Abstract
Background:
Mitochondrial DNA copy number (mtDNA-CN) is an accessible blood-based measurement believed to capture underlying mitochondrial (MT) function. The specific biological processes underpinning its regulation, and whether those processes are causative for disease, is an area of active investigation.
Methods:
We developed a novel method for array-based mtDNA-CN estimation suitable for biobank-scale studies, called ‘automatic mitochondrial copy (AutoMitoC).’ We applied AutoMitoC to 395,781 UKBiobank study participants and performed genome- and exome-wide association studies, identifying novel common and rare genetic determinants. Finally, we performed two-sample Mendelian randomization to assess whether genetically low mtDNA-CN influenced select MT phenotypes.
Results:
Overall, genetic analyses identified 71 loci for mtDNA-CN, which implicated several genes involved in rare mtDNA depletion disorders, deoxynucleoside triphosphate (dNTP) metabolism, and the MT central dogma. Rare variant analysis identified SAMHD1 mutation carriers as having higher mtDNA-CN (beta = 0.23 SDs; 95% CI, 0.18–0.29; p=2.6 × 10-19), a potential therapeutic target for patients with mtDNA depletion disorders, but at increased risk of breast cancer (OR = 1.91; 95% CI, 1.52–2.40; p=2.7 × 10-8). Finally, Mendelian randomization analyses suggest a causal effect of low mtDNA-CN on dementia risk (OR = 1.94 per 1 SD decrease in mtDNA-CN; 95% CI, 1.55–2.32; p=7.5 × 10-4).
Conclusions:
Altogether, our genetic findings indicate that mtDNA-CN is a complex biomarker reflecting specific MT processes related to mtDNA regulation, and that these processes are causally related to human diseases.
Funding:
No funds supported this specific investigation. Awards and positions supporting authors include: Canadian Institutes of Health Research (CIHR) Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award (MC, PM); CIHR Post-Doctoral Fellowship Award (RM); Wellcome Trust Grant number: 099313/B/12/A; Crasnow Travel Scholarship; Bongani Mayosi UCT-PHRI Scholarship 2019/2020 (TM); Wellcome Trust Health Research Board Irish Clinical Academic Training (ICAT) Programme Grant Number: 203930/B/16/Z (CJ); European Research Council COSIP Grant Number: 640580 (MO); E.J. Moran Campbell Internal Career Research Award (MP); CISCO Professorship in Integrated Health Systems and Canada Research Chair in Genetic and Molecular Epidemiology (GP)
Research organism: Human
eLife digest
Our cells are powered by small internal compartments known as mitochondria, which host several copies of their own ‘mitochondrial’ genome. Defects in these semi-autonomous structures are associated with a range of severe, and sometimes fatal conditions: easily checking the health of mitochondria through cheap, quick and non-invasive methods can therefore help to improve human health.
Measuring the concentration of mitochondrial DNA molecules in our blood cells can help to estimate the number of mitochondrial genome copies per cell, which in turn act as a proxy for the health of the compartment. In fact, having lower or higher concentration of mitochondrial DNA molecules is associated with diseases such as cancer, stroke, or cardiac conditions. However, current approaches to assess this biomarker are time and resource-intensive; they also do not work well across people with different ancestries, who have slightly different versions of mitochondrial genomes.
In response, Chong et al. developed a new method for estimating mitochondrial DNA concentration in blood samples. Called AutoMitoC, the automated pipeline is fast, easy to use, and can be used across ethnicities. Applying this method to nearly 400,000 individuals highlighted 71 genetic regions for which slight sequence differences were associated with changes in mitochondrial DNA concentration. Further investigation revealed that these regions contained genes that help to build, maintain, and organize mitochondrial DNA. In addition, the analyses yield preliminary evidence showing that lower concentration of mitochondrial DNA may be linked to a higher risk of dementia.
Overall, the work by Chong et al. demonstrates that AutoMitoC can be used to investigate how mitochondria are linked to health and disease in populations across the world, potentially paving the way for new therapeutic approaches.
Introduction
Mitochondria are semiautonomous organelles present in nearly every human cell that execute fundamental cellular processes including oxidative phosphorylation, calcium storage, and apoptotic signaling. Mitochondrial (MT) dysfunction has been implicated as the underlying cause for many human disorders based on mechanistic in vitro and in vivo studies (Burbulla et al., 2017; Desdín-Micó et al., 2020; Sliter et al., 2018). Complementary evidence comes from recent epidemiological studies that measure mitochondrial DNA copy number (mtDNA-CN), an MT-derived marker that can be conveniently measured from peripheral blood. Since mitochondria contain their own unique set of genomes that are distinct from the nuclear genome, the ratio of mtDNA to nuclear DNA molecules (mtDNA-CN) in a sample serves as an accessible marker of MT DNA abundance per cell (Longchamps et al., 2020). Indeed, observational studies suggest that individuals with lower mtDNA-CN are at higher risk of age-related complex diseases, such as coronary artery disease, sudden cardiac death, cardiomegaly, stroke, portal hypertension, and chronic kidney disease (Tin et al., 2016; Ashar et al., 2017; Zhang et al., 2017; Hägg et al., 2021). Conversely, higher mtDNA-CN levels have been associated with increased cancer incidence (Kim et al., 2015; Hu et al., 2016).
While previous studies demonstrate that mtDNA-CN is associated with various diseases, evidence suggests that it may also play a direct and causative role in human health and disease. For example, in cases of mtDNA depletion syndrome, wherein rare defects in nuclear genes responsible for replicating and/or maintaining mtDNA lead to deficient mtDNA-CN (Gorman et al., 2016), patients manifest with severe dysfunction of energy-dependent tissues (heart, brain, liver, and cardiac and skeletal muscles). So far, 19 genes have been reported to cause mtDNA depletion (Oyston, 1998). In addition to these rare monogenic syndromes, the importance of common genetic variation in regulating mtDNA-CN is an active area of research with approximately 50 common loci identified so far (Cai et al., 2015; Guyatt et al., 2019; Longchamps, 2019; Hägg et al., 2021). In contrast to marked drops in mtDNA-CN by 60–80% as seen in those with rare mtDNA depletion syndromes, the relevance of subtler perturbations in mtDNA-CN in disease risk remains to be determined (Basel, 2020).
Granted, the connection between blood mtDNA-CN and aspects of MT biology remains unclear with many studies showing only moderate correlation between mtDNA-CN and markers of MT function or biogenesis (Frahm et al., 2005; Wachsmuth et al., 2016). Further complicating the interpretation of epidemiological associations between blood mtDNA-CN and disease risk is the fact that (i) blood mtDNA-CN is strongly confounded by blood cell composition, particularly, platelets that are devoid of nuclei and that (ii) blood mtDNA-CN does not correlate well with mtDNA-CN measured in other tissues in which MT dysfunction may be more relevant (Picard, 2021). Accordingly, understanding the genetic determinants of blood mtDNA-CN may provide a better understanding of the etiological processes reflected by this poorly understood MT biomarker in the blood.
To interrogate mtDNA-CN as a potential determinant of human diseases and to better understand its biological relevance to mitochondria, we performed extensive genetic investigations in up to 395,781 participants from the UKBiobank study (Sudlow et al., 2015). We first developed and validated a novel method for biobank-scale mtDNA-CN investigations that leverages single nucleotide polymorphism (SNP) array intensities, called ‘automatic mitochondrial copy (AutoMitoC).’ Leveraging AutoMitoC-based mtDNA-CN estimates, we performed large-scale genome-wide association study (GWAS) and exome-wide association study (ExWAS) to identify common and rare genetic variants contributing to population-level variation in mtDNA-CN. Various analyses were then conducted to build on previous publications regarding the specific genes and pathways underlying mtDNA-CN regulation (Cai et al., 2015; Guyatt et al., 2019; Longchamps, 2019; Hägg et al., 2021). Finally, we applied Mendelian randomization analyses to assess potential causal relationships between mtDNA-CN and disease susceptibility.
Results
AutoMitoC: a streamlined method for array-based mtDNA-CN estimation
We built on an existing framework for processing normalized SNP probe intensities (log2ratio [L2R] values) from genetic arrays into mtDNA-CN estimates known as the ‘MitoPipeline’ (Lane, 2014) (Materials and Methods, Figure 1). The MitoPipeline yields mtDNA-CN estimates that correlate with direct qPCR measurements and has been successfully implemented in several epidemiological investigations (Ashar et al., 2017; Zhang et al., 2017; Figure 1—figure supplements 1–2). We developed a novel method, ‘AutoMitoC,’ which incorporates three amendments to facilitate large-scale investigations of mtDNA-CN (Figure 1). First, AutoMitoC replaces autosomal signal normalization of common variants with globally rare variants which negates the need for linkage disequilibrium pruning (Materials and Methods, Figure 1—figure supplements 3–4). As a result, this simplifies derivation of mtDNA-CN estimates in ethnically diverse cohorts by allowing for use of a single, universal variant set for normalization. Second, to detect potentially cross-hybridizing probes, we empirically assess the association of corrected probe signal intensities with off-target genome intensities (Materials and Methods, Supplementary file 1 – Tab 1–2), rather than relying on sequence homology of probe sequences, which is not always available. Last, the primary estimate of MT signal is ascertained using principal component analysis ([PCA]; as opposed to using the median signal intensity of MT probes as per the MitoPipeline) which improves concordance of array-based mtDNA-CN estimates with those derived from alternative methods (Materials and Methods). A detailed description of the development of the AutoMitoC pipeline is provided in the Materials and Methods.
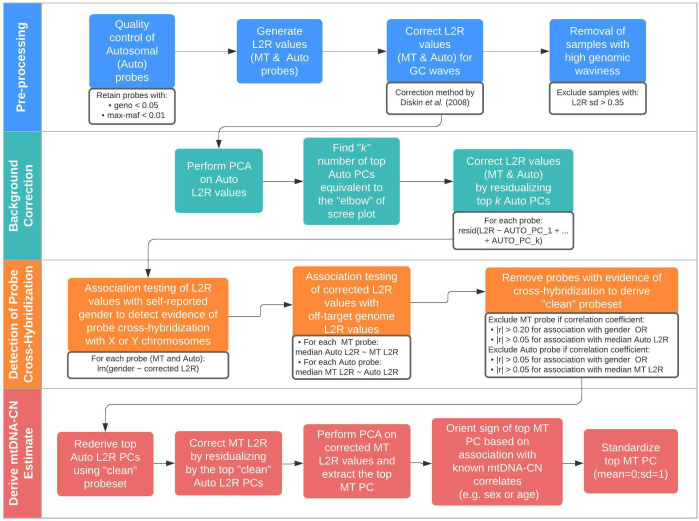
Figure 1. Schematic summary of the automatic mitochondrial copy (AutoMitoC) pipeline.
The AutoMitoC pipeline is comprised of four major steps: (i) preprocessing, (ii) background correction, (iii) detection of probe cross-hybridization, and (iv) final derivation of mitochondrial DNA copy number (mtDNA-CN) estimates. First, preprocessing is simplified by restricting analysis of autosomal variants to those that have low minor allele frequency ( <0.01) and low genotype missingness ( <0.05). For probes passing quality control, MT and autosomal log2ratio (L2R) values undergo an initial correction for guanine cytosine (GC) waves using the method by Diskin et al., 2008. Samples exhibiting high genomic waviness post GC-correction (L2R SD >0.35) are removed. Second, background correction consists of performing principal component analysis of the autosomal probe L2R values and finding the top k principal components (PCs) that correspond to the ‘elbow’ of the scree plot. In our case, ~70% variance in autosomal L2R values was explained by the top k PCs in both UKbiobank and INTERSTROKE datasets. GC-corrected MT and L2R values are then further adjusted for the top autosomal PCs (representing technical background noise) by taking the residuals of the association between the L2R values versus the k autosomal PCs. Third, we derive a ‘clean’ set of autosomal and MT probes without signs of off-target probe cross-hybridization by empirically testing the GC-corrected and background-corrected L2R values for association with either the sample medians of off-target genome L2R values or self-reported gender (to capture off-target hybridization to sex chromosomes). Fourth, using the ‘clean’ probeset, we repeat the autosomal background correction, extract the top MT PC as a crude measure of mtDNA-CN, change the sign of the MT PC according to association of the MT PC with known predictors of mtDNA-CN that are commonly reported (sex or age), and last, standardize the MT PC values as the final AutoMitoC estimate.
Figure 1—figure supplement 1. Intuition behind differentiation of genotypes and determination of mitochondrial DNA copy number (mtDNA-CN).
Figure 1—figure supplement 2. Overview of the MitoPipeline (Source: http://genvisis.org/MitoPipeline/) (Lane, 2014).
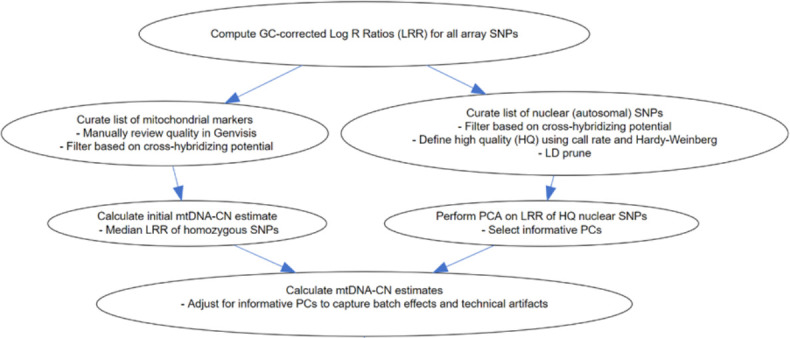
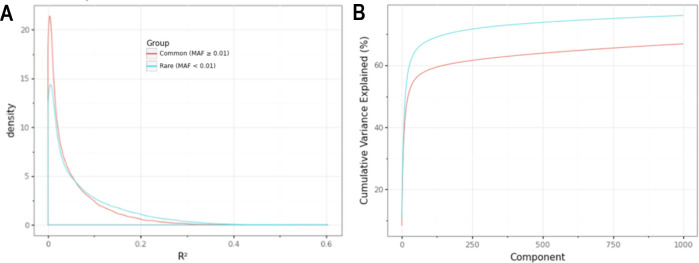
Figure 1—figure supplement 3. Minor allele frequency (MAF)-stratified analyses demonstrating utility of rare vs common autosomal variants for signal normalization.
