Abstract
Objective:
To test the null hypothesis that enamel deproteinization with 10% papain gel does not increase the shear bond strength of orthodontic brackets bonded with resin-modified glass ionomer cement (RMGIC).
Materials and Methods:
One hundred and twenty bovine incisors were used and divided into eight groups: 1) Transbond XT according to the manufacturer's recommendations, 2) Transbond XT deproteinized with 10% papain gel, 3) RMGIC without enamel deproteinization and without etching, 4) RMGIC without enamel etching and with deproteinization with 10% papain gel, 5) RMGIC deproteinized with 10% papain gel and etched with polyacrylic acid, 6) RMGIC deproteinized with 10% papain gel and etched with phosphoric acid, 7) RMGIC deproteinized with 2.5% sodium hypochlorite, and 8) RMGIC etched with polyacrylic acid. After bonding, the mechanical tests were performed in a Universal mechanical test machine. The values obtained were submitted to an analysis of variance and afterward to the Tukey test (P < .05).
Results:
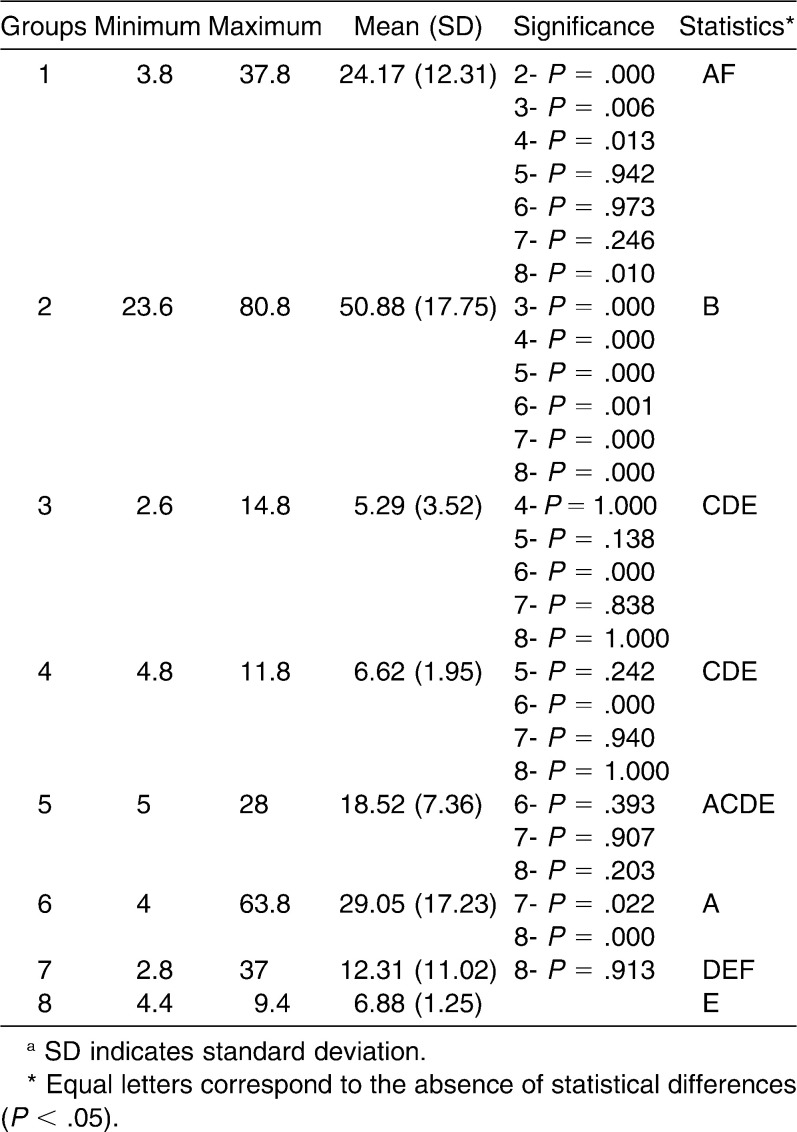
It was demonstrated that group 2 presented the highest shear bond strength value, and this differed statistically from the others; group 3 presented the lowest value and showed no differences from groups 4, 5, 7, and 8. Regarding the Adhesive Remnant Index, groups 2 and 6 presented the best results and groups 3 and 8 the worst. It could be concluded that enamel deproteinization with 10% papain gel increases the shear bond strength, irrespective of the etching agent.
Conclusions:
The hypothesis is rejected. Papain gel was shown to be a new ally in the orthodontic clinic.
