Summary
Background
Poor pregnancy and birth outcomes are common in sub-Saharan Africa and have complex aetiologies. Sulfadoxine-pyrimethamine (SP), given for intermittent preventive therapy of malaria in pregnancy (IPTp), is one of few existing interventions that improves outcomes of both mother and baby despite widespread SP-resistant malaria. Compelling evidence exists that malaria-independent pathways contribute to this protective effect, but the exact sources of non anti-malarial protection remained unknown. We hypothesized that the beneficial effect of SP on birthweight is mediated by SP activity on maternal factors, including increased gestational weight gain and antibiotic activity on pathogens in the maternal gut.
Methods
Expectant mothers from a larger randomized control trial comparing the efficacy of IPTp-SP to IPTp with dihydroartemisinin-piperaquine (DP) were also enrolled in this sub-study study at their first antenatal care visit before commencement of IPTp (n = 105). Participants were followed monthly until delivery. Weights and mid-to-upper-arm circumferences (MUAC) were recorded. Monthly stool samples were collected and screened for five Escherichia coli pathotypes, Shigella spp., Vibrio cholerae, Salmonella, Campylobacter coli/jejuni, and three protozoa (Giardia spp., Entameba histolytica, and Cryptosporidium spp.) using previously validated molecular assays.
Findings
IPTp-SP vs. IPTP-DP was associated with higher maternal gestational weight gain (GWG) and nutritional indicators (MUAC and body-mass index, BMI). GWG was found to be a mediator of the birthweight and IPTp-SP relationship, as the birthweight of SP infants, but not DP infants, varied according to maternal GWG. The burden of maternal enteric infections was high. The three most commonly observed pathogens were enteroaggregative E. coli (EAEC), atypical enteropathogenic E.coli/enterohaemorrhagic E. coli (aEPEC/EHEC), and typical enteropathogenic E.coli (tEPEC). We found that SP reduced the prevalence of EAEC in a dose-dependent manner. After 3 or more doses, SP-recipients were 90% less likely to be infected with EAEC compared to DP-recipients (ORadj = 0.07, CI95 = 0.12, 0.39, p = 0.002). Compared to DP, this coincided with higher maternal gestational weight gain (GWG) and nutritional indicators (MUAC and body-mass index, BMI). The beneficial effect of SP on maternal GWG, MUAC and BMI, was lower if SP mothers had detectable EAEC, aEPEC/EHEC, tEPEC, and LT-ETEC at baseline. Maternal EAEC and tEPEC at baseline associated with lower birthweight for babies of both SP mothers and DP mothers. When comparing IPTp regimens, the positive effect of SP on birthweight compared to DP was only observed for infants of women who did not test positive for EAEC at baseline (adjusted mean birthweight difference SP vs. DP = 156.0 g, CI95 = -18.0 g, 336.9 g, p = 0.087), though confidence intervals crossed the null.
Interpretation
Our findings indicate that in pregnant Malawian women, IPTp-SP vs. IPTp-DP is consistently associated with higher MUAC, BMI, and GWG following the WHO-recommended regimen of at least 3 doses, but carriage of maternal gut pathogens before initiation of IPTp lessens this effect. Because GWG was a mediator of the association between birthweight and SP, we show that SP's previously proven positive effect on birthweight is by promoting maternal weight gain. Overall, our results present one plausible pathway SP exerts malaria-independent protection against poor birth outcomes in the context of its waning antimalarial activity and warrants further investigation.
Funding
A full list of funding bodies that contributed to this study can be found in the Acknowledgements section.
Keywords: Pregnancy, Gestational weight gain, Birthweight, IPTp, Gut pathogens, Sulfadoxine-pyrimethamine
Research in context.
Evidence before this study
Sulfadoxine-pyrimethamine (SP) is widely used for Intermittent Preventive Therapy in pregnancy (IPTp) in countries of sub-Saharan Africa that are endemic for malaria to improve health outcomes of both mother and baby. Malaria parasites resistant to SP are now common, bringing into question whether SP therapy in pregnancy should be discontinued. Paradoxically, SP's positive effects of reduced maternal anaemia and improved birthweight have not been diminished in areas with high rates of SP-resistant malaria parasites. Compelling evidence now indicates that SP has substantial non-malaria beneficial activity, including a recent comprehensive study that quantified the non-malaria effects of SP. Because these non-malaria pathways of protection remain unknown, we hypothesized that the beneficial effect of SP on birthweight is mediated by maternal factors, such as maternal weight gain during pregnancy, which in turn may be influenced by intestinal pathogens.
Added value of this study
Our study is the first step in elucidating that the observed benefits of SP on pregnant women and their babies is mediated by a promotion of maternal weight gain during the second and third trimesters of pregnancy. In addition, our results indicate that this positive weight gain-promoting effect is lessened if intestinal pathogens are detected in the mother's stool prior to IPTp initiation, which in turn may influence birthweight. Our study is innovative because it investigates an entirely new factor (gestational weight gain in the context of gut pathogens) as a determinant for the positive effects of SP therapy.
Implications of all the available evidence
Overall, our study in the context of all available evidence indicates that SP therapy in pregnancy has additional, malaria-independent benefits to both mother and baby. The public health significance of this study is high, because its findings will help inform the decision about whether IPTp with SP should be continued or not.
Alt-text: Unlabelled box
Introduction
Poor birth outcomes, such as preterm birth and low birthweight, are a major public health problem in developing countries and are multifactorial. Malaria is a common and preventable cause of birth complications.1–3 Intermittent preventive therapy during pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP) has been highly effective in preventing the adverse effects of malaria on the mother and foetus. Consequently, the World Health Organization (WHO) recommends that, in malaria-endemic areas, at least three IPTp-SP doses are administered to pregnant women at antenatal care, ideally starting early in the second trimester and continuing at least one month apart until delivery.4
SP-resistant malaria strains have now become widespread.2,5,6 Paradoxically, despite the high prevalence of SP-resistant falciparum malaria in areas such as East Africa, IPTp-SP appears to remain effective at improving pregnancy (e.g., reduces anaemia in mothers) and birth outcomes (e.g., reduces the risk for low birthweight).2,6 At least 2 doses of SP were found to be protective against low birthweight, irrespective of malaria transmission level or geography.3,5, 6, 7 Parasite resistance to SP has catalysed efforts to evaluate new IPTp regimens. Dihydroartemisinin-piperaquine (DP), another long-lasting and potent antimalarial, has emerged as a possible alternative in three randomized trials that have demonstrated its superiority to SP in preventing malaria, but not in preventing low birthweight.8, 9, 10 These data suggest that IPTp-SP may prevent adverse pregnancy and birth outcomes through alternative mechanisms, independent of malaria infection.11
Unlike DP, SP has broad-spectrum antibacterial activity in-vitro12 and is actively absorbed through the gut. In addition, maternal enteric microbial communities have been shown to influence birthweight and infant metabolism in pre-clinical models.13 We therefore hypothesized that SP's mechanism of protection may be mediated by maternal effects, such as the promotion of gestational weight gain and/or effects in the gut where SP may be inhibiting common intestinal pathogens. Here, we report the results of a study that followed expectant mothers at their monthly antenatal visits throughout their second and third trimesters up to delivery. We analysed monthly stool samples collected from pregnant women in Malawi participating in a randomized controlled trial of IPTp-SP vs. IPTp-DP. Our goals were to characterize the burdens of 12 common enteric bacterial and protozoal pathogens (six Escherichia coli pathotypes, Vibrio cholerae, Salmonella, Campylobacter coli/jejuni, Giardia spp., Entamoeba histolytica, and Cryptosporidium spp.), determine if and how they are affected by SP, and ascertain whether SP's effects on maternal factors, like gestational wieght gain, and on intestinal microbial factors might explain its benefit despite waning malaria activity.
Methods
Study population
The present study is an ancillary investigation to an open-label randomized controlled trial (RCT) designed to compare the efficacy and safety of monthly administration of either sulfadoxine-pyrimethamine (SP) or dihydroartemisinin-piperaquine (DP) for intermittent preventive treatment of malaria in pregnancy (IPTp) in Machinga district, Malawi (ClinicalTrials.gov Identifier: NCT03009526). Pregnant women between 16- and 28-weeks gestational age attending their first antenatal care (ANC) visit at Machinga District Hospital were recruited for both the parent and this sub-study. Criteria for inclusion in our sub-study were written informed consent for both parent study and sub-study, viable pregnancy, no history of IPTp use in the current pregnancy, a negative HIV test, residency in the study area, and willingness to provide stool samples at each antenatal visit. Women with high-risk pregnancies or other medical conditions were excluded.
Randomization and masking
Women who consented to the main trial were asked to also participate in the sub-study. The sample size for this sub-study was defined a priori to be 100–110 participants. A total of 105 women consented and were enrolled in this sub-study; 52 were randomized to the SP group and 53 to the DP group using a computer generated list. The investigators at the University of North Carolina at Chapel Hill (UNC at Chapel Hill) remained blinded to study drug allocation until analyses were finalized.
Sample collection, processing, and storage
At enrolment, which was the first antenatal visit for the current pregnancy, mothers were randomized to either the SP or DP group. Thereafter, they were scheduled for follow-up monthly antenatal study visits, each 28–30 days apart. IPTp was administered at enrolment and at each follow-up visit, up to four more study visits. At each study visit, participants were asked whether they were experiencing diarrhoea or abdominal pain at the time of study visit in the 24–48 h preceding the study visit. Upon enrolment prior to drug administration, women were asked to provide an “on the spot” faecal specimen, which was considered the baseline (pre-drug) specimen; women for whom this was not feasible were still enrolled, but they did not have a baseline stool specimen as part of the analytical sample set. For subsequent monthly visits, women used the provided stool collection kit to collect a faecal specimen within 24 h prior to their visit. Some women did not bring stool samples at each visit, but all women received the drug as scheduled. The final study sample was considered as the specimen collected for the last visit prior to delivery, because too few women produced a stool sample at delivery. Upon submission to the study nurse, stool samples were taken to the field laboratory in Machinga, partitioned by laboratory staff into 5 equal aliquots in a biological cabinet using ethanol-treated surfaces and spatulas, and placed in sterile 2 mL polypropylene cryotubes. The specimen aliquots were then placed in a -20 °C freezer for storage. Three of the 5 aliquots were shipped on dry ice to UNC at Chapel Hill, United States for further laboratory procedures. Upon arrival at UNC at Chapel Hill, frozen stool samples were placed in -80 °C freezers until DNA extraction.
