Abstract
Background
Health Technology Assessment (HTA) is a multidisciplinary process that synthesizes, with a systematic, transparent, impartial and robust methodological approach, the main information on the medical, economic, ethical and social implications of the use and dissemination of a health technology. Its aim is to support decision-makers in identifying safe, effective, patient-centered and best-value health policies, in order to promote an equitable, efficient, and high-quality health system. Given the continued application of innovative technologies into clinical practice, healthcare professionals need to be able to adequately evaluate these technologies using evidence-based approaches such as HTA. Therefore, the implementation of training in HTA is crucial. The aim of this study was to investigate existing HTA training initiatives for healthcare professionals provided by international HTA agencies and organizations around the world.
Methods
From March to November 2020, the websites of HTA agencies and organizations belonging to the European network for HTA (EUnetHTA) and to the International Network of Agencies for HTA (INAHTA), and the website of the HTA International (HTAi), were explored for identifying the HTA training initiatives directed to healthcare professionals. In addition, we screened the training initiatives proposed at European level by EUnetHTA as part of its Joint Actions and conducted in collaboration with its public-private partners. Specific keywords were searched in English and adapted to French, Portuguese, Spanish, Italian and German. Data extraction of the retrieved training initiatives was conducted from November 2020 to February 2021 and considered the following information: agency, country, website, coordinator, type of initiative, target, topic, main contents, and language.
Results
Out of 124 agencies/organizations/EUnetHTA public-private partners screened, only 21 provided training initiatives for healthcare professionals. A total of 55 training initiatives were analyzed, 85.5% of which were delivered at the European level and 14.5% at the international level. The countries with a greater number of courses were: Austria, Argentina, Spain, Portugal, and the United Kingdom. Twenty-one training initiatives focused on HTA application and methodology while 34 on specific HTA domains, particularly on the economic one. The technologies covered were mainly drugs.
Conclusions
Our study revealed a limited number of HTA training programs targeting healthcare professionals. HTA supports the decision-making processes concerning the use and application of health technologies with scientific evidence. Indeed, training of healthcare professionals in this field should be a key driver in implementing evidence-based healthcare choices and through rigorous methodological approaches such as HTA, in order to ensure proper health governance and value-based application of technological innovations in clinical practice. Therefore, capacity building of healthcare professionals in this area should be enhanced by using appropriate and effective training initiatives and educational strategies.
Keywords: health technology assessment, training, education, healthcare professionals, HTA
Introduction
In the last 20 years, a growing development of innovative health technologies not associated with an increase in resources, has characterized health contexts around the world (1).
Health technologies include different types of interventions (e.g., drugs, devices, medical and surgical procedures, healthcare organizational, and managerial systems) and represent a major driver of costs for healthcare systems (2). Therefore, to ensure the sustainability of health systems, the interest for “disinvestment” in healthcare increased (3). Indeed, today a necessary goal for modern healthcare systems is to disinvest from low-value health technologies and to reinvest in high value ones (2) and, in order to tackle these challenges, evidence-based approaches, such as Health Technology Assessment (HTA), are needed (2). A planned and systematic evaluation of health technologies is necessary to ensure the introduction and implementation of technological innovations in different healthcare settings and at all levels of health services, in an appropriate way (4). HTA is defined as “a multidisciplinary process that uses explicit methods to determine the value of a health technology at different points in its lifecycle. The purpose is to inform decision-making in order to promote an equitable, efficient, and high-quality health system. A health technology is an intervention developed to prevent, diagnose, or treat medical conditions; promote health; provide rehabilitation; or organize healthcare delivery. The intervention can be a test, device, medicine, vaccine, procedure, program, or system” (5).
HTA plays an essential role to inform stakeholders in the planning and designing of value-based health policies, aiming to promote an equitable, efficient, and high-quality health system (5) and it is recognized internationally as a valuable tool to support policy makers in decision making (6). By maximizing the potential of HTA, policy makers would be able to implement decisions that support the benefits of technologies or interventions, recognize their value and overcome uncertainties, in order to improve population health (7).
Given the application of innovative technologies into clinical practice, healthcare professionals need to be able to properly know and assess these technologies using modern methods of analysis, such as clinical, economic, organizational, comparative effectiveness, ethical and social. To ensure that, a broad range of competencies is needed.
The skills required of healthcare professionals to use the HTA approach appropriately include a range of scientific, analytical and also organizational-managerial competencies (8).
In 2002, an European survey evaluating training and education initiatives in HTA, showed a lack of training/education in this field, in European Union (EU) member countries (9). Only a few countries (Poland, Hungary, Estonia, and Latvia) were more “active” in terms of training initiatives in HTA and many Eastern European countries expressed the need of implementing the knowledge and application of an evidence-based tool such as HTA (9).
Taking into account the scientific and technological advances achieved in the last two decades, it appears necessary that healthcare professionals are appropriately trained to know, recognize and use in a relevant way the information useful for the introduction and implementation of healthcare technologies. Therefore, the implementation of education and training in HTA is strongly recommended.
For these reasons, the aim of our study was to investigate the current state of HTA training for healthcare professionals and to map existing training courses/initiatives in HTA provided by international HTA agencies and organizations all over the world for this target population.
Methods
From March to November 2020, the websites of HTA agencies and organizations belonging to the European network for Health Technology Assessment (EUnetHTA) and to the International Network of Agencies for HTA (INAHTA), and the website of the HTA International (HTAi, an organization representing a variety of stakeholders who have interests in HTA), were explored for identifying the HTA training initiatives directed to healthcare professionals. In addition, we screened the training initiatives proposed at European level by EUnetHTA as part of its Joint Actions (JA) and conducted in collaboration with other public-private partners such as the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and the Innovative Medicines Initiative (IMI).
Search Strategy
Two researchers (C.C., I.H.) independently conducted the online screening process on the websites of EUnetHTA (10), INAHTA (11) and HTAi (12). On HTAi website the search was carried out only for non-profit organizations while for EUnetHTA and INAHTA the websites of all the members belonging to the two HTA networks were consulted, without restrictions on the type of entity (HTA agency, Academy, private or non-profit organization). All websites were accessed and searched entering specific keywords (“course,” “training,” “seminar,” “workshop,” “Massive Open Online Courses – MOOC,” “HTA”) in the query box. The search was conducted in English language and then adapted to French, Portuguese, Spanish, Italian, and German, in order to retrieve courses conducted at national level in different countries. Education and training initiatives on the general HTA methodology or on specific HTA domains were considered eligible for inclusion.
Data Extraction
Data extraction was conducted from November 2020 to February 2021 independently by two researchers (I.H., C.C.) and disagreements were solved through discussion with a third researcher (G.E.C.). Only the initiatives with an available and accessible link were included.
The following data were collected for each training initiative retrieved:
i) information related to the agency providing the course: name, country, website;
ii) information related to the training initiative: title, coordinator, modalities of delivering the courses (if attendance or online course, seminar, workshop), target, topic, objectives, main contents, and language.
Data Synthesis
The included training initiatives were grouped in the following categories:
i) training initiatives provided by European HTA agencies/organizations,
ii) training initiatives provided at EU level by public-private partners of EUnetHTA and its JA,
iii) training initiatives provided by non-EU HTA agencies/organizations.
For each category, the training initiatives were described according to the main topic covered such as principles, general methodology, application of HTA and specific domains of the EUnetHTA core model (13).
Results
A total of 124 HTA agencies/organizations/EUnetHTA public-private partners were screened after removing the duplicates among EUnetHTA, INAHTA and HTAi. Table 1 shows the list. Eighteen HTA agencies/organizations–15 European and three internationals—developed training initiatives in the HTA field. Among the 15 European agencies/organizations, four were in Spain (27%), three in United Kingdom (UK) (20%) and three in Austria (20%), while of the remaining HTA agencies/organizations, five were in Portugal, Italy, the Netherlands, Belgium, and Romania (33%). Internationally, only three agencies/organizations (one in Canada, one in Australia and one in Argentina) developed training initiatives in HTA. Overall, starting from 2009 until 2021, 55 HTA training initiatives (14–67) were retrieved (Table 2), of which 54.5% (n = 30) provided by European HTA agencies/organizations (14–43), 14.5% (n = 8) by international ones (60–67) and, in addition, 31% (n = 17) publicly available at EUnetHTA website (27, 44–59) (Table 3).
Table 1.
