2018). Additionally, in a review of FG associated with SGLT2 inhibitors, 55 cases of FG were identified between 1 March 2013 and 31 January 2019 (3). However, these reviews may have underreported cases of FG as a result of their time frame (mostly covering the time period before the FDA warning was issued) and because of exclusion of reports if no surgical intervention occurred (3,16). The purpose of this article is to report all spontaneous post-marketing cases and case reports of SGLT2 inhibitor–related FG to determine whether there are trends toward certain medications in the class that can indicate increased risk of FG. These findings, in addition to the current literature, confirm the importance of prescriber vigilance when using this class of drugs based on the possible severe genital complications that can occur in high-risk patients.
Research Design and Methods
Health care providers, consumers, and drug manufacturers can report drug adverse events via the FDA Adverse Event Reporting System (FAERS) (17). The public can access and search this database through the FAERS online dashboard (18) for information about adverse events associated with a given drug. We conducted a search of the FAERS database for reported cases of FG associated with the use of any of the four FDA-approved SGLT2 inhibitors (canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin) between 1 March 2013 (the date the first SGLT2 inhibitor, canagliflozin, was approved in the United States) and 30 June 2020. To identify FG cases within the database, we used the reaction search term “Fournier’s gangrene” for each SGLT2 inhibitor, including generic and brand name (canagliflozin [Invokana], dapagliflozin [Farxiga], empagliflozin [Jardiance], and ertugliflozin [Steglatro]). Cases were excluded if the suspected product description included an agent that was not an SGLT2 inhibitor or a combination drug (i.e., SGLT2 inhibitor + biguanide or SGLT2 inhibitor + dipeptidyl peptidase 4 [DPP-4] inhibitor), they were duplicate reports, or they were already reported in the literature. Combination products were excluded because we would be unable to determine whether FG was the result of the SGLT2 inhibitor component alone or whether the second agent could have contributed to this adverse effect.
We conducted a comprehensive literature search in PubMed, Embase, and the Cochrane Library Central Register of Controlled Trials from each index’s inception year (1946, 1947, and 1991, respectively) through 9 October 2020. The search strategy used a combination of search headings and keywords to identify case studies and case series for FG with the use of SGLT2 inhibitors. Search terms included bexaglifozin, canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, glifozin, ipragliflozin, remogliflozin, SGLT 2 inhibitors, sodium-dependent glucose transporter 2 inhibitor, sotagliflozin, tofogliflozin, Fournier gangrene, scrotum infection, perineum infection, perineal, gangrene, gangrenous, and necrosis.
Review of the cases within the FAERS database and review of the literature utilizing Rayyan, a systematic review web application, was completed by two independent reviewers (19). In instances where there were disagreements with case selections or article selection between the two reviewers, a third reviewer was available to make the final decision. The full search strategy of PubMed, Embase, and the Cochrane library is provided in the Supplemental Materials.
Statistical Analysis
No tests of statistical significance were performed in this review. All case descriptive data were compiled in Microsoft Excel 2010 for reporting.
Results
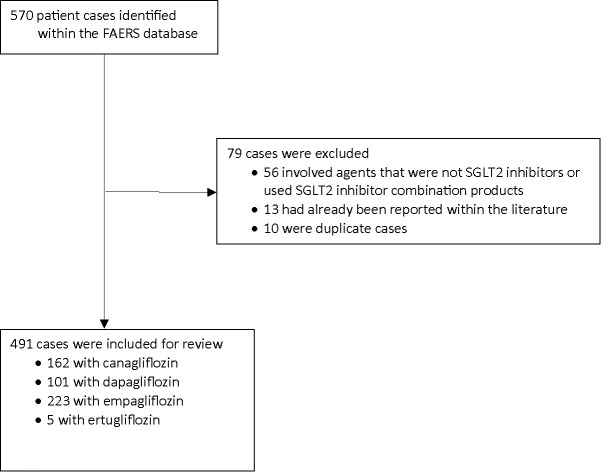
We identified 570 cases of FG associated with SGLT2 inhibitor use within the FAERS database. After excluding cases of SGLT2 inhibitors combined with other antidiabetic therapy (n = 56), duplicate cases (n = 10), and cases already reported in the literature (n = 13), 491 cases were included for review (Figure 1). Of the 491 cases included for review, 162 were reported with canagliflozin, 101 cases with dapagliflozin, 223 cases with empagliflozin, and 5 cases with ertugliflozin. The low number of cases associated with ertugliflozin may be attributed to the fact that it was a latecomer to the U.S. market, having been approved by the FDA in 2017 (8). Although results from the FAERS database depict a higher incidence of FG associated with empagliflozin, correlations regarding differences among the SGLT2 inhibitors remain inconclusive because of unknown prescribing patterns and unreliability of FAERS data. Further prospective studies with appropriate patient randomization are warranted to establish such correlations.