Keywords: Shear strength, Composite resins, Orthodontic appliances, Papain
INTRODUCTION
Elimination of organic substances from the enamel surface before acid etching increases the resistance to orthodontic traction by providing a better acid etching pattern on enamel.1
Deproteinization of the enamel surface before bracket bonding was first proposed by Justus et al.1 This study used 5.25% sodium hypochlorite (NaOCl) for this purpose. According to De-Deus et al.,2 the NaOCl eliminates the organic matter present on the enamel surface by dissolving it. Among substances with similar properties, papain is outstanding.
Papain is extracted from the latex of the Carica papaya, belonging to the Caricaceae family, better known as the papaya (pawpaw).3 It is a proteolytic cysteine enzyme with antibacterial and anti-inflammatory properties, and it acts as an agent for removing debris without any harmful effect on tissues because of the specificity of the enzyme.3–6
In 2003, papain was introduced into dentistry. The product, Papacárie, is used in the chemical removal of caries. It is used with the aim of removing infected tissue without causing any damage to any healthy structure in the mouth, and requires neither instruments with cutting edges nor rotary instruments.5
The aim of the present study was to verify the hypothesis that deproteinization of the enamel surface with 10% papain gel increases the shear bond strength of brackets bonded with resin-modified glass ionomer cement (RMGIC).
MATERIALS AND METHODS
One hundred twenty bovine mandibular permanent incisors, extracted and stored in 10% formaldehyde solution, were used. After 7 days of fixation, they were cleaned and the periodontal tissue adhered to their roots was removed. When cleaning was concluded they were fixed in reduction bushes measuring 25 × 20 (PVC; Tigre, Joinvile, Brazil) with self-polymerizing acrylic resin and stored in water under refrigeration.
Initially, prophylaxis of all the teeth was performed with a pumice stone and water for 15 seconds, followed by washing and drying for an equal length of time. After this, the teeth were randomly divided into eight groups (n = 15) treated as in the following.
Group 1 was etched with 37% phosphoric acid for 15 seconds, followed by primer application and fixation with conventional Transbond XT, (3M/Unitek, Monrovia, Calif) according to the manufacturer's recommendations.
Group 2 was deproteinized with 10% papain gel, (Macela Dourada, Vitória da Conquista, Bahia, Brazil) etched with 37% phosphoric acid for 15 seconds, and underwent primer application and fixation with conventional Transbond XT according to the manufacturer's recommendations;.
Group 3 was fixed with RMGIC (3M/Unitek, Monrovia, Calif) without etching.
Group 4 was deproteinized with 10% papain gel (Figure 1), followed by fixation with RMGIC without etching.
Figure 1.
Commercial presentation of 10% papain gel.
Group 5 was deproteinized with 10% papain gel, fixed with RMGIC, and etched with polyacrylic acid.
Group 6 was deproteinized with 10% papain gel, etched with 37% phosphoric acid, and fixed with RMGIC.
Group 7 was deproteinized with 2.5% sodium hypochlorite, etched with polyacrylic acid, and fixed with RMGIC.
Group 8 was fixed with RMGIC with polyacrylic acid etching.
The polyacrylic acid, phosphoric acid, 10% papain gel, and 2.5% sodium hypochlorite were all applied on the vestibular surface of the enamel with an applicator brush.
The orthodontic brackets were fixed in the center of the crown with Transbond XT and RMGIC. Excess adhesive and glass ionomer were removed from the teeth with a probe, and each bracket was then light polymerized with an LED appliance (LED Radii, SDI, Baywater, Victoria, Australia) (850 Mw/cm2) for 40 seconds (10 seconds on each side). After bonding, all samples were stored in artificial saliva (Apsen, São Paulo, Brazil) in an oven at 37°C for 24 hours.
A custom-made stand was used to stabilize teeth for debonding tests. Each tooth was subjected to a shear load in a Universal Testing Machine, model DL-10.000 (EMIC, São José dos Pinhais, Brazil), applied with a knife-edged blade at a cross-head speed of 0.5 mm/min. The force was applied parallel to the tooth surface on top of each orthodontic bracket base, and the shear load was recorded at the point of failure. The force per unit area required to dislodge the bracket was then calculated and recorded as the shear bond strength (SBS) in megapascals.
The enamel surfaces were examined under a stereomicroscope (Stemi 2000-C; Carl Zeiss, Gottingen, Germany) under 16× magnification to determine the amount of composite remaining and then classified according to the Adhesive Remnant Index (ARI). The ARI scores ranged from 0 to 3, with 0 indicating no composite left on the enamel; 1, less than half of the composite left; 2, more than half of the composite left; and 3, all of the composite remained on the tooth surface.
