Abstract
Background
The choice of surgical incision in the abdomen is determined by access. It has been suggested that other parameters such as recovery and complication rate may be influenced by utilising a transverse or oblique rather than a midline incision. However, there is little consensus in the literature as to whether a particular incision confers any advantage.
Objectives
To determine whether a midline incision or a transverse incision (including oblique incision) confers any recovery advantage to the patient.
Search methods
Search terms include randomised trials containing combinations of the following: 'abdominal', 'incisions', 'horizontal', 'transverse', 'vertical', 'midline', 'laparotomy'
Selection criteria
All prospective randomised trials comparing midline with transverse incisions for abdominal surgery were included. Caesarian sections were excluded.
Data collection and analysis
Two reviewers assessed the methodological quality of potentially eligible trials and independently extracted data from the included trial. A wide range of outcome measures were considered.
Main results
Due to the differences in the method of assessment, the variability of data and the heterogeneity of the participant groups it was difficult to pool some of the outcome data. Despite these limitations and potentially significant biases related to methodological quality there was evidence to suggest that a transverse or oblique incision has less impact on pulmonary function particularly in the early post‐operative period and is less prone to rupture (wound dehiscence/incisional hernia). The data on pain is less clear and should be interpreted with caution but some data suggests a transverse incision is less painful. There was no difference seen in other early or late post‐operative complications and recovery times were similar although the transverse incision may be cosmetically more acceptable.
Authors' conclusions
The analgesia use and the pulmonary compromise may be reduced with a transverse/oblique incision but this does not seem to be significant clinically as pulmonary complication rates and recovery times were the same. The likelihood of wound dehiscence and rupture appears to be reduced with a transverse incision and a transverse incision may look better. The methodological and clinical diversity and the potential for bias also mean that the results in favour of a transverse/oblique incision (particularly with regard to analgesic use) should be treated with caution. The optimal incision for abdominal surgery still remains the preference of the surgeon.
Plain language summary
Transverse verses midline incisions for abdominal surgery
Transverse abdominal access appears to affect pulmonary function less than midline access and may be less prone to rupture. There is a suggestion that a transverse incision is also less painful but this result is less clear. Other recovery and complication rates are similar although the transverse incision may look better.
The choice of abdominal surgical incision is determined largely by access. However, a transverse incision may be superior to a midline incision in terms of recovery and complications. All randomised controlled trials comparing these incisions were identified. Outcomes included analgesic use, pulmonary function, complication rates and hospital stay. Marked variability in methodology made comparison difficult and potential biases in all of the studies suggests results should be treated with caution. Nevertheless a trend was seen toward less analgesic requirement, less effect on pulmonary function and lower wound dehiscence and incisional hernia rates with a transverse incision. However, the lower pain and reduced effect on pulmonary function were not translated into other clinical advantages as recovery times and other complication rates (except cosmetic appearance) were similar.
Background
Major abdominal surgery is an important part of current medical practice. The surgery is common, and is also responsible for significant use of hospital resources both in terms of funding and bed usage. Within England there are around 250,000 abdominal operations performed each year (Hospital Episode Statistics 2003‐4), and although an increasing number of these are performed laparoscopically, a significant proportion, particularly the larger more complex procedures are still performed by an open technique. Even with cholecystectomy, where laparoscopic access is very commonly employed, there still remains advocated of the open technique, albeit via a minimal incision technique (Purkayastha 2007). Although the figures for laparoscopic colorectal resections have increased in England since the last review (Sheldon 2004), the procedure is by no means universal even in centres of excellence. Finally complex pancreatic and vascular procedures are technically difficult to carry out laparoscopically and are still commonly carried out through open incisions.
In clinical practice the choice of incision is usually based on surgical preference rather than any patient criteria. Surgically, ease of access, time to open and close the abdomen, and incidence of post operative complications (burst abdomen, wound infection, postoperative pulmonary complications, incisional hernias) are important. For the patient, however, pain and rapid return to normal function are important. Economically the duration of operation and duration of hospital stay determine cost with time to return to normal activity determining overall cost to the community. The recent interest in accelerated discharge after abdominal surgery highlights the importance of a comparison of incision types as there is an assumption that transverse incisions contribute to more rapid recovery (Kehlet 2003). Surgical practice has evolved to include a variety of incisions to gain access to the abdominal cavity. Midline and transverse (including oblique) incisions are the two commonest forms of incision used. There have been a number of studies proclaiming the benefits of each type of incision. There have however been few direct comparisons of the incisions, with many papers being retrospective, non‐randomised and therefore subject to considerable biases, or under powered to determine differences. There is a lack of heterogeneity within the better designed trials published in this arena with many studies measuring different outcomes in different ways. Attempts at combining the data from these studies in order to see if any incision confers any advantage have been made. In 2001 Grantacharov et al. (Grantcharov 2001) reviewed prospective (N=11) and retrospective (N=7) trials comparing incision types and reported an increased risk of both burst abdomen and incisional hernia following midline incision when compared with transverse incisions. This review included the retrospective data for the analyses which showed significant differences between the two incision types for post operative complications, but excluded them for other analyses. Burger et al. (Burger 2002) published a systemic review of prospective randomised trials of abdominal incision types and complications. In this review transverse, oblique, paramedian and midline incisions were included. They reported an increased rate of incisional hernia with a midline incision, but no differences in infection and dehiscence rates compared with any other incision. This increased incisional hernia rate was only sustained in the group of patients undergoing what they defined as "larger laparotomies". They concluded that the use of the midline incision should be restricted to operations in which unlimited access to the abdominal cavity is useful or necessary, recommending instead the paramedian incision.
The majority of data available compares transverse and oblique with midline incisions. This certainly reflects the commonest incision types for abdominal access utilised in the UK. Although paramedian incisions are still utilised by some, data comparing this incision type has not been included in this review. Instead appropriately designed randomised controlled trials comparing midline with transverse incisions has been included to see if any incision confers any advantage.
Objectives
The primary objectives were the focus of the trials in the literature and concerned factors regarding patient recovery. These factors include; Analgesic requirements Pulmonary function Recovery times (including hospital stay and time to return to work) Complications both early (wound dehiscence with or without evisceration, wound infection and pulmonary complications) and late (incisional hernia)
Secondary objectives concerned comparison of more subjective issues. These factors include; Operator convenience (including time to open and close the wound) Wound cosmesis Quality of life issues
Methods
Criteria for considering studies for this review
Types of studies
Studies in which participants have been prospectively randomised to a midline or a transverse/oblique incision for abdominal surgery were the focus of this study. The following inclusion and exclusion criteria were used;
Inclusion criteria Randomised controlled trials Trials comparing transverse or oblique with midline laparotomy for abdominal surgery Transperitoneal approach
Exclusion criteria Paediatric cases Caesarian sections Retroperitoneal approach
Types of participants
Patients undergoing all open abdominal incisions except caesarian section were included. The participants included all participants undergoing elective surgery as well as those undergoing emergency surgery and abdominal trauma. These participants are at potentially increased risk of wound infections and pulmonary complications but this was taken into account and the data for non‐elective surgery analysed separately.
Types of interventions
Studies that compared transverse and midline incisions were included in this review. Other longitudinal incisions include paramedian incisions. These can be conventional (medial) or lateral paramedian techniques. There are interesting data comparing paramedian techniques with transverse incisions (Ellis 1984,Halasz 1963). These data are not included in the analysis as paramedian incisions are not within the remit of this comparison.
Transverse incisions include true transverse or oblique (subcostal) muscle splitting and muscle cutting techniques and may be bilateral or unilateral. These variations may confound any analysis (Burger 2002) and have been taken into account by analysing subgroups according to the purpose of the operation. Diathermy skin incision compared with scalpel incision may reduce post‐operative pain and enhance recovery (Kearns 2001). Other factors may influence the incidence of complications (particularly wound dehiscence and incisional hernia) and these include the suture technique and suture type (Stone 1983). The importance of these variations in the overall evaluation of incision type has been discussed. In addition, their relevance for inclusion in updates of the current review has been considered.
