Abstract
Background
Muscle pain and muscle weakness, common symptoms among statin-treated patients, may worsen with COVID-19 infection.
Aims
The aim of the paper was to find out if concomitant COVID-19 infections increase the frequency of specific side effects of statins such as muscle pain and muscle weakness.
Method
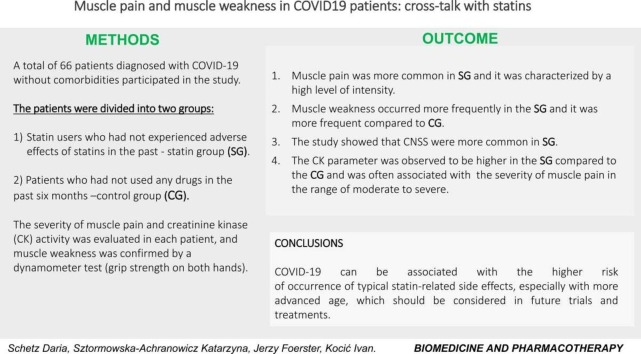
A total of 66 patients diagnosed with COVID-19 without comorbidities participated in the study. The patients were divided into two groups: statin-users who had not experienced adverse effects of statins in the past (statin group (SG)) and patients who had not used any drugs in the past six months (control group (CG)). The severity of muscle pain and creatinine kinase (CK) activity was evaluated in each patient, and muscle weakness was confirmed by a dynamometer test (grip strength on both hands).
Results
In SG, muscle pain was more common and it was characterized by a high level of intensity. Muscle weakness occurred more frequently in the SG and it was more frequent compared to CG. The CK parameter was observed to be higher in the SG compared to the CG and was often associated with the severity of muscle pain in the range of moderate to severe.
Conclusions
Our study indicates that COVID-19 is associated with the higher risk of occurrence of typical statin-related side effects, especially with more advanced age, which should be considered in future trials and treatments.
Keywords: COVID19, Statins, Kinase creatinine, Muscle pain, Muscle weakness
Graphical Abstract
1. Introduction
Hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) have been proposed as useful agents for modulating the host response in COVID-19 [1]. However, the findings from a recent study provide a different conclusion, that they probably have neither a positive nor a negative effect on COVID-related mortality, and may be associated with an 18% increased risk of severe COVID-19 infection [2]. Several cases of myopathy and rhabdomyolysis have been confirmed in patients with COVID-19 [3], [4]. Despite this, it is still unknown whether COVID-19 is associated with the higher risk of occurrence of typical statin-related side effects, such as muscle pain and muscle weakness. Although a case of fatal rhabdomyolysis in a COVID-19 patient on rosuvastatin has been reported [5], further observation, and cohort studies are needed to analyze if SARS-CoV-2 infection exacerbates the development and severity of muscle symptoms in patients on statins.
Statins are drugs effective at lowering the low-density lipoprotein (LDL) cholesterol in the blood, thereby protecting the cardiovascular system against a heart attack and stroke [6], [7]. They share a common mechanism of action, although they differ in terms of their pharmacokinetic profile and efficacy. Atorvastatin, simvastatin, lovastatin, and fluvastatin are relatively lipophilic compounds. Rosuvastatin and pravastatin are more hydrophilic [6]. With the exception of simvastatin and lovastatin, other statins mentioned above are administered as active hydroxy acid [7]. Statins are generally well tolerated, serious adverse drug reactions are rare and myopathy and rhabdomyolysis are the most frequently mentioned among them [8]. Myopathies is a disruption of the structural integrity and metabolic processes of muscle cells that can result from genetic abnormalities, toxins, inflammation, infection, and hormonal and electrolyte imbalances [9]. The risk of myopathy is estimated to occur in 5–10% patients who use statin [9], while it may be lower with fluvastatin XL (extended-release tablets) treatment than with high dosages of other statins [10].
Many statin-treated patients report muscle pain and muscle weakness in clinical practice that contribute to discontinuation of the drug. Some epidemiological data suggest that the risk of adverse side effects associated with statins on muscle is higher in the case of a family history of myopathy, a history of elevated creatine kinase (CK) levels, a previous diagnosis of hypothyroidism or neuromuscular diseases [11], using higher doses and when statins are co-administered with drugs that can cause interactions due to the affected liver cytochrome P450 enzymes [12].
Furthermore, some genetic factors can contribute to the muscle toxicity of statins, such as, for example, a single nucleotide polymorphism in the SLCO1B1 gene, which probably confers an increased risk of myopathy in patients taking simvastatin [13]. The mechanism of statin-induced myopathy is still unknown. It is likely multifactorial [9] and can be minimized by identifying vulnerable patients and eliminating statin interactions.
According to recent reports, statins can affect protein prenylation, post-translational modification of membrane-bound proteins, may adversely influence selenoprotein synthesis, and may interfere with dolichol biosynthesis (the process of protein glycosylation is therefore affected). Furthermore, mitochondrial dysfunction can also be the cause of myopathy after statin use [14].
