Abstract
In developing countries, fermentation is one of the traditional food processing methods for production of relatively safe and nutritious foods. Among many fermented foods in Ethiopia, “Shameta” is one of the locally produced and consumed cereal-based fermented porridge mainly used to support strength and recovery of lactating women after birth. However, even though the product is consumed for years, so far, the nutritional composition and bioactive compounds of the porridge not yet scientifically determined. This study aimed to determine the physicochemical properties, nutritional composition and bioactive compounds of “Shameta” commonly produced and consumed in Western part of the country. A total of 27 “Shameta” samples were collected from houses of lactating mothers residing in different districts of East Wollega zone. Results showed that, “Shameta” sample prepared from blend of maize and barely supplemented with faba bean results in crude protein content of 11.2 g/100g as compared with samples without faba bean, 6.8 g/100g. Samples supplemented with more proportion of rapeseed as oil source resulted in relatively higher crude fat content (12.2 g/100 g) as compared to other samples. From energy point of view, “Shameta” could provide about 85% of the extra energy needs of lactating mothers as compared to staple foods consumed in sample collection areas. It is also confirmed that, the product is a good source of iron and zinc, with the highest scores of 8.1 and 8.6 mg/100g in some samples, respectively, as compared to other mineral elements whose scores were much less than the daily recommended allowances. The average phytate and tannin contents were 0.79 and 0.18 mg/100g, respectively. Even though the Ca, Fe and Zn contents were below the recommended daily allowance, their bioavailability could not be hindered by phytate and tannin. Results also showed that samples have good antioxidant potential to minimize oxidative stresses. It could be deduced that as a sole food for the mothers, the product could not provide sufficient protein and some minerals to meet recommended daily allowance. However, to enhance the importance of the product, it is necessary to optimize the ingredient compositions and processing conditions to meet the nutrient demand of lactating mothers.
Keywords: Fermented porridge, Lactating mothers, Nutrition, “Shameta”
Fermented porridge; Lactating mothers; Nutrition; “Shameta”.
1. Introduction
Fermentation, as a commonly food processing method, has the potential to improve food quality in terms of nutrient content, flavor, taste, and safety (Hasan et al., 2014). Traditional fermented foods are produced from locally available ingredients using household equipment and indigenous knowledge (Abegaz et al., 2002; Binitu et al., 2015). As a result, fermentation is a locally adapted processing method that enables the production of nutritious less expensive foods from locally available resources (Adams, 1990).
In developing countries, the health of lactating mothers and newborns is commonly affected by malnutrition during pregnancy and lactation. In Ethiopia, the dietary intake and nutritional status of lactating mothers are commonly complicated by different socio-economic factors. Because of this, the health and strength of lactating mothers and newborns are critically affected due to insufficient macro-and micro-nutrients, and less daily-recommended calories. According to studies conducted in different parts of the country, 20–40.6% of lactating mothers were underweight (Alemayehu et al., 2015; Kejela et al., 2020) and 52.2% had low dietary intake (Moges et al., 2018). Failure to gain the required weight, strength, and recovery by lactating mothers critically affects mothers’ health and the quality and volume of breast milk for the infants (Soliman et al., 2014).
Particularly, during lactation, due to lactogenesis process the daily nutrient and energy requirement of breastfeeding mothers increases by 30% as compared to the energy requirements at pregnancy time (Butte and King, 2005). Therefore, supporting the health and strength of the mothers' proper maternal nutrition during lactation is important. The type and quality of diet provided to the lactating mother not only determines the mother's health but also the health and growth of the infants. To fill this gap, in most cultures, based upon local knowledge, there are special dietary practices for lactating mothers as compared to the routine diets of the family to support quick recovery and restoration of mothers' body strength.
To prevent deficiencies and to correct nutritional vulnerability of lactating mothers, dietary diversification is one of the recommended nutritional interventions. “Shameta” is one of such traditional fermented porridge exclusively prepared for lactating mothers in the Southwest part of the country (Wollega zones of Oromia region) as an additional diet for delivered mothers. The current practices show that it is locally produced from a mixture of different cereal grains (maize, barley, sorghum, or wheat), with additions of rapeseed oil, different spices, and herbs to impart good flavor and with an assumption as a means to preserve the product. Unlike other fermented products in Ethiopia, ““Shameta”” preparation undergoes a double fermentation process. In the first stage, the main ingredient mixture is allowed to ferment for three days and cooked to make porridge. The cooked porridge once cooled to room temperature is subjected to the second phase of fermentation (14–30 days) after addition of the remaining ingredients (oil source, spices, and herbs). The two-stage fermentation process and intermediate cooking could change the physicochemical properties, nutritional compositions, bioactive contents, and antioxidant capacity of the product unlike other commonly fermented cereal based traditional foods.
Availability of low cost foods produced using local knowledge and resources could contribute to enhance dietary diversity, particularly in low income families. Weldehaweria et al. (2016) indicated that, economic capability is one of the major factors for lack of dietary diversity among lactating women in Aksum town of Northern Ethiopia. For better dietary diversity, it is good to have scientific information about nutritional composition and health promoting factors of locally made foods. In this regard, several studies have been conducted to determine the nutritional compositions of different types of local fermented foods in Ethiopia (Abegaz et al., 2002; Yohannes et al., 2013; Gebrelibanos, 2015). However, the physicochemical properties, nutritional composition, bioactive contents, and antioxidant capacity of “Shameta” commonly consumed by lactating mothers of Wollega Oromo community not yet scientifically addressed. Therefore, this study aimed to determine proximate compositions, minerals contents, anti-nutritional factors, and bioactive compounds of Shameta” collected from homes of lactating mothers. The information generated from this study can be used to guide the local community and other stakeholders on the beneficial use of the product through further optimization of ingredients and fermentation conditions for better nutrition, strength, and recovery of lactating mothers.
2. Materials and methods
2.1. Description of the study area
The study was carried out in the East Wollega zone of Oromia Regional State, located in the western part of Ethiopia at a latitude of 800 31″52 South and longitude of 360007‟51 East with a mean annual rainfall range of 1150–2070 mm/year and average annual temperature range of 18.5 °C–27.5 °C (Figure 1). Based on the Census conducted by the Central Statistical Authority of Ethiopia, this zone has 17 districts and a total population of 1,213,503, of whom 606,379 are men and 607,124 women; with an area of 12,579.77 Km2. Some of the major crops grown in the zone are maize, barley, sorghum, teff (Eragrostis tef), wheat, fava bean, sesame, groundnut, field pea, finger millet, potatoes, tomatoes, and hot-pepper (EWZFDOS, 2018). The zone was intentionally selected based on common use of “Shameta” in the diet of lactating mothers.
Figure 1.
Study area showing regional state, selected zonal administration and districts for the study.
2.2. Study population and sampling of households
“Shameta” is a fermented porridge solely produced and consumed by lactating mothers of Oromo community in Southwest part of Ethiopia. Eastern Wollega zone purposively selected for the study based upon its community rich experience and culture in production and use of “Shameta”. From the zone, four districts (Sibu Sire, Guto Gida, Jimma Arjo, and Wayu Tuka) (Figure 1) were purposefully selected for the study considering popular use of the product by lactating mothers. As inclusion criteria, lactating mothers who gave birth in health centers in between January 2–16, 2021 were considered as study population. This is intentionally done to collect samples having more or less similar fermentation age. Mothers who gave birth before and after specified date of data collection, and those who gave birth outside of health centers were excluded from the study. Significant deviation in processing method and ingredients types out of traditional practice was also considered one of the exclusion criteria. List of mothers from district heath offices who gave births in the specified time were considered as the study population. Accordingly a study population of 103 lactating mothers (35 from Sirbu Sire, 34 from Guto Gida, 23 from Jimma Arjo and 11 from Wayu Tuka) was considered for the study. Based upon proportion sampling method a total of 27 samples were randomly collected from four districts [Sibu Sire (9), Guto Gida (9) Jimma Arjo (6), and Wayu Tuka (3)]. Additional data collections related to ingredients used and other relevant information continues to the end of April 2021. The ethical clearance permit was secured from Jimma University College of Agriculture and Veterinary Medicine Research and Ethical Clearance Board before data and samples collected. Both data and sample collections were performed according to the guidance of the permit.
2.3. Collection of “Shameta” Samples
At the time of collection of samples, efforts were made to gather fresh samples based upon the delivery date of mothers (two weeks interval). As indicated above, 9 samples each from Sibu Sire and Guto Gida, 6 from Jimma Arjo and 3 from Wayu Tuka districts were collected as triplicate measurements. Based upon ingredients composition and fermentation time three similar samples were bulked and thoroughly mixed to catagorize into nine categories (Table 1).
Table 1.
Sample collection sites and ingredient compositions of “Shameta” sample. Each sample collected in triplicate and bulked to similar category based upon ingredients composition and fermentation time differences).
| Sample sources (districts) | Samples code | Major ingredients | Approximate premix added |
|
|---|---|---|---|---|
| Types of spices and herbs added | Rapeseed oil (%) | |||
| Sibu Sire | BMWS | Barley (86%) + Maize (5%) + wheat (5%) | F + BCU + Gr + R (0.5%) | 3.5 |
| BMS | Barley (89%) + Maize (5%) | BC + BC2+R (1.5%) | 4.5 | |
| MS | Maize (95%) | F + WC + Gr + R (0.5%) | 4.5 | |
| Guto Gida | MFG | Maize (86.5%) + Faba bean (5%) | F + BC + BCU + Gr + B (1%) | 7.5 |
| MG | Maize (91%) | G + BC + Gr + B (1%) | 8 | |
| MBFG | Maize (81%) + Barley (5%) + Faba bean (5%) | BC + WC + Gr + R (1.5%) | 7.5 | |
| Jimma Arjo | BMJ | Barley (87%) + Maize (5%) | F + Gr (0.5%) | 7.5 |
| BJ | Barley (96%) | F + Gr (0.5%) | 3.5 | |
| Wayu Tuka | MW | Maize (91%) | BCU + R (1.5%) | 7.5 |
BMWS = Barley (86%) + Maize (5%) + wheat (5%) from Sibu Sire, BMS = Barley (89%) + Maize (5%) from Sibu Sire, MS = Maize (95%) from Sibu Sire, MFG = Maize (86.5%) + Faba bean (5%) from Guto Gida, MG = Maize (91%) from Guto Gida, MBFG = Maize (81%) + Barley (5%) + Faba bean (5%) from Guto Gida, BMJ = Barley (87%) + Maize (5%) from Jimma Arjo, BJ = Barley (96%) from Jimma Arjo, MW = Maize (91%) from Wayu Tuka, F=Fenugreek, BCU = Black cumin, Gr = Garlic bulb, R = Rue leaf, BC = Black cardamom, Gn = Ginger, WC = White cumin, B=Basil leaf (n = 27).
The samples were collected using a sterilized glass bottle with a holding capacity of 500 ML and transported using an icebox. Then after, except for moisture content determinations, samples were dried at 50 °C for 24–30 h to reduce moisture content and converted into flour for further analysis. The flours then packed in moisture-proof plastic bags and stored in air-tight tin containers at 4 °C until analysis. All chemicals and reagents used were analytical grade originated from different suppliers.
