Abstract
Objectives
Corticosteroids were clinically used in the treatment of nonsevere patients with COVID-19, but the efficacy of such treatment lacked sufficient clinical evidence, and the impact of dose had never been studied. This study aimed to evaluate the effect of systemic corticosteroid use (SCU) in nonsevere patients with COVID-19.
Methods
We conducted a multicenter retrospective cohort study in Hubei Province. A total of 1726 patients admitted with nonsevere type COVID-19 were included. Mixed-effect Cox model, mixed-effect Cox model with time-varying exposure, multiple linear regression, and propensity score analysis (inverse probability of treatment weight and propensity score matching) were used to explore the association between SCU and progression into severe type, all-cause mortality, and length of stay.
Results
During the follow-up of 30 days, 29.8% of nonsevere patients with COVID-19 received treatment with systemic corticosteroids. The use of systemic corticosteroids was associated with higher probability of developing severe type (adjusted hazard ratio 1.81; 95% confidence interval 1.47-2.21), all-cause mortality (adjusted hazard ratio 2.92; 95% confidence interval 1.39-6.15) in time-varying Cox analysis, and prolonged hospitalization (β 4.14; P < .001) in multiple linear regression. Analysis with 2 propensity score cohorts displayed similar results. Besides, increased corticosteroid dose was significantly associated with elevated probability of developing severe type (P < .001) and prolonged hospitalization (P < .001).
Conclusions
Corticosteroid treatment against nonsevere patients with COVID-19 was significantly associated with worse clinical outcomes. The higher dose was significantly associated with elevated risk of poor disease progression. We recommend that SCU should be avoided unless necessary among nonsevere patients with COVID-19.
Keywords: COVID-19, corticosteroids, mortality, outcomes
Introduction
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2, has become the most severe global health crisis.1 As of June 2021, it has brought the cumulative numbers to >180 million reported cases and to >4 million deaths globally since the start of the epidemic.2 This global pandemic has brought a great challenge to current global health systems and socioeconomic development. Unfortunately, there is still no proven effective antiviral therapy recommended for severe acute respiratory syndrome coronavirus 2 infection.3, 4, 5 Many therapies, including corticosteroid treatment, are still controversial.6
Cytokine storm is considered to be one of the major causes of acute respiratory distress syndrome and multiple-organ failure. It plays an important role in the process of disease aggravation and even death.7 Effectively suppressing the storm may be an effective way for the treatment of COVID-19.8 Corticosteroids were widely used to treat severe acute respiratory infections of viral etiology because of their excellent pharmacological effects on the suppression of exuberant and dysfunctional systematic inflammation.9 , 10 Corticosteroids were also widely used clinically to treat patients with COVID-19 since the early days of pandemic, despite a lack of clear evidence regarding their safety and efficacy.11
At present, studies of corticosteroid therapy for COVID-19 mainly focused on severe or critical cases in the intensive care unit or required mechanical ventilation and showed positive results.12 , 13 As for nonsevere cases, studies have shown that corticosteroids were used in 11% to 56% of nonsevere COVID-19 cases,14, 15, 16, 17, 18 but the role of corticosteroids remains controversial. Wu et al19 reported that treatment with corticosteroid was not significantly associated with increased in-hospital mortality in noncritically severe cases. Another 2 studies reported that corticosteroid therapy was associated with poor lung injury recovery and worse clinical outcomes in nonsevere patients with COVID-19.17 , 18 The relatively small sample size and retrospective design limited the interpretation of these results. The UK-based Randomized Evaluation of COVID-19 Therapy study, a large-scale and well-designed randomized clinical trial, showed that no benefit of dexamethasone use was observed among those patients who did not require respiratory support.13 In addition, none of these studies explored the relationship between corticosteroid dose and outcomes of patients with COVID-19. The therapeutic efficacy and adverse reactions of corticosteroids are strongly correlated with the dose, and it is not clear whether there is a safe low dose.4 , 20 , 21 Hence, it is necessary to clarify the effect of corticosteroids on outcomes in patients with nonsevere patients with COVID-19.
This study used a multicenter retrospective cohort design to analyze the effects of systemic corticosteroids on mortality rate, disease progression, and length of stay to provide clinical evidence for optimizing the therapy of nonsevere patients with COVID-19.
Methods
Ethical Statement
The Medical Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology approved this study. The ethics committee abandoned the requirements of informed consent. Only the pseudonymized data that cannot be recognized were used for our analysis.
Study Design and Patients
This multicenter retrospective cohort study analyzed information on hospitalized patients with COVID-19 admitted to 4 hospitals in Hubei Province, China. A total of 2501 patients with COVID-19 were admitted during the period from December 31, 2019 to March 31, 2020.
