Summary
There have been major advances in the armamentarium for hepatocellular carcinoma (HCC) since the last official update of the Barcelona Clinic Liver Cancer prognosis and treatment strategy published in 2018. Whilst there have been advances in all areas, we will focus on those that have led to a change in strategy and we will discuss why, despite being encouraging, data for select interventions are still too immature for them to be incorporated into an evidence-based model for clinicians and researchers. Finally, we describe the critical insight and expert knowledge that are required to make clinical decisions for individual patients, considering all of the parameters that must be considered to deliver personalised clinical management.
Keywords: HCC, survival, BCLC, ablation, surgery, liver transplantation TACE, TARE, systemic treatment, ALBI score, AFP
Introduction
There have been major advances in the armamentarium for hepatocellular carcinoma (HCC) since the last official update of the Barcelona Clinic Liver Cancer (BCLC) prognosis and treatment strategy published in 2018 [1–7]. Whilst there have been advances in all areas, we will focus on those that have led to a change in strategy and we will discuss why, despite being encouraging, data for select interventions are still too immature for them to be incorporated into an evidence-based model for clinicians and researchers. Scientific evidence should be properly graded according to study design and while observational studies are informative, their limitations for robust causality inference should be acknowledged [8]. While prior updates were developed solely by BCLC members, we have now included expert authors from beyond the BCLC group to integrate different expertise, insights and knowledge into this update.
Prognosis prediction and patient characterisation
While there is no controversy regarding the current stratification of patients according to tumour burden and cancer-related symptoms [9,10] in prognosis prediction, the evaluation of underlying liver function, for which the Child-Pugh classification [11] was already abandoned in the last BCLC version, warrants a further update. Decompensation of liver disease (jaundice, ascites, encephalopathy) reflects non-preserved liver function irrespective of the Child-Pugh or model for end-stage liver disease (MELD) score [12,13], for which several improvements have been proposed [14], but compensated liver function could be stratified with additional granularity by using the albumin-bilirubin (ALBI) score [15–17], while also adding alpha-fetoprotein (AFP) concentration, irrespective of tumour burden [18,19]. These parameters are now included in the 2022 BCLC model (Fig. 1), but while they may impact prognosis, they may not abolish the treatment benefit if the degree of liver dysfunction does not exceed the established selection criteria for an optimal outcome. Prior variceal bleeding also reflects more advanced liver disease with clinically significant portal hypertension (CSPH)[20–22], but history thereof does not necessarily warrant its incorporation into prognosis prediction in patients with liver cancer, while it could still be an important aspect in defining treatment indication. The degree of ascites and response to therapy also impact prognosis: small radiographic ascites or fluid retention that is controlled by a low sodium diet differs from tense ascites regardless of medical treatment (diuretics and/or paracentesis) with or without the presence/absence of renal failure [23,24]. Regarding performance status (PS) assessment [9,10], it is important to highlight that PS assessment should incorporate tumour-related symptoms but not baseline symptoms already present prior to cancer diagnosis and thus, related to pre-existing comorbidities. This can be difficult to differentiate when PS impairment is related to liver dysfunction, which may or may not be related to tumour burden.
Fig. 1. BCLC staging and treatment strategy in 2022.
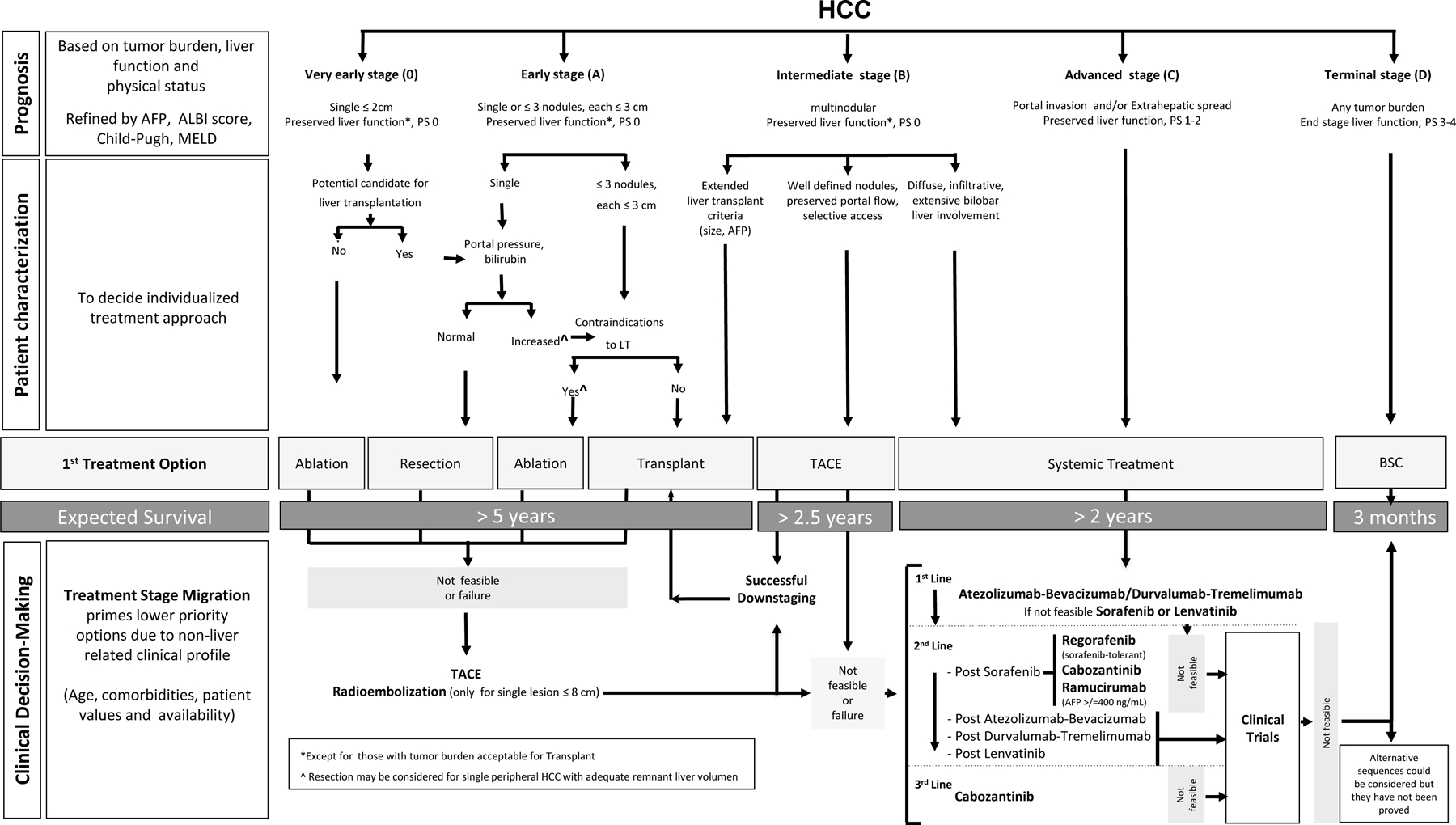
The BCLC system establishes a prognosis in accordance with the 5 stages that are linked to first-line treatment recommendation. The expected outcome is expressed as median survival of each tumour stage according to the available scientific evidence. Individualised clinical decision-making, according to the available data on September 15, 2021, is defined by teams responsible for integrating all available data with the individual patient’s medical profile. Note that liver function should be evaluated beyond the conventional Child-Pugh staging. ++Full availability of the data from the trial testing the combination of tremelimumab and durvalumab may lead to these agents being incorporated as a first-line alternative. AFP, alpha-fetoprotein; ALBI, albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; BSC, best supportive care; ECOG PS, Eastern Cooperative Oncology Group-performance status; LT, liver transplantation; MELD, model of end-stage liver disease; TACE, transarterial chemoembolisation.
Clinical research trials include BCLC staging in the definition of the target population, while at the same time they establish inclusion/exclusion criteria to define the patient’s profile. In clinical practice, the evaluation of a patient’s status incorporates BCLC staging and, simultaneously, the expert and personalised approach of the treating physician and multidisciplinary tumour board who will also consider tumour extension and burden, nutritional status, comorbidities and frailty, age, social status and human values and beliefs. Molecular profiling still cannot predict patient outcomes, risk of recurrence after successful surgery or ablation [25], or the best treatment option [18].