According to many authors, COVID-19 can cause muscle pain and muscle weakness. In one study, a comparison of clinical differences between COVID-19, influenza, and the common cold showed, that individual symptoms in these diseases occur with different frequency [15]. In general, among the very common clinical symptoms of COVID-19, muscular pain occur in 29% of patients and it is not most common symptom. In contrast, among individuals with influenza and common cold, the most frequently reported symptom is muscular pain (94% of the patients in both cases). Taking into account the above, it can be seen that muscular pain is more common in the case of influenza and common cold than in patients with COVID-19 [15].
SARS-CoV-2 is believed to spread through the vascular endothelium or bloodstream causing infection in all tissues containing angiotensin-converting enzyme 2 (ACE2), thus the musculoskeletal system can also be affected, and elevated levels of CK during COVID-19 infection indicate muscle involvement [16]. CK is an intracellular enzyme that is present in the brain, myocardium, and skeletal muscle in the greatest amount. Injury or hypoxia causes disruption of cell membranes, and CK is released from the cellular cytosol into the systemic circulation [17]. Due to this fact, serum CK concentration is measured as a sensitive screening for myopathy [18]. Myopathy is diagnosed when muscle pain or muscle weakness is accompanied by CK levels more than 10 times the upper limit of normal (ULN). It is well known that in patients with rhabdomyolysis elevated levels of CK are noticeable [19], while levels greater than 5000 IU/L indicate its severe course [20]. This issue is crucial because kidney damage is a serious complication of rhabdomyolysis [21]. There are many causes of rhabdomyolysis, for example, drugs, viral infection [22].
In clinical practice, many statin-treated patients report weakness and muscle pain that contribute to discontinuation of the drug [23], [24]. There is also a lack of data to determine whether the risk of muscle symptoms is greater in patients with COVID-19 who use statins.
The purpose of our study was to investigate whether in patients who use statins chronically (for at least 6 months) and who have never reported adverse effects of these drugs, muscle pain, muscle weakness and myopathy are more common in case of COVID-19 infection.
2. Method
In our retrospective study from 10.2020 to 09.2021 The Pomeranian Pharmacovigilance Centre (PPC) of the Department of Pharmacology, Medical University of Gdańsk collected data of patients diagnosed with COVID-19 (a positive result was obtained using the RT-PCR test). We included in the study 66 patients diagnosed with COVID-19 without comorbidities (exceptions: dyslipidemia). The patients were divided into two groups:
-
1.
SG – 33 patients with active COVID-19, who were taking statins (monotherapy) for at least 6 months, who have not experienced adverse effects of statins in the past, such as muscle pain, muscle weakness, elevated CK levels (information obtained on the basis of a previously completed questionnaire).
-
2.
CG – 33 patients with active COVID-19, who did not use statins or other drugs in the past six months.
2.1. Study inclusion criteria
Before inclusion in the study, all patients completed a short questionnaire, in which they answered the questions regarding:
-
1.
Medications used (the names of all drugs used and their doses).
-
2.
Comorbidities.
-
3.
The degree of physical activity just before contracting COVID-19 (Possible answers in the survey: a) as usual, b) reduced, c) slightly increased, d) noticeably increased.
-
4.
Adverse drug reaction of statin in the past (before COVID-19), such as: muscle pain, muscle weakness, elevated CK levels (in SG).
The study included patients who declared that:
-
1.
Only used statins in SG patients.
-
2.
Did not use any medications in CG patients.
-
3.
Have no comorbidities.
-
4.
Did not show more than usual physical activity at least one week before the onset of COVID-19 symptoms.
-
5.
Did not report side effects of statins in SG.
All patients with diagnosed muscular diseases were excluded from the study.
In the study, subjective and objective symptoms (clinically confirmed) were analyzed.
In both groups - statin users and control - patients were examined for the appearance of adverse symptoms twice: the first visit (active COVID-19 disease) and the second visit - follow-up three months after the diagnosis of COVID-19 (healing).
2.2. Variables
Variables that were taken into account in the study: age of the patients, sex, body mass index (BMI), type of statin, the reason for the use of statins.
2.2.1. Subjective symptoms which were taken into account in the study
-
1.
Muscle pain: In each patient, the severity of muscle pain was assessed (subjective pain assessment), based on a Numerical Rating Scale (NRS), in which the patient rates the pain from 0 (no pain) to 10 (worst pain). The obtained results were assigned to one of four categories:
-
•
None: a complete absence of pain.
-
•
Mild
-
•
Moderate
-
•
Severe (a severe pain that limits patients ability to move).
-
2.
Severity of COVID-19 symptoms: All patients completed a short questionnaire, in which they determined whether they had COVID-19 symptoms such as: fever, rhinitis, headache, cough and indicated they severity level: a) none, b) mild, c)moderate, d) severe.
2.2.2 Objective symptoms
2.2.2.1. CK activity
CK activity was measured twice: during the first visit (during active COVID-19 infection) and during the follow-up visit in medical analysis laboratories in Gdańsk.
The activity of CK was assessed and depending on the levels of CK, the values were divided into three groups: normal value (20–200 IU/L), elevated levels (>200–2000 IU/L) and high levels (> 10x the upper limit of normal ULN).
2.2.2.2. Hand grip strength
In all patients a hand grip strength dynamometer test was performed twice:
-
1)
First visit - during active COVID-19 disease.
-
2)
follow up - three months after the diagnosis of COVID-19 (healing).