2.4. Processing method of Shameta
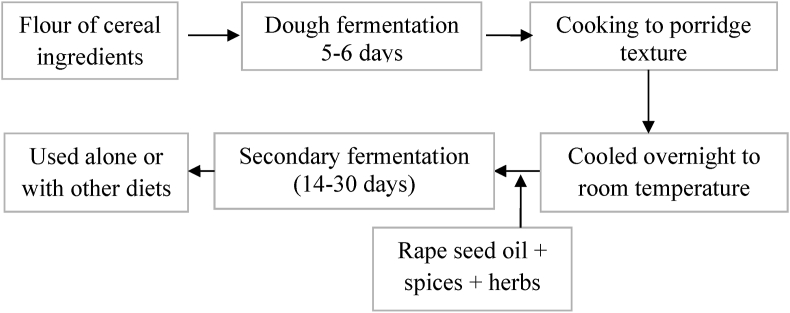
Processing of shameta involves use of cereal grains as major ingredients in three major processing phases. In the first phase, thick dough of major cereal and pulse ingredients subjected to 5–6 days of fermentation in an earthenware pot depending on ambient air temperature. In the second phase, the partially fermented dough thoroughly cooked to make porridge to improve the taste and flavor of the product and to inactivate potential spoilage and pathogenic microorganisms from spontaneous fermentation of the first phase. During cooking grounded rape seed as an oil source and available spices (fenugreek, black cumin seed, black cardamom, and white cumin) and herbs (Rue and Basil leaves) are added to enhance the flavor of the product and as a preservative means to inhibit growth of potential food microorganisms. Cooled porridge to ambient temperature then transferred into thoroughly washed and fumigated clay pot with smoke of dried steam of Olea Africana for 10–20 min. This is done with the assumption that to add more flavor to the product and as a means to kill potential food microorganisms inside the clay pot. Few leaves of the herbs, bulbs of garlic and slices of ginger are placed at different depths of porridge before sealing of the fermentation pot. The pot sealed with its lid and covered tightly with plastic sheet to avoid or minimize migration of outside air into the fermentation pot. Depends upon ingredient types, ambient air temperature and flavor type preferred the second stage of fermentation lasts for two to four weeks. The aroma of the fermented product is an indicator of the degree of maturity of fermented Shameta. Matured Shameta gives a strong cheesy or spicy aroma when the fermentation vessel opened. However, Shameta from immature fermentation results in undesirable type of aroma. Immature Shameta results in poor flavor development, less palatable, and could cause flatulence after consumption. In contrary to this, over fermentation makes the product sour due to excess acid production that results in burning sensation in the stomach and other discomforts to consumers. More summarized processing steps of shameta indicated in Figure 2.
Figure 2.
Follow diagram showing traditional preparation steps of Shameta.
2.5. Measurement of the physico-chemical properties
2.5.1. Measurement of viscosity and electrical conductivity
The viscosity of the “Shameta” samples was determined using a Brookfield Viscometer (Type LV-8 viscometers UK LTD) according to the method of Regenstein and Regenstein (1984), at room temperature using spindle No. 4 probe at a speed of 100 rpm. Readings were taken when the percentage value was around 50%.
The electrical conductivity (EC) of samples was determined (25±2 °C) by dissolving five gram of the sample in 20 ml of distilled water, and then 10 ml of the homogenate was pipette into a beaker. Finally, the sample was measured with a digital multi-parameter (Bante 900-UK) and the corresponding result was recorded (Lee et al., 2013).
2.5.2. Measurement of pH and titratable acidity (TA)
The pH was measured using a digital portable pH meter (pH-013, China) after homogenizing five gram of the sample in 20 ml distilled water followed by pipetting and measuring of 10 ml of the homogenized sample into a beaker (Abegaz, 2007). The TA of “Shameta” was determined by homogenizing 2.5 g of sample in 10 ml of distilled water and filtered through Whatman No. 1 filter paper (Antony and Chandra, 2019). In the filtrate 3 to 5 drops of one gram per 100 ml phenolphthalein indicator was added and samples were titrated with freshly prepared 0.1 mol/L NaOH solution until a faint pink color was persisted for 30 s. The TA value, in terms of lactic acid as a dominant organic acid in the product was determined using the following equation.
where; N = normality of titrant (mEq/ml), VNaOH = Volume of titrant (ml), Eq. wt = Equivalent weight of predominant acid (mg/mEq which is 90.08 for lactic acid), Vs = Volume of sample (ml) and 1,000 = factor relating mg to grams.
2.6. Measurement of nutritional compositions
2.6.1. Measurement of proximate composition
The standard method of the Association of Official Analytical Chemist (AOAC, 2000) was used to determine moisture (method No. 925.09), crude protein (method No 979.09), crude fat (method CAC/RM 55–1976), ash (method No 942.05) and crude fiber (method No 985.29) contents of “Shameta” samples. The total carbohydrate content was determined by difference and the gross energy according to Guyot et al. (2007).
2.6.2. Measurement of minerals content
Calcium, iron, zinc and magnesium contents were determined according to AACC (2000) (method No. 985.35) using atomic absorption spectrophotometer (AA 6800, Japan). To compensate for ionization interferences Lanthanum was used during analysis of Calcium and Magnesium. Sodium determination was conducted according to ISO (1992) (method No 8070:1987) using flame photometer (Cole-palmer, UK) and phosphorus using UV-vis spectrophotometer (AAS 220GF, Buck) at 823 nm using a molybdate-ascorbic acid colorimetric method (AOAC, 2000) (method 986.24).
2.7. Measurement of tannin and phytate contents
2.7.1. Condensed tannin content
Oven-dried “Shameta” from each sample was used for tannin content analysis. The condensed tannins content was determined according to the method described by Maxson and Rooney (1972). In screw caped test tube, one gram was mixed with 10 ml of 1% HCL in absolute methanol and shaken continuously for 24 h at room temperature using mechanical shaker (Hy-2(C), Shanghai, China). After centrifugation (1000 RPM, 5 min) (sigma 2-16KC, UK) one ml of the supernatant was transferred to another test tube and mixed with 5 ml of vanillin–HCl reagent. The D (+)-catechin was used as a standard in a series of concentrations (0, 15, 25, 35, 45, and 55 μg/ml) in 5 ml 1% HCl in methanol. After 20 min of incubation the absorbance of samples and standards was measured at 500 nm using UV-VIS Spectrophotometer (JASCO V-630, Shimadzu Corporation, Tokyo, Japan). The condensed tannins content was determined from a standard curve of catechin as mg/100g.
2.7.2. Phytate content
The method according to Vaintraub and Lapteva (1988) was used to determine the phytate contents of samples. About 0.5 g of the sample was extracted for one hr in 10 ml of HCL (2.4%) using mechanical shaker (Hy-2 (C), Shanghai, China) at room temperature. The clear supernatant of the extract was used after the sample centrifuged (Sigma 2-16KC, UK) at 3000 rpm for 30 min. Sodium salt of phytic acid was used to prepare the standard solution in 0.2 N HCL at series of concentrations (0.0, 5.0, 10.0, 20.0, 30.0, and 40.0 μg/ml of phytic acid). In 3 ml of the supernatant of centrifuged sample one ml of wade reagent was added and thoroughly mixed for 5 s using a vortex. The mix then centrifuged for 10 min and absorbance of the supernatant and standard solutions was measured at 500 nm using UV-VIS spectrophotometer (JASCO V-630, Shimadzu Corporation, Tokyo, Japan) using deionized water as a blank. The phytate content was determined from a standard curve of the sodium salt of phytic acid, and the result was reported in mg/100g.
2.8. Measurement of bioactive compounds
2.8.1. Extraction of samples
To extract the bioactive compounds, 2.5 g of sample was extracted with 25 mL of 80% aqueous methanol. Then, the mixture was placed on a mechanical shaker (Hy-2(C), Shanghai, China) for 24 h and centrifuged at 3000 rpm for 10 min. The clear supernatant was used to determine total phenol and flavonoid contents. Total antioxidant capacities of the samples were also determined from the same extracts.
2.8.2. Total phenols (TPC) contents
The total phenols content of the extract was determined by the Folin–Ciocalteu method (Kaur and Kapoor, 2002). One mg of crude extract was mixed thoroughly with one mL of Folin–Ciocalteu reagent followed by the addition of 0.8 mL of 7.5% (w/v) sodium carbonate (7.5 g of sodium carbonate in 100 ml of methanol). The mixture was allowed to stand for 60 min in the dark, and absorbance was measured at 765 nm using a UV spectrophotometer (Model SP9, PyeUnican UK). The concentrations for the standard calibration curve were 0.0, 5.0, 10.0, 25.0, 50.0 and 100 μg/mL and total phenolic contents was expressed in milligram of gallic acid equivalent (GAE) per gram of sample from the calibration curve (R2 = 0.997).
2.8.3. Total flavonoid (TFC) contents
Total flavonoid content was determined by the method of Olajire and Azeez (2011) using Aluminum chloride. Briefly, crude extract (0.6 mg) of the sample was mixed thoroughly with 4 ml distilled water. At the same time, 0.3 mL of 5% NaNO2 was added to the test tube and 0.3 mL of 10% AlCl3 after 5 min. After 5 min, 2 mL of 1M NaOH was added to the mixture. Then, the volume of the mixture was made to 10 mL by immediately adding 4.4 mL of distilled water. For the developed orange yellowish color, absorbance was measured at 510 nm using a UV spectrophotometer (Model SP9, PyeUnican UK). The concentrations for the standard calibration curve were 0.0, 6.0, 12.0, 25.0, 50.0 and 80.0 μg/mL. The concentration of flavonoid content was expressed in milligram of catechin equivalent (CE) per gram of sample from the calibration curve (R2 = 0.994).
2.8.4. Total antioxidant capacity (TAC)
The antioxidant capacity of the extract was determined by the 1, 1-diphenyl-2-picryl-hydroxyl (DPPH) assays, as described by Villano et al. (2007). One milligram of each extract was mixed with 4 ml solution (0.004 g DPPH in 100 ml methanol) and incubated in the dark at room temperature for one hour. The absorbance of the mixture was measured at 517 nm using a UV spectrophotometer (Model SP9, PyeUnican UK). The concentrations for the standard calibration curve were 0.00, 5.00, 10.00, 25.00, 50.00 and 100.00 μg/mL. The concentration of total antioxidant activities was expressed in milligrams of ascorbic acid equivalent (AAE) per gram of sample from the calibration curve (R2 = 0.998).
2.9. Molar ratios and bioavailability of minerals
The molar ratio values of phytate and minerals was determined by dividing the weight of phytate with atomic weight of specific mineral (phytate: 660 g/mol; Fe: 56 g/mol; Zn: 65g/mol; Ca: 40 g/mol) (FAO, 2018). The ratio values determines bioavailability of the minerals considering their critical ratio limits (Phytate: Calcium <0.17; Phytate: Iron <1 and Phytate: Zinc <5) as indicated in FAO/WHO (2004).
2.10. Experimental setup and data analysis
A complete randomized experimental design was used to conduct one way analysis of variance (ANOVA). This analysis helps to compare significance difference of two sample means for the measured parameters. Samples were determined in triplicates and all the statistical analyses were performed using SAS (SAS Institute and Cary, NC) version 9.3, and the significant difference was considered at p ≤ 0.05. Before analysis data were checked to meet the assumption of ANOVA (normality of data and homogeneity of variances). Fisher's least significant difference (LSD) was used for mean comparison tests to identify significant differences among means (p ≤ .05). The coefficient of variation (CV) of each parameter was determined from the standard deviation and grand mean of the parameter and the results were expressed as mean ± standard deviation.
