Summary
Background
SARS-CoV-2 targets endothelial cells through the angiotensin-converting enzyme 2 receptor. The resulting endothelial injury induces widespread thrombosis and microangiopathy. Nevertheless, early specific markers of endothelial dysfunction and vascular redox status in COVID-19 patients are currently missing.
Methods
Observational study including ICU and non-ICU adult COVID-19 patients admitted in hospital for acute respiratory failure, compared with control subjects matched for cardiovascular risk factors similar to ICU COVID-19 patients, and ICU septic shock patients unrelated to COVID-19.
Findings
Early SARS-CoV-2 infection was associated with an imbalance between an exacerbated oxidative stress (plasma peroxides levels in ICU patients vs. controls: 1456.0 ± 400.2 vs 436 ± 272.1 mmol/L; P < 0.05) and a reduced nitric oxide bioavailability proportional to disease severity (5-α-nitrosyl-hemoglobin, HbNO in ICU patients vs. controls: 116.1 ± 62.1 vs. 163.3 ± 46.7 nmol/L; P < 0.05). HbNO levels correlated with oxygenation parameters (PaO2/FiO2 ratio) in COVID-19 patients (R2 = 0.13; P < 0.05). Plasma levels of angiotensin II, aldosterone, renin or serum level of TREM-1 ruled out any hyper-activation of the renin-angiotensin-aldosterone system or leucocyte respiratory burst in ICU COVID-19 patients, contrary to septic patients.
Interpretation
Endothelial oxidative stress with ensuing decreased NO bioavailability appears as a likely pathogenic factor of endothelial dysfunction in ICU COVID-19 patients. A correlation between NO bioavailability and oxygenation parameters is observed in hospitalized COVID-19 patients. These results highlight an urgent need for oriented research leading to a better understanding of the specific endothelial oxidative stress that occurs during SARS-CoV-2.
Funding
Stated in the acknowledgments section.
Keywords: SARS-CoV-2, Nitric oxide, Angiotensin II, Microvascular thrombosis, Oxidative stress, Endothelial dysfunction
Research in context.
Evidence before this study
We searched PubMed for published article on bioavailability of nitric oxide (NO) and specific markers of vascular endothelial function in confirmed COVID-19 patients without any date or language restrictions, up to September 1, 2021. The search terms were (“COVID-19” or “SARS-CoV-2”) and “bioavailability of nitric oxide”. We found a number of theoretical articles that postulated a decrease of nitric oxide during SARS-CoV-2 infection but no published clinical article reporting specific markers of bioavailability of nitric oxide in patients with COVID-19.
Added value of this study
We demonstrate that the coagulopathy developing at the early stage of COVID-19 is associated with a distinctive NO-dependent endothelial dysfunction and oxidative stress, not explained by an overactivation of the renin angiotensin-aldosterone system or the leucocytes respiratory burst. We used an original technique of low-temperature electron paramagnetic resonance spectroscopy (EPR) to directly measure the 5 α -coordinate-heme nitrosyl-hemoglobin in the T (tense) state (5-α-nitrosyl-hemoglobin or commonly HbNO), which we previously used to quantitatively measure circulating bioavailable NO, to show a direct correlation between NO and respiratory parameters in COVID-19 patients. Our previous work showed that erythrocyte HbNO reflects the bioavailability of NO formed in the vasculature by endothelial NOS with a direct correlation with vascular endothelial function. Septic patients, studied in parallel show distinctive results with disseminated intravascular coagulation (DIC), surprisingly less vascular oxidative stress, but much higher NO, as a consequence of inflammation and iNOS induction (a first demonstration of high circulating NO in human sepsis by state-of-the-art EPR), that, unlike in COVID-19 patients, results in vasoplegia and activation of the renin-angiotensin-aldosterone system (RAAS). Additional detailed analyses of the pulmonary endothelium by electron microscopy (TEM and SEM) in COVID-19 show that the endothelium is not destroyed, but shows clear signs of activation, suggesting that the cell machinery is hijacked towards viral replication, itself a source of additional reactive oxygen species (ROS) production.
Implications of all the available evidence
Even if the number of patients is limited, our observations point to key pathogenic features that should orient future mechanistic studies towards the identification of endothelial ROS production for targeted therapies. Our study also highlights the use of specific biomarkers (e.g. EPR measurements of HbNO) to better stratify patients between early vs. late COVID-19 with infectious complications leading to sepsis.
Alt-text: Unlabelled box
Introduction
SARS-CoV-2 infection predisposes to disproportionate thrombotic events because of a hypercoagulable state despite standard thromboprophylaxis.1 Thrombotic events occur preferentially in ICU COVID-19 patients with an increase of the odds of mortality up to 74%.2 Therapeutic anticoagulation recently showed benefits by improvement of gas exchange and a reduced need for mechanical ventilation3 but failed to improve hospital survival or days free of organ support so far. Endothelial dysfunction is thought to play a major role in the pathogenesis of COVID-19.4 This is supported by histologic observations showing gross alterations of pulmonary vessels, with microangiopathy, alveolar capillary microthrombi and endothelial injury.5 Further, a compromised microcirculatory function has been objectified by measuring an altered sublingual microvascular perfusion6 with increased numbers of leukocytes, red blood cells microaggregates and platelets activation.7 Such microvascular dysfunction, known to be associated with mortality in ICU patients8 may result from endothelial dysfunction that would account for the tissue hypoperfusion, inflammation and procoagulant state observed during COVID-19. Indeed, a previous study revealed reduced plasma nitric oxide (NO) species in COVID-19 patients compared to controls, related to a reduced synthesis or an increased consumption from scavenging by reactive oxygen species.9 Accordingly, others have suggested monitoring nitrite/nitrate levels (NOx) and even supplementation with exogenous nitrate or nitrite in COVID-19.10,11
However, a specific evaluation of the NO-dependent endothelial function under SARS-CoV-2 invasion with specific biomarkers is currently missing. Endothelial nitric oxide (NO) is the pivotal endothelium-derived substance that maintains the vasculature in a quiescent state by inhibition of inflammation, cellular proliferation, and thrombosis.12 Endothelial dysfunction is due, in part, to a decreased NO bioavailability that predisposes the vasculature towards a prothrombotic and a pro-inflammatory state with expression of pro-inflammatory cytokines and adhesion molecules.13 Angiotensin-converting enzyme 2 (ACE2) which allows SARS-CoV-2 endothelial cell invasion14 also acts as a counter-regulator of the renin-angiotensin-aldosterone system (RAAS) by converting angiotensin II (Ang II) into Angiotensin 1-7 (Ang 1-7), limiting Ang II effects via Ang II type 1 receptors (AT1R). On the endothelial cell, the AT1R activation induces the generation of reactive oxygen species (ROS) through activation of the NADPH oxidase (NOX)-2, thus limiting the NO bioavailability.15 Therefore, the RAAS has been involved in the pathogenesis of COVID-19 because of the well-described ACE2 shedding that occurs during SARS-CoV-2 viral invasion. However, previous measurements of plasma Ang II, the primary effector hormone of the RAAS in COVID-19 patients have yielded conflicting results, leaving the issue unsolved.16, 17, 18
We postulated that endothelial SARS-CoV-2 invasion would lead to a severe endothelial dysfunction, reflected by decreased venous erythrocyte levels of the 5-α -nitrosyl-haemoglobin (HbNO) that could participate in the microvascular dysfunction and the hypercoagulable state we observed in COVID-19 patients. Accordingly, measurements of HbNO would allow the early detection of patients at risk of such lethal complications.
ICU COVID-19 patients were compared both with a non-infected control group matched for similar cardiovascular risk factors and with hospitalized, non-ICU COVID-19 patients. We added a comparison with ICU septic shock patients in order to identify distinctive pathogenic factors for the coagulopathy associated with COVID-19.
Materials and methods
Study design and participants
We prospectively enrolled adult COVID-19 patients admitted in ICU (Intensive Care Unit) or in the Infectious disease ward. Control subjects with matched age, sex and co-morbidities similar to ICU-COVID 19 patients were recruited in internal medicine consultation. Septic shock patients were recruited at the same period in ICU. All the patients were included during hospitalization at the Cliniques Universitaires Saint-Luc, Brussels, Belgium (between 27 April and 3 November 2020). Exclusion criteria for patients were as follows: older than 75 years, chronic coagulopathies or cirrhosis. COVID-19 exclusion criteria included also a concomitant proved bacterial infection or ICU admission more than five days after admission on a general ward, in order to avoid any undiagnosed bacterial infection. SARS-CoV-2 infection was confirmed by real-time reverse transcription PCR in nasopharyngeal swabs. Septic shock patients corresponded to refractory hypotension due to a non-SARS-CoV-2-related infection.19 ICU COVID-19 patients and septic shock patients were included within the first day of ICU admission. Non-ICU COVID-19 patients were included within the first two days of hospital admission.
Ethics
The study was approved by the local Ethic Committee of the Cliniques Universitaires Saint-Luc (2020/27AVR/247 and 2020/20AVR/234). All the patients and volunteers have signed an informed consent document.
Procedures
Blood collection from study subjects
Human blood was collected from patients by a venopuncture from the median cubital vein for routine hematological analysis. Measurements of venous and arterial blood O2 concentration were performed by using a blood gas analyser (ABL800 Flex, Radiometer, Copenhagen, Denmark) in samples collected into O2-stop syringes (Smiths Medical ASD, USA) Additional venous blood samples were drawn into O2-stop tubes (BD-Plymouth, Vacutainer) containing (1.8 mg/mL), Lithium Heparin (Starstedt, S-Monovette® Lithium Heparin), Citrate (Starstedt, Citrate S-Monovette® Citrate 1:10) or without additive (Starstedt, S-Monovette® Serum) and were immediately centrifuged (700 × g, 10 min). Plasma and erythrocytes were collected and immediately frozen for the redox status testing. The samples were stored at −80 °C before all the measurements.