FIGURE 1.
Cases from FAERS database meeting inclusion criteria for review.
The characteristics of the reported cases are presented in Table 1. Further review of the data showed that the median age of the patients was 54 years (range 28–87 years); 291 cases involved males, 167 involved females, and 33 did not report patient sex. Regarding outcomes, 281 were hospitalized, 21 died, and no data point was provided for 188 cases. Concomitant medication use was also reviewed and showed that patients were taking a wide array of antihyperglycemic medications, including biguanides (n = 26), sulfonylureas (n = 1), incretin mimetics (n = 2), DPP-4 inhibitors (n = 2), pioglitazone (n = 1), insulin (n = 23), or a combination of antiglycemic drugs (n = 106). In 329 cases, concomitant drugs used were not reported (n = 313) or none were used (n = 16).
TABLE 1.
Characteristics of Cases Identified in FAERS Database (n = 491)
| Characteristics | Canagliflozin, n | Dapagliflozin, n | Empagliflozin, n | Ertugliflozin, n | Total, n (%) |
|---|---|---|---|---|---|
| Number of cases | 162 | 101 | 223 | 5 | 491 (100) |
| Sex Male Female Not specified |
121 35 6 |
20 77 4 |
145 55 23 |
5 0 0 |
291 (59) 167 (34) 33 (7) |
| Age, years 20–29 30–39 40–49 50–59 60–69 70–79 80–89 Not specified |
0 12 38 45 38 12 0 17 |
1 7 13 24 21 5 0 30 |
0 6 19 49 55 19 6 69 |
0 0 1 2 0 0 0 2 |
1 (<1) 25 (5) 71 (14) 120 (24) 114 (23) 36 (7) 6 (1) 118 (24) |
| Outcomes Hospitalized Died Not specified |
109 5 48 |
26 4 71 |
143 12 68 |
4 — 1 |
282 (57) 21 (4) 188 (38) |
| Concomitant antihyperglycemics Biguanides Sulfonylureas Incretin mimetics DPP-4 inhibitors Pioglitazone Insulin Combination* None Not reported |
5 0 1 0 1 2 6 2 145 |
10 1 0 1 0 7 28 6 48 |
11 0 1 1 0 13 71 8 117 |
0 0 0 0 0 1 1 0 3 |
26 (5) 1 (<1) 2 (<1) 2 (<1) 1 (<1) 23 (5) 106 (22) 16 (3) 314 (64) |
Combination indicates more than one antihyperglycemic agent was used in combination with the reported SGLT2 inhibitor.