Types of outcome measures
The primary and secondary outcome measures have been highlighted in the objectives section. Many of the endpoints with regard to analgesia use and pulmonary function are difficult to compare between studies since different scales, tests and timings of the tests are used in each of the studies in which they are employed. Therefore only those tests and those data which are common to more than one study have been analysed. Pulmonary complications were considered only if there was clinical or radiological evidence (pneumonia or atelectasis) requiring medical intervention. Wound infection was considered only if there was purulent discharge from the wound. Incisional hernia was considered absent only if there has been at least one year follow‐up.
Many of the secondary outcome measures were not uniformly available for most studies. The importance of these parameters in the overall evaluation of incision type is discussed. In addition, their relevance for inclusion in updates of the current review have been considered.
Search methods for identification of studies
See: Cochrane Colorectal Cancer Group search strategy
MEDLINE, the Cochrane Library and EMBASE were searched from 1966 to November 2010 for all randomised trials using combinations of the following: 'abdominal', 'incisions', 'horizontal', 'transverse', 'vertical', 'midline', 'laparotomy'. English language was not a restriction in the search. The list of cited references in all included reports also were used to find additional comparative studies.
For the most recent update the following search strategy was used for MEDLINE;
#1 exp Laparotomy/
#2 laparotom*.mp.
#3 exp Abdomen/su [Surgery]
#4 (abdom* and surger*).mp.
#5 1 or 2 or 3 or 4
#6 (horizont* or transvers* or vertic* or midline*).mp.
#7 incision*.mp.
#8 5 and 6 and 7
#9 exp Cesarean Section/
#10 8 not 9
#11 randomised controlled trial.pt.
#12 controlled clinical trial.pt.
#13 randomized.ab.
#14 placebo.ab.
#15 clinical trial.sh.
#16 randomly.ab.
#17 trial.ti.
#18 11 or 12 or 13 or 14 or 15 or 16 or 17
#19 humans.sh.
#20 18 and 19
#21 10 and 20
#22 limit 21 to yr="2007 ‐Current"
For the most recent update the following search strategy was used for EMBASE;
#1 exp LAPAROTOMY/
#2 laparotom*.mp.
#3 exp abdominal surgery/
#4 (abdom* and surger*).mp.
#5 1 or 2 or 3 or 4
#6 exp INCISION/
#7 incision*.mp.
#8 (horizont* or transvers* or vertic* or midline*).mp.
#9 6 or 7
#10 5 and 8 and 9
#11 randomised controlled trial/
#12 randomizations/
#13 controlled study/
#14 multicenter study/
#15 phase 3 clinical trial/
#16 phase 4 clinical trial/
#17 double blind procedure/
#18 single blind procedure/
#19 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).ti,ab.
#20 (random* or cross* over* or factorial* or placebo* or volunteer*).ti,ab.
#21 16 or 13 or 17 or 19 or 12 or 18 or 14 or 11 or 20 or 15
#22 "human*".ti,ab.
#23 (animal* or nonhuman*).ti,ab.
#24 23 and 22
#25 23 not 24
#26 21 not 25
#27 10 and 26
#28 exp cesarean section/
#29 27 not 28
#30 limit 29 to yr="2007 ‐Current"
For the most recent update the following search strategy was used for the Cochrane library;
#1 MeSH descriptor Laparotomy explode all trees
#2 (laparotom*):ti,ab,kw
#3 MeSH descriptor Abdomen explode all trees
#4 (abdom*):ti,ab,kw
#5 (surger*):ti,ab,kw
#6 (#4 AND #5)
#7 (#1 OR #2 OR #3 OR #6)
#8 incision*
#9 horizont* or transvers* or vertic* or midline*
#10 (#7 AND #8 AND #9)
#11 MeSH descriptor Cesarean Section explode all trees
#12 (#10 AND NOT #11)
#13 (#12), from 2007 to 2010
In addition proceedings of relevant meetings were screened for presentations not yet in print, focusing on the last three years. Such meetings included the annual meetings of the Association of Coloproctology of Great Britain and Ireland, European Association of Coloproctology, American Society of Colon & Rectal Surgeons, Association of Surgeons of Great Britain and Ireland, Association of Vascular Surgeons, Royal Society of Medicine (coloproctology section), European Council of Coloproctology. Authors of some published reports were contacted, querying their awareness of ongoing studies.
Data collection and analysis
Trials selection Two reviewers (SB, Peter Goodfellow) examined all the citations and abstracts derived from the electronic searches up to 2008. Jim Tiernan (JT) replaced Peter Goodfellow for this update. Reports of potentially relevant trials were retrieved in full. The reviewers independently applied the selection criteria to trials reports. Reviewers were not blind to the names of authors, institutions or journals. Any disagreements were resolved by discussion.
Quality assessment The methodological quality of identified trials were assessed independently by the two reviewers taking into account the quality of random allocation concealment and the description of dropouts and withdrawals, as well as blinding of the patients and careers to the intervention. Any disagreements were resolved by discussion. Studies were excluded if they were not randomised controlled trials in adults. The excluded studies and the reasons for their exclusion are summarised in the Table of Excluded Studies.
Data extraction Data extraction from the included trial was undertaken independently by the two reviewers. Only published data have been used. Data were processed as described in the Cochrane Collaboration Handbook (Higgins 2008). Any difference of opinion was resolved by discussion between the reviewers. Some data required extraction from figures in the publications. An attempt was made to get missing information from the trial's authors.
Analysis Data were analysed using the RevMan Analyses statistical programme in Review Manager 5.
Odds ratios and 95% confidence intervals were calculated for dichotomous outcomes using the Mantel‐Haenszel method and a fixed effect model. Continuous variables were analysed using fixed effect meta‐analyses of (weighted) mean differences. Continuous variables were processed using mean and standard deviation values. When only means and ranges were available an estimate of the standard deviation was calculated from the range (range X 0.95/4).
Subgroup analysis A subgroup analysis was performed to determine whether the outcomes of length of operation and hospital stay differ according to the type of operation. In addition, the effect of elective and emergency procedures on outcomes was analysed
Sensitivity analysis A sensitivity analysis was performed for certain outcomes (Wound infection, hospital stay, incisional hernia rate and length of operation) in order to test the effect of removing extraordinary studies which differ in some way from the main body of evidence.
Results
Description of studies
Nineteen randomised controlled trials met the inclusion criteria for this review. One trial (Lindell 1976) although meeting the criteria for a part of the trial, had combined the randomised section of the trial with a non randomised series. It was not possible to obtain the raw data and subsequently separate the randomised patients. This trial containing 19 patients was therefore excluded. The total number of patients encompassed by these 19 RCTs was 3464.
Four trials included emergency surgery in the patient data (Garcia‐Valdecasas 1988; Greenall 1980; Greenall 1980a; Stone 1983). In 2 trials the emergency procedure data could not be separated from the elective data (Garcia‐Valdecasas 1988; Greenall 1980). One trial consisted of only emergency procedures (Stone 1983).
Risk of bias in included studies
A power calculation was carried out in only one study (Seiler 2009 ). This was the only study that mentioned 'intention to treat'. Eight studies gave details with regard to withdrawals (Halm 2009; Sehnal 2008; Greenall 1980; Greenall 1980a; Lindgren 2001; Garcia‐Valdecasas 1988; Inaba 2004; Armstrong 1990). In one paper (Seiler 2009) the concept of intention to treat and equivalence was approached in detail. A complex decision tree was created considering 3 populations based on the variance from protocol, particularly regarding analgesic requirements. The population utilised for each outcome measure is discussed in the results.
It is unclear in most studies as to how the patient was randomised. In eight studies the randomisation process was not described. In the remaining studies randomisation was by sealed envelope in seven (Brown 2004; Greenall 1980; Greenall 1980a; Lindgren 2001; Fassiadis 2005; Halm 2009; Seiler 2009), by selection of random digits in one (Stone 1983), by computer generated random numbers in 2 (Salonia 2005; Proske 2005) and by 'block randomisation' in the other (Seenu 1994). Even in those trials that detail the method of randomisation, only two studies gives information that allocation was not known by the operator until at least the point of allocation (Lindgren 2001; Seiler 2009) suggesting potential for selection bias (Figure 1; Figure 2).
1.
