Abstract
Mounting evidence indicates that stress can predispose chickens to disease. The objective of the current study was to develop a method that utilized physiological stress to predispose Ross 308 broiler chickens to acute necrotic enteritis (NE). Stress was mediated through the administration of the stress hormone, corticosterone. At 11 d posthatch (p.h.), corticosterone (20 mg kg−1) administration commenced. At 12 and 13 d p.h., birds were orally inoculated with a virulent strain of Clostridium perfringens, and at 14 d p.h., birds were euthanized. Birds administered corticosterone exhibited decreased weight gain, and birds co-challenged with C. perfringens and corticosterone were affected to a higher degree. Necrotic lesions were present in birds inoculated with C. perfringens (33%), but a substantially higher prevalence of birds treated with C. perfringens and corticosterone in combination exhibited lesions (100%). Clostridium perfringens densities were correlated with necrotic lesion and histopathologic scores. Both C. perfringens and corticosterone challenge altered mRNA immune responses in the small intestine. In this regard, birds infected with the pathogen showed higher relative mRNA concentrations of toll-like receptor 2A (TLR2A), transforming growth factor beta 2 (TGFβ2), and inducible nitric oxide synthase (INOS). Birds co-challenged with C. perfringens and corticosterone showed hindered TLR2A mRNA expression. A reduction in TLR2A responses mediated by corticosterone administration suggests that the glucocorticoid suppresses immune stimulation in jejunal mucosa, which may be the underlying cause for the increased prevalence and intensity of disease observed in corticosterone treated birds. Overall, the corticosterone stress model resulted in levels of NE comparable to other models of NE that currently exist without the use of a co-infection agent. This model may facilitate the exploration of mechanisms of stress-induced NE, and the development of effective alternatives to antibiotics.
Key words: necrotic enteritis model, Clostridium perfringens, corticosterone stress, disease predisposition, intestinal health
INTRODUCTION
The increasing economic impacts of necrotic enteritis (NE) to poultry production have driven the need to develop models of NE. Several predisposing states and conditions in the intestine have been described that contribute to the proliferation and success of infection by Clostridium perfringens. Damaged mucosa, compromised barrier function, and mucus release can promote C. perfringens pathogenesis, and this can be induced by co-challenging birds with Eimeria maxima and C. perfringens (Moore, 2016). Currently, the most common method of inducing NE in a laboratory setting is by utilizing E. maxima as a co-infection agent, in combination with a strain of C. perfringens that expresses cardinal virulence factors (e.g., NetB toxin) (Lee et al., 2011; Shojadoost et al., 2012). Damage sustained to the epithelium by E. maxima can expose extracellular matrices, such as collagen, that C. perfringens can adhere to, and facilitate invasion of epithelial cells (Collier et al., 2008; Moore, 2016). Moreover, C. perfringens possesses enzymes that breakdown mucins, and the bacterium can grow in minimal media containing mucin glycoproteins (MacMillan et al., 2019; Low et al., 2021). Diet components, such as nonstarch polysaccharides and high protein, are also factors that can contribute to NE (Palliyeguru et al., 2010; Shojadoost et al., 2012). Immunosuppression is another predisposing factor to NE. In this regard, infectious bursal disease virus vaccine has been used as a method to promote immunosuppression and induce NE (McReynolds et al., 2004). All of these predisposing conditions in the intestine undoubtedly alter the microbiota (Moore, 2016). Modulations to the microbiota have been described with NE, although factors that contribute to its alteration are complex (Stanley et al., 2012). Research models of NE often fail to reproduce disease as severe as found in production settings, which may be due to the multitude of predisposing conditions to NE that occur on farm. This highlights the need for research to continue identifying factors that predispose birds to NE, and to elucidate mechanisms of disease onset.
Increasingly, research is examining the role that physiological stress plays as a predisposing factor to disease. Stress can promote similar predisposing conditions in the intestine as other co-factors to disease. For example, stress has been implicated to impact barrier function, and may alter mucus secretion (Smirnov et al., 2004; Pearce et al., 2012; Varasteh et al., 2015). It is well recognized that stress, especially chronic stress, can result in the suppression of immune responses and promote the development of disease (Shini et al., 2010). Heat stress has shown variable impacts on NE (Calefi et al., 2016; Tsiouris et al., 2018). For instance, cyclic acute heat stress tended to promote disease, while chronic heat stress reduced macroscopic lesions (Calefi et al., 2016; Tsiouris et al., 2018). Moreover, various stressors encountered in production settings, such as stocking density and cold temperatures, have been shown to increase macroscopic necrotic lesions, although the mechanisms responsible are yet to be determined (Tsiouris et al., 2015a,b).
We previously administered corticosterone in water to layer chickens as a method to mediate a physiological stress response and characterize the impacts of subclinical NE on the host and the enteric microbiota (Zaytsoff et al., 2020a). In the current study, we hypothesize that broiler chickens administered corticosterone will mount a stress response that will predispose them to clinical NE due to a reduction in immune responses. To test this hypothesis, the objective of the current study was to examine microscopic, macroscopic, and molecular changes in the intestines of broiler chickens ± administration of dietary corticosterone and ± C. perfringens challenge. A primary goal of the study was to develop a stress-induced model of NE for future elucidation of mechanisms of stress-incited predisposition of birds to NE, and to develop rationale-based mitigation strategies including alternatives to antibiotics.
