Abstract
Tracking new and emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants has become increasingly important for public health responses, primarily because of variant-dependent transmission, disease severity, and treatment decisions. This evaluation compared Seegene Technologies Novaplex SARS-CoV-2 Variants I, II, and IV (I,II&IV) assays to detect known SARS-CoV-2 variants using traditional spike gene Sanger sequencing results as the gold standard reference. Both RNA extraction and extraction-free protocols were assessed. A total of 156 samples were included in this study. There was 100% (109/109) overall agreement (95% CI, 96.7%–100%) between the spike gene sequencing and the I,II&IV results using extracted RNA for the variants included in the Novaplex assay menus. The RNA extraction-free method was 91.7% (143/156) as sensitive (95% CI, 86.2%–95.5%) as the traditional RNA extraction method. Using the extraction-free method on samples with higher cycle threshold values (>30) resulted in some mutations not being detected, presumably due to lower nucleic acid concentrations in the original samples. In conclusion, the I,II&IV assays provide an accurate, rapid, and less labor-intensive method for detecting SARS-CoV-2 and identifying known variants of interest and concern. The RNA extraction-free method for samples with cycle threshold of <30 could be cost-effective for surveillance purposes. However, spike gene sequencing retains the advantage of detecting more and new variants.
Since the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic in early 2020, new variants of interest and concern have emerged throughout the world.1,2
Once a new variant of concern is detected, it is important to track its spread in real-time and monitor recent genomic changes as part of public health surveillance. Some variants exhibit increased transmissibility and disease severity and may be resistant to vaccine-induced immunity, limiting efficacy.3, 4, 5 Novel variants that evade naturally acquired immunity could also contribute to a surge in cases and increased reinfection.6 For example, in the United States, the Delta variant reversed the downward coronavirus disease 2019 (COVID-19) incidence trend during the summer of 2021 (https://covid.cdc.gov/covid-data-tracker/#variant-proportions and https://www.gisaid.org/hcov19-variants, last accessed October 15, 2021). Delays in genomic sequencing results could negatively impact mitigation efforts. Therefore, rapid variant detection is crucial for impact assessment and implementation of control measures.
Currently, SARS-CoV-2 variants are monitored by spike (S) gene sequencing or whole-genome sequencing. S gene sequencing is most widely used and can detect mutations that may affect antibody binding and angiotensin-converting enzyme 2 receptor affinity in the human body.1 These mutations have been used to characterize the known variants. According to the CDC, variants are described as follows: variants of interest, variants of concern, variants of high consequence, and variants being monitored (https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html, last accessed October 18, 2021). At the time of this study, the only variant of concern in the United States was the Delta (B.1.617.2 and AY lineages) variant, and there were no variants of interest or variants of high consequence. However, after submitting the study, the CDC declared Omicron a new variant of concern. The variants that emerged earlier in the pandemic but are no longer circulating or circulating at low levels are defined as variants being monitored. These include Alpha (B.1.1.7 and Q lineages), Beta (B.1.351 and descendent lineages), Gamma (P.1 and descendent lineages), Epsilon (B.1.427 and B.1.429), Eta (B.1.525), Iota (B.1.526), Kappa (B.1.617.1), Mu (B.1.621 and B.1.621.1), Zeta (P.2), and B.1.617.3 (https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html, last accessed October 18, 2021). However, SARS-CoV-2 is constantly mutating. Therefore, although specific variants may contain many of the same S gene mutations, additional mutations and recombination events in other parts of the genome can be advantageous to the virus.7