The diagnosis and clinical classification of COVID-19 followed the World Health Organization interim guidance and the Diagnosis and Treatment Protocol for COVID-19 Patients released by the National Health Commission of China.22 , 23 All confirmed patients with COVID-19 included in this study were diagnosed by physicians based on epidemiological history, clinical symptoms, laboratory examination, etiology and serology examination, and chest imaging. Based on the definition of the Diagnosis and Treatment Protocol for COVID-19 Patients, there were 4 clinical classifications of patients with COVID-19: (1) mild cases (those with mild clinical symptoms and no evidence of pneumonia in chest radiology), (2) moderate cases (those with fever and respiratory symptoms and the chest radiology suggestive of pneumonia), (3) severe cases (those who met any of the following criteria: respiratory rate of at least 30 breaths per minute, oxygen saturation of 93% or lower in a resting state, ratio of arterial partial pressure of oxygen and oxygen concentration ≤300 mm Hg, >50% lesion progression in lung imaging within 24-48 hours), and (4) critical cases (those who met any of the following criteria: respiratory failure and the mechanical ventilation is needed, other organ failure, shock, or death). Referring to this protocol and the published literature,24, 25, 26 we defined the mild and moderate cases as nonsevere type of patients and the severe and critical cases as severe type of patients in this study.
The inclusion criteria contained patients with COVID-19 who were admitted to the hospitals in Hubei, China, from December 31, 2019 to March 31, 2020. The exclusion criteria contained (1) age of <18 years, (2) pregnancy, (3) acquired immune deficiency syndrome, (4) autoimmune diseases (eg, systemic lupus erythematosus, myasthenia gravis), (5) severe cases within 48 hours of admission, (6) using systemic corticosteroids after progression into severe cases, and (7) the time of systemic corticosteroids initiation from admission was >30 days.
The demographic information (age and sex), clinical symptoms (cough, fever, and anhelation), comorbidities (hypertension, malignancy, diabetes, chronic obstructive pulmonary disease [COPD], and coronary heart disease), treatments (antiviral drugs, nonsteroidal anti-inflammatory drugs [NSAIDs], and antibiotic drugs), and clinical outcomes were obtained from the electronical medical system. Laboratory data on admission (white blood cell count, albumin, and C-reaction protein) were collected from the laboratory information system. Prescription information of corticosteroids, including type, dose, initiation form admission, and duration, was also collected. We converted all preparations to hydrocortisone equivalent doses27 and anonymized personally identifiable information, such as name and identity card number, and generated a new identification for each patient to protect their privacy.
Exposure and Outcomes
The exposure in this study was patients with nonsevere COVID-19 receiving systematic corticosteroids therapy for the first time. Patients in this group were classified as systemic corticosteroid use (SCU) group. Otherwise, patients were defined as the non-SCU (NSCU) group. To further explore the effect of dose on outcomes among patients with COVID-19, the total hydrocortisone equivalent dose since admission was classified as an ordinal categorical variable (grouped by every 500 mg increase). The study outcomes were (1) progressing from nonsevere type COVID-19 to severe type during 30 days of in-hospital follow-up, (2) all-cause death during 30 days of in-hospital follow-up, and (3) length of stay.
Statistical Analyses
Continuous variables were expressed as median and interquartile range (IQR), and categorical variables were expressed as number and percent (%). Statistical differences between 2 groups were analyzed using the Student’s t test or Wilcoxon-Mann-Whitney U test for continuous variables, whereas categorical variables were compared using Pearson’s chi-square test or Fisher’s exact tests. We evaluated the association between SCU and disease progression and mortality rate using 2 approaches. First, the mixed-effect Cox regressions model with site as a random effect was conducted to examine the impact of systemic corticosteroids and dose groups on the clinical outcomes. Multivariable analyses were all adjusted for age, sex, clinical symptoms (cough, fever, and anhelation), comorbidities (hypertension, malignancy, diabetes, COPD, and coronary heart disease), treatments (antiviral drugs, NSAID, and antibiotic drugs), and laboratory data on admission (white blood cell count, albumin, and C-reaction protein). Second, in mixed-effect Cox regressions model, SCU was accounted as a time-varying exposure to mitigate immoral time bias. In time-varying analysis, data were reconstructed according to the time of SCU. The time from admission to time of SCU was classified as unexposed. The hazard ratios (HRs) and 95% confidence intervals (CIs) were reported. The cumulative probability of progression to severe type COVID-19 was analyzed using the Kaplan-Meier method and compared by log-rank test. Multiple linear regression models were used to examine the influence of systemic corticosteroids and dose groups on length of stay. The covariates are the same as the adjusted variables of the mixed-effect Cox regressions model.
To reduce the effect of systemic corticosteroid treatment selection bias and potential confounding in this observational study, we performed rigorous adjustment for differences in baseline characteristics by propensity score (PS)-matched (PSM) and inverse probability of treatment weight (IPTW) analysis, respectively.28 , 29 The PS was calculated using a logistic regression model in which all baseline characteristics were included and the treatment group (SCU vs NSCU) was the dependent variable within each imputed sample. In PSM, 2 cohorts were matched at a ratio of 1:1 with a caliper width of 0.2. In IPTW, for the SCU group, the weight was equal to 1/PS, whereas for the NSCU group, the weight was equal to 1/(1−PS). The balance among covariates was evaluated by estimating the standardized differences between SCU and NSCU groups in PSM and IPTW cohorts. Only those with absolute value <0.1 were considered as qualified.