As known, clinical practice guidelines and algorithms such as the BCLC model [1–7] reveal the current state of knowledge and degree of scientific evidence available for each intervention [26–30], but the ultimate decision must be taken by the responsible physician and tumour board, who need to assimilate all variables and recommend a given path of care for the patient as an individual [31]. The multidisciplinary approach is key from the initial diagnosis and tumour staging through to defining the best initial and sequential treatment strategy. Expert radiologists, interventional radiologists, radiation oncologists, pathologists, nurses, clinicians, surgeons in the field of HCC and palliative care specialists and social workers need to work together for this purpose. Healthcare teams are responsible for performing and/or interpreting imaging techniques and pathology samples, to ultimately integrate all those data with the individual patient’s medical profile. In this regard, the personalised management of patients goes beyond factors related to HCC and liver function. Therefore, clinical decision-making and treatment recommendations should not merely be based on a simplified figure but on a complex process that requires personal insights and expertise.
Treatment
In the following paragraphs we describe the proposed treatment options for each BCLC stage. We also emphasise that while a given option should be considered first, expert evaluation of all the clinical and sociocultural information may result in two important concepts: treatment stage migration (TSM) and untreatable progression [6,32,33]. TSM is applied when a specific patient profile may induce a shift of the recommendation to the option that would be considered a priority for a more advanced stage. Untreatable progression was developed for patients under transarterial chemoembolisaton (TACE) [32,33] but applies to all BCLC stages and treatments. It represents failure of the selected treatment strategy [34]. It emerges when patients present treatment failure or progression but still fit into their initial BCLC stage, thus warranting the consideration of a therapy corresponding to a more advanced stage. The 2022 BCLC treatment strategy (Fig. 1) incorporates a specific section to help guide an individualised approach to clinical decision-making, according to the available data on November 15, 2021.
It is worth stressing that while at first sight the BCLC model (Fig. 1) displays a given option that should be considered first, the specific profile of an individual patient may induce a shift in the recommendation to a treatment considered a priority for a more advanced stage (TSM concept) [6]. In some cases, treatment may shift from that initially recommended for early stage, to that recommended for advanced stage, or even to no treatment.
Very early stage (BCLC 0)
This is defined as a solitary HCC ≤2 cm without vascular invasion or extrahepatic spread in a patient with preserved liver function and no cancer-related symptoms. BCLC-0 management varies according to the potential access to liver transplantation (LT) and specific profiles as depicted in the clinical decision-making section. The potential for LT should be considered because of the high recurrence risk, as already described in the last BCLC model [5]. Therefore, if transplantation is an option and patients fulfil the criteria for surgery, resection should be the first choice. Pathology patterns indicative of increased recurrence risk (microscopic vascular invasion, satellites) may induce the consideration of LT because of such risk [35–37]. However, local regulations for enlistment and priority policies may preclude effective transplant for BCLC-0 until recurrence is apparent. If LT is not feasible, the first treatment approach would be ablation, which is associated with similar survival outcomes to resection [38–41]. Those patients with very small HCCs who present with severe liver dysfunction/decompensation may be considered for LT if they fulfil the enlistment criteria [42–47]. If not eligible for LT due to non-HCC factors, patients should be classified as BCLC stage D because of their dismal predicted survival. Indeed, patients may receive priority because of their end-stage liver status, while the presence of very early HCC is not considered as the reason for enlistment and/or for priority allocation.
Early stage (BCLC-A)
This is defined as solitary HCC irrespective of size or as a multifocal HCC up to 3 nodules (none of them >3 cm), without macrovascular invasion, extrahepatic spread or cancer-related symptoms (PS-0). Liver function must be preserved and not have reached LT criteria, at which point the patient would be classified as BCLC stage D because of their dismal prognosis outside of LT eligibility. As mentioned above, those patients with liver disease deserving LT consideration should be evaluated in the framework of end-stage liver disease. In such settings, a diagnosis of HCC could become an exclusion criterion for LT if exceeding the enlistment criteria [42–47]. If LT is contraindicated due to non-HCC factors, there is no effective option to be offered. Thus, the patient should be classified as BCLC stage D.
The treatment approach for BCLC-A patients varies according to tumour number and degree of liver function impairment.
Solitary HCC
Treatment selection in these patients requires a multiparametric approach.[48] Liver function assessment should be stratified according to the degree of portal hypertension as it has been established that the presence of CSPH (defined by a hepatic venous pressure gradient [HVPG] >10 mmHg) predicts a higher rate of postoperative complications and a lower long-term survival [49–51]. It is important to note that the accuracy of HVPG measurement is controversial in patients with non-alcoholic fatty liver disease [52,53].
In the absence of CSPH, patients are considered for resection, and the recommendation should consider tumour burden and location, as well as potential candidacy for LT if the pathology profile of a resected HCC indicates a high risk of recurrence [35–37]; microvascular invasion and satellites are well-known recurrence predictors and because of such increased risk, LT may be considered if they are present [35–37]. If no decision is to be derived from pathological examination, the survival offered by ablation in patients with HCC ≤3 cm may be competitive with that offered by resection [38–40,54–56]. Because of less invasiveness and cost, ablation could be given priority. However, resection may be preferred for larger nodules and those in high-risk locations for ablation, for instance, adjacent to the gallbladder (see clinical decision-making section) [57].
If patients present with CSPH, surgical resection should be considered of significant risk [50,58] and LT offers improved medium- and long-term survival. However, patients may not be considered for LT because of any characteristic and ablation has limited efficacy for large HCC. Therefore, laparoscopic resection could be considered if HCC is in the appropriate location and there is a minor degree of CSPH [27,48,59–63]. Unfortunately, no portal pressure cut-off value may be given for such a decision and no robust recommendation can be made.
Multifocal within Milan criteria (up to 3 HCC nodules, each ≤3 cm)
Multifocal disease still within the Milan criteria [42] is better served by LT, as ablation and resection are hampered by a high risk of HCC recurrence [64–66]. If LT is not feasible, it is a matter of debate if outcome after surgery or ablation is better than that offered by TACE [67,68]. Prospective clinical research is mandatory to define when surgical resection or ablation should be given priority over TACE for patients with up to 3 nodules. This may provide very competitive survival figures in early-stage patients with preserved liver function and health status.
Intermediate stage (BCLC-B)
This is defined as multifocal HCC (exceeding BCLC-A criteria) with preserved liver function, no cancer-related symptoms (PS 0) and no vascular invasion or extrahepatic spread. As well known, the magnitude of tumour burden may be quite heterogeneous in this stage, and prognosis is also influenced by AFP concentration [69] and the degree of liver function impairment even if still belonging to Child-Pugh class A [16]. However, robust cut-offs are not available. This individualised patient profile may also determine whether LT, TACE or systemic therapy should be favoured [70,71]. The 2022 BCLC version stratifies the BCLC-B stage into 3 groups of patients according to tumour burden and liver function.
The first subgroup within BCLC-B includes patients with well-defined HCC nodules. These patients could be candidates for LT if they meet the ‘Extended Liver Transplant criteria’ according to the criteria of the Institution [72]. Expansion of the criteria for LT has been proposed for several years [42–47]. A minor increase in tumour size or number may provide competitive survival figures, hampered by a slight increase in recurrence that impairs long-term survival [73]. The same has been reported if patients are allowed to develop a limited asymptomatic progression beyond Milan criteria [74]. Survival may be competitive with that of patients within Milan criteria, but always at a cost of higher recurrence and lower long-term survival. Accordingly, the decision to accept extended criteria is defined by the impact of such expansion on access to LT for other indications and the minimal outcome that should be achieved for each indication [75]. Elevated AFP values predict a higher risk of HCC recurrence and thus, lower survival. Several groups have established a concentration limit beyond which LT is not considered [45,46,76]. A 1,000 ng/dl cut-off value is currently applied as an exclusion criterion. While downstaging therapy may induce a reduction of AFP, there are no robust data to define the magnitude and/or duration of reduction required before considering LT [77].
The second subgroup comprises patients without the option of LT but who have preserved portal flow and defined tumour burden, suggesting the feasibility of selective access to feeding tumour arteries. They are candidates for TACE. If patients neither meet the ‘Extended liver transplant criteria’ nor the TACE criteria to secure optimal outcomes [5,27,71], systemic therapy should be considered.
