Abstract
In metropolitan Tokyo, the Ehrlichia muris seropositivity rate of 24 wild mice was 63% in Hinohara Village, but in the surrounding areas, it was 0 to 5%. This finding suggests that the reservoir of E. muris is focal. Among the 15 seropositive mice, ehrlichiae were isolated from 9 Apodemus speciosus mice and 1 A. argenteus mouse, respectively. Five ehrlichial isolates were obtained from 10 ticks (Haemaphysalis flava) collected in Asuke Town, Aichi Prefecture, where the E. muris type strain had been isolated. These new isolates were compared with the E. muris type strain. The mouse virulence and ultrastructure of the new isolates were similar to those of the type strain, and all of them were cross-reactive with each other, as well as with the type strain, by indirect immunofluorescent-antibody test. The levels of similarity of the base sequences of the 16S rRNA gene of one of the A. speciosus isolates and one of the tick isolates to that of the E. muris type strain were 99.79 and 99.93%, respectively. We suggest that all of these isolates are E. muris; that E. muris is not limited to Eothenomys kageus but infects other species of mice; and that E. muris is present at locations other than Aichi Prefecture. It appears that H. flava is a potential vector of E. muris. Twenty (1%) of 1803 humans from metropolitan Tokyo were found to be seropositive for E. muris antibodies. A serological survey revealed that exposure to E. muris or organisms antigenically cross-reactive to E. muris occurred among dogs, wild mice, monkeys, bears, deer, and wild boars in Gifu Prefecture, nearby prefectures, and Nagoya City, central Japan. However, human beings and Rattus norvegicus rats in this area were seronegative. These results indicate broader geographic distribution of and human and animal species exposure to E. muris or related Ehrlichia spp. in Japan.
Ehrlichioses are known as important emerging tick-borne diseases in humans, as well as in domestic animals (18–20), and are caused by infection with Ehrlichia spp. Ehrlichia spp. are obligate intracellular bacteria that belong to the Family Rickettsiaceae. Ehrlichia spp. can be divided into three distinct genetic groups on the basis of their 16S rRNA gene sequences (19, 20). Group 1 includes Ehrlichia canis and E. ewingii isolated from dogs outside Japan, E. chaffeensis, and a Venezuelan human ehrlichia, likely a strain of E. canis, recently isolated from a human (1, 16). In 1983, we isolated an infectious agent inducing splenomegaly in laboratory mice from a wild mouse, Eothenomys kageus, caught in Asuke Town, Aichi Prefecture, Japan. This agent was identified as a member of the genus Ehrlichia on the basis of morphological and antigenic comparisons (9). Analysis of the sequence of its 16S rRNA gene revealed that the agent is a new Ehrlichia sp. designated E. muris (21). E. muris also belongs to group 1. Recently dogs seropositive for E. canis were identified in Japan (24), suggesting the existence of E. canis in Japan, but this has yet to be proven. It is unknown whether E. chaffeensis exists in Japan. Group 2 includes E. equi isolated from horses, E. phagocytophila from sheep and goats, and the human granulocytic ehrlichiosis agent (3). These three organisms are very closely related and probably belong to the same species. The presence in Japan of Ehrlichia spp. from this group has not been examined yet. Group 3 includes E. risticii, E. sennetsu, and the SF agent from Stellantchasmus falcatus. E. sennetsu was isolated from a patient’s blood in 1953 in Japan (15). Sennetsu fever, caused by E. sennetsu, is distributed in southwestern Japan and in Malaysia. The SF agent was isolated from the metacercaria of S. falcatus in Japan (8) and is probably a strain of E. risticii (22).
So far, only one strain of E. muris has been described (9); hence, the strain divergence is unknown. The extent of its geographic distribution, the rodent reservoir, the vector, and the exposure of humans and animals other than wild mice to E. muris are also unknown. In this study, we compared E. muris isolates from wild mice caught in metropolitan Tokyo and from Haemaphysalis flava ticks collected in Aichi, where the E. muris type strain was isolated. We also performed a seroepidemiologic study to assess the extent of exposure of humans and animals to group 1 Ehrlichia spp.
MATERIALS AND METHODS
Isolation of the infectious agent from wild mice and ticks.
In November 1992, 24 wild mice (Apodemus speciosus and A. argenteus) were caught by using Sherman live traps (H. B. Sherman Traps, Inc., Tallahassee, Fla.) with peanuts as bait in Hinohara Village, Nishitama County, metropolitan Tokyo (Fig. 1). The spleens were aseptically removed and homogenized in 10% (wt/vol) sucrose-phosphate-glutamate buffer (0.0038 M KH2PO4, 0.0072 M K2HPO4, 0.0049 M l-glutamate, 0.218 M sucrose, pH 7.2). Each homogenate was inoculated intraperitoneally into two ddY strain mice at 0.2 ml per mouse. Ten isolates were obtained. All isolates were passaged twice in ddY strain mice.
