Abstract
Forty years into the HIV pandemic, approximately 50% of infected individuals still suffer from a constellation of neurological disorders collectively known as ‘neuroHIV.’ Although combination antiretroviral therapy (cART) has been a tremendous success, in its present form, it cannot eradicate HIV. Reservoirs of virus reside within the central nervous system, serving as sources of HIV virotoxins that damage mitochondria and promote neurotoxicity. Although understudied, there is evidence that HIV or the HIV regulatory protein, trans‐activator of transcription (Tat), can dysregulate neurosteroid formation potentially contributing to endocrine dysfunction. People living with HIV commonly suffer from endocrine disorders, including hypercortisolemia accompanied by paradoxical adrenal insufficiency upon stress. Age‐related comorbidities often onset sooner and with greater magnitude among people living with HIV and are commonly accompanied by hypogonadism. In the post‐cART era, these derangements of the hypothalamic‐pituitary‐adrenal and ‐gonadal axes are secondary (i.e., relegated to the brain) and indicative of neuroendocrine dysfunction. We review the clinical and preclinical evidence for neuroendocrine dysfunction in HIV, the capacity for hormone therapeutics to play an ameliorative role and the future steroid‐based therapeutics that may have efficacy as novel adjunctives to cART.
Keywords: HIV‐associated neurocognitive disorder, hypothalamic‐pituitary‐adrenal axis, hypothalamic‐pituitary‐gonadal axis, mitochondria, neurosteroids
The HIV regulatory protein, trans‐activator of transcription (Tat), dysregulates steroidogensis and promotes dysfunction of the hypothalamic‐pituitary‐adrenal stress axis in rodent models, recapitulating clinical HIV endophenotypes. Neuroactive steroids, such as allopregnanolone, ameliorate several Tat‐mediated deficits in cell culture and behavioral impairments in rodents.
1. INTRODUCTION
It has been 40 years since the beginning of the HIV pandemic and an estimated 37.6 million people continue to live with the virus worldwide. 1 The tremendous success of combination antiretroviral therapy (cART) has increased the life expectancies of people living with HIV (PLWH) and has greatly improved morbidity. However, the transformation of HIV to a chronic care illness has also revealed the long‐term consequences of living with infection. Even in the post‐cART era, many individuals continue to suffer from neurological symptoms including an increased prevalence of cognitive impairment, major depression, generalized anxiety disorder, neuropathic pain and motor dysfunction, collectively referred to as “neuroHIV”. 2 Identifying and treating the mechanisms of these neurological deficits is the subject of intense investigation. To this end, the influence of neuroHIV on the neuroendocrine system has become increasingly apparent. In this review, we summarize the recent advances made in our understanding of HIV effects on neuroendocrine function and the potential benefits of steroid‐based therapeutics, including the progesterone metabolite, 5α‐pregnan‐3α‐ol‐20‐one (3α,5α‐THP or allopregnanolone), on neuroHIV and HIV viremia. Taken together, the data support a reciprocal relationship between HIV and neuroendocrine status, in which HIV dysregulates the neuroendocrine axes, facilitating stress‐ and age‐related comorbidities that neuroendocrine‐based therapeutics may help ameliorate.
2. HIV‐MEDIATED NEUROLOGICAL DYSFUNCTION
In the post‐cART era, approximately 50% of PLWH suffer from neuroHIV. 3 Although cART has reduced the incidence of the most severe form of neurocognitive impairment, HIV‐associated dementia (present in ~20% of HIV patients in the pre‐cART era vs. ~2% in the post‐cART era), 3 the proportion of individuals that continue to express any cognitive impairment has remained stable, albeit the symptoms are markedly milder.
2.1. The HIV central nervous system reservoir
HIV enters the central nervous system (CNS) early in infection, likely by the crossing of infected monocytes and monocyte‐derived macrophages through the blood–brain barrier. 4 Within the CNS, microglia (the macrophages of the brain) are thought to be the first cells to contact the virus. Microglia and astrocytes largely comprise the central HIV reservoir (albeit, astrocytes do not infect productively), capable of harboring proviral HIV in a latent state. 5 , 6 There is a paucity of therapeutic treatments for HIV within the CNS. cART does not accumulate well within this compartment, which may partly be a result of active efflux 7 and its accumulation in endothelial cells, 8 nor can cART target the latent reservoirs that harbor virus. 9 , 10 As such, a functional cure for HIV is priority.
2.2. Mechanisms of neuroHIV
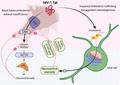
There are multiple mechanisms by which HIV can promote CNS damage and dysfunction (Figure 1). 4 , 11 , 12 , 13 In brief, neuronal damage is largely promoted by both indirect inflammatory mediators and by direct excitotoxic challenges from HIV proteins. The most well‐characterized of these virotoxic proteins are the trans‐activator of transcription (Tat) and glycoprotein (gp120). Tat is an important regulatory protein that drives HIV transcription. It is present in post‐mortem HIV+ brain tissues 14 , 15 and in the cerebrospinal fluid of HIV+ patients, even when virally‐suppressed, 16 , 17 , 18 , 19 supporting its presence within the CNS despite cART treatment. Tat can produce neurotoxicity through a variety of mechanisms including the direct activation of L‐type calcium channels, 20 , 21 direct or low density lipoprotein receptor‐related protein 1‐mediated activation of NMDA receptors, 22 , 23 , 24 and imbalance of intracellular sodium and potassium. 25 , 26 Tat‐mediated cellular pathogenesis is also demonstrated to involve many additional divalent cations beyond Ca2+, 27 as well as via the activation of pro‐apoptotic activator protein 1 and nuclear factor‐kappa B (NF‐κB) signaling, thereby promoting downstream inflammatory cytokine production. 13 These effects may occur alone or in synergy with other HIV proteins including gp120. As a non‐covalently bound glycoprotein comprising the outer HIV envelope, gp120 can be shed to act at chemokine receptors (largely CXCR4 or CCR5, among other HIV co‐receptors), thereby promoting intracellular Ca2+ accumulation and neuroinflammatory cytokine release. 28 , 29 Of great importance when considering HIV virotoxicity in light of endocrine function, Tat, gp120 and additional HIV proteins (Nef, viral protein R) can alter mitochondrial dynamics, biogenesis and membrane potential, as well as glycolytic pathways and ATP production, to promote oxidative stress, mitophagy and apoptosis. 30 , 31 , 32 Thus, there are multiple pathways by which HIV virotoxic proteins can exert neuronal damage or death.
FIGURE 1.
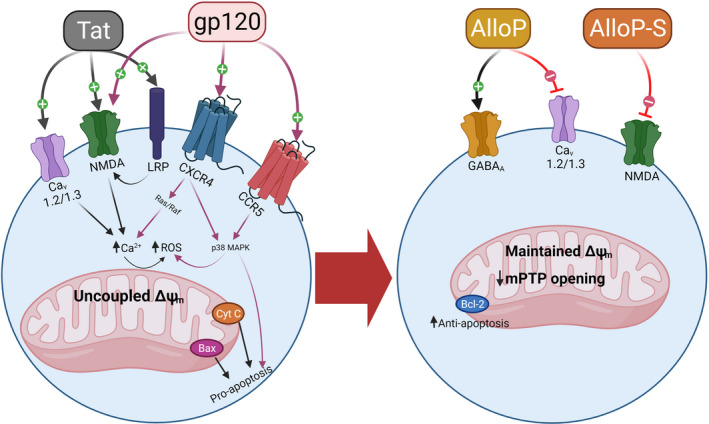
Mechanisms of direct neuronal damage for the HIV proteins, trans‐activator of transcription (Tat) and glycoprotein 120 (gp120). HIV proteins can directly drive intracellular Ca2+ (partly by activation of NMDA receptors, L‐type Ca2+ channels or activation of chemokine receptor‐mediated signaling) or can indirectly activate NMDA receptors via activation of low density lipoprotein receptor‐related protein (LRP). The downstream effects of HIV proteins dysregulate mitochondrial membrane potential, drive the formation of reactive oxygen species (ROS), and promote cell injury and death (left). Allopregnanolone is a potent positive allosteric modulator of GABAA receptors that can antagonize L‐type Ca2+ channels and restore mitochondrial homeostasis, potentially off‐setting the excitotoxic actions of HIV proteins. Allopregnanolone‐sulfate is an antagonist of NMDA receptors (right)
In addition to the use of infectious animal models, the functional effects of single or multiple HIV proteins to promote a neuroHIV‐like phenotype have been elucidated via the use of transgenic rodent models. Understanding the singular or synergistic effects of HIV proteins to promote neuroHIV pathology is an important step in identifying therapeutic targets and testing novel therapeutic strategies. To this end, a Tet‐on transgenic mouse model has been widely used to conditionally‐express Tat from astrocytic sources. Using Tat‐transgenic mice, the functional effects of Tat have been identified to be sufficient to produce cognitive impairment on spatial learning tasks, 33 , 34 , 35 executive learning tasks 34 , 36 , 37 and fear conditioning, 38 as well as to impair sensorimotor gating. 39 Tat is also sufficient to promote affective‐like disturbances in mice, increasing anxiety‐ and depression‐like behavior. 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 Both of these affective phenotypes were associated with increased reactive oxygen species in the CNS of mice 42 , 45 which correlated with anxiety‐like behavior. 45 Peripheral neuropathy is also indicated in this model with allodynia observed in response to mechanical stimuli 49 , 50 and CNS increases in cytokine production. 51 , 52 Notably, sex differences are seen with females demonstrating antinociceptive thresholds that are intractable to morphine. 53 Conversely, greater durations of Tat exposure impair learned motor performance and grip strength among males, but not females, in this model. 54 These findings implicate Tat as an important therapeutic target within the HIV genome.
Although there are limitations to the use of transgenic models, they are an improvement over direct pharmacological manipulations. In particular, accurate i.c.v. infusion of Tat is challenging given that it is a highly basic peptide (pI = ~9 at physiological pH) that readily adsorbs to surfaces, including glass. Nonetheless, i.c.v. infusion of Tat is found to increase depression‐like behavior of Balb/c or C57BL/6J mice. 55 Despite positive findings, this approach suffers from additional drawbacks including the acute nature of the i.c.v. bolus which is unlikely to mimic the virotoxin exposure associated with HIV and the disruption of the blood–brain barrier which produces additional neuroinflammatory confounds.
Constitutive expression of an astrocytic gp120 transgene has also been observed to impair spatial cognitive performance 56 , 57 and increase anxiety‐like behavior. 58 Similarly, transgenic gp120 expression or intrathecal infusion recapitulates peripheral neuropathy in rodents 59 , 60 with mechanical allodynia and cold sensitivity found to be greater in female mice. 61 These data support findings in HIV transgenic rats (which express all HIV proteins with the exception of Gag and Pol) wherein cognitive deficits and increased anxiety‐ and depression‐like behavior have been reported. 62 , 63 , 64 Thus, secreted HIV proteins are likely contributors to the etiology of neuroHIV and may exert separate or interacting effects.
3. STEROIDOGENIC DYSREGULATION BY HIV
The endocrine system has long been known to be perturbed by HIV infection with patients presenting with dysfunction of the adrenals, gonads, pituitary and thyroid. 65 However, it is becoming apparent that the interactions between HIV and the endocrine system are dynamic. Endogenous steroids influence HIV replication and neuropathology and, conversely, HIV virotoxins can influence steroid formation. As such, the relationship between neuroendocrine function and neuroHIV symptomatology is reciprocal.