3. Results and discussion
3.1. Physic-chemical properties
3.1.1. Viscosity and electrical conductivity
“Shameta” is usually a less viscous product at the time of consumption. The highest value of viscosity was observed from the MG sample (3,222.0 cP) and the lowest from MW (655.7 cP) with an average value of 1,329.4 cP. The variation in viscosity might be due to the difference in concentration of beta-glucan and starch in the ingredients, and the volume of water added (Berggren et al., 2017) during preparation. The authors reported that flours with high beta-glucan and starch content lead to a high viscosity due to their ability to absorb water, which corresponds to viscosity observed in “Shameta” sample rich in maize than barely as a major ingredient. Other macro-structural components from different ingredients could also play a role to modify the rheological property of the product. The average viscosity value of the present finding was greater than that of fermented non-alcoholic beverages namely Kunuzaki (10.3 cP), Agidi (84.6 cP), and 'Ogwo', a gruel like product consumed in Nigeria (1115.0 cP) (Zakari et al., 2010; Bede et al., 2015). The reduction in viscosity is beneficial in the sense that less viscous food products can be digested faster than the more viscous ones as it could encourage a higher intake of the food for better energy and recovery (Zakari et al., 2010) of lactating mothers.
The maen electrical conductivity (EC) in the present finding was 1.2 mS/cm with the highest value in MW (2.4 mS/cm) sample and the lowest value in BMS (0.66 mS/cm). The observed variability in electrical conductivity might be due to the variation in the concentration of different ions in the samples such as mineral salts, organic acids, and proteins. The EC value of the present study is in line with the values previously recorded in Korefe (1.06 mS/cm), but greater than Tella (0.69 mS/cm) (Shewakena et al., 2017) and Borde (0.89 ± 0.13) (Nemo and Bacha, 2020) which might be associated with more dilution effect of more water in the latter cases.
3.1.2. pH and titratable acidity (TA)
The pH and titratable acidity (TA) of “Shameta” samples collected for this study are presented in Table 2 pH values range from 3.6 to 4.4, which categorized the product as an acidic food which is expected from fermented products. This pH range is an indicator of the relative safeness of the food against certain low pH intolerant food borne pathogenic microorganisms (Garbutt, 1997; FAO, 1998). During our survey work (unpublished) the relative safeness of the food was also confirmed by the respondents. The highest and lowest pH values were from BMS and MG samples, which correspond with the lowest and highest TA values respectively. The variation in pH and TA of samples might be associated with differences in duration of fermentation, type of ingredients used and, roles and dynamics of microorganisms during the fermentation process (Yao et al., 2009). The average pH (4.0) recorded in the present study was greater than other fermented foods like Azo (pH, 3.81) and Cheka (pH, 3.74) (Gebrelibanos, 2015; Binitu et al., 2018), but in close agreement with Borde (pH < 4.2) (Ashenafi and Mehari, 1995). Similarly, the average TA (0.78%) in the present finding was greater than the value of Azo (0.35%) but lower than Cheka (0.94%) (Gebrelibanos, 2015; Binitu et al., 2018). The more acidic environment could affect the sensorial characteristics of the product and could also initiate stomach discomfort as indicated by the respondents during the survey work.
Table 2.
Physicochemical properties of “Shameta” samples collected from four different districts but grouped into nine categories according to their ingredient composition and fermentation time.
| Coded samples | Viscosity (cP) | pH | Titratable acidity (%) | Electrical conductivity (mS/cm) |
|---|---|---|---|---|
| BMWS | 1,150.2 ± 3.4e | 4.1 ± 0.1d | 0.78 ± 0.01c | 0.96 ± 0.02e |
| BMS | 777.8 ± 2.8f | 4.4 ± 0.01a | 0.57 ± 0.01f | 0.66 ± 0.01f |
| MS | 1,210.0 ± 2.7d | 4.1 ± 0.01d | 0.74 ± 0.02d | 0.77 ± 0.02f |
| MFG | 1,259.6 ± 2.6c | 3.9 ± 0.00e | 0.82 ± 0.01b | 1.4 ± 0.01b |
| MG | 3,222.0 ± 1.7a | 3.6 ± 0.02f | 1.1 ± 0.03a | 1.4 ± 0.1b |
| MBFG | 1,264.1 ± 0.75c | 3.9 ± 0.02e | 0.82 ± 0.00b | 1.3 ± 0.1cd |
| BMJ | 1,274.5 ± 1.3b | 4.2 ± 0.01b | 0.61 ± 0.00e | 0.78 ± 0.01f |
| BJ | 1,151.0 ± 5.9e | 3.9 ± 0.00e | 0.81 ± 0.01b | 1.3 ± 0.1cd |
| MW | 655.7 ± 6.3g | 4.2 ± 0.01c | 0.76 ± 0.01cd | 2.4 ± 0.1a |
| CV | 0.17 | 0.56 | 1.14 | 3.88 |
| MSD | 10.1 | 0.1 | 0.03 | 0.14 |
BMWS = Barley (86%) + Maize (5%) + wheat (5%) from Sibu Sire, BMS = Barley (89%) + Maize (5%) from Sibu Sire, MS = Maize (95%) from Sibu Sire, MFG = Maize (86.5%) + Faba bean (5%) from Guto Gida, MG = Maize (91%) from Guto Gida, MBFG = Maize (81%) + Barley (5%) + Faba bean (5%) from Guto Gida, BMJ = Barley (87%) + Maize (5%) from Jimma Arjo, BJ = Barley (96%) from Jimma Arjo, MW = Maize (91%) from Wayu Tuka, CV = Coefficient of variation, MSD = Minimum significant different. (Each sample collected in triplicate and bulked to similar category based upon ingredients composition and fermentation time differences, n = 27).
3.2. Nutritional compositions
3.2.1. Proximate compositions
The moisture contents (MC) of samples ranged between 62.7-71.3 g/100 g with an average value of 65.3 g/100 g. The variation might be associated with the ingredient used, the volume of water added, and fermentation duration of the product. When the average (65.3 g/100 g) moisture content is compared with other fermented foods, it is lower than that of Azo (79.4 g/100 g) and Borde (87.29 ± 3.21) (Nemo and Bacha, 2020) but greater than teff Injera (59.9%) (Gebrelibanos, 2015; Yegrem, 2019). Moisture-related microbial growth is a key factor contributing to food spoilage (Zambrano et al., 2019). Except for BJ and BMJ, the MC of all other samples was below 66% which can be categorized as one of intermediate-moisture-foods which may be prone to spoilage by less moisture tolerant spoilage or pathogenic microorganisms. However, as an intermediate-moisture-food, together with a relatively lower pH value (<4.4), the possibility of its spoilage by virulent vegetative pathogenic microorganisms could be less.
The crude protein contents of samples ranged between 6.8-11.2 g/100g with an average value of 8.6 g/100g. The value was significantly (p < 0.05) different among collected samples (Table 3) which might be due to variation in terms of ingredients used and duration of fermentation (Elenga et al., 2010; Pranoto et al., 2013). The high level of protein content (11.2 g/100g) from MBFG might be linked with the protein of fava bean (Kebebu et al., 2013). However, the lowest value (6.8 g/100 g) was from the BJ sample without fava bean. The average value in the present finding was greater than other types of fermented products in Ethiopia like Azo (4.5 g/100g) and Cheka (3.8 g/100g) (Gebrelibanos, 2015; Binitu et al., 2018), but in close agreement with Borde (9.6 g/100g) (Ashenafi and Mehari, 1995).
Table 3.
Proximate compositions (g/100g sample, db) of “Shameta” samples collected from four different districts but grouped into nine categories according to their ingredient composition and fermentation time.
| Samples coded | Moisture | Crude protein | Crude fat | Ash content | Fiber content | Carbohydrate content | Gross Energy (kcal/100g) |
|---|---|---|---|---|---|---|---|
| BMWS | 63.6 ± 0.2e | 7.7 ± 0.2e | 4.8 ± 0.1i | 2.3 ± 0.0c | 1.6 ± 0.1c | 83.6 ± 0.2b | 408.1 ± 0.9h |
| BMS | 60.9 ± 0.4g | 7.3 ± 0.1f | 6.8 ± 0.1g | 1.7 ± 0.0g | 2.5 ± 0.0a | 81.8 ± 0.1c | 417.3 ± 0.6f |
| MS | 64.8 ± 0.2d | 10.0 ± 0.1c | 8.7 ± 0.1e | 1.8 ± 0.0f | 2.6 ± 0.1a | 76.9 ± 0.2f | 425.7 ± 0.2d |
| MFG | 65.9 ± 0.4c | 10.4 ± 0.1b | 10.1 ± 0.0b | 2.6 ± 0.0b | 2.1 ± 0.0b | 74.8 ± 0.1h | 431.8 ± 0.2b |
| MG | 63.5 ± 0.1e | 9.7 ± 0.0d | 12.2 ± 0.2a | 1.9 ± 0.0e | 1.9 ± 0.1b | 74.2 ± 0.3i | 445.7 ± 0.6a |
| MBFG | 65.9 ± 0.4c | 11.2 ± 0.1a | 9.0 ± 0.0d | 2.1 ± 0.1d | 2.0 ± 0.1b | 75.7 ± 0.1g | 428.8 ± 0.5c |
| BMJ | 68.7 ± 0.1b | 7.2 ± 0.2f | 9.4±+0.1c | 1.7 ± 0.0g | 1.9 ± 0.1b | 79.8 ± 0.0e | 432.2 ± 0.3b |
| BJ | 71.3 ± 0.1a | 6.8 ± 0.1g | 5.4 ± 0.1h | 1.7 ± 0.0g | 2.1 ± 0.0b | 83.9 ± 0.1a | 411.9 ± 0.7g |
| MW | 62.7 ± 0.2f | 7.2 ± 0.0f | 8.1 ± 0.0f | 2.8 ± 0.0a | 1.3 ± 0.0d | 80.6 ± 0.0d | 423.8 ± 0.2e |
| CV | 0.39 | 2.24 | 1.18 | 1.07 | 3.66 | 0.15 | 0.12 |
| MSD | 0.7 | 0.3 | 0.3 | 0.1 | 0.2 | 0.2 | 1.4 |
BMWS = Barley (86%) + Maize (5%) + wheat (5%) from Sibu Sire, BMS = Barley (89%) + Maize (5%) from Sibu Sire, MS = Maize (95%) from Sibu Sire, MFG = Maize (86.5%) + Faba bean (5%) from Guto Gida, MG = Maize (91%) from Guto Gida, MBFG = Maize (81%) + Barley (5%) + Faba bean (5%) from Guto Gida, BMJ = Barley (87%) + Maize (5%) from Jimma Arjo, BJ = Barley (96%) from Jimma Arjo, MW = Maize (91%) from Wayu Tuka, CV = Coefficient of variation, MSD = Minimum significant different. (Each sample collected in triplicate and bulked to similar category based upon ingredients composition and fermentation time differences, n = 27).