5-coordinate α-HbNO (HbNO) concentration in human erythrocytes
Erythrocytes collected for the HbNO analysis were pre-treated with a mixture of antioxidant solution (sodium ascorbate and N-acetylcysteine, 5 mmol/L each, added into a closed vacutainer using a Micro-Fine™ syringe), centrifuged (10 min, 800xg, at room temperature), then retrieved from the bottom of the vacutainer tube, transferred into a 1 ml syringe and immediately frozen for low-temperature Electron Paramagnetic Resonance (EPR) spectroscopy measurements. The EPR spectra from the frozen erythrocyte samples were recorded on a Bruker X-band EPR spectrometer (EMX-micro) at 77 K using an EPR quartz finger Dewar filled with liquid nitrogen with the following settings: microwave frequency ∼ 9.35 GHz; modulation frequency, 100 kHz; microwave power (MW), 20 mW; modulation amplitude (MA), 0.7 mT. The relative concentration of the heme-FeII nitrosyl-hemoglobin (T-form) was quantified from the intensity of the hyperfine components of the HbNO signal (g-factor 2.01, Ahf=16.8 G,) after subtraction of the overlapping EPR signal of protein free radicals (g = 2.005, linewidth ∼10–15 G) as described previously.20 The absolute HbNO concentration was determined from the calibration curve generated using the HbNO complexes synthesized after incubation of erythrocytes with a NO-donor system in anaerobic condition; the concentration of HbNO was deduced from the signal intensity obtained by spectra double-integration normalized by that of known EPR standards (Tempol, 20–100 µmol/L frozen in 30% glycerol-water solution).
Hemostasis markers
Prothrombin time, fibrinogen and antithrombin were analysed on the ACL-TOP 750 (HemosIL ReadiPlasTin, HemosIL Q.F.A Thrombin, HemosIL Liquid Antithrombin, Werfen Barcelona, Spain). Von Willebrand antigen and activity were determined by immunoturbidimetric latex based assays (HemosIL von Willebrand Factor Antigen, HemosIL von Willebrand Ristocetin Cofactor assay; Werfen) on the ACL-TOP 700. Protein C was analyzed on ACL-TOP 700 (HemosIL Protein C, Werfen). ADAMTS13 was determined with the Technozym® ADAMTS-13 Activity ELISA kit (Technoclone, Wien, Austria). Von Willebrand antigen and activity are expressed in %. This is equivalent to IU/dL since these tests are calibrated against HemosIL Calibration Plasma (Werfen), which is traceable to the current International Standards.
Endothelial biomarkers
All the specific markers of endothelial function were measured in serum and using kits purchased from R&D Systems (Minneapolis, USA). Concentrations of soluble InterCellular Adhesion Molecule (serum, human sICAM-1/CD54 Quantikine Kit, DCD540), Endothelin-1 (serum, human Endothelin-1 Quantikine Kit, DET100), tissue Plasminogen Activator (serum, human t-Plasminogen Activator Quantikine Kit, DTPA00) and Plasminogen activator inhibitor-1 (serum, human Total PAI-1 Quantikine Kit, DTSE100) were measured according to the manufacturer's instructions.
Oxidative stress markers
Nitrite/nitrate concentrations (NOx) and plasma lipids peroxides were measured with a colorimetric assay (NOx: 780001 from Cayman Chemical, Ann Arbor, USA and lipids peroxides: BI-5007, from Biomedica Medizinprodukte GmbN, Wien, Austria) according to the manufacturer's instructions.
RAS metabolites
Plasma aldosterone was measured by radioimmunoassay (Demeditec, Kiel, Germany). Renin was measured using the Renin Kit III (Cis Bio International, Saclay, France). Plasma Ang II was measured by radioimmunoassay after SepPak extraction as described before.21 Detection limits were 14.8 pg/mL, 2 pg/mL and 0.5 fmol/mL, respectively. Serum Angiotensin-Converting Enzyme 2 (ACE2) was measured using Human ACE2 Duo Set ELISA, R&D, DY933-05.
Soluble Triggering receptor expressed on myeloid cells-1 (sTREM-1)
Plasma sTREM-1 levels were measured using an analytically validated ELISA assay according to regulatory requirements (EMA 2011) using a commercially available research use only ELISA assay (Human TREM-1 Quantikine® ELISA kit, R&D Systems).
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) of human pulmonary tissue
We performed lung autopsies on three COVID-19 ICU patients and compared them to lung biopsies obtained from two control patients who had undergone lobectomy for lung cancer, taken at distance from the lesions. Importantly, the delay between autopsies and tissue sampling was short (less than 12 h) and an experienced pathologist confirmed lung tissue quality before the analyses. Lung samples were fixed with glutaraldehyde 2.5% (EM grade, EMS) at 4 °C overnight, washed three times in cacodylate buffer (pH 7.4) and postfixed 1 h at room temperature in 1% osmium (OsO4, EMS), 1.5% potassium ferrocyanide (Sigma-Aldrich) in 0.15M cacodylate buffer. This was immediately followed by a second incubation in OsO4 (1% in distilled water) for 1h at room temperature. After washing in distilled water, samples were stained with 1% uranyl acetate (EMS) and serially dehydrated in increasing ethanol concentrations. Samples were then embedded in epoxy resin (Agar 100 resin, Agar Scientific Ltd, UK) and left to polymerize for 2 days at 60 °C. Ultrathin sections (50–70 nm thick) were collected with a Leica UC6 ultra-microtome on formvar-carbon-coated copper grids and further stained with uranyl acetate and lead citrate by standard procedures. Observations were made on a Tecnai 10 electron microscope (FEI) and images were captured with a Veleta camera and processed with SIS iTEM software (Olympus).
Guideline-directed specific treatments
As soon as the results of the Recovery Trial22 were announced, all ICU COVID-19 patients received Dexamethasone (6mg/day during the first 10 days of hospitalization). Dexamethasone was administered in non-ICU patients presenting symptoms for more than 7 days. At the time of the study, none of the included ICU COVID-19 patients received exogenous (inhaled) nitric oxide. There was no specific diet for COVID-19 patients. All COVID-19 and septic shock patients received thromboprophylaxis with low-molecular-weight heparin (Nadroparin 3800 IU/days subcutaneously).
Statistical analysis
All results are expressed as mean ± SD, SEM or counts and proportions. Raw data were examined for normal distribution using the Shapiro-Wilk test. COVID-19 groups were compared using Z tests. One-way ANOVA with F test was used to compare all groups together, followed by Tukey tests for multiple comparisons, either directly or on log-transformed data when there was a right-skewed distribution. In case of non-normal distribution on log-scale, a Mann-Whitney (both COVID-19 groups) or Kruskal-Wallis tests (all different groups) followed by Dunn's correction for multiple comparisons were used. Chi-square test was used for discrete data. Outliers that exceeded 4 times the standard deviations and/or aberrant values based on confirmed technical failure have been excluded. The sample size of the present study was not formally computed, due to the observational nature of the study. Based on our results, the observed effect size (which is the difference between ICU patients and controls expressed in SD units) was close to 1 for HbNO, which reflects the NO-dependent endothelial function. At the 0.05 significance level and including 30 patients in the ICU group and 15 controls corresponding to a 2 by 1 design, our study had a power of 87.1% to detect a Cohen's effect size of 1 unit in HbNO. Analyses were performed with Graph Pad Prism version 9 (GraphPad Software, San Francisco, California). P-values of < 0.05 was considered as statistically significant.
Role of funding source
The Funder had no active role in study design, data collection, data analyses, interpretation, or writing of this report.
Results
Patient characteristics, comorbidities and treatments
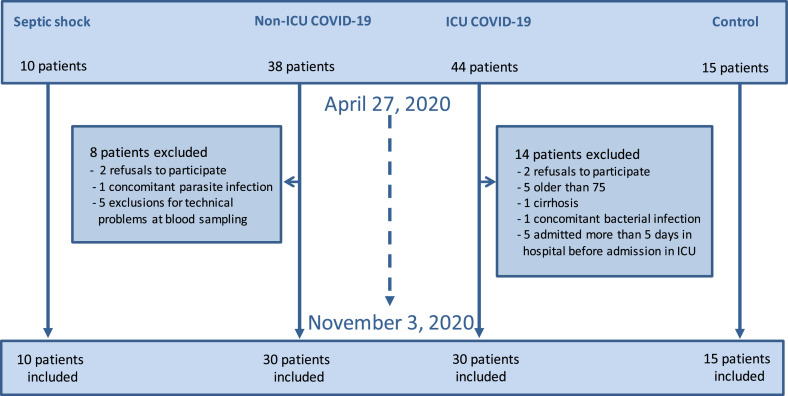
60 adult COVID-19 patients (30 ICU and 30 non-ICU) were finally included in this study cohort. 12 ICU COVID-19 and 6 non-ICU COVID-19 patients were excluded in accordance with the exclusion criteria and 2 refused to participate in both COVID-19 group.
ICU-COVID 19 patients present the same comorbidities as their matched control group but present more comorbidities than non-ICU patients. Few chronic diseases were observed in both hospitalized groups of patients. Septic shock patients present worse severity scores due to multiple organ failure than ICU-COVID 19 patients (APACHE II Score and SOFA score) and all septic shocks were related to bacterial infection treated with antibiotics. Time delay between symptom onset and hospital admission was longer for COVID-19 patients compared with septic shock. A majority of COVID-19 patients benefited from corticosteroids (Dexamethasone 6 mg/day during the first 10 days of hospitalization) compared to a minority of septic shock patients (Hydrocortisone 150 mg/day). Before announcement of the Recovery Trial results, a small proportion of COVID-19 patients received Hydroxychloroquine (400 mg twice daily at day 1 followed by 200 mg twice daily from day 2 to day 5).
At inclusion time, ICU COVID-19 patients exhibited more acute respiratory failure characterized by a lower PaO2 / FiO2 ratio (Partial pressure of arterial oxygen to Fraction of inspired oxygen ratio) than septic shock patients. Contrary to COVID-19 patients, all septic shock patients received antibiotics therapy. The majority of ICU-COVID 19 patients were first under High Flow Nasal Cannula (HFNC) and finally intubated. No vasopressor administration was necessary in ICU-COVID 19 patients before sedation for mechanical ventilation compared to septic shock patients. ICU length of stay differs between ICU-COVID 19 patients and septic shock patients. Hospital length of stay differs between ICU-COVID 19 patients and non-ICU COVID 19 patients and also compared to septic shock patients. Mortality did not differ between the two ICU groups of patients.
The patient's study flow diagram is shown in Figure 1 while the main clinical characteristics, comorbidities, adjunctive treatments and outcomes of all the different groups are reported in Table 1.
Figure 1.
Flow chart of the ENDOCOVID observational study.
Table 1.