3.1. Steroid hormones modulate HIV replication
Gonadal steroids have long been proposed to be ameliorative to HIV viremia. 17β‐estradiol attenuates HIV replication in cultured peripheral blood mononuclear cells (PBMCs) or human fetal astrocytes in an estrogen receptor α‐dependent manner 66 , 67 and inhibits HIV infection of CD4+ T‐cells or monocyte‐derived macrophages (MDMs). 68 Moreover, estradiol was observed to decrease Tat‐driven activation of the HIV long terminal repeat (LTR; essential for efficient HIV replication), but exerted no effects on basal HIV LTR activity, 69 supporting the notion that these effects were relegated to a direct inhibition of Tat peptide or of the Tat‐trans‐activation response element (TAR) complex within the LTR. However, large variability in the capacity for estrogens to alter HIV replication has been observed across donors, HIV clades 70 and the menstrual cycle. 71 Similarly, progesterone has been reported to improve antiretroviral potency in cell culture, 72 as well as to attenuate HIV replication at high concentrations in PBMCs, 73 monocytes and MDMs. 74 These effects may be concentration‐dependent with low concentrations of estradiol or progesterone being permissive of HIV replication in cultured MDMs, and greater concentrations attenuating HIV replication. 75
3.2. Steroid hormones ameliorate HIV‐related neuropathology
Given the critical role that Tat plays in HIV replication and its potent neurotoxic effects, it has been recognized as an important therapeutic target, particularly given that current cART does not target Tat. Several studies report that estradiol ameliorates Tat and/or gp120‐induced oxidative stress in human neuroblastoma cells or rat striatal synaptosomes, 76 as well as Tat‐induced expression of pro‐apoptotic Bax, caspases related to mitochondrial‐driven apoptosis and cell death in human fetal neurons 77 , 78 or neuroblastoma cells. 46 Estradiol was also observed to attenuate Tat‐induced activation of a microglial cell line and the subsequent release of proinflammatory cytokines in these 79 and human endothelial cells. 80 Progesterone also exerts protection against Tat‐mediated cell death in human fetal neurons 78 or neuroblastoma cells, 46 albeit to a lesser extent than estradiol. Rather, we have observed greater neuroprotective capacity exerted by the 3α‐hydroxy/5α‐reduced metabolite of progesterone, allopregnanolone (AlloP). We have observed AlloP (up to 100 nm) to partially attenuate microgliosis and neuronal or microglial influx of intracellular Ca2+, to fully‐attenuate Tat‐mediated depolarization of mitochondrial membranes and to protect human neuroblastoma cells or primary murine neuron/glial co‐cultures against Tat‐mediated cell death (Figure 2). 45 , 81 It is likely that steroid hormones also exert beneficial effects over Tat‐mediated insults given their pleiotropic capacity to attenuate cytokine production. In particular, AlloP is recently observed to attenuate toll‐like receptor signal activation. 82 Thus, estradiol and AlloP exert marked protection against several mechanisms of Tat‐mediated cellular dysfunction and death.
FIGURE 2.
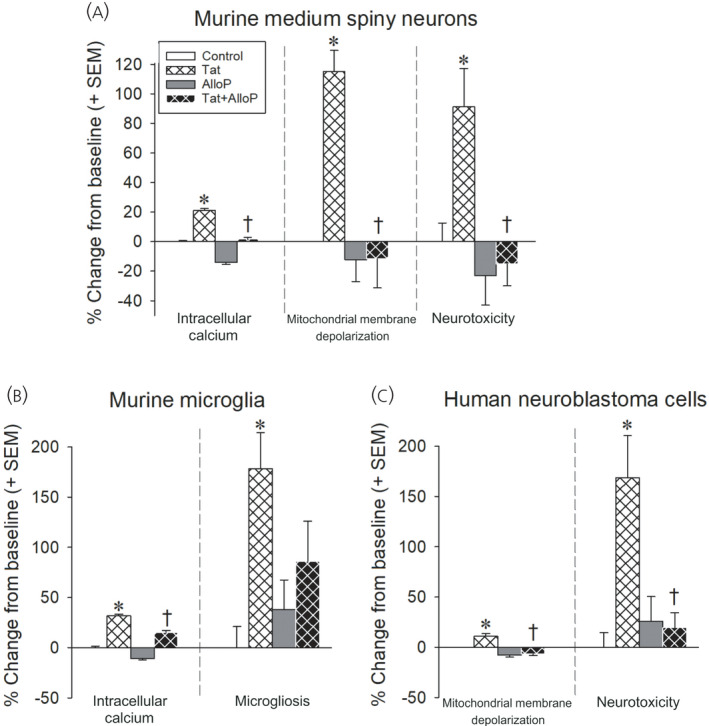
HIV Tat (50–100 nm) increases intracellular calcium, depolarizes mitochondria, and promotes microgliosis and/or neurotoxicity in murine striatal medium spiny neurons (A), murine microglia (B) or differentiated human SH‐SY5Y neuroblastoma cells (C). Pretreatment with allopregnanolone (AlloP; 100 nm) attenuates Tat‐mediated effects. *Significant increase from control following Tat exposure. †Significant AlloP‐mediated rescue from Tat exposure
3.3. Pregnane steroids ameliorate HIV Tat‐induced behavioral pathology
The functional effects of pregnane steroids to ameliorate neuroHIV‐like behavior have been demonstrated using Tat‐transgenic mice. High dose progesterone (4 mg kg–1 per day for 7 days) 44 or a physiological progesterone schedule (4 mg kg–1 once every 5 days for 15 days) 45 rescued the anxiety‐like profile induced by Tat expression in ovariectomized mice. However, the protective effects of progesterone were attenuated when the 5α‐reductase inhibitor, finasteride (50 mg kg–1), was co‐administered, implicating metabolism to AlloP as underlying these beneficial effects. 45 We later observed Tat expression to potentiate the psychomotor effects of opioids and found AlloP to dose‐dependently attenuate this. 81 Notably, estradiol did not significantly improve anxiety‐like performance following Tat exposure in our hands and rather antagonized the beneficial effects of progesterone when co‐administered. 44 However, these studies utilized high, pharmacological estradiol dosing and should be reassessed with physiological concentrations. Thus, AlloP ameliorates neuroHIV‐like behavior in a mouse model, consistent with its anti‐Tat activities observed in vitro.
3.4. HIV disrupts central and circulating steroidogenesis
Given the mitotoxic capacity of HIV virotoxins (Tat, gp120, Nef, viral protein R), it is perhaps not surprising that HIV infection can be associated with perturbations in CNS steroid formation. Tat is also found to alter homeostasis and bioavailability of the steroid precursor, cholesterol (Figure 3), 83 , 84 and to upregulate ceramides that can inhibit steroid‐synthesizing CYP enzymes. 85 However, this has been the subject of surprisingly little investigation. Immunohistochemical observations of post‐mortem brains from PLWH indicated a reduction in P450scc, 5α‐reductase and 3α‐hydroxysteroid dehydrogenase compared to seronegative controls. 86 These findings are intriguing and were supported by parallel studies in human fetal astrocytes that revealed downregulated protein expression of 5α‐reductase and 3α‐hydroxysteroid dehydrogenase following exposure to HIV‐infected supernatants. 86 Changes in neuroactive steroid formation may be particularly important for neuroHIV status. A recent investigation profiled circulating neuroactive steroids in 99 PLWH and found that eight steroids were downregulated in those individuals with high depressive symptoms, including pregnenolone sulfate, dehydroepiandrosterone‐sulfate (DHEA‐S) and 5α‐androstane‐3β,17β‐diol monosulfate. 87 However, a comprehensive profile of CNS steroids in human patients is lacking.
FIGURE 3.
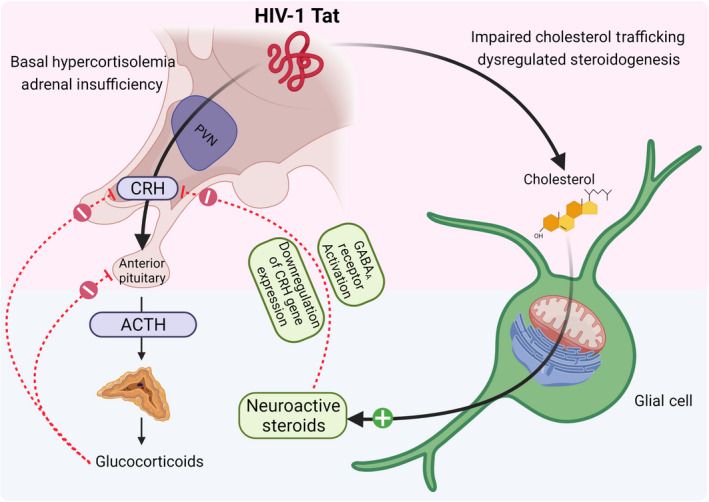
Expression of HIV Tat protein in mice increases basal corticotropin releasing hormone (CRH) and corticosterone. Upon stress, male (but not female) mice demonstrate adrenal insufficiency. The effects of Tat to dysregulate cholesterol homeostasis and alter steroidogenesis may contribute to the dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis. In normative systems, neuroactive steroids such as allopregnanolone restore HPA axis homeostasis, downregulating CRH, adrenocorticotropic hormone (ACTH) and glucocorticoid production
We have begun to address these questions using a mouse model of neuroHIV. We assessed a panel of 23 pregnane steroids in male HIV Tat‐transgenic mice and unexpectedly found Tat to upregulate CNS pregnenolone, AlloP and its 3β isomer (3β,5α‐THP), as well as their 20α‐hydroxylated metabolites. 81 The only steroid found to be downregulated was deoxycorticosterone and no differences were observed in plasma, supporting the notion that Tat may influence neurosteroidogenesis. 81 Although we had anticipated neurosteroidogenesis to be reduced given the clinical observations in post‐mortem brains, the upregulation of 5α‐reduced CNS steroids observed is consistent with findings in models of traumatic brain injury and ischemic stroke, which may indicate a neuroadaptive response to challenge. 88 , 89 , 90 It is also important to note that the HIV+ post‐mortem brains assessed had HIV encephalitis and were compared with seronegative brains of individuals who suffered from stroke, sepsis and leukemia, which may have promoted a high neurosteroid baseline in the control group. Irrespective of changes within the CNS, the capacity for HIV to disrupt circulating steroids is well‐documented in the post‐cART era and may contribute to HIV comorbidities.
4. HIV EFFECTS ON HYPOTHALAMIC‐PITUITARY‐ADRENAL AND ‐GONADAL AXES
HIV can exert profound influence on circulating steroid hormone production. These effects can reduce circulating steroid content via actions at endocrine glands, such as the adrenals or gonads (i.e. primary insufficiency), or via actions targeted to the source of steroid‐promoting corticotropins and gonadotropins in the hypothalamus and anterior pituitary (i.e. secondary insufficiency). The latter is far more common in the post‐cART era, emphasizing the need for HIV therapeutics with efficacy in the CNS. As a result, PLWH are commonly affected by disruptions to the hypothalamic‐pituitary‐adrenal (HPA) and –gonadal (HPG) axes. 91 , 92 Such dysfunction likely contributes to the neuropsychiatric components of neuroHIV and the age‐related comorbidities that are observed earlier in life and to a greater magnitude among PLWH.