Figure 1—figure supplement 4. Distribution of log10 transformed coefficients of determination (r2) from the association between autosomal probe intensities and median mitochondrial (MT) signal with (blue) or without (red) correction for background noise (i.e. 120 autosomal principal components [PCs]).
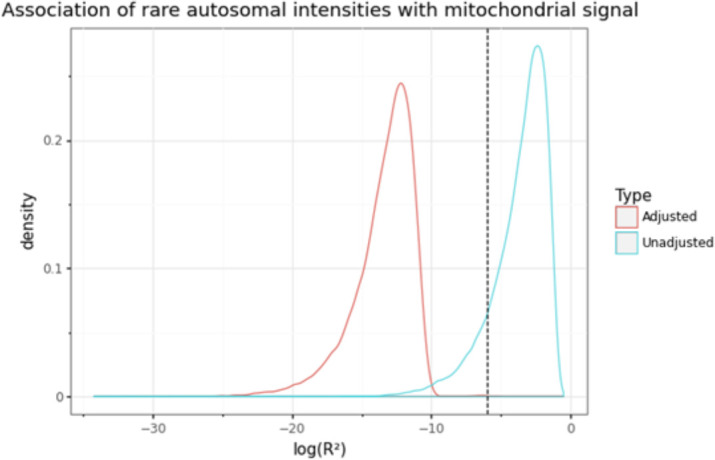
Figure 1—figure supplement 5. Validation of automatic mitochondrial copy in an ethnically diverse cohort with qPCR measurements.
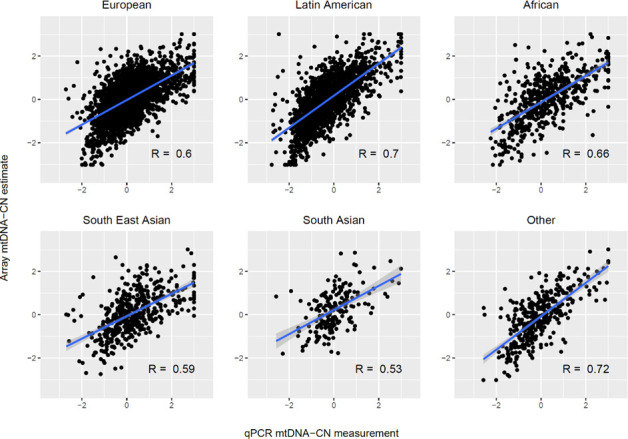
Figure 1—figure supplement 6. Bland Altman plots illustrating the extent of agreement between array and qPCR measurements.
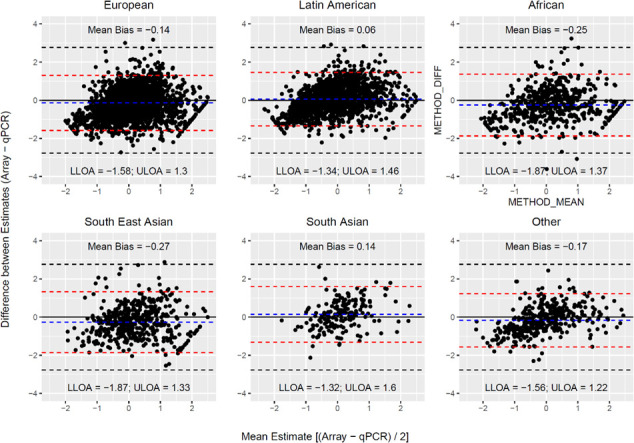
To benchmark performance of AutoMitoC, array-based mtDNA-CN estimates were compared to complementary measures of mtDNA-CN in two independent studies. First, array-based mtDNA-CN estimates were derived in a subset of 34,436 UKBiobank participants with available whole exome sequencing (WES) data. Reference mtDNA-CN estimates were derived from the proportion of WES reads aligned to the MT genome relative to the autosome (Longchamps, 2019). AutoMitoC estimates were significantly correlated with WES estimates (r = 0.45; p<2.23 × 10–308). Since WES data involves enrichment for nuclear coding genes and therefore could result in biased reference estimates for mtDNA-CN, we also performed an independent validation in an ethnically diverse study of 5791 participants where mtDNA-CN was measured using qPCR, the current gold standard assay (Fazzini et al., 2018). Indeed, we observed stronger correlation between AutoMitoC and qPCR-based estimates (r = 0.64; p<2.23 × 10–308; Figure 1—figure supplement 5). Furthermore, AutoMitoC demonstrated robust performance (r > 0.53) across all ethnic strata in the secondary validation cohort including Europeans (N = 2431), Latin Americans (N = 1704), Africans (N = 542), South East Asians (N = 471), South Asians (N = 186), and others (N = 360; ; Figure 1—figure supplement 5). Bland Altman plots also illustrate the extent of agreement between methods (Figure 1—figure supplement 6). For every ethnicity, 95% limits of agreement intervals were smaller than expected by chance. Last, while all analyses hitherto followed the MitoPipeline condition of requiring >40,000 autosomal variants for normalization, we observed comparable performance using even 1000 random very rare probes (MAF <0.001; r = 0.60; p<5 × 10–300) for signal normalization which reduced the runtime from several hours to less than 10 min for these 5791 samples running on 10 CPUs.
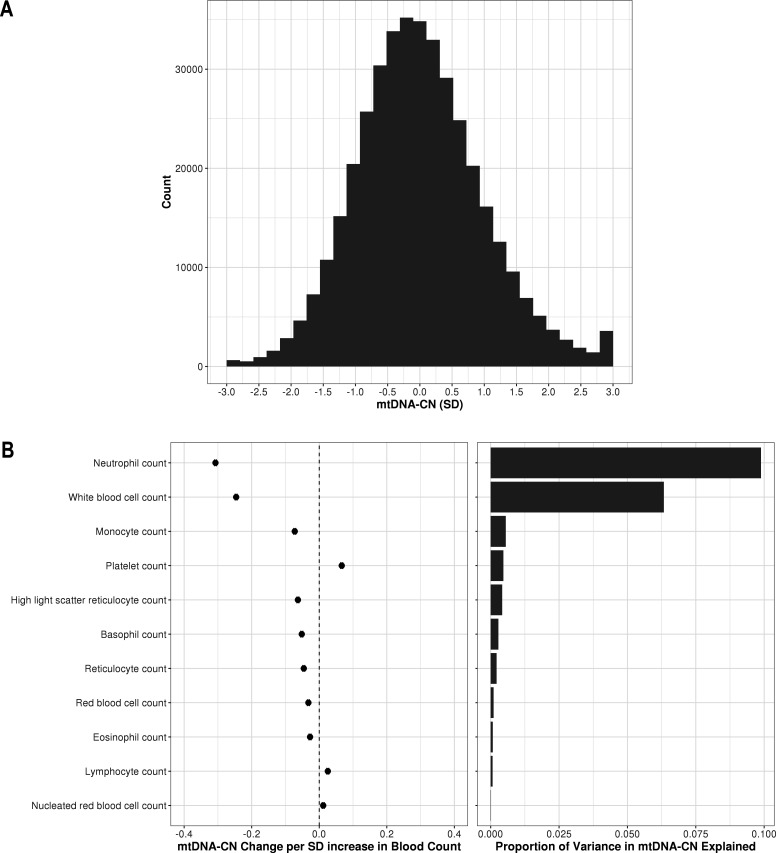
In the larger UKBiobank dataset of 395,781 with suitable array-based estimates, the distribution of mtDNA-CN was approximately normal (Figure 2A). To further verify that AutoMitoC-based estimates were indeed capturing mtDNA-CN, we performed association testing between mtDNA-CN values and known predictors including age, sex, ethnicity, and blood cell composition. Consistent with previous reports, every decade increase in age was associated with lower levels (beta = −0.08 SDs; 95% CI, –0.07 to –0.08; p<2.23 × 10–308), and females had higher levels than males (beta = 0.12 SDs; 95% CI, 0.11–0.12; p=1.67 × 10–299). MtDNA-CN levels also differed between ethnicities with South Asians (beta = −0.18; 95% CI, –0.16 to –0.21; p=2.93 × 10–47) and Africans (beta = −0.18; 95% CI, –0.16 to –0.21; p=8.39 × 10–48) having significantly lower levels than Europeans. Collectively, age, sex, and ethnicity accounted for 0.83% of the phenotypic variance in mtDNA-CN levels. All types of cell counts were significantly associated with mtDNA-CN including the standard set of covariates commonly adjusted for in mtDNA-CN investigations, namely, white blood cells (beta = −0.25 SDs in mtDNA-CN levels per 1 SD increase in cell counts; 95% CI, –0.25 to –0.24; p<2.23–308) and platelets (beta = 0.07; 95% CI, 0.06–0.07; p<2.23 × 10–308) (Figure 2B). White blood cell and platelet counts each accounted for 6.3% and 0.5% of the variance in mtDNA-CN levels, respectively (Figure 2B; Figure 2—source data 1). Notably, neutrophil count (beta = −0.31; 95% CI, –0.31 to –0.30; p<2.23–308) was the strongest predictor of mtDNA-CN levels and accounted for more variance (9.9%) than white blood cell and platelet counts combined (8.2%). Collectively, total white blood cell, platelet, and neutrophil counts explained 12.3% variance in mtDNA-CN levels.
Figure 2. Distribution of automatic mitochondrial copy (AutoMitoC) estimates and the influence of blood cell counts.
(A) Histogram illustrating AutoMitoC mitochondrial DNA copy number (mtDNA-CN) estimates in 395,781 UKBiobank participants expressed per SD change in mtDNA-CN. Associations between blood cell counts with mtDNA-CN levels as conveyed by forest plots illustrating effect estimates (left) and a bar plot showing the proportion of variance in mtDNA-CN explained (right). Models were adjusted for age, age2, sex array type, 20 genetic principal components, and ethnicity. Both blood cell counts and mtDNA-CN levels were standardized (mean = 0; SD = 1).
GWAS identifies 70 common loci for mtDNA-CN
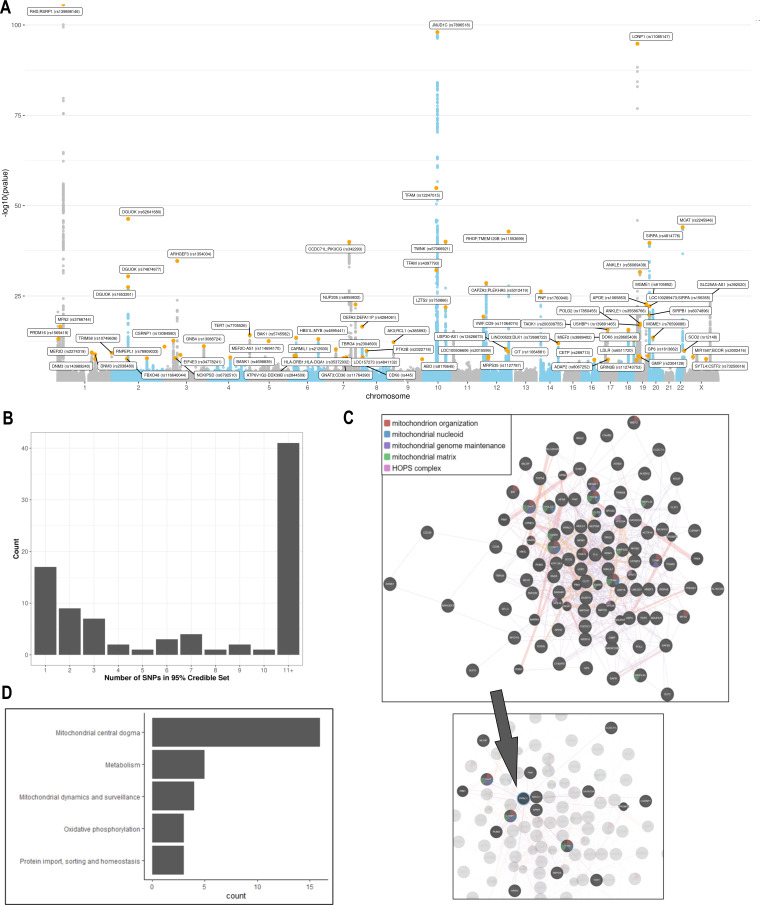
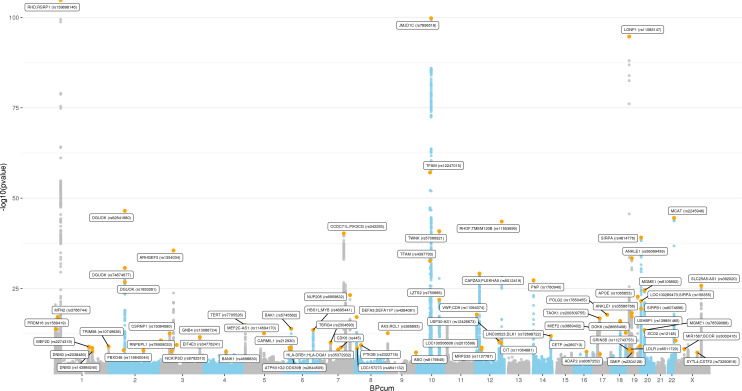
A GWAS was performed testing the association of 11,453,766 common genetic variants (MAF >0.005) with mtDNA-CN in 383,476 UKBiobank participants of European ancestry (Figure 3). In total, 9602 variants were associated with mtDNA-CN at genome-wide significance (Figure 3A; Figure 3—figure supplement 1), encompassing 82 independent signals in 72 loci (Supplementary file 2 – Tab 1; Figure 3—source data 1). The genomic inflation factor was 1.16 and the LD score intercept was 1.036, indicating that most inflation in test statistics was attributable to polygenicity. Sensitivity analyses revealed that nuclear mitochondrial DNA (NUMT) interference may have played a role in two independent signals (two loci), which were subsequently discarded, leading to a total of 80 independent signals in 70 loci. These 80 independent genetic signals explained 1.48% variance in mtDNA-CN levels.
Figure 3. Analyses of common genetic loci associated with mitochondrial DNA copy number (mtDNA-CN).
(A) Manhattan plot illustrating common genetic variant associations with mtDNA-CN. (B) Size distribution of 95% credible sets defined for 80 independent genetic signals. (C) GENE-MANIA-mania protein network interaction exploration showing all DEPICT and gene-mania prioritized genes (top) with functions color coded and a zoom-in highlighting interactors of the key mitochondrial (MT) regulator gene, PPRC1 (bottom). (D) ‘MitoPathway’ counts corresponding to 27 prioritized MitoCarta3 genes encoding proteins with known MT localization.
