Abstract
Objective:
To investigate (1) the relationships between the stages of mandibular second molar calcification and skeletal maturity; and (2) whether second molar calcification stages can be used as a reliable diagnostic tool to determine skeletal maturity.
Materials and Methods:
Samples were derived from panoramic radiographs and lateral cephalograms of 300 subjects (137 males and 163 females) with ages ranging from 9 to 18 years, and estimates of dental maturity (Demirjian Index [DI]) and skeletal maturity (cervical vertebrae maturation indicators [CVMI]) were made.
Results:
A highly significant association (C* = 0.854 for males and 0.866 for females) was found between DI and CVMI. DI stage E corresponded to stage 2 of CVMI (pre–peak of pubertal growth spurt) and DI stages F and G corresponded to stages 3 and 4 of CVMI (peak of pubertal growth spurt). DI stage H was associated with stages 5 and 6 of CVMI (end of pubertal growth spurt).
Conclusion:
A highly significant association exists between DI and CVMI. Mandibular second molar DI stages are reliable indicators of skeletal maturity.
Keywords: Skeletal maturation, Mandibular second molar, Demirjian Index, Cervical vertebrae maturation indicators, Panoramic radiograph, Lateral cephalogram
INTRODUCTION
Assessment of skeletal maturity and dental development is a common clinical practice in many health professions, especially for growth modification in orthodontics and dentofacial orthopedics and for age estimation in forensic sciences. Because of the considerable variations in development among children, chronological age may have little or no role in the determination of the maturation stage of a child1–3 and has led to the concept of biologic or physiologic age. Physiologic age is the rate of progress toward maturity and is estimated by somatic, sexual, skeletal, and/or dental maturity.3–5
The technique for assessing skeletal maturity consists of a visual inspection of the developing bones, including their initial appearance, and their subsequent ossification-related changes in shape and size. An assessment of skeletal development is commonly done using hand-wrist radiographs6–10 and lateral cephalograms.11 Hassel and Farman11 observed the bodies of cervical vertebrae (C2, C3, and C4) in lateral cephalograms to evaluate skeletal maturity (cervical vertebrae maturity indicators [CVMI]).
Dental development has been widely investigated as a potential predictor of skeletal maturity.1,4,5,12–17 Generally, dental development can be assessed by either the phase of tooth eruption or the stage of tooth calcification, with the latter being more reliable.18–20 The ability to assess skeletal maturity by the stages of mandibular second molar calcification through the examination of a panoramic radiograph (which is a routine diagnostic radiograph for dental/orthodontic treatment) would offer an advantage over the conventional hand-wrist radiographic method. No additional exposure to radiation would be necessary if assessment of skeletal maturity were performed through routine radiographs, keeping in mind the ALARA (As Low as Reasonably Achievable) principle.
It was previously reported21–25 that the stages of mandibular second molar calcification showed the highest correlation with the stages of skeletal maturity as compared to other teeth. It has been suggested that racial variations also play a role in the relationship of dental and skeletal maturation.1,24,25 Mappes et al.26 indicated that the predominant ethnic origin of the population, climate, nutrition, socioeconomic levels, and urbanization are causative factors of these racial variations. Therefore, we sought to evaluate the mandibular second molar calcification stages as indicators of skeletal maturity in Indian subjects.
The aims of this study were:
To investigate the relationships between mandibular second molar calcification stages and skeletal maturity, and
To evaluate whether the mandibular second molar calcification stages can be used as a reliable diagnostic tool to assess skeletal maturity.
MATERIALS AND METHODS
We designed this descriptive study as a cross-sectional research project. We obtained panoramic and lateral cephalometric radiographs of 300 Indian subjects (137 male and 163 female) from the pretreatment records of patients attending clinics for orthodontic treatment. The inclusion criteria were as follows:
Chronological age ranging from 9 to 18 years
No serious illness
Normal overall growth and development
Absence of abnormal dental conditions, such as impaction, transposition, and congenitally missing teeth
Absence of previous history of trauma or disease to the face and neck
Absence of orthodontic treatment
No permanent teeth extracted
All radiographic assessments were performed in a darkened room. Any radiograph that showed motion dullness or had poor contrast was discarded. A single radiologist exposed and developed all radiographs.
