Abstract
Commercial sex workers (CSWs) serve as the most important reservoir of sexually transmitted diseases (STD), including gonorrhea. Periodic monitoring of the antimicrobial susceptibility profile of Neisseria gonorrhoeae in a high-risk population provides essential clues regarding the rapidly changing pattern of antimicrobial susceptibilities. A study concerning the prevalence of gonococcal infection among CSWs was conducted in Bangladesh. The isolates were examined with regards to their antimicrobial susceptibility to, and the MICs of, penicillin, tetracycline, ciprofloxacin, cefuroxime, ceftriaxone, and spectinomycin by disk diffusion and agar dilution methods. The total plasmid profile of the isolates was also analyzed. Of the 224 CSWs, 94 (42%) were culture positive for N. gonorrhoeae. There was a good correlation between the results of the disk diffusion and agar dilution methods. Some 66% of the isolates were resistant to penicillin, and 34% were moderately susceptible to penicillin. Among the resistant isolates, 23.4% were penicillinase-producing N. gonorrhoeae (PPNG). 60.6% of the isolates were resistant and 38.3% were moderately susceptible to tetracycline, 17.5% were tetracycline-resistant N. gonorrhoeae, 11.7% were resistant and 26.6% had reduced susceptibility to ciprofloxacin, 2.1% were resistant and 11.7% had reduced susceptibility to cefuroxime, and 1% were resistant to ceftriaxone. All PPNG isolates contained a 3.2-MDa African type of plasmid, and a 24.2-MDa conjugative plasmid was present in 34.1% of the isolates. Since quinolones such as ciprofloxacin are recommended as the first line of therapy for gonorrhea, the emergence of significant resistance to ciprofloxacin will limit the usefulness of this drug for treatment of gonorrhea in Bangladesh.
Despite a sharp decline in the incidence of gonococcal infection in developed countries during the last decade, gonorrhea remains one of the most common venereal diseases in developing countries and a global health problem (7). The problem is compounded by the development of resistance to antimicrobials in N. gonorrhoeae, which is a result of both wide dissemination of resistant clones and the emergence of strains with novel resistance mechanisms (13). Periodic monitoring of the antimicrobial susceptibility profile of N. gonorrhoeae strains prevalent in a high-risk group such as commercial sex workers (CSWs) provides essential clues regarding treatment options and emergence of drug resistance. CSWs sustain very high rates of transmission of sexually transmitted diseases (STD); among them, gonorrhea with multiple antibiotic resistance is common (6). Gonococcal infection has been implicated in facilitating human immunodeficiency virus (HIV) acquisition and transmission (18). This is probably due to mucosal inflammation, which provides greater access for HIV than that provided by normal tissue, and the release of virus particles in semen is significantly greater in HIV-infected patients with gonorrhea than in patients without gonorrhea (31, 27).
Strategies for control of gonorrhea have relied on the use of highly effective and, often, single-dose therapy administered at the time of diagnosis. Penicillin has been used as the first-line therapy for gonorrhea for over 40 years. Due to the appearance and the subsequent increase in the prevalence of penicillinase-producing N. gonorrhoeae (PPNG) and to N. gonorrhoeae isolates with chromosomally mediated resistance to penicillin and tetracycline (CMRNGPT), the Centers for Disease Control, Atlanta, Ga., has advocated broad-spectrum cephalosporins or selected fluoroquinolones, including ciprofloxacin and ofloxacin, as the first-line therapies for uncomplicated gonorrhea (3–5, 16). Ciprofloxacin is being used as the first-line therapy for uncomplicated and suspected gonorrhea cases in Bangladesh. It is also included in syndromic management in cases of suspected gonorrhea.
Since the introduction of fluoroquinolone for the treatment of uncomplicated gonorrhea, ciprofloxacin has been used extensively in Southeast Asia. However, gonococcal strains with reduced susceptibility to the fluoroquinolones have been reported in Asia (25, 6), the United Kingdom (10), North America (15), and Europe (1).
Continuous surveillance of the epidemiology of gonococcal resistance, based on type characterization, and detailed in vitro assessment of the antimicrobial susceptibility of strains are imperative for treatment and prevention (19). The prevalence of gonorrhea and antimicrobial susceptibility of N. gonorrhoeae in a high-risk population is not well documented in Bangladesh. The aim of the present study was to examine the antimicrobial susceptibility and plasmid profile of N. gonorrhoeae isolates from CSWs in Dhaka, Bangladesh.
MATERIALS AND METHODS
Study population.