During lactation time, mothers require 20 g/day more protein than the demand during a pre-pregnancy time (45 g/day). Accordingly “Shameta” from MBFG (11.2 g/100g) could meet close to 50% of the extra protein requirement for lactating mothers. This can supplement the protein demand of lactating mothers along other protein obtained from other staple foods consumed by the mothers and other family members. Consumption of “Shameta” as an additional meal to commonly consumed foods in the family could supplement the protein demand gap where animal products are not affordable and available. Related study indicated that Borde could meet 48% of the protein requirement during lactation (Abegaz et al., 2002) although the recent report made from a different study site (Nemo and Bacha, 2020) is not in support of the latter as it reported total protein content (g/100ml) of only 3.20 ± 0.39. The reasons for this variation in protein values as compared to “Shameta”, could be due to differences in local resources being used and differences during fermentation. Overall, as compared to other supplementary foods made from different ingredients for lactating mothers, “Shameta” can serve as an additional protein source for lactating mothers. Likewise, supplementary food made of wheat flour, milk powder, soybean flour, and carrots in Bangladesh meets the extra needs of protein (19 g/day) for lactating mothers (Mohammed et al., 2016), with protein contents even greater than what reported in this study. The relatively higher protein content in formulation from Bangladesh could be associated with added milk powder and soybean flour. This implies that further home-based fortification works may be necessary to enhance the protein content of MBFG based “Shameta” for better nutrition. However, “Shameta” improved the prevalence of minimum dietary diversity among lactating mothers in Ethiopia, as most of its associated factors are not related to household food insecurity (Fufa and Laloto, 2021).
Dietary fat intake during pregnancy and lactation affects pregnancy outcomes, child growth, development, and health (Koletzko et al., 2007). In the present finding, the contribution of 100 g of “Shameta” to crude fat contents was 12.2 g/100 g from the MG sample (highest) and 4.8 g/100g in the BMWS sample (lowest). The variation in terms of crude fat content might be associated with the volume of rapeseed oil added during the second phase of fermentation. The average value (8.3 g/100 g) in the present finding is greater than other cereal-based fermented beverages in Ethiopia including Borde (6.9 g/100g) and Cheka (1.3 g/100g)) (Ashenafi and Mehari, 1995; Binitu et al., 2018). The result of the study confirms that the consumption of “Shameta” could meet the dietary fat requirement of lactating mothers as compared to other cereal-based staple foods such as Injera (Abiyu et al., 2013; Cherie et al., 2018).
The ash contents of “Shameta” samples ranged from 1.7-2.9 g/100g with an average value of 2.1 g/100g. In the present finding, except for BMJ and BMS samples, the ash contents were significantly (p < 0.05) different from each other. The highest value was observed in the MW sample, while the lowest was in BMS (Table 3). The average value (2.1 g/100g) in the present finding is greater than what was reported from Cheka (0.75 g/100g) and wheat-based Borde (0.78 ± 0.12) (Nemo and Bacha, 2020), but lower than in maize-based Borde (3.7 g/100g) (Ashenafi and Mehari, 1995; Binitu et al., 2018). However, the present result is in close agreement with the ash content of Azo (2.3 g/100g) (Gebrelibanos, 2015). This implies that “Shameta” could contribute more minerals for lactating mothers as compared to Cheka commonly consumed in the southern part of Ethiopia.
The fiber contents ranged from 1.3-2.5 g/100g with an average value of 1.9 g/100g which are not significantly different (P > 0.05) from each other. The average value in the present finding is slightly greater than what was reported from Cheka (1.1 g/100g) (Binitu et al., 2018), but lower than the value reported in teff Injera (2.8 g/100g) (Yegrem, 2019). The dietary fiber has a protective effect against certain gastrointestinal diseases, constipation, colon cancer, obesity, stroke, and cardiovascular diseases (Ötles and Ozgoz, 2014) which can contribute to better health of the mothers.
The carbohydrate contents of “Shameta” samples were significantly (p < 0.05) different from each other as indicated in Table 3. The highest value was recorded from the BJ sample (83.9 g/100 g) and the lowest in the MG sample (74.2 g/100g), with an average value of 79.0 g/100g. The highest value recorded in sample BJ might be due to lower values of other proximate compositions since carbohydrate is determined by difference. The lowest carbohydrate recorded in sample MG might be due to increased microbial activity that requires energy and nutrient during fermentation hence a decrease in carbohydrates as the main source of energy (Simwaka et al., 2017). The result in the present finding is lower than spontaneously fermented Medida (87.1 g/100g) (Kabeir et al., 2004) but higher than other cereal-based fermented foods in Ethiopia such as Azo (16.6 g/100g) and Cheka (9.6 g/100g) (Gebrelibanos, 2015; Binitu et al., 2018). Carbohydrates are a good source of energy for lactating mothers to spare body protein (Thompson and Manore, 2005; Eggert et al., 2011). The Recommended Dietary Allowance (RDA) of carbohydrates for lactating women is 160 g/kg/day (Eggert et al., 2011); therefore, the average result may contribute 49.4% of the recommended requirement per day. Also as “Shameta” is made of different ingredients, it improved the dietary diversity of lactating mothers as minimum dietary diversity is prevalent in Ethiopia (Getacher et al., 2020; Fufa and Laloto, 2021). The result is similar to the carbohydrate value of Injera [spongy baked product made from teff [Eragrostis teff]] which contributes 48.7% of RDA (Cherie et al., 2018).
The gross energy of “Shameta” samples ranged from 408.1 kcal/100g (BMWS) to 445.7 kcal/100g (MG) with an average value of 425.0 kcal/100g (Table 3). The energy requirement for non-lactating mothers is 2300 kcal/day with extra energy (500 kcal/day) demand for exclusive breastfeeding from birth to 6 months postpartum (FAO/WHO, 2004). According to the average value (425.0 kcal/100g), “Shameta” could provide 85% of the extra energy required for lactating mothers. While other fermented cereal-based foods in Ethiopia such as Injera, Azo, and Cheka provides 76.8, 18.3, and 18.8% of the extra energy required for lactating mothers, respectively (Gebrelibanos, 2015; Binitu et al., 2018; Cherie et al., 2018), which are lower than present finding. Therefore, the consumption of “Shameta” with other common foods at different mealtimes would meet the energy requirements of lactating mothers.
3.2.2. Minerals content
The sodium (Na) content varied from 27.2 (BMJ) to 4.0 mg 100g-1 (MG) with average value of 16.8 mg 100g-1 (Table 4). The variation in Na content might be due to the effects of varieties of grains used, fermentation conditions, and other pre-fermentation processing methods (dehulling or removing seed coat, milling) and cooking (Lioger et al., 2007; Ullah et al., 2010; Suri and Sherry, 2016). The average value is lesser than what was reported in Ogi (66.0 mg 100g-1) and Mahewu (74.4 mg 100g-1) (Idowu et al., 2016; Okafor et al., 2017); but greater than that of Kutukutu (0.8 mg100g-1) (Roger et al., 2015). The value meets only 1.68% of the RDA of lactating mothers in the area, although it can be increased by adding table salt or through consumption of other foods rich in sodium. Although sodium-ion determines osmolality and directly contributes to arterial pressure maintenance, excessive dietary consumption has emerged as a major health problem associated with the development of several life-threatening disorders, such as hypertension, chronic kidney disease, and stroke (WHO, 2015).
Table 4.
Mineral contents of “Shameta” (mg/100g, db) samples collected from four different districts but grouped into nine categories according to their ingredient composition and fermentation time.
| Samples coded | Na | Ca | Mg | P | Fe | Zn |
|---|---|---|---|---|---|---|
| BMWS | 6.5 ± 0.2f | 14.8 ± 0.2e | 31.1 ± 0.2d | 400.6 ± 1.2b | 4.5 ± 0.07h | 3.1 ± 0.0f |
| BMS | 25.6 ± 0.3b | 17.0 ± 0.1c | 29.8 ± 0.3e | 83.1 ± 0.2g | 2.5 ± 0.01i | 2.1 ± 0.0g |
| MS | 4.4 ± 0.1g | 11.8 ± 0.1f | 31.1 ± 0.1d | 232.5 ± 0.1f | 5.5 ± 0.02f | 2.0 ± 0.0g |
| MFG | 24.3 ± 0.3c | 15.5 ± 0.2d | 29.1 ± 0.4f | 316.3 ± 0.3c | 7.2 ± 0.03b | 8.6 ± 0.1a |
| MG | 4.0 ± 0.1g | 15.5 ± 0.3d | 30.7 ± 0.3d | 234.0 ± 0.3e | 6.7 ± 0.03d | 1.8 ± 0.0h |
| MBFG | 14.8 ± 0.2e | 18.0 ± 0.1b | 33.6 ± 0.3b | 313.9 ± 0.2d | 7.0 ± 0.02c | 6.7 ± 0.0b |
| BMJ | 27.2 ± 0.3a | 10.0 ± 0.0g | 31.9 ± 0.1c | 233.2 ± 0.2ef | 5.0 ± 0.02g | 4.1 ± 0.0c |
| BJ | 27.0 ± 0.1a | 9.7 ± 0.1g | 29.7 ± 0.3e | 231.9 ± 0.2f | 6.1 ± 0.06e | 3.3 ± 0.0e |
| MW | 17.8 ± 0.2d | 31.6 ± 0.5a | 35.1 ± 0.1a | 483.3 ± 0.1a | 8.1 ± 0.10a | 3.9 ± 0.0d |
| DRA (mg/day)† | 1,500 | 1000 | 270 | 1000 | 9 | 12 |
| CV | 1.35 | 1.36 | 0.79 | 0.6 | 0.16 | 1.24 |
| MSD | 0.6 | 0.6 | 0.7 | 5.3 | 0.2 | 0.1 |
BMWS = Barley (86%) + Maize (5%) + wheat (5%) from Sibu Sire, BMS = Barley (89%) + Maize (5%) from Sibu Sire, MS = Maize (95%) from Sibu Sire, MFG = Maize (86.5%) + Faba bean (5%) from Guto Gida, MG = Maize (91%) from Guto Gida, MBFG = Maize (81%) + Barley (5%) + Faba bean (5%) from Guto Gida, BMJ = Barley (87%) + Maize (5%) from Jimma Arjo, BJ = Barley (96%) from Jimma Arjo, MW = Maize (91%) from Wayu Tuka, db = dry base, DRA = Daily Recommended Allowance, CV = Coefficient of variation, MSD = Minimum significant different. (Each sample collected in triplicate and bulked to similar category based upon ingredients composition and fermentation time differences, n = 27).
Calcium (Ca) is an essential micronutrient during pregnancy and lactation as it is associated with maternal bone mineralization, lower blood pressure, reduction of preterm deliveries, and building of bones and teeth of infants (Chan et al., 2006). In the present finding, the average calcium content of “Shameta” samples is 16.0 mg 100g-1 with the highest value recorded in MW (31.6 mg 100g-1) and the lowest in BJ (9.7 mg 100g-1). The mean calcium content of “Shameta” is lower than the content in Injera (167.7 mg 100g-1) (Yegrem, 2019), but slightly higher than that in Cheka (14.73 mg 100g-1) (Binitu et al., 2018). The present finding suggests that lactating mothers should get additional foods rich in Ca fulfill the requirements in line with the recommended daily allowance.
