Abstract
Background:
Eczema is a common inflammatory skin disease with varying developmental trajectories/patterns that are influenced by different risk factors. The aim of this study was to investigate eczema development from infancy to early adulthood by identifying distinct developmental trajectories that describe disease patterns over time and evaluate the role of prenatal and early life risk factors.
Methods:
The Isle of Wight Birth Cohort (n=1,456) was prospectively assessed at birth, 1, 2, 4, 10, 18, and 26 years. In all assessments, eczema was defined as chronic or chronically relapsing itchy dermatitis lasting >6 weeks with characteristic morphology and distribution in the past 12 months. Developmental trajectories of eczema between 1-or-2 and 26 years were identified separately for males and females by applying semiparametric mixture models. Associations were assessed by applying a modified Poisson regression to estimate adjusted risk ratios (aRR) and 95% confidence intervals (CI).
Results:
In both males and females, the following eczema developmental trajectories were identified: unaffected/transient (males: 77.7% vs. females: 73.0%), mid-onset late-resolving (males: 7.8% vs. females: 4.4%), late-onset (males: 5.2% vs. females: 9.5%), and early-onset persistent (males: 9.3% vs. females: 5.4%). In females, an additional trajectory was identified: early-onset early-resolving (7.7%). Among males, filaggrin gene (FLG) variants (aRR = 2.45, 95% CI: 1.34-4.46) and paternal eczema (2.66, 1.39-5.08) were associated with the early-onset persistent trajectory. Among females, maternal eczema (2.84, 1.42-5.70) and high birthweight (2.25, 1.08-4.69) were associated with the early-onset persistent trajectory.
Conclusions:
Four and five trajectories represented eczema development among males and females, respectively, with different predisposing risk factors. Our results indicate that males and females may experience a different course of eczema.
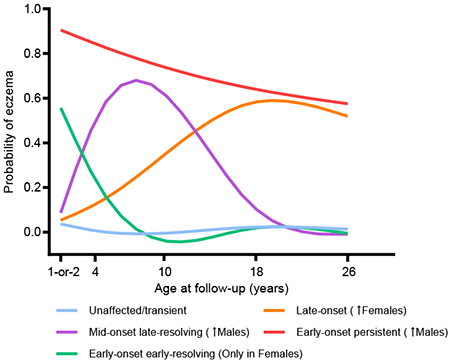
Graphical Abstract
Males and females follow different developmental trajectories of eczema from infancy to 26 years of age, with more males than females following an early-onset persistent trajectory and a mid-onset late-resolving trajectory. In contrast, more females than males followed a late-onset trajectory and only females were in the early-onset early-resolving trajectory. Moreover, males and females have distinct risk factors that influence the developmental trajectories of eczema. Hence, it is important to considered sex-stratified clinical management of the disease.
INTRODUCTION
Eczema, also known as atopic dermatitis and atopic eczema, is a common and chronic inflammatory skin disease that often follows a relapsing-remitting course. It has been estimated that up to 20% of children and 10% of adults are affected by eczema.1,2 The disorder is characterized by a heterogeneous clinical presentation that includes erythematous, scaly, pruritic, and acute oozing and crusting lesions.3,4 The pathophysiology of eczema is multifactorial, with immune system dysregulation and epidermal barrier dysfunction being the major hallmarks of the disease.5,6 Although eczema is not life-threatening, it poses a significant impact on healthcare resources and patients’ and their relatives’ overall health, including quality of life and psychosocial wellbeing.7,8
There is a consensus in the scientific literature that most cases of eczema develop during early childhood7,9,10; however, the long-term natural history of the disease is less understood. It has been widely accepted that most early childhood cases resolve later in childhood, with few cases developing in adolescence and adulthood, but this view has been challenged as adulthood onset is not rare, and some childhood cases may relapse later in life (relapsing-remitting nature) or even follow a persistent course of the disease.11–13 A meta-analysis estimated adult-onset eczema to be 26.1% (95% CI: 16.5-37.2%), i.e., around 1 in 4 adults with eczema report adulthood-onset of their disease.14 Hence, this heterogeneity indicates that different trajectories of eczema development exist that may be influenced by different determinants. One strong risk factor that has been related to eczema development, and a more persistent and severe phenotype is carrying loss-of-function (LOF) variants in the filaggrin gene (FLG), which encodes an important structural protein involved in the epidermal barrier formation and integrity.15–19
Several birth cohorts have investigated the course of eczema during the first two decades of life, and in a few instances up to early adulthood, as synthesized in a prior systematic review, which concluded that the prevalence of eczema was similar before and after childhood, with some observed heterogeneity between studies.20 Of the five studies reporting eczema prevalence beyond 20 years of age, three studies reported that eczema prevalence is lowest in early adulthood,21–23 whereas two studies showed that eczema prevalence was higher in early adulthood13,24 as compared to assessments during adolescence. Few studies have investigated the longitudinal trajectories of eczema development, where distinct developmental trajectories, such as early-onset persistent, early transient, and late-onset, and their determinants have been identified.25–27 These studies have investigated eczema development up to 6 years,25 10 years,27 and 16 years26 and hence, there is a need to investigate eczema trajectories beyond adolescence. Another study has incorporated eczema severity phenotypes while investigating eczema development up to age 14 years.28
The participants of the Isle of Wight Birth Cohort (IOWBC) study have been seen on six occasions over the course of 26 years, at 1, 2, 4, 10, 18, and 26 years, with eczema being assessed at each follow-up.29 Using data from the IOWBC, this study aims to investigate the natural course of eczema development from infancy to early adulthood in terms of identifying distinct developmental trajectories that describe disease patterns over time. Given that male and female subjects experience different course of the disease, with eczema prevalence being equal or slightly higher among males during childhood and becoming preponderant among females after puberty,23,24,30–32 our analysis was stratified by sex. In addition, effects of prenatal and early life risk factors on eczema trajectories were assessed.