Figure 3—figure supplement 1. Minor allele frequency (MAF) and ethnicity-stratified genome-wide association study quantile-quantile plots.
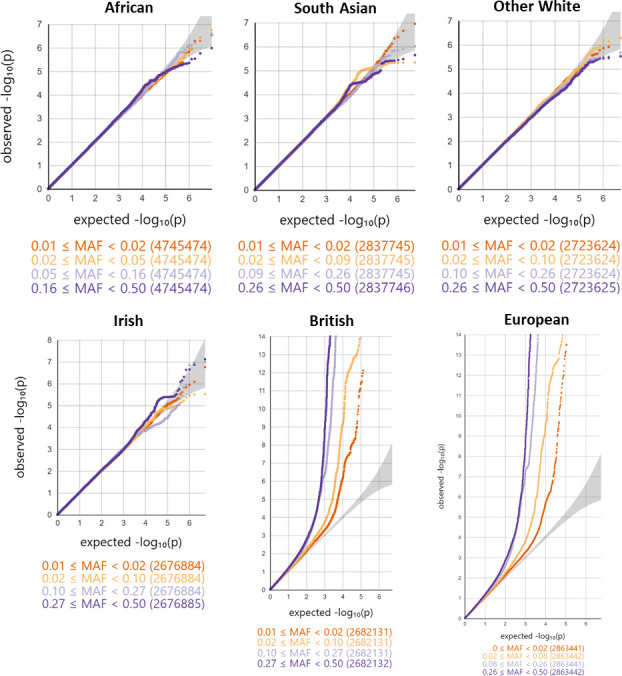
Figure 3—figure supplement 2. Manhattan plot for transethnic genome-wide association study meta-analysis (N = 395,781).
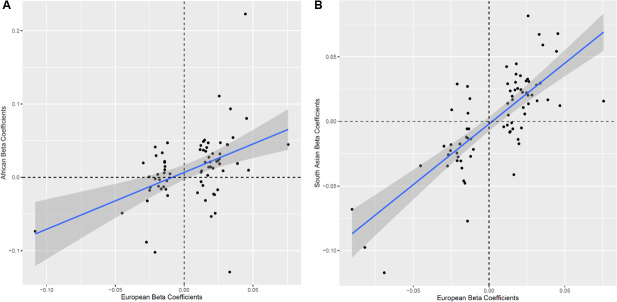
Figure 3—figure supplement 3. Correlation between conditionally independent mitochondrial DNA copy number loci effect estimates derived from European genome-wide association study (GWAS) meta-analyses (x-axes) vs effect estimates from non-European GWAS (y-axes).
Fine-mapping via the FINEMAP algorithm (Benner et al., 2016) yielded 95% credible sets containing 2363 genome-wide significant variants. Of the 80 independent genetic associations, 17 (22%) mapped to a single candidate causal variant; 32 (39%) mapped to 5 or fewer variants, and 42 (51%) mapped to 10 or fewer variants (Figure 3B; Supplementary file 2 – Tab 2). Credible sets for 11 genetic signals overlapped with genes responsible for rare mtDNA depletion disorders including DGUOK (3), MGME1 (2), TFAM (2), TWNK (2), POLG2 (1), and TYMP (1) (Supplementary file 2 – Tab 1 & 2). Several associations mapped to coding variants with high posterior probability. DGUOK associations mapped to a synonymous variant (rs62641680; posterior probability = 1) and a nonsynonymous variant (rs74874677; p=1). TFAM associations mapped to a 5’ untranslated region (UTR) variant (rs12247015; p=1) falling within an ENCODE candidate cis-regulatory element with a promotor-like signature and an intronic variant (rs4397793; p=1) with a proximal enhancer-like signature. Last, POLG2 associations mapped to a nonsynonymous variant (rs17850455; p=1). Beyond the six aforementioned mtDNA depletion genes identified at genome-wide significance, suggestive associations were found for POLG (rs2307441; p=1.0 × 10–7), OPA1 (rs9872432; p=5.2 × 10–7), SLC25A10 (rs62077224; p=1.2 × 10–7), and RRM2B (rs3907099; p=4.7 × 10–6). Given these observations, we hypothesized that mtDNA depletion genes may be generally enriched for common variant associations. Indeed, 10 (53%) of 19 known mtDNA depletion genes (Oyston, 1998) harbored at least suggestive mtDNA-CN associations (p<5 × 10–6).
We also compared our findings to genome-wide significant results from two recent GWAS of mtDNA-CN by Hägg et al., 2021 and Longchamps et al., 2022 to better contextualize our findings. Of the 66 independent signals identified by Hägg et al., 2021, we found varying levels of evidence to support 43 (65%) genetic associations; 41 (62%) variants were detected at Bonferroni significance; 2 (3%) variants were detected using a suggestive significance threshold (p<5 × 10–6) (Supplementary file 2 – Tab 3). For the remaining 23 (35%) variants not detected at suggestive significance, we hypothesized that differences in covariate adjustment strategies. Specifically, Hagg et al. did not adjust for platelets which is an important confounder of blood mtDNA-CN levels which might explain the majority of these discrepancies. As a sensitivity analysis, we repeated association testing without adjusting for blood cell traits and were able to recover 19/23 (83%) of the remaining variants at suggestive significance threshold, which suggests that approximately 1 in 3 associations detected by Hägg et al., 2021 may not be robust to adjustment for platelets (Supplementary file 2 – Tab 3). While we could not perform the reverse lookup of our top GWAS hits within the Hägg et al., 2021 GWAS due to a lack of full genome-wide summary statistics, 48 (60%) of our 80 genetic associations are likely novel relative to Hägg et al., 2021 as these variants were not reported as genome-wide significant nor in linkage disequilibrium (r2 >0.10) with their top hits (Supplementary file 2 – Tab 1). Among the 133 independent signals identified by Longchamps et al., 2022, 119 achieved Bonferroni significance, of which 116 were available in our GWAS (Supplementary file 2 – Tab 4). The remaining three variants (or their proxies; r2 >0.7) did not meet the MAF threshold for inclusion (MAF >0.005). Of the 116 detectable variants, we found varying levels of evidence to support 112 (97%) genetic associations; 70 (60%) variants were detected at Bonferroni significance; 15 (13%) variants were detected using a suggestive significance threshold; 27 (24%) variants were detected at nominal significance with concordant directionality (Supplementary file 2 – Tab 4). While we could not perform the reverse lookup of our top GWAS hits within the Longchamps et al., 2022 GWAS, 11 (14%) of our 80 genetic associations are likely novel as these variants were not reported as genome-wide significant nor in linkage disequilibrium with their top hits (Supplementary file 2 – Tab 1). Notably, these 11 associations were also not reported in Hägg et al., 2021.
Additionally, transethnic meta-analysis inclusive of non-Europeans (N = 395,781) was performed but given the small increase in sample size, GWAS findings remained highly similar (Figure 3—figure supplement 2). However, European effect estimates were significantly and highly correlated with those derived from South Asian (r = 0.97; p=2.2 × 10–15) and African (r = 0.88; p=9.1 × 10–5) GWAS analyses (Figure 3—figure supplement 3).
mtDNA-CN loci influence MT gene expression and heteroplasmy
We postulated that differential expression of genes encoded by the MT genome may trigger changes in copy number, and thus a subset of identified loci may affect mtDNA-CN through MT genome transcription. Ali et al., 2019 recently conducted a GWAS to identify nuclear genetic variants associated with variation in mtDNA-encoded gene expression (i.e. mitochondrial expression quantitative trait loci [mt-eQTLs]) (Ali et al., 2019). Nonsynonymous variants in LONP1 (rs11085147) and TBRG4 (rs2304693), as well as an intronic variant in MRPS35 (rs1127787), were associated with changes in MT gene expression across various tissues (Supplementary file 2 – Tab 5). Nominally associated mtDNA-CN loci were also observed to influence MT gene expression including intronic variants in both PNPT1 (rs62165226; mtDNA-CN p=5.5 × 10–5) and LRPPRC (rs10205130; mtDNA-CN p=1.1 × 10–4). Although differences in MT gene expression may be a consequence rather than a cause of variable mtDNA-CN, the analysis performed by Ali et al., 2019 was corrected for factors associated with global changes in the MT transcriptome (Ali et al., 2019). Moreover, the direction of effect estimates between mtDNA-CN and mt-eQTLs varied depending on gene and tissue context. Altogether, such findings imply that some mtDNA-CN loci may regulate mtDNA-CN by influencing MT gene expression.
Heteroplasmy refers to the coexistence of multiple mtDNA alleles within an individual for a particular variant, which is a function of the multicopy nature of the MT genome. A recent GWAS by Nandakumar et al., 2021 for mean heteroplasmy levels in saliva specimen provided initial evidence supporting a shared genetic basis for heteroplasmy and copy number. To further explore the overlap in genetic determinants of these traits, we searched for the previously reported heteroplasmy loci within our mtDNA-CN GWAS. Of 19 matching variants between the GWAS, 4 heteroplasmy loci were also associated with mtDNA-CN at genome-wide significance including variants nearby or within TINCR/LONP1 (rs12461806; mtDNA-CN GWAS p=7.5 × 10–88), TWNK/MPRL43 (rs58678340; p=1.3 × 10–39), TFAM (rs1049432; p=1.5 × 10–21), and PRKAB1 (rs11064881; p=2.6 × 10–10) genes. Consistent with the initial finding from Nandakumar et al., 2021 that the heteroplasmy-increasing TFAM allele was also associated with higher mtDNA-CN, we also observed concordant directionality for the other three variants. No additional mtDNA heteroplasmy loci were identified to influence mtDNA-CN when using a more liberal suggestive significance threshold. Full genome-wide summary statistics for the heteroplasmy GWAS were not publicly available so we could not perform the reverse lookup to examine whether the 80 mtDNA-CN variants affected heteroplasmy.
Genes and pathways implicated in the regulation of mtDNA-CN
DEPICT analysis led to the prioritization of 91 out of 18,922 genes (false discovery rate (FDR) p<0.05; Supplementary file 2 – Tab 6). Among them, 87 of the genes intersected with the GeneMANIA database and were uploaded to the GeneMANIA platform to identify additional functionally related genes (Warde-Farley et al., 2010). GeneMANIA analysis discovered an additional 20 related genes (Supplementary file 2 – Tab 7). Among the 107 total genes prioritized by DEPICT or GeneMANIA (Figure 3C), MT functions were significantly enriched in gene ontology (GO) terms including mitochondrion organization (coverage: 12/225 genes; FDR p=7.4 × 10–5), MT nucleoid (6/34; FDR p=2.2 × 10–4), MT genome maintenance (4/10; FDR p=6.8 × 10–4), and MT matrix (11/257; FDR p=6.8 × 10–4). Visual inspection of the links between key genes involved in these functions highlights PPRC1, a member of the Peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1A) family of MT biogenesis activators (Scarpulla, 2011), as a potential coordinator of mtDNA-related processes (Figure 3C).
MitoCarta3 is a comprehensive and curated inventory of 1136 human proteins (1120 nuclear) known to localize to the mitochondria based on experiments of isolated mitochondria from 14 nonblood tissues (Rath et al., 2021). We leveraged this recently updated database, that was absent from GeneMANIA, to conduct a complementary set of targeted analyses focused on MT annotations (Supplementary file 2 – Tab 7). First, we hypothesized that prioritized genes would be generally enriched for genes encoding the MT proteome. Overall, 27 (25%) of 107 genes had evidence of MT localization corresponding to a 4.2-fold enrichment (null expectation = 5.9%; p=1.0 × 10–10). Next, given that PPRC1, an activator of MT biogenesis, was prioritized by DEPICT analyses and then linked to central mtDNA regulators in GeneMANIA, we postulated that prioritized genes may be enriched for downstream targets of PGC-1A. PGC-1A induction resulted in a higher mean fold change among prioritized genes (beta = 1.48; 95% CI, 0.60–2.37) as compared to any MT gene (beta = 1.19; 95% CI, –0.76–3.13; t-test p=0.04). Finally, we categorized the 27 MitoCarta3 genes into their respective pathways. Most (16; 57%) genes were members of the ‘Mitochondrial central dogma’ pathway, which represents a nearly threefold enrichment as compared to the frequency of this pathway in the whole MitoCarta3 database (null expectation = 20.7%; p=1.3 × 10–5). Other implicated (albeit not significantly enriched) pathways included ‘Metabolism,’ ‘Mitochondrial dynamics and surveillance,’ ‘Oxidative phosphorylation,’ and ‘Protein import, sorting and homeostasis’ (Figure 3D). Four proteins were annotated as part of multiple pathways including TYMP/SCO2, GTPBP3, MIEF1, and OXA1L (Supplementary file 2 – Tab 7).
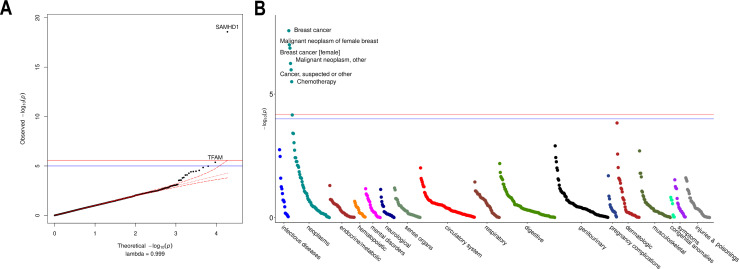
Exome-wide association testing uncovers rare coding SAMHD1 mutations as a determinant of mtDNA-CN levels and breast cancer risk
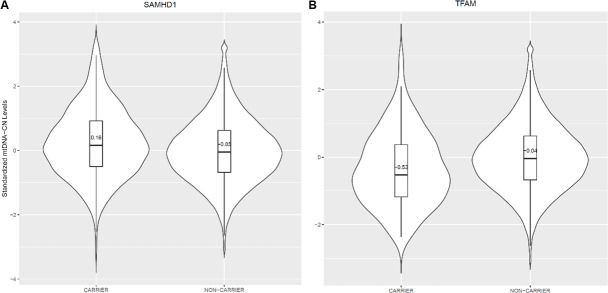
We performed an ExWAS in 147,740 UKBiobank participants with WES data to assess the contribution of rare coding variants. Among 18,890 genes tested, SAMHD1 was the only gene reaching exome-wide significance (Figure 4A; Figure 4—source data 1). The carrier prevalence of rare SAMHD1 mutations was 0.75%, and on average, mutation carriers had higher mtDNA-CN than noncarriers (beta = 0.23 SDs; 95% CI, 0.18–0.29; p=2.6 × 10–19; Figure 4—figure supplement 1). Also, while none of the 19 known mtDNA depletion genes reached Bonferroni significance, a suggestive association was found for TFAM (beta = −0.33; 95% CI, –0.47 to –0.19; p=4.2 × 10–6), and this association was independent of the common TFAM variants (rs12247015; rs4397793) previously identified in the GWAS (beta = −0.33; 95% CI, –0.47 to –0.19; p=8 × 10–6). Rare variants in SAMHD1 and TFAM accounted for 0.06% of the variance in mtDNA-CN levels. Collectively, rare and common loci accounted for 1.55%.