Baseline characteristics, comorbidities, adjunctive treatments and outcomes for COVID-19 and septic shock patients.
| Variables | Matched control n = 15 | Non-ICU COVID-19 n = 30 | ICU COVID-19 n = 30 | Septic shock n = 10 |
|---|---|---|---|---|
| Age (years) | 61.5 ± 8.2 | 52.1 ± 13.5* | 61.3 ± 8.7 | 65.4 ± 8.4 |
| Sex Male - n (%) | 11 (73.3) | 22 (73.3) | 25 (83.3) | 6 (60.0) |
| Cardio-vascular comorbidities | ||||
| BMI (kg/m²) | 29.3 ± 5.1 | 28.7 ± 6 | 30.1 ± 4.2 | 26.4 ± 8.9* |
| Hypertension - n (%) | 10 (66.6) | 9 (30.0)* | 20 (66.6) | 4 (40.0) |
| Diabetes - n(%) | 6 (40.0) | 3 (10.0)* | 13 (43.3) | 3 (30.0) |
| Current smoker - n(%) | 2 (13.3) | 3 (10.0) | 5 (16.6) | 2 (20.0) |
| Chronic renal failure - n(%) | 1 (6.6) | 0 (0.0) | 3 (10.0) | 3 (30.0) |
| Chronic obstructive pulmonary disease - n(%) | 0 (0.0) | 2 (6.6) | 1 (3.3) | 3 (30.0)* |
| Chronic heart failure - n(%) | 1 (6.6) | 0 (0.0) | 2 (6.6) | 2 (20.0) |
| Immunocompromised - n(%) | 0 (0.0) | 1 (3.3) | 2 (6.6) | 5 (50.0)* |
| Cardio-vascular treatment | ||||
| ACE inhibitor - n(%) | 5 (33.3) | 2 (6.6)* | 8 (26.6) | 1 (10.0) |
| Angiotensin receptor blocker - n(%) | 4 (26.6) | 1 (3.3) | 4 (13.3) | 2 (20.0) |
| Delay between onset of symptoms and hospital admission (days) | 7.8 ± 2.5 | 7.3 ± 3.2 | 1.9 ± 1.3* | |
| ICU scores | ||||
| SOFA Score | - | 2.1 ± 0.6* | 4.3 ± 1.3 | 12.1 ± 3.6* |
| APACHE II Score | - | 4.7 ± 2.6* | 12.7 ± 3.7 | 23 ± 7.5* |
| Site of infection | ||||
| Intra-abdominal infection - n(%) | - | - | - | 5 (50.0) |
| Urinary tract infection - n(%) | - | - | - | 2 (20.0) |
| Cutaneous infection - n(%) | - | - | - | 1 (10.0) |
| Pneumonia - n(%) | - | - | - | 2 (20.0) |
| Etiological treatment at inclusion time | ||||
| Hydroxychloroquine - n(%) | - | 3 (10.0) | 2 (6.6) | 0 (0.0) |
| Antibiotics - n(%) | - | 0 (0.0) | 0 (0.0) | 10 (100.0)* |
| Steroid therapy - n(%) | - | 22 (73.3) | 25 (83.3) | 2 (20.0)* |
| PaO2 / FiO2 (mmHg) at inclusion time | - | 268.7 ± 74* | 87.7 ± 28.5 | 226.3 ± 80.5* |
| Respiratory support at inclusion time | ||||
| Conventional oxygen therapy | 30 (100.0)* | 0 (0.0) | 1 (10.0) | |
| NIV | 0 (0.0) | 4 (13.3) | 0 (0.0) | |
| HFNC | 0 (0.0)* | 25 (83.3) | 1 (10.0)* | |
| MV | 0 (0.0) | 1 (3.3) | 8 (80.0)* | |
| Respiratory support during ICU | ||||
| NIV alone - n(%) | - | - | 0 (0.0) | 0 (0.0) |
| HFNC alone - n(%) | - | - | 7 (23.3) | 1 (10.0) |
| HFNC / NIV followed by invasive MV - n(%) | - | - | 22 (73.3) | 0 (0.0)* |
| Invasive MV alone - n(%) | - | - | 1 (3.3) | 8 (80.0)* |
| ECMO - n(%) | 6 (20.0) | 0 (0.0) | ||
| Renal replacement therapy during ICU | - | 5 (16.6) | 2 (20.0) | |
| Vasopressors during ICU | ||||
| Within first 3 days - n(%) | - | - | 6 (20.0) | 10 (100.0)* |
| Before sedation - n(%) | - | - | 0 (0.0) | 10 (100.0)* |
| Outcomes | ||||
| ICU length of stay (days) | - | - | 38.9 ± 6.9 | 7.5 ± 2.4* |
| Hospital length of stay (days) | - | 8.3 ± 3.3* | 49.1 ± 7.3 | 20.6 ± 4.9* |
| Mortality - n(%) | - | 0 (0.0)* | 12 (40.0) | 3 (30.0) |
Data are in mean (± SD) or n (%), days in mean (± SEM). BMI = body-mass index. HIV/AIDS = human immunodeficiency virus / acquired immune deficiency syndrome. ACE = angiotensin converting enzyme. ICU = Intensive Care Unit. SOFA score = sepsis-related organ failure assessment. APACHE II Score = Acute Physiology And Chronic Health Evaluation II Score. PaO2 / FiO2 = Partial pressure of arterial oxygen to Fraction of inspired oxygen ratio. HFNC = High Flow Nasal Cannula. NIV = Non-invasive ventilation. MV = Mechanical Ventilation. ECMO = Extracorporeal membrane oxygenation. Corticosteroids means Dexamethasone (6mg/day during 10 days) for COVID-19 patients and Hydrocortisone (150 mg/day during vasopressors support) for septic shock patients.
P < 0.05 compared to ICU Covid-19 patients by one-way ANOVA followed by Tukey's correction for multiple comparisons, normal distribution for comparisons between all groups, or by unpaired t-test (for continuous data) or Chi-square test (for discrete data) for comparisons between two groups.
High vascular oxidative stress despite unstimulated RAAS in COVID-19 disease, contrary to sepsis
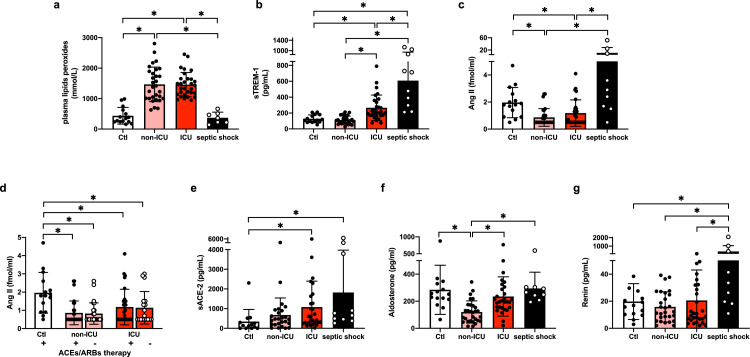
The mean plasma levels of peroxides, reflecting vascular oxidative stress were increased in both COVID-19 groups up to four times the levels observed in patients with septic shock or the control group (Figure 2a). However, plasma soluble Triggering Receptor expressed on Myeloid cell-1 (sTREM-1), an indicator of leucocyte activation was higher in the septic shock group compared with ICU COVID-19 patients, suggesting that high oxidative stress in the latter came from another source (Figure 2b). sTREM-1 levels in non-ICU COVID-19 patients were not different from control subjects. We next measured the activity of the RAAS, as a classical source of vascular oxidative stress. Surprisingly, Ang II levels were low both in ICU and Non-ICU COVID-19 patients compared to the matched control subjects (Figure 2c); this finding was unaffected by excluding patients under angiotensin-converting-enzyme inhibitors (ACE inhibitor) or Ang II receptor blocker (ARB) therapy (Figure 2d). Conversely, Ang II levels were much higher in septic shock patients (Figure 2c). Both septic shock and COVID-19 patients displayed increased levels of soluble ACE2 compared to the control group, with no difference between the two COVID-19 groups (Figure 2e). Plasma levels of aldosterone, a final effector of the RAAS, were similar between control subjects, ICU COVID-19 and septic shock patients, and even lower in non-ICU COVID-19 patients (Figure 2f). The plasma aldosterone to Ang II ratio was lowest in septic shock patients (Table 2). Accordingly, plasma renin levels were highest in septic shock patients, whereas both groups of COVID-19 patients displayed levels comparable to the control group (Figure 2g).
Figure 2.
Oxidative stress in ICU versus non-ICU COVID-19 and septic shock patients.
Datapoints indicate individual measurements whereas horizontal bars show geometric mean (SD) for plasma lipid peroxides (2a), soluble TREM-1 (2b), plasma Ang II (2c), plasma Ang II ± ACEs and ARBs therapy (2d), soluble ACE-2 (2e), Aldosterone (2f) and Renin (2g). * P < 0.05 by one-way ANOVA followed by Tukey's correction for multiple comparisons, normal distribution except plasma Ang II and Aldosterone performed by Kruskal-Wallis corrected by Dunn's correction for multiple comparisons.
Number of subjects for each analysis. 2a: Controls n = 15, non-ICU COVID-19 n = 30, ICU COVID-19 n = 30, Septic shock n = 7 (related to an interference with hyperbilirubinemia in 3 patients). 2b: Controls n = 15, non-ICU COVID-19 n = 30, ICU COVID-19 n = 29, Septic shock n = 10. 2c-d: Controls n = 15, non-ICU COVID-19 n = 29, ICU COVID-19 n = 28, Septic shock n = 9. 2e: Controls n = 14, non-ICU COVID-19 n = 27, ICU COVID-19 n = 28, Septic shock n = 10. 2f: Controls n = 14, non-ICU COVID-19 n = 29, ICU COVID-19 n = 29, Septic shock n = 9. 2g: Controls n = 15, non-ICU COVID-19 n = 28, ICU COVID-19 n = 29, Septic shock n = 10.
Table 2.