METHODS
Study design and participants
The IOWBC is a whole population-based prospective study that recruited all children (n = 1,536, ~98% Caucasian) born between January 1st, 1989 and February 28th, 1990 on the Isle of Wight, UK, to study the natural history and etiology of allergic and respiratory conditions.29,33 Parental informed consent was obtained to enroll 1,456 newborns to participate in the ensuing follow-ups that occurred at the ages of 1, 2, 4, 10, 18, and 26 years, with >80% retention at all assessments up to 18 years and 71% retention at age 26 years.29,34 In the current analysis, participants who had three or more valid eczema status information (i.e., not missing) across the follow-ups were included; hence, 1,381 (94.8%) participants satisfied our inclusion criteria. All participants provided informed consent and ethical approval was obtained from the local/national Research Ethics Committee at recruitment and at each assessment. At age 26, ethical approval was granted by National Research Ethics Committee, West Midlands (15/WM/0071). Phenotypic and environmental information were collected using questionnaires and a wide range of clinical assessments and measurements were carried out as detailed by Arshad et al.29
Eczema ascertainment
In all assessments of the IOWBC, investigators determined the presence of eczema based on symptoms over the last 12 months, which was defined as chronic or chronically relapsing itchy dermatitis lasting more than 6 weeks with characteristic morphology and distribution,29,35 according to the criteria of Hanifin and Rajka.36 Since the 1- and 2-year follow-up data on eczema were collected in a relatively narrow time window, we have combined them for analytic purposes and reported them hereafter as 1-or-2 years.
Prenatal and early life variables
In the current report we have assessed the effect of prenatal and early life risk factors on the development of eczema, including FLG LOF variants, maternal and paternal history of eczema, birthweight (low/normal <4,000 grams; high ≥4,000 grams; the low (<2,500 grams) birthweight group was analyzed with the normal birthweight group due to a small proportion (3.5%, 48/1360) of participants were born with low birthweight), birth order, duration of breastfeeding reported in weeks, and whether the child’s family owned a cat and/or a dog at the time of the child’s birth. The underweight (BMI <18.5) group was analyzed with the normal group due to a small proportion (1.8%) of mothers being underweight. A total of 1150 participants were genotyped for FLG variants R501X, 2282del4, S3247X, 3702delG, and R2447X, with the latter two variants were not informative in our study population due to minor allele frequencies <0.1%. Individuals carrying the minor allele for at least one of the FLG variants R501X, 2282del4, or S3247X were classified as carrying FLG LOF variants. FLG variants genotyping was performed using the GoldenGate Genotyping Assays (Illumina, Inc, SanDiego, CA) on the BeadXpressVeracode platform (Illumina, Inc, SanDiego, CA) per Illumina’s protocol, detailed information is provided by Ziyab et al.37
Measures of disease occurrence
To investigate the natural course of eczema across the five analytical follow-ups (1-or-2, 4, 10, 18, 26 years), different measures of disease occurrence were estimated. The 12-month period prevalence was calculated at each follow-up as the number of existing cases divided by the total number of subjects participating in the respective follow-up. Incidence, to measure newly developed eczema, was defined at the 10, 18, and 26 years follow-ups as the number of new cases at the respective follow-up divided by the total cohort at risk (i.e., disease-free in all preceding follow-ups).38 Remission, to estimate the proportion of cases that outgrew eczema, was defined at the 10, 18, and 26 years follow-ups as the number of cases that outgrew eczema at the respective follow-up divided by the cohort who had eczema in preceding follow-ups.38 Moreover, given the relapsing-remitting course of eczema, disease transition measures were estimated to describe the change in a person’s disease status between two consecutive assessments, regardless of whether it was the first such occurrence or whether prior data were missing.30,38,39 Hence, four transition periods were considered in our analysis: 1-or-2 to 4 years, 4 to 10 years, 10 to 18 years, and 18 to 26 years. Positive transition (change from disease-free to diseased) was defined as the number of eczema cases at the end of the transition period divided by the number of subjects that were disease-free at the beginning of the transition period. Negative transition (change from diseased to disease-free) was defined as the number of eczema-free subjects at the end of the transition period divided by the number of subjects with eczema at the beginning of the transition period. It is worth noting that positive transition may capture some incident cases and negative transition may capture some remitting cases (i.e., some overlap may exist).
Trajectory analysis
Developmental trajectories (i.e., distinct subgroups within the total sample that follow similar disease patterns over time) of eczema across ages 1-or-2, 4, 10, 18, and 26 years were identified separately for males and females by applying semiparametric mixture models using PROC TRAJ macro in SAS 9.4 (SAS Institute, Cary, North Carolina, USA).40,41 See the Methods section in this article’s Supporting Information for more details.
Statistical analysis
All statistical analyses were conducted using SAS 9.4. The statistical significance level was set to α=0.05. Descriptive analyses were conducted to calculate frequencies and proportions of categorical variables and median and interquartile range (IQR) were used to describe continuous variables. Chi-square (χ2) tests were used to assess differences in proportions across sex. To control for false positive results, we applied the false discovery rate (FDR) method to estimate adjusted p-values for the sex comparisons.42 Adjusted associations were assessed by applying a modified Poisson regression with robust variance estimation to estimate and infer risk ratios (RR) and their 95% confidence intervals (CI).43 Adjusted associations between prenatal and early life risk factors (predictors) and eczema trajectories (outcome variable) were assessed separately for males and females. The “unaffected/transient” trajectory was set as the reference group and a series of three and four regression models were evaluated among males and females, respectively, to assess the associations.
RESULTS
Description of the study population
In total, information on eczema status was available for 1,377 (700 males), 1,214 (619 males), 1,359 (688 males), 1,307 (651 males), and 1,029 (469 males) study participants at ages 1-or-2, 4, 10, 18, and 26 years, respectively. The total enrolled study sample and the analytical study sample were similar with respect to all characteristics under study (Table 1). The combined proportion of carrying the minor (loss-of-function) allele(s) of FLG variants R501X, 2282del4, and/or S3247X was 10.3% (116/1,129) in the analytical study sample. No individuals were homozygous for the minor allele of any FLG variants. One person had a compound heterozygous genotype for R501X and 2282del4, and this person was analyzed with the heterozygotes.