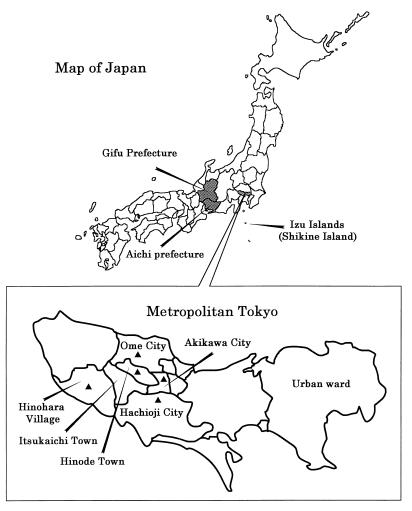
FIG. 1.
The area in metropolitan Tokyo where E. muris isolates were collected and seroepidemiological survey of humans and wild mice was performed. ▴, survey point where wild mice were caught.
Ten H. flava nymphs collected by flagging over vegetation in Asuke Town from June to September 1994 were tested. One H. flava was attached to each of 10 BALB/c mice kept individually in cages placed over a water-filled pan to prevent the ticks from escaping. The five isolates obtained from enlarged spleens were passaged once in mice by inoculating the spleen homogenate as described above. An additional 10 mice with no attached ticks served as controls.
Clinical signs, relative spleen weights, and titers of antibody against the E. muris type strain.
The 10% spleen homogenates of each mouse infected with E. muris, 10 isolates from wild mice caught in Tokyo, and 5 isolates from ticks in Aichi Prefecture were inoculated intraperitoneally at 0.2 ml per mouse into five mice (BALB/c strain). The clinical signs (ruffled fur, inactivity, anorexia, and death) of mice inoculated were observed for up to 20 days postinoculation (p.i.) and compared with those of mice infected with the E. muris type strain. After blood collection, the relative spleen weights (in grams per 100 g of body weight) of all mice inoculated were estimated, and titers of antibody against E. muris were measured by indirect immunofluorescent-antibody assay (IFA) (9).
Serological comparison between E. muris isolates and E. sennetsu.
The E. muris type strain, three isolates (I-268, I-289, and I-306) from A. speciosus, one isolate (I-269) from A. argenteus, one isolate (NA-1) from a tick, and E. sennetsu (Miyayama strain) were used as antigens in the IFA. The antigen and antisera were prepared, and the IFA was performed as previously described (9).
Light and electron microscopic observations.
Smear preparations of peritoneal cells of infected mice killed on day 10 p.i. were stained by the Diff-Quik method (International Reagents Corp., Kobe, Japan). For electron microscopy, peritoneal cells of mice infected with strains I-268 and NA-1 were collected at day 10 p.i. by lavage with Eagle’s minimum essential medium containing heparin (50 IU) and by centrifugation at 800 × g for 10 min. The pellets were fixed overnight at 4°C in a mixture containing 5% glutaraldehyde, 2.5% paraformaldehyde, and 0.03% trinitrophenol in 0.1 M sodium cacodylate buffer (pH 7.4). The cells were washed twice with 0.1 M sodium cacodylate buffer, postfixed with 1% osmium tetroxide in 1.5% potassium ferrocyanide for 1 h, block stained with 1% uranyl acetate, dehydrated in a graded ethanol-propylene oxide series, and embedded in Poly/Bed 812 resin (Polysciences, Inc., Warrington, Pa.). Ultrathin sections were stained with uranyl acetate and lead citrate, and the stained sections were examined with a Philips model 300 electron microscope at 60 kV.
Extraction of DNA.
Spleen homogenates of mice inoculated with strain I-268 isolated from A. speciosus and strain NA-1 isolated from a tick on day 10 p.i. were aseptically prepared at a rate of 10% (wt/vol) in phosphate-buffered saline. The homogenates were centrifuged at 500 × g at 4°C, and the supernatants were centrifuged at 8,000 × g for 20 min at 4°C. TE buffer (10 mM Tris, 1 mM EDTA, pH 8) containing 1% sodium dodecyl sulfate and 20 mg/ml proteinase K (Wako, Osaka, Japan) was added to the pellets, and these were incubated at 50°C for 2 h. The resulting lysed suspensions were extracted twice with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1) and with an equal volume of chloroform. The top layers, the aqueous phases, were transferred to fresh tubes, and 1/10 volume of 3 M sodium acetate (pH 5.2) and 2.5 volumes of cold 95% ethanol were added, mixed, and cooled at −70°C for 1 h to precipitate DNA. The tubes were centrifuged at 8,000 × g for 20 min, and the pellets were washed in 500 μl of ice-cold 70% ethanol and again centrifuged at 8,000 × g for 20 min at 4°C. The ethanol was completely discarded, and the DNA pellets were dissolved in 100 μl of TE buffer.
Analysis of the 16S rRNA gene sequences of isolates.