The interaction between the use of corticosteroids and age, sex, clinical symptoms (cough, fever, and anhelation), comorbidities (hypertension, malignancy, diabetes, COPD, and coronary heart disease), and treatments (antiviral drugs, NSAID, and antibiotic drugs) on developing severe type and all-cause mortality was explored using multivariable logistic regression in the PSM cohort. Data were analyzed in SAS 9.4 (by SAS Institute Inc, Cary, North Carolina) and R4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
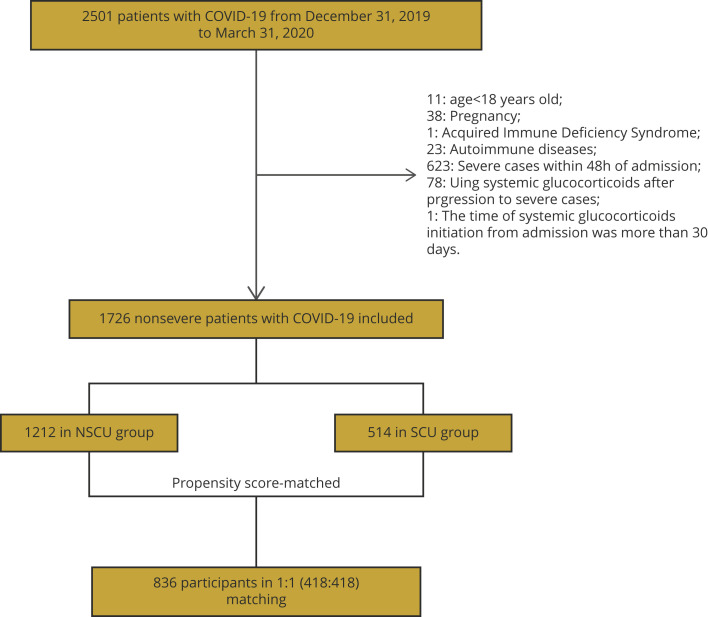
This study cohort included 2501 patients with COVID-19 who were admitted to 4 hospitals in Hubei, China. After excluding 773 participants following our exclusion criteria, 1726 participants were included in the analysis. Among them, 514 (29.78%) patients received systemic corticosteroid treatment (SCU group), and the remaining 1212 (70.22%) did not receive systemic corticosteroid (NSCU group) (Fig. 1 ). Patients in the SCU group were more likely to be younger (51 [IQR 37-64] vs 56 [IQR 41-67] years; P < .001), be male (50.58% vs 43.40%; P = .006), and have a higher proportion of malignancy (7.78% vs 4.62%; P = .009). Of the COVID-19 symptoms, fever was more frequent in the SCU group than the NSCU group (74.32% vs 54.37%; P < .001), whereas anhelation was more frequent in the NSCU group than the SCU group (10.48% vs 7.20%; P = .034). Laboratory findings on admission showed that the median C-reaction protein was higher in the SCU group than in the NSCU group (14.65 [IQR 4.25-38.10] vs 2.60 [IQR 0.70-13.96] mg/liter; P < .001). Moreover, antivirus drugs (95.33% vs 85.48%; P < .001), NSAIDs (48.64% vs 19.06%; P < .001), and antibiotic drugs (95.14% vs 61.22%; P < .001) were more frequently used in the SCU group (Table 1 ).
Figure 1.
Flowchart of the patient enrollment.
NSCU indicates nonsystemic corticosteroid use; PSM, propensity score matching; SCU, systemic corticosteroid use.
Table 1.
Characteristics of 1726 nonsevere patients with COVID-19.
| Parameters | Total∗ (N = 1726) | NSCU∗ (n = 1212) | SCU∗ (n = 514) | P value† |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 55 (39-66) | 56 (41-67) | 51 (37-64) | <.001 |
| Laboratory test | ||||
| White blood cell count (109/liter) | 5.30 (4.10-6.64) | 5.41 (4.28-6.64) | 4.95 (3.55-6.63) | .1642 |
| Albumin(g/liter) | 39.80 (36.50-42.90) | 39.90 (36.60-43.00) | 39.60 (36.20-42.70) | .3659 |
| C-reaction protein (mg/liter) | 4.60 (1.00-22.30) | 2.60 (0.70-13.96) | 14.65 (4.25-38.10) | <.001 |
| Sex | .006 | |||
| Female | 940 (54.46) | 686 (56.60) | 254 (49.42) | |
| Male | 786 (45.54) | 526 (43.40) | 260 (50.58) | |
| Symptom | ||||
| Cough | 799 (46.29) | 561 (46.29) | 238 (46.30) | .995 |
| Fever | 1041 (60.31) | 659 (54.37) | 382 (74.32) | <.001 |
| Anhelation | 164 (9.50) | 127 (10.48) | 37 (7.20) | .034 |
| Comorbidity | ||||
| Hypertension | 539 (31.23) | 384 (31.68) | 155 (30.16) | .531 |
| Malignancy | 96 (5.56) | 56 (4.62) | 40 (7.78) | .009 |
| Diabetes | 258 (14.95) | 183 (15.10) | 75 (14.59) | .787 |
| COPD | 104 (6.03) | 74 (6.11) | 30 (5.84) | .830 |
| Coronary heart disease | 145 (8.40) | 99 (8.17) | 46 (8.95) | .593 |
| Treatment | ||||
| Antivirus drugs | 1526 (88.41) | 1036 (85.48) | 490 (95.33) | <.001 |
| NSAID | 481 (27.87) | 231 (19.06) | 250 (48.64) | <.001 |
| Antibiotic drugs | 1231 (71.32) | 742 (61.22) | 489 (95.14) | <.001 |
| Outcomes | ||||
| Length of stay (days) | 16 (10-24) | 14 (9-20) | 22 (14-30) | <.001 |
| Progression to severe type (30 days) | 509 (29.49) | 328 (27.06) | 181 (35.21) | <.001 |
| All-cause death (30 days) | 43 (2.49) | 25 (2.06) | 18 (3.50) | .079 |
COPD indicates chronic obstructive pulmonary disease; NSAID, nonsteroidal anti-inflammatory drug; NSCU, nonsystemic corticosteroid use; SCU, systemic corticosteroid use.