Magnesium (Mg) values of “Shameta” samples ranged from 29.7 (MW) to 35.1 mg 100g-1 (MFG) with an average value of 31.3 mg 100g-1 which could meet only 11.6% of the RDA for lactating mothers. Similar to the present results, the Mg content of Kutukutu (fermented corn paste) ranged from 28.0-34.1 mg 100g-1 (Roger et al., 2015). However, the value in this study is higher than the content in Burukutu (20.2 mg 100g-1) (Ogbonna et al., 2016). Mg is an essential mineral and a cofactor for hundreds of enzymes (FAO/WHO, 1998) and its inadequate dietary intake could be associated with increased risk of cardiovascular disease, osteoporosis, and metabolic disorders (Volpe, 2019).
The amount of Phosphorus (P) in the samples ranged from 83.1 (BMS)-483.3 mg 100g-1 (MW) with an average value of 281.0 mg 100g-1. The highest value in the MW sample (483.3 mg 100g-1) might be associated with the reduction of anti-nutritional substances such as phytates and tannin to form complexes with minerals (Songre-Oruattara et al., 2008). In present results, the recorded average value (281.0 mg 100g-1) only meets 40.1% of RDA for lactating mothers in the study area. However, the value is greater than the Phosphorus contents of Ogwo (98.0), Ogi (253.4), and Kutukutu (120.8 mg 100g-1) (Adegbehingbe, 2015; Roger et al., 2015; Okafor et al., 2017). The P retention is related to bone mineralization, lean body mass accretion, and protein retention which are very important during growth and development (Rigo et al., 2011).
Iron (Fe) contents in the present finding were significantly (p < 0.05) varied among samples (Table 4). The highest value was observed in MW (8.1 mg/100g) followed by the MFG sample (7.2 mg/100g), while the lowest was in BMS (2.5 mg/100g). The average value of Fe is 5.8 mg 100g-1 which meets 64.4% of RDA for lactating mothers. This value is lower than Fe contents of Cheka (18.3 mg 100g-1) and Injera (15.4 mg 100g-1) (Binitu et al., 2018; Yegrem, 2019), but greater than its content in Ogwo (0.34 mg 100g-1) (Adegbehingbe, 2015). However, it is similar to the iron content of Ogi (5.6 mg 100g-1) (Ojo and Enujiugha, 2018). Iron plays key roles in oxygen transport by red blood cells, energy production, growth, and development, functions particularly important during infancy for hematopoiesis, growth, and development (Brannon and Taylor, 2017). The requirements of iron (Fe) for lactating women is lower than that of pregnant and non-lactating women, because of the expectation that there will be no menstrual losses during the first 6 months postpartum, and the iron accumulated during the prenatal formation of maternal red blood cells can be recycled and used by the mother during postpartum (Thompson et al., 2008). However, iron shortage causes anemia which is affecting 50% of women of childbearing age in developing countries (Stevens et al., 2013). The value in present results showed that Shameta could help to recover the iron lost during birth through bleeding. However, complementing Shameta with iron-rich staple foods like teff-based 'Injera’ could help to meet the RDA for lactating mothers.
Zinc (Zn) contents of the present finding were significantly (p < 0.05) different among samples (Table 4). The highest zinc content was observed from MFG (8.6 mg/100 g) and lowest in MG (1.8 mg 100g-1) samples, with an average value of 4.0 mg 100g-1. The highest value observed in MFG might be due to the effects of double and prolonged fermentation time to release bounded minerals in macro-molecules of the ingredients. Zinc plays an important role in pregnancy and lactation, including fetal development and milk secretion (Khayat et al., 2017). In the present finding, the average value (4.0 mg 100g-1) for samples collected from the home of lactating mothers meets 33.3% of RDA and is relatively better than zinc contents of other fermented foods like Cheka, Injera, and Burukutu with values of 0.92, 2.4 and 1.7 mg 100g-1, respectively (Stephen et al., 2017; Binitu et al., 2018; Yegrem, 2019).
Results in this study also confirmed that, the minerals need of lactating mothers can't be achieved even using “Shameta” as a supplementary food. This might be further aggravated by lack of dietary diversity to meet recommended daily allowance. For instance a study conducted in central part of Ethiopia, showed that, approximately 51.2 % of lactating mothers were prone to micronutrients deficiencies due to lack of dietary diversity (Lemma et al., 2020).
3.3. Anti-nutritional factors and bioavailability of minerals
3.3.1. Phytate and tannin contents
Phytate content of “Shameta” samples ranged from 0.01-1.4 mg100g-1 with an average value of 0.79 mg 100g-1. The current value is lower than values reported from Ogwo (1.4 mg/100g), Kutukutu (12.4 mg/100g), and Lohoh (311.0 mg/100g) (Osman, 2010; Adegbehingbe, 2015; Roger et al., 2015). The variation in the concentration of phytate might be associated with a difference in the composition of ingredients and durations of fermentation. In addition to these, the observed low phytate content in the collected samples as compared to other staple foods could be due to the production and release of phytase and phosphatase enzymes by microorganisms during fermentation which results in hydrolysis of phytates to inositol and orthophosphates (Chaoui et al., 2003; Reale et al., 2004).
Phytate chelates several essential nutrients and digestive enzymes in the gastrointestinal tract of humans making them less bioavailable (Li et al., 1993; Reddy, 2002). Many studies support the hypothesis that phytate negatively impacts calcium, iron, and zinc bioavailability (Fredlund et al., 2006; Schlemmer et al., 2009). An increase by 500 mg/day of dietary phytate leads to a 0.04 mg/day reduction in zinc absorption (Miller et al., 2015); while Ndie and Okaka (2018) reported that levels of phytate between 23.5-130.65 mg/kg is high enough to be associated with health risk. However, as indicated in Table 5, the phytate values of all samples collected from homes of lactating mothers were below 1.5 mg 100g-1 which will not have a significant impact on the chelating of the minerals. Double fermentation before and after cooking could contribute to the degradation of phytate.
Table 5.
Anti-nutritional factors of “Shameta” samples (mg/100g, db) collected from four different districts but grouped into nine categories according to their ingredient composition and fermentation time.
| Samples coded | Phytate content | Tannin |
|---|---|---|
| BMWS | 0.78 ± 0.0c | 0.18 ± 0.0abc |
| BMS | 1.2 ± 0.0b | 0.22 ± 0.0ab |
| MS | 1.4 ± 0.1a | 0.13 ± 0.0c |
| MFG | 1.4 ± 0.1a | 0.15 ± 0.1c |
| MG | 0.1 ± 0.0d | 0.23 ± 0.0a |
| MBFG | 0.1 ± 0.0d | 0.16 ± 0.0bc |
| BMJ | 1.4 ± 0.1a | 0.18 ± 0.0abc |
| BJ | 0.01 ± 0.0d | 0.17 ± 0.0abc |
| MW | 0.7 ± 0.1c | 0.22 ± 0.0ab |
| CV | 7.3 | 12.4 |
| MSD | 0.2 | 0.1 |
BMWS = Barley (86%) + Maize (5%) + wheat (5%) from Sibu Sire, BMS = Barley (89%) + Maize (5%) from Sibu Sire, MS = Maize (95%) from Sibu Sire, MFG = Maize (86.5%) + Faba bean (5%) from Guto Gida, MG = Maize (91%) from Guto Gida, MBFG = Maize (81%) + Barley (5%) + Faba bean (5%) from Guto Gida, BMJ = Barley (87%) + Maize (5%) from Jimma Arjo, BJ = Barley (96%) from Jimma Arjo, MW = Maize (91%) from Wayu Tuka, CV = Coefficient of variation, MSD = Minimum significant different. (Each sample collected in triplicate and bulked to similar category based upon ingredients composition and fermentation time differences, n = 27).
Tannin content ranged from 0.13 (MS)-0.23 mg 100g-1 (MG) with an average values of 0.18 mg 100g-1 (Table 5) with no significant (p > 0.05) difference among most of the samples. This might be associated with double and/or prolonged fermentation which results in a reduction of tannin due to microbial phenyl oxidase action (Emambux and Taylor, 2003) and tannase activity by lactic acid bacteria (Molin, 2008). Intermediate cooking to make porridge could also make an additional contribution to the reduction of initial tannin concentration. The average value (0.18 mg 100g-1) is lower than what is indicated in other fermented products like ogwo (0.39 mg 100g-1) (Adegbehingbe, 2015) but greater than that of Korefe (21.8 mg/L) and Tella (29.7 mg/L) Shewakena et al. (2017).
Tannins are naturally occurring plant polyphenols, which bind and precipitate protein interfering with its digestion and absorption (Reddy and Pierson, 1994). Condensed tannins are known to inhibit several digestive enzymes, including amylases, cellulases, pectinases, lipases, and proteases (Bhat et al., 2013). They have major anti-nutritive properties that can negatively influence the digestibility of lipids, starch, and amino acids (Garcia et al., 2004; Brestensky et al., 2012). Osagie and Eka (1998) reported that a high level of dietary tannin (120 mg/kg) reduces the absorption of protein and damages the intestinal walls; however, Ndie and Okaka (2018) reported that levels of tannins up to 108.3 mg/kg is high enough to be associated with health risk. However, the tannin content of the present results is too low to bring negative effects on the human body. Therefore, as a fermented product, “Shameta” will not limit the bio-availability of minerals and proteins.
3.3.2. Bioavailability minerals
Fermentation increases the bioavailability of minerals like Mg, Ca, Zn, P, and Fe. As indicated above, this is likely due to the degradation of phytates that are complex with minerals thereby reducing their availability (Sripriya et al., 1997; Pranoto et al., 2013). In the present finding, the calculated molar ratio values of Phy: Ca, Phy: Fe, and Phy: Zn for estimation of mineral bioavailability are as shown in Table 6.
Table 6.
Estimated mineral bioavailability of different “Shameta” samples collected from four different districts but grouped into nine categories according to their ingredient composition and fermentation time.
| Samples coded | Phytate:Ca | Phytate:Fe | Phytate:Zn |
|---|---|---|---|
| BMWS | 3.2 × 10−3±2 × 10−4e | 1.5 × 10−2±8 × 10−4d | 2.5 × 10−2±1 × 10−3d |
| BMS | 4.3 × 10−3±1 × 10−4d | 4.1 × 10−2±9 × 10−4a | 5.6 × 10−2 ± 1.2 × 10−3b |
| MS | 7.3 × 10−3±6 × 10−4b | 2.2 × 10−2 ± 1.8 × 10−3c | 7.0 × 10−2±6 × 10−3a |
| MFG | 5.4 × 10−3±3 × 10−4c | 1.6 × 10−2±9 × 10−4d | 1.6 × 10−2±1 × 10−3e |
| MG | 3.0 × 10−4±2 × 10−5g | 9.0 × 10−4±7 × 10−5f | 4.0 × 10−3±3 × 10−4f |
| MBFG | 2.0 × 10−4±3 × 10−5g | 7.0 × 10−4±1 × 10−4f | 9.0 × 10−4±1 × 10−4f |
| BMJ | 8.8 × 10−3±4 × 10−4a | 2.4 × 10−2±9 × 10−4b | 3.4 × 10−2±1 × 10−3c |
| BJ | 1.0 × 10−4±3 × 10−5g | 2.0 × 10−4±8 × 10−5f | 4.0 × 10−4±1 × 10−4f |
| MW | 1.4 × 10−3±1 × 10−4f | 7.8 × 10−3±7 × 10−4e | 1.9 × 10−2±2 × 10−3e |
| CODEX† | <0.17 | <1 (preferable <0.4) | <15 |
| CV | 7.44 | 6.12 | 8.77 |
| MSD | 7 × 10−4 | 2.5 × 10−3 | 6.3 × 10−3 |
BMWS = Barley (86%) + Maize (5%) + wheat (5%) from Sibu Sire, BMS = Barley (89%) + Maize (5%) from Sibu Sire, MS = Maize (95%) from Sibu Sire, MFG = Maize (86.5%) + Faba bean (5%) from Guto Gida, MG = Maize (91%) from Guto Gida, MBFG = Maize (81%) + Barley (5%) + Faba bean (5%) from Guto Gida, BMJ = Barley (87%) + Maize (5%) from Jimma Arjo, BJ = Barley (96%) from Jimma Arjo, MW = Maize (91%) from Wayu Tuka, CV = Coefficient of variation, MSD = Minimum significant different. (Each sample collected in triplicate and bulked to similar category based upon ingredients composition and fermentation time differences, n = 27).