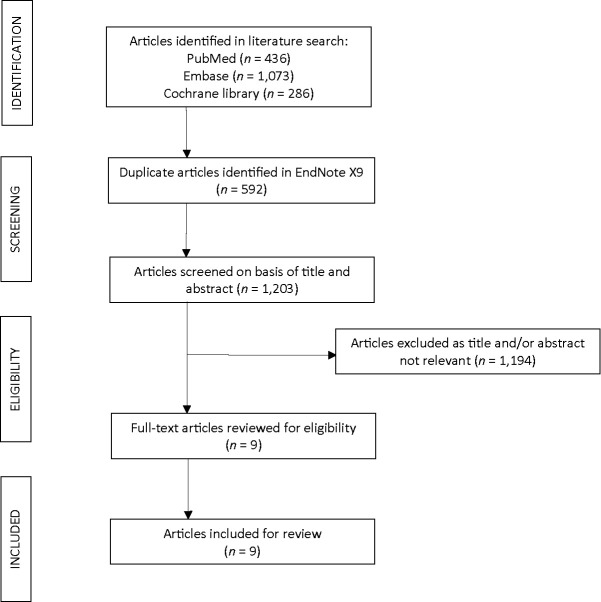
A systematic review of the literature for case reports of FG associated with the use of SGLT2 inhibitors resulted in 1,203 articles, of which nine were identified as case reports of FG associated with the use of an SGLT2 inhibitor (Figure 2) (20–28). Characteristics of the nine case reports are presented in Table 2. Of the nine cases included for review, two were associated with canagliflozin use, four with dapagliflozin use, and three with empagliflozin use. The median age of patients was 52 years (range 34–72 years). Our study yielded similar findings, with 65–75% of patients who developed FG being male, with ages ranging between the fifth and sixth decades of life. The majority of cases in the literature were male (eight of the nine cases). With regard to the clinical course, patients in all cases reported scrotal swelling or pain in the testicles, groin, or gluteal region. All patients had to be admitted to the inpatient setting and required surgical incision, drainage, and/or debridement. Broad spectrum antibiotics were empirically initiated and streamlined based on culture and sensitivity results. Lengths of stay varied between 12 days and 9 weeks, when longer duration of treatment was required either because of prolonged use of antibiotics or other clinical complications. Clinical courses were most often complicated by sepsis, uncontrolled diabetes requiring insulin management, multiple surgical re-explorations with further debridement, or prolonged intravenous antibiotics use (20–28).
FIGURE 2.
Case reports from literature search meeting inclusion criteria for review.
TABLE 2.
Summary of Case Reports Included for Review (n = 9)
| Article | SGLT2 Inhibitor | Sex | Age, years | A1C, % | Time to Development of FG | Concomitant Drugs |
|---|---|---|---|---|---|---|
| Kumar et al., 2017 (20) | Empagliflozin | M | 42 | 11.2 | 14 months | Metformin |
| Omer et al., 2018 (21) | Dapagliflozin | M | 60 | — | 5 months | Glicazide, metformin |
| Onder et al., 2019 (22) | Dapagliflozin | M | 64 | 7.4 | 6 months | Insulin, metformin, vildagliptin |
| Elshimy et al., 2019 (23) | Empagliflozin | M | 57 | — | 10 days | Glipizide, linagliptin, metformin |
| Nagano et al., 2019 (24) | Empagliflozin | M | 34 | 6.5 | 142 days | Glibenclamide, sitagliptin |
| Rodler et al., 2019 (25) | Dapagliflozin | M | 39 | — | 4 years | Amlodipine, levothyroxine, metformin, sitagliptin, valsartan |
| Elbeddini et al., 2020 (26) | Canagliflozin | M | 72 | 7.5 | 6 years | Glargine, metformin, sitagliptin |
| García-García et al., 2020 (27) | Dapagliflozin | M | 68 | 7.8 | 2–3 years | Glargine, metformin, sitagliptin |
| Kasbawala et al., 2020 (28) | Canagliflozin | F | 37 | 9.8 | 1 months | Cetirizine, citalopram, levothyroxine, lisinopril, metformin, pantoprazole, pravastatin, sitagliptin, trazodone, valacyclovir |
The diagnosis in all case reports was type 2 diabetes, and the outcome was hospitalization. F, female; M, male.