Figure 4. Rare variant gene burden association testing with mitochondrial DNA copy number and disease risk.
(A) QQ plot illustrating expected vs observed -log10 p-values for exome-wide burden of rare (MAF <0.001) and nonsynymous mutations. (B) Manhattan plot showing phenome-wide significant associations between SAMHD1 carrier status and cancer-related phenotypes.
Figure 4—figure supplement 1. Effect of rare mutation carrier status for SAMHD1 and TFAM genes on mitochondrial DNA copy number (mtDNA-CN) levels.
To evaluate whether rare SAMHD1 mutations also influenced disease risk, we conducted phenome-wide association testing of 771 diseases within the UKBiobank. At phenome-wide significance, SAMHD1 mutation carrier status was associated with approximately twofold increased risk of breast cancer (OR = 1.91; 95% CI, 1.52–2.40; p=2.7 × 10–8), as well as greater risk of ‘cancer (suspected or other)’ (OR = 1.52; 95% CI, 1.28–1.80; p=1.1 × 10–6; Figure 4B; Figure 4—source data 2). Exclusion of breast cancer cases was attenuated but did not nullify the association with ‘cancer (suspected or other)’ (OR = 1.36; 95% CI, 1.10–1.67; p=0.004) suggesting that SAMHD1 mutations may also increase risk of other cancers, as has been shown for colon cancer (Rentoft, 2019). To understand whether differences in mtDNA-CN levels between SAMHD1 mutation carriers was a consequence of cancer diagnosis, we repeated association testing with mtDNA-CN excluding cancer patients. In this analysis, the association with mtDNA-CN levels was not attenuated (beta = 0.26; 95% CI, 0.19–0.32; p=7.8 × 10–15) suggesting that the effect of rare SAMHD1 variants on mtDNA-CN levels is not driven by its relationship with cancer status. A summary of MT genes and pathways implicated by common and rare loci is provided in Figure 5.
Figure 5. Graphical summary of mitochondrial genes and pathways implicated by genetic analyses.
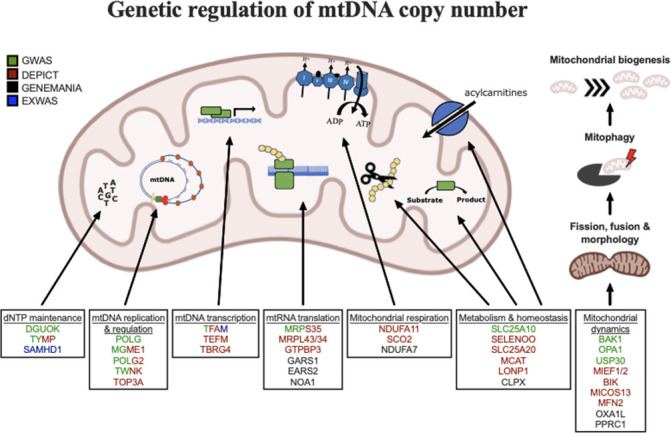
Color coding indicates through which set(s) of analyses genes were identified. The image was generated using BioRender (https://biorender.com/).
Mendelian randomization analysis implicates low mtDNA-CN as a causal mediator of dementia
Given that common variant loci overlapped with several mtDNA depletion genes, we postulated that polygenically low mtDNA-CN might cause a milder syndrome with phenotypically similar manifestations. To assess whether mtDNA-CN may represent a putative mediator of mtDNA depletion-related phenotypes, we conducted Mendelian randomization analyses between genetically determined mtDNA-CN and MT disease phenotypes using summary statistics derived from the FinnGen v4 GWAS dataset (Supplementary file 2 – Tab 8).
After accounting for multiple testing of 10 phenotypes, an association between mtDNA-CN and all-cause dementia was found (OR = 1.94 per 1 SD decrease in mtDNA-CN; 95% CI, 1.55–2.32; p=7.5 × 10–4; Figure 6; Figure 6—source data 1). Sensitivity analyses indicated no evidence of global (MR-PRESSO p=0.51; Q-statistic p=0.51) or directional (Egger Intercept p=0.47) pleiotropy. The 27 selected variants accounted for 0.70% of the variance in mtDNA-CN and 0.13% of the risk for dementia, consistent with a causal effect of mtDNA-CN on dementia risk and not vice versa (Steiger p=1.9 × 10–62). Findings were robust across several different MR methods including the weighted median (OR = 2.47; 95% CI, 1.93–3.00; p=0.001), MR-EGGER (OR = 2.41; 95% CI, 1.71–3.11; p=0.02), and GSMR-HEIDI (OR = 1.95; 95% CI, 1.34–2.84; p=0.001) methods. Results also remained statistically significant when using a broader set of genetic instruments including all genome-wide significant loci irrespective of whether genetic variants were located proximally to MitoCarta3 genes (GSMR-HEIDI OR = 1.31; 95% CI, 1.02–1.68; p=0.04). Furthermore, we replicated the dementia association using a second UKBiobank-independent GWAS dataset derived from the International Genomics of Alzheimer’s Disease Consortium (2013) including 17,008 Alzheimer’s disease patients (OR = 1.41; 95% CI, 1.0001–1.98; p=0.04993) (Lambert et al., 2013).
Figure 6. Association of genetically lower mitochondrial DNA copy number with mitochondrial (MT) disease phenotypes.
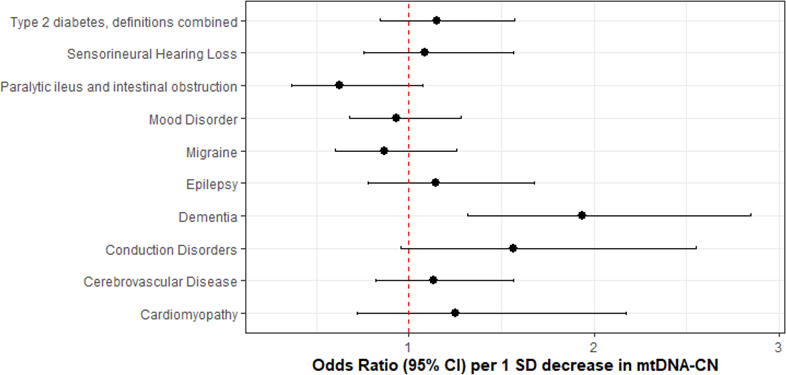
Coefficient plots for Mendelian randomization analyses of MT disease traits. In the absence of heterogeneity (Egger intercept p>0.05; MR-PRESSO global heterogeneity p>0.05), the inverse variance weighted result was reported. In the presence of balanced pleiotropy (MR-PRESSO global heterogeneity p<0.05), the weighted median result was reported. No set of analyses had evidence for directional pleiotropy (Egger intercept p<0.05).
Discussion
We developed a novel method to estimate mtDNA-CN from genetic array data, ‘AutoMitoC,’ and applied it to the UKBiobank study. Extensive genetic investigations led to several key insights regarding mtDNA-CN. First, several novel common and rare genetic determinants of mtDNA-CN were identified, totaling 71 loci. Second, these loci were enriched for MT processes related to dNTP metabolism and the replication, packaging, and maintenance of mtDNA. Third, we observed a strong role for common variation within known mtDNA depletion genes in regulating mtDNA-CN in the general population. Fourth, we found that rare variants in SAMHD1 not only affect mtDNA-CN levels but also confer risk to cancer. Finally, we provided the first Mendelian randomization evidence implicating low mtDNA-CN as a causative risk factor for dementia.
While several investigations for mtDNA-CN have been published, the present study represents the most comprehensive genetic assessment published to date (Cai et al., 2015; Longchamps, 2019; Hägg et al., 2021). Notably, Hägg et al., 2021 recently conducted the largest GWAS for mtDNA-CN in 295,150 UKBiobank participants and identified 50 common loci (Hägg et al., 2021). However, the method developed by Hägg et al., 2021 calibrated SNP probe intensities based on association with WES read depths, which may limit the convenience of the method. In contrast, AutoMitoC only necessitates array probe intensities and does not require any secondary genetic measurements (WES or otherwise) for calibration. In addition, AutoMitoC exhibits superior concordance with WES-based estimates (Hagg r = 0.33; AutoMitoC r = 0.45), which were validated in an independent dataset with gold standard qPCR measurements (AutoMitoC r = 0.64). Further, Hägg et al., 2021 restricted genetic analyses to unrelated European individuals, whereas we incorporated ~100,000 additional individuals and demonstrated consistency in genetic effects between Europeans and non-Europeans (r > 0.88). The greater sample size in combination with more accurate mtDNA-CN estimates may explain the additional 48 (59%) independent common genetic associations identified at genome-wide significance. Furthermore, we took additional precaution to ensure that cataloged NUMTs were not influencing our genetic association results and also to empirically evaluate the robustness of each genetic association against cataloged and uncataloged NUMTs. Finally, in the present study we included complementary explorations of the role of rare variants through ExWAS and phenome-wide association study (PheWAS), as well as, Mendelian randomization analyses to assess disease contexts whereby mtDNA-CN may represent a causal mediator and a potential therapeutic target.
MtDNA-CN has proven to be a biomarker of cardiovascular disease in several large epidemiological studies, with studies often assuming that such relationships are attributable to pathological processes including MT dysfunction, oxidative stress, and inflammation (Tin et al., 2016; Wu et al., 2017; Fazzini et al., 2019; Koller et al., 2020). Consistent with previous GWAS findings, our genetic analyses confirm that mtDNA-CN indeed reflects specific MT functions, but perhaps not the ones commonly attributable to mtDNA-CN (Cai et al., 2015; Guyatt et al., 2019; Hägg et al., 2021). Primarily, differences in mtDNA-CN reflect MT processes related to dNTP metabolism and the replication, maintenance, and organization of mtDNA. Secondarily, genes involved in MT biogenesis, metabolism, oxidative phosphorylation, and protein homeostasis were also identified but do not represent the main constituents. The observed enrichment in common variant associations within mtDNA depletion genes further reinforces the notion that differences in mtDNA-CN first and foremost reflect perturbations in mtDNA-related processes.
No therapy for mtDNA depletion disorders currently exists with treatment mainly consisting of supportive care. Intriguingly, we found that rare variants within SAMHD1 were associated with increased levels of mtDNA-CN. SAMHD1 is a multifaceted enzyme with various functions including tumor suppression through DNA repair activity and maintenance of steady-state intracellular dNTP levels, which has been involved in HIV-1 replication (Baldauf et al., 2012; Kretschmer et al., 2015). Rare homozygous and compound heterozygous loss-of-function mutations in SAMHD1 result in an immune encephalopathy known as Aicardi Goutieres’ syndrome (White et al., 2017). Imbalanced intracellular dNTP pools and chronic DNA damage cause persistent elevations in interferon alpha thus mimicking a prolonged response to HIV-1 infection. While Aicardi Goutieres’ syndrome is a severe recessive genetic disorder, case reports of SAMHD1-related disease often describe heterozygous parents and siblings as being unaffected or with milder disease (familial chilblain lupus 2) (Haskell et al., 2018). In the UKBiobank, the vast majority (99.4%) of individuals possessing SAMHD1 mutations were heterozygote carriers, who had a twofold increased risk of breast cancer. Indeed, our finding that SAMHD1 mutations associate with both elevated mtDNA-CN levels and risk of breast cancer belies the prevailing notion that higher mtDNA-CN is always a protective signature of proper MT function and healthy cells. Such findings may have important clinical implications for genetic screening. First, heterozygous SAMHD1 mutations may be an overlooked risk factor for breast cancer considering that ~1 in 130 UKBiobank participants possessed a genetic mutation conferring twofold elevated risk. Notably, while SAMHD1 mutations have been described previously to be associated with various cancers (Kohnken et al., 2015; Rentoft, 2019), this gene is not routinely screened nor part of targeted gene panels outside the context of neurological disorders (https://www.genedx.com/test-catalog/available-tests/comprehensive-common-cancer-panel/). Second, unaffected parents and siblings of Aicardi Goutieres patients might also present with greater risk of cancer. Third, while SAMHD1 is a highly pleiotropic protein, therapeutic strategies to dampen (but not abolish) SAMHD1 activity might be considered to treat mtDNA depletion disorders caused by defects in nucleotide metabolism. Indeed, Franzolin et al., 2015 demonstrated that siRNA knockdown of SAMHD1 in human fibroblasts with DGUOK mtDNA depletion mutations partially recovered mtDNA-CN (Franzolin et al., 2015).
To our knowledge, we provide the first Mendelian randomization evidence that mtDNA-CN may be causally related to risk of dementia. Although dysfunctional mitochondria have long been implicated in the pathogenesis of Alzheimer’s disease, only recently has mtDNA-CN been tested as a biomarker. Silzer et al., 2019 conducted a matched case-control study of 46 participants and showed that individuals with cognitive impairment had significantly lower blood-based mtDNA-CN (Silzer et al., 2019). Andrews and Goate, 2020 studied the relationship between postmortem brain tissue mtDNA-CN and measures of cognitive impairment in 1025 samples (Andrews and Goate, 2020). Consistent with our findings, a 1 SD decrease in brain mtDNA-CN was associated with lower mini mental state exam (beta = –4.02; 95% CI, –5.49 to –2.55; p=1.07 × 10–7) and higher clinical dementia rating (beta = 0.71; 95% CI, 0.51–0.91). Both studies implicate blood- and brain-based mtDNA-CN as a marker of dementia but were retrospective. In contrast, Yang et al., 2021 observed a significant association between mtDNA-CN and incident risk of neurodegenerative disease (Parkinson’s and Alzheimer’s disease)(Yang et al., 2021). Altogether, our results combined with previous findings suggest that mtDNA-CN represents both a marker and mediator of dementia. Considering that our overall findings suggest that mtDNA-CN reflects numerous MT subprocesses, future studies will be required to disentangle which ones, as reflected by diminished mtDNA-CN, truly mediate dementia pathogenesis.