DNA extraction and molecular detection of enteric pathogens
DNA was extracted from stool samples in batches of 23 with one negative control (sterile PBS). Frozen stool samples were thawed at the time of DNA extraction, which was performed as previously described,14 with modifications as follows: 0.1 mm glass beads were used (BioSpec Products, Cat #11079101), homogenization was performed with a Disruptor Genie (Scientific Industries, Cat # S6001-2-120) using 2 rounds of 60 s beating followed by 5 min resting, i.e. 120 s of total beating) and clean-up of DNA samples was done with the Qiagen QIAamp DNA stool kit. Quality control and quantification of DNA samples was performed with the Qubit dsDNA Broad Range kit (Cat # Q32853) and the Qubit 3.0 Fluorometer (Cat # Q33216). We evaluated the presence of 12 bacterial and protozoan enteric pathogens using a previously validated systematic approach of multiplex quantitative real-time PCR (qPCR).15,16 The pathogens interrogated in this study were: diarrheagenic Escherichia coli species and Shigella (enteroaggregative E. coli [EAEC], typical enteropathogenic E. coli [tEPEC], atypical enteropathogenic E. coli/enterohemorrhagic E. coli [aEPEC/EHEC], heat-labile enterotoxigenic E. coli [LT-ETEC], heat-stable enterotoxigenic E. coli [ST-ETEC], enteroinvasive E. coli, and Shigella spp. [EIEC/Shigella]), Vibrio cholerae, Salmonella spp., Campylobacter coli/C. jejuni, and three protozoan pathogens (Giardia, Entamoeba histolytica, and Cryptosporidium spp.). Commercially available gDNA from reference strains or gDNA from laboratory strains cultured at UNC at Chapel Hill were used in limit of detection experiments and as positive controls alongside test samples: EAEC strain NCDC U14-41 (BEI Cat# NR-3052), tEPEC strain CDC (BEI Cat# NR-3050), EHEC strain EDL933 (BEI Cat# 2648), EIEC strain 1885-77 (BEI Cat# NR-3051), V. cholerae strain 395 (BEI Cat# NR-15694), Salmonella enterica, subsp. enterica, serovar Typhimurium (BEI Cat# NR-4614), Campylobacter coli strain JV20 (BEI Cat# HM-296D), Giardia lamblia H3 (assemblage B) (gDNA extracted in-house from mouse-associated trophozoites obtained from Bartelt laboratory, University of North Carolina at Chapel Hill derived from Waterborne, Inc), Cryptosporidium parvum strain Iowa (ATCC Cat# PRA67DQ) and Entamoeba histolytica strain HM-1:IMSS (ATCC Cat# 30459). The gene targets and primer sequences have been previously published and extensively validated15,16 and are provided in Table S1, along with the configuration of the multiplex detection panels. Details of the qPCR reaction recipes for each panel are given in Table S2 and cycling conditions in Table S3. Definitions of pathogen detections are given in Table S4. The assays were performed with the QuantStudio 6 platform (Life Technologies). Samples were classified as pathogen positive as previously described.15,16 In brief, an analytical cycle threshold (Ct) cut-off of 35 was applied, as detection above this threshold becomes stochastic and less reproducible.
MUAC, BMI and gestational weight gain (GWG) measurements and definitions
At each visit, women were weighed on a digital scale, accurate to the nearest 0.1 kg; height was measured to the nearest 0.1 cm against permanent markings on the wall at enrolment visit only. Mid-upper-arm-circumference (MUAC) was measured at each visit using a flexible tape measure at the mid-point between the tips of the shoulder and elbow. Gestational age was estimated by ultrasound measurement at enrolment. Infants were weighed naked at delivery; weights were recorded to the nearest 1 gm using a digital infant weight scale.
Mid-upper-arm-circumference (MUAC) and body mass index (BMI) were used to define maternal nutritional status, as previously described.1 MUAC is a reliable indicator of maternal malnutrition because it changes little during pregnancy.1 We analyzed MUAC both as a continuous variable and dichotomized at the WHO clinical cut-off of undernutrition of 23 cm, as previously described.1 Because our study did not collect pre-pregnancy measurements (according to WHO, a pre-pregnancy BMI <18.5 kg/m2 is predictive of adverse birth outcomes),17 we used the BMI at the enrolment antenatal visit as our baseline BMI. BMI was analyzed as a continuous variable. As the correlation between BMI and MUAC is not perfect, since BMI is more likely to change during pregnancy than MUAC,1,18, 19, 20, 21, 22, 23 the two indicators were analyzed separately. GWG is an independent and modifiable risk factor for adverse pregnancy and infant neonatal outcomes.19,20,23
We calculated weight difference (kg) between the final visit and the enrolment visit by subtracting the enrolment weight from the final visit weight; to estimate GWG z-scores we performed the following:
-
(1)
the weight difference value (kg) and the 50th centile of weight gain for the corresponding gestational age available from the publicly-available INTERGROWTH table of GWG standards24 (https://media.tghn.org/medialibrary/2017/05/GROW_GWG-nw-ct_Table.pdf) were added to obtain a GWG estimate since the first trimester. For example, for a woman who gained 7 kg throughout the study observation period and had a gestational age at enrolment of 20 weeks, we added the 7 kg that we observed to the average weight gain value of 3.3 kg, which is the 50th centile of weight a mother is expected to gain during the first 20 weeks of gestation, i.e. 12 kg.
-
(2)
Then, GWG z-scores were derived, adjusting for gestational age, using the INTERGROWTH-21 calculator (https://intergrowth21.tghn.org/gestational-weight-gain/#c6), as previously described.24
GWG z-score was used as a continuous variable and dichotomized to below average GWG if z-score < 0 and average or above average GWG if z-score ≥ 0. The analytical population for GWG analyses was chosen based on the following criteria explained in “Statistical analysis”
Statistical analysis
Analytical populations included in the final analyses are as follows. Only women who provided at least one stool specimen during the study were included in the analyses. The baseline analytical sample set included only stool specimens that were produced prior to enrolment and prior first dose of drug. Women without baseline specimens but who provided follow-up specimens were included in analyses of follow-up visits only. Women with baseline and/or follow-up stool specimens for whom MUAC and weight were recorded at the study visit were considered the analytical population for MUAC and BMI analyses at that study visit, as appropriate. Mothers included in final GWG z-score analyses were chosen based on the following criteria: (1) they had a recorded weight at enrolment; (2) gestational age at enrolment was < 23 weeks, because by weeks 23–28 of gestation, GWG is on average 4.9–7.5 kg24 and this gain is too broad to be accurately accounted for in GWG z-score estimates without pre-pregnancy weight; (3) normal BMI (18.5–24.9 kg/m2), as GWG standards for women with higher than average BMI did not exist at the time of our analysis; (4) receipt of ≥ 3 doses of drug, because the greatest protection against low birthweight associated with SP prophylaxis was observed after ≥ 3 doses6–8 and the WHO recommends ≥ 3 dose regimen for maximal protection.4
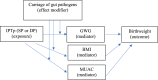
Statistical analyses were performed with STATA16. Differences in participant characteristics at baseline by drug group were assessed using Chi-square (χ2), Fisher's exact tests, or t-tests, as appropriate. Bonferroni corrections were used when multiple comparisons were performed simultaneously. Important covariates were identified a priori by causal assumptions based on background knowledge of covariate relationships25 and by stepwise selection, whereby variables found to be associated with outcomes in unadjusted analyses (p < 0.05) were included in adjusted models.26 When exploring differences between two timepoints or differences between drug groups within timepoints we used linear regression in sub-group analyses. Our primary hypothesis is: maternal nutritional indices (GWG, MUAC, BMI) are mediators of the known relationship between IPTp treatment arm (SP vs. DP) and birthweight. A secondary hypothesis is: carriage of enteric pathogens modifies the relationship between IPTp and maternal indicators, thus impacting birth weight. A directional acyclical graph summarizing our causal assumptions is provided below.
Primary hypothesis analyses
When evaluating if IPTp associated with changes in MUAC and BMI over the course of pregnancy, we used linear mixed models with generalized estimating equations and robust variance. We included all measurements for each woman, fitted an interaction term between drug group and number of IPTp doses given, and adjusted for the known covariates of gravidity and gestational age. Gravidity and pre-pregnancy and pregnancy BMI are known to be associated with GWG.20 Adjusting for these covariates, we assessed whether GWG associated with MUAC and birthweight by drug group using linear regressions (in sub-group analyses or by fitting interaction terms, as appropriate) and non-parametric Mann-Whitney tests when continuous GWG z-scores were used and logistic regression and chi-square (χ2) tests when GWG z-scores were dichotomized to below average (GWG z-score<0) and average or above average (GWG z-score ≥ 0). Mean birthweight differences were estimated with linear regression, controlling for known covariates of gravidity, gestational age at delivery, and maternal MUAC at baseline. We investigated whether GWG was a mediator of the drug and birthweight relationship by using mediation analysis, adjusting for known covariates, and requesting bootstrapped standard errors and confidence intervals with 500 replications.