The third subgroup within BCLC-B includes patients with diffuse, infiltrative, extensive HCC liver involvement. They do not benefit from TACE [6], and systemic therapy should be the recommended option, although there is no strict cut-off for when this is the case.
Advanced stage (BCLC-C)
This stage includes patients presenting with vascular invasion or extrahepatic spread who are still relatively fit, as reflected by a PS ≤2 at staging work-up, and who have preserved liver function. BCLC-C patients should be evaluated for systemic therapy [29]. Different effective options for first-, second- and following lines are currently available if patients fulfil the characteristics defined in the registration trials that led to regulatory approval [29].
The combination of atezolizumab with bevacizumab (Atezo-Bev) is currently the first-choice first-line treatment, as it confers a superior survival benefit compared to sorafenib[78–80], while it has not been evaluated head-to-head vs. lenvatinib [81]. To benefit from Atezo-Bev, patients must present preserved liver function (compensated Child-Pugh A if there is underlying cirrhosis) and absence of high-risk stigmata for bleeding on upper endoscopy, e.g. properly treated oesophageal varices and no history of variceal bleeding, in order to minimise bleeding risk. Additional requirements that may prevent treatment include vascular disorders and arterial hypertension, as well as severe autoimmune disorders and prior transplantation [29]. On October 15, 2021 data from the phase III HIMALAYA trial showed that a single priming dose of tremelimumab added to durvalumab provided a statistically significant survival benefit vs. sorafenib and durvalumab as monotherapy is not inferior to sorafenib in first-line [82]. Hence, availability of all the study data will likely impact on clinical decision-making in this setting. On November 20, the report of the COSMIC 132 trial testing the combination of cabozantinib and atezolizumab showed a significant benefit in progression-free survival (hazard ratio 0.63), but while waiting for the final survival analysis, the interim data do not show a significant survival benefit compared to sorafenib [83].
The treatment landscape following disease progression or toxicity (leading to treatment interruption) has gained complexity [29]. Prior to 2020, sorafenib was the only effective first-line option for which evidence-based sequential treatment could be proposed [84–87]. Patients transitioning to the second-line setting benefit from regorafenib if they are tolerant to sorafenib [84], from cabozantinib irrespective of tolerance to sorafenib [85], or ramucirumab if AFP level is >400 ng/dl and irrespective of tolerance to sorafenib [87]. Cabozantinib is also effective as a third-line treatment [85]. A Western trial comparing pembrolizumab vs. placebo in second-line did not meet its primary overall survival endpoint [88], but an Asian trial in a similar population reported a significant survival improvement [89].
End-stage (BCLC-D)
Patients with major cancer-related symptoms (PS >2) and/or impaired liver function without the option of LT due to HCC burden or non-HCC-related factors present poor short-term survival and belong to the BCLC stage D [1]. Development of HCC in patients with advanced liver disease who would otherwise be considered for LT may mandate their enlistment if tumour burden does not exceed the established criteria. Accordingly, owing to chronic liver disease, treatment of HCC will not change expected survival and would be of no benefit. In such instances, symptomatic management and coordination of palliative care are mandatory.
According to the BCLC proposal, the expected median survival of patients with HCC should be more than 5 years, 2.5 years, 2 years and 3 months for BCLC 0/A, B, C and D, respectively.
Clinical decision-making
The 2022 BCLC strategy incorporates an expert clinical decision-making component (Fig. 1). It highlights the different concepts and parameters that physicians and multidisciplinary tumour boards should integrate into a personalised HCC treatment approach.
BCLC-0 patients
Decisions in BCLC-0 may be modulated by several factors that preclude a certain treatment and justify a different recommendation from that proposed in the patient characterisation block of Fig. 1.
Ablation through radiofrequency (RF) or microwave (MW) is the preferred technique [90,91], while percutaneous ethanol injection is still applied in selected patients when there are technical or safety concerns. If ablation (percutaneous or laparoscopic if needed) is not feasible for any reason (location, availability etc.),[57] the patient may be considered for surgical resection with the feasibility and safety assessment detailed in the BCLC-A section. When this option is not feasible, TACE is the preferred option.[92] Transarterial radioembolisation (TARE) is equally effective [93]. Stereotactic body radiation bears antitumoral activity but further prospective studies are needed to define its role [94,95]. However, while safety and efficacy data are well established for RF and MW, TARE could be considered in patients with single nodules ≤8 cm. This new BCLC recommendation is based on the results of the Legacy study [93], which included patients with singles nodules less than 8 cm, Child-Pugh A and Eastern Cooperative Oncology Group-PS 0/1. It is important to emphasise that the median tumour size of the patients included in that study was 2.6 cm (range 0.9–8.1). If the patient is not a candidate for locoregional treatment, the option of systemic treatment should be considered, but always aligned with the inclusion and exclusion criteria for the available agents with proven survival benefit.
BCLC-A patients
Resection and RF ablation offer the same survival benefit for HCC ≤2 cm [38,39]. Ablation beyond this size is less effective and the lower rate of complete responses and higher rate of local recurrences means that resection should be favoured in such cases. MW achieves more extensive tumour necrosis than RF and is potentially the best option for those patients with HCC ≤4 cm [96–98]. Larger tumours may still benefit from resection as size alone should not be considered a limiting factor for surgical resection, as long as imaging has not identified vascular invasion and the remnant liver volume permits adequate postoperative liver function [99,100]. Radiation lobectomy by TARE may increase remnant liver volume and could be considered in some patients [101]. Major hepatectomy carries excessive risk in patients with cirrhosis and specific tumour locations may also prevent resection, leading to the consideration of LT [48]. In such instances, size may be a limiting factor for LT according to the expanded criteria in place. Finally, large tumours are frequently associated with cancer-related symptoms (e.g. pain) and this portends poor outcomes after resection.
Upon enlisting patients for LT and if the expected waiting time exceeds 6 months, it is recommended to consider treatment to prevent tumour progression that could rule out LT. Ablation, chemoembolisation and TARE are the most widely used options for this purpose.
Laparoscopic/robotic resection allows for adequate margins and is less invasive, with fewer postoperative complications and a potentially non-significant impact on liver function even in patients with CSPH [59–63,102]. These encouraging results may indicate resection in patients who would initially be selected for ablation, but in whom the peripheral tumour location may contraindicate such an approach because of the risk of tract seeding (if punctured without a protective rim of non-tumoural liver) or neighbouring organ damage [60]. While an acceptable increased CSPH value has not been defined, it is worth considering that postoperative mortality increases and 1-year survival decreases in parallel with portal hypertension even if the surgery does not involve the liver [103].
As proposed for BCLC stage 0, if a patient is not a candidate for any of the mentioned approaches the concept of TSM should be applied and treatment with TACE should be considered, as well as TARE in patients who meet the Legacy inclusion criteria [93]. This is a retrospective cohort study in which median tumour size was <3 cm and thus, validation by other groups is eagerly awaited. If TARE is also not feasible, systemic therapy should be considered.
The 2022 version of the BCLC staging system does not recommend resection for multinodular HCC within Milan criteria. Cohort studies of resection report encouraging survival results [104], but prospective data are needed to establish the effectiveness of such an approach compared to locoregional approaches. Thus, TACE is the preferred option if the first treatment option is not feasible. However, large tumours exceeding 8–10 cm are reported to be associated with worse outcomes after TACE [70,105], this potentially being related to the potential impairment of portal venous flow due to invasion or compression, rather than to the impact of major tumour necrosis. Furthermore, patients with large tumours are rarely free from symptoms. If these are present the patients should be classified as BCLC-C. Indeed, survival of symptomatic patients (PS 1) after TACE is significantly lower than that of asymptomatic patients [106].
BCLC-B patients
For patients to be candidates for TACE, liver function has to be well preserved. Increased bilirubin beyond 2 mg or slight fluid retention requiring diuretic treatment are associated with an increased risk of adverse events and suboptimal survival after TACE [70]. It is not possible to define strict evidence-based criteria to recommend TSM (favouring systemic treatment), so expert assessment is key to secure optimal care. Ongoing trials comparing TACE vs. systemic therapy for BCLC-B patients have detailed inclusion and exclusion criteria and may produce very useful information that will guide clinical practice.