Several limitations should be noted. First, mtDNA-CN approximated by array-based methods remains imperfectly accurate as compared to qPCR or whole genome sequencing measurements, though we found strong correlation between AutoMitoC and qPCR-based estimates in this study (r = 0.64; p<2.23 × 10–308). AutoMitoC was benchmarked using multiple comparative mtDNA-CN estimation modalities, in independent cohorts, and in various ethnic groups, but further work can be done to assess the reproducibility and error of mtDNA-CN estimates, calibration to absolute counts, and the robustness to different genotyping platforms. Although whole genome sequencing will eventually supplant array-based mtDNA-CN GWAS, we hypothesize that the improvements made in the areas of speed, portability to ethnically diverse studies, and ease-of-implementation, should greatly increase accessibility of mtDNA-CN research as a plethora of genotyping array data is presently available to reanalyze. Second, we did not detect strong evidence of confounding from population structure (or substructure) in our genetic analyses, and we undertook several precautions to mitigate such biases including (i) stratified GWAS analyses by ethnicity, (ii) adjustment for a large number of intraethnic principal components (PCs), and (iii) restriction of downstream analyses to use of European GWAS summary statistics to harmonize with external databases; however, it is still plausible that population substructure may have influenced our findings. Third, Mendelian randomization analyses were underpowered to conduct a broad survey of diseases in which MT dysfunction may play a causal role, and even among the 10 phenotypes tested, statistical power varied by disease prevalence as evidenced by relatively large CI for the dementia association. Accordingly, future studies including larger case sample sizes are necessary to provide more precise effect estimates. Equally as important, we were unable to differentiate whether specific MT subpathways mediated risk of disease. As additional loci are uncovered, such analyses may be feasible. Fourth, variants and genes implicated in the regulation of mtDNA-CN may be specific to blood samples though findings suggest that many mtDNA-CN loci act through genes that are widely expressed in mitochondria across multiple tissues. Future studies are required to determine whether associations are ubiquitous across mitochondria-containing cells and to investigate the role of mtDNA-CN in other tissues. Lastly, whole blood mtDNA-CN reflects a heterogenous mixture of nucleated and unnucleated cells, and despite adjustment for major known confounding cell types, interindividual differences in cell subpopulations not captured by a standard blood cell count may represent an important source of confounding.
Conclusion
Although commonly viewed as a simple surrogate marker for the number of mitochondria present within a sample, genetic analyses suggest that mtDNA-CN is a highly complex biomarker under substantial nuclear genetic regulation. mtDNA-CN reflects a mixture of MT processes mostly pertaining to mtDNA regulation. Accordingly, the true relationship between mtDNA-CN measured in blood samples with human disease remains to be completely defined though we find evidence for mtDNA-CN as a putative causal risk factor for dementia. Future studies are necessary to decipher if mtDNA-CN is directly involved in the pathogenesis of dementia and other diseases or whether other specific MT processes are truly causative.
Materials and methods
The AutoMitoC number pipeline
Background and rationale
While SNP array data is intended for highly multiplexed determination of genotypes, the raw probe signal intensities used for genotypic inference can also be co-opted to derive estimates of mtDNA-CN. Determining genotypes for a sample at a given variant site relies on contrasting hybridization intensities of allele-specific oligonucleotide probes and then assigning membership to the most probable genotype cluster based on intensity properties (Figure 1—figure supplement 1). Variation in intensities within each genotyping cluster can also be co-opted to deduce variations in copy number. A commonly used metric of probe intensity is the ‘L2R,’ which denotes log2(observed intensity/expected intensity), where the expected intensity is defined as the median signal intensity for a probe conditional on each genotype cluster.
The ‘MitoPipeline’ is a framework for estimating mtDNA-CN from array-based L2R (aka L2R) values developed by Lane, 2014 (Lane, 2014; Zhang et al., 2017). An overview of the MitoPipeline is described in Figure 1—figure supplement 2. To briefly summarize: (i) autosomal and MT L2R values are first corrected for GC waves; (ii) a high-quality set of MT and autosomal markers are selected largely based on visual inspection of genotype clusters and basic local alignment search tool (BLAST) alignment for nonhomologous sequences; (iii) PCA of at least 40,000 autosomal markers is conducted to capture batch effects; (iv) finally, mtDNA-CN is estimated based on median MT L2R value for each sample and then corrected for background noise through residualization of top autosomal PCs.
The MitoPipeline has proven to be effective in estimating mtDNA-CN (correlation coefficient R ~0.5 with direct qPCR estimates) as evidenced by multiple epidemiological studies employing this method (Ashar et al., 2017; Zhang et al., 2017; Fazzini et al., 2019). First, visual inspection of MT probe intensity clusters is recommended to remove probes with poorly differentiated genotype clusters. However, this process is time-consuming (especially for biobank studies that are genotyped across thousands of batches); determination of probes with ‘good’ vs ‘bad’ genotype clustering is subjective; and guidance is only provided for adjudication of polymorphic but not rare or monomorphic markers which may still be informative. Second, in consideration of nuclear and MT sequences with significant sequence similarity due to past and recurrent transposition of MT sequence into the nuclear genome, also known as ‘NUMTs’ (Simone et al., 2011), the MitoPipeline recommends exclusion of MT probes with greater than 80% sequence similarity to the nuclear genome. While determining sequence homology of probes to the nuclear genome may have been feasible with older microarrays wherein probe sequences were often publicized, for many contemporary arrays, including the UKBiobank array, such information is not readily available. Third, LD pruning of common autosomal variants is required to ascertain a set of independent genetic variants, but implementation of this approach within ethnically diverse studies becomes more complex since genetic independence is ancestry dependent. Under the MitoPipeline framework, each ethnicity warrants a unique set of common variants, which not only adds to computational burden but also creates an additional source of variability in terms of performance of the method between ethnicities.
Therefore, we developed a new array-based mtDNA-CN estimation method, which we have dubbed the ‘AutoMitoC’ pipeline, which incorporates three key amendments: (i) autosomal signal normalization utilizes globally rare variants in place of common variants which confer advantages in terms of both speed and portability to ethnically diverse studies, (ii) cross-hybridizing probes are identified by assessing evidence for cross-hybridization via association of signal intensities (rather than using genotype association and identification of homologous sequences through BLAST alignment), and (iii) the primary estimate of MT signal is ascertained using PCA as opposed to using the median signal intensity of MT probes. The rationale underlying these amendments are described in detail in the subsequent sections. An overview of the AutoMitoC pipeline is provided in Figure 1.
Development of AutoMitoC in the UKBiobank study
To develop the AutoMitoC pipeline we used genetic datasets from the UKBiobank. The UKBiobank is a prospective cohort study including approximately 500,000 UK residents (ages 40–69 years) recruited from 2006 to 2010 in whom extensive genetic and phenotypic investigations have been and continue to be done (Sudlow et al., 2015). All UKBiobank data reported in this manuscript were accessed through the UKBiobank data showcase under application #15,525. All analyses involve the use of genetic and/or phenotypic data from consenting UKBiobank participants.
Two main genetic datasets from the UKBiobank were incorporated in the development of AutoMitoC. First, CNV L2R values derived from genetic arrays (i.e. normalized array probe intensities) for 488,264 samples were downloaded using the ukbgene utility, and their corresponding genotype calls (data field: 22418) were downloaded with gfetch. Second, exome alignment maps (EXOME FE CRAM files and indices; data fields 23163 & 23164) from the first tranche of 49,989 samples released in March, 2019 were downloaded with the ‘ukbfetch’ utility. L2R values were used to derive AutoMitoC mtDNA-CN estimates. For WES data, samtools idxstats was used to derive the number of sequence reads aligning to MT and autosomal genomes, from which an estimate of mtDNA-CN was derived according to the procedure by Longchamps, 2019; these complementary WES-based mtDNA-CN estimates served as a comparator to benchmark AutoMitoC performance.
Initial quality control of 488,264 samples and 784,256 directly genotyped variants was executed in PLINK followed that recommended by the MitoPipeline (i.e. sample call rate >0.96; variant call rate >0.98; Hardy Weinberg equilibrium [HWE] p>1 × 10–5; PLINK mishap p>1 × 10–4; genotype association with sex p>0.00001; LD pruning r2 <0.30; MAF >0.01) (Purcell et al., 2007). Variants within 1 Mb of immunoglobulin, T-cell receptor genes, and centromeric regions were removed. After this quality control procedure, 466,093 samples and 86,677 common variants remained. Next, genomic waves were corrected according to Diskin et al., 2008 using the PennCNV ‘genomic_wave.pl’ script (https://github.com/WGLab/PennCNV/blob/master/genomic_wave.pl) (Wang et al., 2007; Diskin et al., 2008). Samples with high genomic waviness (L2R SD >0.35) before and after GC correction were removed resulting in 431,501 samples with array L2R values corresponding to 86,677 common autosomal variants. Last, we excluded samples representing blood cell count outliers ( >3 SDs) as per Longchamps, 2019 which led to 395,781 participants (Longchamps, 2019). Finally, we took the intersection of European samples with both suitable array and WES data resulting in a final testing dataset of 34,436 European participants. To evaluate the possibility of replacing common autosomal variant signal normalization with rare variants, we also analyzed a set of 79,611 rare variants with an MAF <0.01.
Background correlation between autosomal and MT L2R values
Inference of relative mtDNA-CN from array data consists of determining the ratio of MT to autosomal probe signal intensities (or normalized L2R values) within each sample. Technical (e.g. batch and plate effects) and latent sample factors confound raw signal intensities for reasons unrelated to DNA quantity. Such confounders induce strong cross-genome correlation, and therefore, a necessary first step is to remove this background noise from autosomal and MT probe intensities.
Indeed, even after correcting autosomal L2R values for genomic waves, we observed significant correlation between individual autosomal probe intensities and the median sample intensity across the 265 MT variants (Figure 1—figure supplement 3A). The extent of cross-genome intensity correlation varied based on MAF, with rare autosomal variants (MAF <0.01; M = 79,611) showing the strongest correlation. We postulate that intensity properties for rare variants, which have a higher prevalence of homozygous genotypes, more strongly resemble those for MT genotypes, which are predominantly homoplasmic. On this basis, we only use rare autosomal variants to represent autosomal signal. While this approach contrasts with the MitoPipeline, which utilizes common genetic variants to represent autosomal signal, restricting autosomal signal normalization to rare variants confers three major advantages while maintaining the same level of concordance with WES estimates (rcommon = 0.50; rrare = 0.49). First, this allows for further streamlining of the pipeline as this precludes the necessity for common variant filters, such as LD pruning. Second, we show that fewer PCs are necessary to capture the same proportion of total variance in signal intensities with rare as opposed to common variants. Approximately 70% of the total variance in rare autosomal intensities was explained by 120 PCs, whereas the same proportion of variance in common autosomal intensities would necessitate more than 1000 PCs (Figure 1—figure supplement 3B). In the UKBiobank, PCs were derived via the eigendecomposition of the empirical covariance matrix conducted in Python 3.6, using NumPy and SciPy (Harris et al., 2020; Virtanen et al., 2020). Third, the set of autosomal markers used in deriving mtDNA-CN remains independent from the set of common autosomal variants analyzed in subsequent GWAS for mtDNA-CN. Effectively, this ensures that common autosomal variants evaluated for association with mtDNA-CN in downstream GWAS analyses are not directly incorporated into autosomal signal normalization, which could otherwise attenuate GWAS signals.
Empirical detection of off-target probes
After adjustment for 120 autosomal PCs (approximating the elbow of the variance explained curve), there persisted a smaller subset of autosomal probes that were significantly correlated with median MT intensity (Figure 1—figure supplement 4). We hypothesized that such probes either (i) cross-hybridize with the MT genome (i.e. lie within NUMT regions) or (ii) corresponded to genetic loci involved in regulation of mtDNA-CN. As an illustration, Supplementary file 1 – Tab 1 conveys characteristics of the 10 most strongly correlated variants. Four of the top 10 probes were located within 1 Mb of a NUMT region, and in all 4 cases, the sign of the correlation coefficient was negative which might reflect interference of autosomal signal with increasing mtDNA-CN. An additional three probes corresponded to variants within genes that were implicated in MT disorders or regulation of MT processes (Supplementary file 1 – Tab 1).
We further explored whether there was evidence for cross-hybridization between autosomal SNPs and sex chromosomes by regressing adjusted autosomal probe intensities with reported male status. Generally, correlations between autosomal intensities and sex were stronger than those with MT intensities, suggesting that cross-hybridization of autosomal probes to sex chromosomes is more pronounced. For the top 10 sex-associated probes, we performed BLASTn alignment against the human reference genome (GRCh38) using 30 bases surrounding each probe (Supplementary file 1 – Tab 2) (Altschul et al., 1990). All probes had at least one flanking sequence with near-perfect ( >97%) sequence identity to a sex chromosome. For these 10 probes, the sign of the correlation between autosomal intensity and male status was perfectly consistent with homology to X or Y chromosomes, thus supporting the hypothesis that such probes cross-hybridized to sex chromosomes.
Inclusion of autosomal probes with evidence of off-target hybridization to the MT genome or sex chromosomes is problematic. In the former scenario, misattribution of autosomal as MT signal may reduce the effectiveness of normalization. In the latter scenario, inadvertent adjustment for sex through retention of cross-hybridizing autosomal probes may occur if such probes explain substantial variance in autosomal probe intensities and this is particularly problematic given that mtDNA-CN has been robustly shown to differ between genders in epidemiological studies. In preliminary investigations where sex-associated probes were retained, we noticed that several top PCs that perfectly tagged gender. Hypothetically, had these PCs been retained and used for correction of MT signal, then the final mtDNA-CN estimate would have been inadvertently corrected for gender. Therefore, we removed autosomal probes exhibiting at least modest correlation (|r| > 0.05) with reported gender (907; 1.14%) or median MT intensity (193; 0.24%) and then recalculated top autosomal PCs.
PCA-based approach improves concordance with complementary estimates
After correcting MT probes using the updated set of 120 autosomal L2R PCs, we adopted the MitoPipeline’s approach for estimating mtDNA-CN and calculated the median of corrected MT L2R values to denote an individual’s final mtDNA-CN estimate. Using this median-based approach, array mtDNA-CN estimates demonstrated significant correlation with WES mtDNA-CN estimates (r = 0.33; p<2.23 × 10–308). However, we found that performing PCA across all corrected MT L2R values and then extracting the top MT PC for each sample as the final mtDNA-CN estimate resulted in stronger correlation with WES (r = 0.49; p<2.23 × 10–308).