4.1. HPA stress axis dysregulation in people living with HIV
In the pre‐cART era, HIV‐related pathogenesis of the HPA axis was largely primary and associated with direct invasion of opportunistic infections, neoplasms or infiltrative diseases that promoted adrenal atrophy. 65 In the post‐cART era, HPA axis dysfunction is considered to be secondary (mediated at the level of the hypothalamus or pituitary). 93 Estimates vary, but approximately 14–46% of HIV patients demonstrate HPA axis dysfunction. 94 , 95 , 96 , 97 , 98 , 99 This is characterized by elevated basal serum cortisol levels (hypercortisolemia), yet a paradoxical adrenal insufficiency when exposed to a stressor (Figure 3). 95 PLWH already experience a variety of stigma‐related psychosocial stressors that reduce medication adherence, increase the difficulty of receiving a diagnosis and treatment, reduce accessibility to financial aid programs and exacerbate HIV‐related psychological symptomatology. 100 , 101 , 102 , 103 , 104 Although the focus of the current review is on the molecular mechanisms of neuroHIV, it should be appreciated that a number of socioeconomic and environmental stressors may also contribute. Given the importance of glucocorticoids in re‐establishing organismal homeostasis following a stressor, the incapacity to mount a sufficient response further predisposes an individual to a plethora of psychological and physiological disorders. 105
4.2. Potential mechanisms involved in HIV dysregulation of the HPA axis
Hypercortisolemia is observed in many PLWH during both early and late stages of HIV infection. 106 , 107 Potential mechanisms may include a chronic enzymatic shift in the production of adrenal androgens to cortisol, 108 a compensatory increase in response to the decline of other steroids, such as DHEA, 87 , 109 a compensatory response to elevated corticosteroid‐binding globulin as has been noted in the transition to AIDS 110 and/or a reduction in the sensitivity of glucocorticoid receptors in response to their cognate ligands. 111 In support of the latter point, elevated basal glucocorticoid receptor (GR) density has been observed, 111 which may be the product of a glucocorticoid resistant state. Increases in proinflammatory cytokines are found to promote a shift in the GRα to GRβ ratio (GRβ is a dominant negative inhibitor of the bioactive GRα), thus reducing GR mediated inhibition of the negative feedback loop. 112 Moreover, PBMCs collected from women living with HIV demonstrated increased gene expression of FKBP5, a negative regulator of the gene encoding GR, compared to seronegative PBMCs. 113 The phenotypical adrenal insufficiency that is concurrently observed may be indicative of a depletion in the “adrenal reserve” and/or a consequence of the mitotoxic effects promoted by HIV proteins.
There are several virotoxic proteins secreted by HIV‐infected cells that may be involved in HPA dysregulation. Viral protein R is an HIV accessory protein that serves important regulatory functions including viral incorporation, transcription and nuclear translocation of the HIV complex. 114 , 115 However, viral protein R can also act as a co‐activator of the GR and potentiate glucocorticoid actions, perhaps promoting a glucocorticoid resistant state. 116 , 117 In addition, the HIV envelope protein, gp120, is seen to increase plasma adrenocorticotropic hormone (ACTH) and corticosterone levels, with an analogous increase in pituitary ACTH content, when expressed in a transgenic mouse model. 118 Furthermore, gp120 increases CRH mRNA expression and CRH release in ex vivo mouse or rat hypothalamic explants. 119 , 120 Tat may also act in concert with these virotoxins to influence HPA function and has apparent effects to recapitulate the clinical phenotype when expressed in mice.
As described, Tat‐transgenic mice demonstrate several behavioral aspects of neuroHIV (e.g., impairments in cognition and sensorimotor gating, increased anxiety‐ and depression‐like behavior). Our group has assessed HPA function in these mice and found Tat expression to be sufficient in recapitulating the clinical endophenotype. Compared to controls, Tat‐expressing male or female mice demonstrate basal hypercortisolemia (Figure 4). 46 , 47 , 48 We have also observed increased hypothalamic CRF protein expression in females (males have not been assessed). 46 Of interest, males also demonstrate adrenal insufficiency in response to a stressor (15‐min forced swim) or when adrenal corticosterone production is stimulated by pharmacologically‐blocking GRs (Figure 4A); however, females appear to be protected from these effects (Figure 4B). 48 Although the mechanisms by which Tat may induce hypercortisolemia or a sex‐specific adrenal insufficiency are not yet understood, several avenues have potential. Expressing Tat protein in this model promotes the production of cytokines within the CNS. 51 , 52 Several second messengers and transcription factors associated with cytokine production, such as signal transducer and activator of transcription (STAT) 5, p38 mitogen‐activated protein kinase (MAPK) and NF‐κB, inhibit GR signaling. 121 Interleukin (IL)‐1α, IL‐2, IL‐4, or STAT5 phosphorylation are reported to inhibit nuclear translocation of the GR. 122 , 123 , 124 In particular, IL‐2 and IL‐4 can phosphorylate the GR via p38 MAPK signaling, thereby reducing affinity for its cognate ligands. 125 , 126 Together, these actions may contribute to a GR‐insensitive state. In support, we have found Tat to induce glucocorticoid resistance in primary mouse splenocytes in vitro. 81 Thus, Tat, gp120 and/or viral protein R may act alone or in concert to promote glucocorticoid resistance. Given the importance of the stress response in overcoming psychological and physiological challenges, disruption of the HPA axis may be an important contributor to the neuroHIV‐like phenotype observed.
FIGURE 4.
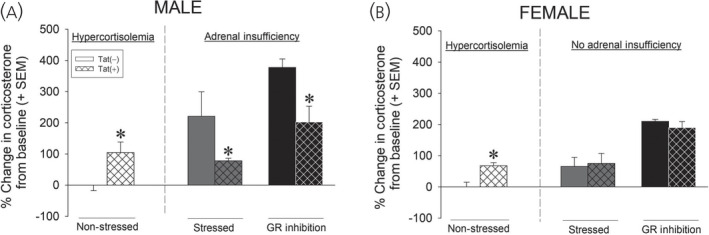
Compared to Tat(−) controls, male Tat(+) mice demonstrate greater corticosterone at baseline with paradoxical adrenal insufficiency in response to a stressor (15‐min forced swim) or pharmacological inhibition of the glucocorticoid receptor (GR) via RU‐486 (A). Compared to controls, female Tat(+) mice also demonstrate increased corticosterone at baseline; however, no differences are observed following a 15‐min forced swim stress or administration of RU‐486 (B). *Significant difference from Tat(−) control 46 , 47 , 48
4.3. HPG stress axis dysregulation in people living with HIV
Consistent with central neuroendocrine dysfunction in the post‐cART era, approximately 10–50% of PLWH present with hypogonadism (defined by low testosterone levels in men and dysregulated estradiol/progestogen levels in women). 127 , 128 , 129 , 130 , 131 The consequences of this phenotype are particularly evident among PLWH that are ≥ 50 years of age, which now comprise the majority of all HIV cases in the US. 132 People living with HIV experience an early occurrence of age‐related complications, including frailty, cardiovascular diseases, renal diseases that are co‐morbid with diabetes and hypertension, cognitive deficits, endocrine disorders, and bone diseases including osteoporosis. 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 Animal models of some of these disorders, such as diabetes, are associated with a downregulation of steroid‐synthesizing enzymes and dysregulated formation of androstane/pregnane steroids, including AlloP. 144 , 145 , 146 , 147 Both men and women living with HIV experience an earlier onset of the climacteric, concurrent with hormonal deficiencies. As such, early hormone therapeutic intervention may provide a particularly salient benefit to this population.
In men living with HIV, modern‐day hypogonadism is mainly secondary and occurs in both young or middle‐aged cART‐treated men (approximately 12–28%). 127 , 130 , 148 , 149 , 150 The incidence of hypogonadism increases with age, HIV duration and lower CD4+ T‐cell counts. 150 , 151 , 152 , 153 Men living with HIV experience a premature transition to andropause associated with a lower level of circulating testosterone, 127 , 131 , 154 , 155 , 156 , 157 , 158 normal or low levels of luteinizing hormone, 127 , 131 , 159 a greater level of sex hormone binding globulin 150 , 155 , 160 and a greater estradiol‐to‐testosterone ratio. 159 Androgen deficiency increases the risk for central fat accumulation, cardiovascular diseases and frailty among HIV‐infected men. 155 , 161
Similarly, women living with HIV display accelerated ovarian aging (transition to peri‐ and post‐menopause) sooner than seronegative women, 162 , 163 , 164 lower circulating androgens 165 , 166 and 17β‐estradiol, 165 and a greater frequency of vasomotor/climacteric symptoms, including hot flashes, depression/anxiety and bone degeneration. 164 , 167 , 168 , 169 , 170 , 171 , 172 , 173 Hormonal replacement therapy may be particularly effective in attenuating these HIV‐associated co‐morbidities. Taken together, the aging HIV+ population faces a myriad of premature and/or accentuated co‐morbidities that are likely to influence the pathogenesis of neuroHIV and age‐related complications. 139 , 142 , 174
4.4. Potential mechanisms involved in HIV dysregulation of the HPG axis
Although the mechanisms of HIV‐mediated neuroendocrine dysfunction are not known, animal models have begun to provide some insights. Recent work has established a link between HIV Tat expression and some age‐related comorbidities. Long‐term Tat expression in middle‐aged mice impaired both short‐ and long‐term memory of males, although only short‐term memory of female mice; motor coordination and balance were impaired in both sexes. 175 Nuanced sex differences in neuropathology were observed, with Tat inducing greater pre‐ and post‐synaptic marker density in the female cortex and lower pre‐synaptic marker density in the cerebellum of males. 175 In addition, global DNA methylation was greater in Tat‐exposed females. 175 These plastic and epigenetic changes may occur in response to CNS challenge. Magnetic resonance imaging reveals increased ventricular volume and decreased motor cortex gray matter volume in Tat‐exposed middle‐aged male mice, concurrent with astrogliosis and elevated proinflammatory cytokines. 85 When aged males and females were directly compared, magnetic resonance spectroscopy revealed Tat exposure to reduce the antioxidants, glutathione and taurine, in aged female mice, but not aged males. 176 However, it is also important to parse the influence of aging and HIV Tat exposure to better understand the source of such sex differences. When stratified by estropause status (pre‐, peri‐ or post‐estropausal), Tat‐exposure and aging were found to exert largely independent effects on behavioral pathology.
Irrespective of Tat exposure, peri‐ and post‐estropausal mice demonstrated greater anxiety‐like behavior and cognitive impairment than pre‐estropausal mice (Figure 5A,5B). 177 Tat exposure independently reduced learning in a radial arm water maze (Figure 5A), as well as grip strength (Figure 5C) and mechanical nociceptive thresholds. 177 Males appeared more resilient to Tat's age‐related effects; however, Tat‐impairment of grip strength was exacerbated with aging (Figure 5C). When endocrine function was assessed, estropausal status and Tat exposure interacted, such that pre‐estropausal Tat(+) mice had a greater estradiol‐to‐testosterone ratio (largely driven by reduced testosterone levels) and post‐estropausal Tat(+) mice had an estradiol reduction not observed in any other group. 177 Similar endocrine interactions were observed when comparing young and middle‐aged male mice exposed to Tat. Tat greatly reduced circulating total testosterone and increased corticosterone in middle‐aged males. Regressions revealed increased corticosterone to be associated with greater anxiety‐like behavior, greater swim speed in a radial arm water maze and poorer grip strength. Among young adult males, Tat increased circulating 17β‐estradiol, the estradiol‐to‐testosterone ratio and progesterone, a profile consistent with an early andropausal transition. AlloP was significantly elevated in the hippocampus of young adult and middle‐aged males and the midbrain of middle‐aged males (with no changes seen in the frontal cortex). Together, these data reveal the separate and interactive constructs on which the aging endocrine system interacts with Tat exposure.