Biochemicals values of patients in different groups at inclusion time.
| Biochemicals values | Standard reference (or mean of the matched group) | Matched control (n = 15) | Non-ICU COVID-19 (n = 30) | ICU COVID-19 (n = 30) | Septic shock (n = 10) |
|---|---|---|---|---|---|
| Blood count | |||||
| Haematocrit | 38–48% | - | 40.5 ± 4.8 | 37.4 ± 5.7 | 29.1 ± 6.6 |
| Neutrophils count | 1.6–7.0 × 10³/µL | - | 4.9 ± 2.3 | 6.1 ± 2.5 | 15.5 ± 9.6*,$ |
| Lymphocytes count | 0.8–5 × 10³/µL | - | 1.1 ± 4.3 * | 0.7 ± 3.1 | 1.0 ± 5.6 |
| NLR | < 1.6 | - | 5.3 ± 3.4 * | 10.4 ± 7.8 | 14.9 ± 8.5 |
| Platelets counts | 150–450 × 10³/µL | - | 221.9 ± 87.2 | 199.3 ± 65.1 | 119.1 ± 107.6*,$ |
| Primary haemostasis | |||||
| vWF: Ag, % | 66–176 | 148.3 ± 50.0*,$ | 341.9 ± 126.8 * | 418.2 ± 112.9 | 421.4 ± 151.1 |
| VWF: RCo, % | 60–200 | 114 ± 45.4*,$ | 268.2 ± 100.7* | 328.5 ± 88.5 | 341.7 ± 107.4 |
| ADAMTS13 protease activity, % | > 40 | 94.2 ± 7.7* | 92.6 ± 6.9* | 78.8 ± 15.0 | 42.6 ± 18.7*,$ |
| vWF: Ag to ADAMTS13 activity ratio | < 1.6 | 1.6 ± 0.6*,$ | 3.7 ± 1.4* | 5.4 ± 1.7 | 13.5 ± 10.2*,$ |
| Coagulation markers | |||||
| APTT, s | 25.1–36.5 | - | 28.0 ± 4.7 | 30.4 ± 3.7 | 48.5 ± 30.5*,$ |
| INR | 0.8–1.2 | - | 1.1 ± 0.1 | 1.2 ± 0.1 | 2.2 ± 0.7*,$ |
| AT, % | 78 – 130 | 108.2 ± 14* | 108.6 ± 19.4* | 91.5 ± 18.9 | 45.6 ± 20.7*,$ |
| C protein, % | 70–130 | 127.6 ± 16.1* | 115.3 ± 29.5* | 94.5 ± 23 | 39.4 ± 18*,$ |
| Fibrinolysis markers | |||||
| D-dimer, ng/mL | < 500 | - | 863 ± 642 | 1169 ± 1442 | 8820 ± 10704*,$ |
| Fibrinogen, mg/dL | 150 – 450 | - | 508.5 ± 106.1⁎ | 623.6 ± 135.0 | 408.1 ± 181.8*,$ |
| tPA, pg/mL | 1058–7576 | 5518 ± 2956*,$ | 10640 ± 6665* | 16697 ± 5781 | 18317 ± 5165$ |
| PAI-1, ng / mL | 2.66–69.3 | 53.6 ± 14.2*,$ | 111.4 ± 37.4 | 92.5 ± 44.3 | 144.2 ± 108.2 |
| Soluble adhesion molecules | |||||
| sICAM-1, ng/mL | 106–337 | 280.8 ± 69.8* | 315.2 ± 94.9* | 418.2 ± 95.3 | 593.6 ± 197.7*,$ |
| Vasomotor tonus | |||||
| ET1, pg/mL | 0.58–2 | 2.1 ± 0.6* | 3.1 ± 1.6* | 4.1 ± 2.6 | 4.6 ± 2.8 |
| HbNO, nmol/L | > 160 | 163.3 ± 46.7* | 161.5 ± 73.7* | 116.1 ± 62.1 | 451 ± 447*,$ |
| NOx, μM/L | > 60 | 61.8 ± 48.3* | 41.4 ± 32.3* | 25.2 ± 28.5 | 96.8 ± 100.9* |
| RAAS axis | |||||
| sACE2, pg/mL | < 350 | 341.9 ± 618.5* | 659.8 ± 863.4 | 1080.3 ± 1317.3 | 1820.1 ± 2150.8 |
| Angiotensin II, fmol/mL | 1 – 20 | 2.0 ± 1.1*,$ | 0.86 ± 0.6 | 1.2 ± 1.0 | 10.9 ± 17.2*,$ |
| Aldosterone, pg/mL | 9–272 | 284.4 ± 181.4 | 120.3 ± 81.9* | 235.1 ± 145.1 | 295.0 ± 119.5$ |
| Aldosterone to Angiotensin II ratio | < 200 | 198.7 ± 165.0 | 181.9 ± 157.9 | 296.4 ± 247.6 | 106.8 ± 121.5*,$ |
| Renin, pg/mL | 2–70 | 19.7 ± 13.2 | 15.8 ± 11.3 | 20.0 ± 22.7 | 375.9 ± 685.2*,$ |
| Endovascular oxidative stress | |||||
| Plasma lipid peroxidation, mmol/L | < 450 | 436 ± 272.1*,$ | 1467.3 ± 566.2 | 1456.0 ± 400.2 | 369.3 ± 187.6*,$ |
| sTREM-1, pg/mL | < 130 | 127.8 ± 49.2* | 111.6 ± 49.8* | 265.3 ± 161.2 | 608 ± 338.3*,$ |
Data are mean (± SD) or n (%). Standard reference refers to standard laboratory values or values previously observed in control subjects. NLR= Neutrophil to Lymphocyte Ratio. vWF = Von Willebrand Factor. vWF: Ag = vWF antigen. VWF:RCo = vWF activity. ADAMTS13 = a disintegrin and metalloprotease with thrombospondin type I repeats-13. APTT= activated partial thromboplastin time. INR= International Normalized Ratio. AT = antithrombin. tPA = Tissue plasminogen activator. PAI-1 = Plasminogen activator inhibitor-1. sICAM-1 = soluble intercellular adhesion molecule-1. ET1 = Endothelin 1. HbNO = 5-α-nitrosyl-hemoglobin. NOx = plasma Nitrite/Nitrate. RAAS = renin-angiotensin-aldosterone system axis. sACE2 = soluble Angiotensin-Converting Enzyme 2. s-TREM-1 = soluble Triggering receptor expressed on myeloid cells-1.
P < 0.05 compared to ICU Covid-19 patients.
P < 0.05 compared to non-ICU Covid-19 patients by one-way ANOVA followed by Tukey's correction for multiple comparisons, normal distribution or by Kruskal-Wallis corrected by Dunn's correction for multiple comparisons for ADAMST13, APTT, sACE2, Ang II and Aldosterone, not normally distributed. All analyses were performed after logarithmic transformation.
Decreased vascular nitric oxide bioavailability in COVID-19 disease, contrary to sepsis
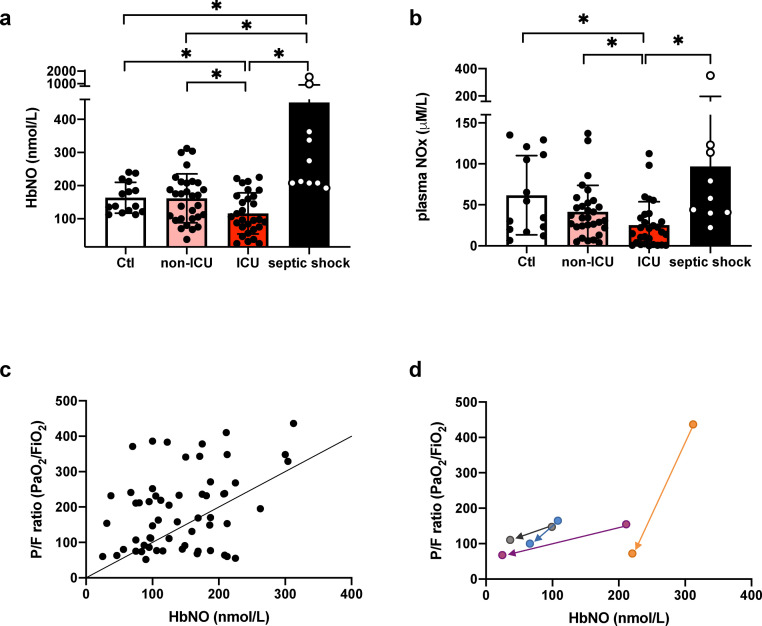
Circulating concentrations of erythrocyte 5-α-nitrosyl-hemoglobin (HbNO), reflective of vascular NO bioavailability23 were decreased in ICU COVID-19 patients while HbNO values were not different between non-ICU COVID-19 patients and the control group. Conversely, an increase of HbNO was observed in septic shock patients (Figure 3a). Similar trends were observed for nitrite/nitrate levels (NOx), the corresponding stable end products of NO oxidation (Figure 3b). HbNO values correlated with an oxygenation index defined by the PaO2/FiO2 ratio in the cohort of COVID-19 patients (R2 = 0.13; P = 0.005) (Figure 3c). In four severe COVID-19 patients that secondarily required respiratory support in the ICU, HbNO values and PaO2/FiO2 ratio decreased over time when compared to admission values in general ward (Figure 3d). Finally, an inverse correlation was observed between HbNO levels and the oxidative stress (defined by the plasma peroxides levels, R2 = 0.07; P = 0.03) (Supplemental Figure 1a).
Figure 3.
NO bioavailability and plasma NOx in ICU versus non-ICU COVID-19 and septic shock patients.
Datapoints indicate individual measurements whereas horizontal bars show geometric mean (SD) for HbNO (3a) and plasma NOx (3b). * P < 0.05 by one-way ANOVA followed by Tukey's correction for multiple comparisons, normal distribution. Linear regression analysis between PaO2/FiO2 ratio and HbNO (R2 = 0.13; P = 0.005) (3c). Decreased HbNO levels and PaO2/FiO2 ratio in 4 COVID-19 patients requiring secondarily ICU support (3d).
Number of subjects for each analysis. 3a: Controls n = 15, non-ICU COVID-19 n = 30, ICU COVID-19 n = 29, Septic shock n = 10. 3b: Controls n = 14, non-ICU COVID-19 n = 30, ICU COVID-19 n = 29, Septic shock n = 10. 3c: non-ICU COVID-19 n = 30, ICU COVID-19 n = 30. 3d: ICU COVID-19 initially admitted to the general ward requiring secondarily ICU support n = 4.
The whole blood count in COVID-19 disease is characterized by lymphopenia resulting with an increased neutrophil to lymphocyte ratio
The incidence of lymphopenia was higher in the ICU compared to non-ICU COVID-19 patients while the degree of lymphopenia was similar in the two ICU groups. Total white cells or neutrophils counts were not increased in both COVID-19 patient groups. In addition, platelets counts were normal in both groups. Conversely, septic shock was associated with thrombocytopenia, and increased counts for both the total white cells including neutrophils. As expected, the neutrophil to lymphocyte ratio (NLR) was increased in ICU-COVID 19 patients compared to non-ICU COVID-19 patients mainly due to the significant lymphopenia observed in ICU patients. The full results are shown in Table 2.