Table 1.
Characteristics of the total enrolled study sample and the analytical study sample
| Characteristic | Enrolled study sample | Analytical study sample* |
|---|---|---|
| Sex, % (n/total) | ||
| Male | 51.2 (786/1,536) | 50.4 (696/1,381) |
| Female | 48.8 (750/1,536) | 49.6 (685/1,381) |
| FLG genotype, % (n/total) | ||
| Loss-of-function | 10.3 (118/1,150) | 10.3 (116/1,129) |
| Maternal eczema, % (n/total) | ||
| Yes | 12.1 (147/1,213) | 12.1 (144/1,191) |
| Paternal eczema, % (n/total) | ||
| Yes | 6.4 (78/1,213) | 6.5 (77/1,191) |
| Birthweight, % (n/total) | ||
| Low/normal† | 88.0 (1,329/1,511) | 87.6 (1,192/1,360) |
| High‡ | 12.0 (182/1,511) | 12.4 (168/1,360) |
| Birth order, % (n/total) | ||
| First | 42.1 (510/1,212) | 42.0 (500/1,189) |
| Second | 34.6 (419/1,212) | 34.5 (410/1,189) |
| Third or more | 23.3 (283/1,212) | 23.5 (279/1,189) |
| Household cat at birth, % (n/total) | ||
| Yes | 32.4 (492/1,516) | 32.6 (448/1,374) |
| Household dog at birth, % (n/total) | ||
| Yes | 29.0 (440/1,516) | 28.8 (396/1,374) |
| Breastfeeding duration, Median (IQR) | ||
| Weeks | 8.0 (1.0-26.0) | 9.0 (2.0-28.0) |
IQR: interquartile range.
Includes participants who have three or more valid eczema status information (i.e., not missing) across the five analytical follow-ups.
Low/normal birthweight: <4000 grams.
High birthweight: ≥4000 grams
Measures of disease occurrence
Estimates of the 12-month period prevalence, positive transition, incidence, negative transition, and remission of eczema from 1-or-2 to 26 years of age are shown in Table 2. The 12-month prevalence of eczema was highest at 1-or-2 years (14.2%) and lowest at 26 years (10.2%). Among males, eczema was most prevalent at 1-or-2 years (14.6%) and lowest at 18 years (8.3% at 18 years) and 26 years (8.5%). Among females, eczema was most prevalent at 18 years (16.3%) and lowest at 26 years (11.6%; Table 2, Figure S1). Only minor differences in the prevalence were detected between males and females up to 10 years of age; however, eczema was statistically significantly more common among females compared to males at age 18 years (16.3% vs. 8.3%, p <0.001, FDR adjusted p <0.001) and slightly higher at 26 years (11.6% vs. 8.5%, p = 0.104, FDR adjusted p = 0.395; Table 2).
Table 2.
Prevalence (12-month), positive transition, incidence, negative transition, and remission of eczema from infancy to 26 years of age in the total study sample and according to sex
| Age at follow-up (years), % (n/total) |
|||||
|---|---|---|---|---|---|
| 1-or-2 | 4 | 10 | 18 | 26 | |
| Prevalence | |||||
| Overall | 14.2 (196/1,377) | 11.9 (145/1,214) | 13.7 (186/1,359) | 12.3 (161/1,307) | 10.2 (105/1,029) |
| Male | 14.6 (102/700) | 12.6 (78/619) | 13.4 (92/688) | 8.3 (54/651) | 8.5 (40/469) |
| Female | 13.9 (94/677) | 11.3 (67/595) | 14.0 (94/671) | 16.3 (107/656) | 11.6 (65/560) |
| P-value† | 0.715 | 0.472 | 0.733 | < 0.001 | 0.104 |
| Positive transition | |||||
| Overall | 5.9 (57/962) | 8.6 (88/1,025) | 6.9 (73/1,063) | 4.9 (42/850) | |
| Male | 6.4 (31/488) | 8.3 (43/518) | 4.3 (23/532) | 4.0 (16/404) | |
| Female | 5.5 (26/474) | 8.9 (45/507) | 9.4 (50/531) | 5.8 (26/446) | |
| P-value† | 0.569 | 0.743 | 0.001 | 0.209 | |
| Incidence | |||||
| Overall | 6.9 (60/865) | 5.7 (42/743) | 3.0 (16/539) | ||
| Male | 6.7 (29/435) | 3.0 (11/365) | 1.6 (4/249) | ||
| Female | 7.2 (31/430) | 8.2 (31/378) | 4.1 (12/290) | ||
| P-value† | 0.754 | 0.002 | 0.125 | ||
| Negative transition | |||||
| Overall | 56.9 (103/181) | 43.8 (56/128) | 57.5 (96/167) | 58.1 (72/124) | |
| Male | 55.4 (51/92) | 45.8 (33/72) | 65.4 (53/81) | 51.3 (20/39) | |
| Female | 58.4 (52/89) | 41.1 (23/56) | 50.0 (43/86) | 61.2 (52/85) | |
| P-value† | 0.685 | 0.590 | 0.044 | 0.300 | |
| Remission | |||||
| Overall | 31.4 (22/70) | 37.2 (16/43) | 33.3 (7/21) | ||
| Male | 34.2 (13/38) | 39.1 (9/23) | 20.0 (2/10) | ||
| Female | 28.1 (9/32) | 35.0 (7/20) | 45.5 (5/11) | ||
| P-value† | 0.585 | 0.780 | 0.362 | ||
Calculated using chi-square (χ2) tests to compare proportions across sex. If any cell count was less than 5 observations, Fisher’s exact test was used.
