Abstract
Background:
Fetal Alcohol Spectrum Disorder (FASD) is caused by prenatal alcohol exposure (PAE), the intake of ethanol (C2H5OH) during pregnancy. Features of FASD cover a range of structural and functional defects including congenital heart defects (CHDs). Folic acid and choline, contributors of methyl groups to one-carbon metabolism (OCM), prevent CHDs in humans. Using our avian model of FASD, we reported that betaine, another methyl donor downstream of choline, prevents CHDs. The CHD preventions are substantial but incomplete. Ethanol causes oxidative stress as well as depleting methyl groups for OCM to support DNA methylation and other epigenetic alterations. To identify more compounds that can safely and effectively prevent CHDs and other effects of PAE, we tested glutathione (GSH), a compound that regulates OCM and is known as a “master antioxidant.”
Methods/Results:
Quail embryos injected with a single dose of ethanol at gastrulation exhibited congenital defects including CHDs similar to those identified in FASD individuals. GSH injected simultaneously with ethanol not only prevented CHDs, but also improved survival and prevented other PAE-induced defects. Assays of hearts at 8 days (HH stage 34) of quail development, when the heart normally has developed 4-chambers, showed that this single dose of PAE reduced global DNA methylation. GSH supplementation concurrent with PAE normalized global DNA methylation levels. The same assays performed on quail hearts at 3 days (HH stage 19–20) of development, showed no difference in global DNA methylation between controls, ethanol-treated, GSH alone, and GSH plus ethanol-treated cohorts.
Conclusions:
GSH supplementation shows promise to inhibit effects of PAE by improving survival, reducing the incidence of morphological defects including CHDs, and preventing global hypomethylation of DNA in heart tissues.
Keywords: anti-oxidants, cardiogenesis, congenital heart defects, prenatal supplements, epigenetic regulation, GSH, heart defects, heart development
Background
Prenatal alcohol exposure (PAE) has a broad range of major structural and functional consequences resulting in Fetal Alcohol Spectrum Disorder (FASD) (Hoyme et al., 2016). Craniofacial and neurobehavioral characteristics define a subset of individuals with PAE as having fetal alcohol syndrome (FAS)(O’Leary, 2004). However, it has been recognized over the years that many systems, including the cardiovascular system, are affected by PAE (Burd et al., 2007; Hoyme et al., 2016; Reid et al., 2019). Research to identify compounds that could protect the embryo from PAE-induced effects has expanded, with much of the clinical focus on neurobehavioral measures (Coles et al., 2015; Young et al., 2014). Our focus has been on preventing PAE-induced congenital heart defects (CHDs) that can result from as little as a single binge drinking episode early during the first trimester in human gestation (Maier and West, 2001; Sayal et al., 2014, 2009). The embryo is at particular risk at this time because most women do not know they are pregnant that early. We modeled this PAE using a single injection of ethanol into quail eggs and discovered that cardiac structures and function are abnormal within 24 hours (Ford et al., 2017b; Karunamuni et al., 2015b, 2014a; Peterson et al., 2017). The defects that resulted from this acute regimen of exposure resembled those reported in FASD individuals (Burd et al., 2007) and are included in the current guidelines for diagnosing FASD (Hoyme et al., 2016). We also found that betaine co-administered with the single bolus of alcohol is capable of preventing these CHDs in a substantial number of embryos in this avian model of PAE (Karunamuni et al., 2017).
PAE disrupts embryogenesis through multiple mechanisms [reviewed in (Ehrhart et al., 2019; Sulik, 2014)]. One mechanism is the disruption of epigenetic regulation. PAE is known to reduce the substrates and alter the enzymes available for one-carbon metabolism (OCM) that are critical for DNA methylation. The most abundant and studied form of DNA methylation is the addition of a methyl group to cytosine at position C5 (Mandal et al., 2017). Direct effects of ethanol on the enzymes involved in OCM have been reported in adult tissues (Auta et al., 2017). In many species, including humans, it has been established that there is a DNA demethylation phase after fertilization followed by rapid DNA methylation postimplantation (Dobbs et al., 2013; Zeng and Chen, 2019). DNA methylation has been associated with a reduction in gene expression (Brandeis et al., 1993; Meehan, 2003; Phillips, 2008). However, depending on the site of DNA methylation, it has also been associated with an increase or a neutral effect on gene expression (Anastasiadi et al., 2018; Lea et al., 2018; Stricker and Götz, 2018). DNA methylation is organism, organ, stage, and tissue specific, ranging from 1–8% of total DNA for 5-mC (Roost et al., 2017).
Early embryogenesis is a time of rapid DNA methylation as cells proliferate and differentiate (Guibert et al., 2009; Li et al., 2016a; Zhou, 2012). At this time the requirement for donors of methyl groups needed for methylation becomes critical. It is well known that deficiencies in the methyl donor folate increase the risk for birth defects with neural tube defects receiving the most attention in research investigations [reviewed in (Beaudin and Stover, 2007; Imbard et al., 2013)]. The folic acid (FA) requirement becomes high during pregnancy and, as if in response, FA is more concentrated in the embryo/fetus compared to the mother (Economides et al., 1992; Hutson et al., 2012; Wallace et al., 2008). Therefore a PAE-induced reduction in methyl group availability during early embryogenesis could lead to hypomethylated DNA, including those loci important for cardiac progenitors (Lan and Evans, 2019; Li et al., 2018; Liu et al., 2009). This is particularly important in neural crest cells that undergo significant differentiation and migration to create valves, septa, and outflow tracts (Keyte and Hutson, 2012). These pluripotent cells greatly influence heart development in many ways (Smith et al., 2014; Waldo et al., 1999).
Another important mechanism for the teratogenic effects of PAE is through the production of excess reactive oxygen species (ROS) (Brocardo et al., 2011; Dennery, 2007; Henderson et al., 1995). Compounds that reduce the negative effects of PAE as supplements include choline, folic acid and betaine (Jacobson et al., 2018; Jiang et al., 2020; Karunamuni et al., 2017, 2014b; Shi et al., 2014). While these are likely to be preventing PAE effects in part through serving as methyl donors to OCM, all are also known antioxidants (Atteia, Bashir Mahmoud Rezk El-Kak, Abd El-Aziz Atteia Lucchesi, Pamela A Delafontane, 2009; Rezk et al., 2011; Wu et al., 2014; Zhang et al., 2016). Among the most abundant endogenous antioxidants in the human body is glutathione (Lushchak, 2012). Glutathione (GSH: gamma-1-glutamyl-L-cysteinyl-glycine) is a tripeptide, made by all types of cells [reviewed in (Forman et al., 2009; Wu et al., 2004)]. Reduced GSH, also known as L-glutathione, is the active form and plays major roles in repairing oxidative damage and preventing cells from excess reactive oxygen species (ROS) (Zitka et al., 2012). GSH also helps in the recycling and functions of other antioxidants in the body, like vitamin C, vitamin E, lipoic acid and CoQ10 (Dringen, 2000; Leopold, 2015; Willcox et al., 2008). GSH protects mitochondria from organic toxins and free radicals produced during metabolic activities, saves cells from adverse effects of ROS, and ensures the much needed energy supply for metabolism (Enns and Cowan, 2017). Oxidized GSH inhibits DNA and histone methylation by inhibiting the activity of S-adenosyl methionine synthetase, a key enzyme in the synthesis of S-adenosyl methionine (SAM) which is a substrate for DNA methyltransferases and histone methyltransferases. Addition of GSH helps recover the activity of S-adenosyl methionine synthetase and maintain the required balance of DNA and histone methylation for gene expression (García-Giménez and Pallardó, 2014; Mato et al., 2002). For this investigation we tested the efficacy of GSH in the prevention of PAE-induced CHDs. GSH has the potential to suppress at least two critical avenues of PAE-induced disruption, epigenetic alterations and oxidative stress (Ducker and Rabinowitz, 2017; Forman et al., 2009). We hypothesized that the CHDs and other early structural defects induced by PAE in our avian model of FASD would be prevented or reduced by administering GSH at the time of ethanol exposure. In this study we also tested whether GSH is able to protect global DNA methylation levels.