From June to November 1997, the prevalence of gonorrhea among the CSWs in the city of Dhaka was studied. All CSWs attending a rehabilitation center at Mirpur, Dhaka, for voluntary rehabilitation under a government rehabilitation program were evaluated for gonococcal infection. CSWs were recruited irrespective of symptoms of STD. The only exclusion criterion for participation in the study was the use of antimicrobial agents in the preceding 2 weeks. Endocervical swabs from 224 consecutively seen CSWs were cultured for N. gonorrhoeae.
Isolation.
Endocervical swabs were streaked on modified Thayer-Martin agar (BBL Microbiology Systems, Cockeysville, Md.). The identity of the organism was confirmed by colony morphology, Gram staining, oxidase and catalase tests, and the sugar fermentation test. Isolates were further confirmed by PCR using primers which amplify a 390-bp region of the gonococcal cryptic plasmid (12). Isolates were kept at −70°C in Trypticase soy broth with 20% glycerol until further studied.
β-Lactamase test.
All penicillin-resistant isolates were tested for β-lactamase production by a paper acidometric method as described earlier (32).
Disk diffusion.
Antimicrobial disks containing penicillin (10 U/disk), tetracycline (30 μg/disk), ciprofloxacin (5 μg/disk), ceftriaxone (30 μg/disk), cefuroxime (30 μg/disk) (all from Oxoid, Hampshire, United Kingdom), and spectinomycin (100 μg/ml) (Becton Dickinson, Cockeysville, Md.) were used for disk diffusion as described earlier (16). Each test was done in triplicate, and the mean value was determined.
MICs.
MICs of penicillin, tetracycline, spectinomycin, ceftriaxone, cefuroxime, and ciprofloxacin were determined by an agar dilution method as described earlier (22). Briefly, GC agar base agar (BBL Microbiology Systems) supplemented with 1% IsoVitaleX and twofold serial dilutions of antibiotics were used. Plates were inoculated with 104 CFU of bacteria and incubated in 5% CO2 for 36 h. The end point was read as the lowest concentration of antimicrobial agent giving complete inhibition of growth. Five N. gonorrhoeae reference strains, WHO A to WHO E, for which the MICs were known, were included for quality control in each test. Each test was repeated three times. The antimicrobial susceptibility was judged by breakpoint criteria defined by the National Committee for Clinical Laboratory Standards (NCCLS) (21). Twofold serial dilutions of antibiotics were used at the following concentrations: penicillin (Sigma, St. Louis, Mo.), 0.03 to 64 μg/ml; tetracycline (Sigma), 0.03 to 64 μg/ml; ciprofloxacin (Bayer, Hampshire, United Kingdom), 0.004 to 4 μg/ml; spectinomycin (Upjohn, Puurs, Belgium), 2.0 to 128 μg/ml; cefuroxime (Sigma), 0.06 to 4 μg/ml; and ceftriaxone (Sigma), 0.004 to 0.5 μg/ml.
Plasmid analysis.
Isolates were grown overnight at 37°C in 5% CO2 on GC agar base with 1% Kellogg’s supplement. The plasmid DNA was extracted by the rapid alkaline lysis method (2). The plasmid profile was determined by electrophoresis at 50 V on a 0.7% agarose gel. The gel was stained with ethidium bromide (0.5 μg/ml) and visualized by UV light transillumination.
Phenotypic characterization.
The criteria used for phenotypic characterization of N. gonorrhoeae based on plasmid and chromosomally mediated resistance to penicillin and tetracycline are shown in Table 1 (26).
TABLE 1.
Phenotypic categories of N. gonorrhoeae based on plasmid and chromosomally mediated resistance to penicillin and tetracycline (n = 94)
| Category | Criteriaa | No (%) of isolates |
|---|---|---|
| 1. Penicillinase-producing N. gonorrhoeae (PPNG) | β-Lactamase +ve; tetracycline MIC of ≤16 μg/ml | 22 (23.4) |
| 2. Plasmid-mediated tetracycline resistant N. gonorrhoeae (TRNG) | β-Lactamase −ve; tetracycline MIC of ≥16 μg/ml | 10 (10.6) |
| 3. PPNG/TRNG | β-Lactamase +ve; tetracycline MIC of ≥16 μg/ml | 0 |
| 4. N. gonorrhoeae with chromosomally mediated resistance to both penicillin and tetracycline (CMRNGPT) | Non-PPNG, non-TRNG with penicillin MIC of ≥2 μg/ml and tetracycline MIC of ≥2 μg/ml | 28 (29.8) |
| 5. N. gonorrhoeae with chromosomally mediated resistance to penicillin (CMRNGP) | Non-PPNG, non-TRNG with penicillin MIC of ≥2 μg/ml and tetracycline MIC of ≤2 μg/ml | 9 (9.6) |
| 6. N. gonorrhoeae with chromosomally mediated resistance to tetracycline (CMRNGT) | Non-PPNG, non-TRNG with tetracycline MIC of ≥2 μg/ml | 14 (14.9) |
| 7. N. gonorrhoeae susceptible to both penicillin and tetracycline (susceptiblePT) | Penicillin and tetracycline MIC of ≤2 μg/ml | 11 (11.7) |
+ve, positive; −ve, negative.