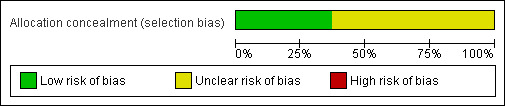
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The process of blinding the patient, the carers and the outcome assessor is obviously difficult in this situation. Nevertheless an attempt was made in two studies (Brown 2004; Seiler 2009). In both studies, the patient, the nursing staff and the medical staff caring for the patient as well as the patient were blinded by the use of strategically placed dressings that were consistent for the two groups of patients and that remained on the patient either for the duration of the hospital stay (Brown 2004) or until the primary end point assessment day (Seiler 2009). These studies aside the results of the other trials, particularly in relation to analgesia use and hospital stay all potentially suffer from substantial performance and detection bias.
Effects of interventions
There were 12 studies that investigated analgesic use. The method of assessment varied from visual analogue scales through to amount of opiate given, proportion of patients reporting pain or number of doses given over a period varying from 24 hours through to 7 days. This makes comparison difficult particularly as the type of procedure also varied. The influence of ward practice and local protocols on the delivery of analgesia as well as the expectations of the patient and the influence of ward staff on that expectation make it essential that carers (and indeed patients) are blinded to the intervention. The variability of analgesia use as well as patient perception of pain are illustrated in two studies that abandoned analogue assessment of pain as part of the trial due to the inconsistent results (Greenall 1980a; Lacy 1994). This element of detection bias seriously weakens the confidence in the trials without blinding of the carer, particularly those that involve 'on demand' analgesia delivered by the carers (Lacy 1994; Ali 1979; Garcia‐Valdecasas 1988; Salonia 2005; Halm 2009). One trial suggested analgesia was given 'on demand' by the carers but that the carers were blinded to the type of incision (Inaba 2004). One trial gave standard analgesia for all and gave no details of extra analgesia given 'on demand' (Proske 2005). Of the 4 remaining trials, 3 (Brown 2004; Armstrong 1990; Seiler 2009) state analgesia was given via a patient controlled device (in the per protocol population for the Seiler 2009 trial). This removes some of the influence of the carers on patient analgesic use but not all. Ward staff may encourage patients to press the patient controlled device and indeed may even press the device themselves. The trial by Lindgren 2001 included a visual analogue assessment with patients receiving strong opioid analgesia only if a score of three or above was recorded. However, this subjective assessment was carried out by someone not blinded to the intervention, again introducing bias. Only two trials state carers and patients were blinded to the intervention (Brown 2004; Seiler 2009).
Bearing in mind the clinical and methodological diversity and the potential for bias it is possible to combine data on the total opioid analgesic delivered to the patient from all studies except Greenall 1980a, Inaba 2004, Proske 2005, Halm 2009 and Salonia 2005. This requires some assumptions including conversion of merepidine (pethidine), piritramide and ketobemidon into dose equivalents of morphine (1mg merepidine=5mg morphine, 1mg of piritramide=0.65mg morphine, 1mg ketobemidon=1mg morphine) and multiplication of dose per kg body weight by mean body weight if given or 70kg if not given. The combined data shows a significant result in favour of the transverse incision (comparison data table 1,1 weighted mean difference ‐16.86; 95% confidence interval ‐20.32 to ‐13.39). However, there is significant statistical heterogeneity (p<0.00001). In an attempt to reduce the methodological diversity 5 trials were excluded from the analysis. The Ali 1979 trial and the Lacy 1994 trial were excluded as they recorded data on analgesic use for the first 72 hours only. Similarly Armstrong 1990's trial gave data only on the first 24 hours before the patient controlled device was taken down and Seiler 2009 recorded data for a 24 hour period only. Data for all opioid analgesia given in these trials may not therefore be included. The Lindgren 2001 trial was excluded because of the significantly different method of analgesia delivery mentioned previously. Combination of the 2 remaining trials that give data for total analgesic use during the hospital stay (Brown 2004; Garcia‐Valdecasas 1988) reduces statistical heterogeneity and also favours a transverse incision (CDT 1,2 WMD(fixed) 95% CI ‐6.28 [‐12.57,0.00]). However, it should be highlighted that the Garcia‐Valdecasas trial included 22 (out of 129) emergency procedures.
Another analgesic comparison that was possible was the VAS score on the first or second post‐operative day. Data on this parameter was available from 4 trials (Lindgren 2001; Proske 2005; Salonia 2005; Seiler 2009). Data was converted to a score of 0‐10. No difference was seen (CDT 1,3 WMD(fixed) 95% CI ‐0.03 [‐0.18,0.12]). Again heterogeneity was highly significant.
Ten trials focused on the effect of abdominal incisions on postoperative pulmonary function (Ali 1979; Armstrong 1990; Becquemin 1985; Garcia‐Valdecasas 1988;Lacy 1994; Lindgren 2001; Massucci 1989; Proske 2005; Salonia 2005; Seiler 2009). The methods of assessment included spirometry (FEV1, VC), arterial blood gas analysis and oscillography (Mean inspiratory and mean expiratory pressures (MIP, MEP) and thoraco‐abdominal respiratory synchronism). The timings of these assessments varied with each study from one assessment on the first post‐operative day through to assessments every day up to 12 days after the operation. Seiler 2009 only gave information on pulmonary function 1 year after surgery and data could therefore not be pooled. This coupled with the varied reporting of the original data makes pooling of the data difficult. One study (Massucci 1989) gave insufficient data for analysis. Four studies failed to show any difference in pulmonary function with incision type (Armstrong 1990; Lacy 1994; Lindgren 2001; Salonia 2005). Of the five studies that showed a significant difference all favoured the transverse approach. Pooling of the data was carried out for spirometry for the first day after the operation and for day 7 or the day nearest day 7 after the operation. Two studies (Massucci 1989; Garcia‐Valdecasas 1988) gave insufficient data for analysis and the authors were not contactable. Pooling the other studies showed that a transverse incision had significantly less effect on vital capacity and FEV1 (CDT 2,1‐2,4). Only one study assessed pulmonary function for those with and without chronic obstructive airways disease, showing a marked effect on spirometry with incision for the chronic obstructive group but no difference for the 'normal' group (Lacy 1994). All the studies analysed included elective patients only.
Complications of surgery included pulmonary disorders. These were assessed in 12trials. However, in one (Ali 1979) it was impossible to separate the data from the unrandomised data in another part of the study. Four trials gave a detailed definition of a pulmonary complication (Becquemin 1985; Garcia‐Valdecasas 1988; Greenall 1980a; Lacy 1994) while others gave data that included pneumonia and atelectasis (Brown 2004; Lindgren 2001; Seenu 1994). In four trials pneumonia was not defined (Inaba 2004; Halm 2009; Seiler 2009; Sehnal 2008). Combining all 11 studies and including all pulmonary complications there was no significant difference between the two interventions (CDT 3.1OR(fixed) 95% CI 1.01[0.76,1.34]. Two studies (Greenall 1980a; Garcia‐Valdecasas 1988) included emergency and elective patients. If emergency patients were excluded from the data there was still no difference in pulmonary complications (CDT 3,2 OR(fixed) 95% CI 0.98 [0.72,1.34].
Wound infection rates were examined in 10 trials (Garcia‐Valdecasas 1988; Greenall 1980; Halm 2009; Lindgren 2001; Seenu 1994; Sehnal 2008; Stone 1983; Seiler 2009; Proske 2005; Inaba 2004). In the Greenall 1980 study the infection rates were very high and possibly more related to the antibiotic prophylaxis regime. Antibiotics were not given at all in some patients (even those considered high risk for infection) and never included metronidazole. Whether this study with quality issues is included or excluded in a sensitivity analysis the infection rate does not significantly differ between interventions (CDT 4,1‐2 OR (fixed) 95% CI 1.10 [0.76, 1.34], 1.04[0.72,1.51]). If emergency patients were excluded from the analysis there was still no difference in wound infection rates (CDT 4,3 OR(fixed) 95% CI 1.09 [0.68,1.76].