Materials and Methods
Quail embryos
Fertilized quail eggs (Cortunix cortunix communis) were obtained from a commercial supplier (Northwest Heritage Quail, Pullman, WA) and incubated in a humidified egg incubator (G.Q.F. Manufacturing Co., Savannah, GA) at 38°C. According to our Institutional Animal Care and Use Committee (IACUC) policy on use of avian embryos, approval is not required for studies unless investigators are using eggs/embryos older than 3 days before hatching. Quails hatch at 17 days of incubation and all of our studies were concluded by 8 days.
Dose response of glutathione on the prevention of PAE consequences
The dose response was tested as previously published (Karunamuni 2017) for different doses of GSH alone (Table 1) and GSH plus ethanol (Table 2). Briefly, GSH (L-Glutathione reduced, cat#G4251, Sigma-Aldrich) was dissolved in phosphate buffered saline (1XPBS diluted from10X PBS, Cat# BP399500, Fisher Scientific) and a range of concentrations was injected in combination with 40 microliters of 50% ethanol solution into fertilized eggs incubated for 21 hours. This ethanol exposure simulates a binge drinking episode during the first trimester of pregnancy as previously described (Karunamuni et al., 2015, 2017). The embryos were assayed after a total of 8 days of incubation when the hearts would have normally septated to form the primitive valve leaflets, divided outflow tracts, and 4 chambers. Survival and external gross defects in head and ventral body wall morphology were noted (Table 2). The optimal dose was chosen as the one with the best survival with the fewest embryos exhibiting gross defects.
Table 1.
Glutathione (GSH) toxicity data. Doses of GSH were injected into quail eggs 24 hours after incubation. At 8 days of incubation, the embryos were examined to determine survival and identify those with gross head and body defects.
| Treatment (n) | Survival rate (%) | Survivors with gross head/body defects (%) |
|---|---|---|
| Saline vehicle control (54) | 48 / 54 (89) | 0 / 48 (0) |
| Uninjected control (82) | 74 / 82 (90) | 2 / 74 (3) |
| 100 nmol GSH (30) | 27 / 30 (90) | 3 / 27 (11) p<0.043 |
| 1 µmol GSH (27) | 21 /27 (78) | 2 / 21 (10) p=0.09 |
| 10 µmol GSH (24) | 22 / 24 (92) | 1 / 22 (5) |
| 100 µmol GSH (24) | 21 / 24 (87) | 2 / 21 (10) p=0.09 |
| 1 mmol GSH (54) | 43 / 54 (80) | 4 / 43 (9) p=0.046 |
| 10 mmol GSH (47) | 45 / 47 (96) | 1 / 45 (2) |
Table 2.
Efficacy of glutathione (GSH) in preventing ethanol-induced death and defects. At 24 hours, quail eggs were injected with 40ul of a solution containing 50% ethanol (EtOH) and range of GSH concentrations. Embryos were examined at 8 days of incubation for survival and gross head and body defects.
| Treatment (n) | Survival rate (%) | Survivors with gross head/body defects (%) |
|---|---|---|
| Saline vehicle control (54) | 48 / 54 (89) | 0 / 48 (0) |
| Uninjected controls (82) | 74 / 82 (90) | 2 / 74 (3) |
| 10 nmol GSH/EtOH (25) | 17 / 25 (68) | 5 / 17 (29) |
| 100 nmol GSH /EtOH (24) | 17 / 24 (71) | 1 / 17 (6) |
| 1 µmol GSH /EtOH (25) | 21 / 25 (84) | 3 / 21 (14) |
| 10 µmol GSH /EtOH (30) | 26 / 30 (87) | 6 / 26 (23) |
| 100 µmol GSH /EtOH (36) | 30 / 36 (83) | 5 / 30 (17) |
| 1 mmol GSH/EtOH (48) | 41 / 48 (85) | 12 / 41 (29) |
| 10 mmol GSH/EtOH (48) | 42/ 48 (88) | 9 / 42 (21) |
The optimal dose was used for the rest of the experiments. Fertilized eggs were injected 21 hours after the initiation of incubation with 40 microliters of 50% ethanol solution. Another cohort of eggs were injected with the optimal 10 µmol of GSH in the ethanol solution; control eggs received GSH alone, saline alone, or no injection. Embryos were staged according to Hamburger and Hamilton (HH) criteria (Hamburger and Hamilton, 1992). These eggs were incubated for a total of 3 (HH stage 19) or 8 days (HH stage 34) and embryonic hearts were analyzed for survival, external defects, and CHDs or rapidly frozen and stored at 80°C for global DNA methylation assays (see below).
Analysis of congenital heart defects by optical coherence tomography (OCT)
Hearts were optically cleared and then analyzed by optical coherence tomography (OCT) as described previously (Karunamuni et al., 2017, 2015b). Briefly, OCT images were generated from the raw data by using Customized MATLAB programs (MathWorks; Natick, MA) and were analyzed with AMIRA software (FEI Visualization Sciences Group; Burlington, MA). Images were analyzed blinded for CHDs by a pediatric cardiologist who specializes in fetal cardiac imaging (JS).
Global DNA Methylation
Global DNA methylation as measured as a percentage of 5-methylcytosine (5-mC %) was assayed in the DNA extracted from hearts from each cohort of 3 day and 8 day embryos. The cohorts were embryos treated in ovo with (1) ethanol, (2) the vehicle saline, (3) GSH alone, and (4) ethanol plus GSH. To obtain adequate DNA from hearts of day 3 (HH stage 19–20) embryos, we pooled 5–6 hearts per sample and analyzed 8 samples per cohort. For the 8 day hearts, one heart was used per sample. DNA was extracted using the FitAmp General Tissue Section DNA Isolation Kit (Epigentek), DNA concentration was determined by NanodropOne (ThermoFisher), and the global DNA methylation assay [Epigentek, Global DNA Methylation (5-mC) ELISA Easy Kit] was used. Samples were measured in duplicate and the average taken to quantify the percentage DNA methylation. The standard curve was generated from the standard methylated DNA provided with the assay kit over the amount of total DNA.
Statistical analyses
Significance between groups (survival, defects, CHDs) was calculated with the Fisher’s exact test using MiniTab (State College, PA, USA), and a p-value of <0.05 was considered significant. 5-mC methylation data were analyzed using Microsoft Excel (version 2016). Data are presented as mean with standard deviation. Independent sample t-tests were conducted between the groups with equal variance assumptions to evaluate the difference significance between their means. A p-value of <0.05 was considered statistically significant. Microsoft Excel was used to create the graphs.
Results
Dose response to glutathione with or without ethanol exposure
A range of GSH concentrations dissolved in PBS was injected into fertilized quail eggs to determine the toxicity of GSH alone in this system (Table 1). The lowest concentration of GSH that resulted in the highest survival at 8 days of incubation with the least percentage of defects was chosen for the next phase of our investigations. Next, we injected these same GSH concentrations with ethanol. We found the lowest quantity of GSH that would best prevent death and congenital defects when injected in quail egg with ethanol was 40 µl of a 10 µM solution (Table 2). For a human, this is equivalent to about 24g of glutathione a day (0.34g/kg in a 70kg woman). We emphasize here that the stability and bioavailability of exogenous GSH is known to be low (Beutler et al., 1963; Jeong et al., 2018; Schmitt et al., 2015).