Correlation of MIC and inhibition zone.
The correlation of the MIC to the zone of inhibition and the correlation coefficient were calculated by the method of least squares, with the zone diameter as the independent variable (x axis) and the MICs as the dependent variable (y axis).
RESULTS
Among the endocervical swabs cultured from 224 consecutively seen CSWs, 94 (42%) were culture positive for N. gonorrhoeae. We examined the antimicrobial susceptibility of the isolates to penicillin, tetracycline, ciprofloxacin, cefuroxime, ceftriaxone, and spectinomycin as well as the MICs of these antimicrobials for the isolates by using disk diffusion and agar dilution methods. The MICs at which 50 and 90% of the isolates were inhibited (MIC50s and MIC90s, respectively) were determined, and the range of MICs of each antibiotic is shown in Table 2.
TABLE 2.
Antimicrobial susceptibilities of N. gonorrhoeae isolates (n = 94) in Dhaka, Bangladesh
| Antimicrobial agent | MIC (μg/ml)
|
||
|---|---|---|---|
| 90% | 50% | Range | |
| Penicillin | 16.0 | 2.0 | 0.25–64.0 |
| Tetracycline | 16.0 | 2.0 | 0.25–32.0 |
| Ciprofloxacin | 1.0 | 0.015 | 0.004–4.0 |
| Cefuroxime | 1.0 | 0.5 | 0.06–4.0 |
| Ceftriaxone | 0.06 | 0.015 | 0.004–0.5 |
| Spectinomycin | 16.0 | 8.0 | 4.0–32.0 |
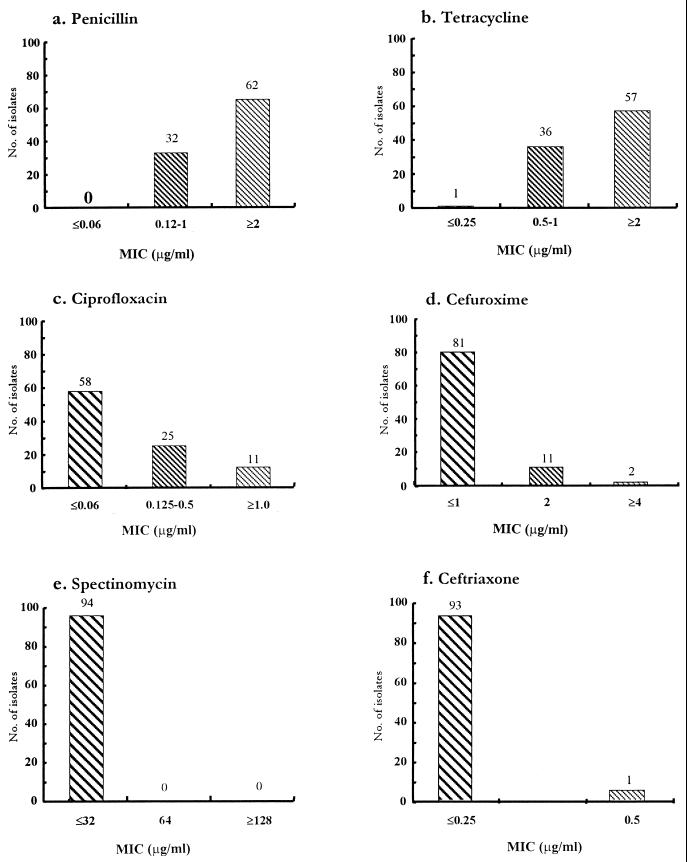
On the basis of susceptibility criteria established by NCCLS, the overall susceptibilities of the isolates were determined and are shown in Fig. 1. Among the isolates, 62 (66%) were resistant (MIC, ≥2 μg/ml) to penicillin, including 22 (23%) PPNG isolates for which the penicillin MICs were 8 to 16 μg/ml (Fig. 1a); 57 (60.6%) were resistant (MIC, ≥2 μg/ml) to tetracycline, including 10 (10.6%) tetracycline-resistant N. gonorrhoeae (TRNG) isolates for which the tetracycline MICs were ≥16 μg/ml (Fig. 1b); and 11 (11.7%) were resistant (MIC, ≥1.0 μg/ml) and 25 (26.6%) had reduced susceptibility (MIC, 0.125 to 0.5 μg/ml) to ciprofloxacin (Fig. 1c). Two (1.2%) isolates were resistant (MIC, ≥4 μg/ml) and 11 (11.7%) isolates were moderately susceptible (MIC, 2 μg/ml) to cefuroxime (Fig. 1d), whereas 1 (1%) was resistant to ceftriaxone (MIC, ≥0.5 μg/ml) (Fig. 1f). This isolate had concomitant resistance to cefuroxime. All isolates were susceptible to spectinomycin (Fig. 1e).