The average value of Phy: Ca, Phy: Fe, and Phy: Zn is 3.4 × 10−3, 1.4 × 10−2, and 2.5 × 10−2, respectively. This implies that all samples collected from homes of lactating mothers have a molar ratios value below the recommended maximum values of FAO/WHO (2004), proof for better minerals bioavailability. Similar to the present finding, Ogi has good bioavailability of Ca, Fe, and Zn with Phy: Ca, Phy: Fe, and Phy: Zn molar ratio values of 4.2 × 10−4, 1.5 × 10−2, and 5 × 10-2, respectively (Ojo and Enujiugha, 2018). The Phy: Fe and Phy: Zn molar ratio values of teff Injera, the other fermented staple food of Ethiopia were 4 and 12, respectively (Maren et al., 2014). According to the authors, Fe in teff Injera is above the threshold (<1) that estimates a good Fe bioavailability and moderate Zn bioavailability as compared to “Shameta”.
3.4. Bioactive compounds in “shameta”
The total phenolic content of “Shameta” samples ranged between 0.39-11.9 mg GAE/g with an average value of 3.9 mg GAE/g. Among analyzed “Shameta” samples, MW has the highest (11.9 mgAAE/g) total phenol content followed by BMS (9.9 mgAAE/g) (Table 7). However, the BMJ sample has the lowest (0.39 mgAAE/g) total phenol content. The variation might be due to the difference in the number of spices and herbs added, pre-fermentation treatments, and duration of fermentation which could result in the transformation of tannins to phenols (Sripriya et al., 1997). The results obtained in this study are relatively greater than the total phenol content of some Ethiopian traditional cereal-based fermented beverages (Keribo, Borde, Tella, and Korefe) (Debebe et al., 2016). Shewakena et al. (2017) also reported lower total phenol contents of 0.31 and 0.44 mg GAE/g for Korefe and Tella, respectively.
Table 7.
Total phenol, total flavonoid and total antioxidant activities of “Shameta” samples collected from four different districts but grouped into nine categories according to their ingredient composition and fermentation time.
| Samples coded | Total phenol contents (mg GAE/g wb) | Total flavonoid content (mg CE/g wb) | Total antioxidant activity (IC50) (mgAAE/g wb) |
|---|---|---|---|
| BMWS | 0.67 ± 0.02e | 0.43 ± 0.04b | 2.9 ± 0.2d |
| BMS | 9.9 ± 0.34b | 0.10 ± 0.01e | 0.68 ± 0.02f |
| MS | 0.59 ± 0.08e | 0.61 ± 0.06a | 6.2 ± 0.1c |
| MFG | 2.0 ± 0.16d | 0.19 ± 0.01d | 2.1 ± 0.1e |
| MG | 2.8 ± 0.08d | 0.10 ± 0.01e | 0.81 ± 0.02f |
| MBFG | 6.0 ± 0.56c | 0.32 ± 0.03c | 0.71 ± 0.02f |
| BMJ | 0.39 ± 0.09e | 0.15 ± 0.01de | 11.9 ± 0.5a |
| BJ | 0.58 ± 0.01e | 0.41 ± 0.02b | 10.0 ± 0.03b |
| MW | 11.9 ± 0.93a | 0.03 ± 0.01f | 0.52 ± 0.01f |
| CV | 9.9 | 9.81 | 4.3 |
| MSD | 1.1 | 0.1 | 0.5 |
BMWS = Barley (86%) + Maize (5%) + wheat (5%) from Sibu Sire, BMS = Barley (89%) + Maize (5%) from Sibu Sire, MS = Maize (95%) from Sibu Sire, MFG = Maize (86.5%) + Faba bean (5%) from Guto Gida, MG = Maize (91%) from Guto Gida, MBFG = Maize (81%) + Barley (5%) + Faba bean (5%) from Guto Gida, BMJ = Barley (87%) + Maize (5%) from Jimma Arjo, BJ = Barley (96%) from Jimma Arjo, MW = Maize (91%) from Wayu Tuka, CV = Coefficient of variation, MSD = Minimum significant different. (Each sample collected in triplicate and bulked to similar category based upon ingredients composition and fermentation time differences, n = 27).
The presence of polyphenols in the maternal diet is very important for the health of both the mother and the infant. Phytochemicals in maternal diet and breast milk protect both mothers and infants from oxidative stress, thereby reducing the risk of different diseases including cancers (Fenga et al., 2016; Fahad and Al-Harbi, 2018). Fahad and Al-Harbi (2018) reported on the positive relationship between the content of polyphenol in maternal diet and the antioxidant capacity of breast milk.
The total flavonoid content of the samples also ranged between 0.03-0.61 mg CE/g with an average value of 0.3 mg CE/g. From among samples collected from consumers, MS has the highest (0.61 mg CE/g) total flavonoid content followed by BMWS (0.43 mg CE/g) (Table 7). The highest value observed may be due to the addition of high amounts of spices and herbs during the preparation of “Shameta” (Derwich et al., 2011; Charles, 2013; Sandhu et al., 2017). In contrast, MW had the lowest (0.03 mg CE/g) total flavonoid content which might be due to the processing methods such as dehulling of grains used for the making of “Shameta” where phytochemicals are normally found at a higher concentration (Liukkonen et al., 2003; Mattila et al., 2005). The average flavonoid value (0.3 mg CE/g) of the present finding is greater than the value reported from other Ethiopian fermented beverages such as Keribo, Borde, Tella, and Korefe (Debebe et al., 2016). Shewakena et al. (2017) also reported an average value of 0.2 mg CE/g for Korefe and Tella which are lower than the present finding.
It has been reported that the flavonoid content of breast milk increases when the mother consumes foods rich in flavonoids. For instance, in nursing mothers who consumed a soy beverage containing 55 mg of total isoflavones for 2–4 days, the isoflavone contents of breast milk increased from 5.1 to 70.7 nmol/L (Franke et al., 2006). Vasallo (2008) also reported the positive correlation between total flavones concentrations in an infant's plasma with the level of isoflavones in the maternal diet.
The benefits of polyphenols and flavonoids in human health are often ascribed to their potential ability to act as antioxidants (Fraga, 2010). The total antioxidant activities of “Shameta” samples ranged between 0.52-11.9 mg AAE/g with average values of 4.0 mg AAE/g (Table 7). MW samples have the highest antioxidant activities followed by BMS, while BMJ has the lowest value. The variation of antioxidant activities in this finding might be due to the volume of spices and herbs added and fermentation processes (Mattila et al., 2005; Charles, 2013). Shewakena et al. (2017) reported the average values of Korefe (492.3 mg AAE/L) and Tella (548.5 mg AAE/L) which are greater than the present finding. Dietary supplementation of lactating mothers with antioxidant-rich foods has a positive impact on the health of lactating mothers and infants (Tsopmo, 2018). Based on the present results, it can be concluded that the consumption of “Shameta” could improve the health of lactating mothers through the provision of different health-promoting bioactive compounds.
3.5. Limitation of the study
The limited sample size is the major limitation of this study due to limited population size. This mainly because of unique nature of the product produced and consumed by a target group in a specific geographic location by specific community. As an inclusion criterion to avoid variability among samples due to variation in fermentation time, the study time was limited to households or mothers who gave birth in health centers in between January 2–16, 202. This resulted in lower number of population of the mothers (103 mothers) as total population in the study areas. The study accounted about 26% of mothers due delicate nature of the work to collect the samples from households. However, as a pioneer work to characterize the product in terms of its nutritional composition, anti-nutrient contents and bioactive components, results generated from this work will help to explore further the potential of the product as an alternative and affordable diet to lactating mothers.
4. Conclusion
In most houses of lactating mothers, “Shameta” is mainly prepared from cereal crops. However, in addition to the use of cereals, the study result showed that the crude protein and fat contents improved by supplementing the cereal's ingredients with faba bean and rapeseed oil. From similar ingredients, as supplementary food, the product could contribute to meeting the daily extra crude protein and energy demand of lactating mothers. In terms of minerals content, the product provides low Na, Ca, and Mg; however, it could provide relatively better Fe, P, and Zn. The double fermentation processes coupled with intermediate cooking could contribute to a lower concentration of anti-nutritional factors with better minerals bioavailability. The product can also contributes to better health and wellbeing of lactating mothers and infants due to its relatively high polyphenols and flavonoids contents. The lower pH value and intermediate moisture content nature of the product in combination with potential inhibitory activities of the added spices and herbs could also contribute to better keeping quality of the product. Subsequent optimization and home–based fortification efforts in terms of ingredients composition and fermentation conditions could further enhance the nutritional value of the product as an affordable supplementary diet for rapid recovery, strength, and health of lactating mothers.
Declarations
Author contribution statement
Daniel A. Kitessa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ketema Bacha, Yetenayet B. Tola: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mary Murimi, Ernest Smith: Analyzed and interpreted the data; Wrote the paper.
Soressa Gershe: Performed the experiments.
Funding statement
This work was supported by Jimma University and VITRAS (Virtual Institute for Trans-disciplinary Research and Scholarship for African Students).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors greatly acknowledge lactating mothers who shared samples for the study, health extension workers involved during samples collection and Jimma University College of Agriculture and Veterinary Medicine for provision of laboratory facilities.