MATERIALS AND METHODS
Ethics Statement
The study was carried out in strict accordance with the Canadian Council on Animal Care Guidelines. The project was reviewed and approved by the Lethbridge Research and Development Centre (LeRDC) Animal Care Committee (Animal Use Protocol Review # 1912) before commencement of the research.
Experimental Design
The study was designed as a factorial experiment with 2 levels of corticosterone administration (±) and 2 levels of C. perfringens challenge (±) arranged as a complete randomized design with 6 replicates. The 4 treatments were: (1) no corticosterone and no C. perfringens (Control); (2) inoculation with C. perfringens and no corticosterone (Cp); (3) corticosterone administration and no C. perfringens (CORT); and (4) C. perfringens inoculation and corticosterone administration (Cp + CORT). The experiment was repeated on 3 separate occasions (i.e., “runs”) with 2 replicates per run.
Animals and Husbandry
Ross 308 broiler chicken eggs for all 3 experimental runs originated from a single broiler breeder farm and were obtained from a local hatchery (Lethbridge, AB). Eggs were incubated in a Brinsea Ovation 56 EX fully automatic digital egg incubator (Brinsea Products Inc., Titusville, FL) according to the manufacturer's guidelines. Briefly, eggs were maintained at 37.5°C at 60% humidity, and were turned hourly for the first 18 d of incubation. Thereafter, eggs were set flat for hatching and the humidity was increased to 70%. Chicks (1-d-old) were placed in pairs within individually ventilated cages (1,862 cm2 floor space; Techniplast, Montreal, QC). These cages were operated in containment mode (i.e., negative air pressure flow) to provide bi-directional HEPA filtered air exchange and protect researchers from pathogens, including C. perfringens. Birds were provided continuous access to a crumble starter diet for the first 10 d, and a pelleted grower diet from d 11 until the end of the experiment (Table 1). Birds had access to water at all times through 2 nipple drinkers per cage. Birds were maintained at 30°C for 2 d, 28°C for 2 d, and then maintained at 26°C for the remainder of the experiment on an 18 h light: 6 h dark cycle. To ascertain daily weight gain, individual birds were weighed each morning throughout the experimental period.
Table 1.
Starter and grower diet ingredients and rations.
| Ingredient | Composition (%) |
|
|---|---|---|
| Starter1 | Grower2 | |
| Corn | 49.53 | 54.68 |
| Soybean meal | 43.06 | 37.31 |
| Canola oil | 2.39 | 3.26 |
| Salt | 0.51 | 0.52 |
| Limestone | 1.52 | 1.41 |
| Dicalcium phosphate | 1.26 | 1.09 |
| Magnesium oxide | 0.10 | 0.15 |
| L-Lysine HCl | 0.11 | 0.12 |
| D,L-Methionine | 0.37 | 0.33 |
| L-Threonine | 0.15 | 0.13 |
| Vitamin premix3 | 0.50 | 0.50 |
| Choline premix | 0.50 | 0.50 |
0 to 10 d-of-age.
11 to 14 d-of-age.
For birds administered corticosterone, the glucocorticoid was mixed in the vitamin premix of the grower diet at a concentration of 20 mg kg−1.
Corticosterone Administration and Diet
Corticosterone was incorporated into the grower diet at a dose of 20 mg kg−1. Fresh feed was provided each morning and afternoon. Administration of corticosterone began on d 11 posthatch (p.h.) and continued until the end of the experiment.
Clostridium perfringens Inoculation
A starter culture of C. perfringens (strain CP1) was grown overnight at 37°C in Heart Infusion Broth (Thermo-Fisher Scientific, Mississauga, ON) and incubated within an anaerobic chamber containing an 85% N2: 10% CO2: 5% H2 atmosphere. The following morning, 2.5 mL of the overnight culture was transferred to 50 mL of Fluid Thioglycolate Medium (BD Difco, Franklin Lakes, NJ) within the anaerobic chamber, and bacterial cells were incubated for 4 h at 37°C. Birds were inoculated with 1 mL of C. perfringens containing 1-2 × 108 colony forming units (CFU) via oral gavage starting at 12 d p.h. for 2 consecutive days. Cell density of C. perfringens was enumerated by diluting the inoculation broth in a 10-fold dilution series with 200 μL spread onto Columbia agar (BD Difco) containing 5% sheep blood. Cultures were maintained in the anaerobic chamber at 37°C for 24 h, and colonies were counted at the dilution yielding 30 to 300 CFU.
Animal Euthanasia and Tissue Collection
At 14 d p.h. birds were humanely euthanized; birds were anesthetized with isoflurane (5% isoflurane; 1 L of O2 min−1) and then euthanized by cervical dislocation. Immediately following euthanasia, the abdomen was opened, and the small intestine was removed aseptically for examination and lesion scoring. Digesta from the jejunum was removed using a sterile wooden splint and stored at -80°C until processed. Jejunal samples collected for RNA analysis were placed in RNAlater Stabilization Solution (Thermo Scientific, Ottawa, ON) and stored at -80°C until processed. Tissues for histopathology were placed in 10% neutral buffered formalin (Leica, Concord, ON).