The time to the development of FG with the use of SGLT2 inhibitors ranged from 10 days (23) to 6 years (26), suggesting that other comorbidities and risk factors played a role in the development of FG. A1C levels were reported in six of the nine cases, with a range of 6.5–11.2%. All patients were diagnosed with type 2 diabetes, were on at least one other antihyperglycemic medication, and were hospitalized with no deaths reported. In one instance, a patient reported an episode of thrush approximately 7 months after initiation of empagliflozin, for which the patient did not seek medical attention but instead self-treated with over-the-counter antifungals (20). However, the patient described having multiple subsequent episodes of thrush with continued use of empagliflozin. After 14 months on therapy, the patient developed scrotal swelling and pain, which prompted a doctor visit and subsequently led to a diagnosis of FG requiring emergency debridement and exploration (20). BMI was reported for six of the nine case reports. Of the six cases with BMI, five were classified as obese, with BMI ranging from 33 to 62 kg/m2, which is consistent with the finding of obesity as a risk factor for the development of FG (20,22–25,28). Smoking, also a risk factor for FG, was identified in three of the nine cases (20,24,25).
Discussion
Our search, which included data from the FAERS database and an independent literature search, identified 500 cases of FG associated with the use of an SGLT2 inhibitor. Diabetes is one of the most common risk factors for development of FG, along with obesity, chronic renal failure, cirrhosis, and impaired host defense (e.g., malignancy or AIDS). When coupled with local trauma such as perineal surgery, periurethral urine leak, or other iatrogenic causes, there is a higher susceptibility for development of FG, especially among males and older patients (29,30).
An analysis by Sorensen et al. (31) of 25 million patients from the State Inpatient Database using International Classification of Diseases, 9th revision, diagnostic codes revealed that, of those with FG, only 2% (391,641) were female (31,32). In contrast, an analysis by Kim et al. (33) of patient data from the American College of Surgeons National Surgical Quality Improvement Program between 2005 to 2009 identified 636 patients with surgery for FG using the Current Procedural Terminology codes, of which 43.4% were female (276 of 636) (32,33). The difference in male:female ratio may lie in how FG is diagnosed in females rather than FG being more common in males.
The infection is typically polymicrobial, involving both aerobic and anaerobic bacteria, requiring broad spectrum antimicrobial coverage with prolonged treatment and surgical intervention (30,34). Commonly isolated pathogens included Bacteroides species, Escherichia coli, Staphyloccocus aureus, Pseudomonas aeruginosa, Streptococcus species, and Prevotella species, with increasing incidence of methicillin-resistant Staphyloccocus aureus (MRSA) (34). Although the exact mechanism for development of FG has not been elucidated, the mechanism of action of the drug class may contribute to its development. SGLT2 inhibitors facilitate glucosuria by excretion of glucose from the proximal convoluted tubule, thereby preventing reabsorption of glucose (35). Patients with type 2 diabetes are generally at a higher risk for urinary tract infections and nonsexually transmitted genital infections because of elevated glucose levels in the urine. This risk may be further increased pharmacologically by the additional urinary glucose excretion brought on by SGLT2 inhibitors (6).
In addition to their glucose-lowering effects, SGLT2 inhibitors have demonstrated many other benefits, including prevention of microvascular and macrovascular complications associated with uncontrolled diabetes, weight loss, reduction of microalbuminuria and other renoprotective effects, and pleiotropic cardiovascular effects leading to reduced cardiac and arterial remodeling and blood pressure and improved outcomes in heart failure (36,37). The first drug in its class to gain FDA approval for reduced risk of cardiovascular death and hospitalization for heart failure in 2019, dapagliflozin led the way for expanded indications (1). In response to the EMPA-REG OUTCOME (BI 10773 [Empagliflozin] Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study, in which empaglifozin demonstrated reduced death from cardiovascular causes, the FDA granted it fast-track designation for additional indications (1). For this reason, increased use of certain SGLT2 inhibitors may also correlate with a rise in reports of the FG side effect (36,37). Until further research on each SGLT2 inhibitor agent is conducted, the correlation remains as a likely class effect.
