Abstract
Immunotherapy has failed to achieve durable remissions in advanced prostate cancer patients. More potent T-cell–redirecting strategies may be needed to overcome the immunologically exclusive and suppressive tumor microenvironment. Clinical trials are underway, seeking to define the optimal target for T-cell redirection, such as PSMA, PSCA, or STEAP-1, as well as the optimal strategy, with CAR or bispecific antibodies. As results continue to emerge from these trials, understanding differential toxicity and efficacy of these therapies based on their targets and functional modifications will be key to advancing these promising therapies toward clinical practice. This review provides a unique depth and breadth of perspective regarding the diverse immunotherapy strategies currently under clinical investigation for men with advanced prostate cancer.
Introduction
Metastatic castration-resistant prostate cancer (mCRPC) remains a lethal disease, causing >30,000 deaths in the United States each year (1). Although many new life- prolonging treatments have been developed over the last decade, all of these eventually fail due to the development of resistance, and durable remissions remain rare beyond first-line therapy. Strategies involving immune-checkpoint inhibitors have been minimally successful (2, 3), and the autologous activated cellular therapy Sipuleucel-T is the only immunotherapeutic that has shown a survival benefit in mCRPC (4). Except for some subgroups yet to be fully defined, mCRPC is regarded as a “cold” tumor characterized by sparse infiltration of lymphocytes and dominance of suppressive immune components including myeloid-derived suppressive cells (5). Thus, additional immunotherapy modalities are needed, and powerful cellular strategies may be key to inducing inflammation in the tumor microenvironment (TME).
Novel immunotherapies that harness the redirection of T lymphocytes against cancer have recently garnered significant attention. In particular, chimeric antigen receptor-modified (CAR) T cells and bispecific antibodies (e.g., bispecific T-cell engagers, referred to as BiTEs), which both utilize single-chain variable fragment (scFv) technologies for the recognition of tumor-associated antigens (TAA), have demonstrated therapeutic promise in hematologic malignancies. Although CAR-T-cell therapies entail an engineered chimeric receptor comprised of an extracellular scFv TAA recognition domain and intracellular CD3ζ and costimulatory domains, BiTE therapies link a TAA scFv with an anti-CD3 scFv, thereby facilitating T-cell–mediated antitumor responses. Importantly, through these novel T-cell redirection approaches, cytolytic T-cell activity may be stimulated in an MHC-independent manner, thus obviating the need for conventional TCR-MHC signaling that may be severely impaired in the “cold” TME of mCRPC.
Both BiTE and CAR-T-cell therapeutic strategies have demonstrated compelling and durable clinical outcomes in a variety of treatment-refractory hematologic malignancies, resulting in several FDA approvals (6–8). More recently, based on this initial success, these T-cell redirection approaches have additionally been applied to the treatment of advanced solid malignancies (9, 10). Both CAR-T-cell and BiTE therapies have been an active area of investigation for the treatment of mCRPC. Indeed, mCRPC offers many potential advantages for the successful development of T-cell–redirecting therapies, including a large unmet clinical need and the availability of a variety of putative TAAs. However, there are several disease-specific aspects of prostate cancer that warrant consideration when developing these therapies. These considerations include the appropriate selection of TAA targets, which vary in their prostate cancer specificity, potential for off- tumor toxicity, functional significance, heterogeneity of expression, and amenability to noninvasive detection. Additionally, the bone-predominant and immune-inhibitory nature of prostate cancer metastases imposes both physical and immunologic barriers to T-cell therapies. Finally, the typical elderly male mCRPC population, often with comorbid medical conditions, may increase the risk for treatment-related toxicities and issues regarding optimal patient selection.
Given the recent high interest in the development and clinical testing of T-cell–redirecting therapies for prostate cancer, this review focuses on BiTE and CAR-T therapies currently under investigation for the treatment of mCRPC. We will specifically discuss current antigen targets, observed barriers to success, and potential future approaches to improve durable remissions for these therapies in mCRPC. In particular, we will review the more well-established cell-surface prostate adenocarcinoma targets, including prostate stem cell antigen (PSCA), prostate-specific membrane antigen (PSMA), and six-transmembrane epithelial antigen of the prostate (STEAP-1), as well as implications for future neuroendocrine prostate cancer (NEPC) targets including carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5, also known as CEA) and Notch ligand delta-like protein 3 (DLL3).
Prostate Tumor-Associated Antigens for Redirected T-Cell Therapies
Prostate stem cell antigen (PSCA)
PSCA is a cell-surface glycosylphosphatidylinositol (GPI)-anchored glycoprotein that belongs to the Thy-1/Ly-6 family, which was identified in 1998 as an overexpressed gene in prostate cancer cells and localized to chromosome 8q24 (11). PSCA is expressed in up to 94% of prostate primaries and 87.5% to 100% of prostate cancer metastases with moderate to strong intensity immunohistochemical staining in 84%, weakly positive staining in 17%, and negative staining in 6% (12, 13). PSCA has additionally been noted to be expressed in urothelial, pancreatic, renal cell and non–small cell lung cancers (14, 15) with limited normal tissue expression in the urinary bladder, kidney, skin, esophagus, brain, and stomach (16–18). A summary of PSCA expression is presented in Table 1, along with the other prostate cancer TAA that are addressed in this review.
Table 1.
TAAs and their expression in type of prostate cancer versus their normal distribution.
| Tumor-associated antigen | Cell/tissue distribution |
|---|---|
| PSCA | Prostate adenocarcinoma, urothelial, skin, esophagus, neuronal, stomach |
| PSMA | Prostate adenocarcinoma, prostate acinar epithelium, proximal tubular cells, glial cells, jejunal brush border cells, salivary glandular cells |
| STEAP-1 | Prostate adenocarcinoma, bladder, ovary, bone marrow, cardiac, respiratory |
| DLL3 | Neuroendocrine prostate cancer (NEPC), neurons, pancreatic islet cells, pituitary |
| CEA | NEPC, urogenital, respiratory, gastrointestinal |
PSCA expression in tumors correlates with disease progression and prognosis in prostate cancer (12, 19–21). For example, PSCA mRNA levels in peripheral blood are also associated with high-grade and extra-prostatic disease (22) and has predictive value for biochemical recurrences and overall survival (23). Therefore, PSCA has been explored as a PET imaging target for cancer detection, staging, and monitoring (24).
The biological function of PSCA is not entirely known; in some studies, PSCA abrogation in cancer cells reduces metastatic potential (25–27), whereas other studies suggest that PSCA suppresses metastasis (28). It is not entirely understood if PSCA is controlled by AR signaling, but studies show androgen-responsive elements in the PSCA gene and the potential for androgen-mediated PSCA expression (29).