Lesion Scoring and Histopathology
The entire length of the small intestine (duodenum to the ileal-cecal junction) was examined for gross lesions and scored 0 to 6 as described by Shojadoost et al. (2012). The proximal jejunum was processed for histopathologic changes; care was taken to ensure that lesions were not sampled. Jejunal tissue was fixed for a minimum 24 h. Samples were dehydrated using a Leica tissue processor (Leica TP1020 Benchtop Tissue Processor, Leica Biosystems, Concord, ON), embedded in paraffin blocks using a Shandon Histocentre 3 Embedding Center (Thermo Scientific), and sectioned (≈5 µm) using a Finesse 325 Manual Rotary Microtome (Thermo Scientific). Slides were deparaffinized with xylene and stained with hematoxylin and eosin. Sections were scored by a pathologist (V.F.B.) blinded to treatments using a modified scoring system based on previously described methods (Gholamiandehkordi et al., 2007; Olkowski et al., 2008; To et al., 2017). Sections were graded from 0 to 4 for villar fusion, villar atrophy, mucosal necrosis, bacterial invasion, and lamina propria changes (Table 2). Total histological scores were determined by calculating the sum of scores from all categories.
Table 2.
Histopathologic scoring criteria.
| Parameter |
|---|
| Villar fusion |
| 1: Occasional fusion of 2 villi in a section |
| 2: Occasional fusion >2 villi or several fusions of 2 villi |
| 3: Multiple areas where >2 villi were fused |
| 4: Large clusters of fused villi throughout |
| Villar atrophy |
| 1: Slight |
| 2: Slight to moderate |
| 3: Moderate to severe |
| 4: Severe |
| Mucosal necrosis |
| 1: Necrosis or sloughing of the mucosal epithelium |
| 2: Scattering of necrotic foci |
| 3: Multiple necrotic foci |
| 4: Coalesced or layered necrosis |
| Bacterial invasion |
| 1: Small number of bacteria |
| 2: Sporadic clumps of bacteria |
| 3: Multiple clumps of bacteria |
| 4: Large clumps of bacteria |
| Lamina propria changes |
| 1: Mild edema and a few cells showing early necrosis |
| 2: Amorphous eosinophillic material in the lamina propria |
| 3: Obliteration of the structural integrity of the lamina propria |
| 4: Coagulative necrosis with band of neutrophils |
Quantification of C. perfringens
DNA was extracted from jejunal digesta using a QIAamp Fast DNA Stool Mini Kit (Qiagen Inc, Toronto, ON). To each 200 mg sample, a 5-mm-diameter glass bead was added at the lysis step. The sample with a bead was vortexed for 3 min, and then homogenized using Qiagen TissueLyser LT (Qiagen Inc.) for 3 min at 50 Hz. A standard curve was generated using a 10-fold dilution series ranging from 107 to 101 copies of the C. perfringens 16S rRNA gene as previously described (Zaytsoff et al., 2020a; Zaytsoff et al., 2020b). Each reaction contained 10 μL QuantiTect SYBR green master mix (Qiagen Inc.), 1 μL of each primer (10 μM), 2 μL of bovine serum albumin (1μg μL−1), 4 μL of DNase-free water, and 2 μL of template DNA (10 ng μL−1). Reaction conditions were: 95°C for 15 min; and 40 cycles of 95°C for 15 s, 55°C for 30 s, and 72°C for 30 s. Melt curve analysis was conducted from 55-95°C. A M3005p thermocycler (Agilent Technologies, Santa Clara, CA) was used to conduct quantitative PCR (qPCR) analysis. Each reaction was run in duplicate, and the mean of the 2 observations was calculated.
Quantification of Jejunal mRNA
RNA was extracted from jejunal tissue using RNeasy Plus Mini Kit (Qiagen Inc.). An Agilent Bioanalyzer (Agilent Technologies, Mississauga, ON) was used access the quality and quantity of RNA, and 1 μg of RNA was reverse transcribed to cDNA using a QuantiTect reverse transcription kit (Qiagen Inc.). A Mx3005p thermocycler (Agilent Technologies) was used to perform qPCR. Each reaction contained 5 μL of Quantitect SYBR green master mix (Qiagen Inc.), 0.5 μL of each primer (10 μM), 3 μL of RNase-free water, and 1 μL of cDNA. Reaction conditions were: 95°C for 15 min; 40 cycles of 95°C for 15 sec, 55-58°C for 30 sec, and 72°C for 30 sec. Melt curve analysis was conducted from 55 to 95°C. Primer sequences and annealing temperature are listed in Supplemental Table S1; primers were designed using the National Center for Biotechnology Information primer Basic Local Alignment Search Tool unless indicated otherwise. Reactions were run in duplicate and the average cycle threshold values were used to calculate mRNA concentrations relative to the reference gene, β-actin, using qBase+ software (Biogazelle, Gent, Belgium) (Hellemans et al., 2007).
Statistical Analyses
Graphpad Prism software (La Jolla, CA, USA) was used to perform statistical analysis. Continuous data (i.e., weight gain, mRNA gene expression) was checked for normality and analyzed by 2-way analysis of variance. Clostridium perfringens densities and mRNA gene expression data were log-transformed to achieve normality. Means were compared using least significance difference test to determine changes between treatments. Nonparametric data (i.e., necrotic lesion and histopathological scoring) was analyzed using the Kruskal-Wallis test with Dunn's test for multiple comparison correction. Correlation analysis to examine the relationship between necrotic lesion and histopathologic scoring was conducted using Spearman's correlation. The relationship between C. perfringens densities and necrotic lesion/histopathologic scores was assessed using point biserial correlation (Tate, 1954). P values of ≤0.050 and ≤0.100 were considered statistically significant and as a trend for biological significance, respectively (Greenland et al., 2016; Amrhein et al., 2017; Ganesh and Cave, 2018).