Evisceration is a rare occurrence with any incision. Only two randomised studies record any individual occurrence (Greenall 1980; Seiler 2009) in 2 patients with a midline incision and 1 patient with a transverse incision suggesting the incidence in the combined series is less than 0.1%. Five studies comment on subcutaneous wound disruption or wound healing by secondary intention (Garcia‐Valdecasas 1988; Lindgren 2001; Inaba 2004; Proske 2005; Sehnal 2008). Again the recorded incidence is too low to comment on whether it is more common with a particular intervention. One further study (Stone 1983) combines the incidence of wound dehiscence, with or without evisceration, occurring in 7 patients in the midline group and 3 in the transverse group. The separated data is not available but the authors comment that no significant difference was seen between the groups. If the incidence of wound dehiscence with or without evisceration is combined there is a trend towards a lower incidence in the transverse group (CDT 5,1 OR(fixed) 95% CI 0.55 [0.25,1.20]). There was not enough data to analyse elective patients as a sub‐group.
Seven trials comment on follow up for incisional hernias (Greenall 1980; Garcia‐Valdecasas 1988; Halm 2009; Inaba 2004; Salonia 2005; Seiler 2009; Fassiadis 2005). There was a significant difference seen in favour of the transverse incision (CDT 6,1 OR(fixed) 95% CI 0.49 [0.26, 1.72]). Follow up ranged from 4 months to 4.4 years but in all studies except three (Fassiadis 2005; Halm 2009; Seiler 2009) the follow up was less than 1 year. This is not long enough follow‐up to pick up all incisional hernias (Hoer 2002). However, the potential for some incisional hernias to not be detected does not explain the significant heterogeneity which remains the same even after exclusion of studies with a short follow up.
Hospital stay was assessed in 10 trials (Armstrong 1990; Brown 2004; Garcia‐Valdecasas 1988; Halm 2009; Lacy 1994; Lindgren 2001; Seenu 1994; Sehnal 2008; Seiler 2009; Proske 2005). Due to the heterogeneity of the surgical procedures carried out it is not appropriate to combine all of the trials. However, four trials looked at cholecystectomy (Armstrong 1990; Garcia‐Valdecasas 1988; Halm 2009; Seenu 1994) One trial (Halm 2009) provided data that was unable to be pooled. This study reported no difference between incision groups. Pooling the other data there was a significant reduction in hospital stay for the transverse incision group (CDT 7,1 WMD(fixed) 95% CI ‐1.35[‐1.57,‐1.14]). However, the study with the greatest influence over hospital stay (Seenu 1994) ) has some statistical issues, the main being lack of data requiring a crude estimate of standard deviation. There was also significant heterogeneity if this study was included (p=0.02). If the Seenu 1994 trial is excluded in a sensitivity analysis no difference was seen (CDT 7,2 WMD(fixed) 95% CI 0.32 [‐0.97, 1.61]). It is not possible to analyse the elective patients separately, although only 22 of the 369 patients with data on hospital stay were emergency procedures.
Combining the 2 trials on right hemicolectomy (Brown 2004; Lindgren 2001) showed no difference in hospital stay (CDT 7.3, WMD(fixed) 95% CI ‐0.69[‐2.03, 0.65]). Combining the two trials on patients undergoing surgery for pancreatic and gastric conditions (Proske 2005, Seiler 2009) was not possible as the Seiler 2009 trial data could not be separated from other data. Individually, no study showed any difference between incisions. The remaining trial participants included those undergoing abdominal aortic surgery (Lacy 1994) and those undergoing hysterectomy and bilateral adnexectomy for patients undergoing gender reassignment (Sehnal 2008). The Seiler 2009 study also included patients undergoing a heterogeneous group of colonic procedures. These trials also showed no significant difference in hospital stay. Like analgesic requirements, hospital stay is heavily influenced by local practice and the absence of blinding of the carers in most trials mean that the results may not be valid and may be an alternative explanation to the statistical heterogeneity.
Data on time taken to complete the operation was given in 12 trials. Again the heterogeneity of the type of operation make comparison difficult but it was possible to combine the trials looking at the same participants with 4 trials looking at patients undergoing cholecystectomy (Ali 1979; Armstrong 1990; Halm 2009; Seenu 1994), two trials looking at patients undergoing right hemicolectomy (Brown 2004; Lindgren 2001) and three trials looking at gastric or pancreatic surgery (Proske 2005; Inaba 2004; Seiler 2009). Of the remaining trials one looked at patients undergoing abdominal aortic surgery (Lacy 1994), one at hysterectomy and bilateral adnexectomy for patients undergoing gender reassignment (Sehnal 2008) and one at patients undergoing surgery for prostate cancer (Salonia 2005). The Seiler 2009 study also included patients undergoing a heterogeneous group of colonic procedures.
Combining the cholecystectomy trials showed no difference in operation time (CDT 8,1 WMD(fixed) 95% CI ‐2.52[‐6.81, 1.78]). Again there were statistical issues with the Seenu 1994 trial. There was also significant heterogeneity if this study was included (p=0.06). Even if this study was excluded in a sensitivity analysis no difference was seen (CDT 8,2 WMD(fixed) 95% CI 6.38[‐2.82, 15.57]). There was no difference in operation time with incision for a right hemicolectomy (CDT 8,3 WMD(fixed) 95% CI 6.18 [‐5.78, 18.14]). Of the trials on gastric or pancreatic surgery all gave data on opening and closing times and one gave data for the whole procedure (Seiler 2009). Unfortunately insufficient data was available to pool the results although all trials suggested the midline procedure took significantly less time to gain abdominal access (Proske 2005; Inaba 2004; Seiler 2009) and to close (Inaba 2004; Seiler 2009). No difference was seen with the remaining three trials for aortic, gynaecological and prostate surgery.
Incision length was investigated in 6 trials (Brown 2004; Halm 2009; Inaba 2004; Proske 2005; Salonia 2005; Seenu 1994). For the Seenu 1994 paper, although the incision was larger in the midline group insufficient data was available for analysis. For all of the other trials the midline incision was significantly longer in two (Brown 2004; Halm 2009) and shorter in 2 (Inaba 2004; Proske 2005). Due to the heterogeneity of the operations it is only reasonable to combine the results of 3 trials requiring upper gastrointestinal access (Halm 2009; Inaba 2004; Proske 2005). This analysis showed the midline incision was significantly shorter (CDT 9,1 WMD(fixed) 95% CI 3.77 [3.67, 3.87]). However, the highly significant heterogeneity seen may be reflective if the slightly different surgical indications particularly pancreatic surgery for the Proske 2005 trial.
A number of trials concluded that both midline and transverse incisions provided equal exposure to most intra‐abdominal structures but this convenience of incision was only subjectively assessed in one trial (Becquemin 1985). In this trial the convenience of incision was found to be poor in only one patient (from the transverse intervention group) of the 25 patients studied.
Other outcomes examined in individual studies included cosmetic score or assessment (Proske 2005; Halm 2009). Different assessment criteria meant that data could not be combined but both studies significantly favoured the transverse incision even though this was significantly longer.
Discussion
The choice of incision for abdominal access is controversial. Although many randomised controlled trials have favoured a transverse or oblique incision over a midline incision in terms of complication rates and recovery, the individual study results are by no means universal. When the data from all of these trials is combined there is a suggestion that a transverse incision may result in less pain. However, the lack of blinding in almost all trials is very likely to have introduced substantial bias. This coupled with the marked clinical and methodological diversity of the individual papers and the fact that there is no subjective evidence of less pain (according to the VAS assessment) suggests that these results should be treated with caution.
Caution when comparing outcomes should also be reinforced by the heterogeneity of the operations and techniques utilised in opening and closing abdominal wounds. Some of these techniques (for instance muscle cutting and bilateral transverse or oblique incisions) are likely to cause more pain and pulmonary compromise than others (muscle splitting and unilateral incisions). Unfortunately, data on access techniques is minimal in most of the included studies (with notable recent exceptions).
The effects on pulmonary function favouring a transverse approach appear to be real. Further data available from more recent trials add to the evidence, with all studies that analysed pulmonary function showing the advantage of a transverse incision approach. However, the clinical consequences of these statistically significant analgesic and pulmonary function differences for incision type were not seen. There were no differences in pulmonary complications or hospital stay with each incision. This perhaps draws into question whether the analgesic and pulmonary function differences are relevant. Indeed, spirometry is only a surrogate marker of pulmonary 'well being' that may not be related to the more important clinical outcomes.