Important to immunotherapy considerations, PSCA is immunogenic. PSCA-derived peptides were shown to induce cytotoxic T cells in HLA-A24+ prostate cancer patients (30). Preclinical studies with PSCA-based vaccines demonstrated long-term protective immunity, without evidence of autoimmunity, in prostate cancer–prone transgenic adenocarcinoma mouse prostate (TRAMP) mice mediated in part by cytotoxic T cells (31). Dendritic cell–based PSCA vaccine approaches yielded similar preclinical antitumor responses (32). A phase I/II trial recently demonstrated safety and immunogenicity of a prostate cancer–specific self-adjuvanted mRNA vaccine with multiple antigens, including PSCA, further supporting the immunogenicity of PSCA (33).
PSCA can also be targeted using MHC-independent T-cell–engineering therapies due to its cell-surface expression profile. Early attempts of engineered T cells showed in vitro targeting of PSCA+ prostate and pancreatic cancer cell lines using first-generation chimeric antigen receptors (CAR) strategies (34, 35). Hillerdal and colleagues demonstrated antitumor efficacy and prolonged survival of mice bearing subcutaneous PSCA+ tumors that were treated with third-generation (containing costimulatory domains of CD28 and OX40) PSCA-CAR T cells (36). Superior antitumor activity of CD28 costimulatory domain-containing second-generation PSCA-CAR T cells, compared with third-generation PSCA-CAR T cells, were shown in human xenograft models of pancreatic cancer (17). Subsequent studies further optimized a second-generation CAR-T- cell containing a 4-1BB costimulatory domain for improved tumor selectivity, potent antitumor activity, and bone-homing ability of PSCA-CAR T cells in human xenograft models of bone metastatic prostate cancer (37).
Our more recent study using a fully immunocompetent mouse model of PSCA+ prostate and pancreatic cancers offered the ability to assess both safety and efficacy of PSCA-CAR T cells. Murad and colleagues demonstrated safety and durable antitumor immune responses with PSCA-CAR T cells (38), even with PSCA expression in various normal tissues including the prostate, bladder, and stomach. Importantly, these preclinical studies also highlight the potential for engaging endogenous immunity in targeting PSCA+ and PSCA− prostate tumors. Two clinical trials of PSCA-targeted CAR-T cells are under way; a summary of ongoing CAR-T and BiTE antibody studies is presented in Table 2.
Table 2.
List of past, current, and future trials for CAR-T and BiTE therapies in prostate cancer.
| NCT# | Biological/drug name | Targets | Status | Phase |
|---|---|---|---|---|
| CAR-T | ||||
| NCT04227275 | CART-PSMA-TGFβRDN | PSMA | Active | Phase I |
| NCT03873805 | Autologous anti-PSCA-CAR-4–1BB/TCRzeta-CD19t-expressing T lymphocytes | PSCA | Recruiting | Phase I |
| NCT04053062 | LIGHT-PSMA-CART | PSMA | Recruiting | Phase I |
| NCT01140373 | Engineered autologous T cells | PSMA | Active | Phase I |
| NCT04249947 | P-PSMA-101 CAR-T cells | PSMA | Recruiting | Phase I |
| NCT04107142 | NKG2DL-targeting chimeric antigen receptor–grafted gamma delta T-cell | NKG2DL | Not yet recruiting | |
| NCT04633148 | UniCAR02-T-pPSMA | PSMA | Recruiting | Phase I |
| NCT02744287 | BPX-601 | PSCA | Recruiting | Phase I |
| NCT04429451 | 4SCAR-PSMA T cells | PSMA | Recruiting | Phase I |
| BiTE | ||||
| NCT04104607 | CC-1 | PSMA x CD3 | Recruiting | Phase I |
| NCT03406858 | HER2 bi-armed activated T cells | HER2 x CD3 | Recruiting | Phase II |
| NCT04702737 | AMG 757 | DLL3 x CD3 | Not yet recruiting | Phase I |
| NCT03792841 | AMG 160 | PSMA x CD3 | Recruiting | Phase I |
| NCT02262910 | ES414 | PSMA x CD3 | Completed | Phase I |
| NCT00635596 | MT110 | EpCAM x CD3 | Completed | Phase I |
| NCT03927573 | GEM3PSCA | PSCA x CD3 | Recruiting | Phase I |
| NCT01723475 | BAY2010112 | PSMA x CD3 | Completed | Phase I |
| NCT04221542 | AMG 509 | STEAP-1 x CD3 | Recruiting | Phase I |
| NCT04631601 | AMG 160 | PSMA x CD3 | Not yet recruiting | Phase I |
| NCT04424641 | GEN1044 | 5T4 x CD3 | Recruiting | Phase I/II |
| NCT03517488 | XmAb20717 | PDL1 x CTLA4 | Recruiting | Phase I |
| NCT03849469 | XmAb22841 | CTLA4 x LAG-3 | Recruiting | Phase I |
Other T-cell–redirecting approaches have been preclinically evaluated that target PSCA, including bispecific T-cell engagers. CD3-PSCA bispecific antibodies have demonstrated potent and selective CD4+ and CD8+ cytolytic T-cell activity in cell culture systems (39). Additionally, various formats of novel bispecific antibodies and antibody-based modular targeting of PSCA have shown efficient cell lysis with an ability to further redirect to different TAAs (40). However, bispecific modalities targeting PSCA have not yet been investigated clinically.
Prostate-specific membrane antigen (PSMA)
For nearly three decades, PSMA, a type II transmembrane protein, has been explored as a biomarker of disease activity, as a method for in vivo imaging, and as a disease-specific therapeutic target in prostate cancer. The PSMA gene was cloned in 1993 and found to encode a 750-amino acid transmembrane protein (2, 3). The extracellular binding domain of PSMA forms a dimer that binds to glutamate and glutamate-like structures and acts as a glutamate carboxypeptidase. Under normal physiologic conditions, PSMA is found with variable low expression in the prostate gland (secretory acinar epithelium), kidney (proximal tubules), nervous system glia (astrocytes and Schwann cells), salivary glandular cells, and the small intestine (jejunal brush border; ref. 41). However PSMA is highly expressed in malignant prostate tissue and directly correlates with both advancing prostate cancer stage/metastases and higher histologic grade (42). PSMA upregulation may serve as a marker for prostate cancer progression and has been independently associated with prostate-specific antigen (PSA) recurrence following local therapy and with the development of the castration-resistant phenotype (43).