16S rRNA gene sequences were amplified with primer EC9 (5′-AAGGATCCTACCTTGTTACGACTT-3′) and EC12 (5′-AATCTAGAGTTTGATCMTGG-3′), which are universal primers for the 16S rRNA gene sequences of prokaryotic cells. PCR was performed as described previously (1). The amplified DNA fragment that was the expected size (approximately 1.5 kb) was purified from a 1% low-melting-temperature agarose gel by using a PCR DNA purification system (Promega, Madison, Wis.). The purified double-stranded DNA was kept at −20°C. The 16S rRNA PCR product was cloned by using the pGEM-T Vector System (Promega), and the insert was sequenced with the ABI PRISM Dye Primer Cycle Sequencing Ready Reaction Kit (Perkin-Elmer, Norwalk, Conn.). The primers used for sequencing were EC9, EC10 (5′-AATCTAGATTAGATACCCTDGTAGTCC-3′, where D is A, T, or G), EC11 (5′-AAGGATCCGGACTACHAGGGTATCTAAT-3′, where H is C, T, or A), EC12, U396 (5′-GAAGGCCTTCGGGTTGTA-3′), U1124 (5′-GATAAACTGGAGGAAGGTGGG-3′), L297 (5′-AGACCGTATCTCAGTTCCAGTG-3′), L696 (5′-CAGTGTCAGTATCGAACCAGA-3′), and L1148 (5′-GGGCCGTGCTGACTTGACATC-3′). The sequences of three clones each were determined on both DNA strands.
Computer analysis of DNA sequences.
The sequence data obtained were prepared for analysis by using AutoAssembler Version 1.4 (Perkin-Elmer). The corrected levels of nucleotide divergence of 16S rRNA genes were calculated by using Genetic Information Processing Software GENETYX-MAC Version 8.0 (Software Development Co., Tokyo, Japan) on a Power Mac 8500. A phylogenetic tree was constructed by the unweighted pair group method analysis using arithmetic averages and data from the distance matrix.
Serum specimens from humans and animals.
Serum samples collected from 24 wild mice used for the isolation of Ehrlichia-like organisms from Hinohara Village, Nishitama County in 1992; 122 samples collected from wild mice in Hinode Town in 1992; 17 samples collected from wild mice in Akikawa City in 1990; 78 samples collected from wild mice in Hachioji City in 1992; and 31 samples collected from wild mice in Oume City in 1992 were used (Table 1). Forty R. norvegicus serum samples from Shikine Island collected in 1990 and 1,487 human serum samples collected in Itsukaichi Town and 316 human serum samples collected in Oume City from 1991 through 1995 were examined. Serum samples collected from 976 humans with various symptoms in the Hospital of Gifu University in 1995; 699 serum samples collected from dogs in Gifu Prefecture and in Tsukuba City Ibaragi Prefecture, from 1981 through 1989; 48 serum samples collected from bears, 70 collected from monkeys, 20 collected from deer, and 18 collected from wild boars in Gifu or a nearby prefecture from 1991 through 1992; and 221 serum samples collected from wild mice and 327 serum samples collected from R. norvegicus in Nagoya City in 1993 were examined.
TABLE 1.
Species of wild mice caught in metropolitan Tokyo and numbers of seropositive mice
| Area | Total no. of wild mice (no. seropositive)
|
|||
|---|---|---|---|---|
| A. speciosus | A. argenteus | E. kageus | Total | |
| Akikawa City | 17 | 17 | ||
| Hinohara Village | 18 (14) | 6 (1) | 24 (15) | |
| Hinode Town | 30 | 70 | 22 | 122 |
| Hachioji City | 73 (4) | 5 | 78 (4) | |
| Oume City | 18 | 13 | 31 | |
Serological test using E. muris antigen.
Titers of antibody against E. muris in the sera were measured by IFA (9). Fluorescein isothiocyanate-conjugated anti-human, -dog, -monkey, -mouse, and -rat antibodies were obtained from Organon Teknika (Durham, N.C.) and used at 1:40 (human, dog, and monkey) and 1:30 (mouse and rat) dilutions for the respective serum samples, and fluorescein isothiocyanate-protein G (Zymed Laboratories, Inc., South San Francisco, Calif.) was used at a 1:30 dilution against bear, deer, and wild-boar specimens as the secondary probe. The positive control sera were mouse anti-E. muris serum, dog anti-E. canis serum, and human anti-E. chaffeensis serum (kindly provided by the Centers for Disease Control and Prevention, Atlanta, Ga.). A cutoff titer of 1:10 was chosen based on the result obtained with negative control sera from laboratory-reared mice, rats, and dogs and our previously obtained data on mice and rats (9, 10). However, for human, monkey, bear, wild boar, and deer specimens, such negative control sera were not available.
Nucleotide sequence accession numbers.
The GenBank nucleotide sequence accession numbers for the 16S rRNAs used for comparison in this study are as follows: E. chaffeensis, M73222; E. canis, M73221; E. sennetsu, M73225; E. muris, U15527. The nucleotide sequences of the 16S rRNAs of strains I-268 and NA-1 have been deposited in the GenBank data library under accession no. AB013008 and AB013009.
RESULTS
Isolation from wild mice and ticks.