In this study, Seegene Technologies Novaplex SARS-CoV-2 Variants I, II, and IV (I,II&IV) assays (Seegene Technologies, Walnut Creek, CA) were compared with S gene sequencing for the detection of known SARS-CoV-2 variants. In addition, differences in the I,II&IV assay results using either standard chloroform-ethanol extracted RNA or extraction-free, heat-released RNA, were investigated. S gene sequencing can detect all known variants, whereas the I,II&IV assays, which rely on multiplex RT-PCR technology, simultaneously confirm the presence of SARS-CoV-2 by detecting the RNA-dependent RNA polymerase gene (RdRp) and the following notable S gene substitutions: H69/V70 deletion, W152C, K417T, K417N, L452R, E484K, N501Y, and P681R. Furthermore, on the basis of the presence/absence of these mutations, the I,II&IV assays together can positively identify B.1.1.7 (Alpha/United Kingdom), B.1.351 (Beta/South Africa), P.1 (Gamma/Brazil), B.1.617.2 (Delta/India), and B.1.427/429 (Epsilon/California) variants. The Seegene software (version 1.0) enables the identification and differentiation of multiple targets in a single channel. It provides cycle threshold (CT) values as well as melting curve analysis for each target. After the Omicron variant was designated as a variant of concern in the United States on November 30, 2021, Seegene Technologies released the Novaplex VII assay for detection of Omicron BA.1 (RdRp, H69/V70 del, E484A, and N501Y) and Omicron BA.2 (RdRp, E484A, and N501Y). Combining an extraction-free processing method with RT-PCR technology to detect known SARS-CoV-2 variants helps overcome the challenges of reagent and specialized equipment availability. It provides laboratories without sequencing capabilities a feasible option for variant detection. The objective of this study is to evaluate the I,II&IV assays to determine the accuracy of variant identification in SARS-CoV-2–positive samples for epidemiologic and surveillance purposes.
Materials and Methods
Samples
A total of 144 SARS-CoV-2–positive nasopharyngeal swabs in viral transport media were used in this study. In addition, 12 unused viral transport media vials spiked with a Beta variant isolate (hCoV-19/USA/MD-HP01542/2021; EPI_ISL_890360), kindly provided by the World Reference Center for Emerging Viruses and Arboviruses (University of Texas Medical Branch, Galveston, TX), were used because Beta variants were not present in the patient population. Detection of SARS-CoV-2 nucleic acid was determined by at least one of the following nucleic acid amplification tests performed in the clinical microbiology laboratory at the University of Texas Medical Branch: Xpert Xpress SARS-CoV-2 by Cepheid GenXpert (Sunnyvale, CA), Aptima SARS-CoV-2 by Hologic Panther System (San Diego, CA), or SARS-CoV-2 assay by Hologic Panther Fusion system.
RNA Extraction
Each specimen (200 μL) was inactivated with 1 mL of TRIzol LS Reagent (Invitrogen; Thermo Fisher Scientific, Waltham, MA) and stored at −80°C overnight. The next day, samples were thawed and vortexed, and 266.6 μL of chloroform was added. Samples were centrifuged at 12,500 × g for 15 minutes at 4°C to separate the phases. The top aqueous phase (500 μL) was aspirated and added to 667 μL of isopropanol containing 2 μL of GlycoBlue Coprecipitant (Invitrogen; Thermo Fisher Scientific), and vortexed for 10 seconds. Samples were incubated at room temperature for 10 minutes and centrifuged at 20,800 × g for 20 minutes at 4°C to pellet RNA. Isopropanol was removed, 1 mL of 75% ethanol was added, and samples were vortexed and inverted several times. RNA was precipitated by centrifugation at 10,000 × g for 10 minutes at 4°C. Ethanol was removed, and the pellet was allowed to air dry for 5 to 10 minutes. The RNA pellet was then resuspended in 20 μL of RNase/DNase-free water.
Extraction-Free Method
A total of 15 μL of viral transport media for each sample was added to 45 μL of nuclease-free molecular-grade water in PCR tubes. The tubes were capped, quickly vortexed, and briefly centrifuged. These were then placed on a thermocycler and incubated at 98°C for 3 minutes, then cooled to 4°C for 5 minutes. The samples were used immediately for the I,II&IV assays.