References
- AACC . 2000. Approved Methods of the American Association of Cereal Chemists. [Google Scholar]
- Abegaz K. Isolation, characterization and identification lactic acid bacteria involved in traditional fermentation of borde, an Ethiopian cereal beverage. Afr. J. Biotechnol. 2007;6(12):1469–1478. [Google Scholar]
- Abegaz K., Fekadu B., Thor L., Judith A.N. Indigenous processing methods and raw materials of borde, an Ethiopian traditional fermented beverage. J. Food Technol. Afr. 2002;7(2):59–64. [Google Scholar]
- Abiyu H.T., Woldegiorgis A.Z., Haki G.D. Preparation of injera from pre-fermented flour: nutritional and sensory quality. Int. J. Sci. Innov. Discov. 2013;3(1):165–175. [Google Scholar]
- Adams M.R. Topical aspects of fermented foods. Trends Food Sci. Technol. 1990;8:140–144. [Google Scholar]
- Adegbehingbe K.T. Effect of starter cultures on the anti-nutrient contents, minerals, and viscosity of ogwo, fermented sorghum-Irish potato gruel. Int. Food Res. J. 2015;22(3):1247–1252. [Google Scholar]
- Alemayehu M., Argaw A., Mariam A.G. Factors associated with malnutrition among lactating women in subsistence farming households from dedo and seqa-chekorsa districts, Jimma zone, 2014. Develop. Countr. Stud. 2015;5(21):117–118. [Google Scholar]
- Antony U., Chandra T.S. Microbial population and biochemical changes in fermenting finger millet (Eleusine coracana) World J. Microbiol. Biotechnol. 2019;13:533–537. [Google Scholar]
- AOAC . eighteenth ed. 2000. Official Methods of Analysis of the AOAC. Washington, DC, USA. [Google Scholar]
- Ashenafi M., Mehari T. Some microbiological and nutritional properties of “borde” and “shamita”, traditional Ethiopian fermented beverages. Ethiop. J. Heal. Dev. 1995;9:105–110. [Google Scholar]
- Bede E.N., Okeke C.E., Amandikwa C. Physicochemical properties and sensory evaluation of kunu-zaki beverage produced by substitution of sweet potatoes with date fruits. IOSR-JESTFT. 2015;9(3):81–84. [Google Scholar]
- Berggren S., Hedren E., Edman K. Linnaeus University; Sweden: 2017. Water Holding Capacity and Viscosity of Ingredients from Oats. The Effect of Beta-Glucan and Starch Content, Particle Size, pH, and Temperature. [Google Scholar]
- Bhat T.K., Kannan A., Singh B., Sharma O.P. Value addition of feed and fodder by alleviating the antinutritional effects of tannins. Agric. Res. 2013;2:189–206. [Google Scholar]
- Binitu B.W., Zewdu A.W., Fekadu H.G. Indigenous processing methods of Cheka: a traditional fermented beverage in southwestern Ethiopia. J. Foodserv. 2015;7(1):1–7. [Google Scholar]
- Binitu B.W., Fekadu H.G., Zewdu A.W. Nutritional and alcoholic contents of Cheka: a traditional fermented beverage in Southwestern Ethiopia. Food Sci. Nutr. 2018:1–7. doi: 10.1002/fsn3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon P.M., Taylor C.L. Iron supplementation during pregnancy and infancy: uncertainties and implications for Research and policy. Nutrients. 2017;9:1327. doi: 10.3390/nu9121327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestenský M., Nitrayová S., Patrás P., Heger J. The quality of sorghum grain in an aspect of utilization amino acids in pigs. J. Microbiol. Biotechnol. Food Sci. 2012;1:1032. [Google Scholar]
- Butte N.F., King J.C. Energy requirements during pregnancy and lactation. Publ. Health Nutr. 2005;8(7A):1010–1027. doi: 10.1079/phn2005793. [DOI] [PubMed] [Google Scholar]
- Chan Gary M., McElligott Kathleen, McNaught Teresa, et al. Effects of dietary calcium intervention on adolescent mothers and newborns: a randomized controlled trial. Obstet. Gynecol. 2006;108(3):565–571. doi: 10.1097/01.AOG.0000231721.42823.9e. [DOI] [PubMed] [Google Scholar]
- Chaoui A., Faid M., Belhcen R. Effect of natural starters used for sourdough bread in Morocco on phytate biodegradation. East. Mediterr. Health J. 2003;9:141–147. [PubMed] [Google Scholar]
- Charles D.J. Springer; New York, NY, USA: 2013. Antioxidant Properties of Spices, Herbs, and Other Sources; p. 612. [Google Scholar]
- Cherie Z., Gregory R.Z., Fekadu H.G., Zewdu A.W. Optimization and modeling of teff-maize-rice-based formulation by simplex lattice mixture design for the preparation of brighter and acceptable injera. Cogent Food Agric. 2018;4:1444–3381. [Google Scholar]
- Debebe A., Singh B.C., Redi-Abshiro M. Total contents of phenolics, flavonoids, tannins and antioxidant capacity of selected traditional Ethiopian alcoholic beverages. Bull. Chem. Soc. Ethiop. 2016;30(1):27–37. [Google Scholar]
- Derwich E., Benziane Z., Chabir R. Aromatic and medicinal plants of Morocco: chemical composition of essential oils of Rosmarinus officinalis and Juniperus Phoenicia. Int. J. Appl. Boil. Pharm. Technol. 2011;2(1):146–153. [Google Scholar]
- Eggert J., Eggert L. 2011. Glob. libr. Women’s med.(ISSN: 1756-2228) [Google Scholar]
- Elenga M., Massamba J., Kobawila S.C., Makosso V.G., Silou T. Assessment and improvement of the nutritional quality of pasta and fermented corn porridge in Congo. Int. J. Biol. Chem. Sci. 2010;3(6):1274–1285. [Google Scholar]
- Emambux M.N., Taylor J.N. Sorghum kafirin interaction with various phenolic compounds. J. Sci. Food Agric. 2003;83:402–407. [Google Scholar]
- EWZFDOS (East Wollega Zone Finance . 2018. Development Office and Socio-Economics. Zonal Abstract Report. Nekemte, Ethiopia. [Google Scholar]
- Fahad J., Al-Harbi . 2018. The Impact of Dietary Polyphenols on the Quality of Expressed Breast Milk.https://www.researchgate.net/publication/322724762 [Google Scholar]
- FAO . FAO Agricultural Services Bulletin; 1998. Fermented Fruits and Vegetables: A Global Perspective by Battcock, M., & Azam-Ali, S. Book. 1998 No.134. [Google Scholar]
- FAO . PhyFoodComp1.0); Rome, Italy: 2018. Global Food Composition Database for Phytate Version 1.0; p. 22. [Google Scholar]
- FAO/WHO . second ed. 1998. Human Vitamin and Mineral Requirements. [Google Scholar]
- FAO/WHO . FAO Food and Nutrition Technical Report Series 1. Food and Agricultural Organisation of the United Nations; Rome: 2004. Human energy requirements: report of a joint FAO/WHO expert consultation. [Google Scholar]
- Fenga C., Costa C., Caruso E., Raffa L., Alibrando C., et al. Review on current evidence on the protective effect of dietary polyphenols on breast cancer. FARMACIA. 2016;64(1):1–9. [Google Scholar]
- Fraga C.G. Wiley; Hoboken, NJ: 2010. Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology. [Google Scholar]
- Franke A.A., Halm B.M., Custer L.J., Tatsumura Y., Hebshi S. Isoflavones in breastfed infants after mothers consume soy. Am. J. Clin. Nutr. 2006;84:406–413. doi: 10.1093/ajcn/84.1.406. [DOI] [PubMed] [Google Scholar]
- Fredlund K., Isaksson M., Rossander-Hulthèn L., Almgren A., Sandberg A.S. Absorption of zinc and retention of calcium: dose-dependent inhibition by phytate. J. Trace Elem. Med. Biol. 2006;20:49–57. doi: 10.1016/j.jtemb.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Fufa D.A., Laloto T.A. Assessment of dietary diversity and associated factors among lactating mothers in Debub bench district. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt J. Hodder Headline Group; London: 1997. Essentials of Food Microbiology. [Google Scholar]
- Garcia R.G., Mendes A.A., Sartori J.R., de Lima Almeida Paz I.C., et al. Digestibility of feeds containing sorghum, with and without tannin, for broiler chickens submitted to three-room temperatures. Braz. J. Poult. Sci. 2004;6:55–60. [Google Scholar]
- Gebrelibanos L. Addis Ababa University; 2015. Microbiological and Physicochemical Study of Azo, A Traditional Fermented Condiment Prepared from Sorghum and Leaves of Endod (Phytolacca Dodecandra) in Kafta Humera, Tigray Regional State. MSc thesis. [Google Scholar]
- Getacher L., Gudina E., Tadesse A., Agegnehu B., Abebaw M., et al. Minimum dietary diversity and associated factors among lactating mothers in Ataye District, North Shoa Zone, Central Ethiopia: a community-based cross-sectional study. Trends Food Sci. Technol. 2020;2020 doi: 10.1155/2020/1823697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot J.P., Rochette I., Treche S. Effect of fermentation by amylolytic lactic acid bacteria, in-process combinations, on characteristics of rice/soybean slurries: a new method for preparing high energy density complementary foods for young children. Food Chem. 2007;100:623–663. [Google Scholar]
- Hasan M.N., Sultan M.Z., Mar-E-Um M. Significance of fermented food in nutrition and food science. J. Sci. 2014;6(2):373–386. [Google Scholar]
- Idowu O.O., Fadahunsi I.F., Onabiyi O.A. Production and nutritional evaluation of Mahewu: a non-alcoholic fermented beverage of South Africa. Int. J. Res. Pharm. Biosci. 2016;3(6):27–33. [Google Scholar]
- Kabeir B.M., Mustafa S., Kharidah M., Suraini A., Abdul M.Y. A nutritious Medida (Sudanese cereal thin porridge) prepared by fermenting malted Brown rice flour with bifidobacterium longum BB 536. Mal. J. Nutr. 2004;10(2):183–193. [PubMed] [Google Scholar]
- Kaur C., Kapoor H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002;37:153–161. [Google Scholar]
- Kebebu A., Whiting S.J., Dahl W.J., Henry C.J., Abegaz K. Formulation of A Complimentary food fortified with broad beans (Vicia faba) in southern Ethiopia. Afr. J. Food Agric. Nutr. Dev. 2013;13(3):7789–7803. [Google Scholar]
- Kejela G., Gebremeskel F., Hassen H., Shewangizaw M., Desalegn M. 2020. Under Nutrition and Associated Factors Among Lactating Mothers in Southern Ethiopia: Institution Based Cross-Sectional Study. [Google Scholar]
- Khayat S., Fanaei H., Ghanbarzehi A. Minerals in pregnancy and lactation: a review article. J. Clin. Diagn. Res. 2017;11(9):1–5. doi: 10.7860/JCDR/2017/28485.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletzko B., Cetin I., Brenna J.T. Dietary fat intakes for pregnant and lactating women. Br. J. Nutr. 2007:1–5. doi: 10.1017/S0007114507764747. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Choi K.H., Kim S.H., Park K.S., Park S.H., et al. Physicochemical characteristics and electric conductivity of various fruit wines. Int. Food Res. J. 2013;20(6):2987–2993. [Google Scholar]
- Lemma G., Gudina E., Tadesse A., Agegnehu B., Abebaw M. Minimum dietary diversity and associated factors among lactating mothers in Ataye district, North shoa zone, Central Ethiopia: a community-based cross-sectional study. J. Nutr. Metab. 2020 doi: 10.1155/2020/1823697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Alli I., Kermasha S. In vitro alpha-amylase inhibitor activity-phytate relationships in proteins from Phaseolus beans. Food Res. 1993;26:195–201. [Google Scholar]