Independent validation of AutoMitoC in the INTERSTROKE study
We additionally validated the AutoMitoC pipeline by deriving array-based mtDNA-CN estimates in the INTERSTROKE study and comparing these with parallel qPCR-based measurements, the current gold standard for measuring mtDNA-CN. INTERSTROKE is an international case-control study of stroke including 26,526 participants from 32 countries and 142 centers (O’Donnell et al., 2010). Blood samples have been collected for a subset of approximately 12,000 individuals, of which 9311 have been successfully genotyped using the Axiom Precision Medicine Research Array (PMRA r3). MtDNA-CN estimates for INTERSTROKE were generated according to the AutoMitoC pipeline as illustrated in Figure 1. A further subset of 5791 samples with both suitable array genotypes have undergone qPCR measurement of mtDNA-CN using the plasmid-normalized protocol from Fazzini et al., 2019. Please see Chong et al., 2021 for further details Chong et al., 2021.
GWAS in the UKBiobank
Data acquisition and quality control
UKBiobank samples were genotyped on either the UK Biobank Array (~450,000) or the UK BiLEVE array (~50,000) for approximately 800,000 variants (Bycroft et al., 2018). Further imputation was conducted by the UKBiobank study team using a combined reference panel of the UK10K and Haplotype Reference Consortium datasets. Imputed genotypes (version 3) for 488,264 UKBiobank participants were downloaded through the European Genome Archive (Category 100319). Samples were removed if they were flagged for any of the UKBiobank-provided quality control annotations (Resource 531; ‘ukb_sqc_v2.txt’) for high ancestry-specific heterozygosity, high missingness, mismatching genetic ancestry, or sex chromosome aneuploidy (‘het.missing.outliers,’ ‘in.white.British.ancestry.subset,’ ‘putative.sex.chromosome.aneuploidy’). Samples were also removed if their submitted gender did not match their genetic sex or if they had withdrawn consent at the time of analysis. Variant quality control consisted of removing variants that had low imputation quality (INFO score <0.30), were rare (MAF <0.005), or were in violation of Hardy Weinberg Equilibrium (HWE p<1 × 10–10). The HWE test was conducted within a subset of unrelated individuals for each ethnic strata, though all related individuals were retained for subsequent GWAS analysis. Lastly, in special consideration of mtDNA-CN as the GWAS phenotype, we also removed variants within ‘NUMTs,’ which refer to regions of the nuclear genome that exhibit homology to the MT genome due to past transposition of MT sequences. Accordingly, NUMTs represent a specific confounder of mtDNA-CN GWAS analyses which may lead to false positive associations. NUMT boundaries were obtained from the UCSC NumtS Sequence (numtSeq) track, which is based on the Reference Human NumtS curated by Simone et al., 2011. All sample and variant quality control of imputed genotypes were executed using qctools and resultant bgen files were indexed with bgenix. Smaller ethnic groups with similar genetic ancestry were consolidated; individuals self-reporting as ‘African’ or ‘Caribbean’ were combined into a larger ‘African’ stratum and individuals self-reporting as ‘Indian’ or ‘Pakistani’ were combined into a ‘South Asian’ stratum.
Association testing
GWAS were initially conducted in an ethnicity-stratified manner for common variants (MAF >0.005). The number of variants tested for association with mtDNA-CN varied for British (M = 10,728,525), Irish (M = 10,707,537), other White (M = 10,894,497), South Asian (M = 11,350,981), and African (M = 18,981,896) study participants, respectively. To allow for genetic relatedness between participants, GWAS were conducted using the REGENIE framework, which consists of two steps (Mbatchou, 2020). In step 1, mtDNA-CN was predicted using a ridge regression model fit on a set of high-quality genotyped SNPs (MAF >0.01, minor allele count (MAC) >100, genotype and sample missingness above 10%, and passing HWE [p>10−15]) across the whole genome in blocks of 1000 SNPs. In step 2, the linear regression model was used to test the association of all SNPs adjusting for age, age2, sex, chip type, 20 genetic PCs, and blood cell counts (white blood cell, platelet, and neutrophil counts), and conditional on the model from step 1.
Blood cell counts were determined for blood specimen collected at the initial assessment visit using Beckman Coulter LH750 analyzers (https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/haematology.pdf). Information on blood cell counts was retrieved from the UKBiobank data showcase. Individuals with missing values for any blood cell counts (~2.5%) were removed from any subsequent analysis involving blood cell counts. Quality control of blood counts was done following the same procedure as Longchamps, 2019 (Longchamps, 2019). Except for platelet counts, all blood cell counts were log transformed and samples exhibiting outlying values were removed (~4% samples). Lastly, values were standardized to have a mean of 0 and SD of 1.
After ethnicity-specific GWAS were performed, results were combined through meta-analysis using METAL (Willer et al., 2010). European (N = 383,476) and transethnic (N = 395,718) GWAS meta-analyses were performed. Due to the high proportion of Europeans (97%), results from the transethnic meta-analyses strongly resembled that of the European meta-analysis. Accordingly, we report results from the European meta-analysis as the primary GWAS but also provide a comparison of regression coefficients to non-European ethnic groups. To summarize statistical associations, Manhattan plots and quantile-quantile plots were generated by uploading summary statistics into the locus zoom web platform (https://my.locuszoom.org/) (Pruim et al., 2010). LD score regression was performed to calculate the LD score intercept by uploading GWAS results to the LDhub test center (http://ldsc.broadinstitute.org/) (Bulik-Sullivan et al., 2015). As per the instructions, all variants within the major histocompatibility (MHC) region on chromosome 6 were removed prior to uploading. Annovar (version date June 07, 2020) was used to functionally annotate genome-wide significant loci based on their proximity (±250 kb) to genes (RefSeq), predicted effect on amino acid sequence, allele frequency in external datasets (1000 Genomes), clinical pathogenicity (Clinvar), and in silico deleteriousness (CADD), and eQTL information (GTEx v8) (1000 Genomes Project Consortium et al., 2012; GTEX, 2014; Landrum et al., 2016; Rentzsch et al., 2019).
NUMT sensitivity analyses
To assess whether genome-wide significant associations could be explained by the interference of cryptic MT pseudogenes (NUMTs), we performed sensitivity analyses as per Nandakumar et al., 2021. The MT genome was divided into thirds, and AutoMitoC estimates were rederived for each region (MT:1–6425; MT:6526–11947; MT:11948–16569) using the corresponding MT variants belonging to these three consecutive regions. Association testing was repeated for each region using REGENIE. For a given variant, if at least one region-based analysis yielded a nonsignificant association (p<0.05), we considered this as evidence of NUMT interference.
Fine-mapping of GWAS signals
We followed a similar protocol to Vuckovic et al., 2020 for fine-mapping mtDNA-CN loci (Vuckovic et al., 2020). Genome-wide significant variants were consolidated into genomic blocks by grouping variants within 250 kb of each other. LDstore was used to compute a pairwise LD correlation matrix for all variants within each block and across all samples included in the European GWAS meta-analysis (Benner et al., 2017). For each genomic block, FINEMAP was used to perform stepwise conditional regression (Benner et al., 2016). The number of conditionally independent genetic signals per genomic block was used to inform the subsequent fine-mapping search parameters. Finally, the FINEMAP random stochastic search algorithm was applied to derive 95% credible sets constituting candidate causal variants that jointly contributed to 95% (or higher) of the posterior inclusion probabilities (Benner et al., 2016).
Mt-eQTL and heteroplasmy look-ups
Among GWAS hits, we searched for mt-eQTLs using information from Ali et al., 2019, ‘Nuclear genetic regulation of the human mitochondrial transcriptome’ (Ali et al., 2019). When mt-eQTLs also had reported effect estimates, the consistency in direction-of-effects between mt-eQTL and mtDNA-CN associations was reported. Genome-wide significant variants from a heteroplasmy GWAS by Nandakumar et al., 2021 were interrogated in the same manner (Nandakumar et al., 2021).
Gene prioritization and pathway analyses
The Data-driven Expression-Prioritized Integration for Complex Traits (DEPICT) v.1.1 tool was used to map mtDNA-CN loci to genes based on shared coregulation of gene expression using default settings (Pers et al., 2015). Genome-wide significant variants from the European GWAS meta-analysis were ‘clumped’ into independent loci using PLINK ‘--clump-p1 5e-8 --clump-kb 500 --clump-r2 0.05’ with LD correlation matrix derived from 1000 Genomes Europeans (Purcell et al., 2007). DEPICT was subsequently run on independent SNPs using default settings. DEPICT identified 91 genes in total at an FDR of 0.05. Of the 91 genes, 4 noncoding genes were excluded from subsequent analyses for lack of a match in the GeneMANIA database (Warde-Farley et al., 2010). The excluded genes include a pseudogene (PTMAP3), an intronic transcript (ALMS1-IT1), and two long noncoding RNAs (SNHG15, RP11-125K10.4). The remaining 87 DEPICT-prioritized genes were uploaded to the GeneMANIA web platform (https://genemania.org/), which mines publicly available biological datasets to identify additional related genes based on functional associations (genetic interactions, pathways, coexpression, colocalization, and protein domain homology). Based on the combined list of DEPICT and GeneMANIA identified genes, a network was formed in GeneMANIA maximizing the connectivity between all input genes, using the default setting, ‘Assigned based on query gene.’ Functional enrichment analysis was then performed to identify overrepresented GO terms among all network genes (Ashburner et al., 2000). All network genes with at least one GO annotation were compared to a background comprising all GeneMANIA genes with GO annotations.
MT annotation-based analyses
To complement the previous pathway analyses, we labeled prioritized genes with MitoCarta3 annotations and performed subsequent statistical enrichment analyses (Rath et al., 2021). MitoCarta3 is an exquisite database of MT protein annotations, which draws from mass spectrophotometry and green fluorescent protein colocalization experiments of isolated mitochondria from 14 different tissues to assign all human genes statuses indicating whether the corresponding proteins are expressed in the mitochondria or not. We tested whether prioritized genes were enriched for the MT proteome by using a binomial test in R. The number of ‘trials’ was set to the total number of DEPICT and GeneMANIA-prioritized genes (107); the number of ‘successes’ was set to the aforementioned gene subset that was labeled as MT proteins by MitoCarta3 (27); finally, the expected probability was set to the number of nuclear-encoded MitoCarta3 genes divided by the total number of genes (1120/18,922). Furthermore, a t-test was used to compare mean PGC-1A induced fold change for the subset of GWAS-prioritized genes expressed in the MT proteome (27) as compared to the mean PGC-1A induced fold change for all 1120 nuclear MitoCarta3-annotated genes. Also, genes were categorized based on MitoCarta3 ‘MitoPathway’ annotations. MitoCarta3 genes with missing values were excluded from this analysis. Lastly, the 27 genes were labeled based on MitoCarta3 ‘MitoPathways.’ Only the top-level pathway (i.e. parent node) was ascribed to each gene.
Genetic analysis of rare variants
Data Acquisition and Quality Control
Population-level WES variant genotypes (UKB data field: 23,155) for 200,643 UKBiobank participants corresponding to 17,975,236 variants were downloaded using the gfetch utility. These data represent the second tranche of WES data released by the UKBiobank and differ from the first tranche (~50 K samples) which was used for the development of the AutoMitoC pipeline. Quality control of WES data was conducted as follows. First, 11 samples which withdrew consent by the time of analysis were removed. Second, 83,700 monomorphic variants were removed. Third, 369,215 variants with nonmissing genotypes present in less than 90% of samples were removed. Fourth, two samples with call rates less than 99% were removed. Fifth, 18 samples exhibiting discordance between genetic and reported sex were removed. Sixth, through visual inspection of scatterplots of the first two genetic PCs, three outlying samples whose locations strongly departed from their putative ethnicity cluster were removed. Seventh, 35,317 variants deviating from HWE were removed. Eighth, 12,765 samples belonging to smaller ethnic groups with less than 5000 samples (South Asian = 3395; African = 3168; other = 6202) were removed. Ninth, we selected for a maximal number of unrelated samples and excluded 14,156 samples exhibiting third degree or closer relatedness. Finally, 12,394,404 noncoding variants were removed, and 5,176,300 protein-altering variants (stopgain, stoploss, startloss, splicing, missense, frameshift, and in-frame indels) were retained in 173,688 samples.
ExWAS to identify rare mtDNA-CN loci
Of the 173,688 individuals, suitable AutoMitoC mtDNA-CN estimates were available for 147,740 samples. Rare variant inclusion criteria consisted of variants which were infrequent (MAF <0.001), nonsynonymous, and predicted to be clinically deleterious by Mendelian clinically applicable pathogenicity v.1.4 scores (or were highly disruptive variant types including frameshift indel, stopgain, stoploss, or splicing) (Jagadeesh et al., 2016). Herein, such variants are referred to as ‘rare variants’ for simplicity. For each gene, rare allele counts were added per sample. A minor allele count threshold of at least 10 rare alleles was applied leading to a total of 18,890 genes analyzed (exome-wide significance p<0.05/18,890 = 2.65 × 10–6). Linear regression was conducted using mtDNA-CN as the dependent variable and the rare alleles counts per gene as the independent variable. The same set of covariates used in the primary GWAS analysis was used in the ExWAS analysis.
Phenome-wide association testing for rare SAMHD1 mutation carrier status
To identify disease phenotypes associated with carrying a rare SAMHD1 mutation, we maximized sample size for phenome-wide association testing by analyzing the larger set of 173,688 WES samples (with or without suitable mtDNA-CN estimates). Disease outcomes were defined using the previously published ‘PheCode’ classification scheme to aggregate ICD-10 codes from hospital episodes (field ID 41270), death registry (field ID 40001 and 40002), and cancer registry (field ID 40006) records (Denny et al., 2013; Wu et al., 2019). Logistic regression was applied to test the association of SAMHD1 mutation carrier status versus 771 PheCodes (phenome-wide significance p<0.05/771 = 6.49 × 10–5) with a minimal case sample size of 300 (Wei et al., 2017). The same set of covariates used in the primary GWAS were also employed in this analysis.