Positive transition (i.e., change in eczema status from ‘no eczema’ to ‘yes eczema’ in two consecutive follow-ups) was highest in males between 4 and 10 years (8.3%) and lowest between 18 and 26 years (4.0%; Table 2). Among females, positive transition was highest between 10 and 18 years (9.4%) and lowest between 1-or-2 and 4 years (5.5%) and 18 and 26 years (5.8%). Positive transition between 10 and 18 years was statistically significantly higher in females compared to males (9.4% vs. 4.3%, p = 0.001, FDR adjusted p = 0.009). Incidence (i.e., the first occurrence of eczema) of eczema decreased over the ages from 6.9% at age 10 to 3.0% at age 26 years. Similar to the finding of positive transition measure, incidence at age 18 years was higher in females than males (8.2% vs. 3.0%, p = 0.002, FDR adjusted p = 0.013), a trend, which partially continued at age 26 years (Table 2).
Negative transition (i.e., change in eczema status from ‘yes eczema’ to ‘no eczema’ in two consecutive follow-ups) was highest in males between 10 and 18 years (65.4%) and highest in females between 18 and 26 years (61.2%; Table 2). On the base of negative transition, males were more likely than females to experience negative transition of eczema between 10 and 18 years (65.4% vs. 50.0%, p = 0.044, FDR adjusted p = 0.187), with no sex differences detected at other negative transition periods. Remission (i.e., outgrowing eczema) was highest in males at age 18 years (39.1%) and highest in females at age 26 years (45.5%; Table 2) without showing statistically significant differences between the sexes.
Developmental trajectories of eczema
Trajectories of eczema development across the first 26 years of age were determined separately for male and female subjects. The optimum number of trajectories was determined based on BIC as shown in Table S1 in this article’s Supporting Information. In males, the development of eczema from infancy to 26 years of age was best represented by four developmental trajectories, labeled as: (1) unaffected/transient (77.7%, n = 541), (2) mid-onset late-resolving (7.8%, n = 54), (3) late-onset (5.2%, n = 36), and (4) early-onset persistent (9.3%, n = 65; Figure 1A). In females, the development of eczema across the first 26 years of life was best represented by five developmental trajectories, labeled as: (1) unaffected/transient (73.0%, n = 500), (2) early-onset early-resolving (7.7%, n = 53), (3) mid-onset late-resolving (4.4%, n = 30), (4) late-onset (9.5%, n = 65), and (5) early-onset persistent (5.4%, n = 37; Figure 1B). The early-onset early-resolving trajectory was identified in females but not in males. Overall, similar proportions made-up the unaffected/transient trajectories in males and females (77.7% vs. 73.0%), but the distribution of the other trajectories were different across sex. For instance, more males than females were in the mid-onset late-resolving trajectory (7.8% vs. 4.4%, p = 0.009) and in the early-onset persistent trajectory (9.3% vs. 5.4%, p = 0.005). Whereas females were more often than males to be in the late-onset trajectory (9.5% vs 5.2%, p = 0.002), which also reflects their higher incidence/positive transition of eczema at age 18 years and the lower negative transition compared to males (Table 2).
Figure 1.
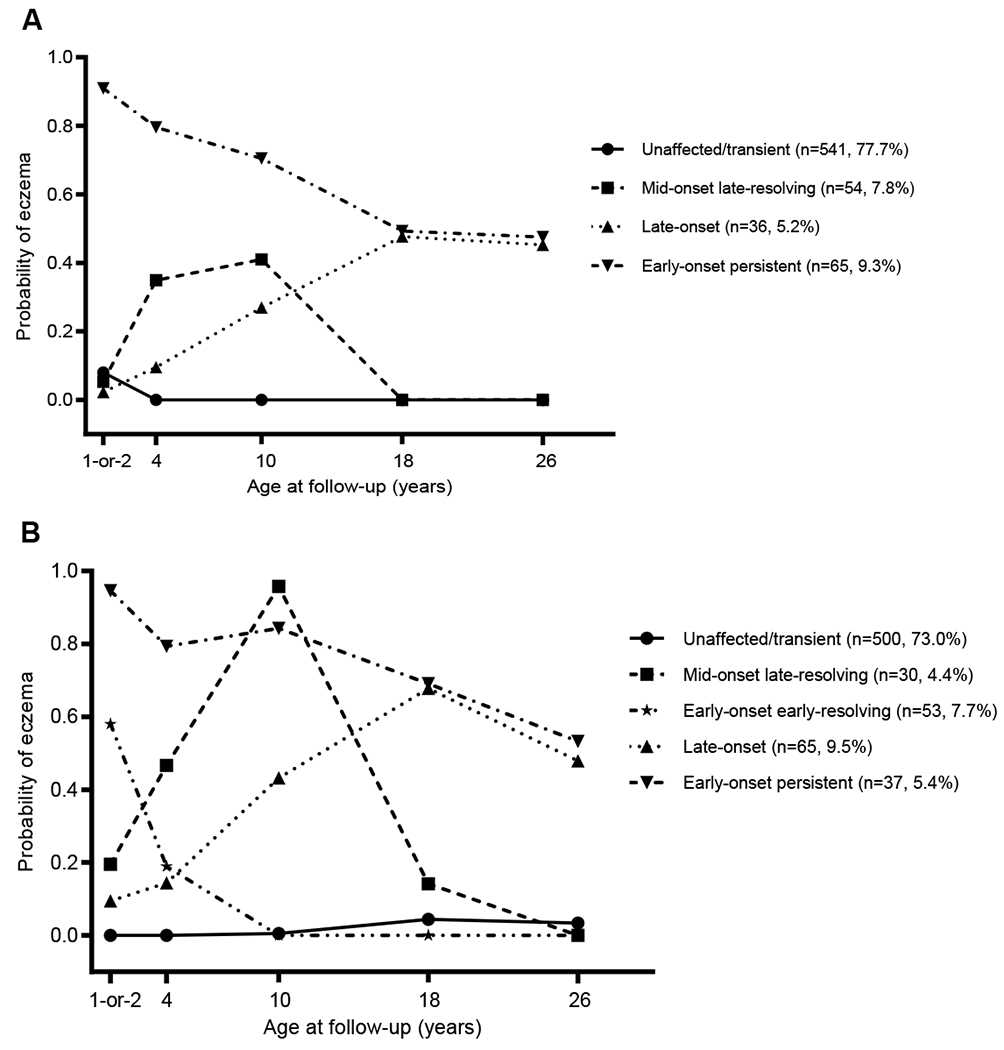
Trajectories of eczema from 1-or-2 to 26 years of age. A) The four trajectories represent the latent growth patterns of eczema from 1-or-2 to 26 years of age in males (n = 696). B) The five trajectories characterize the latent growth patterns of eczema from 1-or-2 to 26 years of age in females (n = 685). The y-axis presents the predicted probability of having eczema among the participants of each trajectory at the different time points.