List of HTA agencies/organizations belonging to INAHTA, HTAi and EUnetHTA network, and public-private partners of EUnetHTA and its JA, which have been consulted.
| Country | HTA agency/organization | INAHTA | HTAi | EUnetHTA |
|---|---|---|---|---|
| Argentina | IECS—Institute for Clinical Effectiveness and Health Policy | x | ||
| Australia | AHTA—Adelaide Health Technology Assessment | x | x | |
| ASERNIP-S—Australian Safety and Efficacy Register of New Interventional | x | |||
| Procedures—Surgical | ||||
| PBAC&MSAC—Pharmaceutical Benefits Advisory Committee | x | |||
| Austria | AIHTA—Austrian Institute for Health Technology Assessment | x | x | |
| UMIT—University for Health Sciences, Medical Informatics and Technology | x | |||
| GOG—Gesundheit Österreich GmbH/Geschäftsbereich | x | x | ||
| HVB—Hauptverband der Österreichischen Sozialversicherungsträger (Association of Austrian Social Insurance Institutions) | x | |||
| Belgium | KCE—Belgian Health Care Centre | x | x | |
| IPH—Scientific Institute of Public Health RIZIV—INAMI- Rijksinstituut voor Ziekte- en Invaliditeitsverzekering |
x x |
|||
| Brazil | ANS—National Regulatory Agency for Private Health Insurance and Plans | x | ||
| CONITEC—National Committee for Technology Incorporation | x | x | ||
| MoH—Ministry of Health of Brazil | x | |||
| Bulgaria | NCPHA—National Center of Public Health and Analyses | x | ||
| Canada | CADTH—Canadian Agency for Drugs and Technologies in Health | x | x | |
| IHE—Institute of Health Economics | x | x | ||
| INESS—Institut national d'excellence en santé et en services sociaux* | x | |||
| OH—Ontario Health | x | |||
| China | CDE—Center for Drug Evaluation, Taiwan | x | ||
| Colombia | IETS—Instituto de Evaluación Tecnológica en Salud | x | ||
| Croatia | MIZ—Ministry of Health of the Republic of Croatia | x | ||
| CHIF—Croatian Health Insurance Fund | x | |||
| CIPH—Croatian Institute of Public Health | x | |||
| Cyprus | MoH Cyprus—Ministry of Health of Cyprus | x | ||
| Czech Republic | MoH Czech—Ministry of Health of the Czech Republic | x | ||
| SUKL—State Institute for Drug Control | x | |||
| Denmark | DEFACTUM (formerly CFK) | x | x | |
| Estonia | UTA—Institute of Family Medicine and Public Health | x | ||
| Finland | FinCCHTA—Finnish Coordinating Center for Health Technology Assessment | x | x | x |
| FIMEA—Finnish Medicines Agency | x | |||
| THL—National Institute for Health and Welfare | x | |||
| France | HAS—French National Authority for Health (Haute Autorité de Santé) | x | x | x |
| AP-HP—Assistance publique- Hopitaux de Paris, FRANCE | x | |||
| Germany | DIMDI—German Institute for Medical Documentation and Information | x | ||
| GBA—Gemeinsamer Bundesausschuss | x | x | ||
| IQWIG—Institute for Quality and Efficiency in Health Care | x | x | ||
| Greece | EKAPTY-NKUA—National and Kapodistrian University of Athens | x | ||
| EKAPTY SA—National Evalution Center of Quality and Technology in S.A.- | x | |||
| EOF—National Organization for Medicines | x | |||
| EOPYY—National Organisation for Healthcare Provision | x | |||
| IFET—Institute of Pharmaceutical Research and Technology | x | |||
| OCSC—Onassis Cardiac Surgery Centre | x | |||
| Hungary | NIPN—National Institute of Pharmacy and Nutrition | x | ||
| SU—Health Services Management Training Center | x | |||
| Indonesia | CEEBM Center for Clinical Epidemiology-Evidence Based Medicine at Cipto Mangunkusumo Hospital | x | ||
| Ireland | HIQA—Health Information and Quality Authority | x | x | x |
| NCPE—National Centre for Pharmacoeconomics, St. James Hospital | x | |||
| Italy | AGENAS—National Agency for Regional Health Services | x | x | |
| UCSC Gemelli- University Hospital A. Gemelli | x | x | ||
| AIFA—Italian Medicines Agency | x | x | ||
| CRUF/AOUIVR—Centro Regionale Unico sul Farmacia del Veneto | x | |||
| DGFDM IT—Sede del Ministro–Ministero della salute | x | |||
| RER—Regione Emilia-Romagna | x | |||
| UVTA/AOP—Unita di Valutazione Technology Assessment | x | |||
| Veneto/CRUF—Regione Del Veneto–Area Sanità e Sociale | x | |||
| Kazakhstan | SK-NRCHD—Salidat Kairbekova National Research Center for Health Development* | x | ||
| Korea | NECA—National Evidence-based healthcare Collaborating Agency* | x | x | |
| Latvia | NVD—National Health Service | x | ||
| Lithuania | HI—The Institute of Hygiene | x | ||
| VASPVT—State Health Care Accreditation Agency | x | |||
| VVKT—State Medicines Control Agency of Lithuania | x | |||
| Malaysia | MaHTAS—Health Technology Assessment Section, Ministry of Health Malaysia* | x | x | |
| Malta | DPA/MoH Malta—Directorate for Pharmaceutical Affairs | x | ||
| Netherlands | EUR—Erasmus Universiteit Rotterdam | x | ||
| UU—Utrecht University | x | |||
| ZIN—National Health Care Institute | x | x | x | |
| ZonMw—The Netherlands Organisation for Health Research and Development* | x | |||
| Norway | NIPH—Norwegian Institute of Public Health | x | ||
| HDIR—Norwegian Directorate of Health | x | |||
| NIPHNO (formerly NOKC) —The Norwegian Institute of Public Health | x | x | ||
| NOMA—Norwegian Medicines Agency | x | |||
| Norwegian Centre for E-health Research | x | |||
| Peru | IETSI—Institute of Health Technology Assessment and Research | x | ||
| Poland | AOTMiT—Agency for Health Technology Assessment and Tariff System | x | x | |
| Portugal | ACSS IP—Administração Central do Sistema de Saúde, I.P. | x | ||
| INFARMED—National Authority of Medicines and Health Products | x | |||
| Romania | NIPHB—Institutu National De Sanatate Publica (INSP) | x | ||
| NSPHMPDB—National School of Public Health, Management and Professional | x | |||
| Development | ||||
| UBB—Babes-bolayi University, Cluj School of Public Health | x | |||
| Russian Federation | CHQA—Center for Healthcare Quality Assessment and Control | x | ||
| HTA Association | x | |||
| Singapore | ACE—Agency for Care Effectiveness | x | ||
| Slovakia | MoH Slovak Republic—Ministry of Health of the Slovak Republic | x | ||
| UniBA FOF—Comenius University in Bratislava | x | |||
| Slovenia | JAZMP—Public Agency of the Republic of Slovenia for Medicinal Products and | x | ||
| Medical Devices | ||||
| MoH Slovenia—Ministry of Health of the Republic of Slovenia | x | |||
| NIJZ—National institute of Public Health (NIJZ) | x | |||
| Spain | AEMPS—Agencia Española de Medicamentos y Productos Sanitarios | x | ||
| AETS-ISCIII—The Instituto De Salud Carlos III | x | |||
| AETSA—Andalusian HTA Agency | x | x | x | |
| AquAS—Agency for Health Quality and Assessment of Catalonia | x | x | ||
| AVALIA FNS—Fundacion Profesor Novoa Santos AVALIA-T—Galician Agency for HTA |
x | x | x x |
|
| BIOEF—Basque Foundation for Health Innovation and Research | x | |||
| DGFPS MSPSI-Directorate General for Pharmacy and Health Care Products | x | |||
| FPS—Fundación Pública Andaluza Progreso y Salud | x | |||
| FUNCANIS—Fundación Canaria de Investigación Sanitaria | x | |||
| IACS—Health Sciences Institute in Aragon, SPAIN | x | |||
| OSTEBA—Basque Office for Health Technology Assessment- Ministry for Health | x | x | x | |
| SESCS—Evaluation AND Planning Unit–Directorate of the Canary Islands Health Service | x | |||
| Sweden | SBU—Swedish Agency for Health Technology Assessment and Assessment of | x | x | |
| Social Services | ||||
| MPA—Medical Products Agency | x | |||
| TLV—Dental and Pharmaceutical Benefits Agency | x | x | ||
| Switzerland | SNHTA—Swiss Network for HTA | x | ||
| SFOPH—Swiss Federal Office of Public Health* | x | x | ||
| Tunisia | INEAS—National Authority for Assessment and Accreditation in Healthcare* | x | x | |
| Ukraine | MoH Ukraine—HTA Department of SEC of Ministry of Health of Ukraine* | x | x | |
| United Kingdom | HTW—Health Technology Wales | x | x | |
| HIS—Healthcare Improvement Scotland | x | x | x | |
| NICE—National Institute for Health and Care Excellence | x | x | x | |
| NIHR—National Institute for Health Research* | x | |||
| AWTTC—All Wales Therapeutics and Toxicology Centre | x | x | x | |
| United States | AHRQ—Agency for Healthcare Research and Quality | x | x | |
| Blue Cross Blue Shield Association | x | |||
| CMTP—Center for Medical Technology Policy | ||||
| ICER—Institute for Clinical and Economic Review | x | |||
| Kaiser Permanente | x | |||
| PCORI—Patient-Centered Outcomes Research Institute (USA) | x | |||
| Uruguay | HAD—Health Assessment Division, Ministry of Public Health | x | ||
| HAD—Health Assessment Division, Ministry of Public Health | x | |||
| EUnetHTA JA2 | x | |||
| Joint Action (JA) and public-private partners of EUnetHTA | ||||
| European Union | IMI—Innovative Medicines Initiative | x | ||
| ISPOR—International Society for Pharmacoeconomics and Outcomes Research | x | |||
Websites not available.
Table 2.