Statistical analyses were performed with the SPSS version 13.0 program (SPSS Inc, Chicago, Ill). Descriptive statistics that included mean, standard deviation, median, and minimum and maximum values were calculated for all eight groups. Analysis of variance was applied to determine whether there were significant differences among the groups. For the post hoc test, the Tukey test was used. Kruskal-Wallis and Mann-Whitney U tests were used for assessing the ARI scores.
RESULTS
The shear bond strength results demonstrated that the highest bond strength values were achieved by group 2, and this differed statistically from the others. The lowest values were obtained by group 3, which did not differ statistically from groups 4, 5, 7, and 8 (Table 1).
Table 1.
Groups, Minimum, Maximum, Mean, and Standard Deviation of the Shear Bond Strength Values and Statistical Analysis of the Groups Evaluateda
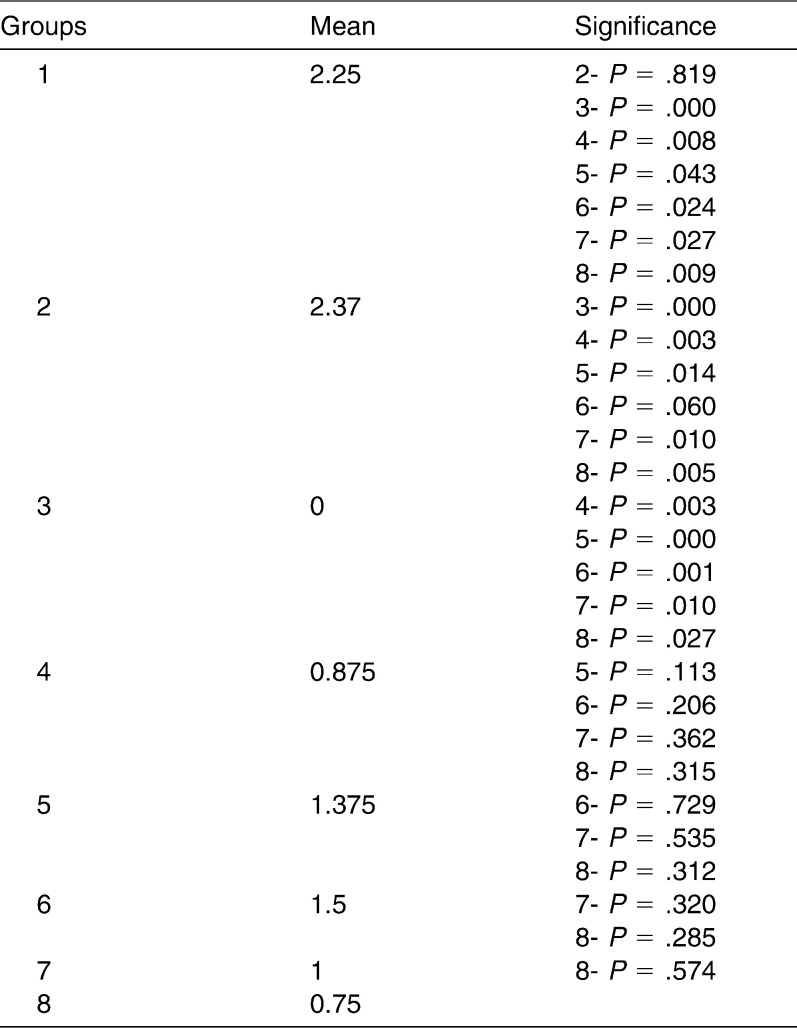
Regarding the ARI, no statistical differences were found between groups 1 and 2 (P = .819), 4 and 5 (P = 1.13), 4 and 6 (P = .206), 4 and 7 (P = .362), 4 and 8 (P = .315), 5 and 6 (P = .729), 5 and 7 (P = .535), 5 and 8 (P = .312), 6 and 7 (P = .320), 6 and 8 (P = .285), and 7 and 8 (P = .574). The mean ARI values in the evaluated groups are shown in Table 2.
Table 2.
Mean IRA Values of the Evaluated Groups
DISCUSSION
The formation of white spot lesions and marginal gingivitis adjacent to fixed orthodontic appliances occurs due to the presence of bacterial biofilm.7 According to Bishara and Ostby,8 decalcification is an important effect of orthodontic therapy on dental enamel.