Secondary hypothesis analyses
We first evaluated the impact of IPTp regimen and number of IPTp doses on pathogen prevalence in two ways: (1) with logistic regression at each study visit independently, adjusting for gravidity, gestational age and nutritional indicators at baseline; (2) to obtain the effect over time, we used log-binomial mixed models with generalized estimating equations and robust variance, fitted an interaction term between drug group and number of doses and adjusted for gravidity, gestational age, and nutritional status at baseline. Pathogen burden (i.e., number of concomitantly infecting pathogens) analyses were conducted with Poisson regression. When evaluating changes of pathogen burden over time, we used a Poisson mixed model with generalized estimating equations and robust variance. We visualized patterns of co-occurring pathogens using UpSet plots generated in R software or with heatmaps of presence/absence of detected pathogens, as appropriate. We evaluated whether pathogen carriage was an effect modifier on the relationship between GWG, birthweight and treatment arm using linear regression, adjusting for important covariates, both in sub-group analyses (pathogen detection at baseline/no pathogen detection at baseline) and by fitting an interaction term between drug group and pathogen detection status at baseline.
Ethics
This study was reviewed and approved by the Institutional Review Boards at the University of North Carolina at Chapel Hill (# 16-1260), at the Centers for Disease Control and Prevention (# 6836), and University of Malawi College of Medicine in Blantyre, Malawi. All participants provided informed consent.
Role of the funding source
The Funders did not have any role in study design, data collection, data analyses, interpretation, or writing of report.
Results
Samples included in final analyses
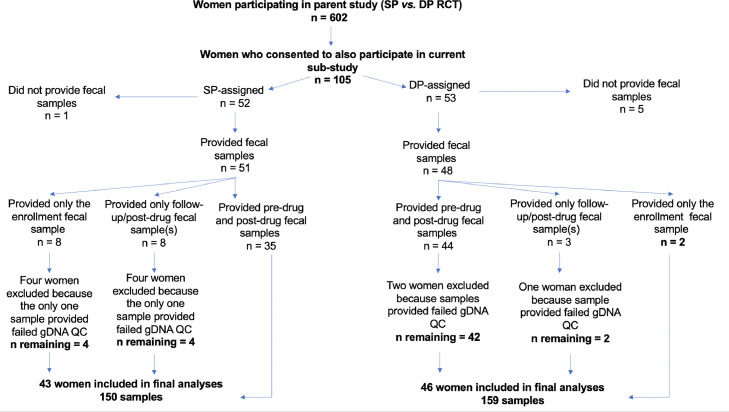
Of 105 women enrolled in our study, 89 women (SP n = 43; DP n = 46) were included in the final analytical dataset. Reasons for exclusion are given in Figure 1. Differences in baseline characteristics between enrolled women (n = 105) and women included in the analysis (n = 89) are given in Table S5. Maternal characteristics of the 89 women at enrolment by IPTp treatment arm are given in Table S6. The mean number of antenatal care visits attended at which stool samples were provided, including the baseline enrolment visit prior to any drug administration, was 3.5 visits (range = 1–5). Sample sizes for each antenatal care visit are given in Table S7. If a participant attended ≥3 study visits, they delivered their baby within an average of 34.1 days of the last visit (range = 1–97 days). A total of 309 stool samples were screened for pathogens (SP n = 150 stools; DP n = 159 stools).
Figure 1.
Study profile and samples included in analyses.
Maternal nutritional indicators (MUAC and BMI) at baseline
Mid-upper-arm-circumference (MUAC) data at enrolment was available for all 89 mothers. The mean MUAC (±SD) at enrolment was 25.8 cm (±2.2 cm). Eight women were classified as undernourished (8.9%) by MUAC < 23cm at the time of enrolment. Height measurements were available for 75/89 (84.3%) of women and thus BMI calculations were only possible for this participant subset. At baseline, the mean body-mass index (BMI, ±SD) was 23.2 kg/m2 (±3.0 kg/m2). All enrolment BMIs were above the cut-off for undernourished (BMI > 18.5 kg/m2), most likely due to gestational weight gain (GWG).24 Three women had a BMI > 30 kg/m2. At baseline, MUAC and BMI did not differ among women between drug groups or by pathogens detection (Table S8).
Gut pathogens at baseline
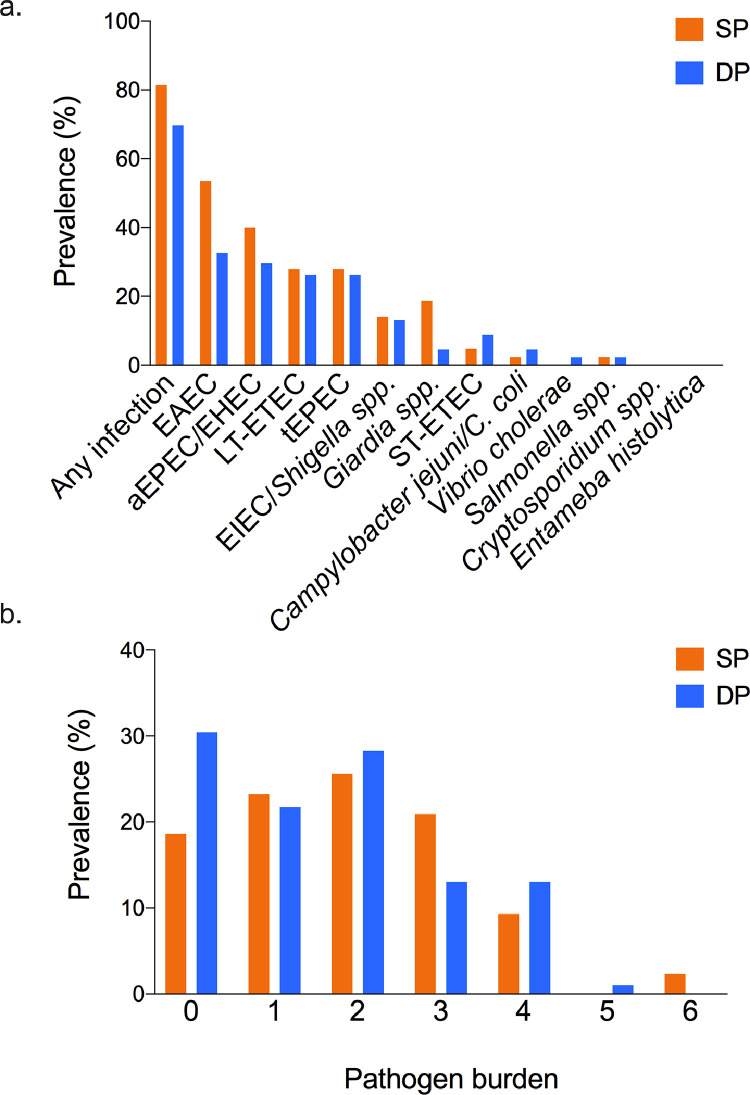
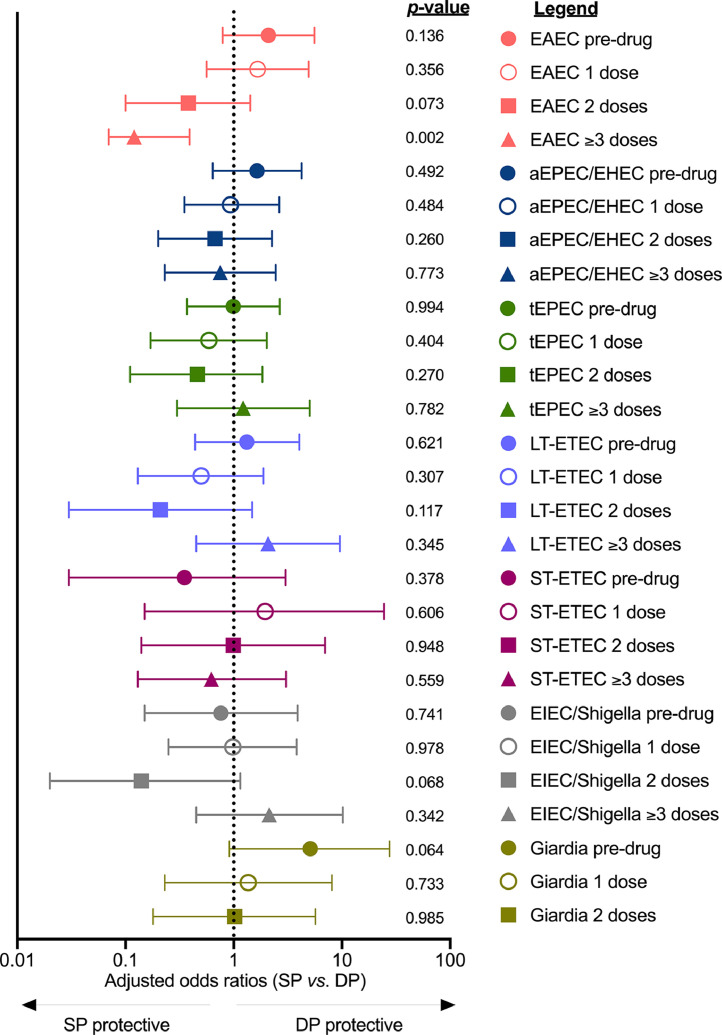
At baseline, 75.3% of all mothers (67/89) had at least one pathogen detected (SP 81.4%; DP 69.6%, χ2 p = 0.196) (Figure 2a). The most frequently observed pathogens were enteroaggregative E. coli (EAEC) and atypical enteropathogenic E. coli/enterohemorrhagic E. coli (aEPEC/EHEC), while Cryptosporidium spp. and Entamoeba histolytica were not observed among baseline samples (Figure 2a). Despite randomization, there were differences between the 2 treatment groups at baseline. EAEC and Giardia spp. were over-represented among women assigned to the SP group (EAEC: 23/43, 53.5%; Giardia spp: 8/43, 18.6%) vs. women assigned to the DP group (EAEC: 15/46, 32.6%; Giardia spp: 2/46, 4.4%) (EAEC χ2p = 0.047, Giardia spp. χ2p = 0.033). More than half of mothers were burdened by at least 2 pathogens (47/89, 52.8%) and a quarter of all mothers (23/89, 25.8%) had three or more concomitant pathogens. The highest pathogen burden observed was six (Figures. 2b, S1). At enrolment, only one participant reported abdominal pain only at the time of study visit and/or in the previous 24-48h. None of the participants reported diarrhoea at enrolment.