Number of HTA training initiatives by year, country, and promoting agency/organization.
| Year | N. of HTA training initiatives | Country | Agency/organization (N. of initiatives) |
|---|---|---|---|
| 2021 | 3 | Austria | UMIT (n. 3) |
| 2020 | 25 | Portugal | INFARMED (n. 2) |
| Spain | AEMPS (n. 1) | ||
| UK | HTW (n. 1) | ||
| Australia | AHTA (n.1) | ||
| Argentina | IECS (n. 6) | ||
| EU | ISPOR* (n. 13) | ||
| EU | IMI (n. 1) | ||
| 2019 | 7 | Italy | UCSC Gemelli (n. 1) |
| Portugal | INFARMED (n. 2) | ||
| Spain | FIISC (n. 1) | ||
| UK | AWTTC (n. 1) | ||
| UK | HTW (n. 1) | ||
| EU | ISPOR* (n. 1) | ||
| 2018 | 3 | Austria | AIHTA (n. 1) |
| Austria | GOG (n. 1) | ||
| EU | ISPOR* (n. 1) | ||
| 2017 | 3 | Austria | AIHTA (n. 1) |
| Austria | GOG (n. 1) | ||
| EU | ISPOR* (n.1) | ||
| 2016 | 7 | Austria | GOG (n. 1) |
| Italy | UCSC Gemelli (n. 1) | ||
| Portugal | INFARMED (n. 1) | ||
| Spain | AEMPS (n. 1) | ||
| Spain | AETSA (n. 1) | ||
| EU | ISPOR* (n. 1) | ||
| EU | EUnetHTA JA2 (n. 1) | ||
| 2015 | 3 | Canada | CADTH (n.1) |
| EU | ISPOR* (n. 1) | ||
| EU | EUnetHTA JA2 (n. 1) | ||
| 2014 | 1 | EU | EUnetHTA JA2 (n. 1) |
| 2013 | 0 | - | - |
| 2012 | 1 | Spain | BIOEF (n. 1) |
| 2011 | 0 | - | - |
| 2010 | 1 | Belgium | KCE (n. 1) |
| 2009 | 2 | Austria | AIHTA (n. 1) |
| Spain | AEMPS (n. 1) |
One training initiative provided by ISPOR was replicated annually for 6 years (2015–2020).
Table 3.
Number of overall training initiatives provided by EU and non-EU HTA agencies/organizations at national/international level.
| Country | Agency/organization | N. of HTA training initiatives |
|---|---|---|
| Argentina | IECS | 6 |
| Australia | AHTA | 1 |
| Austria | AIHTA | 3 |
| UMIT | 3 | |
| GOG | 3 | |
| Belgium | KCE | 1 |
| Canada | CADTH | 1 |
| Italy | UCSC Gemelli | 2 |
| The Netherlands | Erasmus Universiteit Rotterdam | 1 |
| Portugal | INFARMED | 5 |
| Romania | NSPHMPDB | 1 |
| Spain | AEMPS | 3 |
| FIISC | 1 | |
| BIOEF | 1 | |
| AETSA | 1 | |
| United Kingdom | NICE | 1 |
| AWTTC | 2 | |
| HTW | 2 | |
| Public-private partners of EUnetHTA and its JA | ISPOR | 13 |
| EUnetHTA JA2 | 3 | |
| IMI | 1 |
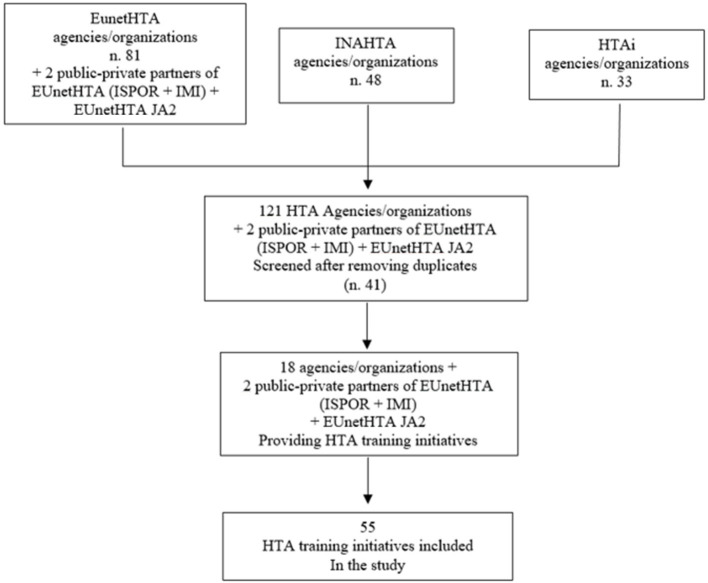
The screening process is shown in Figure 1.
Figure 1.
Flowchart of the selection process.
Overall, Austria (n = 9), Argentina (n = 6), Spain (n = 6), Portugal (n = 5), and UK (n = 5) were the countries with the majority of training initiatives.
Regarding the topic of the training initiatives included in our study, 21 of them (14, 16, 18, 23–26, 31, 32, 36, 37, 39, 43, 56–61, 66, 68) addressed the general methodology and applications of HTA, and 34 focused on specific HTA domains, especially on economic evaluations (n = 22). The technologies covered were the following: health technologies in general (n = 42), drugs (n = 12), genetic therapies (n = 2), and medical devices (n = 1).
Training Initiatives Provided by European HTA Agencies/Organizations
Table 4 shows the training initiatives provided by European HTA agencies/organizations at national level.
Table 4.
Training initiatives provided by EU HTA agencies/organizations at national level.
| Country | Promoting agency/organization | Training initiatives title | Year of delivery | Type of training initiative | Target | Topic | Technology addressed in the course | Language |
|---|---|---|---|---|---|---|---|---|
| Austria | AIHTA—Austrian Institute for Health Technology Assessment | 1st Workshop of the EUnetHTA Task Force on HTA and Medical Devices (14) | 2018 | Workshop | HTA professionals | HTA regulation and legal framework | Medical devices | English |
| Workshop series: Ethik and HTA (15) | 2017 | Workshop | HTA professionals, health policy decision-makers, experts in applied ethics in HTA | Ethical methodology in HTA | Health technology | German | ||
| Workshop Health Technology Assessment (16) | 2009 | Workshop | Healthcare decision makers | HTA methodology and application | Health technology | English/German | ||
| UMIT— University for Health Sciences, Medical Informatics and Technology |
Modeling Approaches for HTA: A Practical Hands-on Workshop (17) | 2021 | Three-day course | Healthcare and health policy organizations, national HTA Agencies; Pharmaceutical and medical device industry; Academia and research institutions; Health insurances/sickness funds; Consultancy organizations. |
HTA methodology | Health technology | English | |
| Introduction to health technology assessment HTADS—program on health technology (1)assessment and decision sciences (18) | 2021 | Four-day course | Healthcare and health policy organizations, national HTA Agencies; Pharmaceutical and medical device industry -Academia and research institutions -Health insurances/sickness funds-Consultancy organizations |
HTA methodology | Health technology | English | ||
| Causal inference for assessing effectiveness in real world data and clinical trials: a practical hands-on workshop (19) | 2021 | Five-day course | Healthcare and health policy organizations, national HTA agencies regulatory agencies; pharmaceutical and medical device industry, academia and research institutions, health insurances, consultancy organizations. | Clinical utility (effectiveness) | Health technology | English | ||
| GOG—Gesundheit Österreich GmbH/Geschäftsbereich | 3rd summer school pharmaceutical pricing and reimbursement policies (20) | 2018 | Summer school/5 days training course | Professionals of public and non-for profit institutions working in the field of pricing and reimbursement of medicines | Economic evaluation | Drugs | English | |
| 2nd summer school pharmaceutical pricing and reimbursement policies (21) | 2017 | Summer school/5 days training course | Professionals of public and non-for profit institutions working in the field of pricing and reimbursement of medicines | Economic evaluation | Drugs | English | ||
| 1st summer school pharmaceutical pricing and reimbursement policies (22) | 2016 | Summer school/5 days training course | Professionals of public and non-for profit institutions working in the field of pricing and reimbursement of medicines | Economic evaluation | Drugs | English, and simultaneous translation into Russian | ||
| Belgium | KCE— belgian health care centre |
HTA workshop | collaboration between HTA agencies in practice: learning from actual experiences (23) | 2010 | Workshop | HTA agencies | HTA methodology | Health technology | English |
| Italy | UCSC Gemelli | Health impact assessment e health technology assessment (24) |
2019 | In attendance course | Researchers and health managers | HTA and HIA methodology | Health technology | Italian |
| Health technology assessment (25) | 2016 | In attendance course | Healthcare professionals | HTA methodology and application | Health technology | Italian | ||
| Netherlands | Erasmus Universiteit Rotterdam |
MOOC health technology assessment (26) | N.A.* | Massive open online course (MOOC) | Health economics policy and law master students, health economics master students and the research master students | HTA methodology and application | Health technology | English |
| Portugal | INFARMED- National Authority of Medicines and Health Products |
Health technology assessment training program (68) | 2016 | Online course | Professionals from government and health insurance funds, HTA bodies, public and private payers and health plans, industry, academia, and patient group representatives; | HTA methodology and application | Health technology | English |
| Pharmacovigilance course (Curso de atualização em farmacovigilância) (28) | 2020 | Online course | Health professionals | Clinical utility (safety) | Drugs | Portuguese | ||
| Curso Pós-Graduado De Atualização: Assessing Therapeutic Efectiveness In Drug Lifecycle (29) | 2019 | In attendance course | Academics and health care professionals, PhD students and master students | Clinical utility (effectiveness) | Drugs | English | ||
| Artificial intelligence in health: governance, accountability and decision-making (Inteligência artificial em saúde: governança, responsabilidade e tomada de decisão) (30) | 2019 | In attendance course | Higher education professionals and students in the fields of health, social sciences, management, computer science and engineering. | Ethical and legal aspects | Digital health technology | Portuguese | ||
| Introduction to value-based health care management (Introdução à Gestão de Cuidados de Saúde Baseada em Valor) (31) | 2020 | b-learning course | Healthcare professionals | HTA application and Value Based Healthcare | Health technology | English | ||
| Romania | NSPHMPDB- The National School of Public Health, Management and Professional Development |
Training programs in both public health and management (32) | N.A.* | In attendance Courses | All categories of staff in public health and management and other areas of the health system | HTA methodology and application | Health technology | Romanian |
| Spain | AEMPS- La Agencia Española de Medicamentos y Productos Sanitarios |
Technical training of biosimilar medicines (Capacitación técnica de medicamentos biosimilares) (33) | 2020 | Online course | Specialized and qualified representatives from seventeen Ibero-American countries. | Legal and regulatory processes | Drugs | Spanish |