Due to its high expression and membrane-bound antigen, PSMA has been proposed as an ideal TAA for targeted prostate cancer therapies. Notably, the expression of PSMA on tumor neovasculature-rich malignancies (ex: renal cell carcinoma and adenoid cystic carcinoma) raise the potential for non–prostate PSMA-directed cancer therapies (44, 45). Although the exact functional significance of PSMA is currently unknown, its extracellular binding domain possesses folate hydrolase activity, and increased PSMA expression may confer a proliferative advantage by increasing the levels of extracellular folate available for prostate cancer cellular import (46). It was recently demonstrated that PSMA interacts with the scaffolding protein receptor for activated C kinase 1 (RACK1), disrupting signaling between the β1 integrin and insulin-like growth factor type 1 receptor (IGF-1R) complex to the mitogen-activated protein kinase (MAPK) pathway, potentiating protein kinase B (AKT) pathway activation instead and thus controlling primary tumor advancement (47). Targeting PSMA-dependent signaling that drives tumor progression may provide a new treatment paradigm due to inhibition of both progression and vascularization, as PSMA blockade with the inhibitor 2-PMPA diminishes integrin activation, endothelial cell adhesion, and angiogenesis (48).
The advent of urea-based small-molecule PSMA ligands has heralded an era of PSMA PET imaging and theranostics. Both 68Ga-labeled (ex: Ga-68 PSMA-11) and 18F-labeled (ex: DCFPyL) PSMA ligands have demonstrated compelling test characteristics for the detection of prostate cancer in men with biochemical recurrence and negative or equivocal conventional imaging, even in patients with low PSA values (49–51). PSMA imaging has paved the way for a theranostic strategy for men with mCRPC—confirming expression of the target antigen radiographically followed by administering a treatment dose radiopharmaceutical. Radiolabeled PSMA ligands (177Lu-PSMA-617) have demonstrated significant antitumor responses, including in cohorts of treatment-refractory mCRPC patients (52, 53). Recently, a large phase III randomized trial demonstrated that 177Lu-PSMA-617 plus standard-of-care treatment improved progression-free and overall survival when compared with standard-of-care treatment alone for men with mCRPC and at least one PSMA PET-avid lesion (54). However, the predictive value of baseline PSMA PET avidity for benefit remains unknown.
A BiTE against PSMA was initially developed in a standard formulation requiring continuous intravenous infusion (AMG212 or pasotuxizumab). A phase I trial (NCT01723475) involving 47 patients showed PSA declines, including two long-term therapeutic responses (55). A reformulation with half-life extension (AMG160) has also been tested in a phase I trial (NCT03792841). This agent yielded PSA50 reductions in 6 of 24 evaluable patients as well as 1 objective radiographic response among 18 patients with measurable disease (56). Two subjects did experience 1 year of cancer control. Toxicity was largely predictable, including cytokine release syndrome in the majority of patients and dry mouth in 28%. Additionally, a PSMA-targeted CAR-T was tested in 5 subjects with mCRPC and evoked PSA declines in 2 men (57). However, the study was stopped due to lack of engraftment. Although sialotoxicity is common with 177Lu-PSMA617 and with AMG160, this has not been reported with PSMA-targeted CAR-T, raising the possibility that on-target off-tumor toxicities may differ between modalities. Ongoing PSMA-targeted immunotherapy trials are summarized in Table 2.
Six-transmembrane epithelial antigen of the prostate-1 (STEAP-1)
STEAP-1 is part of the STEAP family that act as ion channels at cell junctions and serve a metalloreductase role (58). Importantly, STEAP-1 has been shown to be overexpressed in prostate cancer as well as in other hematologic and solid tumors. It is expressed in low levels in some normal tissues such as bladder, ovary, marrow, heart, and lung (59). In terms of diagnostic value of STEAP-1, 89Zr-DFO-MSTP2109A is a radiolabeled antibody targeting STEAP-1 that was well tolerated in studies and showed excellent visualization in mCRPC sites including bone and soft tissue, therefore establishing a potential role as a predictive biomarker for STEAP-1–directed therapy (60). DSTP3086S, a STEAP-1–targeting antibody conjugated with an antimitotic agent monomethyl auristatin E, showed some antitumor activity in mCRPC with an acceptable safety profile in a phase I study (61).
For immunotherapy considerations, STEAP-1 is also immunogenic (62, 63). STEAP-1–derived peptides were shown to induce cytotoxic CD8+ T cells in an HLA-A*0201–restricted manner, which were shown to be reactive in non–small cell lung cancer and prostate cancer patients ex vivo (64).
Vaccine approaches have also demonstrated immunotherapy potential of targeting STEAP-1 (64, 65). As STEAP-1 is primarily expressed at the cell surface, BiTE strategies are also being explored (Table 2).
Carcinocembryonic antigen (CEA) and delta-like protein 3 (DLL3)
Although less is known about immunotherapeutic approaches for the treatment of NEPC, recent studies have identified cell-surface targets that may be used to develop CAR-T-cell and BiTE therapy strategies. Most notable has been the identification that human carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5, also known as CEA) is highly expressed in a large subset of NEPC (66). Lee and colleagues recently demonstrated in vitro support for the rational targeting of NEPC with CAR-T cells. In addition, there have been recent promising in vitro studies showing successful application of anti-CEA CAR-T cells in CEA-positive solid tumors, further supporting CEA as a potential target for NEPC (67).
Another candidate for immunotherapeutic targeting of NEPC is delta-like protein 3 (DLL3), which was originally identified as an overexpressed surface protein in small cell lung cancer. Recent studies have demonstrated that DLL3 is also highly expressed in NEPC (68) and may also be therapeutically evaluated as both a BiTE (69) and CAR-T-cell strategy for late-stage prostate cancer patients. In fact, DLL3 has been studied extensively as a possible therapeutic target for small cell lung cancer with ongoing phase I trials involving AMG 757 (an anti-DLL3 x CD3 bispecific antibody) and AMG 119 (CAR-T cells directed against DLL3; ref. 69). Although the field is at its infancy in realizing the potential for T-cell–redirecting therapies in treating NEPC, this area of research is likely burgeoning in the coming years, and may provide for an arsenal of cellular immunotherapy approaches in targeting advanced heterogeneous prostate cancers.
Advantages and Disadvantages of T-Cell–Redirected Therapies for mCRPC
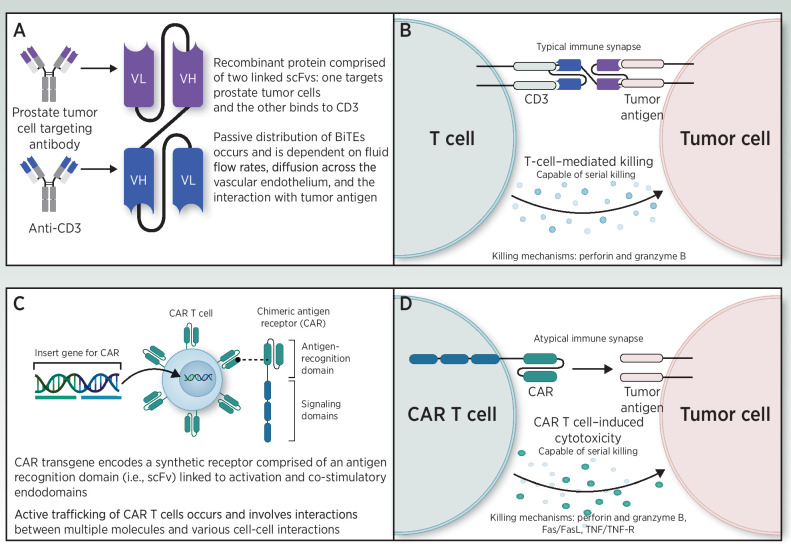
A summary of the mechanism of action of BiTE antibodies and CAR-T cells is presented in Fig. 1.