Novaplex Assays
The Novaplex SARS-CoV-2 Variants I, II, and IV Assays (Seegene Technologies) were performed according to the manufacturer's instructions using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA). In brief, a 20-μL reaction was assembled through the addition of 5 μL of RNA (extracted or extraction free) from each sample and 15 μL of the manufacturer-provided master mix. The PCR protocol was defined by the manufacturer and programmed into the software as follows: i) 50°C, 20 minutes; 95°C, 15 minutes; ii) 95°C, 10 seconds; 60°C, 40 seconds; 72°C, 20 seconds; 3 cycles; iii) 95°C, 10 seconds; 60°C, 15 seconds; 72°C, 10 seconds; 42 cycles; iv) indefinite hold at 4°C. The test results were analyzed with the Seegene software and displayed with the Seegene viewer on the monitor. Table 1 summarizes each assay's substitutions (mutations) and the corresponding SARS-CoV-2 variant identified.
Table 1.
SARS-CoV-2 Variant Information and the Substitutions (Mutations) Detected by the Novaplex SARS-CoV-2 Variants I, II, and IV Assays
| SARS-CoV-2 variant information |
Novaplex SARS-CoV-2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variants I assay |
Variants II assay |
Variants IV assay |
|||||||||
| Pango lineage | WHO label | H69/V70 del | E484K | N501Y | W152C | K417N | K417T | L452R | K417N | L452R | P681R |
| B.1.1.7 | Alpha | Det | Det | ||||||||
| B.1.351 | Beta | Det | Det | Det | Det | ||||||
| P.1 | Gamma | Det | Det | Det | |||||||
| B.1.617.2 | Delta | Det | Det | Det | |||||||
| B.1.427 | Epsilon | Det | |||||||||
| B.1.429 | Epsilon | Det | Det | ||||||||
| P.2 | Zeta | Det | |||||||||
| B.1.525 | Eta | Det | Det | ||||||||
| B.1.526 | Iota | Det | |||||||||
Det, detected; WHO, World Health Organization.
RT-PCR and Sanger Sequencing
Adapted from a previously published protocol, 2 μL of the RNA samples was used for reverse transcription with the SuperScript IV One-Step RT-PCR System (Invitrogen; Thermo Fisher Scientific).8 The set of primers used were as follows: forward primer 5′-TGTTATTTCTAGTGATGTTCTTG-3′ (position 21,521 nucleotides); and reverse primer 5′-CACAATTAAACCGTGCTTTAAC-3′ (position 23,865 nucleotides). A 20-μL reaction was assembled in PCR eight-tube strips through the addition of 5 μL 2× Platinum SuperFi RT-PCR Master Mix (Thermo Fisher, Waltham, MA), 0.1 μL of SuperScript IV RT Mix (Thermo Fisher), 0.5 μL forward primer (10 μmol/L), 0.5 μL reverse primer (10 μmol/L), 2 μL RNA, and 1.9 μL RNase-free water. Reverse transcription and amplification were completed using the following protocol: i) 50°C, 10 minutes; 98°C, 2 minutes; ii) 98°C, 10 seconds; 58°C, 10 seconds; 72°C, 2 minutes; 40 cycles; iii) 72°C, 5 minutes; iv) indefinite hold at 4°C. The presence and size of the desired amplicon were verified with 2 μL of PCR product on a 1% agarose gel. The remaining 18 μL was purified using the QIAquick PCR Purification kit (Qiagen, Germantown, MD), according to the manufacturer's instructions.
Sequences of the purified RT-PCR products were generated using two forward primers: 5′-TGTTATTTCTAGTGATGTTCTTG-3′ (position 21,521 nucleotides) and 5′-TCCACTTTTAAGTGTTATGGAG-3′ (position 22,685 nucleotides) and the BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, Austin, TX). The sequencing reactions were purified using a 96-well plate format (EdgeBio, San Jose, CA) and analyzed on a 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA). The electropherogram quality and sequences alignment was analyzed using MEGA version 6 (https://www.megasoftware.net, last accessed December 20, 2021), and the spike gene mutations were confirmed using the CoVsurver mutation application (https://www.gisaid.org/epiflu-applications/covsurver-mutations-app, last accessed December 20, 2021).