Absence of disseminated intravascular coagulation in COVID-19 patients, contrary to sepsis
Plasma fibrinogen was increased in ICU compared to non-ICU COVID-19 patients while lower values are observed in septic shock patients. D-dimer levels at admission were moderately increased in both COVID-19 groups but highest in the septic shock group. A statistical correlation was observed between the NO-dependent endothelial dysfunction (defined by HbNO values) and the highest D-dimer levels observed during the first 10 days of hospitalization without bacterial reinfection documented (R2 = 0.09; P = 0.02) (Supplemental Figure 1b). In ICU COVID-19 patients, neither thrombocytopenia nor prolonged prothrombin time (APTT; expressed as the International normalized ratio, INR) were observed, while they did occur in septic shock patients. COVID-19 patients had higher levels of vWF antigen (vWF: Ag), and vWF activity (VWF: RCo), compared to control subjects; these parameters also appeared to be associated with disease severity. Compared to controls, ADAMTS13 (A disintegrin and metalloprotease with thrombospondin type 1 repeats) activity was lower in the ICU COVID-19 group but not in non-ICU COVID-19 patients, while lower values were observed in septic shock patients. The vWF: Ag to ADAMTS13 activity ratio was elevated in both COVID-19 groups and related to disease severity and higher values were observed in septic shock. We observed decreased values of the endogenous anticoagulants antithrombin (AT) and protein C proportional to the severity of the COVID-19 disease, albeit with values still within the normal range. These parameters were clearly decreased below the normal range in septic shock patients. Tissue plasminogen activator (tPA) was increased in sepsis and COVID-19 patients in proportion to disease severity. All results are reported in Table 2.
Endothelial cell activation related to severity of COVID-19
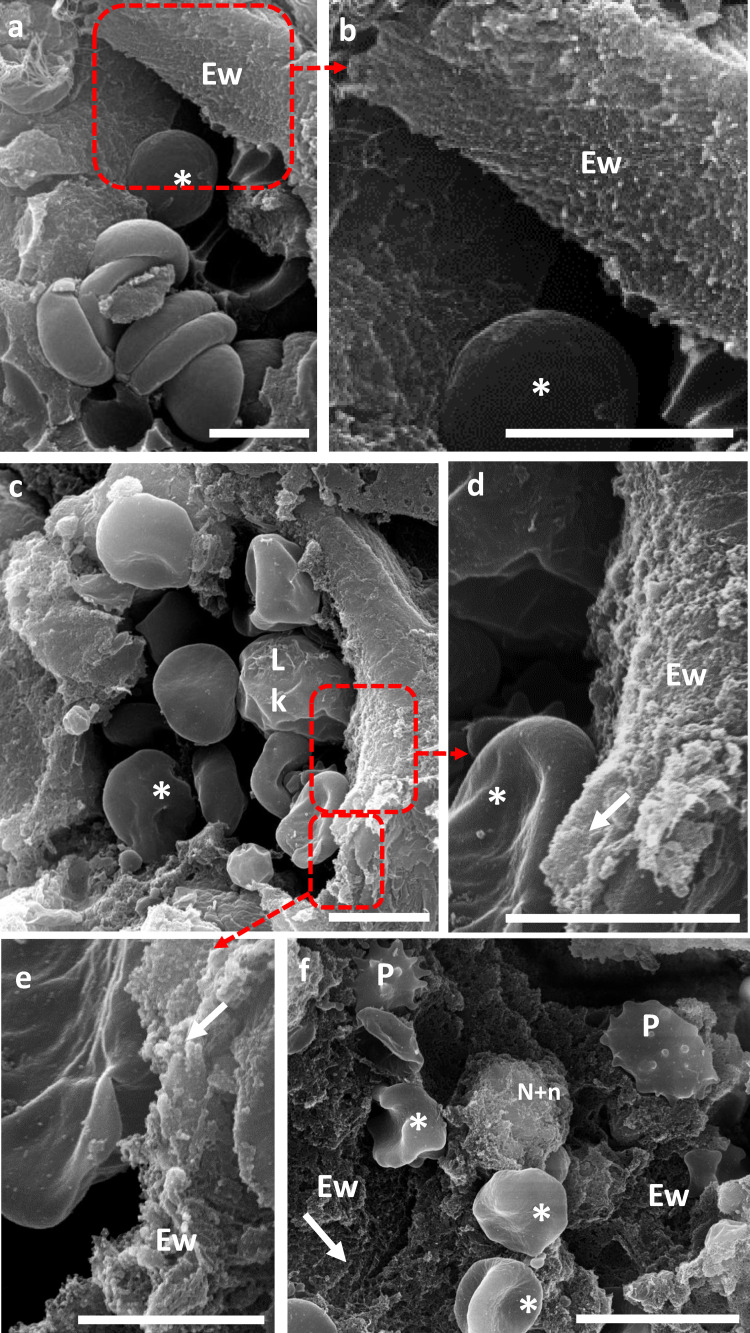
Plasma soluble intercellular adhesion molecule-1(sICAM-1) increased in ICU compared to non-ICU COVID-19 patients, while no difference was observed between this last group and control subjects. Higher values of sICAM-1 were observed in septic shock patients compared to COVID-19 patients. Endothelin-1 (ET-1) levels were higher in ICU COVID-19 patients and septic shock patients compared to controls. Plasminogen activator inhibitor-1 (PAI-1) levels were also higher in both COVID-19 groups and septic shock patients compared to controls. Full results are reported in Table 2. Scanning Electron Microscopy clearly illustrated the SARS-CoV-2-induced endothelial alterations in lung blood vessels of COVID-19 patients (Figure 4). Compared to pneumocytes, Transmission Electron Microscopy imaging suggested damaged but viable endothelial cells characterized by abnormal organelles morphology oriented toward viral protein synthesis (Figure 5). We observed a statistical correlation between the NO-dependent endothelial dysfunction (defined by lower HbNO) and sICAM-1 levels, in hospitalized COVID-19 patients (R2 = 0.21; P < 0.0002) (Supplemental Figure 1c).
Figure 4.
SARS-CoV-2-induced endothelial alterations in lung blood vessels of COVID-19 patients.
4. A, B: Scanning Electron Microscopy (SEM) of a cross section of healthy lung inner vessel with red blood cells (*). The luminal layer is a thin layer, composed of a single continuous layer of endothelial cells, the endothelial wall (Ew) (4A). Zoom of the boxed region showing the smooth surface of this wall (4B).
4. C–F: Cross-section of lung inner vessels of ICU COVID-19 patients. Blood vessel showing the presence of several leukocytes (Lk) and red blood cells (*) with irregular aspect of the endothelial wall (4C). Zooms of the boxed region highlighting the irregular aspect of the endothelial wall (Ew) due to a fibrillar network (arrow) of fibrin depots (4D-E). Large view of a vessel showing activated platelets (P), red blood cells (*), neutrophils and associated NETs and the dramatic fibrillar aspect of the endothelial wall contributed mainly by fibrin depots (4F).
Digital zoom; Bars, A, B, C, D: 5 µm; E: 2 µm: F: 10 µm. *: red blood cells; Lk: leukocytes; Ew: Endothelial wall; P: platelets; arrow heads: fibrin; N+n: Neutrophils and NET.
Figure 5.
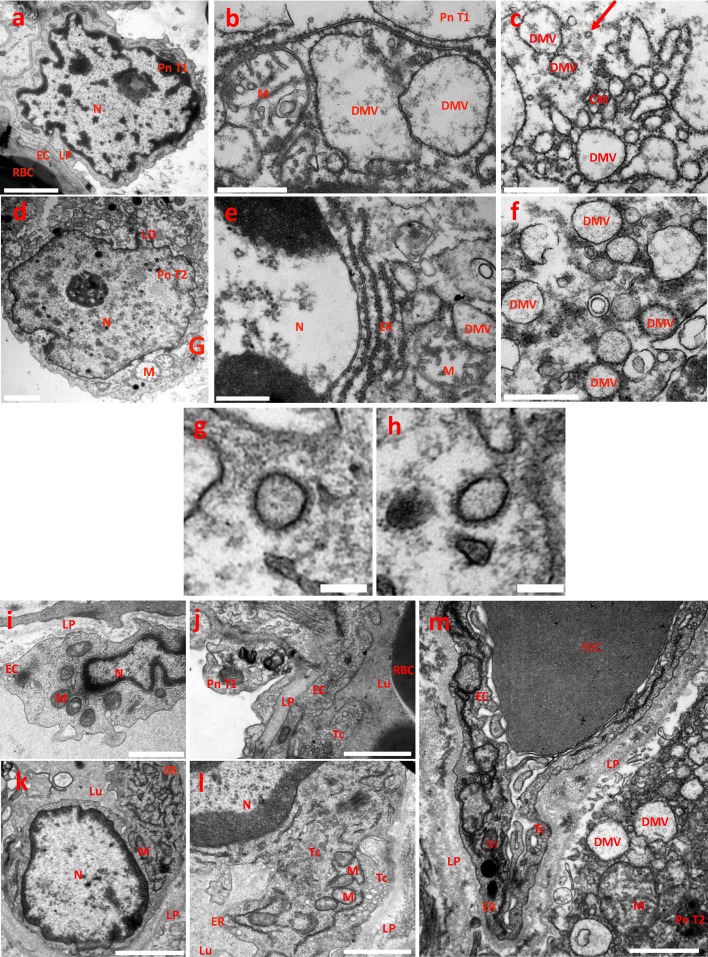
SARS-CoV-2 replication compartments in lung samples of ICU COVID-19 patients.
5. A–F. Pneumocytes. Transmission electron Microscopy (TEM) image of a control sample showing the normal morphology of a pneumocyte type 1 (5A). TEM images of damaged pneumocytes type 1 and type 2 (Pn T1, PnT2, respectively), from patients infected by SARS-CoV-2 coronavirus and showing virus replication compartments (5B to 5F). Pneumocytes contained abnormal swollen mitochondria with loss of cristae (M) (5B-D-E) and lipid droplets (LD) accumulation (5D). Pneumocytes contained typical membranous structures associated to coronavirus replication, such as double-membranes spherical vesicles (DMV), containing fibrous material (dsRNA) in their lumen and attached ribosomes at their external surface; due to their rough-endoplasmic reticulum (ER) origin and intensive genome virus replication (5B-C-E-F). DMVs were bridged by convoluted ER-derived membranes (CM) characteristic of the above-mentioned SARS-CoV-2 replication compartments (5B-C-E-F). Arrow, SARS-CoV-2 virion. Bars, A, D: 2 µm; B, C, E, F: 500 nm.