Of 24 wild mice caught in Hinohara Village, Nishitama County, Tokyo, Japan (Table 1), 15 were seropositive for E. muris, having antibody titers of 1:80 to 1:2,560 (Tables 1; see Table 3). Of 18 A. speciosus and 6 A. argenteus isolates, 9 and 1, respectively, induced splenomegaly. Mice were caught outside the residential area of metropolitan Tokyo. However, not only villagers but also 1 million hikers visit the area annually. Also, five isolates inducing splenomegaly were obtained from 10 H. flava ticks collected in Asuke Town, Aichi Prefecture, where the E. muris type strain had been isolated (9). Ten control mice without attached ticks in the same environment did not develop splenomegaly. These isolation rates were greater than that in our previous study. The agent which induces splenomegaly in laboratory mice was isolated from only one of six wild-mice (E. kageus) caught in Asuke Town in 1983, and after several passages through laboratory mice, the agent was isolated in cell culture, genetically and antigenically characterized, and identified as E. muris (9, 21).
TABLE 3.
Presence of antibody to E. muris in wild mice and humans in Tokyo
| Animal and area | No. of samples | No. of positive sera | % of positive seraa | No. of specimens with following antibody titer (no. of wild mice from which ehrlichia were isolated):
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <10 | 10 | 20 | 40 | 80 | 160 | 320 | 640 | 1,280 | 2,560 | ||||
| Wild mice from: | |||||||||||||
| Akikawa City | 16 | 0 | 0 | 16 | |||||||||
| Hinohara Village | 24 | 15 | 63 | 9 | 1 | 1 (1) | 1 | 6 (3) | 3 (3) | 3 (3) | |||
| Hinode Town | 122 | 0 | 0 | 122 | |||||||||
| Hachiouji City | 78 | 4 | 5 | 74 | 1 | 1 | 1 | 1 | |||||
| Oume City | 31 | 0 | 0 | 31 | |||||||||
| R. norvegicus from: Shikine Island | 40 | 0 | 0 | 40 | |||||||||
| Humans from: | |||||||||||||
| Itsukaichi Town | 1,487 | 16 | 1 | 1,471 | 2 | 7 | 2 | 2 | 3 | ||||
| Oume City | 316 | 4 | 1 | 312 | 1 | 2 | 1 | ||||||
A titer of >1:10 is considered positive.
Clinical signs, relative spleen size, and titers of antibody against E. muris.
All BALB/c mice inoculated with agents inducing splenomegaly, including homogenates from wild mice (10 isolates) and H. flava (5 isolates), developed clinical signs including ruffled fur, inactivity, and anorexia, but no mice died. Relative spleen weights ranged from 2.4 to 3.9% on day 20 p.i., and titers of antibody against E. muris ranged from 1:80 to 1:320 on the same day.
Serological comparison of isolates and E. muris.
Serologic reactivities were tested among E. muris, three isolates (I-268, I-289, and I-306) from A. speciosus, one isolate (I-269) from A. argenteus, one isolate (strain NA-1) from H. flava, and E. sennetsu by IFA. Antisera against the isolates reciprocally reacted with each other and with E. muris. No antisera against any isolate reacted with E. sennetsu and vice versa.
Light and electron microscopic observation.
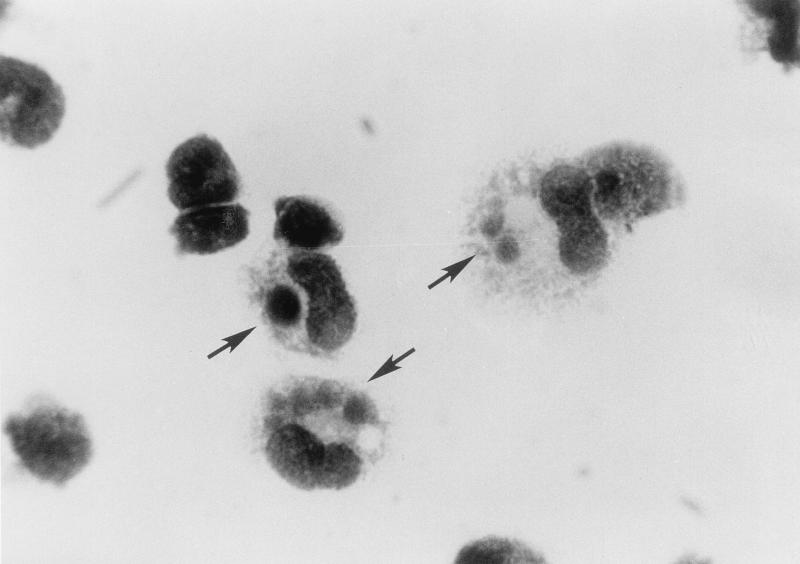
Inclusion bodies were seen in the cytoplasm of peritoneal macrophages of infected BALB/c mice infected with strains I-268 and NA-1 on day 10 p.i. when impression smears were stained with Diff-Quik (Fig. 2). The inclusion bodies (morulae) were compact (1 to 3 μm) and round and stained reddish purple, resembling those of E. muris (9).
FIG. 2.
Morulae (arrows) of strain I-268 (A. speciosus, Tokyo isolate) in the cytoplasm of murine peritoneal cells stained with Diff-Quik. Magnification, ×2,200.