Statistical Analysis
The numerical values of sensitivity and specificity were represented as the proportion (percentage) of true positives and negatives, respectively, that were correctly identified by the evaluated tests when compared with the Sanger sequences. Furthermore, 95% CIs were calculated around proportions. The CT values, a continuous variable, were expressed by box plots showing medians (middle line) and third and first quartiles (boxes), whereas the whiskers showed maximum and minimum range above and below the box. The comparisons between groups were performed with the unpaired, two-sided U-test. All values with P < 0.05 were considered significant (GraphPad Prism version 9.0.1; GraphPad Software, San Diego, CA).
Results
A total of 156 samples were processed with RNA extraction and extraction-free methods. Of those, 109 (109/156) were variants that were identifiable by the I,II&IV assays. There was 100% (109/109) overall agreement (95% CI, 96.7%–100%) between the I,II&IV assays and S gene sequencing (Table 2) in the variants that are listed on the I,II&IV test menus. Of the 35 Alpha variant samples, not all gene mutations associated with the Alpha variant were detected in 4 samples when processed by the extraction-free method. In two of the samples, only the N501Y mutation and RdRp gene were detected. Of the other two samples, only the RdRp gene was detected in one, and only the H69/V70 deletion was detected in the other.
Table 2.
Performance with Known Variants Detectable by Novaplex SARS-CoV-2 Variants I, II, and IV Assays Using RNA Extraction and Extraction-Free Methods
| Variant detection method | WT∗ | Alpha | Beta | Gamma | Delta | Epsilon | Total |
|---|---|---|---|---|---|---|---|
| Spike gene sequencing | 17 | 35 | 12 | 10 | 29 | 6 | 109 |
| Novaplex SARS-CoV-2 Variants I, II, and IV† | |||||||
| Extracted RNA | 17 | 35 | 12 | 10 | 29 | 6 | 109 |
| Extraction free | 17 | 31 | 12 | 10 | 29 | 6 | 105 |
WT, wild type.
WT indicates samples selected with no spike gene mutations other than D614G, which was detected by S gene sequencing.
Variants I, II, and &IV assays cannot differentiate between WT samples and samples without the S gene mutations listed on their test menus.
Table 3 lists 10 samples that had undetectable levels of RNA for S gene sequencing (traditional RT-PCR yielded an undetectable band on gel electrophoresis) but were detected by the I,II&IV assays.
Table 3.
Comparison of RNA Extraction and Extraction-Free Methods on Samples with No Result by Traditional RT-PCR Followed by S Gene Sequencing
| Sample | Extracted RNA | Extraction free |
|---|---|---|
| 1 | RdRp, HV69/70 del | None |
| 2 | RdRp | None |
| 3 | RdRp, N501Y, HV69/70 del | RdRp |
| 4 | RdRp, HV69/70 del | None |
| 5 | RdRp, L452R | None |
| 6 | L452R, P681R | P681R |
| 7 | L452R, P681R | P681R |
| 8 | L452R, P681R | L452R, P681R |
| 9 | L452R, P681R | P681R |
| 10 | L452R, P681R | P681R |
Of the 156 samples, only 17 were identified as wild type by S gene sequencing (Table 2). In addition, 37 samples had mutations detected by S gene sequencing but not detectable by the I,II&IV assays (Table 4). Supplemental Table S1 lists all the mutations detected by S gene sequencing for these 37 samples. They could not be defined as true wild type because of these existing mutations. Taking these data together and analyzing each substitution individually in the 146 samples with S gene sequencing results, both the sensitivities and specificities for all the targets on the I,II&IV assays testing menus were 100%. Supplemental Table S2 provides additional detailed information on the sensitivity and specificity of each mutation detected by the I,II&IV assays.
Table 4.
Results of Samples with Other Mutations
| SARS-CoV-2 variant information |
S sequencing results |
Novaplex SARS-CoV-2 Variants I, II, and IV Assays |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pango lineage | WHO label | Samples, N | H69/V70del | E484K | N501Y | H69/V70del | E484K | N501Y | RdRp |
| B.1 | – | 1 | Det | Det | Det | ||||
| P.2 | Zeta | 2 | Det | Det | Det | ||||
| R.1 | – | 3 | Det | Det | Det | ||||
| Undet | – | 1 | Det | Det | Det | ||||
| Others∗ | – | 30 | Det | ||||||
–, Not available; Det, detected; Undet, undetermined; WHO, World Health Organization.