Although it is unknown whether kidney dysfunction is a risk factor for development of FG, renal failure and kidney disease are associated with increased mortality from FG (15). Regarding SGLT2 inhibitors’ effects on renal function, both empagliflozin and canagliflozin have been shown to preserve renal function compared with placebo (1,2). Consistent with the SGLT2 inhibitor pharmacodynamic class effect, ertugliflozin initially demonstrated reduced eGFR, followed by return to baseline values after continued therapy (38). Because of a lack of data, future studies are needed to assess the association between kidney function and FG with SGLT2 inhibitor use.
Strengths to this review of the literature for case reports of FG associated with SGLT2 inhibitors include the independent and systematic review of the literature using Rayyan software. Previous studies have reported a low number of FG cases within the FAERS database, indicating a potential under reporting of cases based on inclusion/exclusion criteria (3). Our review, with limitations, supports previous findings that there is potential of underreporting. The mention of FG in diagnosis is likely to indicate a true case, as it is such a rare and specialized disease, with reported overall occurrence in 1.6–3.3 of 100,000 patients annually in the United States and an increased risk (three times) in patients with diabetes (12,15,31,39,40). Furthermore, the nine case reports identified from the literature search are more than have been identified in any other article to our knowledge.
This review also included some limitations. The FAERS database is intended to improve access to adverse effects attributed to medications and biologics. First, reported information is voluntary and does not establish causation or rate of occurrence of an adverse effect. Nonetheless, such trends are exigent and warrant further evaluation. For this reason, the FDA issued a warning in 2018 about the rare occurrence of FG associated with SGLT2 inhibitors, requiring an update in prescribing information (12). Additionally, the duration of SGLT2 inhibitor use was not reported in the FAERS database; therefore, medication use cannot be directly correlated to development of FG. Cases may be over-reported within FAERS, although two of the authors independently reviewed the search results to exclude potential duplicate reports. Conversely, the occurrence of FG may have been underreported, since the search terms excluded combination therapies, and the only reaction term to specifically localize the involved tissue was “Fournier’s gangrene.” The broad term “necrotizing fasciitis” was not included.
Other considerations should be taken into account in evaluating the correlation between SGLT2 inhibitor use and development of FG. The addition of an SGLT2 inhibitor to the standard of care therapy for type 2 diabetes (such as metformin) alludes to the patient having uncontrolled diabetes or other comorbidities. It remains unclear, however, whether this may be a direct correlation, since patients who are not taking an SGLT2 inhibitor are not necessarily at a significantly higher risk for development of FG. Patients’ underlying risk factors for FG should be carefully considered before initiation of an SGLT2 inhibitor. The number of cases identified from the FAERS database is consistent with findings reported by Hu et al. (41), reaffirming our concerns of underreporting in the literature. These concerns warrant further investigation.
Conclusion
With recent data to support the use of SGLT2 inhibitors for cardiorenal protective effects, we can expect a significant increase in their use by clinicians, not only in patients with type 2 diabetes, but also in some patients without diabetes. The data presented provide clinicians more evidence regarding the need to be vigilant in prescribing this drug class and its potential to lead to severe genital adverse effects such as FG. Comorbidities such as diabetes play a pivotal role in the survival of patients with FG. SGLT2 inhibitors only reduce A1C by 0.6–0.8%, but their cardiorenal benefits make them more advantageous as add-on therapy in patients with underlying cardiovascular disease or renal dysfunction. Although FG is rare, the mortality rate is as high as 88% in some cases (14). Therefore, patients with preexisting risk factors require careful monitoring to preemptively evaluate signs and symptoms for the development of urinary tract infections and genital infections, especially in patients who are immunocompromised. It is imperative that clinicians weigh the benefits and risks associated with this drug class in patients with high risks of FG (41,42).