Prenatal and early life risk factors and trajectories of eczema
Associations between prenatal and early life risk factors and the identified developmental trajectories of eczema were assessed separately for males (Figure 2A, Table S2) and females (Figure 2B, Table S3). Among males, carrying FLG LOF variants was associated with increased risk for being in the early-onset persistent trajectory (aRR = 2.45, 95% CI: 1.34-4.46), so was paternal eczema (2.66, 1.39-5.08). Moreover, FLG LOF showed positive associations with mid-onset late-resolving (1.84, 0.84-4.03) and late-onset 1.97 (0.79-4.94) trajectories, though these associations did not gain statistical significance. Cat-keeping at child’s birth showed an association with the late-onset trajectory (2.79, 1.24-6.27) among male participants (Figure 2A). Among females, carrying FLG LOF variants was not statistically significantly associated with eczema trajectories (Figure 2B); however, indication for possible association was present with mid-onset late-resolving (1.47, 0.54-3.96) and early-onset persistent (1.58, 0.67-3.75) trajectories. Maternal eczema (2.84, 1.42-5.70) and high birthweight (2.25, 1.08-4.69, Figure 2B) were associated with the early-onset persistent trajectory among females.
Figure 2.
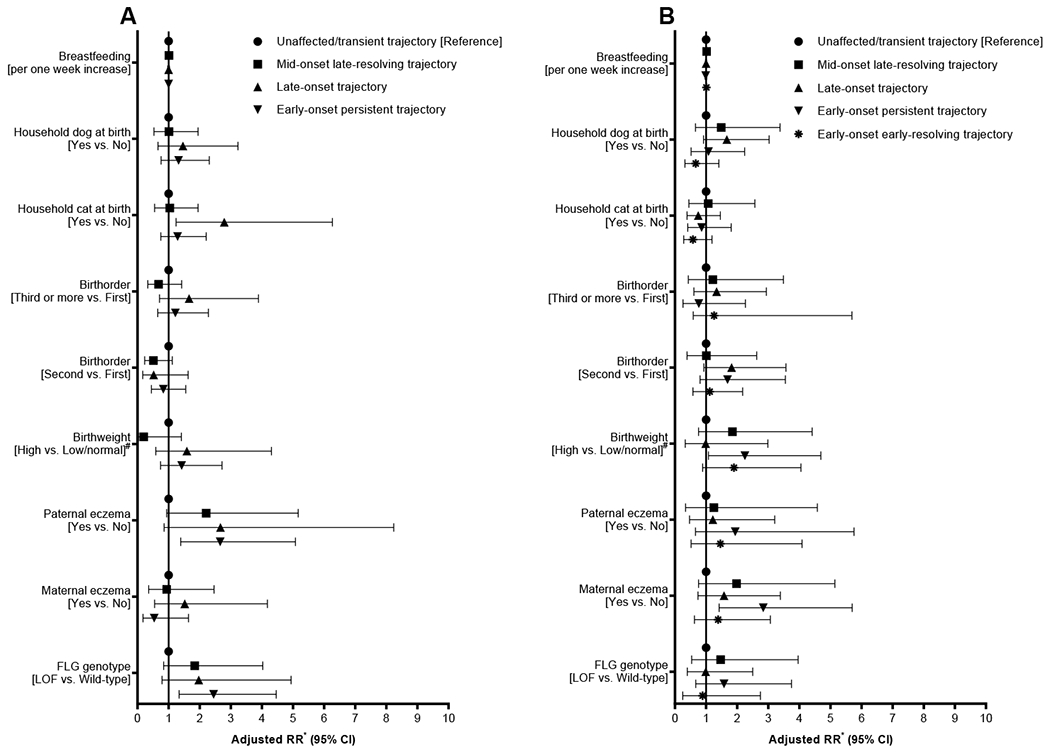
Forest plots showing associations between risk factors and trajectories of eczema among males (A) and females (B). # Low/normal birthweight: <4000 grams, high birthweight: ≥4000 grams; *Adjusted for all variables shown in the figure. LOF: loss-of-function; FLG: filaggrin gene.
DISCUSSION
The current study described the natural course and developmental trajectories of eczema from infancy to early adulthood and examined factors that are associated with eczema development. The developmental trajectories of eczema were best described by four trajectories among males and five trajectories among females. In general, similar developmental patterns of eczema were observed in males and females, with two exceptions. First, an “early-onset early-resolving” trajectory that was only observed among females. Second, more males than females experienced an “early-onset persistent” and “mid-onset late-resolving” developmental trajectories, whereas females were more often than males to be in the “late-onset trajectory.” In terms of risk factors for eczema trajectories, among males, FLG LOF variants and paternal eczema were associated with increased risk for being in the “early-onset persistent” trajectory. In contrast, among females, maternal eczema and high birthweight were associated with higher risk for being in the “early-onset persistent” trajectory. Such results indicate that males and females may experience a different course of eczema and also sex-specific risk factors.