FIG. 1.
Distribution of MICs of penicillin (a), tetracycline (b), ciprofloxacin (c), cefuroxime (d), spectinomycin (e), and ceftriaxone (f) for N. gonorrhoeae isolates. Symbols:  , susceptible;
, susceptible;  , moderately susceptible or strains with reduced susceptibility; ▧, resistant. The breakpoint criteria used for assessing the susceptibility were those recommended previously (21, 22).
, moderately susceptible or strains with reduced susceptibility; ▧, resistant. The breakpoint criteria used for assessing the susceptibility were those recommended previously (21, 22).
Seven (63.6%) ciprofloxacin-resistant isolates and 15 (25.9%) ciprofloxacin-susceptible isolates belonged to the CMRNGPT; 9 (81.8%) ciprofloxacin-resistant isolates had chromosomally mediated resistance to penicillin and/or tetracycline; 12 (48%) of the isolates with reduced susceptibility were PPNG.
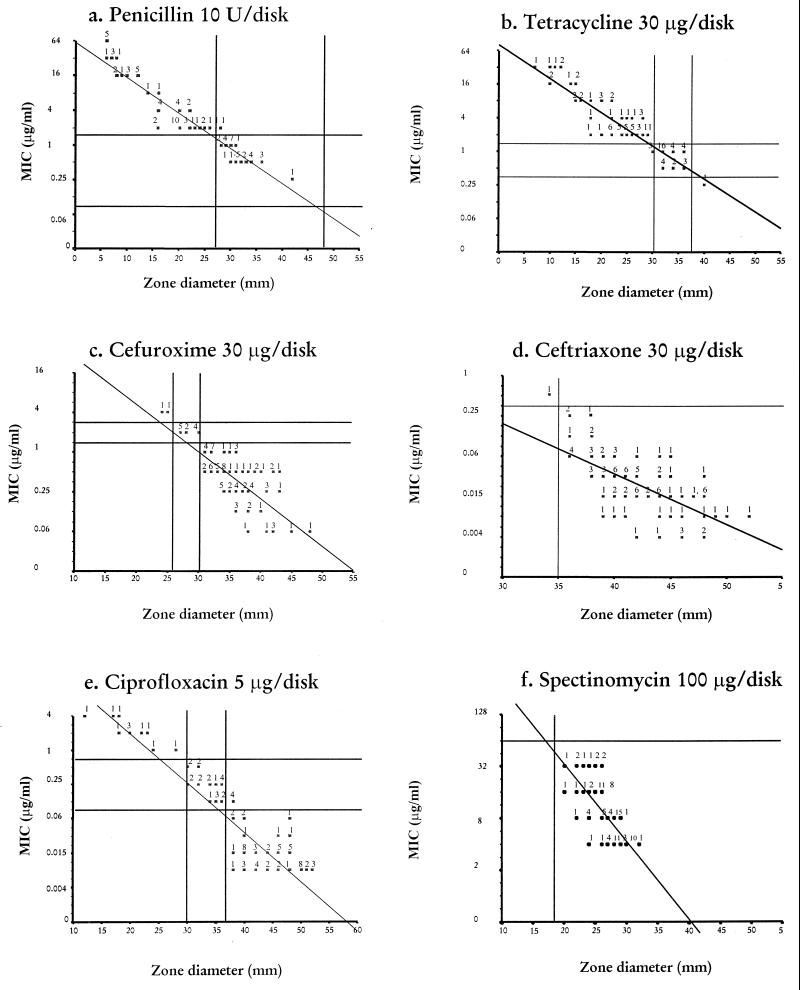
The correlation between MIC and zone diameter is shown in Fig. 2. For cefuroxime (Fig. 2c) and ceftriaxone (Fig. 2d), the zone diameters of inhibition of the isolates were correctly interpreted as belonging to the susceptible, intermediately susceptible, and resistant categories corresponding to the MIC interpretive breakpoints. All isolates were correctly identified as susceptible to spectinomycin when the MIC interpretive breakpoint of ≥128 μg/ml was applied (Fig. 2f). From results for penicillin (Fig. 2a), tetracycline (Fig. 2b), and ciprofloxacin (Fig. 2e), most of the isolates could be identified as belonging to a single susceptibility category. However, for some isolates, the use of neither MIC limits nor zone diameter for susceptibility categories could reproducibly identify isolates as belonging to any single category, since the ranges of the MICs for these isolates were broad.