| 3rd training course in standards of good clinical practice for independent researchers (3° curso de formación en normas de buena práctica clínica para investigadores independientes) (34) | 2009 | In attendance course | Clinical researchers | Ethical and legal aspects | Health technology and drugs | Spanish | ||
| Practical course for conducting pharmacoepideomyology studies with the bifap database (curso practico para la realización de estudios de farmacoepidemiologia con la base de datos bifap) (35) | 2016 | In attendance course | Public researchers | Clinical utility (safety) | Drugs | Spanish | ||
| FIISC—Funcanis_Fundación Canaria de Investigación Sanitaria | Application of molecular biology in the diagnosis and follow-up of chronic lymphocytic leukemia (Aplicación de la Biología molecular en el diagnóstico y seguimiento de la Leucemia Linfocítica crónica) (36) | 2019 | In attendance Course | Graduates and certified lab technicians | HTA application | Genetics, molecular biology and mass sequencing | Spanish | |
| BIOEF— Basque Foundation for Health Innovation and Research |
UPV/EHU summer course on health research and innovation (37) | 2012 | In attendance course | Healthcare professionals, clinical researchers | HTA applications | Health technology | Spanish | |
| AETSA— Andalusian HTA Agency |
Introduction of the economic assessment in the health technology assessment (38) | 2016 | Workshop | Andalusian public health system (SSPA) professionals interested in financial evaluation | Economic evaluation | Health technology and drugs | Spanish | |
| United Kingdom | NICE | Seminars (39) | N.A.* | Seminars; Advanced workshops | Pharmaceutical, medical technology or cell and gene therapy sectors | HTA applications | Health technology, drugs, gene therapy | English |
| AWTTC— All Wales Therapeutics and Toxicology Centre |
AWMSG training day (40) | 2019 | In attendance course | Members and deputies of AWMSG; new medicines group and the all wales prescribing advisory group; medicines and therapeutics committees | Economic evaluation | Orphan medicines | English | |
| Adverse drug reactions: reporting makes medicines safer (41) | N.A.* | Online course | Healthcare professionals | Clinical utility (Safety) | Drugs | English | ||
| HTW— Health Technology Wales |
Health technology assessment and economics (42) | 2019 | Workshop | NHS front line staff; NHS Financial, Medical and Planning Directors; Care commissioners; Workforce managers; Patients; Members of the public; Academia; Technology developers; Industry representatives | Economic evaluation | Health technology | English | |
| Value in health week (43) | 2020 | Webinar | All stakeholders | HTA dimensions and applications | Health technology | English |
The year in which training initiative was provided was Not Available (N.A.).
Training Initiatives Addressing Principles, General Methodology, and Application of HTA
Among the 30 training initiatives (14–43) provided by European HTA agencies/organizations, 47% (n = 14) addressed the general aspects of HTA principles, methodology and application: Austria (n = 3); Spain (n = 2); Portugal (n = 2); UK (n = 2); Italy (n = 2); Romania (n = 1); Belgium (n = 1) and the Netherlands (n = 1).
The Austrian Institute for Health Technology Assessment GmbH (AITHA) organized in 2009 a workshop in English and German for healthcare decision-makers, addressing HTA application and methodology, such as systematic review research, medical statistics and clinical epidemiology appraisal, and decisions in health policy (16). These topics were covered also in two English courses organized in 2021, by The University for Health Sciences, Medical Informatics and Technology (UMIT) in Austria, directed to healthcare professionals and health policy organizations, national HTA agencies, industry, academia and research institution and consultancy organizations (17, 18).
In Spain, a course offered by the Fundación Canaria de Investigación Sanitaria for lab technicians, discussed the HTA applications in genetics and molecular biology, technologies used and mass sequencing (36). In 2016, the course by the Basque Foundation for Health Innovation and Research (BIOEF), for healthcare professionals and clinical researchers, covered HTA applications and the translation from research to clinical practice (37).
Two courses (27, 31) were organized by the National Authority of Medicines and Health Products (INFARMED) in Portugal. The 2016 English course, on HTA principles and multi-perspective approach, was directed to professionals from all relevant sectors, including government and health insurance funds, HTA bodies, public and private payers and industry, academia, and patient group representatives (27). In 2020, a b-learning English course focused on HTA application and Value Based Health care was directed to healthcare professionals (31). The latter were also the topic of a webinar organized in October 2020, by the Health Technology Wales, considering the benefits and challenges of their application in Wales' national health system (43). In UK, HTA principles and application regarding health technology, drugs and gene therapy were part of the NICE seminars/workshops for professionals in the pharmaceutical, medical technology or gene therapy sectors (39).
In Italy, in-attendance courses regarding the HTA methodology in Italian language were provided by Università Cattolica del Sacro Cuore, Policlinico Universitario Agostino Gemelli IRCCS (Rome) for healthcare professionals in 2016 (25) and for researchers and health managers in 2019 (24).
Also, in Romania the National School of Public Health, Management and Professional Development offers periodically several courses in this topic for professionals in public health and management sectors (32). The Belgian Health Care Knowledge Centre (KCE) organized in 2010 a workshop in English addressing professionals working on HTA agencies on HTA applications, collaboration and cooperation (23). In the Netherland, the Erasmus University Rotterdam launched a MOOC in English on the HTA principles and their application in the policy context about new and existing health technologies (26).
Training Initiatives Addressing HTA Specific Domains of the EUnetHTA Core Model
Among the 30 training initiatives provided by European HTA agencies/organizations, 16 (53%) focused on specific HTA domains, as following: 6 initiatives on economic evaluations (20–22, 38, 40, 42); 5 on ethical and legal aspects (14, 15, 30, 33, 34), two on clinical effectiveness (19, 29) and three courses on safety (28, 35, 41).
Economic Evaluations
Health economic evaluation was the topic of 6 courses (20–22, 38, 40, 42): three in Austria (20–22), two in UK (40, 42) and one in Spain (38). The Austrian National Public Health Institute (GOG) organized, for 3 years (2016–2018), a 5-day training course on pharmaceutical pricing and reimbursement policies for professionals of public and non-for profit institutions working in this field (20–22).
In UK, the All Wales Therapeutics and Toxicology Centre agency organized in 2019 an in-attendance course on the health opportunity costs of orphan medicines and the policy implications of decision-making (40). Instead, in October 2020, the Health Technology Wales offered to all stakeholders a webinar on economic evaluation methodology used to understand the cost effectiveness of healthcare technologies and their impact on resources (42).
In Spain, a workshop in Spanish organized by the Andalusian HTA Agency (AETSA) for professionals of the Andalusian public health system was held in 2016 (38). The course topic was the importance and typology of economic evaluation for health technologies and pharmaceuticals.
Ethical and Legal Aspects
Five courses (14, 15, 30, 33, 34) addressed the ethical and legal aspects of health technologies: two courses in Austria (14, 15), two in Spain (33, 34) and one in Portugal (30). In Austria, AIHTA organized a course in German language in 2017 regarding the ethical methodology, targeted for HTA professionals, health policy decision-makers and experts in applied ethics in HTA (15). The other workshop, addressing only HTA professionals, was held in 2018, in English, on HTA regulatory and legal frameworks for medical devices (14). In Spain, the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS), organized in attendance courses in Spanish, for clinical researchers in 2009 (34) and for public researchers in 2020 (33), on the ethical aspects, legal and regulatory processes of drug administration. The ethical and legal implications of the use of digital technologies in healthcare were covered at the Portuguese in-attendance course offered by INFRAMED, addressed to higher education professionals in the fields of health, social sciences, management, computer science and engineering (30).
Clinical Effectiveness
Two courses focused on the effectiveness of health technologies (19, 29). In Portugal, INFRAMED organized in 2019 an in-attendance English course on the therapeutic effectiveness and the evaluation methodology, directed to academic and healthcare professionals (29). In Austria, UMIT provided in 2021 an English course on the methodology for assessing the effectiveness of real world data and clinical trials, to healthcare professionals in policy organizational, national HTA agencies, health industry or academia (19).