RESULTS
Corticosterone Administration Reduced Bird Weight Gain
No difference in weight gain was observed among treatments from d 1 to 10 (Table 3). CORT (P ≤ 0.014) and Cp + CORT (P ≤ 0.003) treatment birds exhibited a reduction in weight gain in comparison to other treatments from d 11 to 14 (i.e., following corticosterone and C. perfringens challenge ).
Table 3.
Average bird weight gain.
| Treatment1 | Average weight gain (g ± standard error of the mean) |
||
|---|---|---|---|
| D 1–5 | D 6–10 | D 11–142 | |
| Control | 91.3 ± 6.4 | 178.5 ± 6.2 | 156.2 ± 5.2a |
| Cp | 95.7 ± 4.9 | 183.0 ± 4.5 | 154.3 ± 5.2a |
| CORT | 94.6 ± 9.0 | 167.7 ± 14.4 | 112.0 ± 11.8b |
| Cp + CORT | 92.0 ± 8.0 | 179.5 ± 9.6 | 103.0 ± 11.0b |
Treatments were: birds not challenged with Clostridium perfringens and not administered corticosterone (Control); birds challenged with C. perfringens but not administered corticosterone (Cp); birds administered corticosterone (20 mg kg−1) but not challenged with C. perfringens (CORT); and birds challenged with C. perfringens and administered corticosterone (Cp + CORT).
Means not followed by the same letter differ (P < 0.050)
Clostridium perfringens Challenge Induced Gross and Histopathologic Changes in the Jejunum
No necrotic lesions were in observed in birds not inoculated with C. perfringens. Necrotic lesions were observed only in birds inoculated with the pathogen. All of the birds inoculated with C. perfringens and administered corticosterone developed lesions, as compared to 33.3% of the birds inoculated with the pathogen alone (Figure 1A–B). Moreover, only birds inoculated with C. perfringens and administered corticosterone exhibited higher lesions scores (P = 0.002) in comparison to the Control and CORT treatment birds (Figure 1C). Villar fusion (P < 0.010), villar atrophy (P < 0.010), mucosal necrosis (P < 0.024), bacterial invasion (P < 0.028), and total histopathologic scores differed (P ≤ 0.033) in C. perfringens challenged birds (Cp and Cp + CORT treatments) (Figure 2A–F).
Figure 1.
Clostridium perfringens and corticosterone incited gross pathologic changes in the jejunum. Birds were untreated (Control), administered 20 mg kg−1 corticosterone in feed (CORT), challenged with C. perfringens (Cp), or received both corticosterone and C. perfringens treatment (Cp + CORT). Corticosterone administration commenced at 11 d posthatch (p.h.). Birds were orally administered 1-2 × 108 colony forming units of C. perfringens on d 12 and 13 p.h., and necropsies were conducted on d 14 p.h. (A) Pictures showing representative necrotic lesions in the proximal jejunum from Cp + CORT treated birds. (B) Prevalence of necrotic lesions (≥ one lesion). (C) Gross necrotic lesion scores; horizontal lines are the median score (n = 6). Lesion scoring was executed as described by Shojadoost et al. (2012). The asterisk indicates a significant increase in lesion scores (P < 0.050) in comparison to the Control and CORT treatments.
Figure 2.
Clostridium perfringens promoted histopathologic changes in the jejunum. Birds were untreated (Control), administered 20 mg kg−1 corticosterone in feed (CORT), challenged with C. perfringens (Cp), or received both corticosterone and C. perfringens treatment (Cp + CORT). Corticosterone administration commenced at 11 d posthatch (p.h.). Birds were orally administered 1-2 × 108 colony forming units of C. perfringens on d 12 and 13 p.h., and necropsies were conducted on d 14 p.h. Histopathological scores for (A) villar fusion, (B) villar atrophy, (C) mucosal necrosis, (D) bacterial invasion, and (E) lamina propria changes. (F) Total scores were calculated by taking the sum of all scores from each category. Horizontal lines are the median score (n = 6). Asterisks indicate a significant increase (P < 0.050) in comparison to the Control and CORT treatments.
Clostridium perfringens Densities were Correlated with Necrotic Lesion and Total Histopathologic Scores
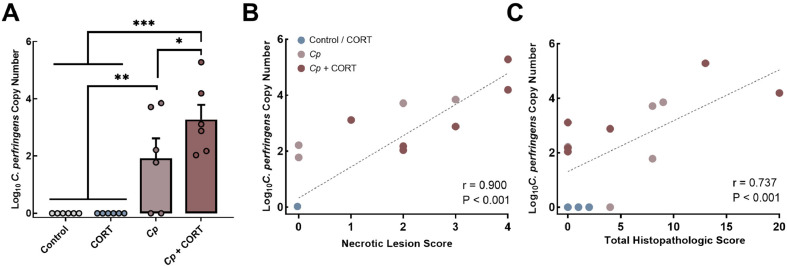
No C. perfringens DNA was detected in the jejunums of birds not inoculated with the pathogen. In contrast, the jejunum of all inoculated birds were colonized (P ≤ 0.001) by the pathogen (Figure 3A). Birds co-challenged with C. perfringens and corticosterone exhibited higher densities (P = 0.038) than birds only challenged with C. perfringens. Point biserial correlation analysis showed that C. perfringens densities were correlated with necrotic lesion scores (r = 0.900; P < 0.001; Figure 3B) and total histopathologic scores (r = 0.737; P < 0.001; Figure 3C).