FIG. 2.
Scattergram of MICs (y axis) and mean zone diameter of inhibition (x axis) around disks of penicillin (10 U) (a), tetracycline (30 μg/disk) (b), cefuroxime (30 μg/disk) (c), ceftriaxone (30 μg/disk) (d), ciprofloxacin (5 μg/disk) (e), and spectinomycin (100 μg/disk) (f) for 94 clinical isolates of N. gonorrhoeae. The solid vertical and horizontal lines indicate the criteria recommended by the NCCLS for the interpretation of the susceptible categories for these agents by disk diffusion and MICs.
Based on plasmid and chromosomally mediated resistance to penicillin and tetracycline, the isolates were phenotypically categorized into seven different groups (Table 1). The susceptibility patterns of the different phenotypic categories of N. gonorrhoeae to ciprofloxacin were further analyzed (Table 3).
TABLE 3.
Susceptibility patterns of different phenotypic categoriesa of N. gonorrhoeae to ciprofloxacin
| Susceptibility pattern | No (%) of isolates
|
|||||
|---|---|---|---|---|---|---|
| PPNG | TRNG | CMRNGPT | CMRNGP | CMRNGT | SusceptiblePT | |
| Susceptible; MIC of ≤0.06 μg/ml (n = 58) | 10 (17.2) | 7 (12.0) | 15 (25.9) | 6 (10.4) | 11 (19.0) | 9 (15.6) |
| Intermediately susceptible; MIC of 0.125 to 0.5 μg/ml (n = 25) | 12 (48.0) | 2 (8.0) | 6 (24.0) | 2 (8.0) | 2 (8.0) | 1 (4.0) |
| Resistant; MIC of ≥1 μg/ml (n = 11) | 0 (0) | 1 (9.1) | 7 (63.0) | 1 (9.1) | 1 (9.1) | 1 (9.1) |
See Table 1 for explanation.
The plasmid profiles of all isolates were analyzed (Table 4). All isolates harbored a 2.6-MDa cryptic plasmid; 22 (23.4%) of the isolates were PPNG, and all of them contained a 3.2-MDa African type PPNG plasmid; 10 (10.6%) of the isolates were TRNG and contained a 25.2-MDa TRNG plasmid. A conjugative plasmid was present in 34.1% of the isolates (36.3% in the PPNG isolates and 32.2% in the others).
TABLE 4.
Plasmid profiles of N. gonorrhoeae isolates (n = 94)
| Resistance category | Plasmid sizes (MDa) | No (%) of strains |
|---|---|---|
| PPNG (n = 22) | 2.6, 3.2 | 14 (14.9) |
| 2.6, 3.2, 24.5 | 8 (8.5) | |
| TRNG (n = 10) | 2.6, 25.2 | 10 (10.6) |
| Isolates without plasmid-mediated resistance (n = 62) | 2.6 2.6, 24.5 | 42 (44.7) 20 (20.3) |
DISCUSSION
Gonorrhea remains a major cause of morbidity in sexually active individuals, and most of the cases are projected to occur in countries of the Southeast Asia region (7, 28). The control of gonococcal infection is important considering the high incidence of acute infections, complications, and sequelae and the role of gonorrhoeae in facilitating HIV acquisition and transmission. N. gonorrhoeae isolates have consistently developed resistance to the antimicrobial agents used for treatment of gonorrhea. Antimicrobial resistance in N. gonorrhoeae has become a major global public health concern. Since the introduction of sulfonamides in the 1930s, N. gonorrhoeae isolates have developed resistance to many of the antibiotics used in the treatment of gonococcal infections. There has been a remarkable increase in antimicrobial resistance among N. gonorrhoeae isolates in many developing countries in recent years (23).
In many western countries, the incidence of gonorrhea has declined since the mid-1970s; during the past year, however, evidence that the incidence of gonorrhea is on the increase again in these countries, as in the developing countries, has emerged. The focal nature of the increased incidence suggests the need for geographically targeted interventions.