Figure 2.
Prevalence of enteric pathogens and pathogen burden at baseline (pre-drug).
(a) At baseline, two thirds of mothers (67/89, 75.2%) had at least one detectable pathogen of the 12 pathogens included in the panel (SP 81.4%; DP 69.6% χ2 p = 0.196). Atypical enteropathogenic E. coli/enterohemorrhagic E. coli (aEPEC/EHEC) and enteroaggregative E. coli (EAEC) were most frequently observed. Cryptosporidium spp. and Entamoeba histolytica were not observed among baseline samples. EAEC and Giardia spp. were over-represented among women assigned to the SP group (EAEC: 23/43, 53.5%; Giardia spp: 8/43, 18.6%) vs. women assigned to the DP group (EAEC: 15/46, 32.6%; Giardia spp: 2/46, 4.4%) (EAEC χ2 p = 0.047, Giardia spp. χ2 p = 0.033).
(b) Multiple concomitant pathogens, up to 6 and defined as “pathogen burden”, were detected per stool. More than half of mothers were burdened by at least 2 pathogens (47/89, 52.8%) and a quarter of all mothers (23/89, 25.8%) had three or more concomitant pathogens.
Primary hypothesis analyses
Our primary hypothesis is: maternal nutritional indices (GWG, MUAC, BMI) are mediators of the known relationship between IPTp treatment arm (SP vs. DP) and birthweight.
Changes in maternal BMI and MUAC during pregnancy and their relationship to IPTp regimen
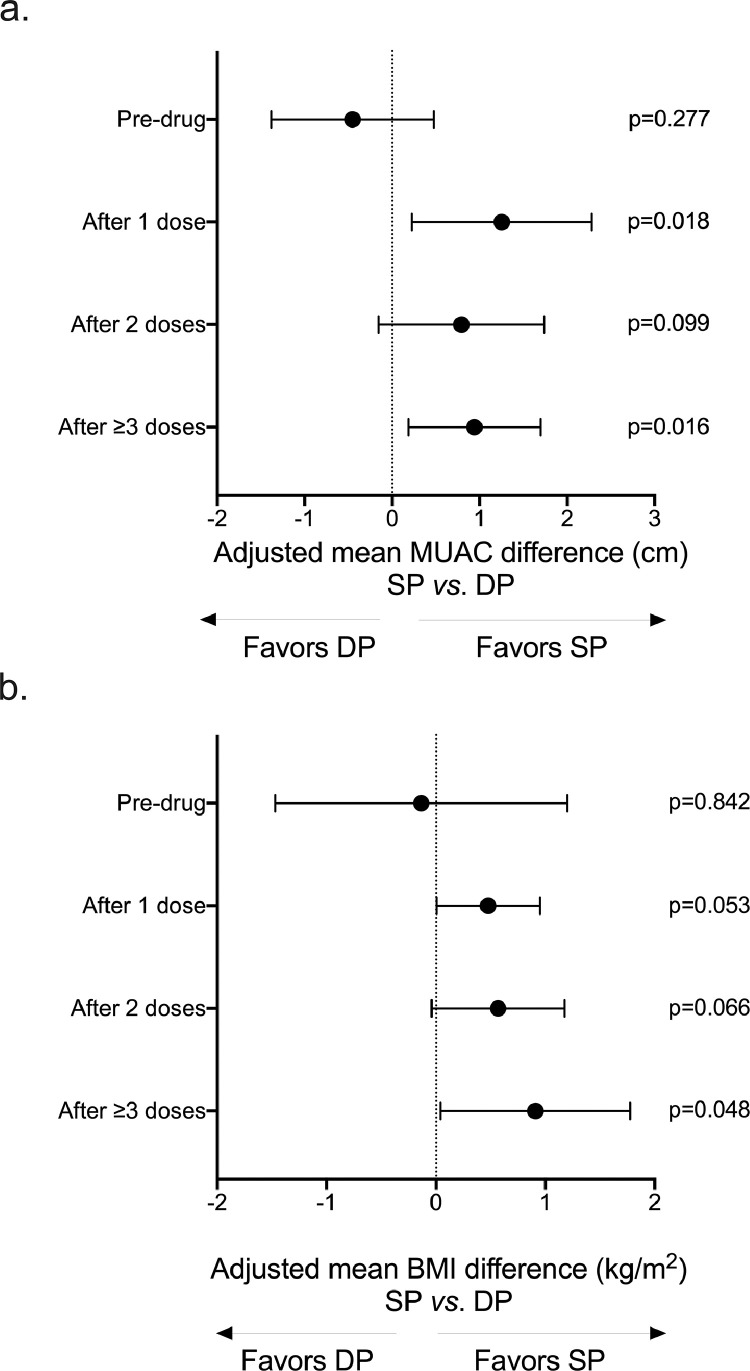
We found that MUAC among SP-treated women increased by an average of 1.1cm after the first dose compared to their baseline measurements (CI95 = 0.5, 1.8 cm, p = 0.001, Fig S2a) using adjusted mixed linear regression with generalized estimating equations. This increase was not seen for DP-treated women (mean MUAC change after 1 dose vs. pre-drug = -0.2 cm, CI95 = -0.9 cm, 0.4cm, p = 0.519, Fig S2a). Next, we found that the MUAC of SP recipients was consistently higher during pregnancy using sub-group linear regressions adjusted for baseline MUAC, gravidity, and gestational age; thus, following the WHO-recommended regimen of ≥ 3 IPTp doses, the MUAC of SP women vs. DP women was higher by an average of 0.94 cm (CI95 = 0.18 cm,1.70 cm, p = 0.016, Figure 3a). While women in both drug groups had increases in BMI during pregnancy (Fig. S2b), women treated with SP had consistently higher BMIs than women treated with DP (Figure 3b), and after ≥ 3 doses, women who received SP had BMIs that was on average 0.9kg/m2 higher than those of DP recipients (CI95 = 0.1 kg/m2, 1.8 kg/m2, p = 0.048, Figure 3b).
Figure 3.
Changes in maternal middle-upper-arm circumference (MUAC) and body-mass index (BMI) following IPTp.
We used linear regressions of MUAC and BMI measurements at each follow-up antenatal visit (baseline before first dose of drug; at follow-up antenatal visit 1, 28–30 days after first dose was given; at follow-up antenatal visit 2, 28-30 days after second dose was given; at follow-up antenatal visit 3, 4, or 5, 28–30 days after the third, fourth, or fifth doses was given). We adjusted for the baseline measurement (MUAC or BMI, as appropriate), gravidity and gestational age.
(a) The MUAC of SP recipients was consistently higher during pregnancy and after the ≥ 3 IPTp-SP doses recommended by the WHO, the MUAC of SP women vs. DP women was higher by an average of 0.94 cm (adjusted linear regression p = 0.016).
(b) The BMI of SP women compared to DP women was higher during pregnancy and after receiving ≥ 3 doses of SP, these women had higher BMI than women treated with ≥ 3 doses of DP (adjusted linear regression p = 0.048).
Gestational wight gain after ≥3 IPTp doses and its relationship to IPTp regimen and birthweight
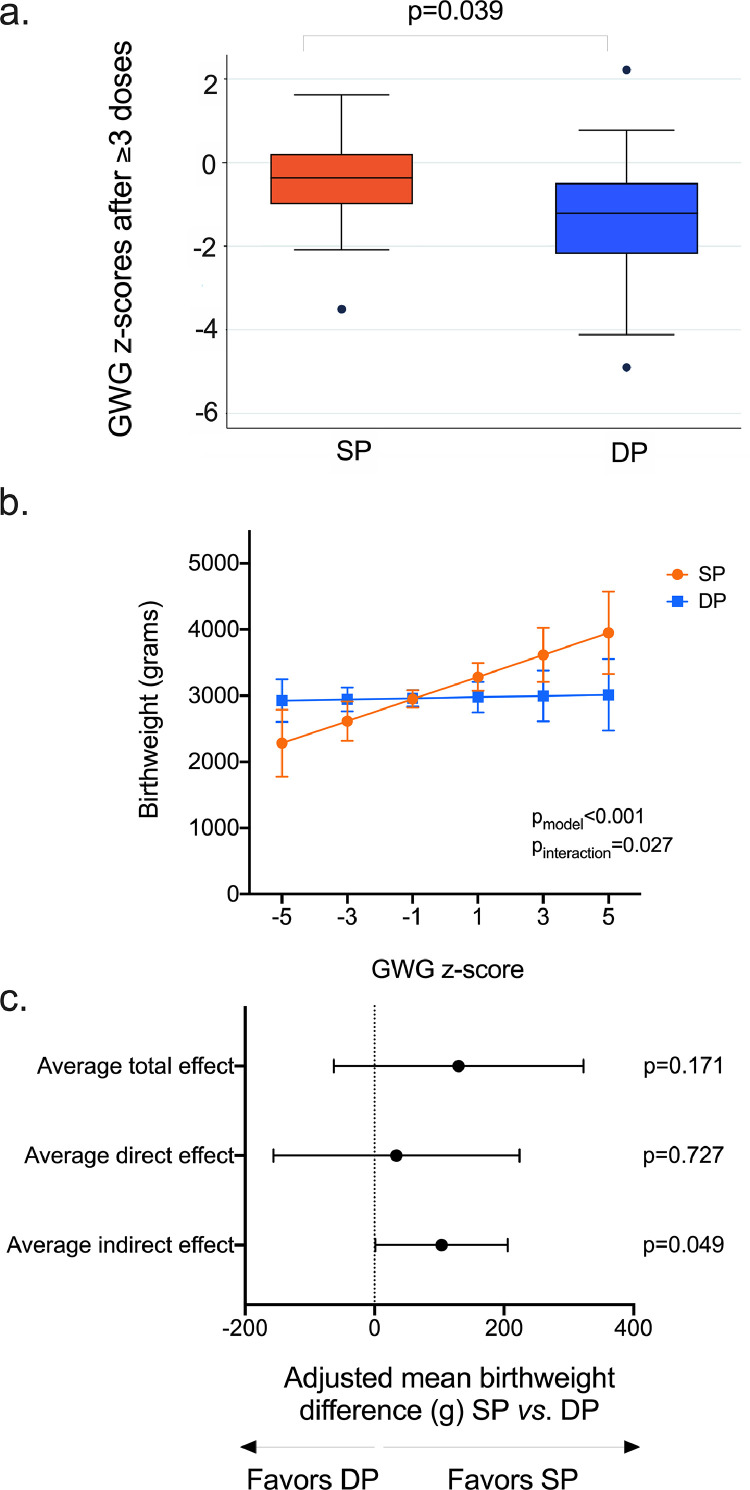
We applied robust criteria for our GWG z-score estimations, as described in methods. As a result, GWG z-scores were available for 56 women (SP n = 27; DP n = 29). Using simple linear regression, we found that GWG z-scores were positively correlated with baseline BMI (mean GWG z-score change = -0.14, CI95 = -0.26, -0.01, p = 0.034), but not baseline MUAC. GWG z-scores of SP-recipients after ≥3 IPTp doses were higher than those of DP-recipients (Mann-Whitney z = 2.4, p = 0.039, Figure 4a) and SP-recipients were more likely to achieve greater or equal to average GWG (i.e., GWG z-score>0) than DP-recipients (44.4% vs. 17.2%, χ2p = 0.027, Fig. S3).