Figure 1.
Mechanisms of action of BiTE antibody (A, B) and CAR-T (C, D) therapies.
CAR T-cell therapy in mCRPC
The transformative promise of CAR-T-cell therapy relates to its unique ability to achieve durable clinical remissions for advanced malignancies following a single administration. Indeed, successful in vivo expansion of adoptive cell transfer raises the potential for long-term engraftment and antitumor activity (70). Treatment-related factors associated with long-term persistence and clinical remissions in patients with hematologic malignancies treated with CD19-directed CAR-T-cell therapy have included increased peak expansion and immunophenotypic signatures of early memory T-cell differentiation (71). An early stem cell memory differentiation state may offer enhanced cell persistence, and the opportunity for a perpetual source of tumor-reactive T cells though their specificity may be limited by TAA expression heterogeneity. Notably, however, the factors required for the optimal expansion, persistence, and bioactivity in solid tumors, including prostate cancer, remain unknown, and recent solid malignancy experiences have lagged behind those in hematologic cancers, which provide better opportunity for high levels of antigen stimulation and more permissive TME.
One aspect of the prostate cancer TME that may limit CAR-T efficacy is the high level of TGFβ, which inhibits T-cell–mediated immunity. In preclinical studies, the antitumor potency of PSMA CAR-T cells could be augmented by the coexpression of a dominant-negative TGFβRII (TGFβRDN). CAR-T cells engineered to express TGFβRDN (CART-PSMA-TGFBRDN) exhibited increased proliferation, enhanced cytokine secretion, resistance to exhaustion, long-term in vivo persistence, and the induction of tumor eradication in aggressive human prostate cancer mouse models (72). In addition, prostate tumor–engrafted mice treated with CART-PSMA-TGFBRDN had significantly higher levels of high-potency central memory CD8+ T cells, compared with mice treated with PSMA CAR-T cells. Although this approach is highly promising, clinical activity has only been observed following infusion of a high number of CART-PSMA-TGFBRDN cells and is tempered by the concomitant induction of cytokine release syndrome, similar to what is observed with CD19-directed CAR-T cells for leukemia. Thus, new strategies are required to improve the persistence of these cells in the toxic TME (Fig. 2), while avoiding dose-limiting toxicities.
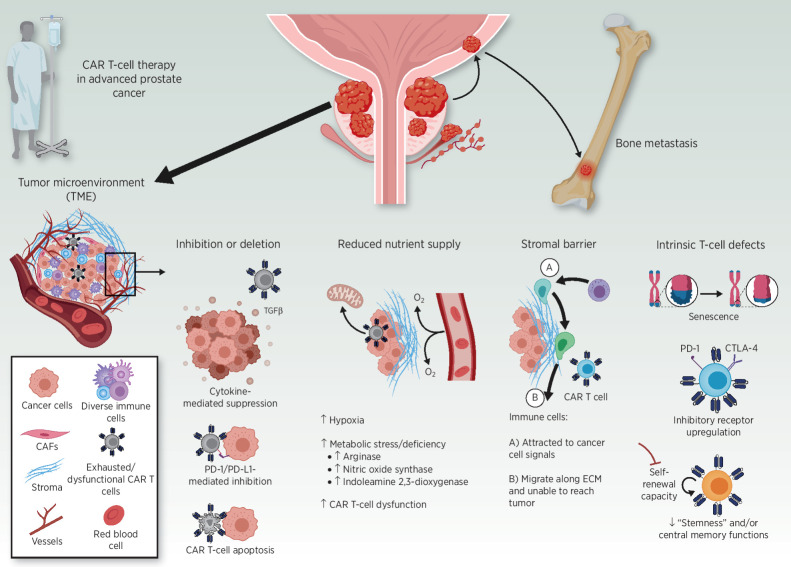
Figure 2.
Immunosuppressive barriers to CAR T-cell function in advanced (metastatic) prostate cancer. Recent studies, including those discussed in this review, suggest that both tumor- and T-cell–intrinsic factors could hamper the efficacy of CAR T cells in prostate cancer. Barriers include elaboration of immunosuppressive cytokines (e.g., TGFβ), increased expression of inhibitory ligands (e.g., PD-L1), apoptosis of CAR T cells, and metabolic stress operative in the TME. A formidable stromal barrier may result in CAR T-cell and bystander immune cell migration along the protumor extracellular matrix (ECM), instead of infiltration into the tumor bed. T cell–intrinsic defects, including replicative senescence, inhibitory receptor upregulation/exhaustion, and reduced early memory T-cell function, may also prevent CAR T cells from eliciting an effective antitumor response.
Even if trafficking and infiltration are successful, T cells can become dysfunctional due to a hostile TME characterized by the presence of soluble inhibitory factors and cytokines, as well as suppressive immune cells or tumor cells that secrete these mediators and overexpress inhibitory ligands. For instance, tumor-infiltrating lymphocytes (TIL) in prostate cancer appear to be defective, in part due to the presence of high levels of arginase, nitric oxide synthase, and indoleamine 2,3-dixoygenase (IDO; refs. 73, 74). IDO inhibitors and other specific combination strategies to overcome the unique TME represent a possible path forward to enhance CAR-T activity in prostate cancer. Even when CD8+ T cells are effective in mediating tumor cell lysis, the localization of cytotoxic T lymphocytes (CTL) within prostate tumors is modest relative to the infiltration of these tumors with myeloid-derived cells (75). The immunosuppressive TME formed by myeloid-derived cells (PMN-MDSC/neutrophils, TAMs, mMDSCs, and suppressive dendritic cells) hampers antitumor immunity and effective treatment in prostate cancer, and this is supported by clinical evidence (76). T-cell–intrinsic defects, such as upregulation of inhibitory receptors (e.g., PD-1 and CTLA-4), a reduction in “stemness”/self-renewal capacity, as well as impaired central memory functions, increased apoptosis sensitivity and augmented replicative senescence are also major barriers to the success of CAR-T cell therapy (77, 78). Accordingly, checkpoint blockade therapy combined with targeted agents that inhibit MDSCs without impacting T-cell function results in robust immunotherapy of mCRPC (79). Another issue of the hostile TME is the accumulation of prostate cell secretion products that can be inhibitor to T cells. In particular, the polyamine spermine has been shown to have immunosuppressive effects on T cells including downregulation of cytokine secretion and decreased cytotoxic activity (80, 81).