Safety
Three courses addressed the safety of health technologies: one course in Portugal (28), one in UK (41) and one in Spain (35). In Portugal, INFARMED provided in 2020 an online course in Portuguese for healthcare professionals on HTA application in pharmacovigilance (28). Pharmacovigilance was the topic also two other courses: an online course organized by the All Wales Therapeutics and Toxicology Centre in UK (41), and an in-attendance course on 2016 by AEPMS in Spain (35).
Training Initiatives Provided at EU Level by Public-Private Partners of EUnetHTA and Its JA
We screened also the training initiatives proposed at European level by EUnetHTA as part of its JA and conducted in collaboration with other public-private partners such as ISPOR and IMI.
Seventeen training initiatives were publicly available at EUnetHTA website (27, 44–59), of which 13 were organized by ISPOR (27, 45–55), three by EUnetHTA JA2 (56–58) and one by IMI (59). These initiatives are reported in Table 5.
Table 5.
Training initiatives* provided at EU level by public-private partners of EUnetHTA.
| Promoter | Training initiatives title | Year of delivery | Type of training initiative | Target | Topic | Technology addressed in the course |
|---|---|---|---|---|---|---|
| ISPOR— International Society for Pharmacoeconomics and Outcomes Research |
Health technology assessment training program (27) | 2015; 2016; 2017; 2018; 2019; 2020 | In attendance course | Users and doers in government - Public and private payers industry, health plans, academia - Patient group representatives |
Economic evaluation (budget impact analysis; cost evaluation) | Health technology |
| Modeling health care costs- part I characteristics of health care costs (44) | 2020 | Virtual training | Healthcare stakeholders | Economic evaluation (budget impact analysis; cost evaluation) | Health technology | |
| Modeling health care costs—Part II: methods and guidelines for estimating health care costs (45) | 2020 | Virtual training | Healthcare stakeholders | Economic evaluation (cost-effectiveness analysis; cost-utility analysis) | Health technology | |
| Modeling health care costs—part III: estimation from censored data (46) | 2020 | Virtual training | Healthcare stakeholders | Economic evaluation (methodology; budget impact analysis) | Health technology | |
| Markov model toolkit: concepts, assumptions and examples (47) | 2020 | Virtual training | Healthcare stakeholders | Economic evaluation (Markov modelling) | Health technology | |
| Introduction to pharmaco-economics (48) | 2020 | Virtual training | Healthcare stakeholders | Economic evaluation (cost-effectiveness analyses) | Drugs | |
| Cost-of-illness/cost-estimation (COI/CE) (49) | 2020 | Virtual training | Healthcare stakeholders | Economic evaluation (cost-of-illness/cost-estimation) | Health technology | |
| Cost-minimization/cost-consequence (CMA/CCA) (50) | 2020 | Virtual training | Healthcare stakeholders | Economic evaluation (cost-of-illness /cost-estimation) | Health technology | |
| Introduction to budget impact analysis (BIA) - part I (51) | 2020 | Virtual training | Healthcare stakeholders | Economic evaluation (budget impact analysis) | Health technology | |
| Introduction to budget impact analysis (BIA) - part II (52) | 2020 | Virtual training | Healthcare stakeholders | Economic evaluation (budget impact analysis) | Health technology | |
| Cost-effectiveness analysis (CEA) and cost-utility analysis (CUA) (53) | 2020 | Virtual training | Healthcare stakeholders | Economic evaluation (cost-effectiveness analysis and cost-utility analysis) | Health technology | |
| Patient reported outcomes: analysis and interpretation (54) | 2020 | Virtual training | Healthcare stakeholders | Patients reported outcomes | Health technology | |
| Patient reported outcomes: instrument development (55) | 2020 | Virtual training | Healthcare providers | Patients reported outcomes | Health technology | |
| EUnetHTA JA2 | HTA core model training course (56) | 2015 | In-attendance Training Course | HTA Agencies | HTA methodology (HTA core model) and application | Health technology |
| Overview of HTA core model training materials (57) | 2016 | b-learning course | EUnetHTA members Agencies | HTA methodology (HTA core model) and application | Health technology | |
| Key principles of HTA or what is meant for HTA (58) | 2014 | Training Course | EUnetHTA stakeholders | HTA methodology and application | Health technology | |
| IMI—Innovative Medicines Initiative | Real-world evidence in medicine development, - getreal (59) | 2020 | Virtual course | Pharmaceutical companies regulatory authorities health technology assessment bodies patients' organizations | HTA methodology and application | Health technology |
All courses were in English language.
Four training initiatives addressed HTA methodology and application (56–59), whereas 13 focused on specific domains, such as economic evaluations (n = 11) (27, 44–53) and Patients Reported Outcomes (PROs) (n = 2) (54, 55).
Among the initiatives on HTA methodology and application, three were organized by EUnetHTA JA2 in English and directed to stakeholders of EUnetHTA agency members (56–58). The first was held in 2014, about the key principles, definition, purpose, history and use of HTA (58). The other two, in 2015 (56) and 2016 (57), focused on HTA core model and applications to produce core HTA information. Moreover, IMI organized the course “Get Real” on the techniques, opportunities and challenges for the use of real-world evidence in medicine development, directed to healthcare professionals in pharmaceutical companies, regulatory authorities, HTA bodies, patients' organizations, consultancy companies, and academia (59).
Training initiatives on specific HTA domains were organized by ISPOR in English and for healthcare stakeholders. Of these 11 courses were on economic evaluations (n = 11) (27, 44–53) and focused on the methodology of cost-effectiveness, cost-utility and budget impact analysis. Two courses were on PROs (n = 2) and addressed the interpretation, analysis and the methodological issues related to the use of PRO tools (54, 55).
Training Initiatives Provided by Non-EU HTA Agencies/Organizations
Eight training initiatives were provided by HTA agencies/organizations at non-European level (60–67): 6 courses from an HTA organization in Argentina (62–67), one in Australia (60) and the other one in Canada (61) (Table 6). Among those, three focused on the HTA principles and methodology, whereas five on the economic evaluations.
Table 6.
Training initiatives provided by non- EU HTA agencies/organizations at international level.
| Country | Promoting agency/organization | Training initiatives title | Year of delivery | Type of training initiative | Target | Topic | Technology addressed in the course | Language |
|---|---|---|---|---|---|---|---|---|
| Australia | AHTA— Adelaide Health Technology Assessment |
Health technology assessment 2020 online course handbook (60) | 2020 | Online course handbook | Health professionals | HTA methodology | Health technology | English |
| Canada | CADTH—Canadian Agency for Drugs and Technologies in Health | Health technology assessment for decision makers HTA institute 2015 (61) | 2015 | Three days intensive course | Decision-makers | HTA methodology | Health technology | English |
| Argentina | IECS— Institute for clinical effectiveness and health policy |
Introducción a las Evaluaciones de Tecnologías Sanitarias y Evaluaciones Económicas (62) | 2020 | Distance learning course | Health professionals working in ministries, secretariats, regulatory agencies, medical directorates, managers of private health systems, pharmaceutical companies and producers of health technologies. | Economic evaluation (cost-effectiveness; cost-utility) | Health technology | Spanish |
| Evaluaciones Económicas: Programación, análisis e interpretación de modelos de decision (63) | 2020 | Distance learning course | Health professionals working in ministries, secretariats, regulatory agencies, medical directorates, managers of private health systems, pharmaceutical companies and producers of health technologies. | Economic evaluation (cost-effectiveness; cost-utility) | Health technology | Spanish | ||
| Estimación de costos para las evaluaciones económicas de programas, servicios y tecnologías en salud (64) | 2020 | Distance learning course | Health professionals working in ministries, secretariats, regulatory agencies, medical directorates, managers of private health systems, pharmaceutical companies and producers of health technologies. | Economic evaluation (cost-analysis) | Health technology | Spanish | ||
| Diseño, programación y análisis de modelos de Markov (65) | 2020 | Distance learning course | Health professionals working in ministries, secretariats, regulatory agencies, medical directorates, managers of private health systems, pharmaceutical companies and producers of health technologies. | Economic evaluation (cost-effectiveness; markov model) | Health technology | Spanish | ||
| Desarrollo e implementación de evaluaciones de tecnologías sanitarias (66) | 2020 | Distance learning course | Health professionals working in ministries, secretariats, regulatory agencies, medical directorates, managers of private health systems, pharmaceutical companies and producers of health technologies. | HTA methodology | Health technology | Spanish | ||
| Análisis de impacto presupuestario (AIP) en salud (67) | 2020 | Distance learning course | Health professionals working in ministries, secretariats, regulatory agencies, medical directorates, managers of private health systems, pharmaceutical companies and producers of health technologies. | Economic evaluation | Health technology | Spanish |
The initiatives on HTA principles and methodology were organized by the Canadian Agency for Drugs and Technologies in Health (CADTH) (61), the Australian Hand Therapy Association (AHTA) (60) and the Institute for Clinical Effectiveness and Health Policy (IECS) (66). In Canada, CADTH organized, in 2015, a 3 days intensive course on to decision-makers (61), whereas in Australia, AHTA offered in 2020 an online course directed to health professionals (60). IECS organized, in 2020 in Argentina, a distance learning course in Spanish, on the tools and knowledge necessary to apply HTA to health decision-making, for health professionals in ministries, regulatory agencies, medical directorates, private health systems and pharmaceutical companies (66). Moreover, IECS provided five distance learning courses, in 2020, targeting the same health professionals, covered the concepts of economic evaluations, such as cost-effectiveness, cost-utility, cost-minimization, and their application (62–65, 67).