Figure 4.
Gestational weight gain (GWG) and birthweight following IPTp.
Gestational weight gain (GWG) z-scores were calculated as described in “Methods”. In brief, we first calculated the mother's weight gain (kg) between enrolment and the visit closest to delivery. This value was added to the 50th centile of weight gain for the gestational age that corresponded to the mother's gestational age at enrolment. The 50th centile of weight gain values are available from the publicly-available INTERGROWTH table of GWG standards24 (https://media.tghn.org/medialibrary/2017/05/GROW_GWG-nw-ct_Table.pdf). Then, GWG z-scores were derived, adjusting for gestational age, using the INTERGROWTH-21 calculator (https://intergrowth21.tghn.org/gestational-weight-gain/#c6), as previously described.24 Only mothers who completed 3 doses of IPTp, were enrolled before 23 weeks gestation, and had normal BMI at enrolment (18.5–24.9 kg/m2) were included in these analyses.
(a) SP-treated women had higher GWG z-scores than DP-treated women (Mann-Whitney p = 0.019; horizontal lines represent the median and 95% confidence intervals).
(b) The GWG, birthweight and IPTp relationship. Birthweight (grams) was recorded for 75 babies (75/89, 84.3%). Using linear regression adjusted for maternal baseline MUAC, gravidity, and gestational age at delivery, we evaluated whether birthweight associated with IPTp regimen according to GWG z-scores, by fitting an interaction term between drug group and GWG z-scores. Shown in this graph is the interaction effect with 95% confidence intervals. We found that birthweight was positively correlated with mothers’ GWG in SP-treated women, but not DP-treated women (pmodel < 0.001, pinteraction = 0.027).
c) We used mediation analysis adjusting for baseline BMI and gravidity and requesting bootstrapped standard errors and confidence intervals with 500 replications to establish whether GWG is a mediator of the relationship between drug and birthweight. The average direct effect of drug on birthweight was not significant (mean birthweight difference SP vs. DP = 33.90g, CI95 = -156.2g, 224.0, p = 0.727), but the indirect effect of drug (SP vs. DP) through GWG was significant: mean birthweight difference = 95.9 g, CI95 = 5.5 g, 209.1, p = 0.049. The proportion of the total effect of IPTp-SP on birthweight that is mediated by GWG, calculated by dividing the indirect effect by the sum of indirect and direct effects is 0.74 (i.e., 95.9/[95.9+33.9] = 0.74).
Birthweight was recorded for 75 babies (84.3%). Mean birthweight (±SD) among all women was 2946.5g (±359.7 g). In unadjusted linear regression, birthweight was positively correlated with mother's enrolment MUAC (mean increase in birthweight for each 1cm increase in MUAC = 38.2 g, CI95 = 0.39g, 76.0g, p = 0.048), gravidity (mean increase in birthweight for each pregnancy = 83.0g, CI95 = -11.0 g, 177.0 g, p = 0.083) and gestational age at delivery (mean increase in birthweight for each 1 week increase in gestational age at delivery = 54.8g, CI95 = 0.11 g, 109.4 g, p = 0.050). When adjusting for these covariates, birthweight was found to be positively associated with GWG and thus birthweight was on average 63.2 g higher for each 1 unit increase in GWG z-score (CI95 = 3.5 g, 122.9 g, p = 0.039). While the birthweight of babies born to mothers who received at least 3 doses did not differ between drug groups in unadjusted linear regression (mean birthweight difference SP vs.DP = -7.5 g, CI95 = -204.7 g, 190.0 g, p = 0.940) or adjusted linear regression (mean birthweight change SP vs. DP = -0.5 g, CI95 = -171.7 g, 170.1 g, p = 0.995), we found that GWG influenced this association by using both sub-group analyses and linear regression with an interaction term between IPTp regimen and GWG z-scores (Figure 4b). In sub-group analyses, mean birthweight of babies born to SP-treated women was 158.9g higher for each 1 unit increase in GWG z-score (CI95 = 27.0 g, 290.1 g, p = 0.021) while there was no positive effect of GWG on birthweight for the babies of DP-treated women (mean birthweight difference for each 1 unit increase in GWG z-score = -2.12 g, CI95 = -79.3 g, 75.0 g, p = 0.954). The results of the adjusted linear regression analysis of birthweight with an interaction term between IPTp regimen and GWG z-scores is shown in Figure 4b (pinteraction = 0.027).
Because GWG was independently associated with both drug and birthweight, mediation analysis was used to establish whether GWG is a mediator of the relationship between drug and birthweight. Using mediation analysis, we found that while the direct effect of drug on birthweight was not significant (mean birthweight difference SP vs. DP = 33.9 g, CI95 = -156.2 g, 224.0, p = 0.727), the indirect effect of drug (SP vs. DP) through GWG was significant: mean birthweight difference = 95.9g, CI95 = 5.5 g, 209.1, p = 0.049 (Figure 4c). Compared to IPTp-DP, the proportion of the total effect of IPTp-SP on birthweight that is mediated by GWG, calculated by dividing the indirect effect by the sum of indirect and direct effects is 0.74 (i.e., 95.9/[95.9+33.9] = 0.74).
Secondary hypothesis analyses
Our secondary hypothesis is: carriage of enteric pathogens mediates or modifies the relationship between IPTp and maternal indicators, thus impacting birthweight.
Changes in gut pathogen carriage following IPTp
Enteroaggregative E. coli (EAEC) was the only pathogen that was strongly associated with drug regimen (Figure 5). We observed a dose-dependent reduction in EAEC in SP-treated women but not DP-treated women (Figs. 5, S4a) using adjusted logistic regression performed for each study visit. SP recipients were more likely than DP recipients to be EAEC negative after ≥3 doses of drug, irrespective of EAEC detection at baseline in adjusted analyses (among EAEC negative women at baseline: aOR SP vs. DP 0.11, CI95 = 0.01, 1.00, p = 0.052; among EAEC positive women at baseline: aOR SP vs. DP = 0.05, CI95 = 0.004, 0.596, p = 0.018). SP was not found to influence aEPEC/EHEC prevalence, the second most common pathogen detected in our study (115/309 stools positive, 37.2%) (Fig. S4b), overall E. coli positivity (Fig. S4c), or overall positivity with any pathogen (Fig. S4d). Neither SP nor DP significantly altered the total number of pathogens harboured by a woman during pregnancy (Poisson regression p = 0.327, Fig. S5). Following enrolment, only three women reported intestinal symptoms: one woman reported diarrhoea only (i.e., absence of abdominal pain) at only one timepoint, and the other two women reported abdominal pain in the absence of diarrhoea at only one study visit.
Figure 5.
Changes in gut pathogen prevalence following IPTp.
We used logistic regression adjusted for gravidity, gestational age and MUAC at baseline to assess the risk of gut pathogens at each study visit independently. The likelihood of carriage in the SP vs. the DP group was estimated for EAEC, aEPEC/EHEC, tEPEC, LT-ETEC, ST-ETEC, EIEC/Shigella at all study visits, but the low prevalence of the others precluded their analysis. We found that only EAEC was strongly associated with IPTp regime. The prevalence of EAEC decreased in a dose-dependent manner in SP-receiving women compared to DP-receiving women. After ≥ 3 doses, SP-treated women were approximately 90% less likely to be infected with EAEC than DP-treated women (p = 0.002).
Associations between BMI and MUAC during pregnancy and IPTp regimen, as a function of gut pathogens
Having found that MUAC and BMI changes were higher among SP-treated than that of DP-treated women during the study period (Figs. 3, S2) and that EAEC was the only pathogen significantly associated with IPTp-SP (Figure 5), we sought to determine whether the carriage of EAEC modified the IPTp and MUAC/BMI relationship. First, the significant increase in MUAC after one dose of SP, but not after one dose of DP (Figure 3), was only observed in the absence of pre-existing EAEC (i.e., EAEC detected at baseline immediately prior to the first dose of drug) (Table S9). Second, MUAC and BMI changes in SP vs. DP recipients after ≥3 doses of drug were higher when EAEC was not detected at baseline (Table S10). For completeness, the other gut pathogens were also evaluated for effect modification of the association. We found a similar effect when other pathogens, including other E. coli pathotypes (aEPEC/EHEC, tEPEC, or LT-ETEC) were considered (Tables S9, S10).