5. G, H: Mature SARS-CoV-2 virions. Typical morphology of coronaviruses with its crown of spike trimers. Diameters including the crowns: G, 191.4 nm; H, 189.3 nm. Bar, 150 nm.
5. I–M: Endothelial cells. TEM image of a control sample showing the normal morphology of a lung endothelial cells (5I, J). TEM images of lung endothelial cells from patients infected by SARS-CoV-2 coronavirus (5K–M). Despite the cell death of subjacent pneumocytes type I and type 2, endothelial cells (EC) retain swollen but active mitochondria with decrease in the electron density of their matrix (M) and transcytosis (Tc) activity, as evidenced by the massive caveolae and vesicle intracellular trafficking. Endothelial cells expressed abundant ribosomes expression associated with a large surface area of rough endoplasmic reticulum and Golgi apparatus (5K-L-M). Virus replication compartments in a type 2 pneumocyte subjacent to an EC (5M); note the detachment of the Pn T2 from the LP and the abnormal mitochondria. Bars, I, J, L, M: 1 µm; K: 500 nm.
LD, lipid droplets; DMV, Double membrane vesicles surrounded by ribosomes; CM, convoluted membranes; M, mitochondrion; N, nucleus; RBC, red blood cell; ER, rough endoplasmic reticulum; LP, lamina propria; Tc, transcytosis; Lu, blood vessel lumen. Pn T1, pneumocytes type 1; Pn T2, pneumocytes type 2; EC, endothelial cells.
Discussion
The main findings from our study are as follows; i. COVID-19 infection is uniquely associated with high vascular oxidative stress, unrelated to hyperactivity of the RAAS or neutrophil activation, contrary to sepsis; ii. COVID-19 patients present with reduced HbNO, reflective of decreased vascular NO bioavailability, that is proportional to disease severity; iii. In these patients, HbNO is reduced beyond levels observed in a control group matched for cardiovascular risk factors, suggesting supplemental vascular aggression (but not destruction), as supported by TEM analysis of the endothelium of COVID-19 patients; iv. These signs of endothelial (NO-dependent) dysfunction may explain, at least in part, the thrombotic microangiopathy complicating ICU COVID-19 and point to a pathophysiology entirely different from the disseminated intravascular coagulation commonly observed in sepsis (Figure 6). Accordingly, measurements of vascular HbNO may help to discriminate between COVID-19 (with low HbNO) and other septic complications (with high HbNO), as well as stratify COVID-19 patients for their risk of vascular complications.
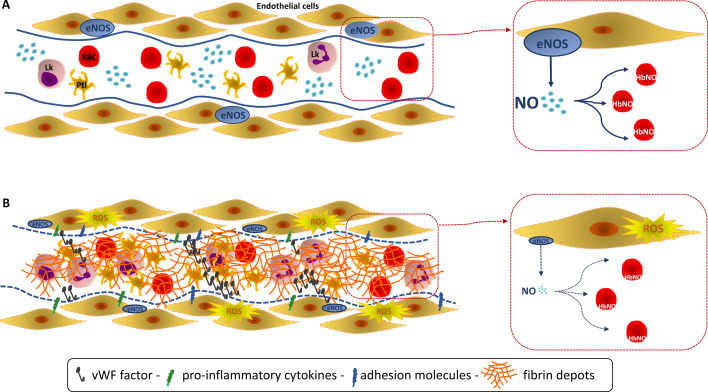
Figure 6.
Endothelial function reflected by the level of the 5-α-nitrosyl-hemoglobin (HbNO).
Endothelial nitric oxide (NO) is the pivotal endothelium-derived substance that reduces platelet aggression and adhesion, inhibits adhesion of leukocytes and expression of pro-inflammatory cytokine genes. 5-α-nitrosyl-hemoglobin (or HbNO) is measured in venous erythrocytes but is mainly influenced by the vascular NO sources (despite expression of an erythrocyte NOS) and is used as a surrogate for the NO-dependent endothelial function. (A, Upper) A healthy endothelium is represented with high venous erythrocyte levels of HbNO. (B, lower) Classically, NO-dependent endothelial dysfunction is defined as an imbalance between the Nitric Oxide (NO) bioavailability and reactive oxidant species (ROS). A decrease of the endothelium-derived NO is observed in response to an endothelial “aggression” related to the SARS-CoV-2 invasion with a pronounced oxidative stress leading to a decreased NO bioavailability. This severe NO-dependent endothelial dysfunction is reflected by decreased venous erythrocyte levels of HbNO. Such alteration could participate to the microvascular dysfunction observed in COVID-19 patients characterized by a hypercoagulable and a pro-inflammatory state with expression of pro-inflammatory cytokines and adhesion molecules, but without DIC as such. RBC: red blood cells - erythrocyte, Lk: leukocyte, Ptl: platelet.
SARS-CoV-2 invades endothelial cells, while subsequent endotheliitis characterized by specific markers of endothelial damage, including von Willebrand factor (VWF) or thrombomodulin released in the circulation, is usually associated with critical illness and death24 and may even predict mortality in COVID-19 patients.25 Before this late stage of endothelial destruction, an endothelial dysfunction develops in response to the endothelial “aggression” (e.g. by SARS-CoV-2) resulting, in part, in a decrease of the endothelium-derived NO.26 Indeed, recent data in COVID-19 patients point to the development of endothelial dysfunction with profound alterations of the microcirculation and the endothelial glycocalyx that were associated with clinical parameters and outcome.27 The early detection of endothelial dysfunction, however, remains a challenge. Our technique of electron paramagnetic resonance spectroscopy that directly measures HbNO as an index of vascular NO bioavailability, allowed us our to confirm a true NO-dependent endothelial dysfunction during COVID-19. This endothelial dysfunction is characterized by a decrease of HbNO proportional to COVID-19 severity associated with an increased oxidative stress, and in the absence of measurable overactivation of the RAAS or extensive endothelial damage.
Our study shows an association of moderate endogenous anticoagulants consumption, increased fibrinogen and D-dimer values that parallel COVID-19 severity, without disseminated intravascular coagulation as such. We confirmed the hypercoagulable state during COVID-19 with an increase of plasma von Willebrand factor (vWF) levels and activity related to COVID-19 severity28 combined with an vWF:Ag to ADAMTS13 activity ratio more pronounced in ICU COVID-19 patients,29 but not to the level of sepsis patients. All these findings support at least a sustained endothelial activation proportional to COVID-19 severity and above all, distinct from systemic acute exacerbated inflammation as observed during sepsis. As already published, ICU COVID-19 patients presented more cardiovascular comorbidities than non-ICU patients.30 However, the pathophysiological link between these comorbidities and the severity of COVID-19 illness is still a matter of debate. Notably, endothelial dysfunction is a common hallmark of all these cardiovascular risk factors31 and a key player in these related coagulopathies.32,33 This prompted us to further investigate the endothelial function during SARS-CoV-2 infection. While a causal link remains to be proven, the additional endothelial aggression related to SARS-CoV-2 infection combined with a pre-existing microcirculatory injury in patients with cardiovascular comorbidities would explain their propensity to develop critical COVID-19.
Endothelial dysfunction and increased thrombogenicity classically stem from an imbalance between nitric oxide (NO) produced by the endothelial nitric oxide synthase (eNOS) and reactive oxidant species (ROS) produced by NADPH oxidase (NOX)-2 or uncoupled eNOS.34 Previous work showed that venous erythrocyte levels HbNO measured by low temperature electron paramagnetic resonance (EPR) spectroscopy mainly reflect the bioavailability of NO formed in the vasculature from vascular endothelial NOS or exogenous NO donors,35 with minor contribution from erythrocyte NOS.36 We previously showed that HbNO measurements are directly correlated with vascular endothelial function measured by digital micro plethysmography.20 While eNOS constitutively produces physiological levels of vascular NO from the endothelium, the inducible NO synthase (iNOS) is expressed under inflammatory stimuli in many parenchymal cells and leucocytes to produce much larger amounts of NO.
Our present observations indicated a significant decrease of venous blood HbNO reflective of endothelial dysfunction that parallels COVID-19 severity. In ICU COVID-19 patients, HbNO decreased below values observed in the control group with similar cardiovascular risk factors, suggesting that these low values cannot solely be explained by these comorbidities. Conversely, patients suffering from septic shock, a paradigmatic disease with cytokine-dependent induction of iNOS, exhibited increased HbNO values. These original data are consistent with the pivotal role of exacerbated NO production, reflected by blood HbNO levels, in sepsis-induced vasoplegia. These high HbNO values in septic shock are in line with experimental studies in animals37,38 but, to our knowledge, were never verified in any human study. Changes in HbNO levels were corroborated by measurements of circulating nitrite/nitrate (NOx) levels which represent an overall estimation of the endothelial NO formation.39 This decreased NO production may also explain the lower need for vasopressor administration in our ICU COVID-19 patients, a finding confirmed by others.40 Moreover, endothelial dysfunction is thought to be involved in the pathogenesis of ARDS (Acute Respiratory Distress Syndrome)41 while we found that the only validated severity marker of hospitalized COVID-19 patients, i.e. hypoxemia, was correlated with HbNO levels. Interestingly, COVID-19 patients initially admitted to the ward and later transferred to the ICU displayed decreasing levels of HbNO, possibly reflective of an evolving endothelial dysfunction over time.
As relevant additional markers of endothelial activation that may participate to the hypercoagulable state, we observed an increase in sICAM1, an endothelial-leukocyte adhesion molecule, but also in PAI-1, the main physiologic inhibitor of fibrinolysis and particularly, ET-1, also known to be under the control of endothelium-derived NO42 in proportion to COVID-19 severity.
From a clinical point of view, endothelial dysfunction during COVID-19 occurs without global hemodynamic instability; rather, it induces a microcirculation failure causing tissue hypoperfusion, inflammation with increased leucocytes trafficking, platelets aggregation and microvascular thrombosis. The lack of benefit observed in the latest therapeutic anticoagulation trials in ICU COVID-19 patients43 could be explained by the degree and the timing of anticoagulation in relation to disease course, but perhaps mostly by its reductive nature limited only to the treatment of hypercoagulability. A more specific, endothelial-targeted strategy to attenuate the oxidative stress and/or increase the NO bioavailability, as previously suggested10,11 may be more efficient, but is currently missing.