Figure 3.
Clostridium perfringens densities in the jejunum were correlated with necrotic lesion and histopathologic scores. Birds were untreated (Control), administered 20 mg kg−1 corticosterone in feed (CORT), challenged with C. perfringens (Cp), or received both corticosterone and C. perfringens treatment (Cp + CORT). Corticosterone administration commenced at 11 d posthatch (p.h.). Birds were orally administered 1-2 × 108 colony forming units of C. perfringens on d 12 and 13 p.h., and necropsies were conducted on d 14 p.h. (A) Densities of C. perfringens were determined by quantitative PCR in jejunal digesta using primers specific for the 16S rRNA gene of C. perfringens. (B and C) Point biserial correlation of (B) necrotic lesion scoring and C. perfringens densities, and (C) total histopathological scores and C. perfringens densities. Vertical lines associated with histogram bars represent standard error of the means (n = 6). Asterisks indicate a significant increase in comparison to the Control and CORT treatments (*P ≤ 0.050, **P < 0.0010, and ***P < 0.0001).
Corticosterone and C. perfringens Modulated Immune Responses in the Jejunum
No differences (P ≥ 0.84) in quantities of interleukin 2 (IL2) or interleukin 17A (IL17A) mRNA were observed among treatments (Figure 4A-B). Although not significant, a trend for higher (P = 0.094) relative mRNA quantities of interleukin 1 beta (IL1β) was observed in birds inoculated with C. perfringens (Figure 4C). Quantities of transforming growth factor beta 2 (TGFβ2) mRNA were higher (P = 0.020) in birds co-challenged with C. perfringens and corticosterone (Figure 4D). Birds treated with corticosterone alone (P = 0.031), with C. perfringens alone (P = 0.038), or co-challenged with C. perfringens and corticosterone (P = 0.0029) exhibited increased inducible nitric oxide synthase (INOS) mRNA expression in comparison to control birds (Figure 4E). An increase in toll-like receptor 2A (TLR2A) mRNA was observed in birds inoculated with C. perfringens (P = 0.010). The administration of corticosterone lowered (P = 0.016) TLR2A mRNA expression in birds co-challenged with C. perfringens and corticosterone.
Figure 4.
Clostridium perfringens and corticosterone challenge modulated relative mRNA gene quantities in the jejunum. Birds were untreated (Control), administered 20 mg kg−1 corticosterone in feed (CORT), challenged with C. perfringens (Cp), or received both corticosterone and C. perfringens treatment (Cp + CORT). Corticosterone administration commenced at 11 d posthatch (p.h.). Birds were orally administered 1-2 × 108 colony forming units of C. perfringens on d 12 and 13 p.h., and necropsies were conducted on d 14 p.h. Relative mRNA expression is presented in comparison to β-actin. Values were log transformed to achieve normality. (A) Interleukin 2 (IL2), (B) interleukin 1 beta (IL1β), (C) interleukin 17A (IL17A), (D) transforming growth factor beta 2 (TGFβ2), (E) inducible nitric oxide synthase (INOS), (F) toll-like receptor 2A (TLR2A). Vertical lines associated with histogram bars represent standard error of the means (n = 6). Asterisks indicate a significant difference (*P < 0.050 and **P < 0.010) among treatments.
DISCUSSION
One goal of the current study was to examine the impact of controlled physiological stress exposure (i.e., as a predisposing factor) on acute NE manifestation in broiler chickens. Birds were administered corticosterone in feed to mediate a stress response, and then challenged with a virulent strain of C. perfringens. Birds were examined for necrotic lesion scoring and histopathologic changes in the small intestine to ascertain the degree of disease. Birds inoculated with C. perfringens and administered corticosterone in combination (i.e. Cp + CORT treatment) exhibited a higher prevalence of necrotic lesions in comparison to both Control treatment birds and birds administered corticosterone alone (i.e., CORT treatment), whereas birds inoculated with C. perfringens but not administered corticosterone did not (i.e. Cp treatment). Densities of C. perfringens in the jejunum were correlated with both necrotic lesion and histopathologic scores. Moreover, both C. perfringens and corticosterone challenge modulated mRNA gene expression in the jejunum.
Corticosterone Administration Impacted Weight Gain
NE imparts economic losses through mortality, but also through poor bird performance in subclinical forms of the disease (Van Immerseel et al., 2004). Our previous research in layer birds showed that subclinical NE was associated with reduced bird weight, but only in birds inoculated with C. perfringens and subjected to corticosterone-mediated stress (Zaytsoff et al., 2020a). In the current study, corticosterone administration was found to impart the greatest impact on bird weight gain, as birds co-challenged with C. perfringens and corticosterone showed the largest reduction in weight gain. This is consistent with other studies that have demonstrated that C. perfringens challenge alone does not affect weight gain, and that other variables in combination with the pathogen are necessary to impact performance (Park et al., 2008; Zaytsoff et al., 2020a). In this regard, co-infection with E. maxima, which can promote immunosuppression and intestinal damage, was necessary to incite infection and result in weight gain losses (McReynolds et al., 2004). These impacts to bird health can contribute to increased disease severity and reduced weight gain (Van Immerseel et al., 2004). Weight loss is an important consideration in chicks, as events such as restricted feed-precipitated weight loss early in life can translate to reduced performance throughout the production period, and ultimately to altered carcass traits at slaughter (Yu et al., 1990; Careghi et al., 2005). As such, our results suggest that birds impacted by corticosterone and C. perfringens early in life will adversely affect overall bird production and carcass characteristics.