The initial review suggested that the other short and long term complications of surgery were no different between incisions. The inclusion of some more recent trials may now confirm the cadaver and animal trials suggesting a transverse incision is more resistant to rupture (Grantcharov 2001). The updated review continues to illustrate a trend to a lower rate of wound dehiscence with transverse incisions. Additionally, bearing in mind the data from Hoer 2002 et al suggesting that it takes more than 2 years for 75% of incisional hernias to occur, the review now includes three trials with longer (but not necessarily sufficient) follow up (Fassiadis 2005; Halm 2009; Seiler 2009).
Cosmesis data is still lacking, but has been examined in 2 studies (Proske 2005; Halm 2009) both suggesting a significant difference in favour of a transverse incision despite a significantly longer incision. It is unfortunate the data cannot be combined to provide further evidence of this subjective outcome. Other outcome measures were similar for each incision. Although the data for convenience of incision is sparse, either incision is likely to give adequate exposure provided it is large enough. . Overall operating time seems to be minimally affected by incision type and the intervention giving the smallest incision seems to vary dependent on the indication for surgery.
To summarise, a transverse incision appears to affect pulmonary function less than a midline incision, although this does not appear to increase the likelihood of pulmonary complications or other recovery parameters. The wound dehiscence and incisional hernia rate appears to be less with a transverse incision and the cosmetic result may be better. Although some data would suggest a transverse incision is less painful, there are too many variables to be sure. All other outcome parameters appear no different.
Authors' conclusions
Implications for practice.
Because of many similar clinical outcomes, the use of a transverse or midline incision remains the choice of the individual surgeon. A midline incision is still the incision of choice in the emergency situation, allowing rapid entry into the peritoneal cavity and access to all organs. It is also the incision of choice in patients where there is an increased probability of re‐laparotomy (Crohn's disease) or where a potential stoma site would be compromised by a transverse incision in a patient who is likely to need one. However, the possible increased pain and compromise on pulmonary function with a midline incision may prompt the operating surgeon to use a transverse incision in high risk patients particularly obese patients or those chronic obstructive airways disease. The increased incidence of wound dehiscence and incisional hernia may also influence the surgeon to favour a transverse incision.
With the increasing use of laparoscopic techniques in all aspects of abdominal surgery, the need for a review on open access incisions could be questioned. However, each update has included several new studies (3 in the last update) and over 1000 more participants, continuing to give testament to the relevance of such a review.
Implications for research.
Larger studies are required that concentrate on specific procedures and specific opening and closing techniques. It is, however, very difficult to standardise these parameters, particularly with the trend of more minimal access techniques.
The effect of incision on patients with chronic obstructive airways disease has not been studied fully. These are the patients most likely to develop respiratory compromise after abdominal surgery and indeed wound rupture and it may be in this group that a large difference in complications is seen.
Pulmonary compromise is undoubtedly related to the cranial extent of the incision with upper midline incisions for gastric surgery, for instance, affecting respiratory function more significantly than incisions for pelvic surgery. This cranial extent of the incision may be an alternative explanation to the heterogeneity seen in some comparisons. This was suggested as a relevant topic for future work in the earlier review particularly if a transverse incision allows a significantly lower incision and a more effective block with the common use of epidurals for pain relief. Such a study has now been carried out (Mimica 2007) and adds to the evidence that upper abdominal incisions are more painful and affect respiratory function more than lower abdominal incisions.
Cosmesis is almost universally quoted in the literature as being an important advantage of minimal access surgery. This outcome is particularly important to the younger body image conscious patient. There has been additional comparison data added from a recent trial but the data still remains limited regarding cosmesis and the transverse or midline incision. .
What's new
| Date | Event | Description |
|---|---|---|
| 3 February 2011 | New search has been performed | Updated after new literature search |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 1 February 2008 | New citation required and conclusions have changed | Substantive amendment |
| 1 January 2008 | Amended | Converted to new review format. |
Acknowledgements
Thanks to Rosie Taylor at the Sheffield University Statistics Department. And Peter B Goodfellow for co work on the protocol and first versions of this review.
Data and analyses
Comparison 1. Analgesic use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recorded total analgesic use (mg morphine equivalent) | 7 | 460 | Mean Difference (IV, Fixed, 95% CI) | ‐16.86 [‐20.32, ‐13.39] |
| 2 Analgesic use (mg morphine equivalent) for total hospital stay | 2 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐6.29 [‐12.57, ‐0.01] |
| 3 VAS scores on the first/second post‐operative day | 4 | 337 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.18, 0.12] |
1.1. Analysis.
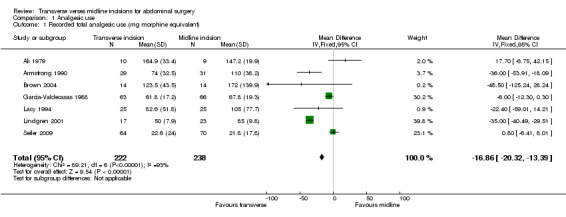
Comparison 1 Analgesic use, Outcome 1 Recorded total analgesic use (mg morphine equivalent).
1.2. Analysis.
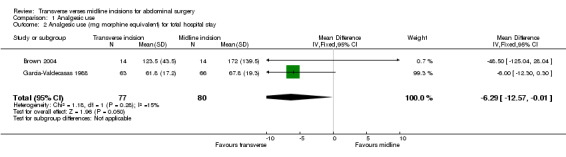
Comparison 1 Analgesic use, Outcome 2 Analgesic use (mg morphine equivalent) for total hospital stay.
1.3. Analysis.
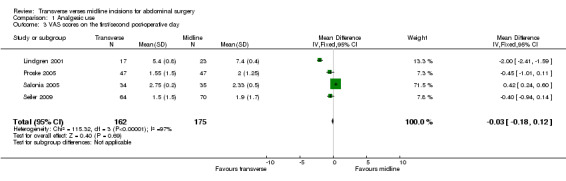
Comparison 1 Analgesic use, Outcome 3 VAS scores on the first/second post‐operative day.
Comparison 2. pulmonary function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percentage change in vital capacity day 1 post‐operative | 5 | 263 | Mean Difference (IV, Fixed, 95% CI) | 8.08 [5.06, 11.10] |
| 2 Percentage change in vital capacity on day 7 | 5 | 210 | Mean Difference (IV, Fixed, 95% CI) | 7.10 [3.21, 10.99] |
| 3 Percentage change in FEV1 day 1 post‐operative | 4 | 244 | Mean Difference (IV, Fixed, 95% CI) | 7.27 [2.90, 11.64] |
| 4 Percentage change in FEV1 last day of measurement | 3 | 140 | Mean Difference (IV, Fixed, 95% CI) | 9.97 [3.46, 16.48] |
2.1. Analysis.
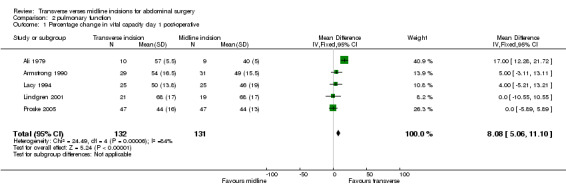
Comparison 2 pulmonary function, Outcome 1 Percentage change in vital capacity day 1 post‐operative.
2.2. Analysis.
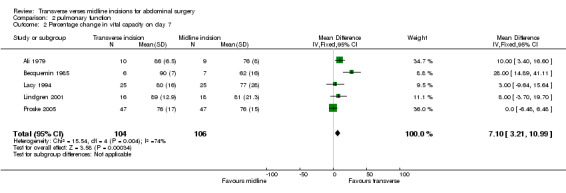
Comparison 2 pulmonary function, Outcome 2 Percentage change in vital capacity on day 7.
2.3. Analysis.
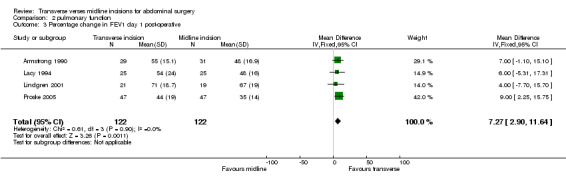
Comparison 2 pulmonary function, Outcome 3 Percentage change in FEV1 day 1 post‐operative.