We have estimated different measures of disease occurrence to better understand the natural course of eczema. The 12-month prevalence captured a decreasing trend in the course of eczema in both males and females, with more females than males being affected at ages 18 and 26 years. Such an observation has been reported in the German birth cohort (the ‘Multicenter Allergy Study’ [MAS]) that showed higher eczema prevalence in females than males at ages 15 and 20 years with an overall decreasing trend.44 This female predominance in eczema prevalence that starts in adolescence and persists into adulthood has been described by several prior studies.32,45,46 In a previous investigation, we reported that more females than males developed eczema between ages 10 and 18 years (positive transition), and more males than females outgrew eczema between age 10 and 18 years (negative transition), which can explain the higher prevalence among adolescent females than males.30 Herein, we showed that between ages 18 and 26 years eczema prevalence did not change among males (8.3% vs. 8.5%), but it decreased among females (16.3% vs. 11.6%). This observation can be explained by the fact that more females than males experienced negative transition and remission at 26 years of age than males. Moreover, newly developed eczema (incident cases) was more common among females than males at ages 18 and 26 years; indicating that females are more likely to experience adolescence- and adult-onset eczema than males. This observation contradicts prior findings that showed adult-onset eczema to be similar among the sexes14,47 and support the idea that it is more common among females than males.31 Moreover, we estimated the incidence of eczema to be 3.0% at age 26 years, which is much less than a previously estimated adult-onset eczema of 26.1%.14
Although few prior studies have investigated the longitudinal development of eczema25–27 (summarized in Table S4 in this article’s Supporting Information), our study is the first to extend such trajectories beyond adolescence, to age 26 years. Overall, our identified trajectories were similar in patterns to previously identified trajectories (see Discussion section in this article’s Supporting Information for more details about the prior studies). None of the studies described in this article’s “Supporting Information Discussion section” have considered analyzing the developmental trajectories separately for males and females. Our analysis identified four eczema developmental trajectories among males and five trajectories among females. Although the developmental patterns of four trajectories were similar among both sexes, we identified an ‘early-onset early-resolving’ trajectory only among females. Nonetheless, the distribution of the common trajectories differed according to sex, with more males than females following an early-onset persistent trajectory (9.3% vs. 5.4%) and a mid-onset late-resolving trajectory (7.8% vs. 4.4%). In contrast, more females than males were in the late-onset trajectory (9.5% vs. 5.2%), which may contribute to the observed higher eczema prevalence at ages 18 and 26 among females compared to males (Table 2). It has been suggested previously that sex hormones may contributed to the observed sex dimorphism, with estrogens inducing pro-inflammatory responses and testosterone being immunosuppressive.48,49 Moreover, we identified different risk factors for male and female trajectories. For example, FLG LOF variants were associated with increased risk for being in the early-onset persistent trajectory among males but such an effect was moderately present among females, which did not gain statistical significance due, possibly, to limited statistical power; this potential sex-specific effect of FLG LOF variants on eczema development has not, up to our knowledge, been reported previously. Such findings indicate that genetic risk factors may influence the long-term development of eczema in a sex-specific manner. Moreover, paternal eczema was associated with early-onset persistent trajectory among males, whereas maternal eczema was associated with early-onset persistent trajectory among females. Hence, indicating sex-dependent effects of parental history of allergy on offspring eczema development. Such a mother-to-daughter and father-to-son effects were reported previously for eczema and asthma in this cohort.50 We also observed that high birthweight to be associated with early-onset persistent eczema among females only. Prior studies have shown that elevated birthweight is associated with eczema development in infancy.51,52 Among males, our analysis identified that cat-keeping at birth was associated with increased risk for late-onset eczema trajectory; this observation has been reported previously.53–55
Comparing different studies on eczema trajectories (see Table S4 in this article’s Supporting Information) suggests that about 10-40% of the eczema cases that develop early in life remain persistent (Generation R: 2%/24% = 8.3%; IOWBC males: 9.3%/22.3% = 41.7%); 60-90% are resolving in late childhood, adolescence, or early adulthood. It is likely that a family history of eczema/allergy or FLG LOF variants can help to distinguish children who have a higher risk of a persisting disease. However, future studies are needed to corroborate these findings. Moreover, the current IOW analysis and the prior investigations have consistently identified a “late-onset” trajectory, but potential risk factors for this trajectory remain to be identified. When comparing different studies (see Table S4 in this article’s Supporting Information) it is obvious, that long-term cohort studies on eczema mainly originated in Europe. Such research and data is deficient in the United States and other parts of the world.
The prospective long-term follow-ups that covered childhood, adolescence, and early adulthood and the high retention are major strengths to our study. In addition, analyzing males and females separately provided novel insights into sex-specific inferences. Nonetheless, splitting the sample by sex might have magnified the influence of random variations on the assignment of males and females into the developmental trajectories. The trajectories we have identified are similar to those reported by previous investigation25–27; hence, further strengthening the consistency of our results. Lack of statistical power due to limited subgroup sample sizes was evident in few instances. For example, among males, FLG LOF variants non-statistically significantly increased the risk for “mid-onset late-resolving” and “late-onset” eczema trajectories. A similar pattern of non-statistically significant effects of FLG LOF variants were seen among females, which can be explained by the limited statistical power among the female subsample. Hence, such findings should not be overlooked solely due to the lack of statistical significance. We have used the Hanifin and Rajka36 criteria to define eczema, which has shown high sensitivity (87.9-96.0%) and specificity (77.6-93.8%) as compared with clinical diagnosis as reference standard.56 Nonetheless, a limitation of our eczema definition is not being age-adaptive to capture the evolvement of eczema over age. Moreover, the large gaps between the follow-ups is a further limitation to our estimated measures of disease occurrence, as such measures might miss cases that develop and resolve in-between the follow-ups.