Article Information
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
B.A.T. and E.S.-B. reviewed data and wrote and edited the manuscript. W.H.U. reviewed data, wrote the introduction, and reviewed/edited the manuscript. K.B. performed the literature search and wrote the Supplementary Materials. B.A.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.15147888
References
- 1. Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 2. Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 3. Bersoff-Matcha SJ, Chamberlain C, Cao C, Kortepeter C, Chong WH. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: a review of spontaneous postmarketing cases. Ann Intern Med 2019;170:764–769 [DOI] [PubMed] [Google Scholar]
- 4. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 6. Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep 2017;7:2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh M, Kumar A. Risks associated with SGLT2 inhibitors: an overview. Curr Drug Saf 2018;13:84–91 [DOI] [PubMed] [Google Scholar]
- 8. Merck Sharp & Dohme . Steglatro [package insert]. Whitehouse Station, NJ, Merck Sharp & Dohme, 2017 [Google Scholar]
- 9. Janssen Pharmaceuticals . Invokana [package insert]. Titusville, NJ, Janssen Pharmaceuticals, 2017 [Google Scholar]
- 10. AstraZeneca Pharmaceuticals . Farxiga [package insert]. Wilmington, DE, AstraZeneca Pharmaceuticals, 2017 [Google Scholar]
- 11. Boehringer Ingelheim Pharmaceuticals . Jardiance [package insert]. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, 2017 [Google Scholar]
- 12. U.S. Food and Drug Administration . FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes. Available from www.fda.gov/Drugs/DrugSafety/ucm617360.htm. Accessed 30 November 2019
- 13. Mallikarjuna MN, Vijayakumar A, Patil VS, Shivswamy BS. Fournier’s gangrene: current practices. ISRN Surg 2012;2012:942437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benjelloun EB, Souiki T, Yakla N, et al. Fournier’s gangrene: our experience with 50 patients and analysis of factors affecting mortality. World J Emerg Surg 2013;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El-Qushayri AE, Khalaf KM, Dahy A, et al. Fournier’s gangrene mortality: a 17-year systematic review and meta-analysis. Int J Infect Dis 2020;92:218–225 [DOI] [PubMed] [Google Scholar]
- 16. Fadini GP, Sarangdhar M, De Ponti F, Avogaro A, Raschi E. Pharmacovigilance assessment of the association between Fournier’s gangrene and other severe genital adverse events with SGLT-2 inhibitors. BMJ Open Diabetes Res Care 2019;7:e000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. U.S. Food and Drug Administration . Questions and answers on FDA’s Adverse Event Reporting System (FAERS). Available from https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers. Accessed 30 November 2019
- 18. U.S. Food and Drug Administration . FDA Adverse Event Reporting System (FAERS) public dashboard. Available from https://www.fda.gov/drugs/questions-and-answers-fdas- adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard. Accessed 1 July 2019
- 19. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan: a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar S, Costello AJ, Colman PG. Fournier’s gangrene in a man on empagliflozin for treatment of type 2 diabetes. Diabet Med 2017;34:1646–1648 [DOI] [PubMed] [Google Scholar]
- 21. Omer T, Dharan SS, Adler A. SLGT-2 inhibitor dapagliflozin and Fournier’s gangrene: a life-threatening severe adverse effect. Poster presented at the Diabetes UK Professional Conference in London, England, 21–23 March 2018 [Google Scholar]
- 22. Onder CE, Gursoy K, Kuskonmaz SM, Kocer U, Culha C. Fournier’s gangrene in a patient on dapagliflozin treatment for type 2 diabetes. J Diabetes 2019;11:348–350 [DOI] [PubMed] [Google Scholar]