Mendelian randomization analysis
Disease outcomes
To assess evidence for a causal role of mtDNA-CN on MT disorder-related traits, we first defined a list of testable disease outcomes related to MT disorders. We cross-referenced a list of 36 clinical manifestations of MT disease to FinnGen consortium GWAS (release 4; November 30, 2020) traits (Gorman et al., 2016; Feng, 2020). Among 2444 FinnGen traits, 10 overlapped with MT disease and had a case prevalence greater than 1% and were chosen for two-sample Mendelian randomization analyses. These 10 traits included type 2 diabetes (N = 23,364), mood disorder (N = 20,288), sensorineural hearing loss (N = 12,550), cerebrovascular disease (N = 10,367), migraine (N = 6687), dementia (5675), epilepsy (N = 4558), paralytic ileus and intestinal obstruction (N = 2999), and cardiomyopathy (N = 2342). FinnGen effect estimates and standard errors were used in subsequent Mendelian randomization analyses to define the effect of selected genetic instruments on disease risk.
Genetic instrument selection
First, genome-wide significant variants from the present European GWAS meta-analysis of mtDNA-CN were chosen (N = 383,476). Second, we matched these variants to the FinnGen v4 GWAS datasets (Feng, 2020). Third, to enrich for variants that directly act through MT processes, we only retained those within 100 kb of genes encoding for proteins that are expressed in mitochondria based on MitoCarta3 annotations (Rath et al., 2021). Fourth, we performed LD pruning in PLINK with 1000 Genomes Europeans as the reference panel to ascertain an independent set of genetic variants (LD r2 <0.01) (Purcell et al., 2007; 1000 Genomes Project Consortium et al., 2012). Lastly, to mitigate potential for horizontal pleiotropy, we further removed variants with strong evidence of acting through alternative pathways by performing a phenome-wide search across published GWAS with Phenoscanner V2 (Kamat et al., 2019). Variants strongly associated with other phenotypes (p<5 × 10–20) were removed unless the variant was a coding mutation located within gene encoding for the MT proteome (MitoCarta3) or had an established MT role based on manual literature review (Rath et al., 2021). Seven genetic variants were removed based on these criteria including rs8067252 (ADAP2), rs56069439 (ANKLE1), rs2844509 (ATP6V1G2-DDX39B), rs73004962 (PBX4), rs7412 (APOE), rs385893 (AK3, RCL1), and rs1613662 (GP6). A total of 27 genetic variants were used to approximate genetically determined mtDNA-CN levels. As sensitivity analyses, a broader set of genetic instruments including variants irrespective of their proximity to MT protein-encoding genes was additionally tested.
Mendelian randomization and sensitivity analyses
Two-sample Mendelian randomization analyses were performed using the ‘TwoSampleMR’ and ‘MR-PRESSO’ R packages (Hemani et al., 2018; Verbanck et al., 2018). Effect estimates and standard errors corresponding to the 27 genetic variants on mtDNA-CN (exposure) and MT disease phenotypes (outcome) were derived from the European GWAS meta-analysis and FinnGen v4 GWAS summary statistics, respectively. Three MR methodologies were employed including inverse variance weighted (primary method), weighted median, and MR-EGGER methods. MR-PRESSO was used to detect global heterogeneity and p-values were derived based on 1000 simulations. If significant global heterogeneity was detected (p<0.05), a local outlier test was conducted to detect outlying SNPs. After removal of outlying SNPs, MR analyses were repeated. In the absence of heterogeneity (Egger-intercept p>0.05; MR-PRESSO global heterogeneity p>0.05), we reported the inverse-variance weighted result. In the presence of balanced pleiotropy (MR-PRESSO global heterogeneity p<0.05) and absence of directional pleiotropy (Egger-intercept p>0.05), we reported the weighted median result. In the presence of directional pleiotropy (Egger-intercept p<0.05), we reported the MR-EGGER result. Also, the Steiger directionality test was employed to ensure that a greater proportion of variance in mtDNA-CN was explained than risk of the outcome. To further examine the robustness of these findings, MR analyses were also conducted using the generalized summary data-based MR (GSMR) method which incorporates HEIDI outlier removal and accounts for the uncertainty in SNP-outcome effect estimates. Finally, to replicate the two-sample MR finding using an independent outcome dataset without UKBiobank participants, we repeated two-sample MR analyses using the International Genomics of Alzheimer’s Disease Consortium (2013) GWAS meta-analysis including 17,008 cases and 37,154 controls (Lambert et al., 2013).
Acknowledgements
We want to acknowledge the participants and investigators of the UKBiobank and FinnGen studies.
Funding Statement
No external funding was received for this work.
Contributor Information
Guillaume Paré, Email: pareg@mcmaster.ca.
Magnus Nordborg, Austrian Academy of Sciences, Austria.
YM Dennis Lo, The Chinese University of Hong Kong, Hong Kong.
Additional information
Competing interests
No competing interests declared.
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review and editing.
Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review and editing.
Formal analysis, Visualization, Writing – review and editing.
Conceptualization, Methodology, Software, Visualization, Writing – review and editing.
Conceptualization, Supervision, Visualization, Writing – review and editing.
Formal analysis, Writing – review and editing.
Data curation, Formal analysis, Writing – review and editing.
Formal analysis, Writing – review and editing.
Formal analysis, Writing – review and editing.
Conceptualization, Methodology, Writing – original draft, Writing – review and editing.
Formal analysis, Writing – review and editing.
Data curation, Validation, Writing – original draft, Writing – review and editing.
Conceptualization, Data curation, Investigation, Resources, Writing – review and editing.
Supervision, Writing – review and editing.
Supervision, Writing – review and editing.
Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – review and editing.
Ethics
Approval was received to use UKBiobank study data in this work under application ID # 15,255 ("Identification of the shared biological and sociodemographic factors underlying cardiovascular disease and dementia risk"). The UKBiobank study obtained ethics approval from the North West Multi-centre Research Ethics Committee which encompasses the UK (REC reference: 11/NW/0382). All research participants provided informed consent.
Additional files
Table 1. Characteristics of top 10 autosomal probes whose background-corrected L2R values remain correlated with median MT L2R. Table 2. Characteristics of top 10 autosomal probes whose background-corrected L2R values remain associated with gender.
Data availability
Individual-level UKBiobank genotypes and phenotypes can be acquired upon successful application (https://bbams.ndph.ox.ac.uk/ams/). All individual-level UKBiobank data was accessed as part of application # 15255. FinnGen summary statistics are freely available to download (https://www.finngen.fi/en/access_results). All data products generated as part of this study will be made publicly accessible. Specifically, the AutoMitoC array-based mtDNA-CN estimation pipeline is available on GitHub (https://github.com/GMELab/AutoMitoC, copy archived at swh:1:rev:5669ae00011aece6ed494a3fde241ab3027c59e2). The mtDNA-CN estimates derived in UKBiobank participants have been returned to the UKBiobank and made accessible to researchers through the data showcase (https://biobank.ndph.ox.ac.uk/showcase/). Summary-level association statistics from GWAS have been made publicly available for download from the GWAS catalogue (https://www.ebi.ac.uk/gwas/publications/35023831). All remaining data are available in the main text or supplementary materials.
The following dataset was generated:
Chong M, Mohammadi-Shemirani P, Perrot N, Nelson W, Morton R, Narula S, Lali R, Khan I, Khan M, Judge C, Machipisa T, Cawte N, O'Donnell M, Pigeyre M, Akhabir L, Pare G. 2022. UKbiobank mtDNA-CN GWAS summary statistics. GWAS Catalogue. 35023831
References
- 1000 Genomes Project Consortium. Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AT, Boehme L, Carbajosa G, Seitan VC, Small KS, Hodgkinson A. Nuclear genetic regulation of the human mitochondrial transcriptome. eLife. 2019;8:e41927. doi: 10.7554/eLife.41927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Andrews SJ, Goate AM. Mitochondrial DNA copy number is associated with cognitive impairment. Alzheimer’s & Dementia. 2020;16:S5. doi: 10.1002/alz.047543. [DOI] [Google Scholar]
- Ashar FN, Zhang Y, Longchamps RJ, Lane J, Moes A, Grove ML, Mychaleckyj JC, Taylor KD, Coresh J, Rotter JI, Boerwinkle E, Pankratz N, Guallar E, Arking DE. Association of Mitochondrial DNA Copy Number With Cardiovascular Disease. JAMA Cardiology. 2017;2:1247–1255. doi: 10.1001/jamacardio.2017.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology The Gene Ontology Consortium. Nature Genetics. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldauf H-M, Pan X, Erikson E, Schmidt S, Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg T, Panitz S, Flory E, Landau NR, Sertel S, Rutsch F, Lasitschka F, Kim B, König R, Fackler OT, Keppler OT. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nature Medicine. 2012;18:1682–1687. doi: 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basel D. Mitochondrial DNA Depletion Syndromes. Clinics in Perinatology. 2020;47:123–141. doi: 10.1016/j.clp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- Benner C, Spencer CCA, Havulinna AS, Salomaa V, Ripatti S, Pirinen M. FINEMAP: efficient variable selection using summary data from genome-wide association studies. Bioinformatics. 2016;32:1493–1501. doi: 10.1093/bioinformatics/btw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner C, Havulinna AS, Järvelin M-R, Salomaa V, Ripatti S, Pirinen M. Prospects of Fine-Mapping Trait-Associated Genomic Regions by Using Summary Statistics from Genome-wide Association Studies. American Journal of Human Genetics. 2017;101:539–551. doi: 10.1016/j.ajhg.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics Consortium. Patterson N, Daly MJ, Price AL, Neale BM. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nature Genetics. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, Santos DP, Blanz J, Obermaier CD, Strojny C, Savas JN, Kiskinis E, Zhuang X, Krüger R, Surmeier DJ, Krainc D. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017;357:1255–1261. doi: 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, Cortes A, Welsh S, Young A, Effingham M, McVean G, Leslie S, Allen N, Donnelly P, Marchini J. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai N, Li Y, Chang S, Liang J, Lin C, Zhang X, Liang L, Hu J, Chan W, Kendler KS, Malinauskas T, Huang G-J, Li Q, Mott R, Flint J. Genetic Control over mtDNA and Its Relationship to Major Depressive Disorder. Current Biology. 2015;25:3170–3177. doi: 10.1016/j.cub.2015.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong MR, Narula S, Morton R, Judge C, Akhabir L, Cawte N, Pathan N, Lali R, Mohammadi-Shemirani P, Shoamanesh A, O’Donnell M, Yusuf S, Langhorne P, Pare G. Mitochondrial DNA Copy Number as a Marker and Mediator of Stroke Prognosis: Observational and Mendelian Randomization Analyses. Neurology. 2021;1:e13165. doi: 10.1212/WNL.0000000000013165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny JC, Bastarache L, Ritchie MD, Carroll RJ, Zink R, Mosley JD, Field JR, Pulley JM, Ramirez AH, Bowton E, Basford MA, Carrell DS, Peissig PL, Kho AN, Pacheco JA, Rasmussen LV, Crosslin DR, Crane PK, Pathak J, Bielinski SJ, Pendergrass SA, Xu H, Hindorff LA, Li R, Manolio TA, Chute CG, Chisholm RL, Larson EB, Jarvik GP, Brilliant MH, McCarty CA, Kullo IJ, Haines JL, Crawford DC, Masys DR, Roden DM. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nature Biotechnology. 2013;31:1102–1110. doi: 10.1038/nbt.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desdín-Micó G, Soto-Heredero G, Aranda JF, Oller J, Carrasco E, Gabandé-Rodríguez E, Blanco EM, Alfranca A, Cussó L, Desco M, Ibañez B, Gortazar AR, Fernández-Marcos P, Navarro MN, Hernaez B, Alcamí A, Baixauli F, Mittelbrunn M. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. 2020;368:1371–1376. doi: 10.1126/science.aax0860. [DOI] [PubMed] [Google Scholar]
- Diskin SJ, Li M, Hou C, Yang S, Glessner J, Hakonarson H, Bucan M, Maris JM, Wang K. Adjustment of genomic waves in signal intensities from whole-genome SNP genotyping platforms. Nucleic Acids Research. 2008;36:e126. doi: 10.1093/nar/gkn556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzini F, Schöpf B, Blatzer M, Coassin S, Hicks AA, Kronenberg F, Fendt L. Plasmid-normalized quantification of relative mitochondrial DNA copy number. Scientific Reports. 2018;8:1–11. doi: 10.1038/s41598-018-33684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzini F, Lamina C, Fendt L, Schultheiss UT, Kotsis F, Hicks AA, Meiselbach H, Weissensteiner H, Forer L, Krane V, Eckardt KU, Köttgen A, Kronenberg F, GCKD Investigators Mitochondrial DNA copy number is associated with mortality and infections in a large cohort of patients with chronic kidney disease. Kidney International. 2019;96:480–488. doi: 10.1016/j.kint.2019.04.021. [DOI] [PubMed] [Google Scholar]
- Feng YCA. Findings and Insights from the Genetic Investigation of Age of First Reported Occurrence for Complex Disorders in the UK Biobank and FinnGen. medRxiv. 2020 doi: 10.1101/2020.11.20.20234302. [DOI]