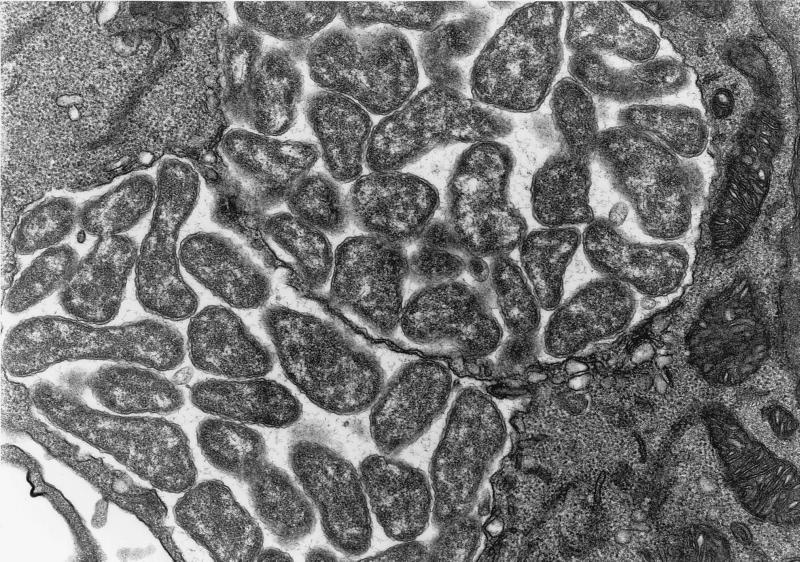
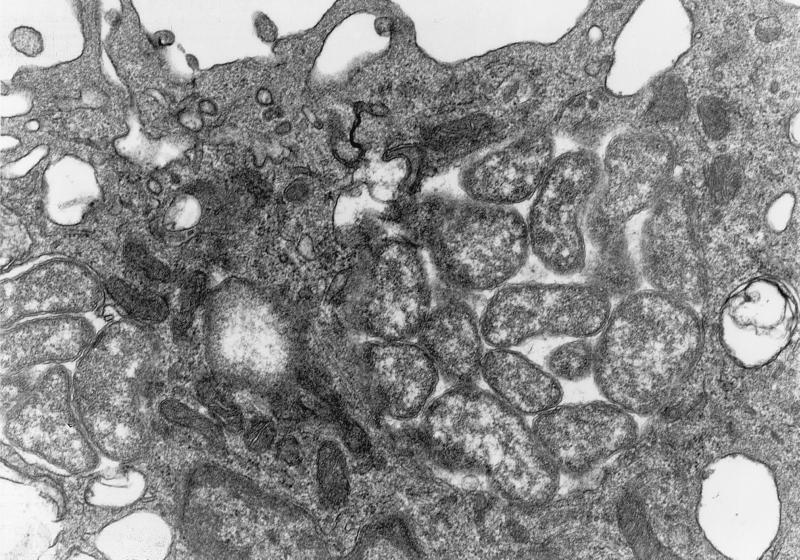
Electron microscopy, on day 10 p.i., of peritoneal macrophages of BALB/c mice infected with strains I-268 and NA-1 revealed that the isolates had an ultrastructural morphology compatible with that of E. muris (9). Numerous tightly packed organisms were observed in membrane-lined cytoplasmic inclusions. The organisms were pleomorphic and variable in size. Each organism was surrounded by an outer membrane and an inner membrane; the outer membrane was often rippled (Fig. 3 and 4).
FIG. 3.
Electron micrograph of morulae of E. muris I-268 (A. speciosus, Tokyo isolate) in the cytoplasm of murine peritoneal cells at day 10 postinfection. Note the numerous pleomorphic coccobacilli enveloped in two layers of membranes embedded in a fine filamentous matrix in the membrane-bound inclusion. Magnification, ×22,100.
FIG. 4.
Electron micrograph of morulae of E. muris NA-1 (H. flava tick, Aichi isolate) in the cytoplasm of murine peritoneal cells at day 10 postinfection. Note the several pleomorphic coccobacilli in the membrane-bound inclusion. Magnification, ×28,100.
Analysis of the 16S rRNA gene base sequence.
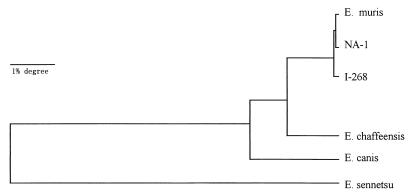
A single DNA fragment (1.5 kb) was amplified by PCR from the genomic DNAs of strains I-268 and NA-1 by using a pair of primers that flanked the 16S rRNA gene. These DNA fragments were sequenced, and a 1,499-base sequence was obtained. The greatest similarities were found among the sequences of E. muris, strain NA-1 isolated from H. flava (99.93%) and strain I-268 isolated from an A. speciosus wild mouse (99.79%) (Table 2). Comparing the 16S rRNA gene sequences of these isolates, the base G at position 331 changed to a C in strain NA-1, the base T at position 102 changed to a C, a base G was inserted at position 382, and the base C at position 829 was absent in strain I-268. The phylogenetic tree obtained from data is shown in Fig. 5. These results suggest that NA-1 and I-268 are strains of E. muris.