The test was carried out in SG and CG. The force was measured in kilograms (0–90) in both hands. Each patient was seated comfortably. The grip strength was measured using the same hand grip dynamometer (Jamar 5030KIT). According to recommendation of The American Society of Hand Therapists (ASHT), All patients were seated with their shoulders adducted, their elbows flexed 90 degree and their forearms in neutral position. In both populations maximum grip strength was assessed (the mean of three trials), for each hand (total 6 times in all), separated by a 30 s rest period. The highest result of the six trials was used as the strength of the grip. The results obtained during the first measurement were compared with the results obtained during follow-up after recovery (second measurement). In the cases where the results of the first measurement were lower than during the follow up, we found a weakening of the muscle strength at the first visit.
2.2.3. Follow-up
Follow-up examinations were performed three months after the diagnosis of COVID-19 in all patients, since according to numerous scientific publications, the majority of statin-induced muscle damage is reversible with most recovery of symptoms occurring within 2–3 months [25].
2.2.4. Statistical analysis
All statistical analyzes were performed with STATISTICA version 13.3 (StatSoft®, Inc. 2017, https: //www.statsoft.com). Categorical variables were expressed as percentages and continuous variables as mean ± SD.
The Shapiro-Wilk test was used to calculate whether a random sample of data comes from a normal distribution. To compare the differences between two independent samples when the sample distributions were not normally distributed, the Mann -Whitney U test or the Kruskal-Wallis ANOVA tests were used. In the case of a normal distribution, the Student's t-test or ANOVA test supplemented with the Newman-Keuls post hoc test was performed. A χ2 test was used for the analysis of qualitative characteristics. Additionally, a correlation analysis was performed using Spearman's rank. A p-value of < 0.05 was accepted as statistically significant.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Declaration of Helsinki of 1975, as revised in 2008.
Written consent was obtained from all subjects/patients and full anonymization of the data was guaranteed.
3. Results
66 patients aged 42–88 (65,7 ± 11,6) participated in the study. The group of patients treated with statins chronically and the control group were of similar age (66,5 ± 12,6 vs 64,9 ± 10,4). There were 17 women in the control group (52%) aged 42–83 (65,8 ± 11,4) and 14 women in the SG (42%) aged 52–74 (63,4 ± 8,8). Among SG, 18 patients were 65 + years old (men: 11, women: 7), while only 4 men were over 79 years old.
In the CG, there were 16 men (48%) aged 43–86 (64,0 ± 10,6), while in the study group there were 19 men (58%) aged 42–88 (68,9 ± 13,8). There were no significant age differences between the sexes in both groups (P > 0.05) [ Table 1].
Table 1.
Clinical characteristics of the SG and CG.
| Variables | Chronic statin users N = 33 (%) | Control group N = 33 (%) | p-value |
|---|---|---|---|
| Age (Mean ± S.D.) | 66,5 ± 12,1 | 64,9 ± 11,2 | 0,57 |
| Females/Men | 14/19 (42,4/57,6) | 17/16 (51,5/48,5) | 0,46 |
| Overweight BMI ≥ 25: Yes/No |
5/28 (15,2/84,8) | – | – |
| Statin type: Atorvastatin/Lovastatin/Rosuvastatin/Simvastatin |
6/2/4/21 (18,2/6,1/12,1/63,6) | – | – |
| Indication for the statin use H/HFH/PCE |
28/3/2 (84,8/9,1/6,1) | – | – |
| Hypertension: Yes/No | 9/24 (27,3/72,7) | – | – |
| Severity of other COVID-19 symptoms: fever, rhinitis, headache, cough. | 13/11/3/6 (39,4/33,3/9,1/18,2) | 8/16/8/1 (24,2/48,5/24,2/3,03) | 0,009* |
| CpK level Normal/Elevated/High/Lack of data |
9/9/5/10 (27,3/27,3/15,1/30,3) |
29/4/-/- (87,9/12,1/-/-) |
<0,0001* |
| Muscle symptoms: Muscle pain: Mild/Moderate/Severe Muscle weakness: Yes/ No |
9/8/16 (27,3/24,2/48,5) 20/13 (60,6/39,4) |
16/15/2 (48,5/45,4/6,1) 5/28 (15,2/84,8) |
0,003* 0,0002* |
Abbreviations used: CpK- Creatine kinase; H – Hypercholesterolaemia; HFH - Homozygous familial hypercholesterolaemia; PCE - Prevention of cardiovascular events.
3.1. Muscle pain
Different levels of severity of muscle pain were observed in the CG and the SG: mild (48,5%; 27,3%), moderate (45,4%; 24,2%), severe (6,1%; 48,5%), respectively.
3.1.1. SG
The number of patients who claimed muscle symptoms with severe pain intensity was significantly higher compared to the CG (P = 0.003). In SG, muscle pain was more common in men and statistically significant (P = 0.0001), compared to women. Furthermore, it was characterized by a high intensity level (P = 0.002) [ Table 1, Table 2].
Table 2.