- Lioger D., Leenhardt F., Demigne C., Remesy C. Sourdough fermentation of wheat fractions rich in fibers before their use in processed food. J. Sci. Food Agric. 2007;87:1368–1373. [Google Scholar]
- Liukkonen K.-H., Katina K., Wilhelmson A., Myllymä ki O., Lampi, et al. Process-induced changes on bioactive compounds in whole grain rye. Proc. Nutr. Soc. 2003;62:117–122. doi: 10.1079/PNS2002218. [DOI] [PubMed] [Google Scholar]
- Maren M., Fischer I.M., Egli I., Richard F.A., Hurrell L. Phytic acid degrading lactic acid bacteria in teff injera fermentation. Int. J. Food Microbiol. 2014;190(3):54–60. doi: 10.1016/j.ijfoodmicro.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Mattila P., Pihlava J.M., Hellstro J. Contents of phenolic acids, alkyl- and alkylresorcinols, and avenanthramides in commercial grain products. J. Agric. Food Chem. 2005;53:8290–8295. doi: 10.1021/jf051437z. [DOI] [PubMed] [Google Scholar]
- Maxson E.D., Rooney L.W. Evaluation of methods for tannin analysis in sorghum grain. Cereal Chem. 1972;49(6):719–728. [Google Scholar]
- Miller L.V., Hambidge K.M., Krebs N.F. Zinc absorption is not related to dietary phytate intake in infants and young children based on modeling combined data from multiple studies. J. Nutr. 2015;145:1763–1769. doi: 10.3945/jn.115.213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moges A., Gudina E., Yadeta D. Harmaya University; Eastern Ethiopia: 2018. Undernutrition and Associated Factors Among Adolescent Pregnant Women in Afdem District, Ethiopian Somali Region. [Google Scholar]
- Mohammed A.Satter, Nusrat Abedin, Jabin S. Absha, Faridul Islam M., et al. A highly nutritive supplementary food: improving the nutritional status of pregnant and lactating mothers. Agro Food Ind. Hi-Tech. 2016;27(1):40–44. [Google Scholar]
- Molin G. In: Handbook of Fermented Functional Foods. second ed. Farnwot E.R., editor. Taylor and Francis Group; Boca Raton: 2008. Lactobacillus Plantarum, the role in foods and in human health. [Google Scholar]
- Ndie E.C., Okaka J.C. Risk assessment of antinutrient consumption of plant foods of southeastern Nigeria. J. Food Sci. Nutr. 2018;1(2):9–12. [Google Scholar]
- Nemo R., Bacha K. Microbial, physicochemical and proximate analysis of selected Ethiopian traditional fermented beverage. LWT Food Sci. Technol. 2020;131(9) [Google Scholar]
- Ogbonna A.C., Abuajah C.I., Umanah I.A. Burukutu: healthy and superior indigenous African traditional opaque beverage. Am. J. Adv. Food Sci. Technol. 2016;4(1):29–37. [Google Scholar]
- Ojo O.D., Enujiugha V.N. Comparative evaluation of ungerminated and germinated Co-fermented instant ‘ogi’ from blends of maize (Zea mays) and ground bean (kerstingiella geocarpa) J. Nutr. Heal. Food Eng. 2018;8(1):2373–4310. [Google Scholar]
- Okafor U.I., Omemu A.M., Obadina A.O., Bankole M.O., Samuel A., Adeyeye O. Nutritional composition and antinutritional properties of maize ogi co-fermented with pigeon pea. Food Sci. Nutr. J. 2017;6:424–439. doi: 10.1002/fsn3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajire A., Azeez L. Total antioxidant activity, phenolic, flavonoid and ascorbic acid contents of Nigerian vegetables. Afr. J. Food Sci. Technol. 2011;2(2):22–29. [Google Scholar]
- Osagie A.U., Eka O.U. University of Benin; 1998. Nutritional Quality of Plant Foods: Post-Harvest Research Unit; p. 62. [Google Scholar]
- Osman M.A. Effect of traditional fermentation process on the nutrient and antinutrient content of pearl millet during the preparation of Lohoh. J. Saudi Soc. Agric. Sci. 2010;10:1–6. [Google Scholar]
- Ötles S., Ozgoz S. Review on health effects of dietary fiber. Acta Sci. Pol. Technol. Aliment. 2014;13(2):191–202. [PubMed] [Google Scholar]
- Pranoto Y., Anggrahini S., Efendi Z. Effect of natural and Lactobacillus Plantarum fermentation on invitro protein and starch digestibilities of sorghum flours. Food Biosci. 2013;2:46–52. [Google Scholar]
- Reale A., Mannina L., Tremonte P., Sobolev A.P., Succi M., et al. Phytate degradation by lactic acid bacteria and yeast during the wholemeal dough fermentation. a 31P NMR study. J. Agric. Food Chem. 2004;52:6300–6305. doi: 10.1021/jf049551p. [DOI] [PubMed] [Google Scholar]
- Reddy N.R. In: Food Phytates, 25–52. Reddy N.R., Sethe S.K., editors. 2002. Occurrence, distribution, content, and dietary intake of phytate. eBook ISBN9780429134883. [Google Scholar]
- Reddy N.R., Pierson M.D. Reduction in antinutritional and toxic components in plant foods by fermentation. Food Res. Int. 1994;27:281290. [Google Scholar]
- Regenstein J.M., Regenstein C.E. Academic Press; Orlando: 1984. Food Protein Chemistry: in an Introduction to Food Science; pp. 286–287. [Google Scholar]
- Rigo J., Mohamed M.W., De Curtis M. In: Fanaroff and Martin Neonatal-Perinatal Medicine. ninth ed. Martin R.J., Fanaroff A.A., Walsh M.C., editors. Elsevier Mosby; St. Louis: 2011. Disorders of calcium, phosphorus, and magnesium. [Google Scholar]
- Roger T., Léopold N.T., Funtong M.C.M. Nutritional properties and anti-nutritional factors of corn paste (Kutukutu) fermented by different strains of lactic acid bacteria. Int. J. Food Sci. 2015;15:13. doi: 10.1155/2015/502910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu K.S., Punia S., Kaur M. Recent Advancements and Developments; 2017. Fermentation of Cereals: A Tool to Enhance Bioactive Compounds. [Google Scholar]
- Schlemmer U., Frølich W., Prieto R.M. Grasses, F. Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role, and analysis. Mol. Nutr. Food Res. 2009;53:S330–S375. doi: 10.1002/mnfr.200900099. [DOI] [PubMed] [Google Scholar]
- Shewakena S., Chandravanshi B.S., Debebe A. Levels of total polyphenol, flavonoid, tannin, and antioxidant activity of selected Ethiopian fermented traditional beverages. Int. Food Res. J. 2017;24(5):2033–2040. http://www.ifrj.upm.edu.my [Google Scholar]
- Simwaka J.E., Chamba M.V.M., Huiming Z., Masamba K.G., Luo Y. Effect of fermentation on physicochemical and antinutritional factors of complementary foods from millet, sorghum, pumpkin, and amaranth seed flours. Int. Food Res. J. 2017;24(5):1869–1879. http://www.ifrj.upm.edu.my [Google Scholar]
- Soliman S.M., Soliman A.M., Bakr M.S. Relationships between maternal nutrition status, quantity and composition of breast milk in Egypt. Afr. J. Agric. Sci. Technol. 2014;2(2):59–64. [Google Scholar]
- Songre-Oruattara L.T., Mouquet-Rivier C., Icard-Verniere C., Humblot C. Enzyme activities of lactic acid bacteria from a pearl millet fermented gruel (ben-saalga) of functional interest in nutrition. Int. J. Food Microbiol. 2008;128(2):395–400. doi: 10.1016/j.ijfoodmicro.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Sripriya G., Antony U., Chandra T.S. Changes in carbohydrate, free amino acids, phytate, and HCL extractability of minerals during germination and fermentation of finger millet (Eleusine coracana) Food Chem. 1997;58:345–350. [Google Scholar]
- Stephen E.C., Daniel N.C.U., Bashir K. Comparative physico-chemical analysis of locally brewed beer (Burukutu) from corn, millet, and sorghum. Am. J. Sci. Technol. 2017;4(3):43–48. [Google Scholar]
- Stevens G.A., Finucane M.M., De-Regil L.M., Paciorek C.J., Flaxman, et al. Vol. 1. 2013. Global, regional, and national trends in hemoglobin concentration and prevalence of total and severe anemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. (1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri D.J., Sherry T.A. Effects of different processing methods on the micronutrient and phytochemical contents of maize. Compr. Rev. Food Sci. Food Saf. 2016;15 doi: 10.1111/1541-4337.12216. [DOI] [PubMed] [Google Scholar]
- Thompson J., Manore M. fifth ed. 2005. Nutrition: an Applied Approach. New York. Published by Pearson (November 13th, 2017). SBN-13: 9780134890586. [Google Scholar]
- Thompson J., Manore M., Vaughan L. second ed. Pearson; San Francisco California: 2008. The Science of Nutrition. [Google Scholar]
- Tsopmo A. Phytochemicals in human milk and their potential Antioxidative protection. Antioxidants. 2018;7:32. doi: 10.3390/antiox7020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah I., Ali M., Farooqi A. Chemical and nutritional properties of some maize varieties grown in NWFP, Pakistan. Pak. J. Nutr. 2010;9(11):1113–1117. [Google Scholar]
- Vaintraub I.A., Lapteva N.A. Colorimetric determination of phytate in unpurified extracts of seeds and the products of their processing. Anal. Biochem. 1988;175(1) doi: 10.1016/0003-2697(88)90382-x. [DOI] [PubMed] [Google Scholar]
- Vasallo N. first ed. Nova Biomedical Publishers; New York: 2008. Polyphenols and Health: New and Recent Advances. [Google Scholar]
- Villano D., Fernandez-Pachon M.S., Moya M.L., Troncoso A.M., Garcıa-Parrilla M.C. The radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007;71:230. doi: 10.1016/j.talanta.2006.03.050. [DOI] [PubMed] [Google Scholar]
- Volpe S.L. sixth ed. Linus Pauling Institute; 2019. Magnesium: Micronutrient Information Center. [Google Scholar]
- Weldehaweria N.B., Misgina K.H., Weldu M.G., Yosef S.G., Berhane H.G., Shewit W.Z., Helen A.N., Higus G.G., Wubrst A. Dietary diversity and related factors among lactating women visiting public health facilities in Aksum town, Tigray, Northern Ethiopia. BMC Nutr. 2016;2:38. [Google Scholar]
- World Health Organization . 2015. WHO|Sodium intake for adults and children. [Online]. Available at http://apps. who.int/iris/handle/10665/77985 (2015) [Google Scholar]
- Yao A.A., Egounlety M., Kouame L.P., Thonart P. Lactic acid bacteria diversity in the fermented wheat hamoum in West Algeria. Annal. M´ed. V´et´er. 2009;153:54–65. [Google Scholar]
- Yegrem L. Haramaya University; Ethiopia: 2019. Evaluation of Nutritional Composition and Sensory Acceptability of Tef (Eragrostis Tef (Zucc.) Trotter) Complemented with Lupine (Lupinus spp.) Injera. M.Sc. Thesis.http://hdl.handle.net/123456789/3315 [Google Scholar]
- Yohannes T., Melak F., Siraj K. Preparation and physicochemical analysis of some Ethiopian traditional alcoholic beverages. Afr. J. Food Sci. 2013;7:86–90. [Google Scholar]
- Zakari U.M., Hassan A., Abbo E.S. Physico-chemical and sensory properties of “Agidi” from pearl-millet (Pennisetum glaucum) and Bambara groundnut (Vigna subterranean) flour blends. Afr. J. Food Sci. 2010;4(10):66. [Google Scholar]
- Zambrano M., Baishali D., Donald G.M., Heather L.M., Marianne F.T., et al. Assessment of moisture content measurement methods of dried food products in small-scale operations in developing countries: A review. Trends Food Sci. Technol. 2019;88:484–496. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.