Body and congenital heart defects were reduced by glutathione supplementation
Exposure to GSH with ethanol significantly increased survival vs. ethanol alone (75% vs 60%) and decreased gross body defects (15% vs 32%), but these were still significantly different from uninjected and saline-injected controls. The percent of embryos with PAE-induced CHDs was similar to our previously reported results at 60% (Karunamuni et al., 2017, 2015a). The number of embryos with structural CHDs after ethanol exposure was significantly reduced by the co-administration of GSH (21% vs 60%) and not significantly different from controls, as seen in (Table 3). Injection of GSH alone did not affect the incidence of CHDs compared to the uninjected or saline injected controls.
Table 3.
Embryo survival and defect rates on day 8. Values for uninjected controls were considered the standard. GSH by itself did not significantly increase gross body and head defects, though it did trend toward significance (p=0.053). Of those imaged for CHDs, GSH alone did not increase the incidence of CHDs. Those given ethanol alone had significantly lower survival and higher numbers of gross defects and CHDs (P<0.001, denoted by **). GSH given with ethanol significantly increased survival and decreased gross defects compared to ethanol (P <0.01, denoted by †), though there were more defects than in controls (P<0.01, denoted by *). Of those imaged for CHDs, there was no difference between controls and those given GSH and ethanol in the incidence of CHDs. The 10 µmol GSH amount was used for the subsequent experiment.
| Treatment | Survival (%) | Gross head/body defects (%) | Imaged hearts with a CHD (%) |
|---|---|---|---|
| Uninjected Control | 86/97 (89) | 2/86 (2) | 3/21 (14) |
| Injected Control | 92/100 (92) | 3/92 (3) | 2/15 (13) |
| GSH (10umol) | 34/38 (89) | 4/34 (12) | 4/21 (19) |
| Ethanol (40ul 50%) | 107/177 (60) ** | 35/107 (32)** | 18/30 (60) ** |
| GSH 10umol + Ethanol 50% | 154/204 (75)*† | 23/153 (15) *† | 7/33 (21) † |
Among the imaged uninjected and saline-injected controls, the few observed CHDs were minor hypoplastic right ventricles and small/atretic pulmonary arteries (Table 4). The CHDs described in the imaged ethanol group were predominantly severely hypoplastic right ventricles (Figure 1B and 1C), though there were other significant numbers of defects associated with valve and septal abnormalities on the left and right side (Figure 1). Those given GSH and ethanol had similar defects to those with ethanol alone, although with significantly lower incidence.
Table 4.
CHDs diagnosed in imaged hearts. The total number of abnormal hearts is indicated, as is the number of hearts with each specified defect. Hearts may have more than one defect, so numbers add up to more than the number of hearts in each group. However, defects included in a syndrome were not counted twice (e.g. VSD was not counted as part of AV Canal, LV hypoplasia not counted in HLHS, etc.). RV – right ventricle, LV – left ventricle, HLHS – hypoplastic left heart syndrome, AV – atrioventricular, VSD – ventricular septal defect, DORV – double outlet right ventricle, TOF – tetralogy of Fallot.
| Uninjected and saline injected Controls (n = 3) | GSH ( n= 4) | Ethanol (n= 18) | GSH + Ethanol (n=7) |
|---|---|---|---|
| Atretic main pulmonary artery (1) Hypoplastic RV (2) |
Undifferentiated Single Ventricle (2) TOF (1) Isolated VSD (1) |
Hypoplastic RV (12) Hypoplastic LV (1) RV Hypertrophy (1) LV Hypertrophy (1) LV Dilatation (2) HLHS (2) DORV (2) Abnormal LVOT (1) Abnormal RVOT (1) VSD (2) AV Canal (1) Noncompaction (1) Conotruncal Defect (1) |
Hypoplastic RV (3) DORV (1) VSD (2) Dilated RV (1) LV Hypertrophy (1) Pulmonary Valve Atresia (1) |
Figure 1: OCT Images of Heart Defects Induced by Ethanol Exposure.
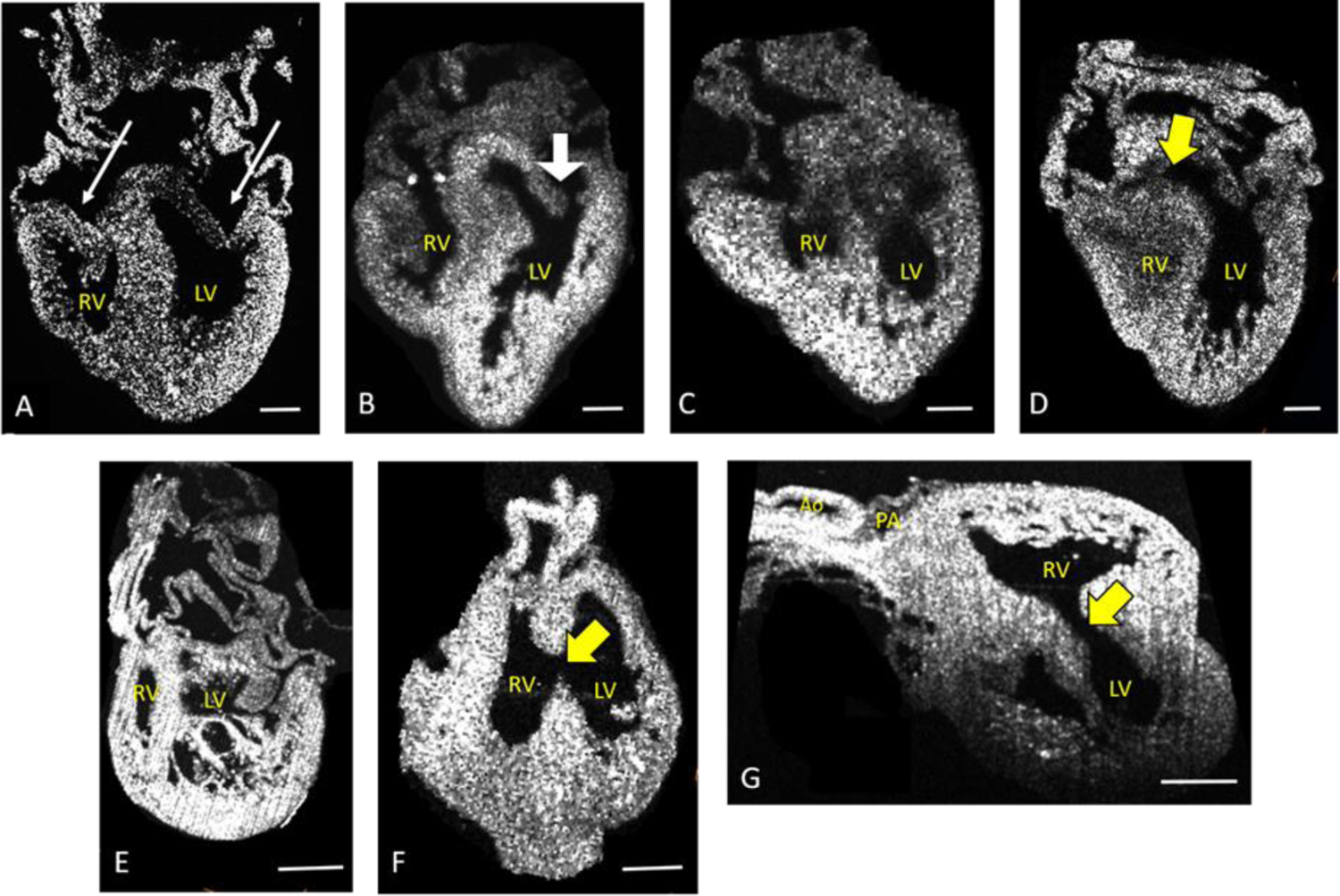
All images are of 8 day, HH stage 34 hearts. Panel (A) is of a normal heart, with long arrows pointing to normal left and right atrioventricular (AV) valve leaflets. Remaining panels show representative abnormalities: (B) Hypoplastic right ventricle and abnormal left AV valve leaflets (large white arrow), (C) Severely hypoplastic right ventricle, (D) ventricular septal defect, (E) left ventricular noncompaction, (F) and (G) different views of the same heart with double outlet right ventricle and large ventricular spetal defect. Yellow arrows point to ventricular septal defects. All scale bars are 50µm. Not all images are standard 4 chamber views, as that view cannot show all defects. RV = right ventricle, LV = Left Ventricle, PA = Pulmonary Artery, Ao = Aorta
Global DNA methylation was reduced by ethanol and preserved by glutathione in 8 day hearts.