Corticosterone Administration Induced a Higher Prevalence of Necrotic Enteritis
Studies developing models of NE rely on the onset and severity of necrotic lesions as a cardinal metric of disease severity. An advantage of lesion scoring, in comparison to scoring histopathologic changes, is the ability to survey the entire small intestine for disease. In the current study, necrotic lesions were observed only in a subset of birds inoculated with C. perfringens, whereas all the birds challenged with C. perfringens and corticosterone showed at least one necrotic lesion. Our finding that physiological stress induction exacerbated disease is supported by other research that showed that stocking density and cold stress increased the prevalence of necrotic lesions following C. perfringens challenge (Tsiouris et al., 2015a,b). Moreover, we observed that co-challenge with C. perfringens and corticosterone resulted in consistent lesion development in all of the birds examined in the current study, which suggests that this stress predisposition model could be useful to elucidate mechanisms of necrotic enteritis (i.e., without the confounding impacts of a co-infection pathogen such as E. maxima) and evaluate mitigation strategies.
Although the majority of published studies use lesion scoring as the sole metric of disease onset, some studies have evaluated the small intestine for histopathologic changes, and this can provide crucial information beyond evaluation of macroscopic lesions. For example, we observed that birds challenged with C. perfringens alone (i.e., and not administered corticosterone) exhibited higher histopathologic scores in the jejunum relative to control treatment animals, despite a limited development of necrotic lesions. This suggests that inflammation on a microscopic level is indicative of subclinical NE. Furthermore, it demonstrates that immune responses necessary to overcome or limit infection were stimulated. We demonstrated that higher histopathologic scores corresponded with elevated immune responses (i.e., TLR2A, INOS, TGFβ2) in C. perfringens challenged birds. From a performance perspective, mounting an immune response (i.e., in the absence of clinical disease) is still catabolically costly, which can adversely affect production. Histopathologic evaluation also allows researchers/clinicians to ascertain why acute disease was not manifested; for example, the ability to rule out poor colonization and/or infection of the host by C. perfringens.
Clostridium perfringens and Corticosterone Challenge Altered Immune Responses in the Jejunum
Six genes involved in immune function were measured in the jejunum. No changes were observed in the expression of the inflammatory genes, IL2, IL17A, and IL1β, although a trend for higher IL1β mRNA quantities was observed in birds challenged with C. perfringens. A relative increase of TGFβ2 mRNA was observed in jejunal tissue following co-challenge of birds with C. perfringens and corticosterone. This is consistent with our observation of microscopic and macroscopic damage to the epithelium in C. perfringens challenged birds, as TGFβ2 functions to promote tissue repair after injury (Roberts and Sporn, 1993). Clostridium perfringens challenge was also associated with increased INOS mRNA expression, which was expected as INOS secretion is stimulated by immune cytokines and bacterial infection (Aktan, 2004). Additionally, co-infection of birds by C. perfringens and E. maxima has been shown to increase INOS expression (Lee et al., 2018). We also observed that the administration of corticosterone alone increased relative quantities of INOS mRNA. This is consistent with other research showing that cold temperature exposures incite increases in INOS mRNA expression in the duodenum of broilers when stress persisted for 5 to 20 d (Zhang et al., 2011). In the current study, TLR2A mRNA was observed to increase following C. perfringens challenge, and this was predicted as TLR2A has previously been shown to be stimulated in intestinal tissue of birds following infection by the pathogen (Cario et al., 2007; Cao et al., 2012). Conversely, we observed that birds treated with corticosterone exhibited decreased quantities of TLR2A mRNA. This corresponds with our previous work in layer chickens, which demonstrated that corticosterone administration was associated with decreased expression of TLR2A and toll-like receptor 15 (TLR15) in the small intestine (Zaytsoff et al., 2020a). Although it is unclear in the current study which cell types expressed TLR2A, the downregulation of this receptor in epithelial or underlying immune cells could be an indication of suppressed immune defenses by the host, which may facilitate the pathogen to incite disease and promote gross pathologies in birds co-challenged with C. perfringens and corticosterone. In this regard, TLR2A is a mediator of nuclear factor κB signaling, which leads to the activation of inflammatory responses (Cario et al., 2007). Therefore, inadequate stimulation of TLR2A in birds administered corticosterone could have resulted in limited incitement of immune responses, including failed recruitment of immune cells to the site of infection. This would permit C. perfringens to grow, colonize, and cause damage to the mucosa, leading to gross pathologies (i.e., necrotic lesions). Additionally, it has previously been demonstrated that TLR2A activation can promote cell survival and inhibit apoptosis in ex vivo murine intestinal epithelial cells (Cario et al., 2007). Thus, an inactivation of TLR2A may promote the opposite trend and facilitate the development of necrosis. Given the evidence of stress-incited predisposition of broiler chickens to NE that was obtained in the current study, further examination of how stress impacts immune response and other factors, such as mucus and tight junction formation, warrants additional investigation.