Modification of the IPTp, gestational weight gain, and birthweight relationship by gut pathogens
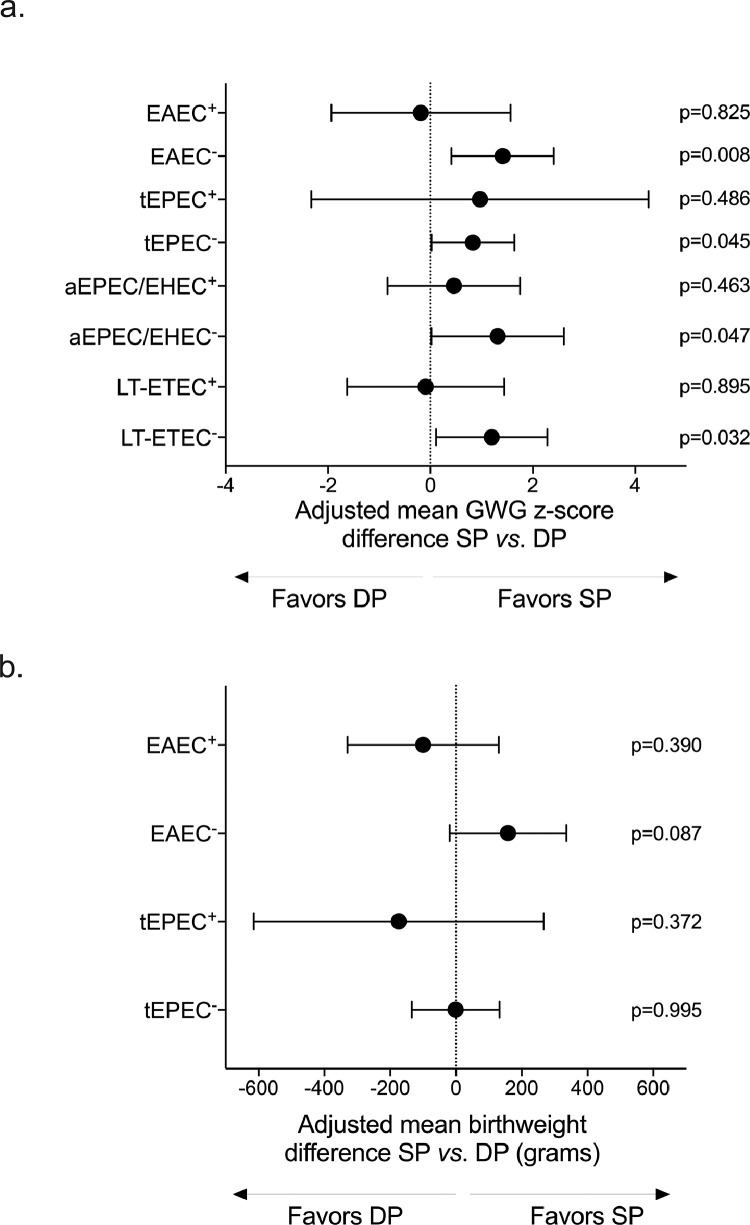
Analyses of birthweight by maternal characteristics and IPTp group are shown in Table S11. Having established that GWG was a mediator of the IPTp-SP and birthweight relationship (Figure 4c) and that the other maternal nutritional indicators (MUAC and BMI) differed between drug groups as a function of pre-existing EAEC and other E. coli pathotypes (Tables S9, S10), we evaluated whether the overall beneficial SP effect on GWG and birthweight varied according to pre-existing pathogens (Table S12 and Table S13, respectively). Among women without pre-existing EAEC, aEPEC/EHEC, tEPEC, or LT-ETEC, SP-treated women had higher GWG z-scores than DP treated women (Table S12). However, when women had pre-existing EAEC, aEPEC/EHEC, tEPEC, or LT-ETEC, this beneficial effect on GWG was not seen (Figure 6a, Table S12).
Figure 6.
The association between IPTp and gestational weight and birthweight is modified by baseline detection of maternal gut pathogens.
(a) Effect modification of pathogens on the GWG and IPTp association. We used sub-group linear regressions adjusted for maternal baseline BMI and gravidity and stratified by pathogen detection at baseline (i.e., pre-existing infection) . A “+” superscript denotes that the pathogen was detected at baseline, while a “-“ superscript denotes the pathogen was not detected at baseline (n EAEC+ = 22; n EAEC− = 32; n tEPEC+ = 15; n tEPEC− = 39; n aEPEC/EHEC+ = 15; n aEPEC/EHEC − = 39; LT-ETEC+ = 20; n tEPEC− = 34). Pre-existing infection with EAEC, tEPEC, aEPEC/EHEC, and LT-ETEC influenced gestational weight gain (GWG) outcomes, as a function of treatment arm, as SP recipients without detectable pathogen at baseline, but not SP recipients with pre-existing pathogen, had higher GWG than DP recipients. Analyses of the other pathogens are provided in Table S11.
(b) Effect modification of pathogens on the birthweight and IPTp relationship. Using sub-group analyses of adjusted linear regression of birthweight, we compared IPTp regimens according to pathogen detections at baseline. We found that pre-existing EAEC and tEPEC influenced how birthweight associated with IPTp regimen. When tEPEC was not detected at baseline among women who received ≥ 3 doses (tEPEC−, n = 34), differences in birthweight between drug groups were not found (adjusted mean birthweight change SP vs. DP = -1.1 g, CI95 = -134.5 g, 132.3 g, p = 0.995). When tEPEC was detected at baseline (tEPEC+, n = 20), differences between drug groups were negligible (adjusted mean birthweight change SP vs. DP = -166.7 g, -618.0 g, 263.1 g, p = 0.362). Compared to DP, SP was associated with higher birthweight when mothers were EAEC negative at baseline (EAEC−, mean adjusted birthweight difference = 156.0 g, CI95 = -18.0 g, 336.9g, p = 0.087, n = 32). This effect was not observed among EAEC positive mothers at baseline (EAEC+, mean birthweight difference SP vs. DP = -99.5 g, CI95 = -329.9 g, 130.9 g, p = 0.390, n = 22).
Birthweight varied according to EAEC and tEPEC (Table S13) only, but in different ways. Pre-existing tEPEC was associated with lower birthweight, irrespective of drug regimen after ≥3 doses (Table S13), as follows: using sub-group analyses of each IPTp group, we used adjusted linear regression and found that the birthweight of babies born to SP mothers who tested positive for tEPEC was on average 262.6 g lower than that of babies born to SP mothers who did not test positive for tEPEC (CI95 = -457.3 g, -67.9 g, p = 0.009, Table S13); similarly, the birthweight of babies born to DP mothers positive for tEPEC at baseline was on average 211.7 g lower than that of babies whose mothers did not have tEPEC at baseline (CI95 = -355.3 g, -68.2 g, p = 0.005, Table S13). With respect to EAEC, we found using adjusted linear regression for each drug group separately that the birthweight of babies born to SP mothers with detectable EAEC at baseline was on average -207.3g lower than that of babies born to SP mothers without prior EAEC (CI95 = -393.2 g, -21.4 g, p = 0.030, Table S13). The birthweight of babies among DP mothers did not differ by pre-existing EAEC infection (Table S13). This led us to next compare the IPTp regimens head-to-head to determine whether SP or DP associated with improved birthweight as a function of EAEC and tEPEC positivity at baseline. We used adjusted linear regressions to compare birthweights between SP and DP recipients for each of the following subgroups: EAEC positive mothers, EAEC negative mothers, tEPEC positive mothers, and tEPEC negative mothers. We found that SP recipients women who were EAEC negative at baseline had infants who were 156.0 g heavier at birth than infants of DP recipients (CI95 = -18.0 g, 336.9 g, p = 0.087, Figure 6b), though confidence intervals crossed the null. This was not observed among women with EAEC at baseline: mean birthweight difference SP vs. DP = -99.5 g, CI95 = -329.9 g, 130.9 g, p = 0.390, Figure 6b). There was no difference in birthweight when comparing IPTp regimens by pre-existing tEPEC (Figure 6b): adjusted mean birthweight SP vs. DP among tEPEC negative women at baseline = -1.1g, CI95 = -134.5 g, 132.3 g, p = 0.995); adjusted mean birthweight SP vs. DP among tEPEC positive women at baseline: -166.7 g CI95 = -618.0 g, 263.1 g, p = 0.372).
Discussion
We conducted an ancillary study to a randomized control trial comparing IPTp with sulfadoxine-pyrimethamine (SP) to IPTp with dihydroartemisinic-piperaquine (DP) in pregnant Malawian women to investigate sources of malaria-independent effects of IPTp-SP on birthweight. Previous observations8–10 and a recent metanalysis of three large randomized controlled trials comparing IPTp-SP and IPTp-DP in Uganda and Kenya11 have indicated that despite widespread SP resistant malaria parasites, IPTp-SP is still effective at preventing low birthweight, likely through malaria-independent effects. We hypothesized that SP's mechanism of protection may be mediated by maternal effects, such as the promotion of gestational weight gain and/or effects in the gut where SP may be inhibiting common intestinal pathogens. We first found that recipients of three doses of SP had higher BMI, MUAC and GWG than recipients of three doses of DP. We also report for the first time that GWG was a mediator of the birthweight and IPTp-SP relationship, as GWG was independently and positively associated with SP therapy, but not DP therapy, and with birthweight in the SP group, but not DP group. This suggests that when the mother is treated with SP, her baby's birthweight is mediated by her own weight gain during the second and third trimesters. These novel findings are in line with previous hypotheses of malaria-independent mechanisms through which SP confers protection from low birthweight, namely via changes to maternal factors.27 Mechanistic studies in mice have found that antibiotic-induced changes in the maternal gut environment led to alterations in lipid and cholesterol metabolism, which increase adiposity28 and that the maternal gut environment plays a key role in the metabolic programming of the offspring.13 In humans, early infant antibiotic use correlates with increased BMI29 and in infants and older children it was shown to increase weight gain; co-trimoxazole, an antifolate drug closely related to SP, is associated with improved growth in paediatric HIV cohorts.30,31 Deciphering how SP promotes maternal weight gain during pregnancy, and how this is linked to infant birthweight, warrants further investigation. A study of the effect of SP on the intestinal microbiomes of these pregnant women is now in progress and will test the hypothesis that SP alters the composition of the maternal faecal microbiome in characteristic ways that are associated with GWG and birthweight (Waltmann et al., in preparation).