In conclusion, we have described the natural course and developmental trajectories of eczema from infancy to early adulthood. Our results showed that males and females may experience different eczema course and different sets of risk factors. Specifically, FLG LOF variants and paternal eczema were associated with a higher risk of an early-onset persistent trajectory among males, whereas maternal eczema and high birthweight were associated with early-onset persistent trajectory among females. Hence, investigations related to the etiology and long-term development of eczema should be sex-stratified to facilitate stratified medicine efforts. Finally, extension of the prior cohorts and their analyses into adulthood and in non-European countries are needed.
Supplementary Material
Key Messages.
Males and females experience different developmental trajectories of eczema, with sex-specific risk factors.
Males are more likely than females to follow an early-onset persistent eczema trajectory.
Females are more likely than males to developed eczema in adolescence and early adulthood.
Acknowledgments
We would like to acknowledge the help of all the staff at The David Hide Asthma and Allergy Research Centre in undertaking all assessments of 1989 Isle of Wight birth cohort study. We are specifically grateful to the research team including Mr Stephen Potter, Mrs Susan Grevatt, Mrs Gill Glasby, Miss Kaisha Bennett, Mrs Debbie Fraser, Mrs Nicky Tongue, and Mrs. Sharon Matthews. Our sincere thanks to the participants and their families who helped us with this project over the last three decades. The Isle of Wight Birth Cohort assessments have been supported by the National Institutes of Health USA (Grant no. R01 HL082925, R01 AI121226, R01 HL132321), Asthma UK (Grant no. 364) and the David Hide Asthma and Allergy Research Trust. The funding bodies had no role in study design, data collection, analysis, and interpretation of data and decision to publish or preparation of the manuscript.
Footnotes
Ethical Statement
All participants provided informed consent and ethical approval was obtained from the local/national Research Ethics Committee at recruitment and at each assessment. At age 26, ethical approval was granted by National Research Ethics Committee, West Midlands (15/WM/0071).
Conflicts of Interest
The authors declare that they have no relevant conflicts of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–360. [DOI] [PubMed] [Google Scholar]
- 2.Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. [DOI] [PubMed] [Google Scholar]
- 3.Brown SJ. Atopic eczema. Clin Med (Lond). 2016;16(1):66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams HC. Atopic dermatitis. N Engl J Med. 2005;352(22):2314–2324. [DOI] [PubMed] [Google Scholar]
- 5.Peng W, Novak N. Pathogenesis of atopic dermatitis. Clin Exp Allergy. 2015;45(3):566–574. [DOI] [PubMed] [Google Scholar]
- 6.Grobe W, Bieber T, Novak N. Pathophysiology of atopic dermatitis. J Dtsch Dermatol Ges. 2019;17(4):433–440. [DOI] [PubMed] [Google Scholar]
- 7.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–1122. [DOI] [PubMed] [Google Scholar]
- 8.Kage P, Simon JC, Treudler R. Atopic dermatitis and psychosocial comorbidities. J Dtsch Dermatol Ges. 2020;18(2):93–102. [DOI] [PubMed] [Google Scholar]
- 9.Illi S, von Mutius E, Lau S, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113(5):925–931. [DOI] [PubMed] [Google Scholar]
- 10.Garmhausen D, Hagemann T, Bieber T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. 2013;68(4):498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abuabara K, Margolis DJ, Langan SM. The Long-Term Course of Atopic Dermatitis. Dermatol Clin. 2017;35(3):291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irvine AD, Mina-Osorio P. Disease trajectories in childhood atopic dermatitis: an update and practitioner’s guide. Br J Dermatol. 2019;181(5):895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams HC, Strachan DP. The natural history of childhood eczema: observations from the British 1958 birth cohort study. Br J Dermatol. 1998;139(5):834–839. [DOI] [PubMed] [Google Scholar]
- 14.Lee HH, Patel KR, Singam V, et al. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J Am Acad Dermatol. 2019;80(6):1526–1532 e1527. [DOI] [PubMed] [Google Scholar]
- 15.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365(14):1315–1327. [DOI] [PubMed] [Google Scholar]
- 16.Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–446. [DOI] [PubMed] [Google Scholar]
- 17.Bohme M, Soderhall C, Kull I, et al. Filaggrin mutations increase the risk for persistent dry skin and eczema independent of sensitization. J Allergy Clin Immunol. 2012;129(4):1153–1155. [DOI] [PubMed] [Google Scholar]
- 18.Brown SJ, Sandilands A, Zhao Y, et al. Prevalent and low-frequency null mutations in the filaggrin gene are associated with early-onset and persistent atopic eczema. J Invest Dermatol. 2008;128(6):1591–1594. [DOI] [PubMed] [Google Scholar]
- 19.Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130(4):912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abuabara K, Yu AM, Okhovat JP, et al. The prevalence of atopic dermatitis beyond childhood: A systematic review and meta-analysis of longitudinal studies. Allergy. 2018;73(3):696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nissen SP, Kjaer HF, Host A, et al. The natural course of sensitization and allergic diseases from childhood to adulthood. Pediatr Allergy Immunol. 2013;24(6):549–555. [DOI] [PubMed] [Google Scholar]
- 22.Finnbogadottir AF, Ardal B, Eiriksson H, et al. A long-term follow-up of allergic diseases in Iceland. Pediatr Allergy Immunol. 2012;23(2):181–185. [DOI] [PubMed] [Google Scholar]
- 23.Gough H, Grabenhenrich L, Reich A, et al. Allergic multimorbidity of asthma, rhinitis and eczema over 20 years in the German birth cohort MAS. Pediatr Allergy Immunol. 2015;26(5):431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burr ML, Dunstan FD, Hand S, et al. The natural history of eczema from birth to adult life: a cohort study. Br J Dermatol. 2013;168(6):1339–1342. [DOI] [PubMed] [Google Scholar]