Analysis of factors influencing the occurrence of adverse muscle symptoms in SG.
| Variables | Muscle symptoms |
|||||
|---|---|---|---|---|---|---|
| Muscle pain |
Muscle weakness |
|||||
| Mild | Moderate | Severe | Yes | No | ||
| Age (Mean ± S.D.) | 59,5 ± 10,5 | 64,2 ± 8,4 | 71,7 ± 12,6 | 70,1 ± 11,8 | 61,1 ± 10,8 | |
| p-value | 0,04* | 0,03* | ||||
| Gender N (%) |
Females | N = 8 (24,2) |
N = 5 (15,1) |
N = 1 (3,0) |
N = 4 (12,1) |
N = 10 (30,3) |
| Men | N = 1 (3,03) |
N = 3 (9,1) |
N = 15 (45,5) |
N = 16 (48,5) |
N = 3 (9,1) |
|
| p-value | 0,0002* | 0,006* | ||||
| Overweight N (%) |
(BMI ≥ 25) | – | N = 1 (3,0) |
N = 4 (12,1) |
N = 5 (15,2) |
– |
| No | N = 9 (27,3) |
N = 7 (21,2) |
N = 12 (36,4) |
N = 15 (45,4) |
N = 1 (39,4) |
|
| p-value | 0,25 | 0,24 | ||||
| Type of statin N (%) |
Atorvastatin | N = 3 (9,1) | – | N = 3 (9,1) |
N = 3 (9,1) |
N = 3 (9,1) |
| Lovastatin | N = 2 (6,1) |
– | – | – | N = 2 (6,1) |
|
| Rosuvastatin | – | N = 2 (6,1) |
N = 2 (6,1) |
N = 3 (9,1) |
N = 1 (3,0) |
|
| Simvastatin | N = 4 (12,1) |
N = 6 (18,2) |
N = 11 (33,3) |
N = 14 (42,4) |
N = 7 (21,2) |
|
| p-value | 0,21 | 0,3 | ||||
| Severity of other COVID-19 symptoms: N (%) |
Mild | N = 2 (6,1) |
N = 7 (21,2) |
N = 4 (12,1) |
N = 7 (21,2) |
N = 6 (18,2) |
| Moderate | N = 1 (3,0) |
N = 1 (3,0) |
N = 9 (27,3) |
N = 9 (27,3) |
N = 2 (6,1) |
|
| Severe | N = 1 (3,0) |
– | N = 2 (6,1) |
N = 2 (6,1) |
N = 1 (3,0) |
|
| None | N = 5 (15,2) |
– | N = 1 (3,0) |
N = 2 (6,1) |
N = 4 (12,1) |
|
| p-value | 0,01* | 0,06 | ||||
| CpK level N (%) |
Normal | N = 5 (15,2) |
N = 2 (6,1) |
N = 2 (6,1) |
N = 4 (9,1) |
N = 5 (15,2) |
| Elevated | – | N = 1 (3,0) |
N = 8 (24,2) |
N = 8 (18,2) |
N = 1 (3,0) |
|
| High | – | – | N = 5 (15,2) |
N = 5 (15,2) |
– | |
| Lack of data | N = 4 (9,1) |
N = 5 (15,2) |
N = 1 (3,0) |
N = 3 (9,1) |
N = 7 (21,2) |
|
| p-value | 0,0002* | 0,06 | ||||
| Hypertension N (%) |
Yes | – | N = 2 (6,1) |
N = 4 (9,1) |
N = 7 (21,2) |
N = 2 (6,1) |
| No | N = 9 (27,3) |
N = 6 (18,2) |
N = 9 (27,3) |
N = 13 (39,4) |
N = 11 (33,3) |
|
| p-value | 0,06 | 0,35 | ||||
| Indication for the statin use N (%) |
H | N = 9 (27,3) |
N = 5 (15,2) |
N = 14 (42,4) |
N = 17 (51,5) |
N = 11 (33,3) |
| HFH | – | N = 2 (6,1) |
N = 1 (3,0) |
N = 2 (6,1) |
N = 1 (3,0) |
|
| PCE | – | N = 1 (3,0) |
N = 1 (3,0) |
N = 1 (3,0) |
N = 1 (3,0) |
|
| p-value | 0,1 | 0,95 | ||||
Abbreviations used: CpK- Creatine kinase; H – Hypercholesterolaemia; HFH - Homozygous familial hypercholesterolaemia; PCE - Prevention of cardiovascular events.
3.1.2. CG
In the CG, muscle pain was more common in men and was statistically significant (P = 0.002) compared to a CG of women, however, unlike the SG it was characterized by a mild degree of intensity of pain (P = 0.02) [Table 2].
3.2. Muscle weakness
3.2.1. SG
Muscle weakness, referred to as an increase in hand grip strength of at least 2% between the first and second measurements (during COVID-19 and three months after COVID-19 diagnosis) occurred significantly more frequently in the chronic statin users (P = 0.0002) than in the CG (60,6% and 15,2%, respectively). Furthermore, muscle weakness was observed to be significantly more frequent in the group of men (P = 0.007) compared to the group of women [Table 1, Table 2].
3.2.2. CG
There were no differences between sex and the appearance of muscle weakness in the CG [Table 1].
3.3. Muscle pain versus muscle weakness
We observed a strong correlation between the intensity of muscle pain and muscle weakness (r = 0.72; P = 0.00003) in patients chronically treated with statins. Statistical significance was also found with increasing age and the severity of muscle pain (P = 0.04) and muscle weakness (P = 0.03) in the SG. Especially in patients over 80 years of age, severe muscle pain and muscle weakness were statistically significant (P < 0.05) [Table 1, Table 2].