A separate cohort of 8 day embryo hearts as described above was assayed for global DNA methylation. The global DNA methylation assay that we used specifically detects the methylation of the fifth carbon on cytosine termed C5-methylcytosine (5-mC). There was a significant reduction (41%, p-value 0.031) in the amount of methylated DNA (CpG) in the ethanol treated embryo hearts compared to all others, including control groups (Figure 2). GSH given with ethanol significantly prevented this decrease in DNA methylation and brought them to a level that was not different from that of the controls. GSH by itself had no effect on DNA methylation levels which remained similar to those of the saline injected controls.
Figure 2: Global methylation on day 8 of development is significantly decreased by ethanol exposure, which is normalized by the addition of glutathione.
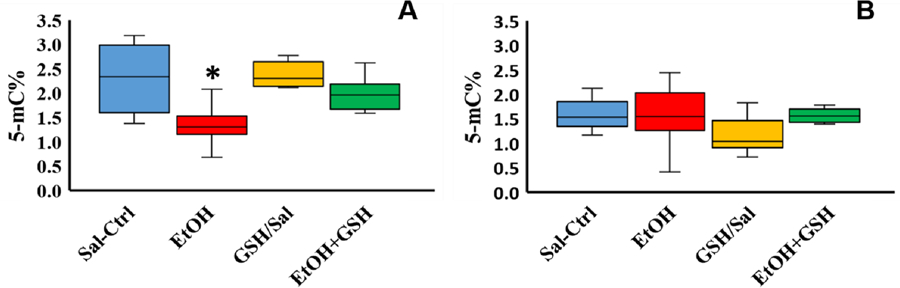
Global DNA Methylation (5-mC) levels were measure in (A) day 8 (stage 34) embryonic hearts (n=6/group) and (B) day 3 (stage 19–20) embryonic hearts (n=8/group). On day 8, Ethanol significantly lowered DNA methylation levels compared to saline and GSH, p=0.031. DNA methylation was normalized when glutathione was co-administered with ethanol. However, in day 3 (stage 19–20) hearts, ethanol did not change DNA methylation levels. All results are reported as percent of DNA with detected 5-mC. Saline = Sal-Ctrl, GSH alone in Saline = GSH/Sal, Ethanol = EtOH, Ethanol + GSH co-injections = EtOH+GSH.
Global DNA methylation levels were not affected by ethanol and glutathione in 3 day hearts
Hearts from 3 day embryos were assayed to detect if global DNA methylation was reduced closer to the time of ethanol exposure. Hearts at 3 days of incubation are tubular, just beginning to loop and form primitive chambers. Our analysis of hearts from 3 day embryos (HH stages 19–20, 2 days after injection) did not show any statistically significant changes in DNA methylation levels even with PAE alone (Figure 2). Likewise, GSH added alone or with ethanol did not result in any differences in global DNA methylation levels compared to the saline-injected controls (Figure 2).
Discussion
We found a compound that prevents PAE induced CHDs, making it a potential therapy that could prevent CHDs in the human population.
The first set of findings from this study show that an early single administration of glutathione (GSH), given at the same time as ethanol, improved quail embryo survival, reduced the number with gross body defects, and reduced the incidence of CHDs (Table 4 and Fig 1). A few studies have used animal models to assess the efficacy of compounds in the prevention of CHDs that result from acute, early PAE. The investigators tested folic acid (Sarmah and Marrs, 2013; Serrano et al., 2010), folic acid plus myoinositol (Serrano et al., 2010), all trans retinoic acid (Twal and Zile, 1997), betaine (Karunamuni et al., 2017), and now in this current study, GSH. Prevention of CHDs in all earlier studies was partial. In the studies from our group, the prevention compounds were only given in a single dose along with a single ethanol exposure. We previously found that betaine improved the percentage of embryos with normal hearts from 27% to 89%, approaching the 98% found in controls. Here, with GSH, the percentage of embryos with normal hearts increased from 40% to 79%, reaching a level (79%) which was not statistically different than controls (86%). Superior prevention of PAE-induced defects may require a different administration regimen with prevention compounds. Some compounds such as folic acid may not reach the therapeutic concentrations required to prevent defects within the embryo unless given preconceptionally (Bortolus et al., 2014; De-Regil et al., 2015; Hodgetts et al., 2015). Alternatively, as each of these compounds act through different pathways (Ducker and Rabinowitz, 2017; Friesen et al., 2007), a combination of prevention compounds may be warranted.
All defects, with the exception of noncompaction and undifferentiated single ventricle, are consistent with valvular and septal defects and similar to our previous data (Ford et al., 2017a; Karunamuni et al., 2015a, 2014b). Ethanol is known to affect the precardiac cells of the second heart field and neural crest cells that greatly influence heart development (Keyte and Hutson, 2012; Sarmah et al., 2020; Sarmah and Marrs, 2017). The second heart field forms endocardial cushions, the right ventricle, and portions of the outflow tract. The endocardial cushions serve as primitive valves while the heart is tubular, and as the heart loops they form mature valves and septa. We found a variety of CHDs resulting from PAE, the majority of which were severely hypoplastic right ventricles. Heart morphology was analyzed on day 8, well before quail eggs hatch (17dys). Our previous data indicate that the embryos with the most severe malformations do not survive to day 8 (Ford et al., 2017b), so we may have missed some CHDs by waiting until 8 days of incubation. However, it is difficult to diagnose CHDs and compare them to clinically significant malformations before the heart has fully septated. Furthermore, a number of these analyzed embryos may not have survived to hatching. Also of clinical importance is that all of the observed CHDs are seen in humans including those with FASD (Burd et al., 2007; Hoyme et al., 2016), and all require surgery and/or life-long monitoring and treatment [e.g.(Dearani et al., 2007; Dos et al., 2009)].
Combinations of compounds rather than one compound alone may be more effective in preventing PAE effects safely.
Most of the studies investigating the prevention of PAE-induced defects have used one compound at a time. The compounds that have shown promise in animal studies are FA, betaine, retinoic acid, and GSH. While they may partially overlap in their modes of action, each is unique in their metabolic roles, and they may be able to complement each other to prevent congenital defects when administered together. In a mouse model, a methyl-supplemented diet consisting of “cofactors and methyl donors for OCM, including methionine, betaine, choline, folic acid, and zinc” was used to prevent a wide range of PAE effects, including the methylation of specific gene loci (Downing et al., 2011). For some of these prevention compounds such as FA, there are already concerns about negative effects of administering high concentrations [e.g., (Tojal et al., 2019) and reviewed in (Liu et al., 2020)]. It may therefore be more effective and safer to use more than one compound at a time to increase efficacy without reaching concentrations of any one compound that might be harmful. Assessment of efficacy and safety of these compounds may require an objective measure, such as modifications in DNA methylation.