FIGURE 5.
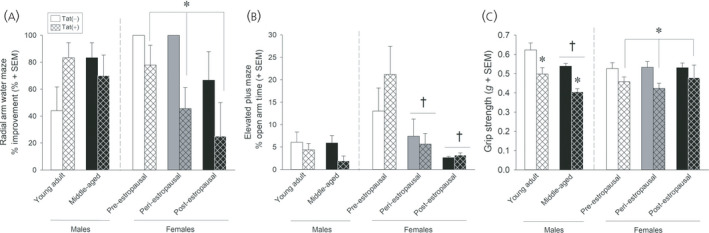
Middle‐aged (16–19 months old) female Tat(+) mice had greater learning deficits on a radial arm water maze than their Tat(−) counterparts, irrespective of estropausal status (A). Anxiety‐like behavior on an elevated plus maze was greater among peri‐ and post‐estropausal mice compared to those that were pre‐estropausal, irrespective of Tat exposure (B). Male or female Tat(+) mice had reduced grip strength compared to Tat(−) controls. Middle‐aged males (11–13 months old) had reduced grip strength compared to young adult males (6–8 months old) (C). *Significant difference from Tat(−) control. †Significant difference from young adult male or pre‐estropausal female group 177
The capacity of Tat to promote circulating steroid deficits may partly involve its toxic actions at mitochondria, the rate‐limiting organelle required for steroid synthesis, 25 , 178 , 179 and may also involve its capacity to alter lipid substrate bioavailability. 83 , 84 Pharmacological maintenance of steroid concentrations may be beneficial. In support, testosterone replacement therapy among men living with HIV improved depression inventory scores, 180 , 181 increased muscle and lean body mass, 181 , 182 and improved sexual function by restoring libido. 182 Administration of testosterone to women living with HIV also improved body weight and quality‐of‐life. 183 Similar benefits for reduced depressive symptomatology have been observed in response to DHEA administration. 184 Thus, steroid intervention may provide a benefit to aged PLWH and the timing for optimal implementation may differ from seronegative aged individuals.
5. STEROID‐BASED THERAPEUTICS FOR THE TREATMENT OF HIV
5.1. Novel adjunct therapeutics for HIV suppression
Antiretroviral therapeutics have dramatically increased the life expectancy of PLWH. However, these drugs are not able to eradicate the virus, nor neuroHIV, given that they cannot target reservoirs such as those within the CNS and do not target certain virotoxins such as Tat. It is notable that several promising leads are based on a steroid‐scaffold. Most notably, didehydro‐cortistatin A (dCA), an analogue of a steroid‐like alkaloid obtained from marine sponge, is demonstrated in vitro to bind to the Tat‐TAR complex, blocking HIV replication without producing cellular toxicity, to attenuate HIV‐mediated cytokine expression and to inhibit behavioral effects promoted by Tat in vivo. 185 , 186 dCA was found to selectively bind the unstructured basic region of Tat. 187 A combination of dCA and cART suppressed active HIV viral replication, reactivation and rebound of the latent viral reservoir. 188 Additional chemical derivatives of dCA were sought to rationalize their ability to dock at specific binding sites of the Tat protein. 187 Given the ability of dCA to inhibit Tat expression during early viral replication and penetrate latent viral reservoirs in the brain with good bioavailability, dCA and its novel steroidal‐based analogs hold potential as future cART adjuncts. 187 , 188 , 189
Additional estrogen‐based therapeutics have also been identified, including selective estrogen receptor β agonists, such as (S)‐equol and several phytoestrogens. (S)‐equol improved sensorimotor gating and motivational deficits in HIV transgenic rats 190 , 191 , 192 and prevented combined cocaine and HIV‐mediated synaptopathy. 193 The phytoestrogens, daidzein and liquiritigenin, restored Tat‐mediated synaptodendritic recovery. 194 Our own work has focused on the potential therapeutic advantages of neurosteroids.
Neurosteroids are synthesized de novo in brain from cholesterol 195 and can also reach the brain from peripheral sources such as the adrenals and gonads. 196 AlloP is perhaps the most well‐characterized neurosteroid with a pharmacodynamic profile that is suitable for potentially offsetting the effects of HIV Tat (Figure 1). Given the excitotoxic profile exerted by Tat, the actions of AlloP as a potent positive allosteric modulator of GABAA receptors are expected to reinstate excitatory–inhibitory balance via the influx of Cl−. Moreover, AlloP may act as an antagonist of L‐type Ca2+ channels, 197 , 198 further attenuating the excitotoxic actions of Tat. We have seen promising evidence for AlloP to offset Tat‐mediated neurotoxicity in vitro (Figure 2) and to attenuate Tat‐mediated behavioral interactions with opioids in vivo. 81 Although AlloP is a small molecule that readily crosses the blood–brain barrier and is well‐tolerated, 199 it is also rapidly re‐distributed from the brain, accumulates in adipose tissue and has a short elimination half‐life. 200 , 201 As such, we are currently working to synthesize AlloP analogues with anti‐Tat and anti‐viremic properties. A ligand structural alignment of dCA, (S)‐Equol and AlloP reveals structural similarities that may help explain their anti‐HIV activities (Figure 6). For example, both terminal ends of the molecules contain polar elements (an oxygen or a nitrogen atom). Induced‐fit docking of dCA in the NMR‐derived structure of Tat (Protein Data Bank: 1K5K) reveals important hydrogen bonding interactions between dCA and the R49 and R52 residues, which are part of the identified motif (the ARM domain) (Figure 7). Induced‐fit docking of AlloP in this binding site also reveals important hydrogen bonding interactions between AlloP and the R49 residue, in addition to strong hydrophobic interactions (Figure 7). These preliminary data suggest that dCA and AlloP may target a shared Tat binding site that may partly underlie their potential anti‐Tat efficacy. In light of this, the development of novel AlloP analogs may hold promise with respect to potential future cART adjunctive therapeutics.
FIGURE 6.
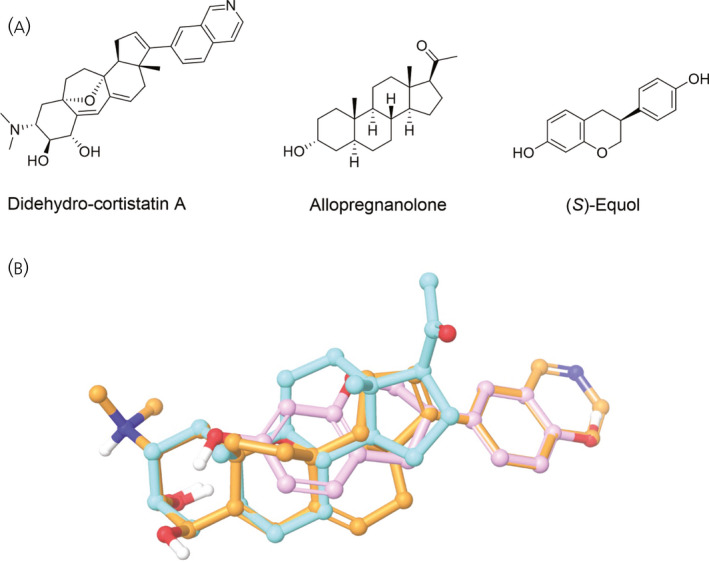
Didehydro‐cortistatin A (orange), allopregnanolone (cyan) and (S)‐equol (pink) demonstrate structural similarities (A) that are evidenced in a ligand‐structure alignment (B)
FIGURE 7.
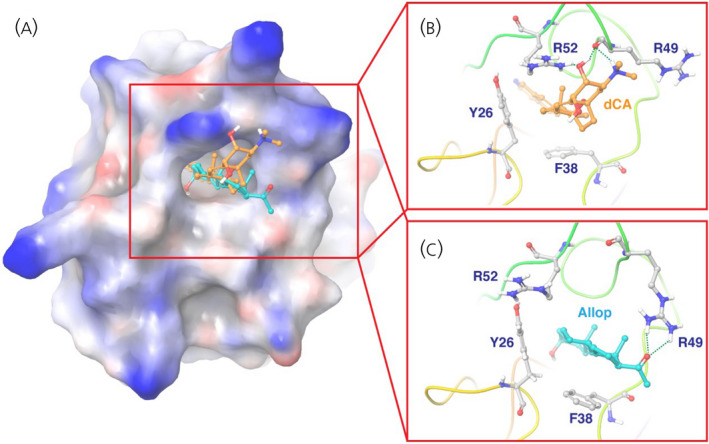
Molecular docking of didehydro‐cortistatin A (dCA) (orange) and allopregnanolone (cyan) in HIV Tat (Protein Data Bank: 1K5K). Tat is shown in a surface representation in the zoom‐out view (A) and in a ribbon representation in the zoom‐in view (B, C). Key residues of Tat for ligand binding are shown in stick representation
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Mohammed F. Salahuddin: Conceptualization; data curation; formal analysis; funding acquisition; investigation; writing‐original draft; writing‐review & editing. Alaa N. Qrareya: Conceptualization; data curation; formal analysis; funding acquisition; investigation; writing‐original draft; writing‐review & editing. Fakhri Mahdi: Data curation; formal analysis; methodology. Emaya Moss: Data curation; formal analysis; funding acquisition. Nicholas S. Akins: Data curation; formal analysis. Jing Li: Conceptualization; data curation; formal analysis; methodology; supervision; writing‐review & editing. Hoang V. Le: Conceptualization; data curation; formal analysis; methodology; supervision; writing‐review & editing. Jason J. Paris: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; writing‐original draft; writing‐review & editing.
ACKNOWLEDGEMENTS
This work was supported by funds from the National Institutes of Health: R00 DA039791 (JJP), R01 DA052851 (JJP) and an administrative supplement from award P30 GM122733 (pilot project to JJP and HVL) and the University of Mississippi (Graduate Student Council awards to MFS and ANQ). EM was supported in part by the Cole‐Eftink Fellowship Program. No figures in the current review were reproduced without permission. Figures 1 and 3 were prepared via Biorender.com.
Salahuddin MF, Qrareya AN, Mahdi F, et al. Allopregnanolone and neuroHIV: Potential benefits of neuroendocrine modulation in the era of antiretroviral therapy. J Neuroendocrinol. 2022;34:e13047. 10.1111/jne.13047
[Correction added on 20 October 2021, after first online publication: The copyright line was changed.]
DATA AVAILABILITY STATEMENT
Data are available from the authors upon suitable request.