Evaluation of Dental Maturity on Panoramic Radiograph
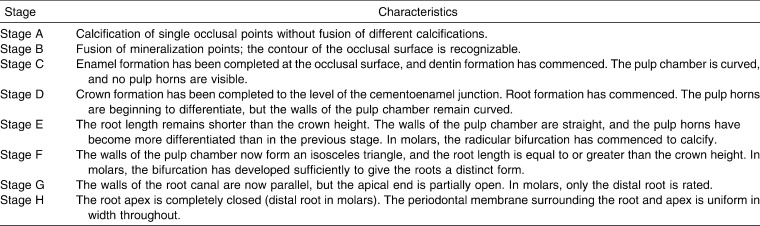
In this study, the mandibular left second molar was used as a sample. Tooth calcification was rated according to the index described by Demirjian et al.18 (Demirjian Index [DI]), in which one of eight stages of calcification (A to H) was assigned to the tooth (Table 1).
Table 1.
Dental Calcification Stages Using Demirjian Index (DI)
Evaluation of Cervical Vertebrae Maturity on Lateral Cephalogram
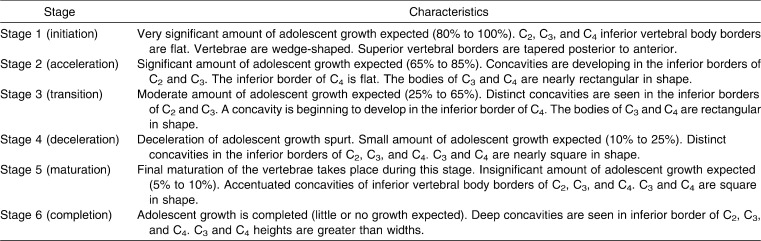
CVMI were evaluated by classifying C2, C3, and C4 into six groups depending on their maturation patterns on the lateral cephalogram using the classification of Hassel and Farman11 (Table 2).
Table 2.
Cervical Vertebrae Maturation Indicators (CVMI)
Statistics
We performed statistical analysis using the Microsoft Office Excel 2007 and SPSS software (version 10, SPSS Inc, Chicago, Ill). We calculated descriptive statistics by determining the means and standard deviations of the chronological ages for the six stages of CVMI. To test the reproducibility of the assessments of DI and CVMI, the same two observers reevaluated randomly selected lateral cephalograms and panoramic radiographs from 10 of the same male and female subjects 8 weeks after the first evaluation. The differences between the interpretations were evaluated statistically. Interobserver and intraobserver agreement for both investigators were determined in terms of the weighted kappa statistics for DI and CVMI.
To study the relationship between DI and the CVMI, the frequency and the percentage distribution of the stages of calcification were recorded for each tooth, and these were calculated separately for male and female subjects. Cross-tabular statistics were performed. The Pearson chi-square test (χ2) value and Sakoda adjusted Pearson contingency coefficient (C*) were estimated to determine the relationships between DI and CVMI.