TABLE 2.
Levels of similarity and evolutionary distances between 16S rRNA gene sequences
| Organism | Nucleotide substitution distances or nucleotide sequence similaritya
|
|||||
|---|---|---|---|---|---|---|
| E. muris | NA-1 | I-268 | E. chaffeensis | E. canis | E. sennetsu | |
| E. muris | 0.0011 | 0.0012 | 0.0103 | 0.0204 | 0.1034 | |
| NA-1 | 99.93 | 0.0021 | 0.0113 | 0.0214 | 0.1044 | |
| I-268 | 99.79 | 99.72 | 0.0104 | 0.0205 | 0.1035 | |
| E. chaffeensis | 97.72 | 97.65 | 97.58 | 0.0155 | 0.0985 | |
| E. canis | 96.13 | 96.06 | 95.99 | 97.44 | 0.1018 | |
| E. sennetsu | 83.75 | 83.68 | 83.61 | 83.86 | 83.40 | |
The values on the upper right are average numbers of substitutions per sequence position (evolutionary distances), adjusted as described by the Kimura two-parameter model for multiple substitutions at individual positions and calculated for 1,428 positions which could be aligned unambiguously. The values on the lower left are levels of fractional nucleotide identity between sequences.
FIG. 5.
Phylogenetic relationships between our isolates of strains NA-1 and I-268 and other members of the tribe Ehrlichieae.
Seroepidemiological survey of humans, wild mice, and R. norvegicus in metropolitan Tokyo.
Although E. muris infection of mice induces vigorous polyclonal activation, antigen-specific immune stimulation is impaired, giving low titers of immunoglobulin G and M antibodies against E. muris (10). There is no antigenic cross-reactivity between other related intracellular bacteria at a 1:10 dilution (9). All of the negative control sera tested were negative at this dilution. Therefore, this low cutoff value was used for this study. The rate of seropositivity for E. muris of wild mice from Hinohara Village was 63% (15 of 24), and the antibody titer was greater than 1:80, with the highest titer measuring 1:2,560 (Table 3). The higher the antibody titer, the greater the E. muris isolation rate (Table 3). On the other hand, samples from wild mice collected in other areas, such as Akikawa City, Hinode Village, and Oume City (Fig. 1), rarely contained antibodies against E. muris, and R. norvegicus rats caught on Shikine Island, off Tokyo, were seronegative. Seropositivity rates in human samples were 1.1% (16 of 1,487) in Itsukaitchi Town and 1.3% (4 of 316) in Oume City, but antibody titers were lower (1:10 to 1:160) than those of wild mice (Table 4). Clinical signs of seropositive humans are unknown but presumably absent or not serious, because these serum specimens were collected as a community service for screening the health status of residents.
TABLE 4.
Antibody to E. muris in animal and human sera detected by IFA
| Animal (capture site or residence) | No. of samples | No. of positive samples | % positive | Range of antibody titer | Mean titer ± SEM |
|---|---|---|---|---|---|
| Dog (Gifu) | 499 | 18 | 3.6 | 10–1,280 | 126 ± 38 |
| Dog (Tsukuba) | 200 | 11 | 5.5 | 10–640 | 62 ± 38 |
| Wild monkey (Gifu) | 70 | 1 | 1.4 | 160 | 160 |
| Wild bear (Gifu) | 48 | 1 | 2.1 | 160 | 160 |
| Wild deer (Gifu) | 20 | 3 | 15.0 | 20–80 | 40 ± 18 |
| Wild boar (Gifu) | 18 | 3 | 17.0 | 40–320 | 101 ± 24 |
| Wild mouse (Nagoya) | 221 | 21 | 9.5 | 10–640 | 50 ± 33 |
| Wild R. norvegicus (Nagoya) | 327 | 0 | 0 | ||
| Human (Gifu) | 976 | 0 | 0 |
Seroepidemiological survey of humans and animals for E. muris in Gifu prefecture and Nagoya City.
The seropositivity rates of dogs for E. muris in Gifu and Tsukuba City were 3.6% (18 of 499) and 5.5% (11 of 200), respectively. Antibody titers were 1:10 to 1:1,280. The seropositivity rates of wild monkeys, bears, deer, and boars were 1.4% (1 of 70), 2.1% (1 of 48), 15% (3 of 20), and 17% (3 of 18), respectively. The range of antibody titers was 1:20 to 1:320. In Nagaya City, the seropositivity rate of wild mice was 9.5% (21 of 221), but no antibody against E. muris was detected in 327 R. norvegicus rats. Rats in Nagoya City were caught in a park, in vegetable gardens, and in fields. Asuke Town is outside the residential area. Other than villagers, no hikers are known to visit this area. All 976 human sera collected at a hospital in Gifu Prefecture were negative (Table 4).