This endothelial dysfunction may well result from the oxidative stress, reflected by increased plasma lipids peroxides measured in COVID-19 groups. Notably, ROS as such also participate to the pathophysiology of ARDS.44 Indeed, an exacerbated imbalance of the redox status, measured as an increased characteristic breathprint of volatile organic compounds related to oxidative stress, was specifically observed in ARDS patients with COVID-19 compared to ARDS from other causes.45 Further, another study observed a complete depletion of the vascular antioxidant enzymatic resources in ICU COVID-19 patients.46 In our study, the plasma lipids peroxides values were well above those observed in control subjects with cardiovascular risk factors, including metabolic syndrome, suggesting a strong oxidative stress during COVID-19. We did not observe high levels of lipid peroxidation in septic shock, commonly associated with massive inflammation-induced ROS production,47 although we did measure a clear increase in sTREM-1 reflective of neutrophilic polymorphonuclear (PMN) activation and phagocytosis. These results are in line with previous studies that found no increase in lipid peroxidation in patients suffering from septic shock.48 sTREM-1 participates in the sepsis-induced activation of NOX-2 during the respiratory burst of activated PMN involved in the host defense against pathogens.49 As we measured low values of sTREM-1, a putative role of respiratory burst, commonly present in classical bacterial infection, in the prevailing oxidative stress in COVID-19 seems unlikely. These results concerning oxidative stress do not exclude an active role of PMN during COVID-19, specifically through the release of neutrophils extracellular traps (NETs).50
Another classical activator of vascular NOX2 is the RAAS system. Our results confirm a decreased plasma angiotensin II levels in ICU COVID-19 patients51 that we now compared to adequately matched control subject using a well-validated, quantitative detection technique. Decreased levels of Ang II may be explained either by a decrease of its upstream generation or increased degradation. A major degradation pathway is ACE2, which is the target of the SARS-CoV-2 Spike protein. Cellular entry of SARS-CoV-1 particles upregulates ACE2 cleavage resulting in transmembrane ACE2 tissue shedding, leading to increased soluble ACE2.52,53 Accordingly, we found an increased concentration of soluble ACE2 in both COVID-19 patients groups. While this could not be measured directly, this finding may reflect a corresponding decrease in membrane-bound ACE2, during acute phase of SARS-Cov-2 infection, which would result in unopposed Ang II signalling in target cells. Our observation that the plasma aldosterone to Ang II ratio in COVID-19 patients is comparable to that in matched control subjects at least indirectly suggests unaltered sensitivity of the AT1R to Ang II. In addition, the known downregulation of the AT1R expression due to Ang II rise associated with relative low aldosterone levels classically described in septic shock54 was not observed in ICU COVID-19 patients. These findings argue against increased Ang II metabolism as a potential explanation of the lowered Ang II levels in COVID-19 patients. The putatively decreased membrane-linked ACE2 may also impact the local formation of protective peptides, Ang 1–7 and Ang 1–9. However, the already low values of plasma Ang II precluded measurement of these peptides as their levels are far below those of Ang II.55
As for upstream activation of the RAAS, data reported a decreased ACE activity correlated to severity in COVID-19 patients56 as in COVID-unrelated ARDS.57 Such attenuated ACE activity that is correlated to pulmonary endothelial function58 could reflect a general endothelial dysfunction as displayed in our ICU COVID-19 patients. We also found unaltered plasma concentrations of renin in COVID-19 patients, arguing against an overall increased RAAS activity. This is compatible with the usual absence of circulatory shock (which would otherwise activate renin production) even in ICU COVID-19 patients and the putatively preserved AT1R sensitivity (maintaining the negative feedback on renin secretion). Conversely, plasma renin was increased in sepsis patients, in parallel with their development of vasoplegia. Likewise, these patients had increased Ang II levels, as well as ACE2 shedding as expected.59 The most plausible explanation of our findings would be a decrease in ACE activity correlated to endothelial dysfunction combined with unaltered renin levels.
Given the absence of RAAS activation in COVID-19 patients, other sources of ROS should be considered. Our finding of increased plasma ET-1 levels in ICU COVID-19 patients, combined with the fact that ET-1 increases ROS production from NADPH oxidase activation and NOS uncoupling,60 would point to ET-1 as a putative player. Furthermore, respiratory viruses including coronavirus also induce endosomal ROS production via NOX2 activation.61 Lipid droplets accumulation as observed in our Transmission Electron microscopic (Figure 5) analysis of both lung microvascular endothelial cells and epithelial cells after SARS-CoV-2 invasion may well promote oxidative stress through increased fatty acid beta-oxidation by dysfunctional mitochondria.62
Limitations of the study
While the monocentric aspect of our trial could be debated, it allows a more homogeneous sample collection, while limiting the risk of batch effects particularly for HbNO measurements. However, the replication of these findings in other populations would be important. The ICU COVID-19 group is not homogenous in terms of specific treatments, as five patients did not receive Dexamethasone because their inclusion was performed before the results of the Recovery Trial. As this is an exploratory study, we did not define an a-priori specific number of patients. The relatively small number of patients included in our trial should also be mentioned. In addition, we limited our analyses to a single time point and were unable to provide any evolution over time (except for HbNO in limited cases for patients requiring secondarily ICU support). Septic shock is known to present specific coagulopathies and was chosen as a comparator to show distinctive pathogenic features during SARS-CoV-2 infection. However, the unbalanced number of patients compared to the two ICU groups could limit the generalizability of some of the results. Given the reduced availability of samples after Ang II dosage in COVID-19 patients, we did not measure other angiotensin metabolites. Also, we did not measure ACE/ACE2 expression in post-mortem lung tissues; however, as this would have entailed confounding effects due to secondary infections related to refractory multiple organ failure, we decided not to do so. Finally, the correlations presented from our observations are modest and suggest, but do not establish, the pathogenic role of the factors involved, which should be demonstrated in future mechanistic studies.
Conclusion
We showed that, at the initial stage of the disease, COVID-19 patients present a hypercoagulable state combined with a pronounced NO-dependent endothelial dysfunction in proportion to disease severity and enhanced oxidative stress that is not associated with an overactivation of the renin-angiotensin-aldosterone system or the respiratory burst. Although our data cannot prove causality, a correlation between NO bioavailability and oxygenation parameters is observed in hospitalized COVID-19 patients. Conversely, patients suffering from sepsis-induced vasoplegia present an opposite hemodynamic profile with exacerbated NO production. These results highlight an urgent need for oriented research to enhance our understanding of the endothelial oxidative stress that may promote both the NO-dependent endothelial dysfunction and the Acute Respiratory Distress Syndrome in severe COVID-19. They also point to the use of HbNO as an early marker of NO-dependent endothelial dysfunction which may be extended in future studies to stratify COVID-19 patients for their risk of vascular and lung complications.
Contributors
VM conceived the original hypothesis and all the experiments, drafted the work, collected all the data, verified the underlying data, performed all the graphs and statistics and was the main contributor in writing all the manuscript.
IL contributed to major technical analyses and extensively revised the manuscript.
LG contributed to the acquisition of the data and extensively revised the manuscript.
MV contributed to the acquisition of the electron microscopy data, was a minor contributor in writing the manuscript and extensively revised the manuscript.
DPM contributed to the acquisition of the electron microscopy data, was a minor contributor in writing the manuscript and extensively revised the manuscript.
TC collected the data and extensively revised the manuscript.
JBM contributed to the acquisition of the data and extensively revised the manuscript.
PH contributed to the acquisition of the data and extensively revised the manuscript.
CC contributed to the acquisition of the data and extensively revised the manuscript.
DG contributed to data collection and extensively revised the manuscript.
MAVD contributed to data collection and extensively revised the manuscript.
AP contributed to the acquisition of the data and extensively revised the manuscript.
CB revised critically for important intellectual content and extensively revised the manuscript.
Mél.D contributed to the acquisition of the data and extensively revised the manuscript.
LB contributed to the acquisition of the data and extensively revised the manuscript.
AR revised all statistical analyses and extensively revised the manuscript.
Marc.D contributed to data collection and extensively revised the manuscript.
PFL contributed to the acquisition of the data and data collection, revised critically for important intellectual content and extensively revised the manuscript.
AHJD contributed to data collection, revised critically for important intellectual content and was a major contributor in reviewing the entire manuscript.
XW contributed to the acquisition of the data, verified the underlying data, revised critically for important intellectual content, and was a major contributor in reviewing the entire manuscript.
JLB contributed to the acquisition of the data, verified the underlying data, revised critically for important intellectual content and was a major contributor in reviewing the entire manuscript.
Additionally, all authors had full access to all the data, approved the final version of this article and agreed to be accountable for all aspects of the work.
Declaration of interests
Marc Derive is a co-founder and employee of Inotrem Company, a drug development company that is developing anti-TREM-1 approaches in septic shock and COVID-19. The other authors have disclosed that they do not have any potential conflicts of interest.
Acknowledgments
Acknowledgments
We thank Lauriane Michel for helping in organizing the figures. We thank Drs. Lucie Pothen, Halil Yildiz and Jean-Cyr Yombi for their advice and support for the inclusion of severe patients and Dr Diego Castanares y Zapatero for his support for the inclusion of ICU patients. We thank Drs Charles Denis, Adil Wiart, Paul Paccaud, Romain Niessen, and also Julien De Poortere and Julie Bodart for their help to perform blood sample measurements, particularly Lucie Jolly for her participation in TREM-1 dosage and Joël Cosse, Delphine De Mulder, Roxane Verdoy and Delphine Hoton for samples preparation. We thank all the nurses of the ICU and Internal Medicine Unit at the Clinique Universitaires Saint-Luc for their help during technical procedures. We thank Dr Catherine Lambert for her advice and reading of the manuscript.
This work was supported by the grants number H.C.046.20F (CUR) from the Fonds National de Recherche Scientifique (FNRS). The CMMI is supported by the European Regional Development Fund and the Walloon Region. VM is a “Specialiste Post-doctorant” of the FNRS; MD is “Specialist Doctorant” of the FNRS; JLB is Senior Investigator of the WELBIO Institute.
Data sharing statement
All data analyzed during this study are included in this published article. Datasets are freely available to reader in the secure online repository Mendeley Data (https://data.mendeley.com//datasets/10.17632/4vsf5hgy6x.5).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103893.