TACE might be performed using chemotherapy emulsified in lipiodol followed by gelfoam or any other material injection (conventional TACE) or using drug-eluting microspheres (DEB-TACE) [105]. The first is associated with a peak of chemotherapy in the systemic circulation that may increase toxicity and lead to higher post-procedural pain, while DEB-TACE has a favourable pharmacokinetic profile.[107] Response rates and survival are not different between the techniques [106,108]. Thus, each team has to define its preference. Available clinical trials comparing bland embolisation to TACE are not informative as the population included does not match the profile of patients for whom TACE would be recommended [109,110]. Meta-analytic assessment is hampered by excessive heterogeneity between trials [111].
Systemic treatment is the recommended option for those BCLC-B patients who are not candidates for TACE for any reason [78–81,84,85,87]. If not candidates for systemic treatment, entry into clinical trials should be considered.
Although the Milan criteria are still largely applied to select patients with HCC for LT, an increasing number of studies have shown that acceptable post-LT survival may be obtained in a selected group of patients at BCLC stage B beyond Milan criteria. Several selection criteria that sought to expand the Milan criteria have been proposed and revised elsewhere [72]. Consensus on expanded criteria for LT in HCC has not been reached. However, composite criteria that consider surrogates of tumour biology (AFP being the most frequently explored) and response to neoadjuvant treatments, are likely to replace conventional morphological criteria for defining transplant feasibility. Several AFP cut-offs have been used to exclude LT [45,46,76,112], with values beyond 1,000 ng/ml widely accepted as a contraindication for LT [45,46,112]. Patients with an AFP >1,000 ng/ml who experienced biochemical response (at least a decrease to less than 500 ng/ml) to locoregional therapies have post-LT outcomes comparable to those reported for patients within Milan criteria [77]. Downstaging has emerged as a reliable tool for selecting patients for LT. The goal of downstaging is to reduce tumour burden in order for residual viable tumours to fall within acceptable LT criteria, with Milan criteria being the commonest endpoint of downstaging [113,114]. The upper limit of where a downstaging approach is considered varies across LT regions. This also affects the specific imaging criteria used to define baseline and post-treatment staging and evaluation of response. Further studies are needed to validate such an approach and to establish how best to apply a downstaging protocol. It is important to note that the need to carefully establish the patient profile that defines a good transplant candidate is due to the shortage of donors. It implies a demand to use the available donors to provide the best outcome for the community and not solely the individual. Live donation may circumvent this challenge, although whether to use the same criteria as for cadaveric donors or to accept a moderate expansion or a downstaging success is still controversial [72]. Again, survival may be competitive but the balance between donor risk and patient benefit is not homogeneously perceived in different cultural settings.
BCLC-C patients
Atezo-Bev provides survival benefit over sorafenib with some patients exhibiting prolonged complete responses. Real-life data will reveal the proportion of patients excluded from the IMbrave150 clinical trial due to comorbidities or associated bleeding risk due to portal hypertension. The combination of tremelimumab and durvalumab has been reported to be superior to sorafenib, adding another first-line treatment option [82]. A significant proportion of patients with advanced HCC may not be appropriate candidates for either Atezo-Bev or tremelimumab and durvalumab or durvalumab as monotheraphy; TKIs (sorafenib or lenvatinib) could still be considered in cases where the previous options are contraindicated.[29]. In this regard, selection of the appropriate option relies on the careful analysis of the clinical, radiological and biochemical profile of the patient, so that they fit into the target population enrolled in the trials where safety and efficacy was demonstrated. Prospective studies of data in real life may broaden the treatment indication, but in its absence, the recommendation is to retain the clinical and biochemical profile defined in registration trials [29]. Furthermore, real-world data may be informative but will never replace the strength of randomised trials as evidence of a survival benefit [115]. Safety in specific populations may be established but survival benefit in the absence of randomised trials will remain speculative.
Even though Atezo-Bev is the preferred first-line option [78], the results of the study on tremelimumab-durvalumab will impact on the choice of first-line treatment. If these options are not feasible for any reason, consideration has to be given to the fact that both lenvatinib and single agent durvalumab are non-inferior to sorafenib[81,82], but recalling that there is a major need to assess if the available second-line alternatives retain their effectiveness in patients initially receiving either of these options. Further, it needs to be evaluated if sorafenib, lenvatinib or durvalumab should be considered as “de facto” second-line options or if their effectiveness could be modified after Atezo-Bev or tremelimumab-durvalumab [29,115]. No robust information is available, thus preventing evidence-based recommendations. Several trials that may clarify some of the current unknowns are ongoing and, they may or may not increase the first-line alternatives and/or change the sequential treatment schedule currently in place [29]. In that sense, as the efficacy of new strategies increases in terms of response and reduction of tumour burden, the registered downstaging may allow some patients to benefit from potentially curative options that were initially discarded because of excessive tumour load [116].
TARE has also been suggested to be as effective as sorafenib in patients with liver-only involvement [117–119]. However, prospective phase III trials comparing it with sorafenib or combining it with sorafenib vs. sorafenib alone have failed to demonstrate its superiority and were not designed to prove non-inferiority [120–122]. Therefore, no evidence-based recommendation can be made until positive trials are available.
Evolutionary events and clinical decision-making process
BCLC staging upon progression after initial diagnosis
It is conventional in oncology to consider tumour progression as a dismal event that is taken as a reflection of treatment failure and the need to transition to another line of therapy [115]. However, it is well established that patients treated with surgical resection, ablation or TACE may present progression at new intrahepatic sites after successful treatment of the first tumour nodule. In some instances, treatment may be repeated, and the tumour again brought under control with potential complete response [6]. Indeed, progression may have different patterns with sharply different meaning in terms of prognosis and potential treatment [123]. This concept was raised years ago for patients treated by TACE [33]. Treatment may be successful, but new tumour sites may appear during follow-up. These may be amenable to new TACE if just intrahepatic and if the patient profile has not changed in terms of liver function and physical status [32]. Contrarily, if progression is due to portal vein invasion or extrahepatic spread or cancer-related symptoms, or if liver function is significantly impaired, new TACE sessions are not recommended. In such instances, the patient is registered as presenting untreatable progression and systemic therapy may be considered [6,33]. This specific progression scenario implies a different prognosis and a different treatment recommendation and hence, offers more clinical insight than the commonly used term “progression”. The heterogeneity of both progression patterns and individual patient profiles at progression mandate multidisciplinary team discussions to identify the best treatment option for a given patient.
The same need to stratify the pattern of progression has emerged in patients undergoing systemic therapy. Prospective studies have demonstrated that prognosis after progression due to increased growth of known tumour sites or new intrahepatic sites is significantly better than progression due to new extrahepatic involvement or vascular invasion [123]. This primed the proposal of a BCLC prognostic model upon progression that is depicted in Fig. 2 [6]. As shown, patients may start systemic therapy either in BCLC stage B or C. Those initially at stage B may progress but remain within the definitions of stage B, being classified as BCLCp-B. Those initially at BCLC stage C may show growth of existing lesions or new intrahepatic sites and be classified as BCLCp-C1 or develop new vascular invasion or extrahepatic spread and then be registered as BCLCp-C2 [6].
Fig. 2. BCLC stratification upon radiological progression.