CONCLUSION
A high prevalence of clinical NE was achieved in broiler chickens inoculated with C. perfringens and administered dietary corticosterone to incite a defined physiological stress response. Birds receiving C. perfringens and corticosterone showed more consistent and severe development of necrotic lesions in comparison to birds challenged only with C. perfringens. Densities of C. perfringens correlated to necrotic lesion and histopathologic scores in the jejunum. Increased relative concentrations of TGFβ2 and INOS mRNA following C. perfringens challenge indicated that infection occurred. The reduction of TLR2A mRNA in birds co-challenged with C. perfringens and corticosterone suggested stress-incited impairment of immune intervention in the small intestinal mucosa, which may be a key mechanism by which stress regulates disease (e.g., manifestation of gross pathologies). This warrants further investigation. Importantly, the corticosterone model generated consistent clinical NE in broilers, and at a comparable level to other NE models. Although the corticosterone model may not be suitable for researchers testing NE vaccines, it does not require a co-infection agent (i.e., Eimeria spp.). Thus, the model may facilitate the elucidation of mechanisms, including on how stress predisposes birds to NE. Moreover, the model may be useful to discovery of biomarkers (e.g., of disease predisposition), and to advance antimicrobial alternatives.
ACKNOWLEDGMENTS
All research was conducted at the Agriculture and Agri-Food Canada (AAFC) Lethbridge Research and Development Centre (LeRDC), and the authors would like to thank the following individuals at AAFC LeRDC: Tara Shelton for her support with animal husbandry and necropsies; Jenny Gusse for ordering supplies and providing assistance with laboratory-based analyses; the staff at the Feed Mill for generating custom diets; and Karen Shamash, Sandra Clarke, and Catherine Brown for aiding with necropsies. We are also grateful to Matt Oryschak, Alberta Agriculture and Forestry for determining the composition of the custom diets, Dr. J. F. Prescott, University of Guelph for providing the strain of C. perfringens used in the study, and to a local broiler hatching egg producer and hatchery for providing the broiler chicks. Financial support was provided in part by grants from Canadian Poultry Research Council (Poultry Science Cluster Project 1373 Activity 14), the Canadian Glycomics Network (ID-04), Alberta Agriculture and Forestry (2019F101R and 2019H001R), and the Alberta Chicken Producers.
DISCLOSURES
The authors have declared no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101726.
Appendix. Supplementary materials
Table S1. List of primers used to quantify Clostridium perfringens and relative mRNA expression.
REFERENCES
- Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Amrhein V., Korner-Nievergelt F., Roth T. The earth is flat (p >0.05): significance thresholds and the crisis of unreplicable research. PeerJ. 2017;5:e3544. doi: 10.7717/peerj.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calefi A.S., da Silva Fonseca J.G., Cohn D.W., Honda B.T., Costola-de-Souza C., Tsugiyama L.E., Quinteiro-Filho W.M., Piantino Ferreira A.J., Palermo-Neto J. The gut-brain axis interactions during heat stress and avian necrotic enteritis. Poult. Sci. 2016;95:1005–1014. doi: 10.3382/ps/pew021. [DOI] [PubMed] [Google Scholar]
- Cao L., Yang X., Li Z., Sun F., Wu X., Yao J. Reduced lesions in chickens with Clostridium perfringens-induced necrotic enteritis by Lactobacillus fermentum 1.2029. Poult. Sci. 2012;91:3065–3071. doi: 10.3382/ps.2012-02548. [DOI] [PubMed] [Google Scholar]
- Careghi C., Tona K., Onagbesan O., Buyse J., Decuypere E., Bruggeman V. The effects of the spread of hatch and interaction with delayed feed access after hatch on broiler performance until seven days of age. Poult. Sci. 2005;84:1314–1320. doi: 10.1093/ps/84.8.1314. [DOI] [PubMed] [Google Scholar]
- Cario E., Gerken G., Podolsky D. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- Collier C., Hofacre C., Payne A., Anderson D., Kaiser P., Mackie R.I., Gaskins H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Ganesh S., Cave V. P-values, p-values everywhere! N. Z. Vet. J. 2018;66:55–56. doi: 10.1080/00480169.2018.1415604. [DOI] [PubMed] [Google Scholar]
- Gholamiandehkordi A.R., Timbermont L., Lanckriet A., Broeck W.V.D., Pedersen K., Dewulf J., Pasmans F., Haesebrouck F., Ducatelle R., Immerseel F.V. Quantification of gut lesions in a subclinical necrotic enteritis model. Avian Pathol. 2007;36:375–382. doi: 10.1080/03079450701589118. [DOI] [PubMed] [Google Scholar]
- Greenland S., Senn S.J., Rothman K.J., Carlin J.B., Poole C., Goodman S.N., Altman D.G. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur. J. Epidemiol. 2016;31:337–350. doi: 10.1007/s10654-016-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J., Mortier G., De Paepe A., Speleman F., Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.W., Lillehoj H.S., Jeong W., Jeoung H.Y., An D.J. Avian necrotic enteritis: experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult. Sci. 2011;90:1381–1390. doi: 10.3382/ps.2010-01319. [DOI] [PubMed] [Google Scholar]
- Lee Y., Lee S., Gadde U., Oh S., Lee S., Lillehoj H. Allium hookeri supplementation improves intestinal immune response against necrotic enteritis in young broiler chickens. Poult. Sci. 2018;97:1899–1908. doi: 10.3382/ps/pey031. [DOI] [PubMed] [Google Scholar]