Categorical variables are presented as number (percent), and continuous variables are presented as median (interquartile range).
P values indicate differences between the NSCU and SCU groups.
After PSM and IPTW (baseline shown in Appendix Tables 1 and 2 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.12.013), the baseline characteristics were balanced between NSCU and SCU groups with absolute value of standard mean differences < 0.1. In PSM data sets, 418 patients in the NSCU group were matched with 418 patients in the SCU group at a ratio of 1:1.
Corticosteroid Use
Among the 514 patients, the most commonly used systemic corticosteroids in clinical treatment was methylprednisolone (93.00%), followed by prednisone (10.31%) and dexamethasone (9.34%), with the least use of hydrocortisone (0.19%). Systemic corticosteroids were initiated within a median of 2 days (IQR 1-4) of hospital admission. The median duration of systemic corticosteroid treatment was 8 days (IQR 4-13), and the median dose was 200 mg (IQR 158.33-227.27) per day of hydrocortisone equivalent (Table 2 ).
Table 2.
Description of corticosteroid used in the SCU group (N = 514).
| Medication variables | Median [IQR] or n (%) |
|---|---|
| Systemic corticosteroids prescribed | |
| Methylprednisolone | 478/514 (93.00) |
| Dexamethasone | 48/514 (9.34) |
| Prednisone | 53/514 (10.31) |
| Prednisolone | 14/514 (2.72) |
| Hydrocortisone | 1/514 (0.19) |
| Duration of systemic corticosteroids, days | 8 [4, 13] |
| Systemic corticosteroids initiation from admission, days | 2 [1, 4] |
| Dose, hydrocortisone equivalents per day (mg) | 200.00 [158.33-227.27] |
IQR indicates interquartile range; SCU, systemic corticosteroid use.
Progression to Severe Type COVID-19
During the follow-up of 30 days, 509 patients progressed into severe type. The proportion of patients progressed into severe type COVID-19 in the SCU group was significantly higher than that in the NSCU group (35.21% [181 of 514] vs 27.06% [328 of 1212]; P < .001). In the mixed-effect Cox model treating site as a random effect, SCU was independently associated with higher probability of developing severe type (adjusted HR 1.27; 95% CI 1.04-1.55). The mixed-effect Cox regressions model with time-varying exposure showed similar results that the SCU group had higher risk of turning into severe type than the NSCU group (adjusted HR 1.81; 95% CI 1.47-2.21) (Tables 1 and 3 ).
Table 3.
Association of systemic corticosteroid use and outcomes in nonsevere patients with COVID-19.
| Progression to severe type |
All-cause death (30 days) |
Length of stay |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cox |
Time-varying Cox |
Cox |
Time-varying Cox |
|||||||
| Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | β (SD) | P value | |
| SCU and NSCU | ||||||||||
| Unmatched | 1.27 (1.04-1.55) | .0212 | 1.81 (1.47-2.21) | <.0001 | 1.93 (0.94-3.97) | .0724 | 2.92 (1.39-6.15) | .0049 | 4.14 (0.43) | <.0001 |
| PSM (1:1)∗ | 1.19 (0.94-1.49) | .1476 | 1.78 (1.41-2.25) | <.0001 | 2.41 (1.01-5.73) | .0470 | 4.16 (1.67-10.34) | .0022 | 4.27 (0.52) | <.0001 |
| IPTW∗ | 1.36 (1.21-1.54) | <.0001 | 2.22 (1.96-2.52) | <.0001 | 1.65 (1.01-2.68) | .0446 | 2.66 (1.61-4.40) | .0001 | 4.52 (0.37) | <.0001 |
| Corticosteroid dose group† | ||||||||||
| Unmatched | 1.06 (1.04-1.08) | <.0001 | 1.08 (1.06-1.09) | <.0001 | 1.06 (1.00-1.13) | .0427 | 1.07 (1.01-1.13) | .0238 | 0.72 (0.06) | <.0001 |
| PSM (1:1)∗ | 1.06 (1.04-1.08) | <.0001 | 1.07 (1.05-1.09) | <.0001 | 1.05 (0.98-1.14) | .1741 | 1.06 (0.99-1.14) | .1018 | 0.72 (0.07) | <.0001 |
| IPTW∗ | 1.09 (1.08-1.10) | <.0001 | 1.10 (1.09-1.11) | <.0001 | 1.05 (1.00-1.10) | .0491 | 1.06 (1.01-1.10) | .0145 | 0.92 (0.05) | <.0001 |
CI indicates confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; IPTW, inverse probability of treatment weight; NSAID, nonsteroidal anti-inflammatory drug; NSCU, nonsystemic corticosteroid use; PSM, propensity score matching; SCU, systemic corticosteroid use; β, regression coefficient.