- 25.Roduit C, Frei R, Depner M, et al. Phenotypes of Atopic Dermatitis Depending on the Timing of Onset and Progression in Childhood. JAMA Pediatr. 2017;171(7):655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paternoster L, Savenije OEM, Heron J, et al. Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. J Allergy Clin Immunol. 2018;141(3):964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu C, Duijts L, Erler NS, et al. Most associations of early-life environmental exposures and genetic risk factors poorly differentiate between eczema phenotypes: the Generation R Study. Br J Dermatol. 2019;181(6):1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulick AR, Mansfield KE, Silverwood RJ, et al. Four childhood atopic dermatitis subtypes identified from trajectory and severity of disease and internally validated in a large UK birth cohort. Br J Dermatol. 2021;185(3):526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arshad SH, Holloway JW, Karmaus W, et al. Cohort Profile: The Isle Of Wight Whole Population Birth Cohort (IOWBC). Int J Epidemiol. 2018;47(4):1043–1044i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziyab AH, Raza A, Karmaus W, et al. Trends in eczema in the first 18 years of life: results from the Isle of Wight 1989 birth cohort study. Clin Exp Allergy. 2010;40(12):1776–1784. [DOI] [PubMed] [Google Scholar]
- 31.Abuabara K, Ye M, McCulloch CE, et al. Clinical onset of atopic eczema: Results from 2 nationally representative British birth cohorts followed through midlife. J Allergy Clin Immunol. 2019;144(3):710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverberg JI. Atopic Dermatitis in Adults. Med Clin North Am. 2020;104(1):157–176. [DOI] [PubMed] [Google Scholar]
- 33.Arshad SH, Patil V, Mitchell F, et al. Cohort Profile Update: The Isle of Wight Whole Population Birth Cohort (IOWBC). Int J Epidemiol. 2020;49(4):1083–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arshad SH, Hodgekiss C, Holloway JW, et al. Association of asthma and smoking with lung function impairment in adolescence and early adulthood: the Isle of Wight Birth Cohort Study. Eur Respir J. 2020;55(3):1900477. [DOI] [PubMed] [Google Scholar]
- 35.Arshad SH, Karmaus W, Kurukulaaratchy R, et al. Polymorphisms in the interleukin 13 and GATA binding protein 3 genes and the development of eczema during childhood. Br J Dermatol. 2008;158(6):1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980;92(44–47. [Google Scholar]
- 37.Ziyab AH, Karmaus W, Yousefi M, et al. Interplay of filaggrin loss-of-function variants, allergic sensitization, and eczema in a longitudinal study covering infancy to 18 years of age. PLoS One. 2012;7(3):e32721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soto-Ramirez N, Ziyab AH, Karmaus W, et al. Epidemiologic methods of assessing asthma and wheezing episodes in longitudinal studies: measures of change and stability. J Epidemiol. 2013;23(6):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porta MS, Greenland S, Hernán M, et al. , A dictionary of epidemiology. Six edition / Edn. Oxford: Oxford University Press, 2014. [Google Scholar]
- 40.Jones BL, Nagin DS, Roeder K. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociological Methods & Research. 2001;29(3):374–393. [Google Scholar]
- 41.Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychological Methods. 1999;4(2):139–157. [DOI] [PubMed] [Google Scholar]
- 42.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 43.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 44.Lau S, Matricardi PM, Wahn U, et al. Allergy and atopy from infancy to adulthood: Messages from the German birth cohort MAS. Ann Allergy Asthma Immunol. 2019;122(1):25–32. [DOI] [PubMed] [Google Scholar]
- 45.Sacotte R, Silverberg JI. Epidemiology of adult atopic dermatitis. Clin Dermatol. 2018;36(5):595–605. [DOI] [PubMed] [Google Scholar]
- 46.Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy. 2018;73(6):1284–1293. [DOI] [PubMed] [Google Scholar]
- 47.Silverberg JI, Vakharia PP, Chopra R, et al. Phenotypical Differences of Childhood- and Adult-Onset Atopic Dermatitis. J Allergy Clin Immunol Pract. 2018;6(4):1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ridolo E, Incorvaia C, Martignago I, et al. Sex in Respiratory and Skin Allergies. Clin Rev Allergy Immunol. 2019;56(3):322–332. [DOI] [PubMed] [Google Scholar]
- 49.Klein SL. Immune cells have sex and so should journal articles. Endocrinology. 2012;153(6):2544–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arshad SH, Karmaus W, Raza A, et al. The effect of parental allergy on childhood allergic diseases depends on the sex of the child. J Allergy Clin Immunol. 2012;130(2):427–434 e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egeberg A, Andersen YM, Gislason G, et al. Neonatal risk factors of atopic dermatitis in Denmark - Results from a nationwide register-based study. Pediatr Allergy Immunol. 2016;27(4):368–374. [DOI] [PubMed] [Google Scholar]
- 52.Kerkhof M, Koopman LP, van Strien RT, et al. Risk factors for atopic dermatitis in infants at high risk of allergy: the PIAMA study. Clin Exp Allergy. 2003;33(10):1336–1341. [DOI] [PubMed] [Google Scholar]
- 53.Brunekreef B, Von Mutius E, Wong G, et al. Exposure to cats and dogs, and symptoms of asthma, rhinoconjunctivitis, and eczema. Epidemiology. 2012;23(5):742–750. [DOI] [PubMed] [Google Scholar]
- 54.AlShatti KA, Ziyab AH. Pet-Keeping in Relation to Asthma, Rhinitis, and Eczema Symptoms Among Adolescents in Kuwait: A Cross-Sectional Study. Front Pediatr. 2020;8(331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rutter CE, Silverwood RJ, Asher MI, et al. Comparison of individual-level and population-level risk factors for rhinoconjunctivitis, asthma, and eczema in the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. World Allergy Organ J. 2020;13(6):100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brenninkmeijer EE, Schram ME, Leeflang MM, et al. Diagnostic criteria for atopic dermatitis: a systematic review. Br J Dermatol. 2008;158(4):754–765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