REFERENCES
- 1. Global HIV and AIDS Statistics‐Fact Sheet . Preliminary UNAIDS 2021 Epidemiological Estimates. https://www.unaids.org/en/resources/fact‐sheet. Accessed June 6, 2021. [Google Scholar]
- 2. Gates TM, Cysique LA. The chronicity of HIV infection should drive the research strategy of neuroHIV treatment studies: a critical review. CNS Drugs. 2016;30(1):53‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saylor D, Dickens AM, Sacktor N, et al. HIV‐associated neurocognitive disorder ‐ pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(5):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zink WE, Zheng J, Persidsky Y, Poluektova L, Gendelman HE. The neuropathogenesis of HIV‐1 infection. FEMS Immunol Med Microbiol. 1999;26(3–4):233‐241. [DOI] [PubMed] [Google Scholar]
- 5. Mbonye U, Karn J. Transcriptional control of HIV latency: cellular signaling pathways, epigenetics, happenstance and the hope for a cure. Virology. 2014;454–455:328‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wallet C, De Rovere M, Van Assche J, et al. Microglial cells: the main HIV‐1 reservoir in the brain. Front Cell Infect Microbiol. 2019;9:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bertrand L, Nair M, Toborek M. Solving the blood‐brain barrier challenge for the effective treatment of HIV replication in the central nervous system. Curr Pharm Des. 2016;22(35):5477‐5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel SH, Ismaiel OA, Mylott WR Jr, et al. Cell‐type specific differences in antiretroviral penetration and the effects of HIV‐1 Tat and morphine among primary human brain endothelial cells, astrocytes, pericytes, and microglia. Neurosci Lett. 2019;712:134475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sengupta S, Siliciano RF. Targeting the latent reservoir for HIV‐1. Immunity. 2018;48(5):872‐895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vanhamel J, Bruggemans A, Debyser Z. Establishment of latent HIV‐1 reservoirs: what do we really know? J Virus Erad. 2019;5(1):3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaskill PJ, Miller DR, Gamble‐George J, Yano H, Khoshbouei HHIV. Tat and dopamine transmission. Neurobiol Dis. 2017;105:51‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8(1):33‐44. [DOI] [PubMed] [Google Scholar]
- 13. Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV‐1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878‐892. [DOI] [PubMed] [Google Scholar]
- 14. Hudson L, Liu J, Nath A, et al. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol. 2000;6:145‐155. [DOI] [PubMed] [Google Scholar]
- 15. Wiley CA, Baldwin M, Achim CL. Expression of HIV regulatory and structural mRNA in the central nervous system. AIDS. 1996;10:843‐847. [DOI] [PubMed] [Google Scholar]
- 16. Cysique LA, Jugé L, Lennon MJ, et al. HIV brain latency as measured by CSF BcL11b relates to disrupted brain cellular energy in virally suppressed HIV infection. AIDS. 2019;33(3):433‐441. [DOI] [PubMed] [Google Scholar]
- 17. Gerena Y, Menéndez‐Delmestre R, Delgado‐Nieves A, et al. Release of soluble insulin receptor from neurons by cerebrospinal fluid from patients with neurocognitive dysfunction and HIV infection. Front Neurol. 2019;10:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henderson LJ, Johnson TP, Smith BR, et al. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS. 2019;33(Suppl 2):S145‐S157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson TP, Patel K, Johnson KR, et al. Induction of IL‐17 and nonclassical T‐cell activation by HIV‐Tat protein. Proc Natl Acad Sci U S A. 2013;110(33):13588‐13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Napier TC, Chen L, Kashanchi F, Hu XT. Repeated cocaine treatment enhances HIV‐1 Tat‐induced cortical excitability via over‐activation of L‐type calcium channels. J Neuroimmune Pharmacol. 2014;9(3):354‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu XT. HIV‐1 tat‐mediated calcium dysregulation and neuronal dysfunction in vulnerable brain regions. Curr Drug Targets. 2016;17(1):4‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eugenin EA, King JE, Nath A, et al. HIV‐tat induces formation of an LRP‐PSD‐95‐ NMDAR‐nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci U S A. 2007;104(9):3438‐3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krogh KA, Wydeven N, Wickman K, Thayer SA. HIV‐1 protein Tat produces biphasic changes in NMDA‐evoked increases in intracellular Ca2+ concentration via activation of Src kinase and nitric oxide signaling pathways. J Neurochem. 2014;130(5):642‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nath A, Psooy K, Martin C, et al. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70(3):1475‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fitting S, Knapp PE, Zou S, et al. Interactive HIV‐1 Tat and morphine‐induced synaptodendritic injury is triggered through focal disruptions in Na⁺ influx, mitochondrial instability, and Ca2+ overload. J Neurosci. 2014;34(38):12850‐12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khan N, Lakpa KL, Halcrow PW, et al. BK channels regulate extracellular Tat‐mediated HIV‐1 LTR transactivation. Sci Rep. 2019;9(1):12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khan N, Chen X, Geiger JD. Role of divalent cations in HIV‐1 replication and pathogenicity. Viruses. 2020;12(4):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Irollo E, Luchetta J, Ho C, Nash B, Meucci O. Mechanisms of neuronal dysfunction in HIV‐associated neurocognitive disorders. Cell Mol Life Sci. 2021;78(9):4283‐4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mocchetti I, Campbell LA, Harry GJ, Avdoshina V. When human immunodeficiency virus meets chemokines and microglia: neuroprotection or neurodegeneration? J Neuroimmune Pharmacol. 2013;8(1):118‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fields JA, Ellis RJ. HIV in the cART era and the mitochondrial: immune interface in the CNS. Int Rev Neurobiol. 2019;145:29‐65. [DOI] [PubMed] [Google Scholar]
- 31. Teodorof‐Diedrich C, Spector SA. Human immunodeficiency virus TYPe 1 gp120 and Tat induce mitochondrial fragmentation and incomplete mitophagy in human neurons. J Virol. 2018;92(22):e00993‐e1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Godai K, Takahashi K, Kashiwagi Y, et al. Ryanodine receptor to mitochondrial reactive oxygen species pathway plays an important role in chronic human immunodeficiency virus gp120MN‐induced neuropathic pain in rats. Anesth Analg. 2019;129(1):276‐286. [DOI] [PubMed] [Google Scholar]
- 33. Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV‐Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res. 2012;229(1):48‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marks WD, Paris JJ, Schier CJ, et al. HIV‐1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J Neurovirol. 2016;22(6):747‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marks WD, Paris JJ, Barbour AJ, et al. HIV‐1 Tat and morphine differentially disrupt pyramidal cell structure and function and spatial learning in hippocampal area CA1: continuous versus interrupted morphine exposure. eNeuro. 2021;8(3):ENEURO.0547‐20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kesby JP, Fields JA, Chang A, et al. Effects of HIV‐1 TAT protein and methamphetamine exposure on visual discrimination and executive function in mice. Behav Brain Res. 2018;349:73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nookala AR, Schwartz DC, Chaudhari NS, et al. Methamphetamine augment HIV‐1 Tat mediated memory deficits by altering the expression of synaptic proteins and neurotrophic factors. Brain Behav Immun. 2018;71:37‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raybuck JD, Hargus NJ, Thayer SA. A GluN2B‐selective NMDAR antagonist reverses synapse loss and cognitive impairment produced by the HIV‐1 protein Tat. J Neurosci. 2017;37(33):7837‐7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paris JJ, Singh HD, Carey AN, McLaughlin JP. Exposure to HIV‐1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav Brain Res. 2015;291:209‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hahn YK, Paris JJ, Lichtman AH, et al. Central HIV‐1 Tat exposure elevates anxiety and fear conditioned responses of male mice concurrent with altered mu‐opioid receptor‐mediated G‐protein activation and β‐arrestin 2 activity in the forebrain. Neurobiol Dis. 2016;92(Pt B):124‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joshi CR, Stacy S, Sumien N, Ghorpade A, Borgmann K. Astrocyte HIV‐1 Tat differentially modulates behavior and brain MMP/TIMP balance during short and prolonged induction in transgenic mice. Front Neurol. 2020;11:593188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McLaughlin JP, Paris JJ, Mintzopoulos D, et al. Conditional human immunodeficiency virus transactivator of transcription protein expression induces depression‐like effects and oxidative stress. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(7):599‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety‐like behavior of mice produced by conditional central expression of the HIV‐1 regulatory protein, Tat. Psychopharmacology. 2014a;231(11):2349‐2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paris JJ, Fenwick J, McLaughlin JP. Progesterone protects normative anxiety‐like responding among ovariectomized female mice that conditionally express the HIV‐1 regulatory protein, Tat, in the CNS. Horm Behav. 2014;65(5):445‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paris JJ, Zou S, Hahn YK, Knapp PE, Hauser KF. 5α‐reduced progestogens ameliorate mood‐related behavioral pathology, neurotoxicity, and microgliosis associated with exposure to HIV‐1 Tat. Brain Behav Immun. 2016;55:202‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salahuddin MF, Qrareya AN, Mahdi F, et al. Combined HIV‐1 Tat and oxycodone activate the hypothalamic‐pituitary‐adrenal and ‐gonadal axes and promote psychomotor, affective, and cognitive dysfunction in female mice. Horm Behav. 2020;119:104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Salahuddin MF, Mahdi F, Paris JJ. HIV‐1 Tat dysregulates the hypothalamic‐pituitary‐adrenal stress axis and potentiates oxycodone‐mediated psychomotor and anxiety‐like behavior of male mice. Int J Mol Sci. 2020;21(21):8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salahuddin MF, Mahdi F, Sulochana SP, Paris JJ. HIV‐1 Tat protein promotes neuroendocrine dysfunction concurrent with the potentiation of oxycodone's psychomotor effects in female mice. Viruses. 2021;13(5):813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Han MM, Frizzi KE, Ellis RJ, Calcutt NA, Fields JA. Prevention of HIV‐1 TAT protein‐induced peripheral neuropathy and mitochondrial disruption by the antimuscarinic pirenzepine. Front Neurol. 2021;12:663373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wodarski R, Bagdas D, Paris JJ, et al. Reduced intraepidermal nerve fibre density, glial activation, and sensory changes in HIV type‐1 Tat‐expressing female mice: involvement of Tat during early stages of HIV‐associated painful sensory neuropathy. Pain Rep. 2018;3(3):e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fitting S, Zou S, Chen W, Vo P, Hauser KF, Knapp PE. Regional heterogeneity and diversity in cytokine and chemokine production by astroglia: differential responses to HIV‐1 Tat, gp120, and morphine revealed by multiplex analysis. J Proteome Res. 2010;9(4):1795‐1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gonek M, McLane VD, Stevens DL, et al. CCR5 mediates HIV‐1 Tat‐induced neuroinflammation and influences morphine tolerance, dependence, and reward. Brain Behav Immun. 