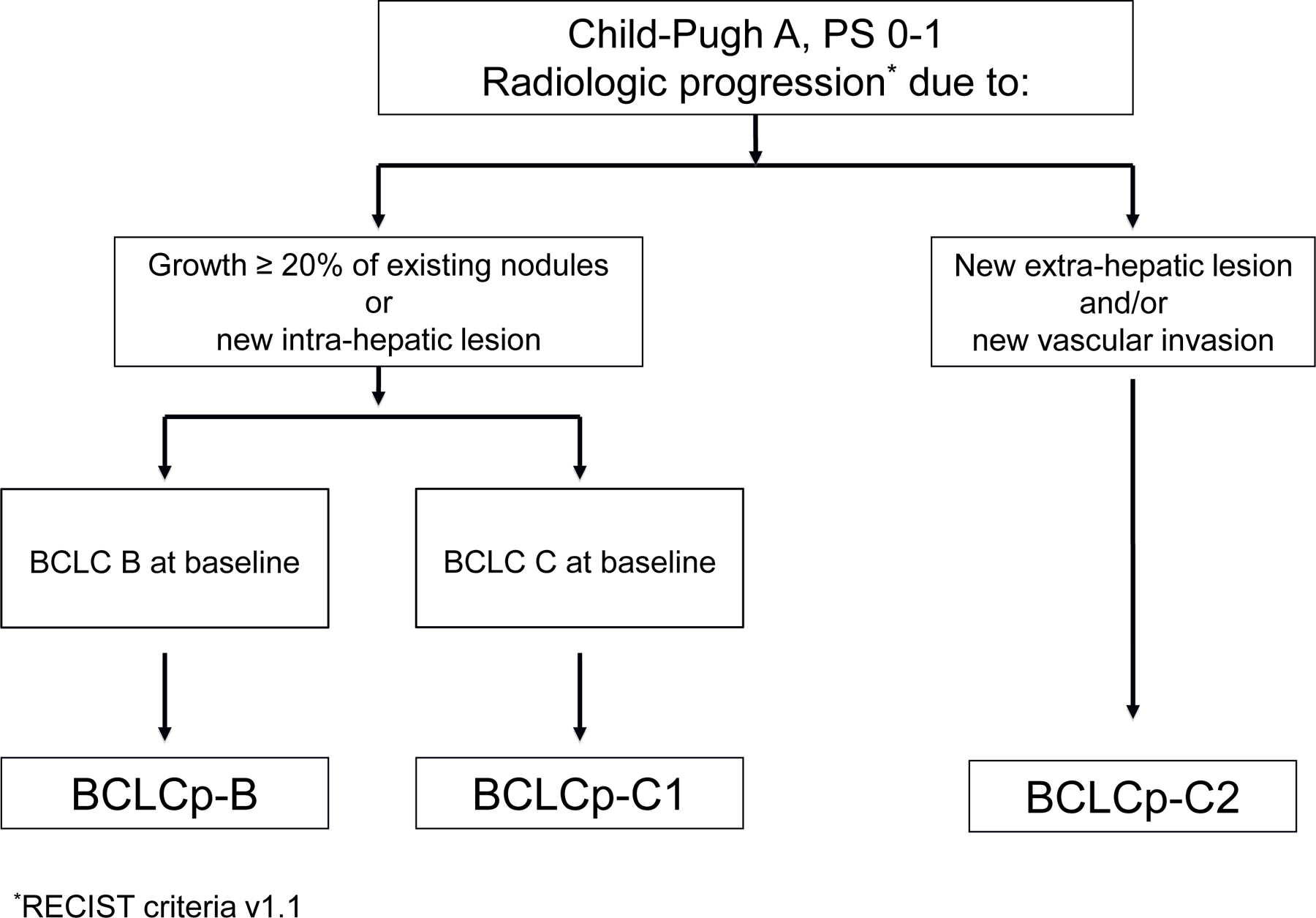
This “BCLC upon progression” (BCLCp) proposal classifies as BCLCp-B those patients who present radiological progression due to growth of existing nodules (≥20%) or new intrahepatic sites but are still within BCLC-B because of the absence of vascular invasion or extrahepatic spread or cancer-related symptoms (PS 0). Those patients who present radiological progression and evolve to BCLC-C or progress within BCLC-C are divided at the time of progression into: BCLCp-C1: those patients who present radiological progression due to growth of existing nodules (≥20%) or new intrahepatic sites, and BCLCp C2: those patients who present progression due to new extrahepatic lesion and/or vascular invasion. BCLC, Barcelona Clinic Liver Cancer; PS, performance status.
The prognostic stratification offered by the classification of patients according to their pattern of progression has been repeatedly validated [124–127]. While pattern of progression is not a predictor of treatment benefit, it has become a relevant parameter to inform patients and to design and analyse clinical trials where a sound survival assumption is key to establish the potential impact of new agents on life expectancy [115].
In summary, herein, we have updated the BCLC strategy for prognosis and treatment. This update has incorporated several advances in the clinical management of HCC and clearly delineated the 3 key steps for patients diagnosed with this cancer. Initial staging serves to stratify patients according to their evolutionary status and is linked to the first treatment recommendation. This should be based on the amalgamation of all the different patient characteristics that are key to choosing the option that is expected to provide the best survival. While the initial recommendations are based on robust scientific evidence, the “clinical decision-making” section highlights the complexity of management at the individual level and the need to personalise decisions at the tumour board level, incorporating the concepts of TSM and untreatable progression. However, no algorithm should be expected to provide exhaustive guidance for each patient. A multiparametric evaluation should be in place for every patient and this should be integrated into multidisciplinary tumour boards where all partners involved in care are actively involved. For an effective output from such boards, it is key to have a clearly established initial approach from where to reach individual decisions. The updated BCLC model and its regular update serves this purpose.
Key points.
The updated BCLC strategy for prognosis prediction and treatment recommendation has been updated according to advances in knowledge.
Stratification of patients into stages has maintained the systems simplicity but further refined the model according to patient characterisation.
Staging is linked to the first option to be considered according to scientific evidence.
Personalised treatment indications are established according to an expert clinical decision-making process where all dimensions of a patient’s profile are taken into account.
The treatment of BCLC-B patients has to be tailored according to tumour burden and effective downstaging may allow for liver transplantation.
Evaluation and management of patients with liver cancer has to integrate baseline patient profiles and evolutionary events.
Tumour progression and/or treatment-related adverse events may lead to treatment recommendations that would usually be for a more advanced stage even if BCLC stage has not changed (treatment stage migration).
Acknowledgement
Some of the authors of this article are members of the European Reference Network on Hepatological Diseases (ERN RARE-LIVER).
Financial support
MR: Dr. Reig’s research is partially supported by Instituto de Salud Carlos III (PI15/00145 and PI18/0358) and from the Spanish Health Ministry (National Strategic Plan against Hepatitis C). AF: Dr. Forner’s research is partially supported by Instituto de Salud Carlos III (PI13/01229 and PI18/00542). JR: Dr. Rimola’s research is partially supported by grant from European Association for the Study of the Liver (EASL). BS: Dr. Sangro’s research is partially supported by ISCIII/EU TRANSCAN-2 (AC16/00065), and Instituto de Salud Carlos III (PI19/00742). AS: Dr. Singal’s research is supported by National Institute of Health R01 R01 MD012565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JB: Dr. Bruix’s research is partially supported by Instituto de Salud Carlos III (PI18/00768), the Spanish Health Ministry (National Strategic Plan against Hepatitis C) and AECC (PI044031). CIBERehd: is funded by the Instituto de Salud Carlos III.
Abbreviations
- AFP
alpha-fetoprotein
- Atez-Bev
atezolizumab with bevacizumab
- BCLC
Barcelona Clinic Liver Cancer
- CSPH
clinically significant portal hypertension
- HCC
hepatocellular carcinoma
- HVPG
hepatic venous pressure gradient
- LT
liver transplantation
- MELD
model for end-stage liver disease
- MW
microwave
- PS
performance status
- RF
radiofrequency
- TACE
transarterial chemoembolization
- TARE
transarterial chemoembolization
- TSM
treatment stage migration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
MR: reports consultancy from Bayer, BMS, Roche, Ipsen, Astra Zeneca, Boston Science and Lilly; lecture fees from Bayer, BMS, Gilead, Lilly, Roche and UniversalDX and travel support from Bayer, BMS, Lilly and Astra Zeneca. Received research funding (to institution) from Bayer and Ipsen. AF: reports lecture fees from Bayer, Boston Science, Gilead, and MSD, consultancy fees from Bayer, Astra Zeneca, Roche, SIRTEX, AB Exact Science and Guerbert. JR: reports lectures and travel grants from Bayer and Roche. JFF: reports lecture fees from Bayer. MB: reports lectures and/or travel support from Boston Science, Terumo, Guerbet and Bayer. AGC: reports lectures fee from Boston Science, Terumo. KRK: reports consultancy fees (to self) from Exact Sciences, Genentech/Roche, Gilead. Received travel support from Ipsen. Received research funding (to institution) from Agios, Astra Zeneca, Bayer, BMS, Eli Lilly, EMD Serono, Exelixis, Genentech/Roche, Merck, Novartis, Partner Therapeutics, QED, Relay Therapeutics, Surface Oncology, Taiho. PRG: consultancy fees and/or travel support from Bayer, Boston Scientific, AstraZeneca, Adaptimmune, BMS, MSD, Sirtex, Lilly, Roche, Guerbet, Ipsen, Eisai. VM: Nothing to disclose. RS: reports consultancy fees from Boston Scientific, Cook, Bard, Genentech, Astrazeneca, Eisai, Sirtex, Siemens, research support from Boston Scientific. BS: reports consultancy fees from Adaptimmune, Astra Zeneca, Bayer, BMS, Boston Scientific, BTG, Eisai, Eli Lilly, H3 Biomedicine, Ipsen, Novartis, Merck, Roche, Sirtex Medical, Terumo; speaker fees from Astra Zeneca, Bayer, BMS, BTG, Eli Lilly, Ipsen, Novartis, Merck, Roche, Sirtex Medical, Terumo; research grants (to Institution) from BMS and Sirtex Medical. AS: has served on advisory boards or consulted for Genentech, Bayer, Eisai, AstraZeneca, BMS, and Exelixis. AV: Speaker, consultancy and advisory role: Amgen, Roche, Bayer, Sanofi, BMS, Lilly, Novartis, EISAI, AstraZeneca, Merck, Incyte, Ipsen, PierreFabre, MSD, Sirtex, BTG, Servier, Terumo, GSK. JFu: Nothing to disclose. CA: reports lectures fee from Bayer. JB: has consulted for Arqule, Bayer-Shering Pharma, Novartis, BMS, BTG-Biocompatibles, Eisai, Kowa, Terumo, Gilead, Bio-Alliance, Roche, AbbVie, MSD, Sirtex, Ipsen, Astra-Medimmune, Incyte, Quirem, Adaptimmune, Lilly, Basilea, Nerviano, Sanofi and UniversalDX; and received research/educational grants from Bayer, and lecture fees from Bayer-Shering Pharma, BTG-Biocompatibles, Eisai, Terumo, Sirtex, Ipsen.