PSM and IPTW were conducted to adjust for basic demographic characteristics (age and sex), symptoms (cough, fever, and anhelation), comorbidities (hypertension, diabetes, malignancy, COPD, and coronary heart disease), treatments (antiviral drugs, NSAID, and antibiotic drugs), and laboratory data on admission (white blood cell count, albumin, and C-reaction protein).
Corticosteroid dose group: grouped by increasing hydrocortisone equivalent dose per 500 mg.
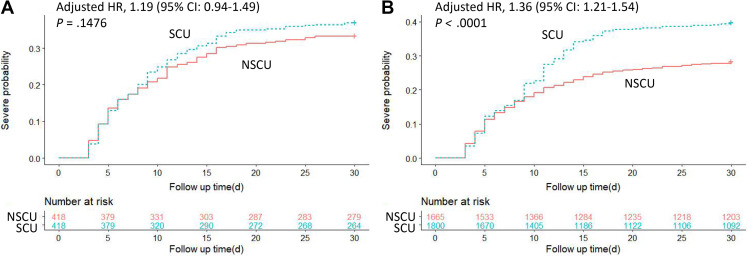
Further analysis was done with PSM and IPTW data sets to balance measured baseline characteristics across treatment groups. In the mixed-effect Cox regressions model with time-varying exposure, SCU was also independently associated with a higher probability of developing severe type after both PS analyses (adjusted HR 1.78; 95% CI 1.41-2.25 in PSM; adjusted HR 2.22; 95% CI 1.96-2.52 in IPTW) (Table 3). Kaplan-Meier curves after PSM and IPTW are shown in Figure 2 A,B. Furthermore, there was a statistically significant interaction between the SCU and NSAID use with the respect to risk of developing severe type (adjusted OR = 2.544; P = .0045 for interaction). There was no significant interaction between the effect of systemic corticosteroids and other covariates on developing severe type (P > .05 for interaction) (Appendix Table 3 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.12.013).
Figure 2.
Kaplan-Meier curves for cumulative probability of progression to severe type COVID-19 during 30-day follow-up duration in SCU/NSCU groups in (A) PSM and (B) IPTW cohorts.
CI indicates confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weight; NSCU, nonsystemic corticosteroid use; PSM, propensity score matching; SCU, systemic corticosteroid use.
In addition, all of these mixed-effect Cox models showed that increased corticosteroid dose was significantly associated with elevated probability of developing severe type. The adjusted HR of corticosteroid dose group indicated that every 500-mg increase in dose was associated with an additional 8% risk of progression to severe type (adjusted HR 1.08; 95% CI 1.06-1.09) in the mixed-effect Cox model with time-varying exposure (Table 3).
All-Cause Mortality During 30 Days of In-Hospital Follow-Up
During a 30-day follow-up period, 43 died of the 1726 patients with COVID-19 admitted with nonsevere type. There was no significant difference between NSCU and SCU groups (2.06% [25 of 1212] vs 3.50% [18 of 514]; P = .079) in the risk of all-cause mortality during 30 days of in-hospital follow-up. In the mixed-effect Cox model with time-varying exposure, the SCU was associated with a higher probability of all-cause mortality (adjusted HR 2.92; 95% CI 1.39-6.15). In 2 PS cohorts, the SCU group had a higher 30-day all-cause mortality risk in the time-varying exposure model (adjusted HR 4.16; 95% CI 1.67-10.34 in PSM; adjusted HR 2.66; 95% CI 1.61-4.40 in IPTW) (Kaplan-Meier curves after PSM and IPTW are shown in Appendix Figure 1 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.12.013). Furthermore, there was no significant interaction between the effect of systemic corticosteroids and other covariates on all-cause mortality (P > .05 for interaction) (Appendix Table 3 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.12.013). In the mixed-effect Cox models grouped by corticosteroid dose, the result showed a tendency toward the association between corticosteroid dose and 30-day mortality. Nevertheless, some of those showed no statistical significance (Tables 1 and 3).
Length of Stay
The median length of stay was 14 days (IQR 9-20) in the NSCU group and 22 days (IQR 14-30) in the SCU group (P < .001). Multiple linear regression showed that, compared with the NSCU group, the SCU group increased the average length of hospital stay by 4.14 days (P < .001). Analysis with PSM and IPTW cohorts displayed similar results (β 4.27; P < .001 in PSM; β 4.52; P < .001 in IPTW). In addition, all of these cohorts showed that corticosteroid dose was significantly associated with length of stay. For every 500-mg increase in corticosteroid dose, the length of stay increased by 0.72 (P < .001) days in the PSM cohort and 0.92 (P < .001) days in the IPTW cohort (Tables 1 and 3).