2.4. Analysis.
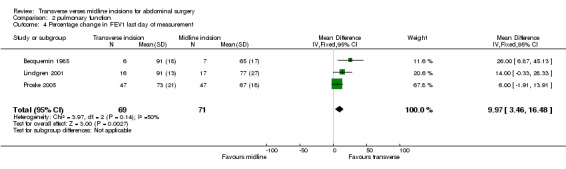
Comparison 2 pulmonary function, Outcome 4 Percentage change in FEV1 last day of measurement.
Comparison 3. pulmonary complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 pulmonary complications | 11 | 1812 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| 2 Pulmonary complications for elective patients | 10 | 1554 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.72, 1.34] |
3.1. Analysis.
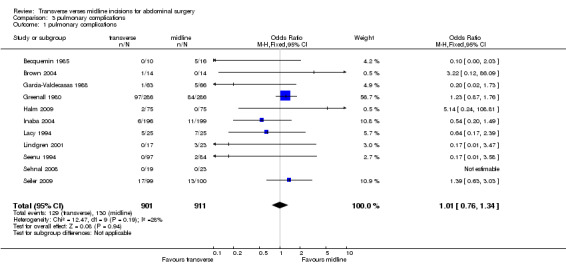
Comparison 3 pulmonary complications, Outcome 1 pulmonary complications.
3.2. Analysis.
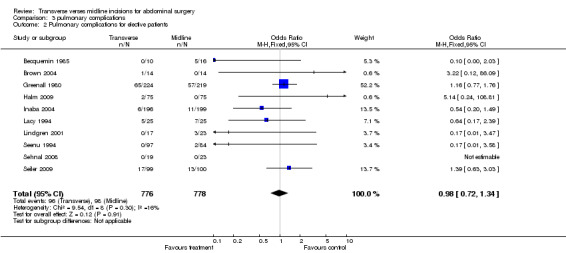
Comparison 3 pulmonary complications, Outcome 2 Pulmonary complications for elective patients.
Comparison 4. wound infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 wound infection | 10 | 2326 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.84, 1.43] |
| 2 Wound infection excluding Greenall study | 9 | 1779 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.51] |
| 3 Wound infection in elective patients | 7 | 1101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.68, 1.76] |
4.1. Analysis.
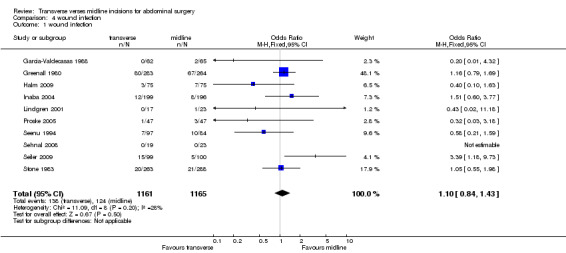
Comparison 4 wound infection, Outcome 1 wound infection.
4.2. Analysis.
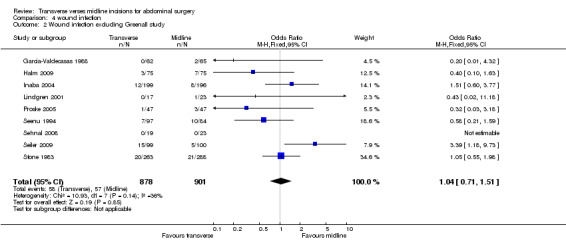
Comparison 4 wound infection, Outcome 2 Wound infection excluding Greenall study.
4.3. Analysis.
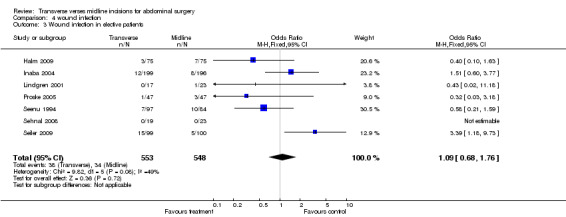
Comparison 4 wound infection, Outcome 3 Wound infection in elective patients.
Comparison 5. Wound dehiscence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 wound dehiscence with or without evisceration | 8 | 1793 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.20] |
5.1. Analysis.
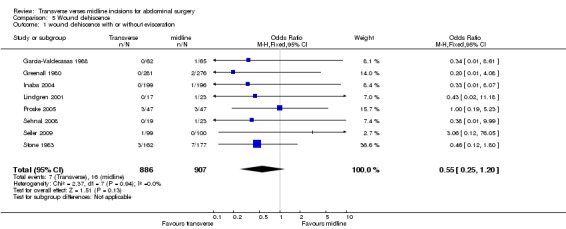
Comparison 5 Wound dehiscence, Outcome 1 wound dehiscence with or without evisceration.
Comparison 6. Incisional hernia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 incisional hernia rate | 7 | 1366 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.79] |
| 2 Incisional hernia rate (studies with >1 year follow up) | 3 | 308 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.14, 0.62] |
6.1. Analysis.
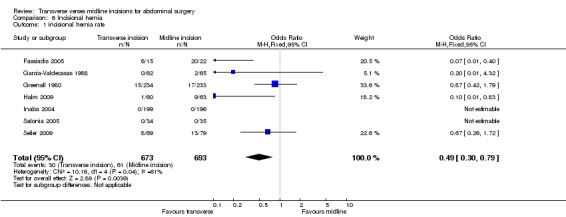
Comparison 6 Incisional hernia, Outcome 1 incisional hernia rate.
6.2. Analysis.
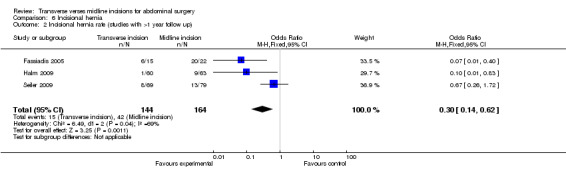
Comparison 6 Incisional hernia, Outcome 2 Incisional hernia rate (studies with >1 year follow up).
Comparison 7. hospital stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital stay for gallbladder surgery | 3 | 369 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐1.57, ‐1.14] |
| 2 Hospital stay forgallbladder surgery (excluding Seenu trial) | 2 | 188 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.97, 1.61] |
| 3 Hospital stay for right hemicolectomy | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐2.03, 0.65] |
7.1. Analysis.
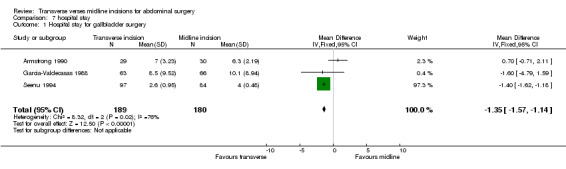
Comparison 7 hospital stay, Outcome 1 Hospital stay for gallbladder surgery.
7.2. Analysis.
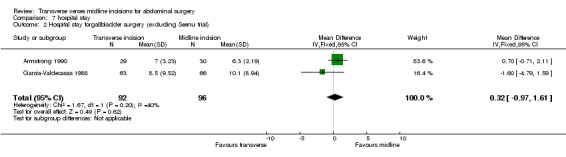
Comparison 7 hospital stay, Outcome 2 Hospital stay forgallbladder surgery (excluding Seenu trial).
7.3. Analysis.
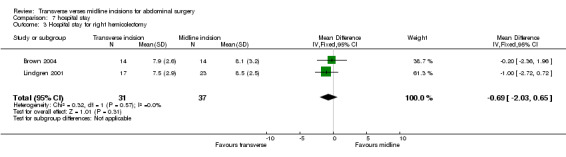
Comparison 7 hospital stay, Outcome 3 Hospital stay for right hemicolectomy.
Comparison 8. Operation time.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 operation time for gallbladder surgery | 3 | 260 | Mean Difference (IV, Fixed, 95% CI) | ‐2.52 [‐6.81, 1.78] |
| 2 Operation time for gallbladder sugery (excluding Seenu) | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | 6.38 [‐2.82, 15.57] |
| 3 Operation time for right hemicolectomy | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | 6.18 [‐5.78, 18.14] |
8.1. Analysis.
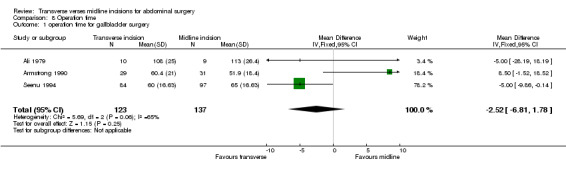
Comparison 8 Operation time, Outcome 1 operation time for gallbladder surgery.