Mutations detected: S98F, G142S, E180V, I468T, T478K, A520S, K558N, D614G, Q675H, Q677H, Q677P, P681H, and T732A.
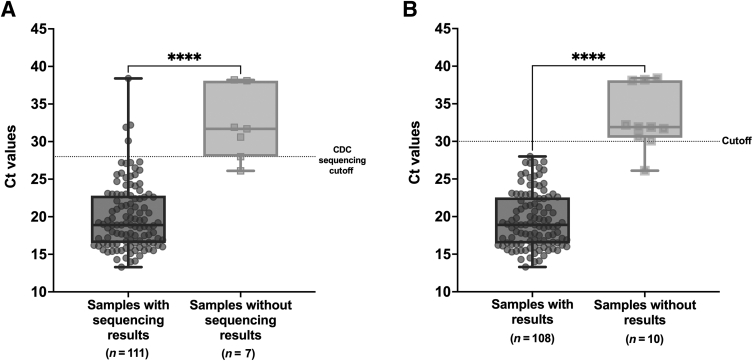
The two different extraction protocols (chloroform-ethanol RNA extraction and extraction-free, heat-release method) were compared using the I,II&IV assays. Overall, there was a 91.7% (143/156) agreement (95% CI, 86.2%–95.5%) between the two (Table 5). Of the total 156 samples, 118 had positive CT values generated by either the Xpert Xpress SARS-CoV-2 (Cepheid GenXpert) or the SARS-CoV-2 Panther Fusion (Hologic). Of the 109 samples with variants on the I,II&IV menu, 85 had available CT values. Using the extraction-free method on samples with CT values of >30 resulted in some mutations not being detected, presumably due to a lower concentration of viral nucleic acid in the original specimens. Of the samples that did not have the expected targets detected when using the extraction-free method compared with using extracted RNA, 90% (9/10) had CT values >30 (Figure 1A), except one sample had a CT value of 26.1. Furthermore, 100% (108/108) of the samples that had 100% target agreement with both extraction techniques had CT values <30 (Figure 1B).
Table 5.
Two-by-Two Table Comparing RNA Extraction and Extraction-Free Methods on Novaplex SARS-CoV-2 Variants I, II, and IV Assays
| Sample processing method | Extracted RNA |
|
|---|---|---|
| D | ND | |
| Extraction free | ||
| D | 143 | 0 |
| ND | 13 | 0 |
D, detected; ND, not detected or partial detection (some genes but not all).
Figure 1.
Box plots showing CT values in the samples with and without SARS-CoV-2 S gene sequencing results using the RNA extraction method (A) and CT values in the samples with and without results by variants I, II, and IV assays using the extraction-free method (B). The box plots show medians (middle line) and third and first quartiles (boxes), whereas the whiskers show maximum and minimum ranges above and below. The numbers of samples (n) are shown underneath. P values were determined with unpaired, two-sided U-test. ∗∗∗∗P ≤ 0.0001.
Discussion
This comparative evaluation of the Seegene Technologies Novaplex I, II, & IV assays demonstrated excellent analytical sensitivity and specificity for the detection and differentiation of known SARS-CoV-2 variants when compared with S gene sequencing. In 10 samples known to be SARS-CoV-2–positive, the I,II&IV assays detected SARS-CoV-2 RNA, when traditional RT-PCR followed by S gene sequencing could not, indicating increased sensitivity in the I,II&IV assays. In addition, in 37 samples with other mutations not on the I,II&IV assays testing menus but detected by S gene sequencing, the I,II&IV assays did not call out the wrong mutations, indicating the assays were specific.