RESULTS
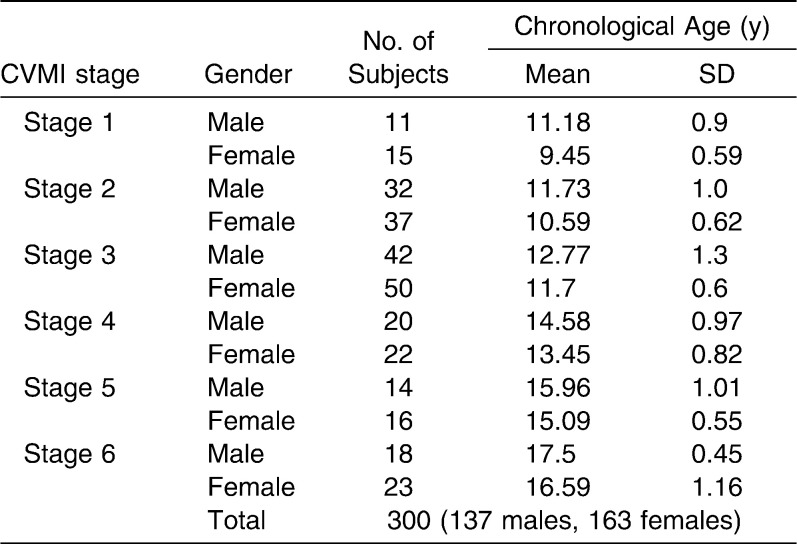
Table 3 shows the distribution of sex and chronological ages for all the subjects, grouped by CVMI stage. Each stage appeared earlier in female subjects than in male subjects. The reproducibility of all the assessments was good. For interobserver agreement, the weighted kappa statistics for DI assessments and CVMI assessments were 0.84 and 0.87, respectively. The kappa statistics for intraobserver agreement were 0.95 for DI assessments and 0.97 for CVMI assessments. It is clear, therefore, that there was almost perfect27 interobserver and intraobserver agreement for both DI and CVMI assessments.
Table 3.
Distribution of Chronological Ages for All Subjects Grouped by CVMI Stages
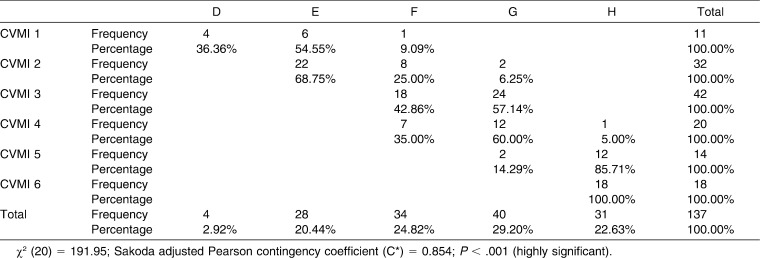
Table 4 shows the associations between DI and CVMI for male subjects. The value of χ2 was highly significant at 191.95 with 20 degrees of freedom (P < .001). The value of C* was 0.854 and showed a highly significant association between DI and CVMI for male participants. From Table 4 it is also clear that lower stages of DI were associated with lower CVMI stages. Again, the higher the DI stage, the higher the CVMI stage. Stage E included the highest percentage distribution (68.75%) at stage 2 of the CVMI. Stages F and G were almost equally distributed for CVMI stage 3. DI stages F and G also included a large percentage of CVMI stage 4 subjects. Stage H displayed a high percent distribution with stage 5 (85.71%) and 100% distribution with CVMI stage 6.
Table 4.
Association Between CVMI and DI for Male Subjects (Contingency Table)
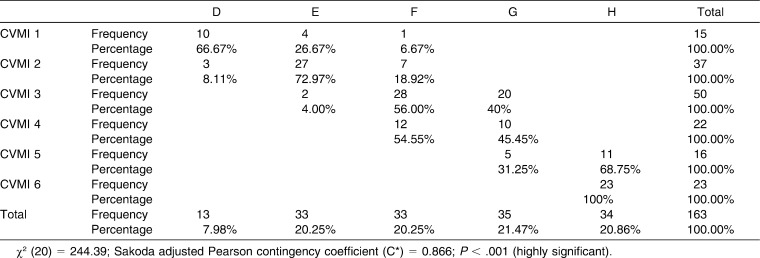
Table 5 shows the associations between DI and CVMI for female subjects. The value of χ2 was 244.39 at 20 degrees of freedom (P < .001). The value for C* was highly significant at 0.866, showing a highly significant association between CVMI and DI for female participants. All the values for female subjects were higher than those for male participants, which indicated that the statistical association was stronger for female subjects than for male subjects. From Table 5 it is clear that lower CVMI stages were more frequently associated with lower DI stages. Conversely, the higher the CVMI stage, the higher the DI stage for female subjects. The individual relationships between DI and CVMI showed a trend similar to that seen for the male subjects. A comparison of Tables 4 and 5 shows that the DI in male subjects was more advanced than in female subjects with respect to CVMI stage.