8.2. Analysis.
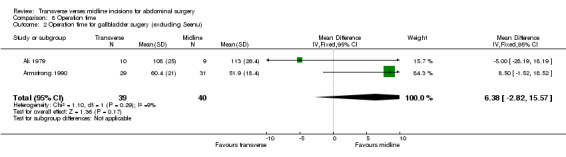
Comparison 8 Operation time, Outcome 2 Operation time for gallbladder sugery (excluding Seenu).
8.3. Analysis.
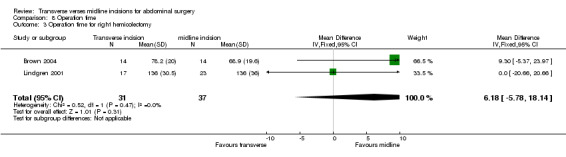
Comparison 8 Operation time, Outcome 3 Operation time for right hemicolectomy.
Comparison 9. Incision length.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of incision for upper abdominal surgery | 3 | 638 | Mean Difference (IV, Fixed, 95% CI) | 3.77 [3.67, 3.87] |
9.1. Analysis.
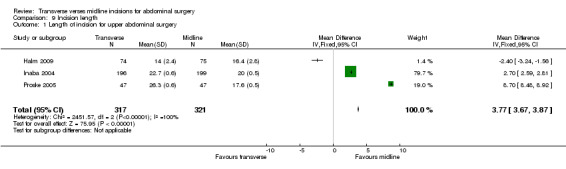
Comparison 9 Incision length, Outcome 1 Length of incision for upper abdominal surgery.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ali 1979.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing open elective cholecystectomy | |
| Interventions | Midline incision or right subcostal incision | |
| Outcomes | Pulmonary complications, spirometry, arterial p02 | |
| Notes | Pulmonary complications = >3 of :temp >38° C >48 hrs, clinical signs of pulmonary consolidation, abnormal CXR, >2+ pus with bacteria in 2 consecutive sputums | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Armstrong 1990.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing open elective cholecystectomy | |
| Interventions | Midline incision or transverse incision | |
| Outcomes | Spirometry, analgesia requirements, hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Becquemin 1985.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing aorta‐iliac vascular procedures | |
| Interventions | Midline incision or transverse incision | |
| Outcomes | Spirometry, pulmonary complications, surgical convenience | |
| Notes | Pulmonary complications defined as purulent sputum +/‐ fever +/‐ Xray evidence lasting >48 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Brown 2004.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing elective right hemicolectomy | |
| Interventions | Midline incision or right transverse (skin crease) incision | |
| Outcomes | Analgesia requirements, hospital stay, complications, length of incision | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Fassiadis 2005.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing elective abdominal aortic aneurysm repair | |
| Interventions | Midline incision or supra umbilical transverse incision | |
| Outcomes | Incisional hernia rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Garcia‐Valdecasas 1988.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing open elective and emergency cholecystectomy | |
| Interventions | Midline incision or right subcostal incision | |
| Outcomes | Pulmonary complications, spirometry, analgesia | |
| Notes | Pulmonary complications defined as symptoms and/or Xray evidence needing medical treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Greenall 1980.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing elective or emergency open laparotomy | |
| Interventions | Midline incision or transverse/oblique incision | |
| Outcomes | Blood loss, wound sepsis, burst abdomen, incisional hernia | |
| Notes | Wound sepsis defined as discharge of pus | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Greenall 1980a.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing elective or emergency open laparotomy | |
| Interventions | Midline incision or transverse/oblique incision | |
| Outcomes | Pulmonary complications | |
| Notes | Scoring system for definition and severity of pulmonary complication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Halm 2009.
| Methods | RCT(Randomised Controlled Trial) | |
| Participants | Female patients undergoing elective cholecystectomy (+/‐cholangiography +/‐ choledochostomy) via an open incision | |
| Interventions | Midline incision or transverse (muscle cutting) incision | |
| Outcomes | Pain and analgesics, wound sepsis, burst abdomen, incisional hernia, length of stay, length of incision and operation, cosmesis | |
| Notes | No complication definitions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A‐Adequate |
Inaba 2004.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing gastrectomy for gastric cancer | |
| Interventions | Upper midline incision or transverse incision (2cm below subcostal margin) | |
| Outcomes | Analgesia (doses) Complications Blood loss Surgical time Incision length | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Lacy 1994.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing elective abdominal aortic surgery | |
| Interventions | Midline incision or transverse incision | |
| Outcomes | Pulmonary complication, operating time, ICU, ventilation and hospital duration, analgesia requirements | |
| Notes | Pulmonary complication if 2 of Xray or clinical evidence or temperature >38° C. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lindgren 2001.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing elective right hemicolectomy for colorectal cancer | |
| Interventions | Midline incision or transverse incision | |
| Outcomes | Spirometry, analgesic requirements | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Massucci 1989.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing elective abdominal aortic surgery | |
| Interventions | Midline incision or supra umbilical transverse incision | |
| Outcomes | Spirometry, p02 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Proske 2005.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing elective major laparotomy for disorders of the pancreas or stomach | |
| Interventions | Midline incision or subcostal concave incision towards umbilicus | |
| Outcomes | Pulmonary function Pain Wound complications Operation time Hospital stay Mortality Incision length Cosmesis score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Salonia 2005.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing open radical retropubic prostatectomy | |
| Interventions | Midline incision or Pfannensteil transverse laparotomy | |
| Outcomes | Pain p02 Haemodynamics and biochemistry Complications Recovery parameters Surgical time Incision length | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Seenu 1994.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing open elective cholecystectomy | |
| Interventions | Midline incision or transverse incision | |
| Outcomes | Operating time, length of incision, hospital stay, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sehnal 2008.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing elective hysterectomy and bilateral adnexectomy for gender reassignment | |
| Interventions | Lower midline incision versus transverse (Pfannenstiel) incision | |
| Outcomes | Blood loss, length of operation, hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B‐ Unclear |
Seiler 2009.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing elective primary laparotomy for stomach, colonic and pancreatic procedures | |
| Interventions | Midline incision or transverse incision | |
| Outcomes | Pain intensity and analgesic use, early onset complications (burst abdomen, pulmonary complications, wound infections) and late onset complications (pulmonary function, incisional hernias), procedure time, length of hospital stay. | |
| Notes | No definition of complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A‐Adequate |
Stone 1983.
| Methods | RCT (Randomised Controlled Trial) | |
| Participants | Patients undergoing emergency laparotomy for abdominal trauma | |
| Interventions | Midline incision or transverse incision | |
| Outcomes | Wound infection rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ellis 1984 | Groups compared are transverse incision patients with paramedian incisions and median with paramedian incisions |
| Halasz 1963 | Groups compared are subcostal incision patients with paramedian incisions |
| Leohapensang 2005 | Groups compared were left retroperitoneal approach with transperitoneal midline approach |
| Lindell 1976 | Although 19 patients are randomised to subcostal and muscle splitting (transverse incisions) or midline incisions these are combined with a group of 25 non randomised patients. The raw data is not obtainable. |
| Mimica 2007 | Comparison of upper versus lower midline and transverse abdominal incisions |
Contributions of authors
Both reviewers were involved in the literature search, analysis and writing of this review.