The extraction-free RNA isolation protocol proved to be a time-saving alternative to standard chloroform-ethanol RNA extraction for samples with CT values of <30. It was able to generate results at least 1 day sooner. A major limiting factor for molecular SARS-CoV-2 assays is the lack of availability of RNA extraction reagents, and conventional extraction remains a time-consuming part of molecular diagnosis of SARS-CoV-2. Extraction-free methods have become a reliable high-throughput diagnostic approach.9, 10, 11, 12, 13 According to the CDC COVID-19 Breakthrough Case Investigations and Reporting, sequencing is recommended only for samples with a CT value ≤28.0, because sequencing is not feasible with higher CT values (https://www.cdc.gov/vaccines/covid-19/health-departments/breakthrough-cases.html, last accessed October 15, 2021). Therefore, although moderately lower sensitivity (91%) was observed with the extraction-free method than conventional extraction, it still represents a viable alternative.
S gene sequencing retains the ability to detect new variants and additional genetic mutations in the spike gene and follow the evolution of SARS-CoV-2. However, S gene sequencing does not comprehensively describe all mutations because other parts of the SARS-CoV-2 genome are likely to have mutations affecting disease severity and transmissibility. Therefore, whole-genome sequencing is necessary to identify all mutations.7 Thus, RT-PCR assays can be tailored to include additional representative genes as different variants emerge, and sequencing results can be used to update the assays. Because of the increase in SARS-CoV-2 variants globally, and the resulting decrease in vaccine efficacy for some,1 it may be necessary to reformulate vaccines in the future. Some variants have also been shown to decrease the efficacy of available therapeutics, such as monoclonal antibodies, as most recently witnessed with the Omicron variant surge. Predominant variants need to be well characterized and expeditiously tracked to facilitate vaccine and therapeutic development and help protect public health by establishing appropriate control and mitigation measures. Therefore, although many laboratories do not have sequencing capabilities, commercial RT-PCR–based assays allow for more accessible variant detection and monitoring to inform public health and treatment decisions.
The Omicron variant is currently surging rapidly, with increasing interest and concern. However, this study was performed before the Omicron variant was detected for the first time in the population. Although the Allplex SARS-CoV-2 Master Assay for screening of SARS-CoV-2 and Omicron variant and Novaplex SARS-CoV-2 Variants VII Assay for identification of Omicron-specific mutations (Seegene Technologies) are available now, these two new assays are not within the scope of evaluation in this study. However, a summary table (Supplemental Table S3) shows the potential capabilities of different assays developed by Seegene Technologies. Another limitation of the study is that because of the absence of Beta variants in the patient population, a laboratory-grown SARS-CoV-2 Beta variant was spiked into the viral transport media. Serial dilutions were made to mimic the different viral concentrations in the population, as expected. The most salient disadvantage of using spiked samples is the difficulty of ensuring no difference in analytical behavior between the spiked and the biological samples.
In conclusion, the Novaplex SARS-CoV-2 Variants I, II, & IV assays demonstrated exceptional agreement when compared with the S gene sequencing and showed reliable detection of the emerging Delta variant and other variants of interest/variants being monitored. The assays offer a suitable alternative for surveillance of circulating variants and can be widely applied in laboratories with real-time RT-PCR technologies. This approach can help public health departments establish control measures to investigate breakthrough cases and variant-dependent effects on treatment efficacy and disease severity.
Acknowledgment
We thank Seegene Technologies for providing Novaplex SARS-CoV-2 Variants I, II, and IV assay kits used in this study.
Footnotes
Supported by Fundação de Amparo à Pesquisa do Estado de São Paulo project 2019/27803-2 (R.R.G.M.); the Sealy and Smith Foundation; and NIH grant R24 AI120942.
M.C.N. and R.R.G.M. contributed equally to this study.
Disclosures: Novaplex SARS-CoV-2 Variants I, II, and IV assay kits used in this study were provided by Seegene Technologies.
Supplemental material for this article can be found at http://doi.org/10.1016/j.jmoldx.2022.02.001.