3.4. Severity of COVID-19 symptoms
The occurrence of symptoms of COVID-19, such as fever, rhinitis, headache, cough, was found to differ statistically significantly between the CG (P = 0.009) and the SG [Table 1].
3.4.1. Study group
In the SG, other symptoms of COVID-19, especially of a moderate type, had a significant effect (P = 0.02) on the intensity of muscle pain, (especially severe pain) (P = 0.03), compared to less severe pain [Table 1, Table 2].
3.5. CK
In SG, 9 patients have a normal CK value (20–200 IU/L). Elevated levels (> 200–250 IU/L) were observed in 9 cases, while 5 patients had high levels (> 250–2000 IU/L) of CK. None of the patients showed very high levels of CK (> than 10 times the upper limit of normal ULN).
In the control group, the normal CK value (20–200 IU/L) was observed in 29 patients, while elevated levels were observed in 4 participants. None of the patients showed higher levels of CK values.
The CK parameter was observed to be statistically significantly different in the SG (P < 0.0001) compared to the CG. Normal CK values were significantly more frequent (P = 0.001) in the CG (87.9%) compared to the SG (27.3%). Additionally, a significant increase (P = 0.03) in CK concentration was observed in the SG (5%) compared to the CG (0%). Furthermore, in the study group, the CK parameter had a statistically significant effect (P = 0.0002) on the severity of muscle pain in the range of moderate (0.5 ± 0.75) and severe (2.06 ± 0.85) pain compared to mild pain (0.55 ± 0.52).
The CK parameter was found to differ statistically significantly in men in the SG (P = 0.005) compared to the group of women, which was not found in the CG. [Table 1, Table 2].
3.6. The type of statin and unwanted symptoms
In the SG, one of four statins was used chronically: atorvastatin (F: 33.3%; M: 66.6%), lovastatin (F: 100%), rosuvastatin (M: 100%) and simvastatin (F: 47.6%; M: 52.4%). Although simvastatin was the most commonly used statin, no significant differences (P > 0.05) were observed between the type of drug and the severity of myopathy. Additionally, the differences between the type of statin and the sex were statistically insignificant (P > 0.05) [Table 2].
3.7. Risk factors: overweight, hypertension, the reason for using statins
There were no statistically significant differences (P < 0.05) differences between the parameters mentioned above and muscle pain intensity and muscle weakness [Table 1, Table 2].
4. Discussion
Our study has shown that muscle pain occurred during the course of COVID-19 infection, however, what is important, its severity intensity was much more common in SG (48,5% of statin users). This tendency was especially observed in men, whose pain was more often of greater intensity than in women. Furthermore, in patients with COVID-19 who did not use statins, muscle pain was most often described as mild, while in statin users its intensity was increased. Therefore, it can be assumed that statin use may be associated with increases the risk of muscle pain in COVID-19.
In addition to muscle pain, in our patients, a common symptom was muscle weakness. As other researchers have shown, statin-induced muscle symptoms are the main reason for discontinuation of statins [11], [26], however, in randomized controlled trials, moderate muscle symptoms appeared to occur as frequently in statin users as in placebo [27]. This observation shows that the patient's subjective symptoms may not be reliable. Precisely for this reason, we assessed the phenomenon of muscle weakness on the basis of a hand grip strength dynamometer test. According to the literature, the measurement of dynamometric grip strength is an objective measurement of total body strength [28], therefore, we were able to objectively confirm the presence of muscle weakness.
Both muscle strength and muscle mass vary throughout the lifetime of the patient and decrease with ageing. Maximal levels are reached in individuals up to 40 years of age, and they are higher in men than in women [29]. Noticeable loss of muscle mass is observed 1–2 per year and a decrease in strength 1.5–5% after 50 years of age [30]. Furthermore, a recent survey showed that reduction in muscle strength was associated with COVID-19 [31]. In our study, muscle weakness occurred much more often in SG (60,6% of patients) than in the CG and was more common in men.
In the study, we were unable to determine the score of hand grip strength before contracting COVID-19. Therefore, we decided to state muscle weakness when the second score of the strength of the hand grip (follow-up examination in healers) was higher than in the first examination (first visit, in patients with active COVID-19). On the one hand, this may be a limitation in our study because we were unable to determine what was the baseline score of hand grip strength (before falling ill in healthy patients), but thanks to this methodology, we were able to observe regressions of muscle weakness, and thus find some improvement in COVID-19 healers after 3 months of follow-up. Therefore, we can assume that in SG with COVID-19, muscle weakness can often be visible, but is not irreversible. These conclusions seem to be particularly important, especially compared to the results of other researchers who stated the possibility of chronic damage to skeletal muscle by SARS-CoV-2 in older adults [31].