With the intention of minimizing and preventing white spot lesions, there is awareness about the use of new fluoride-releasing materials.7 Glass ionomer cements are outstanding among all the others available on the market. Developed by Wilson and Kent,9 glass ionomer cements allow chemical bonding to enamel, dentin, and other surfaces, in addition to releasing fluoride.
However, these cements have lower bond strength to the enamel surface in comparison with orthodontic composites. With the intention of combining important characteristics of the two materials, such as shear bond strength and fluoride release, RMGICs were developed, which release fluoride without compromising the bond strength to the tooth surface.10–12
The bond strength between orthodontic accessories and enamel may be compromised by the presence of the acquired pellicle, which covers the soft and hard tissues in the oral region, particularly the surface of tooth enamel. This membrane is a biofilm free of bacterial colonization, and its most abundant components are proteins, glycoproteins, enzymes, and mucins or their derivatives.13 These organic elements make it difficult for composites to adhere to the tooth enamel surface, diminishing its shear bond strength.
With the purpose of eliminating the influence of the organic matrix on the adhesion of composites to the enamel surface, Justus et al.1 suggested the use of 5.25% sodium hypochlorite for 60 seconds as a deproteinizing agent before etching with 37% phosphoric acid, showing good results.
In the environment there are various substances present with the characteristic of deproteinizing surfaces, among these, papain.
Papain is an alkaloid enzyme extracted from the papaya. It is obtained from the latex in the leaves and skin of the mature fruit. It has a proteolytic action and presents antibacterial and anti-inflammatory properties, acting as an agent for the removal of necrotic remains, without cytotoxic effects on the tissues.3,5,6
Among the various natural enzymes found, papain has some advantages, such as the enzymatic quality and activity as well as stability under the adverse conditions of temperature, humidity, and atmospheric pressure.6
The aim of the present study was to verify the hypothesis that deproteinization of the enamel surface with 10% papain gel for 60 seconds increases the shear bond strength of brackets bonded with RMGIC.
Papain presented in gel form is available under the brand name of Papacárie which, differently from the product used in the present study, is not produced from pure papain. Papacárie, in addition to papain, is composed of chloramine—a compound of chloride and ammonia, used for root canal irrigation—and toluidine blue coloring, a photosensitizer with antimicrobial properties.6 In this study, the gel was used, because in this presentation, in addition to being easy to manipulate, one has better control of the application.
The results found in the present study indicate that the group deproteinized with papain and etched with phosphoric acid obtained the best shear bond strength result in comparison with the group in which Transbond XT was used according to the manufacturer's recommendations. As regards the RMGIC, the group in which papain was used as the deproteinizing agent obtained the best shear bond strength result when compared with the group deproteinized with 2.5% sodium hypochlorite, both etched with polyacrylic acid. This proves that the papain gel is a better enamel surface deproteinizing agent than 2.5% NaOCl, irrespective of the etching agent.
The groups in which no etching agent was used (4, 7, and 8) showed no statistical differences between each other. In addition, they obtained lower results than those etched with phosphoric acid, which may be explained by the lack of mechanical retention between the material and tooth, since only chemical bonding occurred, as observed by Pithon et al.7
Groups 1, 2, and 6 were etched with 37% phosphoric acid and, in addition to showing no statistical difference among them, obtained the best results for shear bond strength, as did Ulker et al14 in that study.
Groups 5, 7, and 8 presented no statistical differences between each other; however, they obtained lower results than those presented by the groups etched with phosphoric acid (Groups 1, 2, and 6). This is explained by the fact that the polyacrylic acid etching agent used in these groups has an etching pattern with less extension and depth than that of phosphoric acid, as observed in several other studies.7–14
In a study conducted by Smith and Cartz,15 etching with polyacrylic acid caused little demineralization of the enamel surface. Bishara and Ostby8 also observed that teeth etched with polyacrylic acid had a significantly lower shear bond strength than teeth etched with 37% phosphoric acid before being bonded with RMGIC, which was proved in the present study.
The ARI shown with the use of Transbond XT and RMGIC, both used with papain gel as deproteinizing agent and phosphoric acid as etching agent, obtained the best results. In contrast, when using the RMGIC without etching, and even when etched with polyacrylic acid, the worst results were obtained, proving that the 10% papain gel is an efficient deproteinizing agent, increasing the bond of the materials to the enamel surface.
CONCLUSIONS
Enamel deproteinization with 10% papain gel increases the shear bond strength, irrespective of the etching agent. Therefore, the gel form of papain becomes an efficient alternative for deproteinization of the tooth enamel surface before bonding orthodontic brackets with RMGIC.