It has recently been shown that conditioning chemotherapy, commonly referred to as “lymphodepletion,” plays a critical role in modulating the TME for CAR-T cell effect. In particular, cyclophosphamide administration increased intratumoral PSCA-targeted CAR-T cell accumulation and expansion in vivo (38). Gene ontology enrichment analysis identified T-cell migration and IFNγ production as key processes enhanced by cyclophosphamide pretreatment. In addition, Alzubi and colleagues showed significant inhibition of tumor growth in prostate cancer xenograft models when they were treated with PSMA-targeted CAR-T cells in combination with low-dose docetaxel, compared with each component individually (82). Further work will elucidate which immunosuppressive components these chemotherapy regimens help to overcome, which will inform exploration of additional strategies to modulate TME for enhanced success with cellular immunotherapy. However, conditioning chemotherapy regimens are not without their own side effects including myelosuppression, leading to potentially life-threatening infections (83). Study of CAR-T cells alone, without conditioning chemotherapy, and vigilance toward the contribution of chemotherapy to the overall toxicity of the treatment will be keys to maximizing patient safety.
Finally, important practical and toxicity considerations must be addressed during the development of CAR-T therapies in advanced prostate cancer. The collection, manufacture, release testing, and administration of autologous CAR-T therapies pose limitations to patient selection in prostate cancer, particularly when heavily pretreated patients may develop rapid disease progression. In addition, the unpredictable and potentially severe treatment-related toxicities associated with CAR-T therapy (84) raise concerns in an elderly prostate cancer population. Similar to the experience with anti-CD19 and other CAR-T therapies for hematologic malignancies, the solid tumor experience to date has reported high-grade cytokine release syndrome (CRS), neurotoxicity (ICANS), on-target/off-tumor effects, and severe allergic reactions (85, 86). Moreover, in the mCRPC experience to date, the reported time course for onset of high-grade CRS, neurotoxicity, and other severe inflammatory events has varied (within 6 hours of infusion to several weeks after infusion), which may limit the ability to reduce inpatient and outpatient observation period (87). Additionally macrophage activation syndrome has been a serious toxicity in prostate cancer CAR-T trials (86), but can be abrogated, indicating a need for highly specialized treatment teams in order to optimize patient safety, especially during early-phase trials. Moving forward, the development of predictive models for CAR-T–related CRS and other toxicities, using disease-related variables and early cytokine inputs, may provide needed guidance for prostate cancer–specific CAR-T toxicity identification and management.
BiTE therapy
Bispecific antibody therapies, including BiTEs, have seen an explosion of clinical development over the past several years (88). A major advantage of BiTE therapy is the fact that it is not an individually produced cellular product, making it easier to scale for widespread use (89). Another advantage perhaps is the different tumor penetrating capabilities of antibody therapies as compared with cellular therapies. It is likely that BiTEs arrive at the tumor site either through direct biodistribution to tumors or through “backpacking” via TILs. BiTE therapies also differentiate from CAR-T cells in the kinetics and severity of CRS, which have been relatively predictable and manageable with mitigation strategies and may result in less need for ICU-level care or tocilizumab administration (90).
A major disadvantage of BiTE antibody therapy is that, in its more common format, it requires repeated dosing either weekly or bimonthly, though this is feasible and preferable to continuous infusion. Although toxicity is predictable, the frequency and severity of CRS seen with AMG160, for instance, necessitated dexamethasone premedication for the first several doses (56). There is concern that steroids may blunt the full potential of a T-cell–mediated antitumor response (91). Furthermore, efficacy has been correlated to the level of TILs, and therefore success may require an already immunologically “warm” tumor. And finally, BiTE antibodies may also generate antidrug antibodies; the extent to which this occurs and how much this limits efficacy remains to be determined (56).
Future Directions
Based on our early experiences, it is likely that combination therapies will be needed to induce significant and durable remissions with solid tumor T-cell–redirected therapies. These combination strategies will require identification of resistance mechanisms, ideally from patient blood and biopsy samples, though preclinical models may be helpful in preliminary mechanistic studies. For instance, adding immune-checkpoint inhibitors would be considered if there is evidence of T-cell exhaustion in patient samples after exposure to a T-cell–redirected therapy, whereas something to reverse the immunosuppressive TME and promote tumor infiltration of T cells in advance of CAR-T-cell or BiTE therapy may be required if inadequate trafficking of T cells into the tumor is identified. Additionally, structural modifications to circumvent stromal barriers may be needed to enhance antitumor activity of CAR-T cell therapies. For example, CAR-T cells redirected toward fibroblast activation protein-α, which is highly expressed on cancer-associated fibroblasts, demonstrated target cell lysis and therapeutic synergy with TAA-redirected CAR-T cells for solid tumors (92). Additionally, CAR-T cells engineered to express heparanase, which is involved in the degradation of heparan sulfate proteoglycans within the extracellular matrix, aided TILs and antitumor activity (93).
Additionally, heterogeneity of neoantigen expression or dedifferentiation is often seen in late-stage or heavily pretreated mCRPC. Epigenetic studies have identified differences in tumor RNA splicing among men of differing ethnic backgrounds (94), which may lead to racially described differences in neoantigen expression and outcomes, but it is likely that specific molecular diagnostics to characterize an individual's expression of the target antigen will be needed to enrich clinical trials to maximize benefit. Multitargeting approaches may also be required to account for tumor antigen heterogeneity; future strategies include next-generation CAR-T cells designed to target two or more antigens simultaneously (i.e., tandem scFvs on CARs, multiple BiTE coinfusions), or BiTE-secreting CAR-T cells (95). There is also increased NEPC after the application of more potent antiandrogen therapies. This has been noted incidentally on some on-study and posttreatment biopsy samples in our experience, which makes dual targeting of traditional plus neuroendocrine antigens attractive.
As discussed extensively in this review, novel immune therapies provide a promising new avenue of treatment for advanced prostate cancer with the possibility of sustained, durable responses. Further modifications to CAR-T cells, or the addition of adjunctive therapies, may be needed to overcome the immune-suppressive microenvironment and physical barriers unique to prostate cancer. Although preclinical models can reveal potential BiTE and CAR-T-cell potency-enhancing or toxicity-mitigating strategies in the presence of a target antigen-expressing tumor, we acknowledge that there are many limitations of preclinical models, and these findings therefore may not translate fully into improved activity during future clinical applications. Well-defined toxicity management protocols will be crucial in order for these treatments to be offered more broadly. Certification or at least robust training will be needed to maintain safety of these therapies and even regionalization to specialized center may end up being necessary to ensure their proper use. Despite all of this, we are optimistic that these therapies may become a significant therapeutic advance in the future.