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ali 1979 {published data only}
- Ali J, Ali Khan T. The comparative effects of muscle transsection and median upper abdominal incisions on postoperative pulmonary function. Surgery, Gynaecology and Obstetrics 1979;148:863‐66. [PubMed] [Google Scholar]
Armstrong 1990 {published data only}
- Armstrong PJ, Burgess RW. Choice of incision and pain following gallbladder surgery. Br J Surg 1990;77:746‐748. [DOI] [PubMed] [Google Scholar]
Becquemin 1985 {published data only}
- Becquemn J‐P, Piquet J, Becquemin M‐H, Melliere D, Harf A. Pulmonary function after transverse or midline incision in patients with obstructive pulmonary disease. Intensive Care Med 1985;11:247‐251. [DOI] [PubMed] [Google Scholar]
Brown 2004 {published data only}
- Brown SR, Goodfellow PB, Adam IJ, Shorthouse AJ. A prospective randomised comparison of right upper quadrant incision verses midline laparotomy for right hemicolectomy. Tech Coloproctol. 2004;8:815‐8. [DOI] [PubMed] [Google Scholar]
Fassiadis 2005 {published data only}
- Fassiadis N, Roidl M, Hennig M, South LM, Andrews SM. Randomised clinical trial of vertical or transverse laparotomy for abdominal aortic anuerysm repair. British Journal of Surgery 2005;92(10):1208‐1211.. [DOI] [PubMed] [Google Scholar]
Garcia‐Valdecasas 1988 {published data only}
- Garcia‐Valdecasas JC, Almenara R, Cabrer C, Lacy AM, Sust M, Taura P, Fuster J, Grande L, Pera M, Sentis J, Visa J. Subcostal incision verses midline laparotomy in gallstone surgery: a prospective and randomised trial. Br J Surg 1988;75:473‐475. [DOI] [PubMed] [Google Scholar]
Greenall 1980 {published data only}
- Greenall MJ, Evans M, Pollock AV. Midline or transverse laparotomy? A random controlled clinical trial. Part 1: Influence on healing. Br J Surg 1980;67:188‐190. [DOI] [PubMed] [Google Scholar]
Greenall 1980a {published data only}
- Greenall MJ, Evans M, Pollock AV. Midline or transverse laparotomy? A random controlled clinical trial.Part II. Influence on postoperative pulmonary complications.. Br J Surg 1980;67:191‐194. [DOI] [PubMed] [Google Scholar]
Halm 2009 {published data only}
- Halm JA, Lip H, Schmitz PI, Jeekel J. Incisional hernia after upper abdominal surgery: a randomised controlled trial of midline versus transverse incision.. Hernia 2009;13:275‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inaba 2004 {published data only}
- Inaba T, Okinaga K, Fukushima R, Iinuma H, Ogihara T, Ogawa F, Iwasaki K, Tanaka M, Yamada H. Prospective randomized study of two laparotomy incisions for gastrectomy: midline incision versus transverse incision. Gastric cancer 2004;7:167‐171. [DOI] [PubMed] [Google Scholar]
Lacy 1994 {published data only}
- Lacy PD, Burke PE, O'Regan M, Cross S, Sheehan SJ, Henir D, Colgan M_P, Moore DJ, Shanik GD. The comparison of type of incision for transperitoneal abdominal aortic surgery based on postoperative respiratory complications and morbidity. Eur J Vasc Surg 1994;8:52‐55. [DOI] [PubMed] [Google Scholar]
Lindgren 2001 {published data only}
- Lindgren PG, Nordgren SR Oresland T, Hulten L. Midline or transverse abdominal incision for right sided colon cancer‐a randomised trial. Colorectal Disease 2001;3:46‐50. [DOI] [PubMed] [Google Scholar]
Massucci 1989 {published data only}
- Massucci M, Lauri D, Faraglia V, Speziale F, Santis F, Taurino M, Dotta F, Galantino A, Guerricchio R, Fiorani P. Approach to the abdominal aorta: impairment of respiratory function after supraumbilical transverse and midline laparotomy. The Italian Journal of Surgical Sciences 1989;19(3):247‐253. [PubMed] [Google Scholar]
Proske 2005 {published data only}
- Proske JM, Zieren J, Muller JM. Transverse versus midline incision for upper abdominal surgery. Surgery Today 2005;35:117‐121. [DOI] [PubMed] [Google Scholar]
Salonia 2005 {published data only}
- Salonia A, Suardi N, Crescenti A, Zanni G, Fantini GV, Gallina A, Ghezzi M, Colombo R, Montorsi F, Rigatti P. Pfannensteil versus vertical laparotomy in patients undergoing radical retropubic prostatectomy with spinal anaesthesis: results of a prospective, randomized trial. European Urology 2005;47:202‐208. [DOI] [PubMed] [Google Scholar]
Seenu 1994 {published data only}
- Seenu V, Misra MC. Mini‐lap cholecystectomy‐an attractive alternative to conventional cholecystectomy. Tropical Gastroenterology 1994;15(1):29‐31. [PubMed] [Google Scholar]
Sehnal 2008 {published data only}
- B. Sehnal, O. Sottner, J. Zahumensky, P. Maresova, P. Holy, M. Halaska. Comparison of Three Hysterectomy Methods in a Set of Female to Male Transsexuals [Vergleich von drei Hysterektomiemethoden bei Frau‐zu‐Mann‐Transsexuellen]. The journal of sexual medicine 2008;68:625‐628. [Google Scholar]
Seiler 2009 {published data only}
- Seiler CM, Deckert A, Diener MK, Knaebel H‐P, Weigand MA, Victor N, Buchler MW. Midline versus transverse incision in major abdominal surgery. A randomized double‐blind equivalence trial.. Annals of Surgery 2009;249:913‐920. [DOI] [PubMed] [Google Scholar]
Stone 1983 {published data only}
- Stone HH, Hoefling SJ, Strom PR, Dunlop WE, Fabian TC. Abdominal incisions: transverse vs vertical placement and continuous vs interrupted closure. Southern Medical Journal 1983;76(6):1106‐1108. [PubMed] [Google Scholar]
References to studies excluded from this review
Ellis 1984 {published data only}
- Ellis H, Coleridge‐Smith PD, Joyce AD. Abdominal incisions‐ vertical or transverse. Postgraduate Medical Journal 1984;60:407‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halasz 1963 {published data only}
- Halasz NA, Calif T. Vertical vs horizontal laparotomies. Arch Surg 1964;88:911‐914. [DOI] [PubMed] [Google Scholar]
Leohapensang 2005 {published data only}
- Laohapensang K, Rerkasem K, Chotirosniramit N. Left retroperitoneal versus midline transperitoneal approach for abdominal aortic aneurysms (AAAs) repair.. Journal of the Thailand Medical Assocation 2005;88(5):601‐6.. [PubMed] [Google Scholar]
Lindell 1976 {published data only}
- Lindell P, Hedenstierna G. Ventilation efficiency after different incisions for cholecystectomy. Acta Chir Scand 1976;142:561‐565. [PubMed] [Google Scholar]
Mimica 2007 {published data only}
- Mimica Z, Pogorelic Z, Perko Z, Srsen D, Stipic R, Dujmovic D. Effect of surgical incision on pain and respiratory function after abdominal surgery: A randomized clinical trial.. Hepatogastroenterology 2007;54:2216‐2220. [PubMed] [Google Scholar]
Additional references
Burger 2002
- Burger JWA, van't Reit M, Jeekel J. Abdominal incisions: techniques and postoperative complications. Scandinavian Journal of Surgery 2002;91:315‐321. [DOI] [PubMed] [Google Scholar]
Grantcharov 2001
- Grantcharov TP, Rosenberg J. Vertical compared with transverse incisions in abdominal surgery. Eur J Surg 2001;167:260‐267. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2008.
Hoer 2002
- Hoer J, Stumpf M, Rosch R, Klinge U, Schumpelick V. Prevention of incisional hernia. Chirurg 2002;73(5):474‐480. [DOI] [PubMed] [Google Scholar]
Kearns 2001
- Kearns SR, Connolly EM, McNally S, McNamara DA, Deasy J. Randomised clinical trial of diathermy verses scalpel incision for elective midline laparotomy. British Journal of Surgery 2001;88:41‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kehlet 2003
- Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery.. Lancet 2003;362:921‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Purkayastha 2007
- Purkayastha S, Tilney HS, Georgiou P, et al. Laparoscopic cholecystectomy versus minim‐laparotomy cholecystectomy; a meta‐analysis of randomised control trials.. Surgical Endoscopy 2007;8:1294‐1300. [DOI] [PubMed] [Google Scholar]
Sheldon 2004
- Sheldon TA, Cullum N, Dawson D, Lankshear A, Lowson K, Watt I, West P, Wright D, Wright J. What's the evidence that NICE guidance has been implemented? Results from a national evaluation using time series analysis, audit of patients' notes, and interviews.. British Medical Journal 2004;329:999. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