References
- [1].Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329–38. [DOI] [PubMed] [Google Scholar]
- [2].Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907–17. [DOI] [PubMed] [Google Scholar]
- [3].Forner A, Reig M, Rodriguez De Lope C, Bruix J, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61–74. 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- [4].Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- [5].Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;31:1301–14. 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- [6].Reig M, Darnell A, Forner A, Rimola J, Ayuso C, Bruix J. Systemic Therapy for Hepatocellular Carcinoma: The Issue of Treatment Stage Migration and Registration of Progression Using the BCLC-Refined RECIST. Semin Liver Dis 2014;34:444–55. 10.1055/s-0034-1394143. [DOI] [PubMed] [Google Scholar]
- [7].Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016;150:835–53. 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- [8].Hernán MA. Methods of Public Health Research - Strengthening Causal Inference from Observational Data. N Engl J Med 2021;385. 10.1056/NEJMp2113319. [DOI] [PubMed]
- [9].Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–93. [DOI] [PubMed] [Google Scholar]
- [10].Sorensen JB, Klee M, Palshof T, Hansen HH. Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer 1993;67:773–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–9. [DOI] [PubMed] [Google Scholar]
- [12].Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464–70. [DOI] [PubMed] [Google Scholar]
- [13].Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and Mortality among Patients on the Liver-Transplant Waiting List. N Engl J Med 2008;359:1018–26. 10.1056/nejmoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim WR, Mannalithara A, Heimbach JK, Kamath PS, Asrani SK, Biggins SW, et al. MELD 3.0: The Model for End-stage Liver Disease Updated for the Modern Era. Gastroenterology 2021. 10.1053/j.gastro.2021.08.050. [DOI] [PMC free article] [PubMed]
- [15].Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550–8. 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pinato DJ, Sharma R, Allara E, Yen C, Arizumi T, Kubota K, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol 2017;66:338–46. 10.1016/j.jhep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- [17].Scheiner B, Pomej K, Kirstein M, Hucke F, Finkelmeier F, Waidmann O, et al. Predicting the outcome of patients with hepatocellular carcinoma treated with immunotherapy-the CRAFITY score. J Hepatol 2021;75:S236–7. [Google Scholar]
- [18].Singal AG, Hoshida Y, Pinato DJ, Marrero J, Nault JC, Paradis V, et al. International Liver Cancer Association (ILCA) White Paper on Biomarker Development for Hepatocellular Carcinoma. Gastroenterology 2021;160:2572–84. 10.1053/J.GASTRO.2021.01.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cabibbo G, Maida M, Genco C, Parisi P, Peralta M, Antonucci M, et al. Natural history of untreatable hepatocellular carcinoma: A retrospective cohort study. World J Hepatol 2012;4:256–61. 10.4254/wjh.v4.i9.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].de Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension. J Hepatol 2015;63:743–52. 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- [21].D’Amico G, Morabito A, D’Amico M, Pasta L, Malizia G, Rebora P, et al. Clinical states of cirrhosis and competing risks. J Hepatol 2018;68:563–76. 10.1016/j.jhep.2017.10.020. [DOI] [PubMed] [Google Scholar]
- [22].Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology 2010;51:1445–9. 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tonon M, Piano S, Gambino CG, Romano A, Pilutti C, Incicco S, et al. Outcomes and Mortality of Grade 1 Ascites and Recurrent Ascites in Patients With Cirrhosis. Clin Gastroenterol Hepatol 2021;19:358–366.e8. 10.1016/j.cgh.2020.03.065. [DOI] [PubMed] [Google Scholar]
- [24].Llach J, Ginès P, Arroyo V, Rimola A, Titó L, Badalamenti S, et al. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology 1988;94:482–7. 10.1016/0016-5085(88)90441-6. [DOI] [PubMed] [Google Scholar]
- [25].Pinyol R, Montal R, Bassaganyas L, Sia D, Takayama T, Chau G-Y, et al. Molecular predictors of prevention of recurrence in HCC with sorafenib as adjuvant treatment and prognostic factors in the phase 3 STORM trial. Gut 2019;Jun;68:1065–1075. 10.1136/gutjnl-2018-316408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vogel A, Cervantes A, Chau I, Daniele B, Llovet J, Meyer T, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2018;29:iv238–55. 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- [27].Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- [28].Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–80. 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- [29].Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma. An EASL position paper. J Hepatol 2021;Oct(75):960–74. 10.1016/j.jhep.2021.07.004. [DOI] [PubMed] [Google Scholar]
- [30].Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol 2020;38:4317–45. 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- [31].Pocock SJ, Stone GW. The Primary Outcome Is Positive — Is That Good Enough? N Engl J Med 2016;375:971–9. 10.1056/nejmra1601511. [DOI] [PubMed] [Google Scholar]
- [32].Forner A, Gilabert M, Bruix J, Raoul J-L. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol 2014;11:525–35. 10.1038/nrclinonc.2014.122. [DOI] [PubMed] [Google Scholar]
- [33].Bruix J, Reig M, Rimola J, Forner A, Burrel M, Vilana R, et al. Clinical decision making and research in hepatocellular carcinoma: Pivotal role of imaging techniques. Hepatology 2011;54:2238–44. 10.1002/hep.24670. [DOI] [PubMed] [Google Scholar]
- [34].Templeton AJ, Amir E, Tannock IF. Informative censoring — a neglected cause of bias in oncology trials. Nat Rev Clin Oncol 2020;17:327–8. 10.1038/s41571-020-0368-0. [DOI] [PubMed] [Google Scholar]
- [35].Sala M, Fuster J, Llovet JM, Navasa M, Sole M, Varela M, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl 2004;10:1294–300. [DOI] [PubMed] [Google Scholar]
- [36].Fuks D, Dokmak S, Paradis V, Diouf M, Durand F, Belghiti J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology 2012;55:132–40. 10.1002/hep.24680. [DOI] [PubMed] [Google Scholar]
- [37].Ferrer-Fàbrega J, Forner A, Liccioni A, Miquel R, Molina V, Navasa M, et al. Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology 2016;63:839–49. 10.1002/hep.28339. [DOI] [PubMed] [Google Scholar]
- [38].Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology 2010;51:1284–90. 10.1002/hep.23466. [DOI] [PubMed] [Google Scholar]
- [39].Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013;59:300–7. 10.1016/j.jhep.2013.04.009. [DOI] [PubMed] [Google Scholar]
- [40].Izumi N, Hasegawa K, Nishioka Y, Takayama T, Yamanaka N, Kudo M, et al. A multicenter randomized controlled trial to evaluate the efficacy of surgery vs. radiofrequency ablation for small hepatocellular carcinoma (SURF trial). J Clin Oncol 2019;37:4002–4002. 10.1200/JCO.2019.37.15_SUPPL.4002. [DOI] [Google Scholar]
- [41].Doyle A, Gorgen A, Muaddi H, Aravinthan AD, Issachar A, Mironov O, et al. Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J Hepatol 2019;70:866–73. 10.1016/j.jhep.2018.12.027. [DOI] [PubMed] [Google Scholar]