Table 5.
Association Between CVMI and DI for Female Subjects (Contingency Table)
DISCUSSION
Many methods for precise prediction of growth have been suggested.3–5 Dental maturity, in particular, has the advantage of easy evaluation during routine dental treatment. Radiation exposure time and dose are high when specialized radiographs are used (hand-wrist radiographs or lateral cephalograms), making their use questionable according to the ALARA principle. The ALARA principle is especially important for children and young adults, and, hence, high-radiation methods should not be used frequently to assess growth.
The ease of recognizing the stages of dental development and the availability of panoramic radiographs are practical reasons for attempting to assess physiologic maturity without resorting to hand-wrist or lateral cephalometric radiographs. Others have questioned these latter approaches from the radiation safety point of view. In addition, the cost of the equipment that is required for these radiographs is high, making them expensive.
Many studies have reported high correlations between tooth calcification stages and skeletal maturity indicators, which would probably allow clinicians to more easily identify pubertal growth stages from panoramic radiographs.4,5,13,15,17,21–26,28,29 On the other hand, Lewis and Garn,14 Garn et al.,30 and Tanner31 reported low or insignificant correlations between skeletal and dental maturation. The lack of agreement among previous studies is a result, at least in part, of the different methods used for assessing skeletal and dental maturity.
Findings from a study done by Chertkow1 indicated that the completion of root formation in the mandibular canine prior to apical closure may be used clinically as a maturity indicator of the pubertal growth spurt with a degree of confidence similar to that of some of the other indicators described for hand-wrist radiographs among white children. Previously it was reported21–25 that tooth calcification stages from panoramic radiographs might be clinically useful as a maturity indicator of pubertal growth and that mandibular second molar calcification showed the highest correlation with skeletal maturity versus other teeth. Racial variations in the relationships between the calcification stages of individual teeth and skeletal maturity also have been reported.1,24–26
Most studies of the dentition have used either mandibular canines1,13,28 or third molars30,32 for dental age assessment, but these two parameters exhibit some drawbacks. Root formation and apex closure of mandibular canines are completed by the age of 13 years, but most children exhibit active growth up to the age of 16 to 17 years. Third molars, on the other hand, are the most commonly missing teeth in the human dentition, making them unreliable for age assessment. The present radiographic study was therefore taken up to assess the reliability of using the developmental stages of mandibular second molars as an indicator of maturity. This tooth offers an advantage over other teeth because its development tends to continue over a longer period and until a later age. Apex closure generally extends up to the age of 16 years in normal children.