Supplemental Data
References
- 1.Boehm E., Kronig I., Neher R.A., Eckerle I., Vetter P., Kaiser L. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin Microbiol Infect. 2021;27:1109–1117. doi: 10.1016/j.cmi.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castonguay N., Zhang W., Langlois M.-A. Meta-analysis and structural dynamics of the emergence of genetic variants of SARS-CoV-2. Front Microbiol. 2021;12:676314. doi: 10.3389/fmicb.2021.676314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., Robertson D.L. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell F., Archer B., Laurenson-Schafer H., Jinnai Y., Konings F., Batra N., Pavlin B., Vandemaele K., Van Kerkhove M.D., Jombart T., Morgan O., le Polain de Waroux O. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance. 2021;26:2100509. doi: 10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies N., Abbott S., Barnard R., Jarvis C., Kucharski A., Munday J., Pearson C., Russell T., Tully D., Washburne A., Wenseleers T., Gimma A., Waites W., Wong K., van Zandvoort K., Silverman J., Diaz-Ordaz K., Keogh R., Eggo R., Funk S., Jit M., Atkins K., Edmunds W. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyson L., Hill E.M., Moore S., Curran-Sebastian J., Tildesley M.J., Lythgoe K.A., House T., Pellis L., Keeling M.J. Possible future waves of SARS-CoV-2 infection generated by variants of concern with a range of characteristics. Nat Commun. 2021;121:1–13. doi: 10.1038/s41467-021-25915-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaworski E., Langsjoen R., Mitchell B., Judy B., Newman P., Plante J., Plante K., Miller A., Zhou Y., Swetnam D., Sotcheff S., Morris V., Saada N., Machado R., McConnell A., Widen S., Thompson J., Dong J., Ren P., Pyles R., Ksiazek T., Menachery V., Weaver S., Routh A. Tiled-ClickSeq for targeted sequencing of complete coronavirus genomes with simultaneous capture of RNA recombination and minority variants. Elife. 2021;10:e68479. doi: 10.7554/eLife.68479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y., Liu J., Plante K.S., Plante J.A., Xie X., Zhang X., Ku Z., An Z., Scharton D., Schindewolf C., Widen S.G., Menachery V.D., Shi P.-Y., Weaver S.C. The N501Y spike substitution enhances SARS-CoV-2 infection and transmission. Nature. 2022;602:294–299. doi: 10.1038/s41586-021-04245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barza R., Patel P., Sabatini L., Singh K. Use of a simplified sample processing step without RNA extraction for direct SARS-CoV-2 RT-PCR detection. J Clin Virol. 2020;132:104587. doi: 10.1016/j.jcv.2020.104587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merindol N., Pépin G., Marchand C., Rheault M., Peterson C., Poirier A., Houle C., Germain H., Danylo A. SARS-CoV-2 detection by direct rRT-PCR without RNA extraction. J Clin Virol. 2020;128:104423. doi: 10.1016/j.jcv.2020.104423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smyrlaki I., Ekman M., Lentini A., Rufino de Sousa N., Papanicolaou N., Vondracek M., Aarum J., Safari H., Muradrasoli S., Rothfuchs A., Albert J., Högberg B., Reinius B. Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-PCR. Nat Commun. 2020;11:4812. doi: 10.1038/s41467-020-18611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruce E.A., Huang M.L., Perchetti G.A., Tighe S., Laaguiby P., Hoffman J.J., Gerrard D.L., Nalla A.K., Wei Y., Greninger A.L., Diehl S.A., Shirley D.J., Leonard D.G.B., Huston C.D., Kirkpatrick B.D., Dragon J.A., Crothers J.W., Jerome K.R., Botten J.W. Direct RT-qPCR detection of SARS-CoV-2 RNA from patient nasopharyngeal swabs without an RNA extraction step. PLoS Biol. 2020;18:e3000896. doi: 10.1371/journal.pbio.3000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron A., Pecora N.D., Pettengill M.A. Extraction-free methods for the detection of SARS-CoV-2 by reverse transcription-PCR: a comparison with the cepheid xpert xpress SARS-CoV-2 assay across two medical centers. J Clin Microbiol. 2021;59:e02643-20. doi: 10.1128/JCM.02643-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.