- [42].Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–9. [DOI] [PubMed] [Google Scholar]
- [43].Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394–403. [DOI] [PubMed] [Google Scholar]
- [44].Mazzaferro V, Llovet JMJM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35–43. 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- [45].Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:985–6. https://doi.org/S0016-5085(12)00941-9 [pii] 10.1053/j.gastro.2012.05.052. [DOI] [PubMed] [Google Scholar]
- [46].Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death Following Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018;154:128–39. 10.1053/j.gastro.2017.09.025. [DOI] [PubMed] [Google Scholar]
- [47].Herrero JI, Sangro B, Pardo F, Quiroga J, Inarrairaegui M, Rotellar F, et al. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transpl 2008;14:272–8. [DOI] [PubMed] [Google Scholar]
- [48].Citterio D, Facciorusso A, Sposito C, Rota R, Bhoori S, Mazzaferro V. Hierarchic Interaction of Factors Associated With Liver Decompensation After Resection for Hepatocellular Carcinoma. JAMA Surg 2016;151:846. 10.1001/jamasurg.2016.1121. [DOI] [PubMed] [Google Scholar]
- [49].Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434–40. [DOI] [PubMed] [Google Scholar]
- [50].Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology 2015;61:526–36. 10.1002/hep.27431. [DOI] [PubMed] [Google Scholar]
- [51].Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996;111:1018–22. [DOI] [PubMed] [Google Scholar]
- [52].Ferrusquía-Acosta J, Bassegoda O, Turco L, Reverter E, Pellone M, Bianchini M, et al. Agreement between wedged hepatic venous pressure and portal pressure in non-alcoholic steatohepatitis-related cirrhosis. J Hepatol 2021;74:811–8. 10.1016/j.jhep.2020.10.003. [DOI] [PubMed] [Google Scholar]
- [53].Baffy G, Bosch J. Overlooked subclinical portal hypertension in non-cirrhotic NAFLD: Is it real and how to measure it? J Hepatol 2021. 10.1016/j.jhep.2021.09.029. [DOI] [PubMed]
- [54].Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ng KKC, Chok KSH, Chan ACY, Cheung TT, Wong TCL, Fung JYY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg 2017;Dec;104:1775–84. 10.1002/bjs.10677. [DOI] [PubMed] [Google Scholar]
- [56].Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794–802. https://doi.org/S0168-8278(12)00361-3 [pii] 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- [57].Nault J-C, Sutter O, Nahon P, Ganne-Carrié N, Séror O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol 2018;68:783–97. 10.1016/j.jhep.2017.10.004. [DOI] [PubMed] [Google Scholar]
- [58].Berardi G, Morise Z, Sposito C, Igarashi K, Panetta V, Simonelli I, et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J Hepatol 2020;Jan(1):75–84. [DOI] [PubMed] [Google Scholar]
- [59].Sposito C, Battiston C, Facciorusso A, Mazzola M, Muscarà C, Scotti M, et al. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg 2016;103:871–80. 10.1002/bjs.10137. [DOI] [PubMed] [Google Scholar]
- [60].Molina V, Sampson-Dávila J, Ferrer J, Fondevila C, Díaz del Gobbo R, Calatayud D, et al. Benefits of laparoscopic liver resection in patients with hepatocellular carcinoma and portal hypertension: a case-matched study. Surg Endosc 2018;32:2345–54. 10.1007/s00464-017-5930-1. [DOI] [PubMed] [Google Scholar]
- [61].Witowski J, Rubinkiewicz M, Mizera M, Wysocki M, Gajewska N, Sitkowski M, et al. Meta-analysis of short- and long-term outcomes after pure laparoscopic versus open liver surgery in hepatocellular carcinoma patients. Surg Endosc 2019;33:1491. 10.1007/S00464-018-6431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Morise Z, Ciria R, Cherqui D, Chen K-H, Belli G, Wakabayashi G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J Hepatobiliary Pancreat Sci 2015;22:342–52. 10.1002/jhbp.215. [DOI] [PubMed] [Google Scholar]
- [63].Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative Shortterm Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761–77. 10.1097/SLA.0000000000001413. [DOI] [PubMed] [Google Scholar]
- [64].Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol 2013;59:89–97. 10.1016/j.jhep.2013.03.009. [DOI] [PubMed] [Google Scholar]
- [65].N’Kontchou G, Mahamoudi A, Aout M, Ganne-Carrie N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009;50:1475–83. 10.1002/hep.23181. [DOI] [PubMed] [Google Scholar]
- [66].Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012;107:569–77. 10.1038/ajg.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cucchetti A, Djulbegovic B, Tsalatsanis A, Vitale A, Hozo I, Piscaglia F, et al. When to perform hepatic resection for intermediate-stage hepatocellular carcinoma. Hepatology 2015;61:905–14. 10.1002/hep.27321. [DOI] [PubMed] [Google Scholar]
- [68].Forner A, Gilabert M, Bruix J, Raoul J-L. Heterogeneity of intermediate-stage HCC necessitates personalized management including surgery. Nat Rev Clin Oncol 2014;12:10–10. 10.1038/nrclinonc.2014.122-c2. [DOI] [PubMed] [Google Scholar]
- [69].Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006;131:461–9. https://doi.org/S0016-5085(06)01075-4 [pii] 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- [70].Galle PR, Tovoli F, Foerster F, Wörns MA, Cucchetti A, Bolondi L. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol 2017;67:173–83. 10.1016/j.jhep.2017.03.007. [DOI] [PubMed] [Google Scholar]
- [71].Kloeckner R, Galle PR, Bruix J. Local and Regional Therapies for Hepatocellular Carcinoma. Hepatology 2021;73:137–49. 10.1002/hep.31424. [DOI] [PubMed] [Google Scholar]
- [72].Mehta N, Bhangui P, Yao FY, Mazzaferro V, Toso C, Akamatsu N, et al. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020:1136–42. 10.1097/TP.0000000000003174. [DOI] [PubMed]
- [73].Kwong A, Mehta N. Expanding the Limits of Liver Transplantation for Hepatocellular Carcinoma: Is There a Limit? Clin Liver Dis 2021;25:19–33. 10.1016/J.CLD.2020.08.002. [DOI] [PubMed] [Google Scholar]
- [74].Ferrer-Fàbrega J, Sampson-Dávila J, Forner A, Sapena V, Díaz A, Vilana R, et al. Limited tumour progression beyond Milan criteria while on the waiting list does not result in unacceptable impairment of survival. J Hepatol 2021. 10.1016/J.JHEP.2021.06.015. [DOI] [PubMed]
- [75].Navasa M, Bruix J. Multifaceted perspective of the waiting list for liver transplantation: the value of pharmacokinetic models. Hepatology n.d;51:12–5. 10.1002/hep.23332. [DOI] [PubMed] [Google Scholar]
- [76].Toso C, Meeberg G, Hernandez-Alejandro R, Dufour J-F, Marotta P, Majno P, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015;62:158–65. 10.1002/hep.27787. [DOI] [PubMed] [Google Scholar]
- [77].Mehta N, Dodge JL, Roberts JP, Hirose R, Yao FY. Alpha‐fetoprotein Decrease from >1000 to <500 ng/ml in Patients with Hepatocellular Carcinoma Leads to Improved Post‐Transplant Outcomes. Hepatology 2019;Mar;69:1193–205. 10.1002/hep.30413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894–905. 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- [79].Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- [80].Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- [81].Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163–73. 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- [82]. https://www.astrazeneca.com/media-centre/press-releases/2021/imfinzi-and-tremelimumab-improved-os-in-liver-cancer.html n.d.