Again in this study, we used the mandibular second molar as a sample because estimation errors occur more frequently in calculating the maturation of maxillary molars than that of mandibular molars. Sometimes the maxillary molar roots overlap with anatomic structures such as the palate, the inferior border of the zygomatic arch, or the maxillary sinus septum. This makes it difficult to observe the roots.32
It has long been contended that dental eruption, which is the most conspicuous and easily determined indicator of dental maturation, is much more variable in its timing than skeletal maturation.19,33 According to Nolla,19 dental eruption has also been reported to be more variable than the calcification sequence in the dentition. Dental eruption is a fleeting event that is under greater environmental influence.18 In the present study, calcification stages of teeth, rather than eruption, were preferred because tooth formation is proposed as a more reliable criterion for determining dental maturation.19 Therefore, the dental maturity assessment stages of Demirjian et al.18 were used. This method also shows high accuracy when applied to Indian populations.34 This method's criterion consists of distinct details based on shape criteria and proportions of root length, using the relative value versus crown height, rather than on absolute length. Therefore, foreshortened or elongated projections of developing teeth will not affect the reliability of assessment.24 Recent studies have verified that Demirjian's classification system shows low intraexaminer and interexaminer error and a high correlation with biological age.35
Several studies21–25 have indicated that each CVMI stage consistently appears earlier in girls than in boys. Thus, the observations of the present study are in line with earlier studies (Table 3). We considered the DI, relative to CVMI, separately for male and female subjects. The findings of Krailassiri et al.24 and Uysal et al.25 indicated that the maturation patterns of tooth development in male subjects tend to be more advanced versus female subjects in relation to skeletal maturity stages. Chertkow1 reported that a markedly more advanced trend in tooth calcification was evident among both black and white boys. In this study, it was determined that at the same CVMI, male subjects had a more advanced trend in DI, and the opposite pattern was present in female subjects. These findings confirm previous reports.1,24,25
The present study revealed a highly significant association between the DI of mandibular second molars and the CVMI (Tables 4 and 5). The relationship between skeletal maturity and peak height velocity (PHV) is well established.3,8,9,36 Fishman,8 Hägg and Taranger,9 and Bjork and Helm36 found that the appearance of the adductor sesamoid of the thumb indicate the beginning of the pubertal growth spurt (onset of PHV), which corresponds to stage 2 of CVMI.37 For both sexes, DI stage E showed the highest percent distribution at stage 2 of CVMI, which signifies the pre–peak of pubertal growth spurt or onset of PHV. Bjork and Helm36 found that the MP3cap stage heralds the peak of the pubertal growth spurt, which corresponds to Fishman's skeletal maturity indicator 6 (stage 3 of the CVMI37). In the present study, for both male and female subjects, stages F and G corresponded to CVMI stages 3 and 4, which infers that DI stages F and G represent the peak of the pubertal growth spurt. This finding supports the suggestions of previous studies.21,25 Fishman's skeletal maturity indicator 11 corresponds to CVMI stage 5,37 and the fusion of the epiphysis and diaphysis of the radius (which signifies the end of growth) corresponds to CVMI stage 6.37 Stage H displayed a higher percent distribution with stage 5 and 100% distribution with stage 6 of the CVMI. DI stage H suggests insignificant/no remaining adolescent growth.
The unique and significant findings from the present study imply that the stages of mandibular second molar calcification as observed on panoramic radiographs give fairly accurate results and can be considered reliable indicators of skeletal maturity with the methodology suggested by Demirjian et al.18
CONCLUSIONS
The appearance of each CVMI stage was consistently earlier in female than in male subjects. However, the DI stages were more advanced in male subjects as compared with female subjects in relation to CVMI stages.
A highly significant association (C* = 0.854 for male subjects and 0.866 for female subjects) was found between DI and CVMI.
DI stage E corresponded to stage 2 of CVMI (pre–peak of pubertal growth spurt) and DI stages F and G corresponded to stages 3 and 4 of CVMI (peak of pubertal growth spurt). DI stage H was associated with CVMI stages 5 and 6 (end of pubertal growth spurt).
Mandibular second molar DI stages are a reliable indicator of skeletal maturity.