Discussion
Our study presents an overview of HTA training initiatives provided to healthcare professionals by international HTA agencies and organizations. The results of our screening showed that to date only a part of the international HTA agencies/organizations carry out training on HTA for healthcare professionals. In fact, out of 124 agencies/organizations consulted only 18 delivered specific training initiatives on HTA for our target population in recent years. Starting from 2009, until 2021, we identified only 55 publicly available courses, the majority of which organized in the last 5 years. Most of the courses were offered by European HTA agencies/organizations, particularly in Austria, Spain, Portugal and UK. Overall, the economic evaluations and general HTA methodology were the main focus of the identified training initiatives.
The first courses were organized in the period 2009–2012, by European agencies/organizations in Austria, Belgium, and Spain, on HTA methodology and ethical principles. This could be related to the EUnetHTA publication, in 2008, of the Handbook on HTA Capacity Building (69). This document highlighted the need to train the internal staff of HTA organizations, through effective educational tools, in accordance with organization and staff qualifications (69). As proposed by EUnetHTA in its handbook, training in HTA should focus on two main aspects: (1) understanding the results of HTA and implementing them in evidence-based health policies and (2) providing continuing training in HTA for its greater application in the healthcare.
National HTA capacity building was one of the focal points of the EUnetHTA JA2 (70), which since 2014, organized training courses on HTA methodology and its key concepts, for 3 consecutive years. In this regard, training of potential HTA agency members, as the practical users, improves general understanding of the HTA impact in decision-making and strengthens the practical application of tools and approaches for a sustainable cross-border HTA collaboration.
Following such initiatives, to address the probable shortage of HTA specialists in front of the large number of new and existing technologies, other European and non-European HTA agencies organized courses focusing on HTA procedures and methodologies. The HTA approach varied among counties according to national healthcare system, organization (central vs. regional); funding, insurance and reimbursement schemes (tax-based vs. social insurance-based); or the perspective used in HTA (health system vs. societal) (71).
Probably due to the fact that HTA is not mandatory in the decision-making process of health policy (69), its application in different countries is very heterogeneous, leading to a different prioritization at a global level of health technologies to bring into market. In fact, the national socio-economic perspective is one of the main drivers of decision-making, and decision-makers mostly prioritize cost-effectiveness analysis and clinical utility when introducing a new technology (72). However, the decision-making process, to be effective, should be based on multi-stakeholder cooperation and should encompass all dimensions of health technology evaluation such as medical, economics, social, legal and ethical (5). The results of our study show that organizational, ethical, social and legal aspects are less addressed in training courses than the economic domain, confirming also in the training field a greater interest in the economic aspects related to health technologies to be evaluated. However, training on the general HTA methodology is also crucial and this is also evident from the results of our study. In fact, out of 55 identified training initiatives, 21 were focused on the general methodology of HTA and its application. Scientific evidence produced with the HTA approach is needed to understand the key concepts of any health technology (drugs, medical device or public health interventions) and a basic knowledge of the HTA methodology should be “at hand” of the healthcare professionals and all stakeholders of health system. Another important finding emerged from our study is the greater consideration, also in the training field, of pharmaceutics compared to other health technologies. In fact, in the courses identified the technologies considered were mainly pharmaceutics, two genetic therapies and one medical devices. Over the years, the HTA methodology was mainly applied to pharmaceutics evaluation. Taking into account the rapid developments in particular in pharmaceutical sector, such as the oncology, over the last decade, HTA was used to support mostly decision-making related to drugs, aiming to assess their therapeutic value and the economic impact on health systems (73).
However, the application of HTA will must be implemented as well as training in this field, also for medical devices and for other health technologies such as digital ones, in relation to the disruptive innovation of recent years and the near future (74). On December 2021, the new Regulation on HTA has been adopted. The Regulation on HTA (75) enters into force in January 2022 and applies as of January 2025. It contributes to improving the availability of innovative health technologies—such as medicines, certain medical devices, medical equipment, and prevention and treatment methods—for EU patients, it ensures efficient use of resources and strengthens the quality of HTA across the Union. Furthermore, it provides a transparent and inclusive framework by establishing a Coordination Group of HTA national or regional authorities, a stakeholder network and by laying down rules on the involvement in joint clinical assessments and joint scientific consultations of patients, clinical experts and other relevant experts. It will also reduce duplication of efforts for national HTA authorities and industry, facilitate business predictability and ensure the long-term sustainability of EU HTA cooperation (75).
Obviously, the application of the new Regulation on HTA further imposes training programs for healthcare professionals and for all actors of the health system in the HTA field, in order to ensure its correct application throughout the EU.
Over the years, the lack of training opportunities was considered the main challenge for the implementation of the HTA application (76, 77). The 2015 global survey of national HTA authorities reported the lack of qualified human resources, information, knowledge and methodology, as main barriers in undertaking and using HTA in nearly 59 countries (72). Despite the various efforts made by several countries, the scarcity of training programs represents a critical issue concerning the necessity to encounter training needs of healthcare professionals using the HTA approach.
HTA requires multi-disciplinary skills and core competencies, which should be synergistically involved and empowered. A recent article on capacity building in HTA agencies reported the ability to design, conduct, evaluate and to understand HTA applications to decision making, as the main training needs for an efficient and effective HTA process (78). According to the proposal by the HTAi Scientific Development and Capacity Building Committee, HTA capacity building represents a much broader suite of activities than simply training of core HTA staff in technical competencies (79).
Our study is the first that, to our knowledge, mapped the existing training initiatives in HTA for healthcare professionals, provided by international HTA agencies and organizations around the world, underlining the need for greater training in HTA for this target population. Training that should be focused, in particular, on the general HTA methodology and on all dimensions related to health technology, not just the economic one.
However, the results of the present work should be interpreted in the light of some methodological limitations. The desk research only explored the websites of EUnetHTA, INAHTA, and HTAi members, suggesting that other HTA agencies/organizations, not affiliated with these networks, might not were captured. Although the search was extensive in English, French, Portuguese, Spanish, Italian, and German, training initiatives in other national languages might not were retrieved, thus indicating a potential publication bias. Moreover, given that not all the courses were publicly available, we could not extract all their information. In this overview, it was not even possible to verify which ones were paid and which were free. However, price information could be very useful as some training courses can be very expensive and this could be an obstacle, for example, for participants from low-income countries.
However, our search strategy was extensive and conducted rigorously, providing a wide overview of the training initiatives provided by HTA agencies/organizations at European and international level.
In conclusion, considering that HTA represents a bridge between scientific evidence and policy decision-making, the training of healthcare professionals in this field should be a key driver for increasing the correct use of HTA, as an applicable tool for health governance and keeping up with technological innovations. From our work emerged the need for developing a structured HTA capacity-building, providing a basic knowledge in HTA principles and methodology, and enhancing the evaluation of all domains of the health technology assessment process. Future skills implementation programs will need to pay particular attention to the training needs of all healthcare professionals involved in the use of health technologies and in their assessment process.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
SB and GC critically reviewed the manuscript and conceived the study critically reviewed the manuscript. IH and CC identified the HTA training initiatives through a search of websites of EUnetHTA, INAHTA and HTAi members, performed the data extraction, and contributed equally to the drafting of the paper. GC supervised IH and CC. GC, IH, and CC critically discussed and interpreted the results of the desk research. All authors contributed to the article and approved the final version.
Funding
This work was supported by the National Center for Disease Prevention and Control (CCM), Italian Ministry of Health (CUP J54I20000350001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Jakovljevic M, Roshi EE, Rancic N, Gutiérrez-Ibarluzea I, Chiumente M, Dauben H-P. The life cycle of health technologies. Challenges and ways forward. Front Pharmacol. (2017) 1:14. 10.3389/fphar.2017.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calabrò GE, Torre G La, De Waure C, Villari P, Federici A, Ricciardi W, et al. Disinvestment in healthcare: an overview of HTA agencies and organizations activities at European level. BMC Health Serv Res. (2018) 18:148. 10.1186/s12913-018-2941-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elshaug AG, Hiller JE, Moss JR. Exploring policy-makers' perspectives on disinvestment from ineffective healthcare practices. Int J Technol Assess Health Care. (2021) 24:1–9. 10.1017/S0266462307080014 [DOI] [PubMed] [Google Scholar]
- 4.Nolte E, Kluge H, Figueras J. World health Organization (WHO)/European Observatory on Health Systems Policies. How Do We Ensure that Innovation in Health Service Delivery Organization Is Implemented, Sustained Spread? (2018). p. 1–28. Available online at: http://www.euro.who.int/en/about-us/partners/%0A; http://www.euro.who.int/_data/assets/pdf_file/0004/380731/pb-tallinn-03-eng.pdf?ua=1
- 5.HTAi . HTA Definition. Available online at: https://htai.org/about-htai/ (accessed May 1, 2021).