In this study, we found a substantial burden of enteric pathogens in Malawian pregnant women from our study area. At their first antenatal care visit (16-28 weeks gestation), two thirds of mothers were infected with at least one detected pathogen and a quarter were burdened by three or more (with a maximum of six) concurrent pathotypes. Enteroaggregative E. coli (EAEC) and atypical enteropathogenic/enterohemorrhagic E. coli (aEPEC/EHEC) were the two most frequently detected, with all mixed infections harbouring at least one of them. While neither IPTp arm led to reduced overall pathogen burden over the course of pregnancy, SP was found to be strongly associated with reduced odds of EAEC carriage only, in a dose-dependent manner. After ≥3 doses of SP, as recommended by the WHO4 for IPTp, SP recipients were approximately 90% less likely to be infected with EAEC than mothers who received 3 doses of DP. While the significant effect of SP on EAEC after 3 doses in our study could reflect chance and our current data cannot prove mechanistically that SP is targeting EAEC, there are several lines of evidence that make it plausible that SP's effect on EAEC is a biological finding and may explain, at least partially, why in our study SP did not influence the other E. coli pathotypes. First, EAEC is thought to adhere to both the small and large bowel epithelia in biofilms.38,39 This characteristic aggregation at the mucosal epithelium may be reminiscent of sequestering malaria parasites at the epithelium of the intervillous spaces of the placenta.40,41 Secondly, there are overlaps in human ligands between EAEC and P. falciparum. The human receptor of EAEC was recently defined as heparan sulphate.42 Heparan sulphate are a group of carbohydrate chains that contain sulphate modifications and can be carried by Syndecan-1 proteins in the intestine.43 Syndecan-1 is not only found on the syncytiotrophoblast, the specialized epithelia layer that covers interior of the villous of the placenta40,41 where P. falciparum parasites during pregnancy sequester, but it also carries the known human ligand of P. falciparum during pregnancy, namely chondroitin sulphate A (CSA).4 Third, sulphonamides, like SP, have been shown to be effective at disrupting biofilms. Previous studies have demonstrated the inhibitory effect of antifolates sulfamethoxazole-trimethoprim and sulfadiazine-pyrimethamine against cryptococcal biofilms44 and to some extent staphylococcal biofilms.45
Notably, SP's beneficial effect on GWG, MUAC, and BMI was not seen or was lessened when prior to IPTp initiation mothers had detectable EAEC, tEPEC, aEPEC/EHEC or LT-ETEC. This led us to evaluate whether the maternal carriage of pathogens also influenced birthweight. Using sub-group analyses among SP recipients and DP recipients separately, we found a significant negative effect on birthweight when mothers of either IPTp group tested positive for tEPEC at baseline. In similar analyses comparing EAEC positive mothers to EAEC negative mothers at enrolment for each IPTp group separately, we found that EAEC among SP mothers was associated with a significant negative effect on birthweight. These results raised the question which IPTp regimen favoured increased birthweight in the context of maternal carriage of EAEC and tEPEC. Compared to DP, the beneficial effect of SP on birthweight appeared to be modified by the pre-treatment detection of EAEC only, as babies born to SP-recipients without detectable EAEC at baseline appeared to be heavier than babies born to DP-recipients (mean increase in birthweight SP vs. DP = 156 g, CI95 = -18.0, 336.9 g, p = 0.087). These results suggest that SP's beneficial effect on birthweight via maternal GWG coincides with undetectable levels of EAEC at the enrolment (pre-treatment) visit, which is strengthened by our finding that compared to DP, SP mothers without prior EAEC had the highest GWG.
Thus, overall our results found several significant associations: we detected a positive effect of SP vs. DP on nutritional indicators (BMI, MUAC) and GWG, which coincided with a significant reduction in the odds of carrying EAEC throughout pregnancy in SP mothers vs. DP mothers; we found that GWG was a mediator of the IPTp-SP and birthweight relationship and detected that EAEC, tEPEC, aEPEC/EHEC or LT-ETEC positivity prior to the first dose of IPTp-SP lessened SP's benefit on increased GWG; we also detected significant negative effects on birthweight by EAEC and tEPEC when conducting sub-group analyses of SP mothers and DP mothers separately. This is the first study, to our knowledge, of the effects of multiple maternal gut pathogens during pregnancy and birthweight, but it has limitations, particularly when comparing IPTp groups with respect to birthweight as a function of maternal EAEC positivity at enrolment, for which we were likely underpowered. Low birthweight is multifactorial with several factors likely occurring simultaneously, posing a limitation on inferring individual effects, including factors that were unaccounted for in our analysis (e.g., socioeconomic status, pre-pregnancy weight or maternal sexually-transmitted infections7). In addition, the heterogeneity of EAEC strains may be an important confounder when investigating the relationship between EAEC and health outcomes. Additional data from larger studies are needed to formally test the hypothesis that maternal carriage of EAEC in the second and third trimesters prior to IPTp initiation is associated with decreased birthweight in the context of IPTp-SP. If this is found to be the case, further mechanistic studies may also consider exploring whether this is due to long-lasting damage by EAEC at the intestinal mucosa, consistent with known aspects of EAEC pathogenesis (i.e., biofilm formation)38,39 or due to a prophylactic, rather than curative, mode of action preventing new EAEC infections, which would be consistent with SP's antifolate activity7,12 and its long half-life.32
Though EAEC carriage during pregnancy and its effect on gestational weight gain and birthweight have not been investigated previously and the unknown clinical relevance of EAEC in this context warrants further investigation, in children, however, the deleterious effect of EAEC on growth is well documented.33,34 It is worth noting that several of the other pathogens in our panel are also associated with childhood growth faltering.35 EAEC is commonly detected in the gut of malnourished children and is considered a contributor to growth shortfalls during the first two years of life, even in the absence of diarrhoea.34 EAEC is associated with intestinal inflammation markers in humans, specifically higher levels of faecal myeloperoxidase (MPO),33,36 which in turn correlate with poor linear growth among children in Brazil, Bangladesh, and the Gambia.36 Recent mechanistic studies in mice have shown that EAEC mediates intestinal inflammation and impairs growth.37 Whether these effects by EAEC also occur in utero remains to be seen.
Taken together, our findings that gestational weight gain of Malawian pregnant women mediates the relationship between IPTp-SP and improved birthweight are significant in the context of SP's waning antimalarial activity but continued protection against low birthweight.2,6 Generalisability of these findings remains to be determined. Currently available data from other studies would allow for assessment of this association and should be pursued. In addition, follow-up metagenomic, proteomic, and metabolic studies will shed light on the mechanism of enhanced maternal weight gain, and reveal how specific pathogens like EAEC or tEPEC, which we found to be associated with lower gestational weight gain and birthweight in the SP group, the host immune system at the intestinal and placental mucosa, and the microbiota are interrelated to affect gestational weight gain and birthweight.
Declaration of interests
The authors have no competing interests to disclose.
Acknowledgments
Contributors
Conceptualization, funding acquisition, writing – review & editing, project administration: JRG, SRM. Investigation, data curation, formal analysis, methodology, validation, visualization, writing – original draft, writing – review & editing: AW. Conceptualization, funding acquisition, writing – review & editing, resources, methodology: IC. Writing – review & editing, resources, methodology, supervision: LAB. Investigation, project administration, writing – review & editing: JC. Resources, methodology, writing – review & editing: ETRM, DJO. Investigation, project administration, writing – review & editing: MI, DPM. Investigation, project administration: EM. Investigation: MK. Investigation, data curation: SMP. Methodology, supervision: JJJ.
Acknowledgments
This study and its findings are dedicated to the amazing scientist and mentor that made it all happen, Steven R Meshnick. Your wisdom and support will never be forgotten. Vale Steve. We would like to sincerely extend our gratitude to the study participants, their families, and their communities. We are thankful to Dr. José Villar, the Principal Investigator of INTERGROWTH-21, for his valuable advice and best practices in estimating gestational weight gain for the present study. We are thankful to the reviewers involved in the peer review process, whose thoughtful and careful examination of the data strengthened our manuscript. The findings and conclusions presented in this manuscript are those of the authors and do not necessarily reflect the official position of the U.S. Centers for Disease Control and Prevention. The research leading to these results (data collection, analysis and interpretation) has received support from the National Institutes of Health (5R21AI125800-02). The parent comparing IPTp-SP to IPTp-DP, with activities relevant to the current study (patient recruitment, specimen, and data collection) was funded by the US President's Malaria Initiative through CDC Cooperative agreement U01GH001206 to the Malaria Alert Centre.
Data sharing statement
Deidentified participant data used in this study and the study protocol will be made available upon reasonable request and approval by Don P. Mathanga and Julie R. Gutman. Requests can be made by email to the corresponding author.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103871.