REFERENCES
- 1.Chertkow S. Tooth mineralization as an indication of the pubertal growth spurt. Am J Orthod. 1980;77:79–91. doi: 10.1016/0002-9416(80)90226-2. [DOI] [PubMed] [Google Scholar]
- 2.Fishman L. S. Chronological versus skeletal age, an evaluation of craniofacial growth. Angle Orthod. 1979;49:181–189. doi: 10.1043/0003-3219(1979)049<0181:CVSAAE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Demirjian A, Buschang P. H, Tanguay R, Patterson D. K. Interrelationships among measures of somatic, skeletal, dental, and sexual maturity. Am J Orthod. 1985;88:433–438. doi: 10.1016/0002-9416(85)90070-3. [DOI] [PubMed] [Google Scholar]
- 4.Chertkow S, Fatti P. The relationship between tooth mineralization and early evidence of the ulnar sesamoid. Angle Orthod. 1979;49:282–288. doi: 10.1043/0003-3219(1979)049<0282:TRBTMA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Sierra A. M. Assessment of dental and skeletal maturity. A new approach. Angle Orthod. 1987;57:194–198. doi: 10.1043/0003-3219(1987)057<0194:AODASM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Bjork A. Timing of interceptive orthodontic measures based on stages of maturation. Trans Eur Orthod Soc. 1972;48:61–74. [PubMed] [Google Scholar]
- 7.Grave K. The use of the hand and wrist radiograph in skeletal age assessment; and why skeletal age assessment is important. Aust Orthod J. 1994;13:96. [PubMed] [Google Scholar]
- 8.Fishman L. S. Radiographic evaluation of skeletal maturation. Angle Orthod. 1982;52:88–112. doi: 10.1043/0003-3219(1982)052<0088:REOSM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Hägg U, Taranger J. Skeletal stages of the hand and wrist as indicators of the pubertal growth spurt. Acta Odontol Scand. 1980;38(3):187–200. doi: 10.3109/00016358009004719. [DOI] [PubMed] [Google Scholar]
- 10.Grave K. G. Physiological indicators in orthodontic diagnosis and treatment planning. Aust Orthod J. 1978;5:114–122. [PubMed] [Google Scholar]
- 11.Hassel B, Farman A. G. Skeletal maturation evaluation using cervical vertebrae. Am J Orthod Dentofac Orthop. 1995;107:58–66. doi: 10.1016/s0889-5406(95)70157-5. [DOI] [PubMed] [Google Scholar]
- 12.Anderson D. L, Thompson G. W, Popovich F. Interrelationships of dental maturity, skeletal maturity, height and weight from age 4 to 14 years. Growth. 1975;39(4):453–462. [PubMed] [Google Scholar]
- 13.Coutinho S, Buschang P. H, Miranda F. Relationship between mandibular canine calcification stages and skeletal maturity. Am J Orthod Dentofacial Orthop. 1993;104:262–268. doi: 10.1016/S0889-5406(05)81728-7. [DOI] [PubMed] [Google Scholar]
- 14.Lewis A. B, Garn S. M. The relationship between tooth formation and other maturational factors. Angle Orthod. 1960;30:70–77. [Google Scholar]
- 15.Demisch S, Wartmann C. Calcification of mandibular third molar and its relationship to skeletal and chronological age in children. Child Dev. 1956;27:459–473. doi: 10.1111/j.1467-8624.1956.tb04824.x. [DOI] [PubMed] [Google Scholar]
- 16.Green L. J. Interrelationship among height, weight and chronological, dental and skeletal age. Angle Orthod. 1961;31:189–193. [Google Scholar]
- 17.Engström C, Engström H, Sagne S. Lower third molar development in relation to skeletal maturity and chronological age. Angle Orthod. 1983;53:97–106. doi: 10.1043/0003-3219(1983)053<0097:LTMDIR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Demirjian A, Goldstein H, Tanner J. M. A new system of dental age assessment. Human Biol. 1973;45:211–227. [PubMed] [Google Scholar]
- 19.Nolla C. M. The development of the permanent teeth. J Dent Child. 1960;27:254–263. [Google Scholar]
- 20.Hotz R, Boulanger G, Weisshaupt H. Calcification time of permanent teeth in relation to chronological and skeletal age in children. Helv Odontol Acta. 1959;3:4–9. [Google Scholar]