Discussion
In our study, 29.8% of nonsevere patients with COVID-19 received systemic corticosteroids. To assess the potential impact of corticosteroids on nonsevere patients, we adjusted for time factors and baseline differences that might affect the disease severity. We found that systemic corticosteroid treatment was associated with the risk of progression to severe type, all-cause mortality, and prolonged hospitalization in nonsevere patients with COVID-19. Besides, with the increase in corticosteroid dose, patients with COVID-19 had an increased risk of progression from nonsevere to severe and prolonged length of stay.
Although the anti-inflammatory effects of corticosteroids may benefit patients with severe COVID-19, the risks of immunosuppression and delayed virus clearance in nonsevere patients with COVID-19 cannot be ignored.14 , 30 , 31 Some evidence suggested that adverse effects might offset the benefits of corticosteroid treatment. Once the virus attacks the body, the immune system is activated to produce a series of immune responses. Corticosteroids are believed to reduce concentrations of proinflammatory cytokines and upregulate anti-inflammatory mediators by inhibiting the transcriptional effect of nuclear factor kappa B.32 Meanwhile, corticosteroids could prevent the migration of inflammatory cells from the circulation to issues by suppressing the synthesis of chemokines and cytokines and inhibiting immune responses mediated by T cells and B cells.33 , 34 Therefore, the alterations in immune reactions caused by corticosteroids might lead to reactivation of latent viruses and delay viral clearance, ultimately increasing the risk of mortality.32 In addition, the results showed a significant interaction between the effect of systemic corticosteroids and NSAID on developing severe type, suggesting that the combination use of systemic corticosteroids and NSAID had a higher risk of developing severe type than the use of corticosteroids alone. This may be due to the fact that NSAID could suppress hyperinflammatory responses and increase the activity of angiotensin-converting enzyme-2, promoting the progression of the disease.35 , 36
Although some studies have suggested that low-dose corticosteroid therapy appeared to have a beneficial role in managing patients with COVID-19, the results have been controversial.37 , 38 It is now widely believed that low-dose corticosteroids effectively reduced the risk of mortality, respiratory failure, and delayed viral clearance of patients with severe COVID-19.13 , 39 , 40 In a study evaluating the effects of low dose of corticosteroids, it was found that, in patients with severe COVID-19, the use of corticosteroids was associated with less likelihood of mortality and care escalation and significantly shorter hospitalizations.14 Nevertheless, the researches evaluating the efficacy of low-dose corticosteroids in nonsevere patients with COVID-19 remain relatively insufficient, and the results are controversial. In a prospective cohort study of nonsevere patients with COVID-19, early, low-dose, and short-term corticosteroid therapy was associated with progression to severe disease, a longer virus clearance time, and a longer hospital stay.17 A randomized clinical trial study by Tang et al41 also found that the patients with nonsevere COVID-19 may not obtain any clinical benefits from low-dose methylprednisolone treatment, which even prolonged the virus clearance. On the contrary, some studies showed that low-dose corticosteroid therapy is associated with better clinical outcomes in nonsevere patients with COVID-19. In a retrospective cohort study with nonsevere COVID-19, the overall mortality, C-reactive protein value, and length of stay in the corticosteroids group were significantly lower than the noncorticosteroid group.42 Another study demonstrated that low-dose corticosteroids could benefit patients with mild COVID-19 without affecting the final clearance of viral nucleic acid.43 Moreover, in some studies, low-dose corticosteroid therapy showed no effect on in-hospital mortality, mechanical ventilation, and viral clearance in patients with nonsevere COVID-19.44, 45, 46 Thus, clinicians should carefully consider the risk versus benefit ratio and optimal dose of corticosteroid use for nonsevere patients with COVID-19.
In addition, the risks of developing secondary bacterial infections, invasive fungal infections, and critical illness are increased in corticosteroid-treated patients because of the immunosuppressive effects of corticosteroids.47 Previous research had shown that corticosteroid treatment was significantly associated with more superinfections in critically ill patients with influenza A/H1N1.48 In fact, the adverse effects of corticosteroids therapy are related to the dose. Our study also confirmed that increased corticosteroid dose was significantly associated with elevated probability of developing severe type and increasing length of stay in patients with nonsevere COVID-19. Zhou et al4 reported that >240 mg of hydrocortisone equivalent dose or an excessive cumulative dose was considered to be able to generate some side effects, including hyperglycemia, psychosis, secondary infection, and avascular necrosis. One study found that every 10-mg increase in dose was associated with an additional 4% mortality risk in critically ill patients with COVID-19.49 What’s more, the average duration of corticosteroid treatment reported in our study was 8 days, which was much higher than the recommended duration of corticosteroid use (3∼5 days) by the guideline of Diagnosis and Treatment Protocol for COVID-19 Patients.23 Thus, the abovementioned mechanisms may contribute to explaining why corticosteroids therapy could increase the risk of poor outcomes in nonsevere patients with COVID-19.