Appendix. Supplementary materials
References
- 1.Cates J.E., Unger H.W., Briand V., et al. Malaria, malnutrition, and birthweight: a meta-analysis using individual participant data. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai M., Gutman J., Taylor S.M., et al. Impact of sulfadoxine-pyrimethamine resistance on effectiveness of intermittent preventive therapy for malaria in pregnancy at clearing infections and preventing low birth weight. Clin Infect Dis. 2016;62:323–333. doi: 10.1093/cid/civ881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger H.W., Ome-Kaius M., Wangnapi R.A., et al. Sulphadoxine-pyrimethamine plus azithromycin for the prevention of low birthweight in Papua New Guinea: a randomised controlled trial. BMC Med. 2015;13:9. doi: 10.1186/s12916-014-0258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO . World Health Organization; 2014. WHO Policy Brief for the Implementation of Intermittent Preventive Treatment in Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP) Geneva. January 2014. [Google Scholar]
- 5.Chico R.M., Cano J., Ariti C., et al. Influence of malaria transmission intensity and the 581G mutation on the efficacy of intermittent preventive treatment in pregnancy: systematic review and meta-analysis. Trop Med Int Health. 2015;20:1621–1633. doi: 10.1111/tmi.12595. [DOI] [PubMed] [Google Scholar]
- 6.van Eijk A.M., Larsen D.A., Kayentao K., et al. Effect of plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of intermittent preventive therapy for malaria in pregnancy in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19:546–556. doi: 10.1016/S1473-3099(18)30732-1. [DOI] [PubMed] [Google Scholar]
- 7.Chico R.M., Chaponda E.B., Ariti C., Chandramohan D. Sulfadoxine-pyrimethamine exhibits dose-response protection against adverse birth outcomes related to malaria and sexually transmitted and reproductive tract infections. Clin Infect Dis. 2017;64:1043–1051. doi: 10.1093/cid/cix026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai M., Gutman J., L'Lanziva A., et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet. 2015;386:2507–2519. doi: 10.1016/S0140-6736(15)00310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kajubi R., Ochieng T., Kakuru A., et al. Monthly sulfadoxine-pyrimethamine versus dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnancy: a double-blind, randomised, controlled, superiority trial. Lancet. 2019;393:1428–1439. doi: 10.1016/S0140-6736(18)32224-4. [DOI] [PubMed] [Google Scholar]
- 10.Kakuru A., Jagannathan P., Muhindo M.K., et al. Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N Engl J Med. 2016;374:928–939. doi: 10.1056/NEJMoa1509150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roh M.E., Kuile F.O.T., Rerolle F., et al. Overall, anti-malarial, and non-malarial effect of intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine on birthweight: a mediation analysis. Lancet Glob Health. 2020;8:e942. doi: 10.1016/S2214-109X(20)30119-4. e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capan M., Mombo-Ngoma G., Makristathis A., Ramharter M. Anti-bacterial activity of intermittent preventive treatment of malaria in pregnancy: comparative in vitro study of sulphadoxine-pyrimethamine, mefloquine, and azithromycin. Malar J. 2010;9:303. doi: 10.1186/1475-2875-9-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura I., Miyamoto J., Ohue-Kitano R., et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science. 2020:367. doi: 10.1126/science.aaw8429. [DOI] [PubMed] [Google Scholar]
- 14.Carroll I.M., Ringel-Kulka T., Keku T.O., et al. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;301:G799–G807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J., Gratz J., Amour C., et al. A laboratory-developed TaqMan array card for simultaneous detection of 19 enteropathogens. J Clin Microbiol. 2013;51:472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J., Kabir F., Manneh J., et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: a multicentre study. Lancet Infect Dis. 2014;14:716–724. doi: 10.1016/S1473-3099(14)70808-4. [DOI] [PubMed] [Google Scholar]
- 17.Kelly A., Kevany J., de Onis M., Shah P.M. A WHO collaborative study of maternal anthropometry and pregnancy outcomes. Int J Gynaecol Obstet. 1996;53:219–233. doi: 10.1016/0020-7292(96)02652-5. [DOI] [PubMed] [Google Scholar]
- 18.Cates J.E., Westreich D., Unger H.W., et al. Intermittent preventive therapy in pregnancy and incidence of low birth weight in malaria-endemic countries. Am J Public Health. 2018;108:399–406. doi: 10.2105/AJPH.2017.304251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferraro Z.M., Barrowman N., Prud'homme D., et al. Excessive gestational weight gain predicts large for gestational age neonates independent of maternal body mass index. J Matern Fetal Neonatal Med. 2012;25:538–542. doi: 10.3109/14767058.2011.638953. [DOI] [PubMed] [Google Scholar]
- 20.Unger H.W., Wangnapi R.A., Ome-Kaius M., et al. Azithromycin-containing intermittent preventive treatment in pregnancy affects gestational weight gain, an important predictor of birthweight in Papua New Guinea - an exploratory analysis. Matern Child Nutr. 2016;12:699–712. doi: 10.1111/mcn.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamo K.B., Ferraro Z.M., Brett K.E. Can we modify the intrauterine environment to halt the intergenerational cycle of obesity? Int J Environ Res Public Health. 2012;9:1263–1307. doi: 10.3390/ijerph9041263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferraro Z.M., Contador F., Tawfiq A., Adamo K.B., Gaudet L. Gestational weight gain and medical outcomes of pregnancy. Obstet Med. 2015;8:133–137. doi: 10.1177/1753495X15591320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nohr E.A., Vaeth M., Baker J.L., Sorensen T., Olsen J., Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008;87:1750–1759. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- 24.Cheikh Ismail L., Bishop D.C., Pang R., et al. Gestational weight gain standards based on women enrolled in the fetal growth longitudinal study of the INTERGROWTH-21st Project: a prospective longitudinal cohort study. BMJ. 2016;352:i555. doi: 10.1136/bmj.i555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenland S., Pearl J., Robins J.M. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 26.Hernan M.A., Hernandez-Diaz S., Werler M.M., Mitchell A.A. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 27.Gutman J., Slutsker L. Intermittent preventive treatment with sulfadoxine-pyrimethamine: more than just an antimalarial? Am J Trop Med Hyg. 2017;96:9–10. doi: 10.4269/ajtmh.16-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho I., Yamanishi S., Cox L., et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dingens A.S., Fairfortune T.S., Reed S., Mitchell C. Bacterial vaginosis and adverse outcomes among full-term infants: a cohort study. BMC Pregnancy Childbirth. 2016;16:278. doi: 10.1186/s12884-016-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boettiger D.C., Muktiarti D., Kurniati N., et al. Early height and weight changes in children using cotrimoxazole prophylaxis with antiretroviral therapy. Clin Infect Dis. 2016;63:1236–1244. doi: 10.1093/cid/ciw514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prendergast A., Walker A.S., Mulenga V., Chintu C., Gibb D.M. Improved growth and anemia in HIV-infected African children taking cotrimoxazole prophylaxis. Clin Infect Dis. 2011;52:953–956. doi: 10.1093/cid/cir029. [DOI] [PubMed] [Google Scholar]
- 32.Weidekamm E., Plozza-Nottebrock H., Forgo I., Dubach U.C. Plasma concentrations in pyrimethamine and sulfadoxine and evaluation of pharmacokinetic data by computerized curve fitting. Bull World Health Organ. 1982;60:115–122. [PMC free article] [PubMed] [Google Scholar]
- 33.Platts-Mills J.A., Taniuchi M., Uddin M.J., et al. Association between enteropathogens and malnutrition in children aged 6-23 mo in Bangladesh: a case-control study. Am J Clin Nutr. 2017;105:1132–1138. doi: 10.3945/ajcn.116.138800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogawski E.T., Guerrant R.L., Havt A., et al. Epidemiology of enteroaggregative Escherichia coli infections and associated outcomes in the MAL-ED birth cohort. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.M-EN. Investigators Childhood stunting in relation to the pre- and postnatal environment during the first 2 years of life: The MAL-ED longitudinal birth cohort study. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerrant R.L., Leite A.M., Pinkerton R., et al. Biomarkers of environmental enteropathy, inflammation, stunting, and impaired growth in children in northeast Brazil. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartelt L.A., Bolick D.T., Mayneris-Perxachs J., et al. Cross-modulation of pathogen-specific pathways enhances malnutrition during enteric co-infection with Giardia lamblia and enteroaggregative Escherichia coli. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hicks S., Candy D.C., Phillips A.D. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun. 1996;64:4751–4760. doi: 10.1128/iai.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nataro J.P., Kaper J.B., Robins-Browne R., Prado V., Vial P., Levine M.M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987;6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Baston-Bust D.M., Gotte M., Janni W., Krussel J.S., Hess A.P. Syndecan-1 knock-down in decidualized human endometrial stromal cells leads to significant changes in cytokine and angiogenic factor expression patterns. Reprod Biol Endocrinol. 2010;8:133. doi: 10.1186/1477-7827-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayres Pereira M., Mandel Clausen T., Pehrson C., et al. Placental sequestration of plasmodium falciparum malaria parasites is mediated by the interaction between VAR2CSA and chondroitin sulfate A on syndecan-1. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajan A., Robertson M.J., Carter H.E., et al. Enteroaggregative E. coli Adherence to human heparan sulfate proteoglycans drives segment and host specific responses to infection. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexopoulou A.N., Multhaupt H.A., Couchman J.R. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol. 2007;39:505–528. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 44.de Aguiar Cordeiro R., Mourao C.I., Rocha M.F., et al. Antifolates inhibit Cryptococcus biofilms and enhance susceptibility of planktonic cells to amphotericin B. Eur J Clin Microbiol Infect Dis. 2013;32:557–564. doi: 10.1007/s10096-012-1774-8. [DOI] [PubMed] [Google Scholar]
- 45.El Haj C., Murillo O., Ribera A., et al. Evaluation of linezolid or trimethoprim/sulfamethoxazole in combination with rifampicin as alternative oral treatments based on an in vitro pharmacodynamic model of staphylococcal biofilm. Int J Antimicrob Agents. 2018;51:854–861. doi: 10.1016/j.ijantimicag.2018.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.