Global DNA methylation was reduced by PAE as shown by others and was protected by our compounds.
Many mechanisms have been proposed to explain how PAE disturbs developmental processes. One proposal is that PAE disrupts the normal course of DNA methylation during early development, leading to altered gene expression at a critical stage (Kaminen-Ahola et al., 2010; Mandal et al., 2017). PAE has been shown to reduce global and specific DNA methylation in whole mouse or rat fetuses, embryos, liver and brain tissues (Garro et al., 1991; Lussier et al., 2017; Ungerer et al., 2013). Here, we have shown that even acute PAE during gastrulation can reduce global DNA methylation in the heart much later in development (incubation day 8). We also found that a single administration of GSH at the same time as the PAE significantly blocked the effect of PAE on reducing global DNA methylation in 8 day hearts. These results correlated with the normalization of the structural abnormalities described in the first part of this study. A causal relationship between DNA methylation changes and CHDs has yet to be made.
To determine how early ethanol exposure reduced global DNA methylation, we assayed two days after ethanol injections (incubation day 3), when quail hearts are at the looping stage. No significant differences in global DNA methylation were detected in these younger heart tissues with any of the treatments, including ethanol. This latter result may mean that the sensitivity of our assay was inadequate to detect the changes in the low levels of 5-mC in the heart at this stage. Additionally, the precardiac cells of the second heart field are outside the heart on day 3, and so we may have missed DNA methylation changes outside of the heart tube itself. Another study that assayed 5-mC in chicken embryos found that 9 day hearts had a 5-mC level between 4–5% (Li et al., 2016b), which was slightly higher than what we detected in 8 day quail hearts (approximately 2.1%). The difference may be due to the different stage and species used. However, they also used HPLC, that is more sensitive than our ELISA-based assay. They did not assay 3 day hearts separately, but 5-mC from the whole embryo was 3–4% at 3 days and increased to 5% by 8 days. This is consistent with our data, demonstrating lower 5-mC at 3 days of incubation.
As a key epigenetic modification, DNA methylation has been widely studied to investigate its role in the etiology of several diseases, including FASD (Cobben et al., 2019; Robertson, 2005; Vukic and Daxinger, 2019). Methylation encodes information not by affecting DNA sequences, but by influencing regulatory protein binding to target sites, which in turn controls nucleosome structure and chromatin accessibility. These events directly or indirectly affect cell differentiation, development, growth, and phenotypic expression, and they may lead to disease. Recent studies have linked epigenetic changes to neurologic and cardiovascular diseases and cancer (Jin and Liu, 2018; Lan and Evans, 2019; Mandal et al., 2017). These studies have focused on the causes of DNA methylation alterations as potential therapeutic targets. DNA methylation modification is possible with currently available pharmacological agents, making targeted therapies an attractive strategy for treating diseases (Graça et al., 2016; Hamm and Costa, 2015; Kelly et al., 2010). Comparative genomics and other studies have revealed that genes critical to development and epigenetic modifications are highly conserved across different vertebrate species, including humans, mice, rats, and chickens (Jiang et al, 2014; Zhou et al, 2017). Cytosine-guanine dinucleotides (CpG) islands marked by methylation are consistently enriched in functional regions of the genome, and these regions are also highly conserved (Jiang et al., 2014). Conserved epigenetics and genes make the rapid and economic quail model ideal for exploring PAE and possible prevention compounds.
Our results confirm that ethanol affects global DNA methylation in the developing heart and that GSH is able to protect against PAE-induced global hypomethylation. Our future studies will probe the mechanisms by which GSH protects the heart from hypomethylation. It is also important to determine which genes have altered expression with PAE and which sites are protected by GSH. Following the details of the epigenetic alterations could determine whether the DNA methylation directly defined the trajectory to CHDs and indicate which pathways are critical for proper heart formation and function.
What about the future of using these compounds in humans?
The administration of compounds during pregnancy shows promise in mitigating PAE effects in humans [e.g., (Jacobson et al., 2018)]. Most of the clinical studies are retrospective with much of the attention paid to improvements in the neurodevelopmental consequences (Coles et al., 2015; Kable et al., 2015; Young et al., 2014). Even in the best prospective studies of humans, there is no control over the PAE amount or pattern of ingestion, although it is possible to encourage and monitor the amount of supplement that is ingested during pregnancy as was done by Jacobson and colleagues (Jacobson et al., 2018). In their study, a high dose of choline throughout pregnancy reduced the severity and incidence of PAE induced effects on growth, eye-blink conditioning (reflecting autonomic function conditioning), and learning and memory. Even with these improvements, choline did not reduce the incidence of FASD. They also did not assay specifically for CHDs in these infants or children. Except for severe cases, CHD detection usually requires specialized imaging and expertise.
The success of maternal prenatal choline intake in alleviating PAE behavioral effects in humans is a promising step in the prevention of PAE consequences, suggesting that supplementation with compounds other than folate may prevent PAE-induced defects. Both betaine and GSH are sold as over the counter supplements for adults, and they appear to be fairly safe (Cholewa et al., 2018; Hwang et al., 2018; Schmitt et al., 2015; Turck et al., 2019). We also know that it is critical to administer these compounds early, even before conception, and certainly when alcohol is being consumed in the first trimester. However, in order to consider the administration of GSH or betaine as prenatal supplements to prevent PAE-induced defects including CHDs, we need more information. What dose is most efficacious and also safe? Is it safe when taken throughout pregnancy or only during specific stages in development? What form and method of administration is best? Is administration of N-acetylcysteine (NAC) a better way to induce higher GSH levels? What is the best way to test for adequate levels of GSH? What combinations of compounds would be most efficacious and safe? Our quail model is a rapid and economical means to obtain preliminary answers to many of these questions, which can then be further confirmed in other animal models such as mice and rats.
Acknowledgements
Research was supported by the National Institutes of Health (NIH) award numbers R01HL083048; R01HL126747; R21HL115373. Ganga Karunamuni was supported by14POST19960016 from the American Heart Association (AHA). Caitlyn Gillespie was supported by the Northern Ohio Alliance for Graduate Education and the Professoriate (NOA-AGEP) funded by NSF-AGEP. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the AHA.