- [83].Kelley RK, Yau T, Cheng A-L, Kaseb A, Qin S, Zhu AX, et al. VP10–2021: Cabozantinib (C) plus atezolizumab (A) versus sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): Results from the randomized phase III COSMIC-312 trial. Ann Oncol 2021;0. 10.1016/J.ANNONC.2021.10.008. [DOI] [Google Scholar]
- [84].Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;Jan 7;389:56–66. 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- [85].Abou-Alfa GK, Meyer T, Cheng A-L, El-Khoueiry AB, Rimassa L, Ryoo B-Y, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54–63. 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Finn RS, Merle P, Granito A, Huang Y-H, Bodoky G, Pracht M, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol 2018;69:353–8. 10.1016/j.jhep.2018.04.010. [DOI] [PubMed] [Google Scholar]
- [87].Zhu AX, Kang Y-K, Yen C-J, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282–96. 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- [88].Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2020;38:193–202. 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- [89]. https://www.merck.com/news/merck-announces-keytruda-pembrolizumab-met-primary-endpoint-of-overall-survival-os-in-patients-with-advanced-hepatocellular-carcinoma-previously-treated-with-sorafenib/ n.d.
- [90].Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122–30. [DOI] [PubMed] [Google Scholar]
- [91].Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgro G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol 2010;52:380–8. https://doi.org/S0168-8278(09)00805-8 [pii] 10.1016/j.jhep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- [92].Burrel M, Reig M, Forner A, Barrufet M, Lope CRD, Tremosini S, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol 2012;56:1330–5. 10.1016/j.jhep.2012.01.008. [DOI] [PubMed] [Google Scholar]
- [93].Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, et al. Yttrium‐90 Radioembolization for the Treatment of Solitary, Unresectable Hepatocellular Carcinoma: The LEGACY Study. Hepatology 2021;Nov;74:2342–52. 10.1002/hep.31819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Chen CP. Role of External Beam Radiotherapy in Hepatocellular Carcinoma. Clin Liver Dis 2020;24:701–17. 10.1016/j.cld.2020.07.006. [DOI] [PubMed] [Google Scholar]
- [95].Shanker MD, Moodaley P, Soon W, Liu HY, Lee YY, Pryor DI. Stereotactic ablative radiotherapy for hepatocellular carcinoma: A systematic review and meta-analysis of local control, survival and toxicity outcomes. J Med Imaging Radiat Oncol 2021. 10.1111/1754-9485.13309. [DOI] [PubMed]
- [96].Yu J, Yu X, Han Z, Cheng Z, Liu F, Zhai H, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut 2017;66:1172–3. 10.1136/gutjnl-2016-312629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Vietti Violi N, Duran R, Guiu B, Cercueil J-P, Aubé C, Digklia A, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol 2018;3:317–25. 10.1016/S2468-1253(18)30029-3. [DOI] [PubMed] [Google Scholar]
- [98].Han J, Fan YC, Wang K. Radiofrequency ablation versus microwave ablation for early stage hepatocellular carcinoma: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e22703. 10.1097/MD.0000000000022703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol 2018;Dec(69):1284–93. 10.1016/j.jhep.2018.08.027. [DOI] [PubMed] [Google Scholar]
- [100].Vitale A, Burra P, Frigo AC, Trevisani F, Farinati F, Spolverato G, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol 2015;62:617–24. 10.1016/j.jhep.2014.10.037. [DOI] [PubMed] [Google Scholar]
- [101].Vouche M, Lewandowski RJ, Atassi R, Memon K, Gates VL, Ryu RK, et al. Radiation lobectomy: Time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol 2013;59:1029. 10.1016/J.JHEP.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Troisi RI, Berardi G, Morise Z, Cipriani F, Ariizumi S, Sposito C, et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child–Pugh B cirrhosis: multicentre propensity score-matched study. Br J Surg 2021;108:196–204. 10.1093/BJS/ZNAA041. [DOI] [PubMed] [Google Scholar]
- [103].Reverter E, Cirera I, Albillos A, Debernardi-Venon W, Abraldes JG, Llop E, et al. The prognostic role of hepatic venous pressure gradient in cirrhotic patients undergoing elective extrahepatic surgery. J Hepatol 2019;71:942–50. 10.1016/j.jhep.2019.07.007. [DOI] [PubMed] [Google Scholar]
- [104].Fukami Y, Kaneoka Y, Maeda A, Kumada T, Tanaka J, Akita T, et al. Liver Resection for Multiple Hepatocellular Carcinomas: A Japanese Nationwide Survey. Ann Surg 2020;272:145–54. 10.1097/SLA.0000000000003192. [DOI] [PubMed] [Google Scholar]
- [105].Raoul J-L, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev 2019;Jan;72:28–36. 10.1016/j.ctrv.2018.11.002. [DOI] [PubMed] [Google Scholar]
- [106].Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111:255–64. 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007;46:474–81. 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- [108].Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Interv Radiol 2010;33:41–52. 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Meyer T, Kirkwood A, Roughton M, Beare S, Tsochatzis E, Yu D, et al. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br J Cancer 2013;108:1252–9. 10.1038/bjc.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Brown KT, Do RK, Gonen M, Covey AM, Getrajdman GI, Sofocleous CT, et al. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone. J Clin Oncol 2016;34:2046–53. 10.1200/JCO.2015.64.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Facciorusso A, Bellanti F, Villani R, Salvatore V, Muscatiello N, Piscaglia F, et al. Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: A meta-analysis of randomized trials. United Eur Gastroenterol J 2017;5:511–8. 10.1177/2050640616673516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945–51. 10.1002/lt.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging: a randomised, controlled, phase 2/3 trial. Lancet Oncol 2020;Jul;21:947–56. [DOI] [PubMed] [Google Scholar]
- [114].Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, et al. Downstaging of hepatocellular cancer before liver transplant: Long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968–77. 10.1002/hep.27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Bruix J End-points in clinical trials for liver cancer and their value in evidence based clinical decision making: an unresolved Gordian knot. J Hepatol 2021;Jun;74:1483–8. 10.1016/j.jhep.2021.01.033. [DOI] [PubMed] [Google Scholar]
- [116].Ho WJ, Zhu Q, Durham J, Popovic A, Xavier S, Leatherman J, et al. Neoadjuvant cabozantinib and nivolumab convert locally advanced hepatocellular carcinoma into resectable disease with enhanced antitumor immunity. Nat Cancer 2021;2:891–903. 10.1038/s43018-021-00234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868–78. 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- [118].Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 2013;57:1826–37. 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- [119].Hilgard P, Hamami M, Fouly A El, Scherag A, Müller S, Ertle, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010;52:1741–9. 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- [120].Chow PKH, Gandhi M, Tan S-B, Khin MW, Khasbazar A, Ong J, et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol 2018;36(19):1913–21. 10.1200/JCO.2017.76.0892. [DOI] [PubMed] [Google Scholar]
- [121].Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux G-P, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017;Dec;18:1624–36. 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- [122].Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol 2019;Dec;71:1164–74. 10.1016/j.jhep.2019.08.006. [DOI] [PubMed] [Google Scholar]
- [123].Reig M, Rimola J, Torres F, Darnell A, Lope CR-, Forner A, et al. Post-progression survival of patients with advanced hepatocellular carcinoma. Rationale for second line trial design. Hepatology 2013;58:2023–31. 10.1002/hep.26586. [DOI] [PubMed] [Google Scholar]
- [124].Iavarone M, Cabibbo G, Biolato M, Della Corte C, Maida M, Barbara M, et al. Predictors of survival of patients with advanced hepatocellular carcinoma who permanently discontinued sorafenib. Hepatology 2015;Sep;62:784–91. 10.1002/hep.27729. [DOI] [PubMed] [Google Scholar]
- [125].Reig M, Galle PR, Kudo M, Finn R, Llovet JM, Metti AL, et al. Pattern of progression in advanced hepatocellular carcinoma treated with ramucirumab. Liver Int 2021;41:598–607. 10.1111/liv.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rimassa L, Assenat E, Peck-Radosavljevic M, Pracht M, Zagonel V, Mathurin P, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol 2018;May;19:682–93. 10.1016/S1470-2045(18)30146-3. [DOI] [PubMed] [Google Scholar]
- [127].de la Torre-Aláez M, Jordán-Iborra C, Casadei-Gardini A, Bilbao JI, Rodriguez-Fraile M, Sancho L, et al. The Pattern of Progression Defines Post-progression Survival in Patients with Hepatocellular Carcinoma Treated with SIRT. Cardiovasc Intervent Radiol 2020;43:1165–72. 10.1007/s00270-020-02444-2. [DOI] [PubMed] [Google Scholar]