2018;69:124‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bagdas D, Paris JJ, Carper M, et al. Conditional expression of HIV‐1 tat in the mouse alters the onset and progression of tonic, inflammatory and neuropathic hypersensitivity in a sex‐dependent manner. Eur J Pain. 2020;24(8):1609‐1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hahn YK, Masvekar RR, Xu R, Hauser KF, Knapp PE. Chronic HIV‐1 Tat and HIV reduce Rbfox3/NeuN: evidence for sex‐related effects. Curr HIV Res. 2015;13(1):10‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lawson MA, Kelley KW, Dantzer R. Intracerebroventricular administration of HIV‐1 Tat induces brain cytokine and indoleamine 2,3‐dioxygenase expression: a possible mechanism for AIDS comorbid depression. Brain Behav Immun. 2011;25(8):1569‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kesby JP, Markou A, Semenova S, Translational Methamphetamine AIDS Research Center (TMARC) Group . Cognitive deficits associated with combined HIV gp120 expression and chronic methamphetamine exposure in mice. Eur Neuropsychopharmacol. 2015;25(1):141‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kesby JP, Heaton RK, Young JW, et al. Methamphetamine exposure combined with HIV‐1 disease or gp120 expression: comparison of learning and executive functions in humans and mice. Neuropsychopharmacology. 2015;40(8):1899‐1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bachis A, Forcelli P, Masliah E, Campbell L, Mocchetti I. Expression of gp120 in mice evokes anxiety behavior: co‐occurrence with increased dendritic spines and brain‐derived neurotrophic factor in the amygdala. Brain Behav Immun. 2016;54:170‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fields JA, Swinton MK, Carson A, et al. Tenofovir disoproxil fumarate induces peripheral neuropathy and alters inflammation and mitochondrial biogenesis in the brains of mice. Sci Rep. 2019;9(1):17158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shi Y, Yuan S, Tang SJ. Morphine and HIV‐1 gp120 cooperatively promote pathogenesis in the spinal pain neural circuit. Mol Pain. 2019;15:1744806919868380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guindon J, Blanton H, Brauman S, Donckels K, Narasimhan M, Benamar K. Sex differences in a rodent model of HIV‐1‐associated neuropathic pain. Int J Mol Sci. 2019;20(5):1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nemeth CL, Glasper ER, Harrell CS, Malviya SA, Otis JS, Neigh GN. Meloxicam blocks neuroinflammation, but not depressive‐like behaviors, in HIV‐1 transgenic female rats. PLoS One. 2014;9(10):e108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rowson SA, Harrell CS, Bekhbat M, et al. Neuroinflammation and behavior in HIV‐1 transgenic rats exposed to chronic adolescent stress. Front Psychiatry. 2016;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vigorito M, LaShomb AL, Chang SL. Spatial learning and memory in HIV‐1 transgenic rats. J Neuroimmune Pharmacol. 2007;2(4):319‐328. [DOI] [PubMed] [Google Scholar]
- 65. Zaid D, Greenman Y. Human immunodeficiency virus infection and the endocrine system. Endocrinol Metab (Seoul). 2019;34(2):95‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Heron PM, Turchan‐Cholewo J, Bruce‐Keller AJ, Wilson ME. Estrogen receptor alpha inhibits the estrogen‐mediated suppression of HIV transcription in astrocytes: implications for estrogen neuroprotection in HIV dementia. AIDS Res Hum Retroviruses. 2009;25(11):1071‐1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Szotek EL, Narasipura SD, Al‐Harthi L. 17β‐Estradiol inhibits HIV‐1 by inducing a complex formation between β‐catenin and estrogen receptor α on the HIV promoter to suppress HIV transcription. Virology. 2013;443(2):375‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rodriguez‐Garcia M, Biswas N, Patel MV, et al. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV‐infection. PLoS One. 2013;8(4):e62069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wilson ME, Allred KF, Bisotti AJ, Bruce‐Keller A, Chuahan A, Nath A. Estradiol negatively regulates HIV‐LTR promoter activity in glial cells. AIDS Res Hum Retroviruses. 2006;22(4):350‐356. [DOI] [PubMed] [Google Scholar]
- 70. Ragupathy V, Devadas K, Tang S, et al. Effect of sex steroid hormones on replication and transmission of major HIV subtypes. J Steroid Biochem Mol Biol. 2013;138:63‐71. [DOI] [PubMed] [Google Scholar]
- 71. Asin SN, Heimberg AM, Eszterhas SK, Rollenhagen C, Howell AL. Estradiol and progesterone regulate HIV type 1 replication in peripheral blood cells. AIDS Res Hum Retroviruses. 2008;24(5):701‐716. [DOI] [PubMed] [Google Scholar]
- 72. Lee AW, Mitra D, Laurence J. Interaction of pregnancy steroid hormones and zidovudine in inhibition of HIV type 1 replication in monocytoid and placental Hofbauer cells: implications for the prevention of maternal‐fetal transmission of HIV. AIDS Res Hum Retroviruses. 1997;13(14):1235‐1242. [DOI] [PubMed] [Google Scholar]
- 73. Muñoz LD, Serramía MJ, Fresno M, Muñoz‐Fernández MA. Progesterone inhibits HIV‐1 replication in human trophoblast cells through inhibition of autocrine tumor necrosis factor secretion. J Infect Dis. 2007;195(9):1294‐1302. [DOI] [PubMed] [Google Scholar]
- 74. Chapuy‐Regaud S, Subra C, Requena M, et al. Progesterone and a phospholipase inhibitor increase the endosomal bis(monoacylglycero)phosphate content and block HIV viral particle intercellular transmission. Biochimie. 2013;95(9):1677‐1688. [DOI] [PubMed] [Google Scholar]
- 75. Devadas K, Biswas S, Ragupathy V, Lee S, Dayton A, Hewlett I. Modulation of HIV replication in monocyte derived macrophages (MDM) by steroid hormones. PLoS One. 2018;13(1):e0191916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120‐ and tat1‐72‐induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59(1):51‐60. [DOI] [PubMed] [Google Scholar]
- 77. Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. ER‐β mediates 17β‐estradiol attenuation of HIV‐1 Tat‐induced apoptotic signaling. Synapse. 2010;64(11):829‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kendall SL, Anderson CF, Nath A, et al. Gonadal steroids differentially modulate neurotoxicity of HIV and cocaine: testosterone and ICI 182,780 sensitive mechanism. BMC Neurosci. 2005;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bruce‐Keller AJ, Barger SW, Moss NI, Pham JT, Keller JN, Nath A. Pro‐inflammatory and pro‐oxidant properties of the HIV protein Tat in a microglial cell line: attenuation by 17 beta‐estradiol. J Neurochem. 2001;78(6):1315‐1324. [DOI] [PubMed] [Google Scholar]
- 80. Lee YW, Eum SY, Nath A, Toborek M. Estrogen‐mediated protection against HIV Tat protein‐induced inflammatory pathways in human vascular endothelial cells. Cardiovasc Res. 2004;63(1):139‐148. [DOI] [PubMed] [Google Scholar]
- 81. Paris JJ, Liere P, Kim S, et al. Pregnane steroidogenesis is altered by HIV‐1 Tat and morphine: physiological allopregnanolone is protective against neurotoxic and psychomotor effects. Neurobiol Stress. 2020;12:100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Balan I, Aurelian L, Schleicher R, Boero G, O'Buckley T, Morrow AL. Neurosteroid allopregnanolone (3α,5α‐THP) inhibits inflammatory signals induced by activated MyD88‐dependent toll‐like receptors. Transl Psychiatry. 2021;11(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cotto B, Natarajaseenivasan K, Ferrero K, Wesley L, Sayre M, Langford D. Cocaine and HIV‐1 Tat disrupt cholesterol homeostasis in astrocytes: implications for HIV‐associated neurocognitive disorders in cocaine user patients. Glia. 2018;66(4):889‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mohseni Ahooyi T, Shekarabi M, Torkzaban B, et al. Dysregulation of neuronal cholesterol homeostasis upon exposure to HIV‐1 Tat and cocaine revealed by RNA‐sequencing. Sci Rep. 2018;8(1):16300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dickens AM, Yoo SW, Chin AC, et al. Chronic low‐level expression of HIV‐1 Tat promotes a neurodegenerative phenotype with aging. Sci Rep. 2017;7(1):7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Maingat FG, Polyak MJ, Paul AM, et al. Neurosteroid‐mediated regulation of brain innate immunity in HIV/AIDS: DHEA‐S suppresses neurovirulence. FASEB J. 2013;27(2):725‐737. [DOI] [PubMed] [Google Scholar]
- 87. Mukerji SS, Misra V, Lorenz DR, et al. Low neuroactive steroids identifies a biological subtype of depression in adults with human immunodeficiency virus on suppressive antiretroviral therapy. J Infect Dis. 2021;223(9):1601‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guennoun R, Labombarda F, Gonzalez Deniselle MC, Liere P, De Nicola AF, Schumacher M. Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection. J Steroid Biochem Mol Biol. 2015;146:48‐61. [DOI] [PubMed] [Google Scholar]
- 89. Schumacher M, Mattern C, Ghoumari A, et al. Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Prog Neurogibol. 2014;113:6‐39. [DOI] [PubMed] [Google Scholar]
- 90. Zhu X, Fréchou M, Liere P, et al. A role of endogenous progesterone in stroke cerebroprotection revealed by the neural‐specific deletion of its intracellular receptors. J Neurosci. 2017;37:10998‐11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. George MM, Bhangoo A. Human immune deficiency virus (HIV) infection and the hypothalamic pituitary adrenal axis. Rev Endocr Metab Disord. 2013;14(2):105‐112. [DOI] [PubMed] [Google Scholar]
- 92. Gomes AC, Aragüés JM, Guerra S, Fernandes J, Mascarenhas MR. Hypogonadotropic hypogonadism in human immunodeficiency virus‐infected men: uncommonly low testosterone levels. Endocrinol Diabetes Metab Case Rep. 2017;2017:17‐0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Raffi F, Brisseau JM, Planchon B, Rémi JP, Barrier JH, Grolleau JY. Endocrine function in 98 HIV‐infected patients: a prospective study. AIDS. 1991;5(6):729‐733. [DOI] [PubMed] [Google Scholar]
- 94. Afreen B, Khan KA, Riaz A. Adrenal insufficiency In Pakistani HIV infected patients. J Ayub Med Coll Abbottabad. 2017;29(3):428‐431. [PubMed] [Google Scholar]
- 95. Chrousos GP, Zapanti ED. Hypothalamic‐pituitary‐adrenal axis in HIV infection and disease. Endocrinol Metab Clin North Am. 2014;43(3):791‐806. [DOI] [PubMed] [Google Scholar]
- 96. González‐González JG, de la Garza‐Hernández NE, Garza‐Morán RA, et al. Prevalence of abnormal adrenocortical function in human immunodeficiency virus infection by low‐dose cosyntropin test. Int J STD AIDS. 2001;12(12):804‐810. [DOI] [PubMed] [Google Scholar]
- 97. Marik PE, Kiminyo K, Zaloga GP. Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit Care Med. 2002;30(6):1267‐1273. [DOI] [PubMed] [Google Scholar]
- 98. Prasanthai V, Sunthornyothin S, Phowthongkum P, Suankratay C. Prevalence of adrenal insufficiency in critically ill patients with AIDS. J Med Assoc Thai. 2007;90(9):1768‐1774. [PubMed] [Google Scholar]