As mentioned above, viral infections can cause myopathy or even increase the risk of rhabdomyolysis. In the case of both, influenza and COVID-19 myalgia can occur, although muscle pain is a typical symptom of influenza [28]. Influenza B is the most commonly known to cause myopathy, accounting for up to 42% of viral muscle pain [32], while influenza A more often causes rhabdomyolysis [33]. Although the underlying mechanism of this phenomenon remains unclear, two possibilities are considered: direct viral invasion of muscle (although skeletal muscle biopsies do not reveal direct viral infection) [34] or immune-mediated action [33]. It is quite possible that the increase in the severity of muscle pain and muscle weakness in active SARS-CoV-2 infection in SG, which is noticeable in our study, was associated with some kind of pathopharmacological interaction between this virus and statins. We observed that in patients who had other moderate or severe symptoms of COVID-19 (severe cough, high fever, severe headache, severe rhinitis), muscle pain defined as severe was more common in SG. Therefore, we can assume that the more severe COVID-19 is, the greater the risk of interactions with statins. Unfortunately, the exact cause of this phenomenon requires further investigation.
We tried to assess the risk of developing myopathy or rhabdomyolysis in patients using statins with COVID-19. For this purpose, we used the CK parameter. In one study it has been shown that SARS-CoV-2 may influence on voluntary muscle, and CK levels should be measured in COVID-19 patients admitted to hospital, due to the fact that there is an independent relationship between the level of CK at admission and the clinical outcome [35].
In chronic statin users with COVID-19, elevated CK values can be common, however, very high values are not to be expected as frequently as low to moderately elevated values. Therefore, discontinuation of statins was not necessary in any case. However, monitoring the CK level can be a very useful parameter in statin users who report adverse muscle symptoms in the course of COVID-19 [19], [20], [21].
We want to strongly emphasize that although we found a greater tendency towards myopathy in the SG (muscle pain, muscle weakness, elevated CK levels), these drugs did not need to be discontinued routinely. In the follow-up examination, we found that all symptoms resolved after the patients recovered. Due to the above, we suggest that statins should not be discontinued routinely in patients with COVID-19 with accompanying muscle pain and muscle weakness, when CK values are moderately elevated.
Based on our study, it could be concluded that the patient groups that should be particularly monitored for the risk of developing severe myopathy and perhaps rhabdomyolysis are elderly patients, especially men over 80 years of age, and in their case, the CK level examination is highly recommended. Such findings are surprising because the gender of women is included among factors that increase the risk of myopathy in statin users [11]. However, we would like to point out that in our study, in the SG, no women over 79 years of age were included; therefore, such a comparison is not valid. The conduct of a new study to assess the risk of developing myopathy in patients 79 + years of age in both sexes could provide valuable data. Interestingly, muscle pain was more common in men compared to women (in all age groups) and was characterized as more severe. Furthermore, the CK parameter in men in the SG differed significantly compared to the group of women, which was not found in the CG.
In one study, the authors stated that increased serum CK after exercise is a sex-linked phenomenon and is grater in men. They drew the hypothesis that possibly female muscles are less susceptible to damage by adverse factors [36]. It appears to be related to differences between sexes in body composition, although in previous clinical studies such differences did not explain all racial-gender differences [37], but in a recent large study, carried out in an asymptomatic Asian population (free of ongoing myocardial damage), CK values increased with greater body mass, increased blood pressure, and more advanced age, thus it can be expected, that such patient populations will be particularly vulnerable to higher CK values also after exposure to harmful factors, e.g. statin interaction with SARS-CoV-2 [38].
Although no significant differences were observed between the type of statin and the severity of myopathy, the limitation of our study is that simvastatin was the most commonly used statin in SG. There is a need to conduct a new study to assess whether the risk of side effects of statins in patients with COVID-19 is greater when a given statin and dose is used.
We are aware of some limitations of our study, such as the small size of the test group, however we were forced to exclude from the study all patients who were taking medications other than statins and with comorbidities (exceptions: dyslipidemia) or for whom other known factors may have caused muscle symptoms. The study aimed to highlight the problem of reporting statin-specific side effects in COVID-19 patients and shows that they are not only subjectively perceived by the patient, but can be confirmed objectively e.g. in laboratory tests (CK). In our opinion the muscle adverse symptoms in SARS-CoV-2 infection in statin users may be caused by some kind of pathopharmacological interaction between the virus and drugs, however, the exact mechanism of this phenomenon needs to be clarified. It is essential to carry out a risk-benefit analysis in each case when considering withdrawal of a statin in COVID-19 positive patients. We believe that determining the level of CK facilitates the making of this clinical decision, especially in elderly patients.
5. Conclusions
To conclude, our study indicates that COVID-19 is associated with the higher risk of occurrence of typical statin-related side effects, especially with more advanced age, which should be considered in future trials and treatments.
CRediT authorship contribution statement
Daria Schetz: Conceptualization, Investigation, Methodology, Formal analysis, Writing – original draft, Supervision. Sztormowska-Achranowicz Katarzyna: Statistical analysis, Writing – review & editing. Foerster Jerzy: Investigation, Methodology. Kocić Ivan: Writing – Methodology, Writing – review & editing, Supervision.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
The study was funded by the Medical University of Gdańsk (www.gumed.edu.pl), ST-22, MUG. The sponsor had no role in study design, data collection and analysis, decision to publish or manuscript preparation.
Footnotes
The authors confirm that the Principal Investigator for this paper is Daria Schetz and that she had direct clinical responsibility for patients.