Footnotes
The authors have no conflict of interest to declare
References
- Anastasiadi D, Esteve-Codina A, Piferrer F (2018) Consistent inverse correlation between DNA methylation of the first intron and gene expression across tissues and species. Epigenetics Chromatin 11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atteia Bashir Mahmoud Rezk El-Kak, Abd El-Aziz Atteia Lucchesi, Pamela A Delafontane P (2009) Antioxidant activity of folic acid: From mechanism of action to clinical application. FASEB Jl 23:103.7. [Google Scholar]
- Auta J, Zhang H, Pandey SC, Guidotti A (2017) Chronic alcohol exposure differentially alters one-carbon metabolism in rat liver and brain. Alcohol Clin Exp Res 41:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin AE, Stover PJ (2007) Folate-mediated one-carbon metabolism and neural tube defects: Balancing genome synthesis and gene expression. Birth Defects Res Part C Embryo Today Rev 81:183–203. [DOI] [PubMed] [Google Scholar]
- Beutler E, Duron O, Kelly BM (1963) Beutler E, Duron 0 & Kelly B M. Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888. [PubMed] [Google Scholar]
- Bortolus R, Blom F, Filippini F, van Poppel MNM, Leoncini E, de Smit DJ, Benetollo PP, Cornel MC, de Walle HEK, Mastroiacovo P, Compagni A, Rigotti E, di Lannoy AN, Coati M, Manfrè S, Barbazza R, Zanconato G, Zenorini MT, Travagliati V, Mantovani E, Angeli A, Cavaliere E, Cherubini G, Negretto A, Lavarini E, Ozzi M, Papadopoulos N, Di Mambro E, Vessella M, Ettore G, Bianca S, Barone C, Cosmi E, Visentin S, Camerin M, Lanza P, Marinangeli S, Negrini G, Ottaviani A, Zivelonghi L, Baffoni A, Bertezzolo M, Pistolato M, Ioverno E, Somigliana E, Scarduelli C, Alagna F, Santi G, Cesari E, Zanini P, Morandini A, Cetin I, Laoreti A, Tresso C, Grazia Salviato M, Matterazzo M, Failli C, Marzolini M, Casaro A, Balestreri D, Benassi E, Caloi E, Libero Giorgino F, Schiavo A, Pietro Piazza G, Ruffini R, Jorizzo G, Cirelli G, Arcidiacono F, De Toni A, Rusconi S, Guaraldi C, Rosi P, Mortaro G, Valotto L, Guido A, Zanni G, Vernier C, Sandri A, Minisci N (2014) Prevention of congenital malformations and other adverse pregnancy outcomes with 4.0 mg of folic acid: Community-based randomized clinical trial in Italy and the Netherlands. BMC Pregnancy Childbirth 14:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis M, Ariel M, Cedar H (1993) Dynamics of DNA methylation during development. BioEssays 15:709–713. [DOI] [PubMed] [Google Scholar]
- Brocardo PS, Gil-Mohapel J, Christie BR (2011) The role of oxidative stress in fetal alcohol spectrum disorders. Brain Res Rev 67:209–225. [DOI] [PubMed] [Google Scholar]
- Burd L, Deal E, Rios R, Adickes E, Wynne J, Klug MG (2007) Congenital heart defects and fetal alcohol spectrum disorders. Congenit Heart Dis 2:250–255. [DOI] [PubMed] [Google Scholar]
- Chamberlain AA, Lin M, Lister RL, Maslov AA, Wang Y, Suzuki M, Wu B, Greally JM, Zheng D, Zhou B (2014) DNA methylation is developmentally regulated for genes essential for cardiogenesis. J Am Heart Assoc 3:e000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholewa JM, Hudson A, Cicholski T, Cervenka A, Barreno K, Broom K, Barch M, Craig SAS (2018) The effects of chronic betaine supplementation on body composition and performance in collegiate females: a double-blind, randomized, placebo controlled trial. J Int Soc Sports Nutr 15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobben JM, Krzyzewska IM, Venema A, Mul AN, Polstra A, Postma AV, Smigiel R, Pesz K, Niklinski J, Chomczyk MA, Henneman P, Mannens MMAM (2019) DNA methylation abundantly associates with fetal alcohol spectrum disorder and its subphenotypes. Epigenomics 11:767–785. [DOI] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, Pashtepa AO, Chambers CD, CIFASD (2015) Dose and timing of prenatal alcohol exposure and maternal nutritional supplements: Developmental effects on 6-month-old infants. Matern Child Health J 19:2605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, Rayco-Solon P (2015) Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev 2015. [DOI] [PMC free article] [PubMed]
- Dearani JA, Connolly HM, Martinez R, Fontanet H, Webb GD (2007) Caring for adults with congenital cardiac disease: successes and challenges for 2007 and beyond. Cardiol Young 17:87–96. [DOI] [PubMed] [Google Scholar]
- Dennery PA (2007) Effects of oxidative stress on embryonic development. Birth Defects Res Part C Embryo Today Rev 81:155–162. [DOI] [PubMed] [Google Scholar]
- Dobbs KB, Rodriguez M, Sudano MJ, Ortega MS, Hansen PJ (2013) Dynamics of DNA methylation during early development of the preimplantation bovine embryo. PLoS One 8:e66230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos L, Dadashev A, Tanous D, Ferreira-González IJ, Haberer K, Siu SC, Van Arsdell GS, Oechslin EN, Williams WG, Silversides CK (2009) Pulmonary valve replacement in repaired tetralogy of Fallot: Determinants of early postoperative adverse outcomes. J Thorac Cardiovasc Surg 138:553–559. [DOI] [PubMed] [Google Scholar]
- Downing C, Johnson TE, Larson C, Leakey TI, Siegfried RN, Rafferty TM, Cooney CA (2011) Subtle decreases in DNA methylation and gene expression at the mouse Igf2 locus following prenatal alcohol exposure: effects of a methyl-supplemented diet. Alcohol 45:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–671. [DOI] [PubMed] [Google Scholar]
- Ducker GS, Rabinowitz JD (2017) One-carbon metabolism in health and disease. Cell Metab 25:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economides DL, Ferguson J, MacKenzie IZ, Darley J, Ware II, Holmes-Siedle M (1992) Folate and vitamin B12 concentrations in maternal and fetal blood, and amniotic fluid in second trimester pregnancies complicated by neural tube defects. BJOG An Int J Obstet Gynaecol 99:23–25. [DOI] [PubMed] [Google Scholar]
- Ehrhart F, Roozen S, Verbeek J, Koek G, Kok G, van Kranen H, Evelo CT, Curfs LMG (2019) Review and gap analysis: molecular pathways leading to fetal alcohol spectrum disorders. Mol Psychiatry 24:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns GM, Cowan TM (2017) Glutathione as a redox biomarker in mitochondrial disease—Implications for therapy. J Clin Med 6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SM, McPheeters M, Wang Y, Ma P, Gu S, Strainic J, Snyder C, Rollins A, Watanabe M, Jenkins M (2017a) Increased Regurgitant Flow Causes Endocardial Cushion Defects in an Avian Embryonic Model of Congenital Heart Disease. Congenit Heart Dis 12:322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SM, McPheeters MT, Wang YT, Ma P, Gu S, Strainic J, Snyder C, Rollins AM, Watanabe M, Jenkins MW (2017b) Increased regurgitant flow causes endocardial cushion defects in an avian embryonic model of congenital heart disease. Congenit Heart Dis 12:322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman HJ, Zhang H, Rinna A (2009) Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med 30:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen RW, Novak EM, Hasman D, Innis SM (2007) Relationship of dimethylglycine, choline, and betaine with oxoproline in plasma of pregnant women and their newborn infants. J Nutr 137:2641–2646. [DOI] [PubMed] [Google Scholar]
- García-Giménez JL, Pallardó FV. (2014) Maintenance of glutathione levels and its importance in epigenetic regulation. Front Pharmacol 5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro AJ, McBeth DL, Lima V, Lieber CS (1991) Ethanol consumption inhibits fetal DNA methylation in mice: implications for the fetal alcohol syndrome. Alcohol Clin Exp Res 15:395–8. [DOI] [PubMed] [Google Scholar]