- 99. Sharma N, Sharma LK, Anand A, et al. Presence, patterns & predictors of hypocortisolism in patients with HIV infection in India. Indian J Med Res. 2018;147(2):142‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV‐related stigma on treatment adherence: systematic review and meta‐synthesis. J Int AIDS Soc. 2013;16(3 Suppl 2):18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nyblade L, Stockton MA, Giger K, et al. Stigma in health facilities: why it matters and how we can change it. BMC Med. 2019;17(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Siefried KJ, Mao L, Kerr S, et al. PAART study investigators. Socioeconomic factors explain suboptimal adherence to antiretroviral therapy among HIV‐infected Australian adults with viral suppression. PLoS One. 2017;12(4):e0174613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tomori C, Kennedy CE, Brahmbhatt H, et al. Barriers and facilitators of retention in HIV care and treatment services in Iringa, Tanzania: the importance of socioeconomic and sociocultural factors. AIDS Care. 2014;26(7):907‐913. [DOI] [PubMed] [Google Scholar]
- 104. Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav. 2017;21(1):283‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55‐89. [DOI] [PubMed] [Google Scholar]
- 106. Lortholary O, Christeff N, Casassus P, et al. Hypothalamo‐pituitary‐adrenal function in human immunodeficiency virus‐infected men. J Clin Endocrinol Metab. 1996;81:791‐796. [DOI] [PubMed] [Google Scholar]
- 107. Christeff N, Gherbi N, Mammes O, et al. Serum cortisol and DHEA concentrations during HIV infection. Psychoneuroendocrinology. 1997;22(Suppl 1):S11‐S18. [DOI] [PubMed] [Google Scholar]
- 108. Villette JM, Bourin P, Doinel C, et al. Circadian variations in plasma levels of hypophyseal, adrenocortical and testicular hormones in men infected with human immunodeficiency virus. J Clin Endocrinol Metab. 1990;70(3):572‐577. [DOI] [PubMed] [Google Scholar]
- 109. Qiao S, Li X, Zilioli S, et al. Hair measurements of cortisol, DHEA, and DHEA to cortisol ratio as biomarkers of chronic stress among people living with HIV in China: known‐group validation. PLoS One. 2017;12(1):e0169827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Schürmeyer TH, Müller V, von zur Mühlen A, Schmidt RE. Thyroid and adrenal function in HIV‐infected outpatients. Eur J Med Res. 1997;2(5):220‐226. [PubMed] [Google Scholar]
- 111. Norbiato G, Bevilacqua M, Vago T, et al. Cortisol resistance in acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74(3):608‐613. [DOI] [PubMed] [Google Scholar]
- 112. Webster JC, Oakley RH, Jewell CM, Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci U S A. 2001;98(12):6865‐6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bekhbat M, Mehta CC, Kelly SD, et al. HIV and symptoms of depression are independently associated with impaired glucocorticoid signaling. Psychoneuroendocrinology. 2018;96:118‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Andersen JL, Planelles V. The role of Vpr in HIV‐1 pathogenesis. Curr HIV Res. 2005;3(1):43‐51. [DOI] [PubMed] [Google Scholar]
- 115. Sawaya BE, Khalili K, Gordon J, Taube R, Amini S. Cooperative interaction between HIV‐1 regulatory proteins Tat and Vpr modulates transcription of the viral genome. J Biol Chem. 2000;275(45):35209‐35214. [DOI] [PubMed] [Google Scholar]
- 116. Kino T, Chrousos GP. Human immunodeficiency virus type‐1 accessory protein Vpr: a causative agent of the AIDS‐related insulin resistance/lipodystrophy syndrome? Ann N Y Acad Sci. 2004;1024:153‐167. [DOI] [PubMed] [Google Scholar]
- 117. Kino T, Gragerov A, Kopp JB, Stauber RH, Pavlakis GN, Chrousos GP. The HIV‐1 virion‐associated protein vpr is a coactivator of the human glucocorticoid receptor. J Exp Med. 1999;189(1):51‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Raber J, Toggas SM, Lee S, Bloom FE, Epstein CJ, Mucke L. Central nervous system expression of HIV‐1 Gp120 activates the hypothalamic‐pituitary‐adrenal axis: evidence for involvement of NMDA receptors and nitric oxide synthase. Virology. 1996;226(2):362‐373. [DOI] [PubMed] [Google Scholar]
- 119. Costa A, Nappi RE, Polatti F, Poma A, Grossman AB, Nappi G. Stimulating effect of HIV‐1 coat protein gp120 on corticotropin‐releasing hormone and arginine vasopressin in the rat hypothalamus: involvement of nitric oxide. Exp Neurol. 2000;166(2):376‐384. [DOI] [PubMed] [Google Scholar]
- 120. Pozzoli G, Tringali G, Dello Russo C, Vairano M, Preziosi P, Navarra P. HIV‐1 Gp120 protein modulates corticotropin releasing factor synthesis and release via the stimulation of its mRNA from the rat hypothalamus in vitro: involvement of inducible nitric oxide synthase. J Neuroimmunol. 2001;118(2):268‐276. [DOI] [PubMed] [Google Scholar]
- 121. Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Goleva E, Kisich KO, Leung DY. A role for STAT5 in the pathogenesis of IL‐2‐induced glucocorticoid resistance. J Immunol. 2002;169(10):5934‐5940. [DOI] [PubMed] [Google Scholar]
- 123. Pariante CM, Pearce BD, Pisell TL, et al. The proinflammatory cytokine, interleukin‐1alpha, reduces glucocorticoid receptor translocation and function. Endocrinology. 1999;140(9):4359‐4366. [DOI] [PubMed] [Google Scholar]
- 124. Raddatz D, Toth S, Schwörer H, Ramadori G. Glucocorticoid receptor signaling in the intestinal epithelial cell lines IEC‐6 and Caco‐2: evidence of inhibition by interleukin‐1beta. Int J Colorectal Dis. 2001;16(6):377‐383. [DOI] [PubMed] [Google Scholar]
- 125. Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM. p38 Mitogen‐activated protein kinase‐induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid‐insensitive asthma. J Allergy Clin Immunol. 2002;109(4):649‐657. [DOI] [PubMed] [Google Scholar]
- 126. Leung DY, Martin RJ, Szefler SJ, et al. Dysregulation of interleukin 4, interleukin 5, and interferon gamma gene expression in steroid‐resistant asthma. J Exp Med. 1995;181(1):33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Crum‐Cianflone NF, Bavaro M, Hale B, et al. Erectile dysfunction and hypogonadism among men with HIV. AIDS Patient Care STDS. 2007;21(1):9‐19. [DOI] [PubMed] [Google Scholar]
- 128. Dobs AS, Dempsey MA, Ladenson PW, Polk BF. Endocrine disorders in men infected with human immunodeficiency virus. Am J Med. 1988;84(3 Pt 2):611‐616. [DOI] [PubMed] [Google Scholar]
- 129. Grinspoon S, Corcoran C, Miller K, et al. Body composition and endocrine function in women with acquired immunodeficiency syndrome wasting. J Clin Endocrinol Metab. 1997;82(5):1332‐1337. [DOI] [PubMed] [Google Scholar]
- 130. Rietschel P, Corcoran C, Stanley T, Basgoz N, Klibanski A, Grinspoon S. Prevalence of hypogonadism among men with weight loss related to human immunodeficiency virus infection who were receiving highly active antiretroviral therapy. Clin Infect Dis. 2000;31(5):1240‐1244. [DOI] [PubMed] [Google Scholar]
- 131. Wunder DM, Bersinger NA, Fux CA, et al. Hypogonadism in HIV‐1‐infected men is common and does not resolve during antiretroviral therapy. Antivir Ther. 2007;12(2):261‐265. [PubMed] [Google Scholar]
- 132. Centers for Disease Control and Prevention . HIV Surveillance Report, 2018 [Reviewed May 27, 2021]. http://www.cdc.gov/hiv/library/reports/hiv‐surveillance.html. Accessed June 10, 2021. [Google Scholar]
- 133. Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241‐4247. [DOI] [PubMed] [Google Scholar]
- 134. Durand M, Chartrand‐Lefebvre C, Baril JG, et al. The Canadian HIV and aging cohort study ‐ determinants of increased risk of cardio‐vascular diseases in HIV‐infected individuals: rationale and study protocol. BMC Infect Dis. 2017;17(1):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB. Functional impairment, disability, and frailty in adults aging with HIV‐infection. Curr HIV/AIDS Rep. 2014;11(3):279‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Maciel RA, Klück HM, Durand M, Sprinz E. Comorbidity is more common and occurs earlier in persons living with HIV than in HIV‐uninfected matched controls, aged 50 years and older: a cross‐sectional study. Int J Infect Dis. 2018;70:30‐35. [DOI] [PubMed] [Google Scholar]
- 137. Warriner AH, Burkholder GA, Overton ET. HIV‐related metabolic comorbidities in the current ART era. Infect Dis Clin North Am. 2014;28(3):457‐476. [DOI] [PubMed] [Google Scholar]
- 138. Greene M, Covinsky KE, Valcour V, et al. Geriatric syndromes in older HIV‐infected adults. J Acquir Immune Defic Syndr. 2015;69(2):161‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Guaraldi G, Orlando G, Zona S, et al. Premature age‐related comorbidities among HIV‐infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120‐1126. [DOI] [PubMed] [Google Scholar]
- 140. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long‐standing suppression of viremia. AIDS. 2010;24(9):1243‐1250. [DOI] [PubMed] [Google Scholar]
- 141. Schouten J, Wit FW, Stolte IG, et al. Cross‐sectional comparison of the prevalence of age‐associated comorbidities and their risk factors between HIV‐infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59(12):1787‐1797. [DOI] [PubMed] [Google Scholar]
- 142. Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69(7):833‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Rivera MJG, Hashmi MF. HIV Nephropathy. [Updated 2021 Aug 14]. StatPearls [Internet]. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK559134/ [Google Scholar]
- 144. Nna VU, Bakar ABA, Ahmad A, Mohamed M. Down‐regulation of steroidogenesis‐related genes and its accompanying fertility decline in streptozotocin‐induced diabetic male rats: ameliorative effect of metformin. Andrology. 2019;7(1):110‐123. [DOI] [PubMed] [Google Scholar]
- 145. Chabrolle C, Jeanpierre E, Tosca L, Ramé C, Dupont J. Effects of high levels of glucose on the steroidogenesis and the expression of adiponectin receptors in rat ovarian cells. Reprod Biol Endocrinol. 2008;19(6):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Romano S, Mitro N, Diviccaro S, et al. Short‐term effects of diabetes on neurosteroidogenesis in the rat hippocampus. J Steroid Biochem Mol Biol. 2017;167:135‐143. [DOI] [PubMed] [Google Scholar]
- 147. Romano S, Mitro N, Giatti S, et al. Diabetes induces mitochondrial dysfunction and alters cholesterol homeostasis and neurosteroidogenesis in the rat cerebral cortex. J Steroid Biochem Mol Biol. 2018;178:108‐116. [DOI] [PubMed] [Google Scholar]
- 148. Bajaj S, Sonkar KK, Verma S, Varma S, Singh AK. Assessment of glycemic status, insulin resistance and hypogonadism in HIV infected male patients. J Assoc Physicians India. 2020;68(8):43‐46. [PubMed] [Google Scholar]
- 149. Lachâtre M, Pasquet A, Ajana F, et al. HIV and hypogonadism: a new challenge for young‐aged and middle‐aged men on effective antiretroviral therapy. AIDS. 2017;31(3):451‐453. [DOI] [PubMed] [Google Scholar]
- 150. Moreno‐Pérez O, Escoín C, Serna‐Candel C, et al. The determination of total testosterone and free testosterone (RIA) are not applicable to the evaluation of gonadal function in HIV‐infected males. J Sex Med. 2010;7(8):2873‐2883. [DOI] [PubMed] [Google Scholar]
- 151. Bajaj S, Pathak Y, Varma S, Verma S. Metabolic status and hypogonadism in human immunodeficiency virus‐infected males. Indian J Endocrinol Metab. 2017;21(5):684‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Postel N, Wolf E, Balogh A, et al. Functional hypogonadism and testosterone deficiency in aging males with and without HIV‐infection. Exp Clin Endocrinol Diabetes. 2021. 10.1055/a-1210-2482 [DOI] [PubMed] [Google Scholar]