This study evaluated the use of systemic corticosteroids among nonsevere patients with COVID-19 with multiple statistical methods and provided evidence-based support for optimizing SCU guidelines in patients with COVID-19. The study also has some limitations. First, because of the intrinsic defects of retrospective study, the confounding factors could not be addressed completely. Although we attempted to minimize the selection bias for corticosteroid use among different groups with PS analysis, we could only adjust for known and measurable confounding factors. Second, we cannot assess the effect of corticosteroids on viral clearance because of the lack of continuous observational data on viral RNA.
Conclusions
This study found that systemic corticosteroid treatment against nonsevere patients with COVID-19 was significantly associated with the higher risk of progression from nonsevere to severe, all-cause mortality, and prolonged length of stay. Furthermore, increased systemic corticosteroid dose was significantly associated with elevated risk of poor disease progression. Given the abovementioned effects of corticosteroid use, we recommend that SCU should be avoided unless absolutely necessary among nonsevere patients with COVID-19, but more cautious treatment strategies and clinical adverse drug reaction monitoring are considered if necessary.
Article and Author Information
Author Contributions:Concept and design: Chen, Gong
Acquisition of data: Tan, Li, Gong
Analysis and interpretation of data: Chen, Wang, Jiang, Tian, Li, Gong
Drafting of the manuscript: Chen, Yin, Wang, Jiang
Critical revision of the paper for important intellectual content: Yin, Tian
Statistical analysis: Tan, Wang, Jiang, Tian, Li
Provision of study materials or patients: Tan, Lu, Xiong
Obtainingfunding: Yin
Administrative, technical, or logisticsupport: Lu, Xiong
Supervision: Lu, Xiong
Conflict of Interest Disclosures: Dr Yin reported receiving funding from Huazhong University of Science and Technology. No other disclosures were reported.
Funding/Support: This work was supported by grants 2020kfyXGYJ073 from the Fundamental Research Funds for the Central Universities, Huazhong University of Science and Technology.
Role of theFunder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgment
The authors thank all patients and their families involved in the study and all healthcare workers who are working against COVID-19.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.jval.2021.12.013.
Supplemental Materials
References
- 1.Pascarella G., Strumia A., Piliego C., et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. World Health Organization. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/Publised2020
- 3.Khalili M., Chegeni M., Javadi S., Farokhnia M., Sharifi H., Karamouzian M. Therapeutic interventions for COVID-19: a living overview of reviews. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620976021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou W., Liu Y., Tian D., et al. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther. 2020;5(1):18. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawoud D.M., Soliman K.Y. Cost-effectiveness of antiviral treatments for pandemics and outbreaks of respiratory illnesses, including COVID-19: a systematic review of published economic evaluations. Value Health. 2020;23(11):1409–1422. doi: 10.1016/j.jval.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C., Huang S., Zheng F., Dai Y. Controversial treatments: an updated understanding of the coronavirus disease 2019. J Med Virol. 2020;92(9):1441–1448. doi: 10.1002/jmv.25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39(7):2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zha L., Li S., Pan L., et al. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med J Aust. 2020;212(9):416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J.W., Yang L., Luo R.G., Xu J.F. Corticosteroid administration for viral pneumonia: COVID-19 and beyond. Clin Microbiol Infect. 2020;26(9):1171–1177. doi: 10.1016/j.cmi.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne J.A.C., Murthy S., et al. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RECOVERY Collaborative Group. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadel R., Morrison A.R., Vahia A., et al. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2020;71(16):2114–2120. doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majmundar M., Kansara T., Lenik J.M., et al. Efficacy of corticosteroids in non-intensive care unit patients with COVID-19 pneumonia from the New York Metropolitan region. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0238827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y., Zeng H., Zhan Z., et al. Corticosteroid use in the treatment of COVID-19: a multicenter retrospective study in Hunan, China. Front Pharmacol. 2020;11:1198. doi: 10.3389/fphar.2020.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q., Li W., Jin Y., et al. Efficacy evaluation of early, low-dose, short-term corticosteroids in adults hospitalized with non-severe COVID-19 pneumonia: a retrospective cohort study. Infect Dis Ther. 2020;9(4):823–836. doi: 10.1007/s40121-020-00332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan M., Xu X., Xia D., et al. Effects of corticosteroid treatment for non-severe COVID-19 pneumonia: a propensity score-based analysis. Shock. 2020;54(5):638–643. doi: 10.1097/SHK.0000000000001574. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Huang J, Zhu G, et al. Systemic corticosteroids show no benefit in severe and critical COVID-19 patients in Wuhan, China: a retrospective cohort study. Posted online Mar 14, 2020. medRxiv. 2020.05.11.20097709. https://doi.org/10.1101/2020.05.11.20097709.