Authors' Disclosures
T.B. Dorff reports personal fees from Janssen outside the submitted work. V. Narayan reports personal fees from Regeneron and Amgen; grants from TMunity and Bristol Myers Squibb; and grants and personal fees from Pfizer, Merck, and Janssen outside the submitted work. S.J. Forman reports grants from Mustang Bioscience during the conduct of the study as well as personal fees from Allogene outside the submitted work; in addition, S.J. Forman has a patent for PSCA-CAR issued and licensed. J.A. Fraietta reports grants from Tmunity Therapeutics during the conduct of the study. C.H. June reports other support from Tmunity during the conduct of the study, as well as a patent pending to Tmunity (IPR licensed by University of Pennsylvania). S.J. Priceman reports grants from Prostate Cancer Foundation and Department of Defense Prostate Cancer Research Program during the conduct of the study as well as grants and personal fees from Imugene Ltd. and Mustang Therapeutics and personal fees from Bayer and Adicet Bio outside the submitted work; in addition, S.J. Priceman has a patent for PSCA-CAR issued, licensed, and with royalties paid from Mustang Therapeutics. No disclosures were reported by the other authors.
Acknowledgments
This work was supported by the Prostate Cancer Foundation (PCFYOUNG18 to V. Narayan), the National Cancer Institute [P30CA033572 and P50CA092131 (COH)], and the Department of Defense (W81XWH-17-1-0208 and W81XWH-2110354 to S.J. Priceman).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol 2020;38:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–22. [DOI] [PubMed] [Google Scholar]
- 5. Mehra N, Seed G, Lambros M, Sharp A, Fontes MS, Crespo M, et al. Myeloid-derived suppressor cells (MDSCs) in metastatic castration-resistant prostate cancer (CRPC) patients (PTS). Ann Oncol 2016;27:vi257. [Google Scholar]
- 6. Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 2017;376:836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 9. O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 2017;9:eaaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 2016;375:2561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, et al. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci U S A 1998;95:1735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gu Z, Thomas G, Yamashiro J, Shintaku IP, Dorey F, Raitano A, et al. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene 2000;19:1288–96. [DOI] [PubMed] [Google Scholar]
- 13. Lam JS, Yamashiro J, Shintaku IP, Vessella RL, Jenkins RB, Horvath S, et al. Prostate stem cell antigen is overexpressed in prostate cancer metastases. Clin Cancer Res 2005;11:2591–6. [DOI] [PubMed] [Google Scholar]
- 14. Cheng L, Reiter RE, Jin Y, Sharon H, Wieder J, Lane TF, et al. Immunocytochemical analysis of prostate stem cell antigen as adjunct marker for detection of urothelial transitional cell carcinoma in voided urine specimens. J Urol 2003;169:2094–100. [DOI] [PubMed] [Google Scholar]
- 15. Argani P, Rosty C, Reiter RE, Wilentz RE, Murugesan SR, Leach SD, et al. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res 2001;61:4320–4. [PubMed] [Google Scholar]
- 16. Saeki N, Gu J, Yoshida T, Wu X. Prostate stem cell antigen: a Jekyll and Hyde molecule? Clin Cancer Res 2010;16:3533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abate-Daga D, Lagisetty KH, Tran E, Zheng Z, Gattinoni L, Yu Z, et al. A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum Gene Ther 2014;25:1003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ono H, Sakamoto H, Yoshida T, Saeki N. Prostate stem cell antigen is expressed in normal and malignant human brain tissues. Oncol Lett 2018;15:3081–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han KR, Seligson DB, Liu X, Horvath S, Shintaku PI, Thomas GV, et al. Prostate stem cell antigen expression is associated with Gleason score, seminal vesicle invasion and capsular invasion in prostate cancer. J Urol 2004;171:1117–21. [DOI] [PubMed] [Google Scholar]
- 20. Zheng K, Chen Z, Tian Y, Hao G. Association between PSCA mRNA expression levels and rs2294008 polymorphism in transitional cell cancer of the bladder. Oncol Lett 2015;9:557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wente MN, Jain A, Kono E, Berberat PO, Giese T, Reber HA, et al. Prostate stem cell antigen is a putative target for immunotherapy in pancreatic cancer. Pancreas 2005;31:119–25. [DOI] [PubMed] [Google Scholar]
- 22. Fawzy MS, Mohamed RH, Elfayoumi AR. Prostate stem cell antigen (PSCA) mRNA expression in peripheral blood in patients with benign prostatic hyperplasia and/or prostate cancer. Med Oncol 2015;32:74. [DOI] [PubMed] [Google Scholar]
- 23. Suh YS, Joung JY, Kim SH, Kim JE, Choi MK, Park WS, et al. Prostate stem cell antigen mRNA in blood is a predictor of survival after radical prostatectomy in patients with high-risk prostate cancer. Oncotarget 2018;9:26291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lepin EJ, Leyton JV, Zhou Y, Olafsen T, Salazar FB, McCabe KE, et al. An affinity matured minibody for PET imaging of prostate stem cell antigen (PSCA)-expressing tumors. Eur J Nucl Med Mol Imaging 2010;37:1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saffran DC, Raitano AB, Hubert RS, Witte ON, Reiter RE, Jakobovits A. Anti-PSCA mAbs inhibit tumor growth and metastasis formation and prolong the survival of mice bearing human prostate cancer xenografts. Proc Natl Acad Sci U S A 2001;98:2658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kang R, Zhao S, Liu L, Li F, Li E, Luo L, et al. Knockdown of PSCA induces EMT and decreases metastatic potentials of the human prostate cancer DU145 cells. Cancer Cell Int 2016;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao Z, Li E, Luo L, Zhao S, Liu L, Wang J, et al. A PSCA/PGRN-NF-kappaB-integrin-alpha4 axis promotes prostate cancer cell adhesion to bone marrow endothelium and enhances metastatic potential. Mol Cancer Res 2020;18:501–13. [DOI] [PubMed] [Google Scholar]
- 28. Wang L, Sang Y, Tang J, Zhang RH, Luo D, Chen M, et al. Down-regulation of prostate stem cell antigen (PSCA) by Slug promotes metastasis in nasopharyngeal carcinoma. J Pathol 2015;237:411–22. [DOI] [PubMed] [Google Scholar]
- 29. Tang S, Mishra M, Frazier DP, Moore ML, Inoue K, Deora R, et al. Positive and negative regulation of prostate stem cell antigen expression by Yin Yang 1 in prostate epithelial cell lines. PLoS One 2012;7:e35570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsueda S, Yao A, Ishihara Y, Ogata R, Noguchi M, Itoh K, et al. A prostate stem cell antigen-derived peptide immunogenic in HLA-A24-prostate cancer patients. Prostate 2004;60:205–13. [DOI] [PubMed] [Google Scholar]