- Graça I, Pereira-Silva E, Henrique R, Packham G, Crabb SJ, Jerónimo C (2016) Epigenetic modulators as therapeutic targets in prostate cancer. Clin Epigenetics 8:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibert S, Forné T, Weber M (2009) Dynamic regulation of DNA methylation during mammalian development. Epigenomics 1:81–98. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL (1992) A series of normal stages in the development of the chick embryo. 1951. Dev Dyn 195:231–72. [DOI] [PubMed] [Google Scholar]
- Hamm CA, Costa FF (2015) Epigenomes as therapeutic targets. Pharmacol Ther 151:72–86. [DOI] [PubMed] [Google Scholar]
- Henderson GI, Devi BG, Perez A, Schenker S (1995) In Utero Ethanol Exposure Elicits Oxidative Stress in the Rat Fetus. Alcohol Clin Exp Res 19:714–720. [DOI] [PubMed] [Google Scholar]
- Hodgetts V, Morris R, Francis A, Gardosi J, Ismail K (2015) Effectiveness of folic acid supplementation in pregnancy on reducing the risk of small-for-gestational age neonates: a population study, systematic review and meta-analysis. BJOG An Int J Obstet Gynaecol 122:478–490. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA (2016) Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics 138:e20154256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson JR, Stade B, Lehotay DC, Collier CP, Kapur BM (2012) Folic acid transport to the human fetus is decreased in pregnancies with chronic alcohol exposure. PLoS One 7:e38057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang P, Morales Marroquín FE, Gann J, Andre T, McKinley-Barnard S, Kim C, Morita M, Willoughby DS (2018) Eight weeks of resistance training in conjunction with glutathione and L-Citrulline supplementation increases lean mass and has no adverse effects on blood clinical safety markers in resistance-trained males. J Int Soc Sports Nutr 15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbard A, Benoist JF, Blom HJ (2013) Neural tube defects, folic acid and methylation. Int J Environ Res Public Health 10:4352–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Carter RC, Molteno CD, Stanton ME, Herbert JS, Lindinger NM, Lewis CE, Dodge NC, Hoyme HE, Zeisel SH, Meintjes EM, Duggan CP, Jacobson JL (2018) Efficacy of Maternal Choline Supplementation During Pregnancy in Mitigating Adverse Effects of Prenatal Alcohol Exposure on Growth and Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol Clin Exp Res 42:1327–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong EM, Yoon J-H, Lim J, Shin J-W, Cho AY, Heo J, Lee KB, Lee J-H, Lee WJ, Kim H-J, Son YH, Lee S-J, Cho S-Y, Shin D-M, Choi K, Kim I-G (2018) Real-time monitoring of glutathione in living cells reveals that high glutathione levels are required to maintain stem cell function. Stem cell reports 10:600–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Wang L, Chen J, Wang L, Leach L, Luo Z (2014) Conserved and divergent patterns of DNA methylation in higher vertebrates. Genome Biol Evol 6:2998–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Lu D, Wang F, Zhang Y, Cao L, Gui Y, Sun S (2020) Folic acid supplement rescues ethanol-induced developmental defects in the zebrafish embryos. Acta Biochim Biophys Sin (Shanghai) 52:536–545. [DOI] [PubMed] [Google Scholar]
- Jin Z, Liu Y (2018) DNA methylation in human diseases. Genes Dis 5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminen-Ahola N, Ahola A, Maga M, Mallitt KA, Fahey P, Cox TC, Whitelaw E, Chong S (2010) Maternal ethanol consumption alters the epigenotype and the phenotype of offspring in a mouse model. PLoS Genet 6:e1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunamuni G, Gu S, Doghman YQ, Noonan AI, Rollins AM, Jenkins MW, Watanabe M, Ganga Karunamuni, Shi Gu, Yong Qiu Doughman, Noonan Amanda I., Rollins Andrew M., Michael W. Jenkins MW, Karunamuni G, Gu S, Doughman YQ, Noonan AI, Rollins AM, Jenkins MW, Watanabe M (2015a) Using optical coherence tomography to rapidly phenotype and quantify congenital heart defects associated with prenatal alcohol exposure. Dev Dyn 244:607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunamuni G, Gu S, Doughman YQ, Noonan AI, Rollins AM, Jenkins MW, Watanabe M (2015b) Using optical coherence tomography to rapidly phenotype and quantify congenital heart defects associated with prenatal alcohol exposure. Dev Dyn 244:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunamuni G, Gu S, Doughman YQ, Peterson LM, Mai K, McHale Q, Jenkins MW, Linask KK, Rollins AM, Watanabe M (2014a) Ethanol exposure alters early cardiac function in the looping heart: A mechanism for congenital heart defects? Am J Physiol - Hear Circ Physiol 306:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunamuni G, Sheehan MM, Doughman YQ, Gu S, Sun J, Li Y, Strainic JP, Rollins AM, Jenkins MW, Watanabe M (2017) Supplementation with the methyl donor betaine prevents congenital defects induced by prenatal alcohol exposure. Alcohol Clin Exp Res 41:1917–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunamuni GH, Gu S, Ford MR, Peterson LM, Ma P, Wang YT, Rollins AM, Jenkins MW, Watanabe M (2014b) Capturing structure and function in an embryonic heart with biophotonic tools. Front Physiol 5:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TK, De Carvalho DD, Jones PA (2010) Epigenetic modifications as therapeutic targets. Nat Biotechnol 28:1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyte A, Hutson MR (2012) The neural crest in cardiac congenital anomalies. Differentiation 84:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Evans T (2019) Epigenetic regulation of cardiac development and disease through DNA methylation. J life Sci (Westlake Village, Calif) 1:1–10. [PMC free article] [PubMed] [Google Scholar]
- Lea AJ, Vockley CM, Johnston RA, Del Carpio CA, Barreiro LB, Reddy TE, Tung J (2018) Genome-wide quantification of the effects of DNA methylation on human gene regulation. Elife 7:e37513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold JA (2015) Antioxidants and coronary artery disease. Coron Artery Dis 26:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Luo XM, Peng BH, Yang CJ, Peng C (2018) Interactive regulatory effect of histone H3K9ac acetylation and histone H3K9me3 methylation on cardiomyogenesis in mice. Chinese J Contemp Pediatr 20:950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhu Y, Zhi L, Han X, Shen J, Liu Y, Yao J, Yang X (2016a) DNA methylation variation trends during the embryonic development of chicken. PLoS One 11:e0159230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gao A, Wang Y, Chen M, Peng J, Yan H, Zhao X, Feng X, Chen D (2016b) Alcohol exposure leads to unrecoverable cardiovascular defects along with edema and motor function changes in developing zebrafish larvae. Biol Open 5:1128–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H-Y, Liu S-M, Zhang Y-Z (2020) Maternal folic acid supplementation mediates offspring health via DNA methylation. Reprod Sci 27:963–976. [DOI] [PubMed] [Google Scholar]
- Liu Y, Balaraman Y, Wang G, Nephew KP, Zhou FC (2009) Alcohol exposure alters DNA methylation profiles in mouse embryos at early neurulation. Epigenetics 4:500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak VI (2012) Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 2012:736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier AA, Weinberg J, Kobor MS (2017) Epigenetics studies of fetal alcohol spectrum disorder: Where are we now? Epigenomics 9:291–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SE, West JR (2001) Drinking patterns and alcohol-related birth defects. Alcohol Res Health 25:168–74. [PMC free article] [PubMed] [Google Scholar]