- 23. Elshimy G, Correa R, Alsayed M, Jyothinagaram S. Early presentation of a rare complication of sodium-glucose cotransporter-2 inhibitors 10 days after initiation: case report and literature review. Cureus 2019;11:e5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagano Y, Yakame NK, Aoki H, Yamakawa T, Kondo NI. Fournier’s gangrene in a patient with type 2 diabetes mellitus treated with empagliflozin: a case report. Drug Saf Case Rep 2019;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodler S, Weig T, Finkenzeller C, Stief C, Staehler M. Fournier’s gangrene under sodium-glucose cotransporter 2 inhibitor therapy as a life-threatening adverse event: a case report and review of the literature. Cureus 2019;11:e5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elbeddini A, Gallinger J, Davey M, et al. A case of Fournier’s gangrene in a patient taking canagliflozin for the treatment of type II diabetes mellitus. Am J Case Rep 2020;21:e920115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. García-García A, Galeano-Valle F, Nuevo-González JA, Demelo-Rodríguez P. Fournier’s gangrene and SGLT2 inhibitors: a case study. Endocrinol Diabetes Nutr (Engl Ed) 2020;67:423–425 [in Spanish] [DOI] [PubMed] [Google Scholar]
- 28. Kasbawala K, Stamatiades GA, Majumdar SK. Fournier’s gangrene and diabetic ketoacidosis associated with sodium glucose co-transporter 2 (SGLT2) inhibitors: life-threatening complications. Am J Case Rep 2020;21:e921536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vyas HG, Kumar A, Bhandari V, Kumar N, Jain A, Kumar R. Prospective evaluation of risk factors for mortality in patients of Fournier’s gangrene: a single center experience. Indian J Urol 2013;29:161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang T, Patel SM, Hickman A, et al. SGLT2 inhibitors and the risk of hospitalization for Fournier’s gangrene: a nested case-control study. Diabetes Ther 2020;11:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sorensen MD, Krieger JN, Rivara FP, et al. Fournier’s Gangrene: population based epidemiology and outcomes. J Urol 2009;181:2120–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Voelzke BB, Hagedorn JC. Presentation and diagnosis of Fournier gangrene. Urology 2018;114:8–13 [DOI] [PubMed] [Google Scholar]
- 33. Kim SY, Dupree JM, Le BV, Kim DY, Zhao LC, Kundu SD. A contemporary analysis of Fournier gangrene using the National Surgical Quality Improvement Program. Urology 2015;85:1052–1057 [DOI] [PubMed] [Google Scholar]
- 34. Bjurlin MA, O’Grady T, Kim DY, et al. Causative pathogens, antibiotic sensitivity, resistance patterns, and severity in a contemporary series of Fournier’s gangrene. Urology 2013;81:752–758 [DOI] [PubMed] [Google Scholar]
- 35. Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther 2014;5:355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chim C, Newaz S. SGLT2 inhibitors and heart failure outcomes. US Pharm 2020;45:18–22 [Google Scholar]
- 37. Pioli MR, Ritter AMV, Modolo R. Unsweetening the heart: possible pleiotropic effects of SGLT2 inhibitors on cardio and cerebrovascular alterations in resistant hypertensive subjects. Am J Hypertens 2018;31:274–280 [DOI] [PubMed] [Google Scholar]
- 38. Cherney DZI, Heerspink HJL, Frederich R, et al. Effects of ertugliflozin on renal function over 104 weeks of treatment: a post hoc analysis of two randomised controlled trials. Diabetologia 2020;63:1128–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sorensen MD, Krieger JN, Rivara FP, Klein MB, Wessells H. Fournier’s gangrene: management and mortality predictors in a population based study. J Urol 2009;182:2742–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sorensen MD, Krieger JN. Fournier’s gangrene: epidemiology and outcomes in the general US population. Urol Int 2016;97:249–259 [DOI] [PubMed] [Google Scholar]
- 41. Hu Y, Bai Z, Tang Y, et al. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: a pharmacovigilance study with data from the U.S. FDA Adverse Event Reporting System. J Diabetes Res 2020;2020:3695101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tenório CEL, Lima SVC, Albuquerque AV, Cavalcanti MP, Teles F. Risk factors for mortality in fournier’s gangrene in a general hospital: use of simplified founier gangrene severe index score (SFGSI). Int Braz J Urol 2018;44:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]