- 21.Mittal S, Singla A, Virdi M, Sharma R, Mittal B. Co-relation between determination of skeletal maturation using cervical vertebrae and dental calcification stages. Internet J Forensic Sci. 2011;4(2) http://www.ispub.com/journal/the_internet_journal_of_forensic_science/volume_4_number_2_59/article/co-relation-between-determination-of-skeletal-maturation-using-cervical-vertebrae-and-dental-calcification-stages.html . [Google Scholar]
- 22.Rai B. Relationship of dental and skeletal radiograph: maturity indicator. Internet J Biol Anthropol. 2008;2(1) http://www.ispub.com/journal/the_internet_journal_of_biological_anthropology/volume_2_number_1_8/article/relationship_of_dental_and_skeletal_radiograph_maturity_indicator.html . [Google Scholar]
- 23.Rai B, Anand S. C. Relationship of hand wrist and panoramic radiographs. Internet J Forensic Sci. 2008;3(1) http://www.ispub.com/journal/the_internet_journal_of_forensic_science/volume_3_number_1_58/article/relationship_of_hand_wrist_and_panoramic_radiographs.html . [Google Scholar]
- 24.Krailassiri S, Anuwongnukroh N, Dechkunakorn S. Relationship between dental calcification stages and skeletal maturity indicators in Thai individuals. Angle Orthod. 2002;72:155–166. doi: 10.1043/0003-3219(2002)072<0155:RBDCSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Uysal T, Sari Z, Ramoglu S. I, Basciftci F. A. Relationships between dental and skeletal maturity in Turkish subjects. AngleOrthod. 2004;74:657–664. doi: 10.1043/0003-3219(2004)074<0657:RBDASM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Mappes M. S, Harris E. F, Behrents R. G. An example of regional variation in the tempos of tooth mineralization and hand-wrist ossification. Am J Orthod Dentofacial Orthop. 1992;101:145–151. doi: 10.1016/0889-5406(92)70006-V. [DOI] [PubMed] [Google Scholar]
- 27.Landis J. R, Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 28.Hareesha K. B, Babu N. C. Co-relation between mandibular canine calcification stages and skeletal maturity. J Int Oral Health. 2010;2(3):41–47. [Google Scholar]
- 29.Lauterstein A. M. A cross-sectional study in dental development and skeletal age. J Am Dent Assoc. 1961;62:161–167. doi: 10.14219/jada.archive.1961.0032. [DOI] [PubMed] [Google Scholar]
- 30.Garn S. M, Lewis A. B, Bonne B. Third molar formation and its developmental course. Angle Orthod. 1962;44:270–276. [Google Scholar]
- 31.Tanner J. M. Growth at Adolescence 2nd ed. Oxford, UK: Blackwell Scientific Publications; 1962. pp. 55–93. [Google Scholar]
- 32.Cho S. M, Hwang C. J. Skeletal maturation evaluation using mandibular third molar. Korean J Orthod. 2009;39(2):120–129. [Google Scholar]
- 33.Van der Linden F. P. Transition of the Human Dentition. Ann Arbor, Mich: Center for Human Growth and Development, University of Michigan; 1979. [Google Scholar]
- 34.Rai B, Kaur J, Anand S, Jain R, Sharma A, Mittal S. Accuracy of the Demirjian Method for the Haryana Population. Internet J Dent Sci. 2008;6(1) http://www.ispub.com/journal/the_internet_journal_of_dental_science/volume_6_number_1_7/article/accuracy_of_the_demirjian_method_for_the_haryana_population.html . [Google Scholar]
- 35.Olze A, Bilang D, Schmidt S, Wernecke K. D, Geserick G, Schmeling A. Validation of common classification systems for assessing the mineralization of third molars. Int J Legal Med. 2005;119:22–26. doi: 10.1007/s00414-004-0489-5. [DOI] [PubMed] [Google Scholar]
- 36.Bjork A, Helm S. Prediction of the age of maximum pubertal growth in body height. Angle Orthod. 1967;37:134–143. doi: 10.1043/0003-3219(1967)037<0134:POTAOM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Kamal M, Ragini and Goyal S. Comparative evaluation of hand wrist radiographs with cervical vertebrae for skeletal maturation in 10–12 years old children. J Indian Soc Pedod Prev Dent. 2006;24(3):127–135. doi: 10.4103/0970-4388.27901. [DOI] [PubMed] [Google Scholar]