- 20.Tang J. What can we learn about corticosteroid therapy as a treatment for COVID-19? Osteoporos Int. 2020;31(8):1595. doi: 10.1007/s00198-020-05487-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill J.S., Breeze J.L., Simopoulos T.T. Pain management best practices from multispecialty organizations during the COVID-19 pandemic and public health crises-evaluating the risk of infection associated with corticosteroid injections. Pain Med. 2020;21(8):1730–1731. doi: 10.1093/pm/pnaa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. World Health Organization. https://apps.who.int/iris/handle/10665/330893
- 23.Diagnosis and treatment protocol for COVID-19 patients (tentative 8th edition). National Health Commission of the People’s Republic of China. http://www.gov.cn/zhengce/zhengceku/2020-08/19/content_5535757.htm
- 24.Tian J., Yuan X., Xiao J., et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsunaga N., Hayakawa K., Terada M., et al. Clinical epidemiology of hospitalized patients with coronavirus disease 2019 (COVID-19) in Japan: report of the COVID-19 Registry Japan. Clin Infect Dis. 2021;73(11):e3677–e3689. doi: 10.1093/cid/ciaa1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S., Wang J., Liu F., et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. 2020;43(8):824–831. doi: 10.1038/s41440-020-0485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang B., Chen J. People’s Medical Publishing House; Beijing, China: 2018. Pharmacology. 9th version. [Google Scholar]
- 28.Ali M.S., Groenwold R.H., Belitser S.V., et al. Reporting of covariate selection and balance assessment in propensity score analysis is suboptimal: a systematic review. J Clin Epidemiol. 2015;68(2):112–121. doi: 10.1016/j.jclinepi.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Mansournia M.A., Altman D.G. Inverse probability weighting. BMJ. 2016;352:i189. doi: 10.1136/bmj.i189. [DOI] [PubMed] [Google Scholar]
- 30.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammed El Tabaa M., Mohammed El Tabaa M. Targeting neprilysin (NEP) pathways: a potential new hope to defeat COVID-19 ghost [published correction appears in Biochem Pharmacol. 2020;182:114249] Biochem Pharmacol. 2020;178:114057. doi: 10.1016/j.bcp.2020.114057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adcock I.M. Corticosteroids: limitations and future prospects for treatment of severe inflammatory disease. Drug Discov Today Ther Strateg. 2004;1(3):321–328. [Google Scholar]
- 33.Annane D. Pro: the illegitimate crusade against corticosteroids for severe H1N1 pneumonia. Am J Respir Crit Care Med. 2011;183(9):1125–1126. doi: 10.1164/rccm.201102-0345ED. [DOI] [PubMed] [Google Scholar]
- 34.Ni Y.N., Chen G., Sun J., Liang B.M., Liang Z.A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis [published correction appears in Crit Care. 2020;24(1):376] Crit Care. 2019;23(1):99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sodhi M., Etminan M. Safety of ibuprofen in patients with COVID-19: causal or confounded? Chest. 2020;158(1):55–56. doi: 10.1016/j.chest.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhaskar S., Sinha A., Banach M., et al. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crisan Dabija R., Antohe I., Trofor A., Antoniu S.A. Corticosteroids in SARS-COV2 infection: certainties and uncertainties in clinical practice. Expert Rev Anti Infect Ther. 2021;19(12):1553–1562. doi: 10.1080/14787210.2021.1933437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang X., Mei Q., Yang T., et al. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infectol. 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S., Hu Z., Song X. High-dose but not low-dose corticosteroids potentially delay viral shedding of patients with COVID-19. Clin Infect Dis. 2021;72(7):1297–1298. doi: 10.1093/cid/ciaa829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cano E.J., Fonseca Fuentes X., Corsini Campioli C., et al. Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest. 2021;159(3):1019–1040. doi: 10.1016/j.chest.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang X., Feng Y.M., Ni J.X., et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration. 2021;100(2):116–126. doi: 10.1159/000512063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almas T., Ehtesham M., Khan A.W., et al. Safety and efficacy of low-dose corticosteroids in patients with non-severe coronavirus Disease 2019: a retrospective cohort study. Cureus. 2021;13(1) doi: 10.7759/cureus.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu H.Y., Luo Y., Gao J.P., et al. Effects of short-term low-dose glucocorticoids for patients with mild COVID-19. BioMed Res Int. 2020;2020 doi: 10.1155/2020/2854186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikeda S., Misumi T., Izumi S., et al. Corticosteroids for hospitalized patients with mild to critically-ill COVID-19: a multicenter, retrospective, propensity score-matched study. Sci Rep. 2021;11(1):10727. doi: 10.1038/s41598-021-90246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z., Li X., Fan G., et al. Low-to-moderate dose corticosteroids treatment in hospitalized adults with COVID-19. Clin Microbiol Infect. 2021;27(1):112–117. doi: 10.1016/j.cmi.2020.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu K., Chen Y., Yuan J., et al. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020;71(15):799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin Y.Y., Zhou Y.H., Lu Y.Q., et al. Effectiveness of glucocorticoid therapy in patients with severe coronavirus disease 2019: protocol of a randomized controlled trial. Chin Med J (Engl) 2020;133(9):1080–1086. doi: 10.1097/CM9.0000000000000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S.H., Hong S.B., Yun S.C., et al. Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: analytic strategy using propensity scores. Am J Respir Crit Care Med. 2011;183(9):1207–1214. doi: 10.1164/rccm.201101-0110OC. [DOI] [PubMed] [Google Scholar]
- 49.Lu X., Chen T., Wang Y., Wang J., Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit Care. 2020;24(1):241. doi: 10.1186/s13054-020-02964-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.