In developing countries where the prevalence of bacterial STD remains high and laboratory facilities are limited, the World Health Organization has recommended that a syndromic approach based on clinical symptoms and signs be used and the Centers for Disease Control have recommended a first-line therapeutic regimen based on fluoroquinolones and cephalosporin. Resistance to fluoroquinolone in neisseriae occurs as a result of point mutations in the DNA gyrase (gyrA) gene and the topoisomerase IV (parC) gene. High-level quinolone resistance indicates adaptive mutations of N. gonorrhoeae under selective antibiotic pressure. N. gonorrhoeae has rapidly developed resistance to most antimicrobial agents and, most recently, to quinolones used in treatment of the diseases. Inappropriate therapy due to self-medication and poor compliance is common among patients with gonorrhea, and these are the factors that enhance the development of resistance. Fluoroquinolones have been recently recommended for the primary treatment of uncomplicated gonorrhea in areas where multiresistant strains are common, such as Southeast Asia and central Africa. As a consequence of the large-scale use of this group of antimicrobials in areas where over-the-counter availability of drugs without prescription is common, a substantial increase of resistant strains may occur. The appearance of a significant number of resistant isolates (11.7% resistant and 26.6% with reduced susceptibility) in our study indicates that CSWs in Bangladesh are exposed to fluoroquinolones and that resistance has developed under selective antibiotic pressure. The fact that the isolates in this study were collected randomly from the CSWs in the city of Dhaka suggests that they are reasonably representative of gonococcal strains in the CSWs.
Ciprofloxacin resistance among gonococci was found to be 1.3% in the United States (8) and 10% in Hong Kong and the Philippines (17). Resistance to ciprofloxacin is chromosomally mediated, affects all the members of the fluoroquinolone group of antibiotics, and is apparently incremental (30). Of the 11 resistant isolates in the present study, 9 (82%) had chromosomally mediated resistance to penicillin and/or tetracycline, indicating that ciprofloxacin-resistant strains are more prevalent in isolates having chromosomally mediated resistance to penicillin and tetracycline. A similar pattern has also been reported earlier in the United States and Thailand (8, 17). The frequent appearance of elevated ciprofloxacin MICs for strains with chromosomally mediated resistance to penicillin and tetracycline remains unexplained because the mechanism of ciprofloxacin resistance is different from those of penicillin and tetracycline.
Resistance to penicillin and tetracycline is either chromosomally mediated or plasmid mediated. Chromosomally mediated resistance to penicillin is a low-level resistance and the result of the additive effect of mutations at multiple loci, including penA, mtr, and penB (29), while plasmid-mediated resistance is due to PPNG (the 3.2-MDa African type or the 4.4-MDa Asian type) encoding a TEM-1 type β-lactamase. The high-level penicillin resistance observed in our study may be due to a consequence of penicillin therapy for unrelated illnesses or self-medication by CSWs with penicillin or penicillin congeners. Self-medication among sex workers in the Philippines has been shown to play a major role in the development of antimicrobial resistance, and a similar practice is also common in Bangladesh (11). Plasmid analysis showed that all PPNG isolates contained a 3.2-MDa African type of plasmid. Transfer of a penicillinase plasmid can occur between the N. gonorrhoeae strains by conjugation, but it requires the presence of a 24.5-MDa conjugative plasmid in the donor to mobilize the transfer (20). About one-third of the isolates we studied harbored a 24.5-MDa conjugative plasmid, and an equal number of PPNG isolates also had this plasmid.
High-level plasmid-mediated resistance to tetracycline is due to acquisition of the tetM determinant by the conjugative plasmid (24.5 MDa) of N. gonorrhoeae, resulting in a 25.2-MDa plasmid (9). This plasmid is self-mobilizable and can move between N. gonorrhoeae strains and other genera. Tetracycline-resistant N. gonorrhoeae isolates are likely to spread more quickly than PPNG isolates because of the presence of the tetM plasmid in other flora found in the genital tract that may act as a reservoir. Approximately 60% of the isolates were tetracycline resistant, which indicates selective pressure resulting from its use for STD and other illnesses.
The extended-spectrum cephalosporin (cefuroxime) and the broad-spectrum cephalosporin (ceftriaxone) demonstrate very high levels of activity against these gonococcal isolates, showing 86.1 and 98.9% susceptibility, respectively. Of two isolates for which the cefuroxime MIC was 4 μg/ml, one was resistant to ceftriaxone (MIC, 0.5 μg/ml) by the in vitro test. We do not know the clinical significance of these isolates and whether infections caused by these strains would fail to respond to the currently recommended treatment with those drugs. However, a therapeutic index (ratio of peak serum concentration to MIC) of 3:1 to 4:1 has been recommended for reliable cure of gonococcal urethritis (14), and a 500-mg intramuscular dose produces a peak serum concentration of 42 to 45 μg/ml with a half-life in serum of 6.0 to 8.3 h (24). Gonococcal isolates for which the ceftriaxone MIC was 0.5 μg/ml and those for which the cefuroxime MIC was 4 μg/ml have been reported in the United States and Thailand, respectively (8, 17). All the isolates in the present study were susceptible to spectinomycin (MIC, ≤32 μg/ml), which remains a valuable alternative.