Appendix. Supplementary materials
References
- 1.Xiong X., Chi J., Gao Q. Prevalence and risk factors of thrombotic events on patients with COVID-19: a systematic review and meta-analysis. Thromb J. 2021;19(1):32. doi: 10.1186/s12959-021-00284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemos A.C.B., do Espirito Santo D.A., Salvetti M.C., et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID) Thromb Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P., Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damiani E., Carsetti A., Casarotta E., et al. Microvascular alterations in patients with SARS-COV-2 severe pneumonia. Ann Intensiv Care. 2020;10(1):60. doi: 10.1186/s13613-020-00680-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Favaron E., Ince C., Hilty M.P., et al. Capillary leukocytes, microaggregates, and the response to hypoxemia in the microcirculation of coronavirus disease 2019 patients. Crit Care Med. 2021;49(4):661–670. doi: 10.1097/CCM.0000000000004862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scorcella C., Damiani E., Domizi R., et al. MicroDAIMON study: microcirculatory daily monitoring in critically ill patients: a prospective observational study. Ann Intensiv Care. 2018;8(1):64. doi: 10.1186/s13613-018-0411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominic P., Ahmad J., Bhandari R., et al. Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19. Redox Biol. 2021;43 doi: 10.1016/j.redox.2021.101982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamasaki H. Blood nitrate and nitrite modulating nitric oxide bioavailability: potential therapeutic functions in COVID-19. Nitric Oxide. 2020;103:29–30. doi: 10.1016/j.niox.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green S.J. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microbes Infect. 2020;22(4-5):149–150. doi: 10.1016/j.micinf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deanfield J.E., Halcox J.P., Rabelink T.J. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 13.Vanhoutte P.M. Endothelium and control of vascular function. State of the art lecture. Hypertension. 1989;13(6 Pt 2):658–667. doi: 10.1161/01.hyp.13.6.658. [DOI] [PubMed] [Google Scholar]
- 14.Lan J., Ge J., Yu J., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 15.Mehta P.K., Griendling K.K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292(1):C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y., Yang Y., Zhang C., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z., Hu R., Zhang C., Ren W., Yu A., Zhou X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit Care. 2020;24(1):290. doi: 10.1186/s13054-020-03015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry B.M., Benoit S., Lippi G., Benoit J. Letter to the editor - circulating plasma levels of angiotensin ii and aldosterone in patients with coronavirus disease 2019 (COVID-19): a preliminary report. Prog Cardiovasc Dis. 2020;63(5):702–703. doi: 10.1016/j.pcad.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhodes A., Evans L.E., Alhazzani W., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensiv Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 20.Lobysheva I.I., Biller P., Gallez B., Beauloye C., Balligand J.L. Nitrosylated hemoglobin levels in human venous erythrocytes correlate with vascular endothelial function measured by digital reactive hyperemia. PLoS One. 2013;8(10):e76457. doi: 10.1371/journal.pone.0076457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balcarek J., Seva Pessoa B., Bryson C., et al. Multiple ascending dose study with the new renin inhibitor VTP-27999: nephrocentric consequences of too much renin inhibition. Hypertension. 2014;63(5):942–950. doi: 10.1161/HYPERTENSIONAHA.113.02893. [DOI] [PubMed] [Google Scholar]
- 22.Group R.C., Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dikalov S., Fink B. ESR techniques for the detection of nitric oxide in vivo and in tissues. Methods Enzymol. 2005;396:597–610. doi: 10.1016/S0076-6879(05)96052-7. [DOI] [PubMed] [Google Scholar]
- 24.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–ee82. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philippe A., Chocron R., Gendron N., et al. Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis. 2021;24(3):505–517. doi: 10.1007/s10456-020-09762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao J.K. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest. 2013;123(2):540–541. doi: 10.1172/JCI66843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rovas A., Osiaevi I., Buscher K., et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24(1):145–157. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward S.E., Curley G.F., Lavin M., et al. Von Willebrand factor propeptide in severe coronavirus disease 2019 (COVID-19): evidence of acute and sustained endothelial cell activation. Br J Haematol. 2021;192(4):714–719. doi: 10.1111/bjh.17273. [DOI] [PubMed] [Google Scholar]
- 29.Mancini I., Baronciani L., Artoni A., et al. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J Thromb Haemost. 2021;19(2):513–521. doi: 10.1111/jth.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y., Yang Q., Chi J., et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: a systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carr M.E. Diabetes mellitus: a hypercoagulable state. J Diabetes Complicat. 2001;15(1):44–54. doi: 10.1016/s1056-8727(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 33.Lip G.Y., Li-Saw-Hee F.L. Does hypertension confer a hypercoagulable state? J Hypertens. 1998;16(7):913–916. doi: 10.1097/00004872-199816070-00003. [DOI] [PubMed] [Google Scholar]
- 34.Farah C., Michel L.Y.M., Balligand J.L. Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol. 2018;15(5):292–316. doi: 10.1038/nrcardio.2017.224. [DOI] [PubMed] [Google Scholar]
- 35.Tejero J., Shiva S., Gladwin M.T. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol Rev. 2019;99(1):311–379. doi: 10.1152/physrev.00036.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dei Zotti F., Lobysheva II, Balligand J.L. Nitrosyl-hemoglobin formation in rodent and human venous erythrocytes reflects NO formation from the vasculature in vivo. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0200352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plonka P.M., Chlopicki S., Wisniewska M., Plonka B.K. Kinetics of increased generation of (.)NO in endotoxaemic rats as measured by EPR. Acta Biochim Pol. 2003;50(3):807–813. [PubMed] [Google Scholar]
- 38.Westenberger U., Thanner S., Ruf H.H., Gersonde K., Sutter G., Trentz O. Formation of free radicals and nitric oxide derivative of hemoglobin in rats during shock syndrome. Free Radic Res Commun. 1990;11(1-3):167–178. doi: 10.3109/10715769009109680. [DOI] [PubMed] [Google Scholar]
- 39.Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta. 1999;1411(2-3):273–289. doi: 10.1016/s0005-2728(99)00020-1. [DOI] [PubMed] [Google Scholar]
- 40.Raschke R.A., Agarwal S., Rangan P., Heise C.W., Curry S.C. Discriminant accuracy of the SOFA score for determining the probable mortality of patients with COVID-19 pneumonia requiring mechanical ventilation. JAMA. 2021 doi: 10.1001/jama.2021.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vassiliou A.G., Kotanidou A., Dimopoulou I., Orfanos S.E. Endothelial damage in acute respiratory distress syndrome. Int J Mol Sci. 2020;21(22) doi: 10.3390/ijms21228793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boulanger C., Luscher T.F. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990;85(2):587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Investigators R.C., Investigators AC-a, Investigators A., Goligher E.C., Bradbury C.A., McVerry B.J., et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med. 2021;385(9):777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chow C.W., Herrera Abreu M.T., Suzuki T., Downey G.P. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol. 2003;29(4):427–431. doi: 10.1165/rcmb.F278. [DOI] [PubMed] [Google Scholar]
- 45.Grassin-Delyle S., Roquencourt C., Moine P., et al. Metabolomics of exhaled breath in critically ill COVID-19 patients: a pilot study. EBioMedicine. 2021;63 doi: 10.1016/j.ebiom.2020.103154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yaghoubi N., Youssefi M., Jabbari Azad F., Farzad F., Yavari Z., Zahedi Avval F. Total antioxidant capacity as a marker of severity of COVID-19 infection: possible prognostic and therapeutic clinical application. J Med Virol. 2021 doi: 10.1002/jmv.27500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biswal S., Remick D.G. Sepsis: redox mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2007;9(11):1959–1961. doi: 10.1089/ars.2007.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ware L.B., Fessel J.P., May A.K., Roberts L.J. Plasma biomarkers of oxidant stress and development of organ failure in severe sepsis. Shock. 2011;36(1):12–17. doi: 10.1097/SHK.0b013e318217025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baruah S., Murthy S., Keck K., et al. TREM-1 regulates neutrophil chemotaxis by promoting NOX-dependent superoxide production. J Leukoc Biol. 2019;105(6):1195–1207. doi: 10.1002/JLB.3VMA0918-375R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cavalcante-Silva L.H.A., Carvalho D.C.M., Lima E.A., et al. Neutrophils and COVID-19: the road so far. Int Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozkan S., Cakmak F., Konukoglu D., et al. Efficacy of serum angiotensin II levels in prognosis of patients with coronavirus disease 2019. Crit Care Med. 2021;49(6):e613–ee23. doi: 10.1097/CCM.0000000000004967. [DOI] [PubMed] [Google Scholar]
- 52.Lambert D.W., Yarski M., Warner F.J., et al. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J Biol Chem. 2005;280(34):30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haga S., Yamamoto N., Nakai-Murakami C., et al. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105(22):7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Correa T.D., Takala J., Jakob S.M. Angiotensin II in septic shock. Crit Care. 2015;19:98. doi: 10.1186/s13054-015-0802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lawrence A.C., Evin G., Kladis A., Campbell D.J. An alternative strategy for the radioimmunoassay of angiotensin peptides using amino-terminal-directed antisera: measurement of eight angiotensin peptides in human plasma. J Hypertens. 1990;8(8):715–724. doi: 10.1097/00004872-199008000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Z., Cai T., Fan L., et al. The potential role of serum angiotensin-converting enzyme in coronavirus disease 2019. BMC Infect Dis. 2020;20(1):883. doi: 10.1186/s12879-020-05619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orfanos S.E., Armaganidis A., Glynos C., et al. Pulmonary capillary endothelium-bound angiotensin-converting enzyme activity in acute lung injury. Circulation. 2000;102(16):2011–2018. doi: 10.1161/01.cir.102.16.2011. [DOI] [PubMed] [Google Scholar]
- 58.Orfanos S.E., Langleben D., Khoury J., et al. Pulmonary capillary endothelium-bound angiotensin-converting enzyme activity in humans. Circulation. 1999;99(12):1593–1599. doi: 10.1161/01.cir.99.12.1593. [DOI] [PubMed] [Google Scholar]
- 59.Patel V.B., Clarke N., Wang Z., et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 60.Loomis E.D., Sullivan J.C., DA O., Pollock D.M., Pollock J.S. Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J Pharmacol Exp Ther. 2005;315(3):1058–1064. doi: 10.1124/jpet.105.091728. [DOI] [PubMed] [Google Scholar]
- 61.To E.E., Vlahos R., Luong R., et al. Endosomal NOX2 oxidase exacerbates virus pathogenicity and is a target for antiviral therapy. Nat Commun. 2017;8(1):69. doi: 10.1038/s41467-017-00057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dias S.S.G., Soares V.C., Ferreira A.C., et al. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 2020;16(12) doi: 10.1371/journal.ppat.1009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.