- 31. Garcia-Hernandez Mde L, Gray A, Hubby B, Klinger OJ, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res 2008;68:861–9. [DOI] [PubMed] [Google Scholar]
- 32. Xiao L, Joo KI, Lim M, Wang P. Dendritic cell-directed vaccination with a lentivector encoding PSCA for prostate cancer in mice. PLoS One 2012;7:e48866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kubler H, Scheel B, Gnad-Vogt U, Miller K, Schultze-Seemann W, Vom Dorp F, et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J Immunother Cancer 2015;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morgenroth A, Cartellieri M, Schmitz M, Gunes S, Weigle B, Bachmann M, et al. Targeting of tumor cells expressing the prostate stem cell antigen (PSCA) using genetically engineered T-cells. Prostate 2007;67:1121–31. [DOI] [PubMed] [Google Scholar]
- 35. Katari μL, Keirnan JM, Worth AC, Hodges SE, Leen AM, Fisher WE, et al. Engineered T cells for pancreatic cancer treatment. HPB 2011;13:643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hillerdal V, Ramachandran M, Leja J, Essand M. Systemic treatment with CAR-engineered T cells against PSCA delays subcutaneous tumor growth and prolongs survival of mice. BMC Cancer 2014;14:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Priceman SJ, Gerdts EA, Tilakawardane D, Kennewick KT, Murad JP, Park AK, et al. Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. Oncoimmunology 2018;7:e1380764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murad JP, Tilakawardane D, Park AK, Lopez LS, Young CA, Gibson J, et al. Pre-conditioning modifies the TME to enhance solid tumor CAR T cell efficacy and endogenous protective immunity. Mol Ther 2021;29:2335–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feldmann A, Stamova S, Bippes CC, Bartsch H, Wehner R, Schmitz M, et al. Retargeting of T cells to prostate stem cell antigen expressing tumor cells: comparison of different antibody formats. Prostate 2011;71:998–1011. [DOI] [PubMed] [Google Scholar]
- 40. Jureczek J, Bergmann R, Berndt N, Koristka S, Kegler A, Puentes-Cala E, et al. An oligo-His-tag of a targeting module does not influence its biodistribution and the retargeting capabilities of UniCAR T cells. Sci Rep 2019;9:10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997;3:81–5. [PubMed] [Google Scholar]
- 42. Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer 1998;82:2256–61. [DOI] [PubMed] [Google Scholar]
- 43. Wright GL Jr, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology 1996;48:326–34. [DOI] [PubMed] [Google Scholar]
- 44. Rowe SP, Gorin MA, Hammers HJ, Som Javadi M, Hawasli H, Szabo Z, et al. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted (1)(8)F-DCFPyL PET/CT. Ann Nucl Med 2015;29:877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klein Nulent TJW, Valstar MH, Smit LA, Smeele LE, Zuithoff NPA, de Keizer B, et al. Prostate-specific membrane antigen (PSMA) expression in adenoid cystic carcinoma of the head and neck. BMC Cancer 2020;20:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yao V, Bacich DJ. Prostate specific membrane antigen (PSMA) expression gives prostate cancer cells a growth advantage in a physiologically relevant folate environment in vitro. Prostate 2006;66:867–75. [DOI] [PubMed] [Google Scholar]
- 47. Caromile LA, Dortche K, Rahman MM, Grant CL, Stoddard C, Ferrer FA, et al. PSMA redirects cell survival signaling from the MAPK to the PI3K-AKT pathways to promote the progression of prostate cancer. Sci Signal 2017;10:eaag3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Conway RE, Rojas C, Alt J, Novakova Z, Richardson SM, Rodrick TC, et al. Prostate-specific membrane antigen (PSMA)-mediated laminin proteolysis generates a pro-angiogenic peptide. Angiogenesis 2016;19:487–500. [DOI] [PubMed] [Google Scholar]
- 49. Rowe SP, Campbell SP, Mana-Ay M, Szabo Z, Allaf ME, Pienta KJ, et al. Prospective evaluation of PSMA-targeted (18)F-DCFPyL PET/CT in men with biochemical failure after radical prostatectomy for prostate cancer. J Nucl Med 2020;61:58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Calais J, Czernin J, Cao M, Kishan AU, Hegde JV, Shaverdian N, et al. (68)Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med 2018;59:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morris MJ, Carroll PR, Saperstein L, Pouliot F, Josephson D, Wong JYC, et al. Impact of PSMA-targeted imaging with 18F-DCFPyL-PET/CT on clinical management of patients (pts) with biochemically recurrent (BCR) prostate cancer (PCa): Results from a phase III, prospective, multicenter study (CONDOR). J Clin Oncol 2020;38:5501. [Google Scholar]
- 52. Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol 2018;19:825–33. [DOI] [PubMed] [Google Scholar]
- 53. Hofman MS, Emmett L, Sandhu SK, Iravani A, Joshua AM, Goh JC, et al. TheraP: a randomised phase II trial of 177Lu-PSMA-617 (LuPSMA) theranostic versus cabazitaxel in metastatic castration resistant prostate cancer (mCRPC) progressing after docetaxel: initial results (ANZUP protocol 1603). J Clin Oncol 2020;38:5500. [Google Scholar]
- 54. Sartor AO, deBono J, Chi K, Fizazi K, Herrman K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med 2021;385:1091–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hummel H-D, Kufer P, Grüllich C, Deschler-Baier B, Chatterjee M, Goebeler M-E, et al. Phase 1 study of pasotuxizumab (BAY 2010112), a PSMA-targeting Bispecific T cell Engager (BiTE) immunotherapy for metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2019;37:5034. [Google Scholar]
- 56. Tran B, Horvath L, Dorff TB, Greil R, Machiels JPH, Roncolato F, et al. Interim results from a phase I study of AMG 160, a half-life extended bispecific T-cell engager (HLE BiTE immune therapy) targeting prostate-specific membrane antigen, in patients with metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol 2020;31:S507. [Google Scholar]
- 57. Junghans RP, Ma Q, Rathore R, Gomes EM, Bais AJ, Lo AS, et al. Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate 2016;76:1257–70. [DOI] [PubMed] [Google Scholar]
- 58. Burnell SEA, Spencer-Harty S, Howarth S, Bodger O, Kynaston H, Morgan C, et al. Utilisation of the STEAP protein family in a diagnostic setting may provide a more comprehensive prognosis of prostate cancer. PLoS One 2019;14:e0220456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moreaux J, Kassambara A, Hose D, Klein B. STEAP1 is overexpressed in cancers: a promising therapeutic target. Biochem Biophys Res Commun 2012;429:148–55. [DOI] [PubMed] [Google Scholar]