There is a need for a simple and reliable routine susceptibility test for gonococci for both epidemiological surveillance and for patient management. A standardized disk diffusion test gives reproducible results and is easy to perform by routine laboratories. Interpretive MIC breakpoints for gonococcal susceptibility categories have been based on single-dose treatment for most drugs, and these limits are accordingly lower than those for other bacteria. The results of the disk diffusion test for most of the isolates tested in this study were in agreement with MIC breakpoints for gonococcal susceptibility categories.
The data presented here indicate that N. gonorrhoeae strains resistant to the commonly used antimicrobial agents have increased, and consequently penicillin and tetracycline can no longer be recommended for the treatment of gonorrhea. Ciprofloxacin may soon approach the end of its utility as a first-line drug for treatment of gonorrhea in Bangladesh, where 11.7% of N. gonorrhoeae isolates are currently resistant.
ACKNOWLEDGMENTS
Our research was funded by the Institute of Post Graduate Medicine and Research and the International Centre for Diarrhoeal Disease Research, Bangladesh, which is supported by countries and agencies that share its concern for the health problems of developing countries. Current donors providing unrestricted support include the aid agencies of the governments of Australia, Bangladesh, Belgium, Canada, Saudi Arabia, Sweden, Switzerland, the United Kingdom, and the United States and international organizations including the United Nations Children’s Fund (UNICEF). One fellowship, to Bahar Uddin Bhuiyan from Jahurul Islam Medical College Hospital, is gratefully acknowledged.
We are grateful to Joseph Bogaerts and Anowar Hossain for their cooperation.
REFERENCES
- 1.Birley H, MacDonald P, Carey P, Fletcher J. High-level ciprofloxacin resistance in Neisseria gonorrhoeae. Genitourin Med. 1994;70:292–293. doi: 10.1136/sti.70.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control. Penicillinase producing Neisseria gonorrhoeae. Morbid Mortal Weekly Rep. 1976;23:261. [Google Scholar]
- 4.Centers for Disease Control. Penicillinase producing Neisseria gonorrhoeae—United States, worldwide. Morbid Mortal Weekly Rep. 1979;28:85–87. [Google Scholar]
- 5.Centers for Disease Control. 1993 sexually transmitted diseases treatment guidelines. Morbid Mortal Weekly Rep. 1993;42(RR-14):4–5. [PubMed] [Google Scholar]
- 6.Clendennen T E, Echeverria P, Saengeur S, Kees E S, Boslego J W, Wignall F S. Antibiotics susceptibility survey of Neisseria gonorrhoeae in Thailand. Antimicrob Agents Chemother. 1992;36:1682–1687. doi: 10.1128/aac.36.8.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Schryver A, Meheus A. Epidemiology of sexually transmitted diseases: the global picture. Bull WHO. 1990;68:639–654. [PMC free article] [PubMed] [Google Scholar]
- 8.Fox K K, Knapp J S, Holmes K K, Hook E W, Judson F N, Thompson S E, Washington J A, Whittington W I. Antimicrobial resistance in Neisseria gonorrhoeae in the United States, 1988–1994: the emergence of decreased susceptibility to the fluoroquinolones. J Infect Dis. 1997;175:1396–1403. doi: 10.1086/516472. [DOI] [PubMed] [Google Scholar]
- 9.Gasocyne-Binzi D M, Heritage J, Hawkey P M. Nucleotide sequences of the tet(M) genes from the American and Dutch type tetracycline resistance plasmid of Neisseria gonorrhoeae. J Antimicrob Chemother. 1993;32:667–676. doi: 10.1093/jac/32.5.667. [DOI] [PubMed] [Google Scholar]
- 10.Gransden W R, Warren C, Philips I. 4-Quinolone-resistant Neisseria gonorrhoeae in the United Kingdom. J Med Microbiol. 1991;34:23–27. doi: 10.1099/00222615-34-1-23. [DOI] [PubMed] [Google Scholar]
- 11.Harrison W O, Wignall F S, Kerbs S B J, Berg S W. Oral rosoxacin for treatment of penicillin-resistant gonorrhoea. Lancet. 1984;i:566. doi: 10.1016/s0140-6736(84)90962-0. [DOI] [PubMed] [Google Scholar]