- Mandal C, Halder D, Jung KH, Chai YG (2017) Gestational alcohol exposure altered DNA methylation status in the developing fetus. Int J Mol Sci 18:1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato JM, Corrales FJ, Lu SC, Avila MA (2002) S-Adenosylmethionine: a control switch that regulates liver function. FASEB J 16:15–26. [DOI] [PubMed] [Google Scholar]
- Meehan RR (2003) DNA methylation in animal development. Semin Cell Dev Biol 14:53–65. [DOI] [PubMed] [Google Scholar]
- O’Leary C (2004) Fetal alcohol syndrome: Diagnosis, epidemiology, and developmental outcomes. J Paediatr Child Health 40:2–7. [DOI] [PubMed] [Google Scholar]
- Peterson LM, Gu S, Karunamuni G, Jenkins MW, Watanabe M, Rollins AM (2017) Embryonic aortic arch hemodynamics are a functional biomarker for ethanol-induced congenital heart defects [Invited]. Biomed Opt Express 8:1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T (2008) The Role of Methylation in Gene Expression. Nat Educ 1:116. [Google Scholar]
- Reid N, Akison LK, Hoy W, Moritz KM (2019) Adverse health outcomes associated with fetal alcohol exposure: A systematic review focused on cardio-renal outcomes. J Stud Alcohol Drugs 80:515–523. [PubMed] [Google Scholar]
- Rezk B, El-Kak AE-A, Delafontane P, Lucchesi P (2011) Antioxidant activity of folic acid in cardiovascular diseases. Free Radic Biol Med 51:S49–S50. [Google Scholar]
- Robertson KD (2005) DNA methylation and human disease. Nat Rev Genet 6:597–610. [DOI] [PubMed] [Google Scholar]
- Roost MS, Slieker RC, Bialecka M, van Iperen L, Gomes Fernandes MM, He N, Suchiman HED, Szuhai K, Carlotti F, de Koning EJP, Mummery CL, Heijmans BT, Chuva de Sousa Lopes SM (2017) DNA methylation and transcriptional trajectories during human development and reprogramming of isogenic pluripotent stem cells. Nat Commun 8:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah S, Marrs JA (2017) Embryonic Ethanol Exposure Affects Early- and Late-Added Cardiac Precursors and Produces Long-Lasting Heart Chamber Defects in Zebrafish. Toxics 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah S, Marrs JA (2013) Complex cardiac defects after ethanol exposure during discrete cardiogenic events in zebrafish: Prevention with folic acid. Dev Dyn 242:1184–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah S, Srivastava R, McClintick JN, Janga SC, Edenberg HJ, Marrs JA, Warren KR, Flentke GR, Smith SM, Sarkar DK, Gangisetty O, Wozniak JR, Eckerle JK, Georgieff MK, Foroud TM, Wetherill L, Wertelecki W, Chambers CD, Riley E, Zymak-Zakutnya N, Yevtushok L, Hutson JR, Stade B, Lehotay DC, Collier CP, Kapur BM, Zhang S, Wang L, Yang T, Chen LL, Zhao L, Wang T, Chen LL, Ye Z, Zheng Z, Qin J, Greenberg JA, Bell SJ, Stamm RA, Houghton LA, Antony AC, Young J, Giesbrecht H, Eskin M, Aliani M, Suh M (2020) Parental alcohol consumption and the risk of congenital heart diseases in offspring: An updated systematic review and meta-analysis. Nutrients 27:675–692. [DOI] [PubMed] [Google Scholar]
- Sayal K, Heron J, Draper E, Alati R, Lewis SJ, Fraser R, Barrow M, Golding J, Emond A, Davey Smith G, Gray R (2014) Prenatal exposure to binge pattern of alcohol consumption: mental health and learning outcomes at age 11. Eur Child Adolesc Psychiatry 23:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayal K, Heron J, Golding J, Alati R, Smith GD, Gray R, Emond A (2009) Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: Longitudinal population-based study. Pediatrics 123:e289–e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt B, Vicenzi M, Garrel C, Denis FM (2015) Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol 6:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Han M, Brinez P, Linask KK (2010) Fetal alcohol syndrome: cardiac birth defects in mice and prevention with folate. Am J Obstet Gynecol 203:75.e7–75.e15. [DOI] [PubMed] [Google Scholar]
- Shi Y, Li J, Chen C, Gong M, Chen Y, Liu Y, Chen J, Li T, Song W (2014) 5-Methyltetrahydrofolate rescues alcohol-induced neural crest cell migration abnormalities. Mol Brain 7:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Garic A, Flentke GR, Berres ME (2014) Neural crest development in fetal alcohol syndrome. Birth Defects Res Part C Embryo Today Rev 102:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker SH, Götz M (2018) DNA-methylation: Master or slave of neural fate decisions? Front Neurosci 12:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulik KK (2014) Fetal alcohol spectrum disorder In: Handbook of Clinical Neurology, pp 463–475. [DOI] [PubMed]
- Tojal A, Neves C, Veiga H, Ferreira S, Rodrigues I, Martel F, Calhau C, Negrão R, Keating E (2019) Perigestational high folic acid: impact on offspring’s peripheral metabolic response. Food Funct 10:7216–7226. [DOI] [PubMed] [Google Scholar]
- Turck D, Castenmiller J, De Henauw S, Hirsch-Ernst KI, Kearney J, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Engel KH, Frenzel T, Heinonen M, Marchelli R, Neuhäuser-Berthold M, Pöting A, Poulsen M, Sanz Y, Schlatter JR, van Loveren H, Turla E, Knutsen HK (2019) Safety of betaine as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J 17:e05658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twal WO, Zile MH (1997) Retinoic acid reverses ethanol-induced cardiovascular abnormalities in quail embryos. Alcohol Clin Exp Res 21:1137–1143. [PubMed] [Google Scholar]
- Ungerer M, Knezovich J, Ramsay M (2013) In utero alcohol exposure, epigenetic changes, and their consequences. Alcohol Res 35:37–46. [PMC free article] [PubMed] [Google Scholar]
- Vukic M, Daxinger L (2019) DNA methylation in disease: Immunodeficiency, Centromeric instability, Facial anomalies syndrome. Essays Biochem 63:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo K, Zdanowicz M, Burch J, Kumiski DH, Stadt HA, Godt RE, Creazzo TL, Kirby ML (1999) A novel role for cardiac neural crest in heart development. J Clin Invest 103:1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JMW, Bonham MP, Strain J, Duffy EM, Robson PJ, Ward M, McNulty H, Davidson PW, Myers GJ, Shamlaye CF, Clarkson TW, Molloy AM, Scott JM, Ueland PM (2008) Homocysteine concentration, related B vitamins, and betaine in pregnant women recruited to the Seychelles Child Development Study. Am J Clin Nutr 87:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox BJ, Curb JD, Rodriguez BL (2008) Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol 101:75D–86D. [DOI] [PubMed] [Google Scholar]
- Wu G, Fang Y-Z, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–92. [DOI] [PubMed] [Google Scholar]
- Wu P, Jiang W-D, Liu Y, Chen G-F, Jiang J, Li S-H, Feng L, Zhou X-Q (2014) Effect of choline on antioxidant defenses and gene expressions of Nrf2 signaling molecule in the spleen and head kidney of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 38:374–382. [DOI] [PubMed] [Google Scholar]
- Young JK, Giesbrecht HE, Eskin MN, Aliani M, Suh M (2014) Nutrition implications for fetal alcohol spectrum disorder. Adv Nutr 5:675–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Chen T (2019) DNA methylation reprogramming during mammalian development. Genes (Basel) 10:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhang H, Li H, Lai F, Li X, Tang Y, Min T, Wu H (2016) Antioxidant mechanism of betaine without free radical scavenging ability. J Agric Food Chem 64:7921–7930. [DOI] [PubMed] [Google Scholar]
- Zhou FC (2012) DNA methylation program during development. Front Biol (Beijing) 7:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitka O, Skalickova S, Gumulec J, Masarik M, ADAM V, Hubalek J, Trnkova Li, Kruesova J, Eckschlager T, Kizek R (2012) Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett 4:1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]