Data Availability
Data supporting the findings of this study are available from the corresponding author on a reasonable request.
References
- 1.Ayeh S.K., Abbey E.J., Khalifa B.A.A., Nudotor R.D., Osei A.D., Chidambaram V., Osuji N., Khan S., Salia E.L., Oduwole M.O., Yusuf H.E., Lasisi O., Nosakhare E., Karakousis P.C. Statins use and COVID-19 outcomes in hospitalized patients. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.025689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Versace V., Sebastianelli L., Ferrazzoli D., Saltuari L., Kofler M., Löscher W., Uncini A. Case report: myopathy in critically Ill COVID-19 patients: a consequence of hyperinflammation? Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.625144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meegada S., Muppidi V., Wilkinson D.C., 3rd, Siddamreddy S., Katta S.K. Coronavirus disease 2019-induced rhabdomyolysis. Cureus. 2020;12(8) doi: 10.7759/cureus.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anklesaria Z., Frankman J., Gordin J., Zhan J., Liu A.K. Fatal rhabdomyolysis in a COVID-19 patient on rosuvastatin. Cureus. 2020;12(10) doi: 10.7759/cureus.11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Nava Guillermo, Trelles-Garcia Daniela Patricia, Yanez-Bello Maria Adriana, et al. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU. A retrospective cohort study. Crit. Care BioMed. Cent. 2020 doi: 10.1186/s13054-020-03154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McTavish D., Pravastatin E.M. Sorkin. A review of its pharmacological properties and therapeutic potential in hypercholesterolaemia. Drugs. 1991;42:65–89. doi: 10.2165/00003495-199142010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Kajinami K., Mabuchi H., Saito Y. NK-104: a novel synthetic HMG-CoA reductase inhibitor. Expert Opin. Investig. Drugs. 2000;9:2653–2661. doi: 10.1517/13543784.9.11.2653. [DOI] [PubMed] [Google Scholar]
- 8.Black D.M. A general assessment of the safety of HMG CoA reductase inhibitors (statins) Curr. Atheroscler. Rep. 2002;4:34–41. doi: 10.1007/s11883-002-0060-0. [DOI] [PubMed] [Google Scholar]
- 9.Rallidis Loukianos S., Fountoulaki Katerina, Anastasiou-Nana Maria. Managing the underestimated risk of statin-associated myopathy. Int. J. Cardiol. 2012;159(3):169–176. doi: 10.1016/j.ijcard.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 10.Bruckert E., Hayem G., Dejager S., Yau C., Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc. Drugs Ther. 2005;19(6):403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 11.Stroes E.S., Thompson P.D., Corsini A., Vladutiu G.D., Raal F.J., Ray K.K., Roden M., Stein E., Tokgözoğlu L., Nordestgaard B.G., Bruckert E., De Backer G., Krauss R.M., Laufs U., Santos R.D., Hegele R.A., Hovingh G.K., Leiter L.A., Mach F., März W., Newman C.B., Wiklund O., Jacobson T.A., Catapano A.L., Chapman M.J., Ginsberg H.N., European Atherosclerosis Society Consensus P. European atherosclerosis society consensus panel. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel statement on assessment, aetiology and management. Eur. Heart J. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson P.D., Clarkson P., Karas R.H. Statin-associated myopathy. JAMA. 2003;289:1681–1689. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 13.Link E., Parish S., Armitage J., Bowman L., Heath S., Matsuda F., Gut I., Lathrop M., Collins R. SLCO1B1 variants and statin-induced myopathy – a genomewide study. New Engl. J. Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 14.Mollazadeh H., Tavana E., Fanni G., Bo S., Banach M., Pirro M., von Haehling S., Jamialahmadi T., Sahebkar A. Effects of statins on mitochondrial pathways. J. Cachex Sarcopenia Muscle. 2021;12(2):237–251. doi: 10.1002/jcsm.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zubak J., Stolarczyk K., Orzeł A., Frączek M., Zatoński T. Comparison of the clinical differences between COVID-19, SARS, influenza, and the common cold: a systematic literature review. Adv. Clin. Exp. Med. 2021;30(1):109–114. doi: 10.17219/acem/129573. [DOI] [PubMed] [Google Scholar]
- 16.Pergolizzi J.V., Jr., Coluzzi F., Colucci R.D., Olsson H., LeQuang J.A., Al-Saadi J., Magnusson P. Statins and muscle pain. Expert Rev. Clin. Pharmacol. 2020;13(3):299–310. doi: 10.1080/17512433.2020.1734451. Epub 2020 Feb 27. [DOI] [PubMed] [Google Scholar]
- 17.Moghadam-Kia S., Oddis C.V., Aggarwal R. Approach to asymptomatic creatine kinase elevation. Cleve Clin. J. Med. 2016;83(1):37–42. doi: 10.3949/ccjm.83a.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawson E.S., Clarkson P.M., Tarnopolsky M.A. Perspectives on exertional rhabdomyolysis. Sports Med. 2017;47(Suppl 1):33–49. doi: 10.1007/s40279-017-0689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavez L.O., Leon M., Einav S., Varon J. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Crit. Care. 2016;20(1):135. doi: 10.1186/s13054-016-1314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aujla Ravinder S., Patel Roshan. StatPearls Publishing; Treasure Island (FL): 2020. Creatine Phosphokinase. StatPearls [Internet] [PubMed] [Google Scholar]