DISCUSSION
Based on pathologic findings, electron microscopic observations, serological cross-reactivity testing, and sequencing of 16S rRNA genes, 10 isolates from wild mice (A. speciosus and A. argenteus) caught in Hinohara Village, metropolitan Tokyo, and 5 isolates from H. flava ticks collected in Aichi Prefecture belong to E. muris. The geographic distributions of E. kageus and A. speciosus overlap in Japan. A. speciosus is the most common mouse in mountainous areas of Japan, while E. kageus is rare. The isolation of E. muris from A. speciosus and A. argenteus wild mice caught in Tokyo, about 320 miles away from Aichi Prefecture, where the type strain of E. muris had been isolated from an E. kageus wild mouse, suggests that E. muris is widely distributed among different species of wild mice in Japan. The seroprevalence rate among wild mice in Hinohara Village, where 10 strains of E. muris were isolated, was very high (63%), whereas the seroprevalence rates in the surrounding areas were very low. Thus, this small area appears to be a hot spot for E. muris infection similar to the prevalence observed for Orientia tsutsugamushi (11).
Antibodies against E. muris were detected in human sera collected in Itsukaichi Town and Oume City, Tokyo. Although titers were lower than those in wild mice, this is the first report in Japan of serologic evidence of human exposure to an Ehrlichia sp. other than E. sennetsu. There is a possibility that these people were infected with an Ehrlichia sp. belonging to group 1, including E. chaffeensis, E. canis, or E. muris. Antigenic cross-reactivity is small among different groups of Ehrlichia spp. Since we did not detect this antibody in any of human sera collected in Gifu Prefecture, the seropositive reactions do not appear to be nonspecific. Further investigation is needed to determine whether this difference is related to geographic factors.
Dogs seropositive for E. canis in western Japan were previously reported, and the positivity rate was 1.9%, and the highest reported antibody titer was 1:360 (24). In the current study, the rate of seropositivity for E. muris was 3.6% in dogs in Gifu Prefecture and 5.5% in dogs in Tsukuba City. The highest antibody titer was 1:1,280. This result suggests that using E. muris as an IFA antigen is more effective for screening of dogs exposed to group 1 Ehrlichia spp. than using E. canis in Japan. Dogs were shown to be susceptible to infection with E. chaffeensis (6), and of 38 dogs from southeastern Virginia, 8 were positive by E. chaffeensis-specific PCR (5). Therefore, as in the United States, some of these dogs in Japan may be infected with E. chaffeensis rather than E. canis or E. muris. Again, isolation of the organisms from dogs is needed to confirm these observations and characterize the infectious agent. Furthermore, several other wild animals also had antibodies against E. muris, but their highest titers were lower than those in dogs.
A total of 9.5% of the wild mice caught in Nagoya City had antibodies against E. muris. The existence of this antibody in wild mice caught in Nagoya City indicates that Ehrlichia spp. exist not only in mountainous areas but also in urban areas. We did not detect antibodies against E. muris in any rats caught on Shikine Island, in metropolitan Tokyo, or in Nagoya City, and these results agree with our previous experiments, which found that rats are resistant to E. muris infection (9). In the United States, the major wild-animal reservoir of E. chaffeensis is white-tailed deer (13), and eight species of wild rodents from the southeastern United States, where E. chaffeensis infections of deer and humans have been confirmed, are negative for antibodies to E. chaffeensis (14). In Japan, it remains to be determined which animal species are reservoirs for potential human ehrlichial infection.
The isolation of E. muris from H. flava ticks suggests that H. flava is a potential vector for E. muris transmission among wild rodents. According to the available literature, H. flava is seen only in Japan and Korea (23). In Japan, H. flava is found on all four major islands. H. flava adults take blood meals on hares and dogs, but they have also been found in considerable numbers on cows, horses, wild boar, deer, and bears. Immature forms of the tick were found on the same hosts as adults, as well as on birds and small rodents. All of the rodents described above (A. argenteus, A. speciosus, and E. kageus) are bitten by immature stages of H. flava, which is one of the most common ticks found in mountainous areas of Japan. From a public health viewpoint, H. flava is one of the most important ticks because of its probable role in the epidemiology of tularemia on Honshu Island in Japan. Whether these E. muris-infected H. flava nymphs serve as vectors for human transmission remains to be studied. By screening 140 pools of 1,579 total ticks consisting of Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis by E. chaffeensis-specific PCR, Anderson et al. (2) found positive reactions in A. americanum adults but not in nymphs or in the other two species of ticks. This report suggests either that transstadial transmission is very inefficient or that it does not occur and that adult ticks which are infected as nymphs transmit E. chaffeensis to humans. Rhipicephalus sanguineus ticks infected as nymphs were shown to transmit E. canis to dogs (12). Anaplasma marginale, which belongs to Ehrlichia sp. group 2, is more efficiently transmitted to cattle by transstadial transmission by adult male Dermacentor andersoni ticks than by adult ticks infected as nymphs (7). Whether transstadial transmission also occurs in ehrlichial infection remains to be studied.