- 12.Ho B S W, Feng W G, Wong B K C, Egglestone S I. Polymerase chain reaction for the detection of Neisseria gonorrhoeae in clinical samples. J Clin Pathol. 1992;45:439–442. doi: 10.1136/jcp.45.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ison C A, Pepin J, Roope N S, Demba E, Secka O, Easmon C S F. The dominance of a multiresistant strain of Neisseria gonorrhoeae among prostitutes and STD patients in The Gambia. Genitourin Med. 1992;68:356–360. doi: 10.1136/sti.68.6.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe H W, Schroeter A L, Reynolds G H, Zaidi A A, Martin J E, Thayer J D. Pharmacokinetic determinants of penicillin cure of gonococcal urethritis. Antimicrob Agents Chemother. 1979;15:587–591. doi: 10.1128/aac.15.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knapp J S, Ohye R, Neal S W, Parekh M C, Higa H, Rice R J. Emerging in vitro resistance to quinolones in penicillinase-producing Neisseria gonorrhoeae strains in Hawaii. Antimicrob Agents Chemother. 1994;38:2200–2203. doi: 10.1128/aac.38.9.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knapp J S, Hale J A, Neal S W, Wintersheid K, Rice R J, Whittington W L. Proposed criteria for interpretation of susceptibilities of strains of Neisseria gonorrhoeae to ciprofloxacin, ofloxacin, enoxacin, lomefloxacin, and norfloxacin. Antimicrob Agents Chemother. 1995;39:2442–2445. doi: 10.1128/aac.39.11.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knapp J S, Fox K K, Trees D L, Whittington W L. Fluoroquinolone resistance in Neisseria gonorrhoeae. Emerg Infect Dis. 1997;3:33–39. doi: 10.3201/eid0301.970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laga M, Nzila A, Goeman J. The interrelationship of sexually transmitted diseases and HIV infection; implication for the control of both epidemics in Africa. AIDS. 1991;5(Suppl. 1):S55–S63. [PubMed] [Google Scholar]
- 19.Lind I. Gonorrhoeae. Curr Probl Dermatol. 1996;24:12–19. doi: 10.1159/000424878. [DOI] [PubMed] [Google Scholar]
- 20.Morse S A, Johnson S R, Biddle J W, Roberts M C. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob Agents Chemother. 1986;30:664–670. doi: 10.1128/aac.30.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing: fifth informational supplement. Document M 100-55. 14, no. 16. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1994. [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Standard methods for dilution antimicrobial susceptibility tests for bacteria, which grow aerobically. Approved standard M7-A2. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 23.Nissinen A, Helina J, Liimatainen O, Jahkola M, Huovinen P the Finnish Study Group for Antimicrobial Resistance. Antimicrobial resistance in Neisseria gonorrhoeae in Finland, 1976–1995. Sex Transm Dis. 1997;24:576–580. doi: 10.1097/00007435-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Patel I H, Kaplan S A. Pharmacokinetic profile of ceftriaxone in man. Am J Med. 1984;77(Suppl. 4C):17–25. [PubMed] [Google Scholar]
- 25.Putnam S D, Lavin B S, Stone J R, Oldfield E C, Hooper D G. Evaluation of the standardized disk diffusion and agar dilution antibiotic susceptibility test methods by using strains of Neisseria gonorrhoeae from the United States and Southeast Asia. J Clin Microbiol. 1993;30:974–980. doi: 10.1128/jcm.30.4.974-980.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice R J, Knapp J S. Antimicrobial susceptibilities of Neisseria gonorrhoeae strains representing five distinct resistance phenotypes. Antimicrob Agents Chemother. 1994;38:155–158. doi: 10.1128/aac.38.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Royce R A, Sena A, Cates W, Cohen M S. Sexual transmission of HIV. N Engl J Med. 1997;10:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 28.Sherrard J S, Bingham J S. Gonorrhoea now. Int J STD AIDS. 1995;6:162–166. doi: 10.1177/095646249500600304. [DOI] [PubMed] [Google Scholar]
- 29.Sparling P F, Sarubbi F A, Blackman E. Inheritance of low level resistance to penicillin, tetracycline and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975;124:740–749. doi: 10.1128/jb.124.2.740-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tapsall J W. Surveillance of antibiotic resistance in Neisseria gonorrhoeae and implications for the therapy of gonorrhoea. Int J STD AIDS. 1995;6:233–236. doi: 10.1177/095646249500600402. [DOI] [PubMed] [Google Scholar]
- 31.Wasserheit J N. Epidemiological synergy: interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:16. [PubMed] [Google Scholar]
- 32.World Health Organization. Bench level laboratory manual for sexually transmitted diseases. WHO/VDT/89.443. Geneva, Switzerland: World Health Organization; 1989. pp. 6–24. [Google Scholar]