- 153. Pongener N, Salam R, Ningshen R, Visi V, Wairokpam T, Devi LS. A study on hypogonadism in male HIV patients in northeastern part of India. Indian J Sex Transm Dis AIDS. 2019;40(1):20‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Aggarwal J, Taneja RS, Gupta PK, Wali M, Chitkara A, Jamal A. Sex hormone profile in human immunodeficiency virus‐infected men and it's correlation with CD4 cell counts. Indian J Endocrinol Metab. 2018;22(3):328‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Gomes AR, Souteiro P, Silva CG, et al. Prevalence of testosterone deficiency in HIV‐infected men under antiretroviral therapy. BMC Infect Dis. 2016;16(1):628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Slama L, Jacobson LP, Li X, et al. Longitudinal changes over 10 years in free testosterone among HIV‐infected and HIV‐uninfected men. J Acquir Immune Defic Syndr. 2016;71(1):57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Monroe AK, Dobs AS, Palella FJ, Kingsley LA, Witt MD, Brown TT. Morning free and total testosterone in HIV‐infected men: implications for the assessment of hypogonadism. AIDS Res Ther. 2014;11(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Cohan GR. HIV‐associated hypogonadism. AIDS Read. 2006;16(7):341‐345, 348, 352‐354. [PubMed] [Google Scholar]
- 159. Rochira V, Zirilli L, Orlando G, et al. Premature decline of serum total testosterone in HIV‐infected men in the HAART‐era. PLoS One. 2011;6(12):e28512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pezzaioli LC, Quiros‐Roldan E, Paghera S, et al. The importance of SHBG and calculated free testosterone for the diagnosis of symptomatic hypogonadism in HIV‐infected men: a single‐centre real‐life experience. Infection. 2021;49(2):295‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Rochira V, Diazzi C, Santi D, et al. Low testosterone is associated with poor health status in men with human immunodeficiency virus infection: a retrospective study. Andrology. 2015;3(2):298‐308. [DOI] [PubMed] [Google Scholar]
- 162. Clark RA, Cohn SE, Jarek C, et al. Perimenopausal symptomatology among HIV‐infected women at least 40 years of age. J Acquir Immune Defic Syndr. 2000;23(1):99‐100. [DOI] [PubMed] [Google Scholar]
- 163. Schoenbaum EE, Hartel D, Lo Y, et al. HIV infection, drug use, and onset of natural menopause. Clin Infect Dis. 2005;41(10):1517‐1524. [DOI] [PubMed] [Google Scholar]
- 164. Boonyanurak P, Bunupuradah T, Wilawan K, et al. Age at menopause and menopause‐related symptoms in human immunodeficiency virus‐infected Thai women. Menopause. 2012;19(7):820‐824. [DOI] [PubMed] [Google Scholar]
- 165. Karim R, Mack WJ, Kono N, et al. Gonadotropin and sex steroid levels in HIV‐infected premenopausal women and their association with subclinical atherosclerosis in HIV‐infected and ‐uninfected women in the women's interagency HIV study (WIHS). J Clin Endocrinol Metab. 2013;98(4):E610‐E618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Mylonakis E, Koutkia P, Grinspoon S. Diagnosis and treatment of androgen deficiency in human immunodeficiency virus‐infected men and women. Clin Infect Dis. 2001;33(6):857‐864. [DOI] [PubMed] [Google Scholar]
- 167. Fantry LE, Zhan M, Taylor GH, Sill AM, Flaws JA. Age of menopause and menopausal symptoms in HIV‐infected women. AIDS Patient Care STDS. 2005;19(11):703‐711. [DOI] [PubMed] [Google Scholar]
- 168. Miller SA, Santoro N, Lo Y, et al. Menopause symptoms in HIV‐infected and drug‐using women. Menopause. 2005;12(3):348‐356. [DOI] [PubMed] [Google Scholar]
- 169. Sherr L, Molloy A, Macedo A, Croome N, Johnson MA. Ageing and menopause considerations for women with HIV in the UK. J Virus Erad. 2016;2(4):215‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Yin MT, Zhang CA, McMahon DJ, et al. Higher rates of bone loss in postmenopausal HIV‐infected women: a longitudinal study. J Clin Endocrinol Metab. 2012;97(2):554‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Looby SE, Shifren J, Corless I, et al. Increased hot flash severity and related interference in perimenopausal human immunodeficiency virus‐infected women. Menopause. 2014;21(4):403‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Looby SE, Psaros C, Raggio G, et al. Association between HIV status and psychological symptoms in perimenopausal women. Menopause. 2018;25(6):648‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Yang J, Sharma A, Shi Q, et al. Improved fracture prediction using different fracture risk assessment tool adjustments in HIV‐infected women. AIDS. 2018;32(12):1699‐1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Capeau J. Premature aging and premature age‐related comorbidities in HIV‐infected patients: facts and hypotheses. Clin Infect Dis. 2011;53(11):1127‐1129. [DOI] [PubMed] [Google Scholar]
- 175. Zhao X, Fan Y, Vann PH, Wong JM, Sumien N, He JJ. Long‐term HIV‐1 Tat expression in the brain led to neurobehavioral, pathological, and epigenetic changes reminiscent of accelerated aging. Aging Dis. 2020;11(1):93‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Paris JJ, Chen X, Anderson J, et al. In vivo proton magnetic resonance spectroscopy detection of metabolite abnormalities in aged Tat‐transgenic mouse brain. Geroscience. 2021. 10.1007/s11357-021-00354-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Qrareya AN, Mahdi F, Kaufman MJ, Ashpole NM, Paris JJ. HIV‐1 Tat promotes age‐related cognitive, anxiety‐like, and antinociceptive impairments in female mice that are moderated by aging and endocrine status. Geroscience. 2021;43(1):309‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Rozzi SJ, Avdoshina V, Fields JA, et al. Human immunodeficiency virus promotes mitochondrial toxicity. Neurotox Res. 2017;32(4):723‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Norman JP, Perry SW, Kasischke KA, Volsky DJ, Gelbard HA. HIV‐1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. J Immunol. 2007;178(2):869‐876. Erratum in: J Immunol. 2007;178(5):3334. [DOI] [PubMed] [Google Scholar]
- 180. Grinspoon S, Corcoran C, Stanley T, Baaj A, Basgoz N, Klibanski A. Effects of hypogonadism and testosterone administration on depression indices in HIV‐infected men. J Clin Endocrinol Metab. 2000;85(1):60‐65. [DOI] [PubMed] [Google Scholar]
- 181. Rabkin JG, Wagner GJ, Rabkin R. A double‐blind, placebo‐controlled trial of testosterone therapy for HIV‐positive men with hypogonadal symptoms. Arch Gen Psychiatry. 2000;57(2):141‐147; discussion 155–6. [DOI] [PubMed] [Google Scholar]
- 182. Zhou T, Hu ZY, Zhang HP, et al. Effects of testosterone supplementation on body composition in HIV patients: a meta‐analysis of double‐blinded randomized controlled trials. Curr Med Sci. 2018;38(1):191‐198. [DOI] [PubMed] [Google Scholar]
- 183. Miller K, Corcoran C, Armstrong C, et al. Transdermal testosterone administration in women with acquired immunodeficiency syndrome wasting: a pilot study. J Clin Endocrinol Metab. 1998;83(8):2717‐2725. [DOI] [PubMed] [Google Scholar]
- 184. Rabkin JG, McElhiney MC, Rabkin R, McGrath PJ, Ferrando SJ. Placebo‐controlled trial of dehydroepiandrosterone (DHEA) for treatment of nonmajor depression in patients with HIV/AIDS. Am J Psychiatry. 2006;163(1):59‐66. [DOI] [PubMed] [Google Scholar]
- 185. Mediouni S, Jablonski J, Paris JJ, et al. Didehydro‐cortistatin A inhibits HIV‐1 Tat mediated neuroinflammation and prevents potentiation of cocaine reward in Tat transgenic mice. Curr HIV Res. 2015;13(1):64‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Moranguinho I, Valente ST. Block‐and‐lock: new horizons for a cure for HIV‐1. Viruses. 2020;12(12):1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Mediouni S, Chinthalapudi K, Ekka MK, et al. Didehydro‐cortistatin a inhibits HIV‐1 by specifically binding to the unstructured basic region of Tat. MBio. 2019;10(1):e02662‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kessing CF, Nixon CC, Li C, et al. In vivo suppression of HIV rebound by didehydro‐cortistatin A, a “block‐and‐lock” strategy for HIV‐1 treatment. Cell Rep. 2017;21(3):600‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Mousseau G, Clementz MA, Bakeman WN, et al. An analog of the natural steroidal alkaloid cortistatin A potently suppresses Tat‐dependent HIV transcription. Cell Host Microbe. 2012;12(1):97‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. McLaurin KA, Moran LM, Booze RM, Mactutus CF. Selective estrogen receptor β agonists: a therapeutic approach for HIV‐1 associated neurocognitive disorders. J Neuroimmune Pharmacol. 2020;15(2):264‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. McLaurin KA, Li H, Cook AK, Booze RM, Mactutus CF. S‐EQUOL: a neuroprotective therapeutic for chronic neurocognitive impairments in pediatric HIV. J Neurovirol. 2020b;26(5):704‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. McLaurin KA, Bertrand SJ, Illenberger JM, Harrod SB, Mactutus CF, Booze RM. S‐Equol mitigates motivational deficits and dysregulation associated with HIV‐1. Sci Rep. 2021;11(1):11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Bertrand SJ, Hu C, Aksenova MV, Mactutus CF, Booze RM. HIV‐1 Tat and cocaine mediated synaptopathy in cortical and midbrain neurons is prevented by the isoflavone Equol. Front Microbiol. 2015;6:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Bertrand SJ, Mactutus CF, Aksenova MV, Espensen‐Sturges TD, Booze RM. Synaptodendritic recovery following HIV Tat exposure: neurorestoration by phytoestrogens. J Neurochem. 2014;128(1):140‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23(8):963‐987. [DOI] [PubMed] [Google Scholar]
- 196. Charlier TD, Cornil CA, Patte‐Mensah C, Meyer L, Mensah‐Nyagan AG, Balthazart J. Local modulation of steroid action: rapid control of enzymatic activity. Front Neurosci. 2015;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Earl DE, Tietz EI. Inhibition of recombinant L‐type voltage‐gated calcium channels by positive allosteric modulators of GABAA receptors. J Pharmacol Exp Ther. 2011;337(1):301‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hu AQ, Wang ZM, Lan DM, et al. Inhibition of evoked glutamate release by neurosteroid allopregnanolone via inhibition of L‐type calcium channels in rat medial prefrontal cortex. Neuropsychopharmacology. 2007;32(7):1477‐1489. [DOI] [PubMed] [Google Scholar]
- 199. Hernandez GD, Solinsky CM, Mack WJ, et al. Safety, tolerability, and pharmacokinetics of allopregnanolone as a regenerative therapeutic for Alzheimer's disease: a single and multiple ascending dose phase 1b/2a clinical trial. Alzheimers Dement (N Y). 2020;6(1):e12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Timby E, Balgård M, Nyberg S, et al. Pharmacokinetic and behavioral effects of allopregnanolone in healthy women. Psychopharmacology. 2006;186(3):414‐424. [DOI] [PubMed] [Google Scholar]
- 201. Porcu P, Barron AM, Frye CA, et al. Neurosteroidogenesis today: novel targets for neuroactive steroid synthesis and action and their relevance for translational research. J Neuroendocrinol. 2016;28(2):12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the authors upon suitable request.