REFERENCES
- 1.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Sims K G, Olson J G, Childs J E, Piesman J F, Happ C M, Maupin G O, Johnson B J B. Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Med Hyg. 1993;49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- 3.Chen S-M, Dumler J S, Bakken J S, Walker D. Identification of granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:585–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson J E, Anderson B E, Fishbein D B, Sanchez J L, Goldsmith C S, Wilson K H, Duntley C W. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson J E, Biggie K L, Warner C K, Cookson K, Jenkins S, Levine J F, Olson J G. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am J Vet Res. 1996;57:1175–1179. [PubMed] [Google Scholar]
- 6.Dawson J E, Ewing S A. Susceptibility of dogs to infection with Ehrlichia chaffeensis, causative agent of human ehrlichiosis. Am J Vet Res. 1992;53:1322–1327. [PubMed] [Google Scholar]
- 7.Eriks I S, Steller D, Pulmer G H. Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J Clin Microbiol. 1993;31:2091–2096. doi: 10.1128/jcm.31.8.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda T, Sasahara T, Kito T. Studies on the causative agent of “Hyuganetsu” disease. XI. Characteristics of rickettsia-like organism isolated from metacercaria of Stellantchasmus falcatus. J Jpn Assoc Infect Dis. 1973;53:713–716. doi: 10.11150/kansenshogakuzasshi1970.47.474. . (in Japanese.) [DOI] [PubMed] [Google Scholar]
- 9.Kawahara M, Suto C, Rikihisa Y. Characterization of ehrlichial organisms isolated from a wild mouse. J Clin Microbiol. 1993;31:89–96. doi: 10.1128/jcm.31.1.89-96.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawahara M, Suto C, Shinichiro S, Futohashi M, Rikihisa Y. Impaired antigen specific responses and enhanced polyclonal stimulation in mice infected with Ehrlichia muris. Microbiol Immunol. 1996;40:575–581. doi: 10.1111/j.1348-0421.1996.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura A. Colonization of R. tsutsugamushi-positive trombicula. In: Kawamura A, Tanaka H, Tamura A, editors. Tsutsugamushi disease. Tokyo, Japan: University of Tokyo Press; 1995. pp. 224–231. [Google Scholar]
- 12.Lewis G E, Ristic M, Smith R D, Lincoln T, Stephenson E H. The brown dog tick Rhipicephalus sanguineus and the dog as experimental hosts of Ehrlichia canis. Am J Vet. 1977;38:1953–1955. [PubMed] [Google Scholar]
- 13.Lockhart J M, Davidson W R, Stallknecht D E, Dawson J E, Howerth E W. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681–1686. doi: 10.1128/jcm.35.7.1681-1686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockhart J M, Davidson W R, Stallknecht D E, Dawson J E. Lack of seroreactivity to Ehrlichia chaffeensis among rodent populations. J Wildl Dis. 1995;34:392–396. doi: 10.7589/0090-3558-34.2.392. [DOI] [PubMed] [Google Scholar]
- 15.Misao T, Kobayashi Y. Studies on infectious mononucleosis (glandular fever). I. Isolation of etiologic agent from blood, bone marrow and lymph node of a patient with infectious mononucleosis by using mice. Kyushu J Med Sci. 1955;6:145–152. [Google Scholar]
- 16.Perez M, Rikihisa Y, Wen B. Ehrlichia canis-like agent isolated from a man in Venezuela: antigenic and genetic characterization. J Clin Microbiol. 1996;34:2133–2139. doi: 10.1128/jcm.34.9.2133-2139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rikihisa Y, Perry B D, Cordes D O. Ultrastructural study of ehrlichial organisms in the large colons of ponies infected with Potomac horse fever. Infect Immun. 1985;50:911–916. doi: 10.1128/iai.49.3.505-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rikihisa Y. The tribe Ehrlichiae and ehrlichial disease. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rikihisa Y. Proceedings of the 5th International Symposium on Rickettsiae and Rickettsial Diseases. Bratislava, Slovak Republic: International Society of Rickettsiae and Rickettsial Disease; 1996. Ehrlichieae; pp. 272–286. [Google Scholar]
- 20.Walker D H, Dumler J S. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen B, Rikihisa Y, Mott J, Fuerst P A, Kawahara M, Suto S. Ehrlichia muris sp. nov., identified on the basis of 16S rRNA base sequences, serological, morphological, and biological characteristics. Int J Syst Bacteriol. 1995;45:250–254. doi: 10.1099/00207713-45-2-250. [DOI] [PubMed] [Google Scholar]
- 22.Wen B, Rikihisa Y, Yamamoto S, Kawahata N, Fuerst P A. Characterization of the SF agent, an Ehrlichia sp. isolated from the fluke Stellantchasmus falcatus, by 16S rRNA base sequence, serological, and morphological analyses. Int J Syst Bacteriol. 1996;46:149–154. doi: 10.1099/00207713-46-1-149. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi N, Tipton V J, Keegan H L, Toshioka S. Ticks of Japan, Korea, and the Ryukyu Islands. Brigham Young Univ Sci Bull Biol Ser. 1971;XV:1–226. [Google Scholar]
- 24.Yamamoto S, Honda M, Ashida Y, Nishimura Y, Niizeki H, Rikihisa Y. Detection of antibody to Ehrlichia canis in dogs. J Jpn Vet Assoc. 1994;47:765–768. [Google Scholar]