- Frahm T, Mohamed SA, Bruse P, Gemünd C, Oehmichen M, Meissner C. Lack of age-related increase of mitochondrial DNA amount in brain, skeletal muscle and human heart. Mechanisms of Ageing and Development. 2005;126:1192–1200. doi: 10.1016/j.mad.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Franzolin E, Salata C, Bianchi V, Rampazzo C. The Deoxynucleoside Triphosphate Triphosphohydrolase Activity of SAMHD1 Protein Contributes to the Mitochondrial DNA Depletion Associated with Genetic Deficiency of Deoxyguanosine Kinase. The Journal of Biological Chemistry. 2015;290:25986–25996. doi: 10.1074/jbc.M115.675082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, Suomalainen A, Thorburn DR, Zeviani M, Turnbull DM. Mitochondrial diseases. Nature Reviews. Disease Primers. 2016;2:16080. doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- GTEX The Genotype-Tissue Expression (GTEx) project The. Nature Genetics. 2014;45:580–585. doi: 10.1038/ng.2653.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt AL, Brennan RR, Burrows K, Guthrie PAI, Ascione R, Ring SM, Gaunt TR, Pyle A, Cordell HJ, Lawlor DA, Chinnery PF, Hudson G, Rodriguez S. A genome-wide association study of mitochondrial DNA copy number in two population-based cohorts. Human Genomics. 2019;13:1–17. doi: 10.1186/s40246-018-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägg S, Jylhävä J, Wang Y, Czene K, Grassmann F. Deciphering the genetic and epidemiological landscape of mitochondrial DNA abundance. Human Genetics. 2021;140:849–861. doi: 10.1007/s00439-020-02249-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, Wieser E, Taylor J, Berg S, Smith NJ, Kern R, Picus M, Hoyer S, van Kerkwijk MH, Brett M, Haldane A, Del Río JF, Wiebe M, Peterson P, Gérard-Marchant P, Sheppard K, Reddy T, Weckesser W, Abbasi H, Gohlke C, Oliphant TE. Array programming with NumPy. Nature. 2020;585:357–362. doi: 10.1038/s41586-020-2649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell GT, Mori M, Powell C, Amrhein TJ, Rice GI, Bailey L, Strande N, Weck KE, Evans JP, Berg JS, Kishnani P. Combination of exome sequencing and immune testing confirms Aicardi-Goutières syndrome type 5 in a challenging pediatric neurology case. Cold Spring Harbor Molecular Case Studies. 2018;4:a002758. doi: 10.1101/mcs.a002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7:e34408. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Yao X, Shen Y. Altered mitochondrial DNA copy number contributes to human cancer risk: evidence from an updated meta-analysis. Scientific Reports. 2016;6:1–11. doi: 10.1038/srep35859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeesh KA, Wenger AM, Berger MJ, Guturu H, Stenson PD, Cooper DN, Bernstein JA, Bejerano G. M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nature Genetics. 2016;48:1581–1586. doi: 10.1038/ng.3703. [DOI] [PubMed] [Google Scholar]
- Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, Butterworth AS, Staley JR. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35:4851–4853. doi: 10.1093/bioinformatics/btz469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Bassig BA, Seow WJ, Hu W, Purdue MP, Huang W-Y, Liu C-S, Cheng W-L, Männistö S, Vermeulen R, Weinstein SJ, Lim U, Hosgood HD, Bonner MR, Caporaso NE, Albanes D, Lan Q, Rothman N. Mitochondrial DNA copy number and chronic lymphocytic leukemia/small lymphocytic lymphoma risk in two prospective studies. Cancer Epidemiology, Biomarkers & Prevention. 2015;24:148–153. doi: 10.1158/1055-9965.EPI-14-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnken R, Kodigepalli KM, Wu L. Regulation of deoxynucleotide metabolism in cancer: novel mechanisms and therapeutic implications. Molecular Cancer. 2015;14:1–11. doi: 10.1186/s12943-015-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller A, Fazzini F, Lamina C, Rantner B, Kollerits B, Stadler M, Klein-Weigel P, Fraedrich G, Kronenberg F. Mitochondrial DNA copy number is associated with all-cause mortality and cardiovascular events in patients with peripheral arterial disease. Journal of Internal Medicine. 2020;287:569–579. doi: 10.1111/joim.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer S, Wolf C, König N, Staroske W, Guck J, Häusler M, Luksch H, Nguyen LA, Kim B, Alexopoulou D, Dahl A, Rapp A, Cardoso MC, Shevchenko A, Lee-Kirsch MA. SAMHD1 prevents autoimmunity by maintaining genome stability. Annals of the Rheumatic Diseases. 2015;74:e17. doi: 10.1136/annrheumdis-2013-204845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Morón FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fiévet N, Huentelman MW, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuiness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossù P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Deniz Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannefelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O’Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltuenen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nöthen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P, Cohorts for Heart and Aging Research in Genomic Epidemiology Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nature Genetics. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Hoover J, Jang W, Katz K, Ovetsky M, Riley G, Sethi A, Tully R, Villamarin-Salomon R, Rubinstein W, Maglott DR. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Research. 2016;44:D862–D868. doi: 10.1093/nar/gkv1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J. MitoPipeline: Generating Mitochondrial copy number estimates from SNP array data in Genvisis. 2014. [June 1, 2020]. http://genvisis.org/MitoPipeline
- Longchamps RJ. EXPLORING THE ROLE OF MITOCHONDRIAL DNA QUANTITY AND QUALITY. bioRxiv. 2019 doi: 10.1101/2021.01.25.428086. [DOI]
- Longchamps RJ, Castellani CA, Yang SY, Newcomb CE, Sumpter JA, Lane J, Grove ML, Guallar E, Pankratz N, Taylor KD, Rotter JI, Boerwinkle E, Arking DE. Evaluation of mitochondrial DNA copy number estimation techniques. PLOS ONE. 2020;15:e0228166. doi: 10.1371/journal.pone.0228166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longchamps RJ, Yang SY, Castellani CA, Shi W, Lane J, Grove ML, Bartz TM, Sarnowski C, Liu C, Burrows K, Guyatt AL, Gaunt TR, Kacprowski T, Yang J, De Jager PL, Yu L, Bergman A, Xia R, Fornage M, Feitosa MF, Wojczynski MK, Kraja AT, Province MA, Amin N, Rivadeneira F, Tiemeier H, Uitterlinden AG, Broer L, Van Meurs JBJ, Van Duijn CM, Raffield LM, Lange L, Rich SS, Lemaitre RN, Goodarzi MO, Sitlani CM, Mak ACY, Bennett DA, Rodriguez S, Murabito JM, Lunetta KL, Sotoodehnia N, Atzmon G, Ye K, Barzilai N, Brody JA, Psaty BM, Taylor KD, Rotter JI, Boerwinkle E, Pankratz N, Arking DE. Genome-wide analysis of mitochondrial DNA copy number reveals loci implicated in nucleotide metabolism, platelet activation, and megakaryocyte proliferation. Human Genetics. 2022;141:127–146. doi: 10.1007/s00439-021-02394-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbatchou J. Computationally Efficient Whole Genome Regression for Quantitative and Binary Traits. bioRxiv. 2020 doi: 10.1101/2020.06.19.162354. [DOI] [PubMed]
- Nandakumar P, Tian C, O’Connell J, 23andMe Research Team. Hinds D, Paterson AD, Sondheimer N. Nuclear genome-wide associations with mitochondrial heteroplasmy. Science Advances. 2021;7:1–10. doi: 10.1126/sciadv.abe7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyston J. Online Mendelian Inheritance in Man. Anesthesiology. 1998;89:811–812. doi: 10.1097/00000542-199809000-00060. [DOI] [PubMed] [Google Scholar]
- O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez-Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener H-C, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S, INTERSTROKE investigators Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- Pers TH, Karjalainen JM, Chan Y, Westra HJ, Wood AR, Yang J, Lui JC, Vedantam S, Gustafsson S, Esko T, Frayling T, Speliotes EK, Boehnke M, Raychaudhuri S, Fehrmann RSN, Hirschhorn JN, Franke L, Genetic Investigation of ANthropometric Traits GIANT Consortium Biological interpretation of genome-wide association studies using predicted gene functions. Nature Communications. 2015;6:5890. doi: 10.1038/ncomms6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M. Blood mitochondrial DNA copy number: What are we counting? Mitochondrion. 2021;60:1–11. doi: 10.1016/j.mito.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath S, Sharma R, Gupta R, Ast T, Chan C, Durham TJ, Goodman RP, Grabarek Z, Haas ME, Hung WHW, Joshi PR, Jourdain AA, Kim SH, Kotrys AV, Lam SS, McCoy JG, Meisel JD, Miranda M, Panda A, Patgiri A, Rogers R, Sadre S, Shah H, Skinner OS, To T-L, Walker MA, Wang H, Ward PS, Wengrod J, Yuan C-C, Calvo SE, Mootha VK. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Research. 2021;49:D1541–D1547. doi: 10.1093/nar/gkaa1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentoft M. Erratum: Heterozygous colon cancer-associated mutations of SAMHD1 have functional significance (Proceedings of the National Academy of Sciences of the United States of America. PNAS. 2019;116:4723–4728. doi: 10.1073/pnas.1519128113). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Research. 2019;47:D886–D894. doi: 10.1093/nar/gky1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochimica et Biophysica Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silzer T, Barber R, Sun J, Pathak G, Johnson L, O’Bryant S, Phillips N. Circulating mitochondrial DNA: New indices of type 2 diabetes-related cognitive impairment in Mexican Americans. PLOS ONE. 2019;14:e213527. doi: 10.1371/journal.pone.0213527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone D, Calabrese FM, Lang M, Gasparre G, Attimonelli M. The reference human nuclear mitochondrial sequences compilation validated and implemented on the UCSC genome browser. BMC Genomics. 2011;12:517. doi: 10.1186/1471-2164-12-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, Burman JL, Li Y, Zhang Z, Narendra DP, Cai H, Borsche M, Klein C, Youle RJ. Parkin and PINK1 mitigate STING-induced inflammation. Nature. 2018;561:258–262. doi: 10.1038/s41586-018-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLOS Medicine. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tin A, Grams ME, Ashar FN, Lane JA, Rosenberg AZ, Grove ML, Boerwinkle E, Selvin E, Coresh J, Pankratz N, Arking DE. Association between Mitochondrial DNA Copy Number in Peripheral Blood and Incident CKD in the Atherosclerosis Risk in Communities Study. Journal of the American Society of Nephrology. 2016;27:2467–2473. doi: 10.1681/ASN.2015060661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature Genetics. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E, Peterson P, Weckesser W, Bright J, van der Walt SJ, Brett M, Wilson J, Millman KJ, Mayorov N, Nelson ARJ, Jones E, Kern R, Larson E, Carey CJ, Polat İ, Feng Y, Moore EW, VanderPlas J, Laxalde D, Perktold J, Cimrman R, Henriksen I, Quintero EA, Harris CR, Archibald AM, Ribeiro AH, Pedregosa F, van Mulbregt P, SciPy 1.0 Contributors SciPy 1.0: fundamental algorithms for scientific computing in Python. Nature Methods. 2020;17:261–272. doi: 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuckovic D, Bao EL, Akbari P, Lareau CA, Mousas A, Jiang T, Chen M-H, Raffield LM, Tardaguila M, Huffman JE, Ritchie SC, Megy K, Ponstingl H, Penkett CJ, Albers PK, Wigdor EM, Sakaue S, Moscati A, Manansala R, Lo KS, Qian H, Akiyama M, Bartz TM, Ben-Shlomo Y, Beswick A, Bork-Jensen J, Bottinger EP, Brody JA, van Rooij FJA, Chitrala KN, Wilson PWF, Choquet H, Danesh J, Di Angelantonio E, Dimou N, Ding J, Elliott P, Esko T, Evans MK, Felix SB, Floyd JS, Broer L, Grarup N, Guo MH, Guo Q, Greinacher A, Haessler J, Hansen T, Howson JMM, Huang W, Jorgenson E, Kacprowski T, Kähönen M, Kamatani Y, Kanai M, Karthikeyan S, Koskeridis F, Lange LA, Lehtimäki T, Linneberg A, Liu Y, Lyytikäinen L-P, Manichaikul A, Matsuda K, Mohlke KL, Mononen N, Murakami Y, Nadkarni GN, Nikus K, Pankratz N, Pedersen O, Preuss M, Psaty BM, Raitakari OT, Rich SS, Rodriguez BAT, Rosen JD, Rotter JI, Schubert P, Spracklen CN, Surendran P, Tang H, Tardif J-C, Ghanbari M, Völker U, Völzke H, Watkins NA, Weiss S, VA Million Veteran Program. Cai N, Kundu K, Watt SB, Walter K, Zonderman AB, Cho K, Li Y, Loos RJF, Knight JC, Georges M, Stegle O, Evangelou E, Okada Y, Roberts DJ, Inouye M, Johnson AD, Auer PL, Astle WJ, Reiner AP, Butterworth AS, Ouwehand WH, Lettre G, Sankaran VG, Soranzo N. The Polygenic and Monogenic Basis of Blood Traits and Diseases. Cell. 2020;182:1214–1231. doi: 10.1016/j.cell.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsmuth M, Hübner A, Li M, Madea B, Stoneking M. Age-Related and Heteroplasmy-Related Variation in Human mtDNA Copy Number. PLOoS Genetics. 2016;12:e1005939. doi: 10.1371/journal.pgen.1005939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hadley D, Liu R, Glessner J, Grant SFA, Hakonarson H, Bucan M. PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome Research. 2007;17:1665–1674. doi: 10.1101/gr.6861907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD, Morris Q. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Research. 2010;38:W214–W220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei WQ, Bastarache LA, Carroll RJ, Marlo JE, Osterman TJ, Gamazon ER, Cox NJ, Roden DM, Denny JC. Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic health record. PLOS ONE. 2017;12:e175508. doi: 10.1371/journal.pone.0175508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TE, Brandariz-Nuñez A, Martinez-Lopez A, Knowlton C, Lenzi G, Kim B, Ivanov D, Diaz-Griffero F. A SAMHD1 mutation associated with Aicardi-Goutières syndrome uncouples the ability of SAMHD1 to restrict HIV-1 from its ability to downmodulate type I interferon in humans. Human Mutation. 2017;38:658–668. doi: 10.1002/humu.23201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu I-C, Lin C-C, Liu C-S, Hsu C-C, Chen C-Y, Hsiung CA. Interrelations Between Mitochondrial DNA Copy Number and Inflammation in Older Adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2017;72:937–944. doi: 10.1093/gerona/glx033. [DOI] [PubMed] [Google Scholar]
- Wu P, Gifford A, Meng X, Li X, Campbell H, Varley T, Zhao J, Carroll R, Bastarache L, Denny JC, Theodoratou E, Wei W-Q. Mapping ICD-10 and ICD-10-CM Codes to Phecodes: Workflow Development and Initial Evaluation. JMIR Medical Informatics. 2019;7:e14325. doi: 10.2196/14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Castellani CA, Longchamps RJ, Pillalamarri VK, O’Rourke B, Guallar E, Arking DE. Blood-derived mitochondrial DNA copy number is associated with gene expression across multiple tissues and is predictive for incident neurodegenerative disease. Genome Research. 2021;31:349–358. doi: 10.1101/gr.269381.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guallar E, Ashar FN, Longchamps RJ, Castellani CA, Lane J, Grove ML, Coresh J, Sotoodehnia N, Ilkhanoff L, Boerwinkle E, Pankratz N, Arking DE. Association between mitochondrial DNA copy number and sudden cardiac death: findings from the Atherosclerosis Risk in Communities study (ARIC) European Heart Journal. 2017;38:3443–3448. doi: 10.1093/eurheartj/ehx354. [DOI] [PMC free article] [PubMed] [Google Scholar]