- Low K.E., Smith S.P., Abbott D.W., Boraston A.B. The glycoconjugate-degrading enzymes of Clostridium perfringens: tailored catalysts for breaching the intestinal mucus barrier. Glycobiology. 2021;31:681–690. doi: 10.1093/glycob/cwaa050. [DOI] [PubMed] [Google Scholar]
- MacMillan J.L., Vicaretti S.D., Noyovitz B., Xing X., Low K.E., Inglis G.D., Zaytsoff S.J., Boraston A.B., Smith S.P., Uwiera R.R.E., Selinger L.B., Zandberg W.F., Abott D.W. Structural analysis of broiler chicken small intestinal mucin O-glycan modification by Clostridium perfringens. Poult. Sci. 2019;98:5074–5088. doi: 10.3382/ps/pez297. [DOI] [PubMed] [Google Scholar]
- McReynolds J., Byrd J., Anderson R., Moore R., Edrington T., Genovese K., Poole T., Kubena L., Nisbet D. Evaluation of immunosuppressants and dietary mechanisms in an experimental disease model for necrotic enteritis. Poult. Sci. 2004;83:1948–1952. doi: 10.1093/ps/83.12.1948. [DOI] [PubMed] [Google Scholar]
- Moore R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016;45:275–281. doi: 10.1080/03079457.2016.1150587. [DOI] [PubMed] [Google Scholar]
- Olkowski A., Wojnarowicz C., Chirino-Trejo M., Laarveld B., Sawicki G. Sub-clinical necrotic enteritis in broiler chickens: novel etiological consideration based on ultra-structural and molecular changes in the intestinal tissue. Res. Vet. Sci. 2008;85:543–553. doi: 10.1016/j.rvsc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Palliyeguru M., Rose S., Mackenzie A. Effect of dietary protein concentrates on the incidence of subclinical necrotic enteritis and growth performance of broiler chickens. Poult. Sci. 2010;89:34–43. doi: 10.3382/ps.2009-00105. [DOI] [PubMed] [Google Scholar]
- Park S.S., Lillehoj H.S., Allen P.C., Park D.W., FitzCoy S., Bautista D.A., Lillehoj E.P. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008;52:14–22. doi: 10.1637/7997-041707-Reg. [DOI] [PubMed] [Google Scholar]
- Pearce S., Mani V., Boddicker R., Johnson J., Weber T., Ross J., Baumgard L., Gabler N. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J. Anim. Sci. 2012;90:257–259. doi: 10.2527/jas.52339. [DOI] [PubMed] [Google Scholar]
- Roberts A.B., Sporn M.B. Physiological actions and clinical applications of transforming growth factor-β (TGF-β) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Shini S., Huff G.R., Shini A., Kaiser P. Understanding stress-induced immunosuppression: exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult. Sci. 2010;89:841–851. doi: 10.3382/ps.2009-00483. [DOI] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 2012;43:74. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov A., Sklan D., Uni Z. Mucin dynamics in the chick small intestine are altered by starvation. J. Nutr. 2004;134:736–742. doi: 10.1093/jn/134.4.736. [DOI] [PubMed] [Google Scholar]
- Stanley D., Keyburn A.L., Denman S.E., Moore R.J. Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Vet. Microbiol. 2012;159:155–162. doi: 10.1016/j.vetmic.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Tate R.F. Correlation between a discrete and a continuous variable. Point-biserial correlation. Ann. Math. Stat. 1954;25:603–607. [Google Scholar]
- To H., Suzuki T., Kawahara F., Uetsuka K., Nagai S., Nunoya T. Experimental induction of necrotic enteritis in chickens by a netB-positive Japanese isolate of Clostridium perfringens. J. Vet. Med. Sci. 2017;79:350–358. doi: 10.1292/jvms.16-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiouris V., Georgopoulou I., Batzios C., Pappaioannou N., Ducatelle R., Fortomaris P. The effect of cold stress on the pathogenesis of necrotic enteritis in broiler chicks. Avian Pathol. 2015;44:430–435. doi: 10.1080/03079457.2015.1083094. [DOI] [PubMed] [Google Scholar]
- Tsiouris V., Georgopoulou I., Batzios C., Pappaioannou N., Ducatelle R., Fortomaris P. High stocking density as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. 2015;44:59–66. doi: 10.1080/03079457.2014.1000820. [DOI] [PubMed] [Google Scholar]
- Tsiouris V., Georgopoulou I., Batzios C., Pappaioannou N., Ducatelle R., Fortomaris P. Heat stress as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. 2018;47:616–624. doi: 10.1080/03079457.2018.1524574. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., De Buck J., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.W., Robinson F.E., Clandinin M.T., Bodnar L. Growth and body composition of broiler chickens in response to different regimens of feed restriction. Poult. Sci. 1990;69:2074–2081. [Google Scholar]
- Zaytsoff S.J., Lyons S.M., Garner A.M., Uwiera R.R., Zandberg W.F., Abbott D.W., Inglis G.D. Host responses to Clostridium perfringens challenge in a chicken model of chronic stress. Gut Pathog. 2020;12:1–16. doi: 10.1186/s13099-020-00362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaytsoff S.J., Uwiera R.R., Inglis G.D. Physiological stress mediated by corticosterone administration alters intestinal bacterial communities and increases the relative abundance of Clostridium perfringens in the small intestine of chickens. Microorganisms. 2020;8:1518. doi: 10.3390/microorganisms8101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Lv Z., Li J., Li S., Xu S., Wang X. Effects of cold stress on nitric oxide in duodenum of chicks. Poult. Sci. 2011;90:1555–1561. doi: 10.3382/ps.2010-01333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of primers used to quantify Clostridium perfringens and relative mRNA expression.