- 6.Mitton C, Seixas BV, Peacock S., Michael B., Bryan S. Health technology assessment as part of a broader process for priority setting and resource allocation. Appl Health Econ Health Policy. (2019) 17:573–6. 10.1007/s40258-019-00488-1 [DOI] [PubMed] [Google Scholar]
- 7.Carlos RC, Goeree R. Introduction: health technology assessment in diagnostic imaging. J Am Coll Radiol. (2009) 6:297–8. 10.1016/j.jacr.2009.01.025 [DOI] [PubMed] [Google Scholar]
- 8.Sorenson C, Drummond M, Kanavos P. Ensuring Value for Money in Health Care: The Role of Health Technology Assessment in the European Union. Copenhagen: European Observatory on Health Systems Policies (2008). p. 14–32. Available online at: https://apps.who.int/iris/bitstream/handle/10665/107886/9789289071833-eng.pdf?sequence=4&isAllowed=y
- 9.Douw K, Vondeling H, Bakketeig LS, Gabbay J, Hansen NW, Kristensen FB. HTA education and training in Europe. Int J Technol Assess Health Care. (2021) 18:808–19. 10.1017/S0266462302000612 [DOI] [PubMed] [Google Scholar]
- 10.EUnetHTA . EUnetHTA Network. Available online at: https://EUnetHTA.eu/about-EUnetHTA/EUnetHTAnetwork/ (accessed February 2021).
- 11.INAHTA . INAHTA Members List. Available online at: https://www.inahta.org/members/members_list/ (accessed February 2021).
- 12.HTAi . HTAi Organizational Members. Available online at: https://htai.org/membership/organizational-members/ (accessed February 2021).
- 13.EUnetHTA . HTA Core Model. Available online at: https://www.EUnetHTA.eu/hta-core-model/ (accessed February 2021).
- 14.AIHTA . Agenda of the 1st Workshop of the EUnetHTA Task Force on HTA and Medical Devices. Vienna: AIHTA; (2018). [Google Scholar]
- 15.AIHTA . Workshop-Reihe: Ethik und HTA. (2017). Available online at: https://aihta.at/uploads/ckEditor/fields_body_translation_de/workshops-ethik-und-hta-2017-final.pdf (accessed February 2021).
- 16.AIHTA . Workshop Health Technology Assessment. Krems: AIHTA; (2009). [Google Scholar]
- 17.UMIT . Modeling Approaches for HTA: A Practical Hands-on Workshop. (2021). Available online at: https://www.umit-tirol.at//page.cfm?vpath=departments/public_health/htads-continuing-education-program/modeling-for-hta (accessed February 2021).
- 18.UMIT . Introduction to Health Technology Assessment. (2021). Available online at: https://www.umit-tirol.at//page.cfm?vpath=departments/public_health/htads-continuing-education-program/introduction-to-hta (accessed January 26, 2022).
- 19.UMIT . Causal Inference for Assessing Effectiveness in Real World Data and Clinical Trials: A Practical Hands-on Workshop. (2021). Available online at: https://www.umit-tirol.at//page.cfm?vpath=departments/public_health/htads-continuing-education-program/causal-inference (accessed February 2021).
- 20.PPRI . 3rd Summer School Pharmaceutical Pricing and Reimbursement Policies. (2018). Available online at: https://ppri.goeg.at/summerschool2018 (accessed February 2021).
- 21.PPRI . 2nd Summer School Pharmaceutical Pricing and Reimbursement Policies. (2017). Available online at: https://ppri.goeg.at/summerschool2017 (accessed February 2021).
- 22.PPRI . 1st Summer School Pharmaceutical Pricing and Reimbursement Policies. (2016). Available online at: https://ppri.goeg.at/summerschool2016 (accessed February 2021).
- 23.KCE . HTA Workshop Collaboration Between HTA Agencies in Practice : Learning from Actual Experiences. (2010). Available online at: https://kce.fgov.be/en/event/hta-workshop-collaboration-between-hta-agencies-in-practice-learning-from-actual-experiences-b (accessed February 2021).
- 24.UCSC-Gemelli . Health Impact Assessment (HIA) e Health Technology Assessment (HTA). Available online at: https://roma.unicatt.it/hta.pdf (accessed July 2, 2021)
- 25.UCSC-Gemelli . Health Technology Assessment. (2016). Available online at: https://roma.unicatt.it/2016-health-technology-assessment (accessed February 2021).
- 26.Rotterdam EU . MOOC Health Technology Assessment. Available online at: https://www.eur.nl/en/about-eur/vision/community-learning-and-innovation/innovation-projects/mooc-health-technology-assessment (accessed February 2021).
- 27.ISPOR . Health Technology Assessment Training Program. Available online at: https://www.ispor.org/conferences-education/education-training/hta-training-program (accessed February 2021).
- 28.INFARMED . Pharmacovigilance Course (Curso de atualização em farmacovigilância). (2020). Available online at: https://escolaverao.med.up.pt/cursos/curso-de-atualizacao-em-farmacovigilancia/ (accessed February 2021).
- 29.Faculty of Pharmacy of Universidade de Lisboa . Curso Pós-Graduado de Atualização: Assessing Therapeutic Efectiveness in Drug Lifecycle. (2019). Available online at: https://www.ff.ulisboa.pt/ensino/ensino-cursos/curso-curso-nao-conferentes-de-grau/curso-pos-graduado-de-atualizacao-assessing-therapeutic-efectiveness-in-drug-lifecycle-2/ (accessed February 2021).
- 30.Escola de Verao . Artificial Intelligence in Health: Governance, Accountability Decision-Making (Inteligência artificial em saúde: governança, responsabilidade e tomada de decisão). (2019). Available online at: https://escolaverao.med.up.pt/cursos/inteligencia-artificial-em-saude-governanca-responsabilidade-e-tomada-de-decisao/ (accessed February 2021).
- 31.MM Faculdade de Medicina U do P . Introduction to Value-Based Health Care Management (Introdução à Gestão de Cuidados de Saúde Baseada em Valor). (2020). Available online at: https://sigarra.up.pt/fmup/pt/cur_geral.cur_view?pv_curso_id=20061 (accessed February 2021).
- 32.The National School of Public Health M PD (NSPHMPDB) . Training Programs in Both Public Health and Management. Available online at: http://www.snspms.ro/en/activity-domains/training (accessed February 2021).
- 33.AEMPS LAE de M y PS . Technical Training of Biosimilar Medicines (Capacitación técnica de medicamentos biosimilares). (2020). Available online at: https://www.aemps.gob.es/informa/notasinformativas/laaemps/2020-laaemps/la-aemps-y-la-red-eami-apuestan-por-el-fortalecimiento-de-los-procesos-de-evaluacion-de-medicamentos-biosimilares-en-iberoamerica/ (accessed January 26, 2022).
- 34.AEMPS LAE de M y PS . 3rd Training Course in Standards of Good Clinical Practice for Independent Researchers (3o curso de formación en normas de buena práctica clínica para investigadores independientes). (2009). Available online at: https://www.aemps.gob.es/la-aemps/3o-curso-de-formacion-en-normas-de-buena-practica-clinica-para-investigadores-independientes/ (accessed January 26, 2022).
- 35.AEMPS LAE de M y PS . Practical Course for Conducting Pharmacoepideomyology Studies With the Bifap Database (curso practico para la realización de estudios de farmacoepidemiologia con la base de datos bifap). (2016). Available online at: https://sigade.isciii.es/publico/actual/VerCurso.asp?ID=4&CodProp=2808&CodEd=2906 (accessed February 2021).
- 36.Funcanis Fundación Canaria de Investigación Sanitaria FIISC . Aplicación de la Biología molecular en el diagnóstico y seguimiento de la Leucemia Linfocítica crónica. Las Palmas de Gran Canaria: Funcanis Fundación Canaria de Investigación Sanitaria FIISC. (2019). [Google Scholar]
- 37.BIOEF BF for HI R . Curso de Verano de la UPV/EHU sobre la investigación y la innovación en el ámbito sanitario. (2012). Available online at: https://www.bioef.org/es/2012/07/12/curso-de-verano-de-la-upvehu-sobre-la-investigacion-y-la-innovacion-en-el-ambito-sanitario/ (accessed February 2021).
- 38.AETSA AHA . Introduction of the Economic Assessment in the Health Technology Assessment. (2016). Available online at: https://www.aetsa.org/formacion/cursos-y-talleres/#1478716683347-cebd4e3b-4e24 (accessed February 2021).
- 39.NICE NI for HC E . Seminars. Available online at: https://www.nice.org.uk/about/what-we-do/life-sciences/scientific-advice/scientific-advice-education-and-training-/seminars (accessed February 2021).
- 40.AWMSG . AWMSG Training Day. All Wales Therapeutics & Toxicology Centre. (2019). Available online at: https://www.awttc.org/news/awmsg-training-day-2019 (accessed July 2, 20221).
- 41.AWTTC AWT TC . Adverse Drug Reactions: Reporting Makes Medicines Safer. Available online at: https://www.awttc.org/yccwales/education#general-for-all-healthcare-professionals- (accessed February 2021).
- 42.Health Technology Wales . Health Technology Assessment and Economics. (2019). Available online at: https://www.healthtechnology.wales/wp-content/uploads/2019/02/20190130-HTA-HE-101-Brochure-English-1.pdf (accessed February 2021).
- 43.Health Technology Wales . Value in Health Week. (2020). Available online at: https://lshubwales.com/value-health-week-programme (accessed February 2021).
- 44.ISPOR . Modeling Health Care Costs- Part I Characteristics of Health Care Costs. (2020). Available online at: https://www.ispor.org/conferences-education/education-training/virtual/distance-learning/modeling-health-care-costs-part-i-characteristics-of-health-care-costs (accessed February 2021).