- 60. Carrasquillo JA, Fine BM, Pandit-Taskar N, Larson SM, Fleming SE, Fox JJ, et al. Imaging patients with metastatic castration-resistant prostate cancer using (89)Zr-DFO-MSTP2109A anti-STEAP1 antibody. J Nucl Med 2019;60:1517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Danila DC, Szmulewitz RZ, Vaishampayan U, Higano CS, Baron AD, Gilbert HN, et al. Phase I study of DSTP3086S, an antibody-drug conjugate targeting six-transmembrane epithelial antigen of prostate 1, in metastatic castration-resistant prostate cancer. J Clin Oncol 2019;37:3518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rodeberg DA, Nuss RA, Elsawa SF, Celis E. Recognition of six-transmembrane epithelial antigen of the prostate-expressing tumor cells by peptide antigen-induced cytotoxic T lymphocytes. Clin Cancer Res 2005;11:4545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alves PM, Faure O, Graff-Dubois S, Cornet S, Bolonakis I, Gross DA, et al. STEAP, a prostate tumor antigen, is a target of human CD8+ T cells. Cancer Immunol Immunother 2006;55:1515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Garcia-Hernandez ML, Gray A, Hubby B, Kast WM. In vivo effects of vaccination with six-transmembrane epithelial antigen of the prostate: a candidate antigen for treating prostate cancer. Cancer Res 2007;67:1344–51. [DOI] [PubMed] [Google Scholar]
- 65. Gray A, de la Luz Garcia-Hernandez M, van West M, Kanodia S, Hubby B, Kast WM. Prostate cancer immunotherapy yields superior long-term survival in TRAMP mice when administered at an early stage of carcinogenesis prior to the establishment of tumor-associated immunosuppression at later stages. Vaccine 2009;27:G52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee JK, Bangayan NJ, Chai T, Smith BA, Pariva TE, Yun S, et al. Systemic surfaceome profiling identifies target antigens for immune-based therapy in subtypes of advanced prostate cancer. Proc Natl Acad Sci U S A 2018;115:E4473–E82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cha S, Yazaki P, Brown C, Shively J. Abstract PO083: treatment of CEA-positive solid tumors with anti-CEA chimeric antigen receptor T-cells in CEA transgenic mice. Cancer Immunol Res 2021;9:PO083. [Google Scholar]
- 68. Puca L, Gavyert K, Sailer V, Conteduca V, Dardenne E, Sigouros M, et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci Transl Med 2019;11:eaav0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hipp S, Voynov V, Drobits-Handl B, Giragossian C, Trapani F, Nixon AE, et al. A bispecific DLL3/CD3 IgG-like T-cell engaging antibody induces antitumor responses in small cell lung cancer. Clin Cancer Res 2020;26:5258–68. [DOI] [PubMed] [Google Scholar]
- 70. Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med 2018;24:1499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018;24:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF, et al. Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther 2018;26:1855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods 2009;348:9–17. [DOI] [PubMed] [Google Scholar]
- 74. Toso A, Revandkar A, Di Mitri D, Guccini I, Proietti M, Sarti M, et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep 2014;9:75–89. [DOI] [PubMed] [Google Scholar]
- 75. Lopez-Bujanda Z, Drake CG. Myeloid-derived cells in prostate cancer progression: phenotype and prospective therapies. J Leukoc Biol 2017;102:393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Santegoets SJ, Stam AG, Lougheed SM, Gall H, Jooss K, Sacks N, et al. Myeloid derived suppressor and dendritic cell subsets are related to clinical outcome in prostate cancer patients treated with prostate GVAX and ipilimumab. J Immunother Cancer 2014;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kasakovski D, Xu L, Li Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J Hematol Oncol 2018;11:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tschumi BO, Dumauthioz N, Marti B, Zhang L, Lanitis E, Irving M, et al. CART cells are prone to Fas- and DR5-mediated cell death. J Immunother Cancer 2018;6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lu X, Horner JW, Paul E, Shang X, Troncoso P, Deng P, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017;543:728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Peng Q, Wong CY, Cheuk IW, Teoh JY, Chiu PK, Ng CF. The emerging clinical role of spermine in prostate cancer. Int J Mol Sci 2021;22:4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Soda K. The mechanisms by which polyamines accelerate tumor spread. J Exp Clin Cancer Res 2011;30:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Alzubi J, Dettmer-Monaco V, Kuehle J, Thorausch N, Seidl M, Taromi S, et al. PSMA-directed CAR T cells combined with low-dose docetaxel treatment induce tumor regression in a prostate cancer xenograft model. Mol Ther Oncolytics 2020;18:226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018;131:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res 2013;1:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Poseida. Poseida Therapeutics announces clinical hold lifted on phase 1 autologous CAR-T study in prostate cancer. News Release. [cited 2020 Nov 2]. Available from: https://www.prnewswire.com/news-releases/poseida-therapeutics-announces-clinical-hold-lifted-on-phase-i-autologous-car-t-study-in-prostate-cancer-301164397.html.
- 87. Narayan V, Gladney W, Plesa G, Vapiwala N, Carpenter E, Maude SL, et al. A phase I clinical trial of PSMA-directed/TGFβ-insensitive CAR-T cells in metastatic castration-resistant prostate cancer. J Clin Oncol 2019;37:TPS347. [Google Scholar]
- 88. Sheridan C. Bispecific antibodies poised to deliver wave of cancer therapies. Nat Biotechnol 2021;39:251–4. [DOI] [PubMed] [Google Scholar]
- 89. Slaney CY, Wang P, Darcy PK, Kershaw MH. CARs versus BiTEs: a comparison between T cell-redirection strategies for cancer treatment. Cancer Discov 2018;8:924–34. [DOI] [PubMed] [Google Scholar]
- 90. Thakur A, Huang M, Lum LG. Bispecific antibody based therapeutics: strengths and challenges. Blood Rev 2018;32:339–47. [DOI] [PubMed] [Google Scholar]
- 91. Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 2014;20:119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kakarla S, Chow KKH, Mata M, Shaffer DR, Song X-T, Wu M-F, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther 2013;21:1611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med 2015;21:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Al Abo M, Hyslop T, Qin X, Owzar K, George DJ, Patierno SR, et al. Differential alternative RNA splicing and transcription events between tumors from African American and White patients in The Cancer Genome Atlas. Genomics 2021;113:1234–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol 2019;37:1049–58. [DOI] [PubMed] [Google Scholar]