REFERENCES
- 1.Justus R, Cubero T, Ondarza R, Morales F. A new technique with sodium hypochlorite to increase bracket shear bond strength of fluoride-releasing resin-modified glass ionomer cements: comparing shear bond strength of two adhesive systems with enamel surface deproteinization before etching. Semin Orthod. 2010;16:66–75. [Google Scholar]
- 2.De-Deus G, Souza E. M, Marins J. R, Reis C, Paciornik S, Zehnder M. Smear layer dissolution by peracetic acid of low concentration. Int Endod J. 2011;44:485–490. doi: 10.1111/j.1365-2591.2010.01847.x. [DOI] [PubMed] [Google Scholar]
- 3.Bruno L. H, Guedes C. C, Motta L. J, Santos E. M, Bussadori S. K. Comparación entre la utilización de elementos rotatorios de baja velocidad y tratamiento químico mecánico de caries dentinal en dentición decidua. Acta Odontológica Venezolana. 2009;47:1–9. [Google Scholar]
- 4.Piva E, Ogliari F. A, Moraes R. R, Cora F, Henn S, Correr-Sobrinho L. Papain-based gel for biochemical caries removal: influence on microtensile bond strength to dentin. Braz Oral Res. 2008;22:364–370. doi: 10.1590/s1806-83242008000400014. [DOI] [PubMed] [Google Scholar]
- 5.Motta L. J, Martins M. D, Porta K. P, Bussadori S. K. Aesthetic restoration of deciduous anterior teeth after removal of carious tissue with Papacarie. Indian J Dent Res. 2009;20:117–120. doi: 10.4103/0970-9290.49060. [DOI] [PubMed] [Google Scholar]
- 6.Silva L. R, Murillo J. H, Santos E. M, Guedes-Pinto A. C, Bussadori S. K. Utilización del gel de la papaya para la remoción de la caries. Acta Odontológica Venezolana. 2005;43:1–5. [Google Scholar]
- 7.Pithon M. M, Dos Santos R. L, de Oliveira M. V, Ruellas A. C, Romano F. L. Metallic brackets bonded with resin-reinforced glass ionomer cements under different enamel conditions. Angle Orthod. 2006;76:700–704. doi: 10.1043/0003-3219(2006)076[0700:MBBWRG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Bishara S. E, Ostby A. W. White spot lesions: formation, prevention, and treatment. Semin Orthod. 2008;14:174–182. [Google Scholar]
- 9.Wilson A. D, Kent B. E. A new translucent cement for dentistry. The glass ionomer cement. Br Dent J. 1972;132:133–135. doi: 10.1038/sj.bdj.4802810. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler A. W, Foley T. F, Mamandras A. Comparison of fluoride release protocols for in-vitro testing of 3 orthodontic adhesives. Am J Orthod Dentofacial Orthop. 2002;121:301–309. doi: 10.1067/mod.2002.120160. [DOI] [PubMed] [Google Scholar]
- 11.Bishara S. E, Ostby A. W, Laffoon J. F, Warren J. Shear bond strength comparison of two adhesive systems following thermocycling. A new self-etch primer and a resin-modified glass ionomer. Angle Orthod. 2007;77:337–341. doi: 10.2319/0003-3219(2007)077[0337:SBSCOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Bishara S. E, Ostby A. W, Laffoon J, Warren J. J. A self-conditioner for resin-modified glass ionomers in bonding orthodontic brackets. Angle Orthod. 2007;77:711–715. doi: 10.2319/070606-280.1. [DOI] [PubMed] [Google Scholar]
- 13.Hannig C, Hannig M, Attin T. Enzymes in the acquired enamel pellicle. Eur J Oral Sci. 2005;113:2–13. doi: 10.1111/j.1600-0722.2004.00180.x. [DOI] [PubMed] [Google Scholar]
- 14.Ulker M, Uysal T, Ramoglu S. I, Ucar F. I. Bond strengths of an antibacterial monomer-containing adhesive system applied with and without acid etching for lingual retainer bonding. Eur J Orthod. 2009;31:658–663. doi: 10.1093/ejo/cjp037. [DOI] [PubMed] [Google Scholar]
- 15.Smith D. C, Cartz L. Crystalline interface formed by polyacrylic acid and tooth enamel. J Dent Res. 1973;52:1155. doi: 10.1177/00220345730520053201. [DOI] [PubMed] [Google Scholar]