- 45.ISPOR . Modeling Health Care Costs – Part II: Methods and Guidelines for Estimating Health Care Costs. ISPOR: (2020). [Google Scholar]
- 46.ISPOR . Modeling Health Care Costs – Part III: Estimation from Censored Data. ISPOR: (2020). [Google Scholar]
- 47.ISPOR . Markov Model Toolkit: Concepts, Assumptions and Examples. (2020). Available online at: https://www.ispor.org/conferences-education/education-training/virtual/distance-learning/markov-model-toolkit-concepts-assumptions-and-examples (accessed February 2021).
- 48.ISPOR . Introduction to Pharmaco-Economics. (2020). Available online at: https://www.ispor.org/conferences-education/education-training/virtual/distance-learning/introduction-to-pharmacoeconomics (accessed February 2021).
- 49.ISPOR . Cost-of-Illness/Cost-Estimation (COI/CE). (2020). Available online at: https://www.ispor.org/conferences-education/education-training/virtual/distance-learning/cost-of-illness-cost-estimation-coi-ce (accessed February 2021).
- 50.ISPOR . Cost-Minimization/Cost-Consequence (CMA/CCA). (2020). Available online at: https://portal.ispor.org/eweb/DynamicPage.aspx?Action=Add&ObjectKeyFROM=1A83491A-9853-4C87-86A4-F7D95601C2E2&WebCode=ProdDetailAdd&DoNotSave=yes&ParentObject=CentralizedOrderEntry&ParentDataObject=Invoice Detail&ivd_formkey=69202792-63d7-4ba2-bf4e-a0da412 (accessed February 2021).
- 51.ISPOR . Introduction to Budget Impact Analysis (BIA) - Part I. (2020). Available online at: https://www.ispor.org/conferences-education/education-training/virtual/distance-learning/introduction-to-budget-impact-analysis-bia-part-i (accessed February 2021).
- 52.ISPOR . Introduction to Budget Impact Analysis (BIA) - Part II. (2020). Available online at: https://www.ispor.org/conferences-education/education-training/virtual/distance-learning/introduction-to-budget-impact-analysis-bia-part-ii (accessed February 2021).
- 53.ISPOR . Cost-Effectiveness Analysis (CEA) and Cost-Utility Analysis (CUA). (2020). Available online at: https://www.ispor.org/conferences-education/education-training/virtual/distance-learning/cost-effectiveness-analysis-cea-and-cost-utility-analysis-cua (accessed February 2021).
- 54.ISPOR . Patient Reported Outcomes: Analysis and Interpretation. (2020). Available online at: https://www.ispor.org/conferences-education/education-training/virtual/distance-learning/patient-reported-outcomes-analysis-and-interpretation (accessed February 2021).
- 55.ISPOR . Patient Reported Outcomes: Instrument Development. (2020). Available online at: https://www.ispor.org/conferences-education/education-training/virtual/distance-learning/patient-reported-outcomes-instrument-development (accessed February 2021).
- 56.EUnetHTA JA2 . HTA Core Model Training Course. (2015). Available online at: https://www.EUnetHTA.eu/wp-content/uploads/2018/01/HTACoreModelTrainingCourse2015pv_revised.pdf (accessed February 2021).
- 57.EUnetHTA JA2 . Overview of HTA Core Model Training Materials. (2016). Available online at: https://www.EUnetHTA.eu/wp-content/uploads/2018/01/HTACoreModel_TrainingMaterials_Overview.pdf (accessed February 2021).
- 58.EUnetHTA JA2 . Key Principles of HTA or What Is Meant for HTA. (2014). Available online at: https://www.EUnetHTA.eu/wp-content/uploads/2018/01/Training_SF2014_key_principles_of_hta.pdf (accessed February 2021).
- 59.IMI . GetReal Training: Real-World Evidence in Medicine Development. (2015). Available online at: https://rwe-navigator.eu/homepage/what-is-the-imi-getreal-project/getreal-training-courses-on-real-world-evidence-in-medicines-development/ (accessed February 2021).
- 60.AHTA AHTA . Health Technology Assessment 2020. Online Course Handbook. (2020). Available online at: https://www.adelaide.edu.au/ahta/htacourse/ (accessed February 2021).
- 61.CADTH . Health Technology Assessment for Decision Makers HTA Institute. (2015). Available online at: https://www.cadth.ca (accessed February 2021).
- 62.IECS . Introducción a las Evaluaciones de Tecnologías Sanitarias y Evaluaciones Económicas. (2020). Available online at: https://www.iecs.org.ar/wp-content/uploads/Programa-Analitico-ETS0-modificado.pdf (accessed February 2021).
- 63.IECS . Evaluaciones Económicas: Programación, análisis e interpretación de modelos de decisión. (2020). Available online at: https://educacion.iecs.org.ar/educacion/evaluaciones-economicas-programacion-analisis-e-interpretacion-de-modelos/ (accessed February 2021).
- 64.IECS . Estimación de costos para las evaluaciones económicas de programas, servicios y tecnologías en salud. (2020). Available online at: https://educacion.iecs.org.ar/educacion/curso-estimacion-de-costos-para-las-evaluaciones-economicas-de-programas-servicios-y-tecnologias-en-salud/ (accessed February 2021).
- 65.IECS . Diseño, programación y análisis de modelos de Markove. (2020). Available online at: https://educacion.iecs.org.ar/educacion/diseno-programacion-y-analisis-de-modelos-de-markov-en-microsoft-excel/ (accessed February 2021).
- 66.IECS . Desarrollo e implementación de evaluaciones de tecnologías sanitarias. (2020). Available online at: https://educacion.iecs.org.ar/educacion/desarrollo-e-implementacion-de-evaluaciones-de-tecnologias-sanitarias/ (accessed February 2021).
- 67.IECS . Análisis de impacto presupuestario (AIP) en salud. (2020). Available online at: https://educacion.iecs.org.ar/educacion/curso-analisis-de-impacto-presupuestario-en-salud/ (accessed February 2021).
- 68.National Authority of Medicines Health Products (INFARMED) . Health Technology Assessment Training Program. (2016). Available online at: https://www.infarmed.pt/web/infarmed/infarmed?p_p_id=101&p_p_lifecycle=0&p_p_state=maximized&p_p_mode=view&_101_struts_action=%2Fasset_publisher%2Fview_content&_101_assetEntryId=1129957&_101_type=document&inheritRedirect=false&redirect=https%3A%2F%2Fwww.i (accessed February 2021).
- 69.Network E Health FOR Assessment T . Handbook on Hta Capacity Building. Health Technology Assessment. (2008). Available online at: http://www.aatrm.net (accessed June 25, 2021).
- 70.EUnetHTA . JA2 WP8 – Maintenance of HTA Core Model infrastructure to Support Shared Production and Sharing of HTA Information – EUnetHTA. Available online at: https://www.EUnetHTA.eu/ja2-archive/ja2-wp8-maintenance-of-hta-core-model-infrastructure-to-support-shared-production-and-sharing-of-hta-information/ (accessed June 25, 2021).
- 71.Angelis A, Lange A, Kanavos P. Using health technology assessment to assess the value of new medicines: results of a systematic review and expert consultation across eight European countries. Eur J Heal Econ. (2018) 19:123–52. 10.1007/s10198-017-0871-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.WHO . Global Survey on Health Technology Assessment by National Authorities. (2015). Available online at: https://www.who.int/health-technology-assessment/MD_HTA_oct2015_final_web2.pdf (accessed February 2021).
- 73.Wang T, McAuslane N, Liberti L, Gardarsdottir H, Goettsch W, Leufkens H. companies' health technology assessment strategies and practices in Australia, Canada, England, France, Germany, Italy and Spain: an industry metrics study. Front Pharmacol. (2020) 11:594549. 10.3389/fphar.2020.594549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moshi MR, Tooher R, Merlin T. Development of a health technology assessment module for evaluating mobile medical applications. Int J Technol Assess Health Care. (2020) 36:252–61. 10.1017/S0266462320000288 [DOI] [PubMed] [Google Scholar]
- 75.European Parliament the Council of the European Union . RegulatioN (EU) 2021/2282 on health technology assessment and amending Directive 2011/24/EU. Official Journal of the European Union. 22.12.2021. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R2282&from=EN
- 76.De Labry Lima AO, Mochón LG, Martínez AC, Ruiz EM, Balbino JE. Mapping capacity to conduct health technology assessment in Central, Eastern and South-Eastern Europe. Croat Med J. (2016) 57:66–70. 10.3325/cmj.2016.57.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma M, Teerawattananon Y, Dabak SV, Isaranuwatchai W, Pearce F, Pilasant S, et al. A landscape analysis of health technology assessment capacity in the Association of South-East Asian Nations region. Heal Res Policy Syst. (2021) 19:19. 10.1186/s12961-020-00647-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mueller D, Gutierrez-Ibarluzea I, Chiumente M, Oortwijn W. Toward a common understanding of competencies for health technology assessment: enhancing educational and training programs around the globe. Int J Technol Assess Health Care. (2020) 37:1–10. 10.1017/S0266462320001919 [DOI] [PubMed] [Google Scholar]
- 79.Pichler F, Oortwijn W, Ruether A, Trowman R. Defining capacity building in the context of HTA: a proposal by the HTAi scientific development and capacity building committee. Int J Technol Assess Health Care. (2019). 35:62–6. 10.1017/S0266462319000631 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.