- 21.Patel D.R., Gyamfi R., Torres A. Exertional rhabdomyolysis and acute kidney injury. Phys. Sportsmed. 2009;37(1):71–79. doi: 10.3810/psm.2009.04.1685. [DOI] [PubMed] [Google Scholar]
- 22.Ju Yanghua, Qiao Hongmei, Zhang Ying, Li Yanan. Three cases of rhabdomyolysis induced by viral infections in children and literature review. Chin. Med. Sci. J. 2020;35(4):383–386. doi: 10.24920/003663. [DOI] [PubMed] [Google Scholar]
- 23.Yosef Y., Schurr D., Constantini N. Statins and muscle pain. Harefuah. 2014;153(7):423–427. 431. Hebrew. [PubMed] [Google Scholar]
- 24.Mohassel P., Mammen A.L. The spectrum of statin myopathy. Curr. Opin. Rheumatol. 2013;25(6):747–752. doi: 10.1097/01.bor.0000434673.85515.89.]. [DOI] [PubMed] [Google Scholar]
- 25.Cham S., Evans M.A., Denenberg J.O., Golomb B.A. Statin-associated muscle-related adverse effects: a case series of 354 patients. Pharmacotherapy. 2010;30(6):541–553. doi: 10.1592/phco.30.6.541. PMID: 20500044; PMCID: PMC4729295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaum D., Dallongeville J., Sabouret P., Bruckert E. Discontinuation of statin therapy due to muscular side effects: a survey in real life. Nutr. Metab. Cardiovasc. Dis. 2013;23(9):871–875. doi: 10.1016/j.numecd.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Finegold J.A., Manisty C.H., Goldacre B., Barron A.J., Francis D.P. What proportion of symptomatic side effects in patients taking statins are genuinely caused by the drug? Systematic review of randomized placebo-controlled trials to aid individual patient choice. Eur. J. Prev. Cardiol. 2014;21(4):464–474. doi: 10.1177/2047487314525531. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz B.P., Tollin R., Cassidy G. Grip strength. Collection of normative data with community dwelling elders. Phys. Occup. Ther. Geriatr. 1997;15:53–64. doi: 10.1080/J148v15n01_04. [DOI] [Google Scholar]
- 29.Del Brutto O.H., Mera R.M., Pérez P., Recalde B.Y., Costa A.F., Sedler M.J. Hand grip strength before and after SARS-CoV-2 infection in community-dwelling older adults. J. Am. Geriatr. Soc. 2021;69:2722–2731. doi: 10.1111/jgs.17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crum-Cianflone N.F. Bacterial, fungal, parasitic, and viral myositis. Clin. Microbiol. Rev. 2008;21:473–494. doi: 10.1128/CMR.00001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agyeman P., Duppenthaler A., Heininger U., Aebi C. Influenza-associated myositis in children. Infection. 2004;32:199–203. doi: 10.1007/s15010-004-4003-2. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira A.S. Influenza A (H1N1) induced-myopathy: an important extrapulmonary complication. Arq. Neuropsiquiatr. 2012;70(5):316–318. doi: 10.1590/s0004-282×2012000500002. [DOI] [PubMed] [Google Scholar]
- 33.Orsucci D., Trezzi M., Anichini R., Blanc P., Barontini L., Biagini C., Capitanini A., Comeglio M., Corsini P., Gemignani F., Giannecchini R., Giusti M., Lombardi M., Marrucci E., Natali A., Nenci G., Vannucci F., Volpi G. Increased creatine kinase may predict a worse COVID-19 outcome. J. Clin. Med. 2021;10(8):1734. doi: 10.3390/jcm10081734. PMID: 33923719; PMCID: PMC8073399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrara F., Vitiello A. The advantages of drug treatment with statins in patients with SARS-CoV- infection. Wien. Klin. Woche. 2021;133:958–965. doi: 10.1007/s00508-021-01845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitiello A., Ferrara F. Plausible positive effects of statins in COVID-19 patient. Cardiovasc. Toxicol. 2021;21:781–789. doi: 10.1007/s12012-021-09674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shumate J.B., Brooke M.H., Carroll J.E., Davis J.E. Increased serum creatine kinase after exercise: a sex-linked phenomenon. Neurology. 1979;29(6):902–904. doi: 10.1212/wnl.29.6.902. [DOI] [PubMed] [Google Scholar]
- 37.Cook J.C., Wong E., Haywood L.J. Creatine kinase: race-gender differences in patients hospitalized for suspected myocardial infarction. J. Natl. Med. Assoc. 1990;82(4):249–254. [PMC free article] [PubMed] [Google Scholar]
- 38.Yen C.-H., Wang K.-T., Lee P.-Y., Liu C.-C., Hsieh Y.-C., Kuo J.-Y., Bulwer B.E., Hung C.L., Chang S.C., Shih S.C., Hu K.C., Yeh H.I., Lam C. Gender-differences in the associations between circulating creatine kinase, blood pressure, body mass and non-alcoholic fatty liver disease in asymptomatic asians. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0179898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author on a reasonable request.