Abstract
Despite the availability of established treatments, heart failure (HF) is associated with a poor prognosis and its management is suboptimal, highlighting the need for new options for treatment and prevention. Patients with type 2 diabetes (T2D) often experience cardiovascular (CV) complications, with HF being one of the most frequent. Consequently, several CV outcome trials have focused on glucose-lowering therapies and their impact on CV outcomes. An established treatment for T2D, sodium-glucose cotransporter-2 inhibitors (SGLT-2is; canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin) have demonstrated beneficial effects on CV outcomes in long-term studies of patients with T2D with established CV disease and/or a broad range of CV risk factors. Recent studies have extended these findings to patients with HF, with and without T2D, finding that SGLT-2is (particularly dapagliflozin and empagliflozin) are effective therapeutic interventions for the treatment and prevention of HF. This narrative review article discusses the use of SGLT-2is in the treatment and prevention of HF in patients with and without T2D. Dapagliflozin was the first SGLT-2i to receive US Food and Drug Administration (FDA) approval for treatment of HF, to reduce the risk of CV death and hospitalization for HF in adults with HF with reduced ejection fraction (HFrEF) with and without T2D. Recently, the FDA also approved empagliflozin for this indication. Given the new HFrEF indications for dapagliflozin and empagliflozin, and the likelihood of similar approvals for other SGLT-2is, cardiology guidelines are beginning to integrate SGLT-2is into a standard-of-care treatment regimen for patients with HFrEF. The utility of SGLT-2is in HF with preserved EF (HFpEF) shows promise based on data from the EMPEROR-Preserved study of empagliflozin in patients with HFpEF. Further clinical trial evidence may lead to more widespread use and further integration of SGLT-2is into standard-of-care regimens for the treatment and management of HF in patients with and without T2D.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01989-z.
Keywords: Canagliflozin, Cardiovascular, Dapagliflozin, Empagliflozin, Ertugliflozin, Heart failure, Sodium-glucose cotransporter 2 inhibitor, Sotagliflozin, Type 2 diabetes
Plain Language Summary
Heart failure is a medical condition in which the heart cannot pump enough blood. Several types of drugs have been used to treat heart failure, but these may not work for every patient, and heart failure can get worse over time even with treatment. That is why new drugs are needed to treat and prevent heart failure. People with diabetes (type 2 diabetes) often have other conditions related to the heart (cardiovascular system), heart failure being one of the most common. Because of this, there have been studies (clinical trials) in people with diabetes to see if diabetes drugs can also treat and/or reduce the risk of cardiovascular disease. In clinical trials, a type of diabetes drug, sodium-glucose cotransporter-2 inhibitors (SGLT-2is, including canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin), has helped people with both diabetes and cardiovascular disease. Recent clinical trials of dapagliflozin and empagliflozin showed they were effective for treating and preventing heart failure in people without diabetes as well as in those with diabetes. Based on these studies, the US Food and Drug Administration approved dapagliflozin and empagliflozin for heart failure in patients with or without diabetes. These drugs can be prescribed for adults with or without diabetes to treat and prevent a type of heart failure, heart failure with reduced ejection fraction, in which the heart is too weak to pump enough blood to the body. Several clinical studies are ongoing that will provide more information about these drugs, SGLT-2is, which will help healthcare providers to treat people with heart failure.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01989-z.
Key Summary Points
| Heart failure (HF) has a poor prognosis, and its management is suboptimal, necessitating new therapeutic options for its treatment and management. |
| In patients with type 2 diabetes, HF is one of the most frequent cardiovascular (CV) complications. |
| The sodium-glucose cotransporter-2 inhibitor (SGLT-2i) class of antidiabetic drugs has shown beneficial effects on CV outcomes, including HF. |
| Specifically, the SGLT-2is dapagliflozin and empagliflozin have been demonstrated to be effective for the treatment and prevention of HF with reduced ejection fraction. |
| Additional evidence from clinical studies may lead to further integration of SGLT-2is into a standard-of-care regimen for the treatment and management of HF. |
Introduction
Heart failure (HF) is considered a significant healthcare issue, affecting over 6 million people in the US, and this number is expected to increase to more than 8 million by 2030 [1]. Significant healthcare costs are associated with HF, particularly direct costs arising from hospitalization and medication [2]. Current HF prevention guidelines recommend aggressive management of risk factors, including treatment of asymptomatic left ventricular dysfunction (with angiotensin-converting enzyme inhibitors [ACEis]), hypercholesterolemia (with statins), and hypertension (with diuretics, ACEis, and angiotensin receptor blockers [ARBs]), smoking cessation, and reduction of alcohol intake [3, 4]. Management strategies include the use of ACEis, ARBs, angiotensin receptor-neprilysin inhibitors (ARNis), beta blockers, and mineralocorticoid/aldosterone receptor antagonists in patients with HF with reduced ejection fraction (HFrEF) [3–5], whereas effective treatments are limited for HF with preserved ejection fraction (HFpEF) [4]. Overall, HF has a poor prognosis, and management remains suboptimal.
HF is one of the most frequent cardiovascular (CV) complications in patients with type 2 diabetes (T2D), with a prevalence of 10–23% [6]. Coronary artery disease and hypertension are considered important contributing factors to HF in T2D [7]. Given the important impact of CV disease (CVD) in patients with T2D, there has been a focus on the effects of established glucose-lowering therapies on CV outcomes. An improvement in glycemic control could be expected to have CV benefit because T2D is a risk factor for CVD. However, some glucose-lowering therapies (such as sodium-glucose cotransporter-2 inhibitors [SGLT-2is] and glucagon-like peptide-1 receptor agonists) have shown CVD benefit beyond glycemic control [8]. Moreover, SGLT-2is have been shown to have beneficial effects on both CV and renal outcomes in patients with T2D [9–16], and SGLT-2is are the first class of antidiabetic drugs to have demonstrated beneficial effects on HF outcomes and, thus, may serve as a novel treatment option for patients without T2D with or at risk of HF.
As with general CV and renal effects, the beneficial effects of SGLT-2is on HF outcomes specifically are thought to be due to nonglycemic mechanisms [9, 11]. The cardioprotective effects of SGLT-2is have been in part attributed to SGLT-2i–associated reductions in proximal renal tubule burden and central sympathetic overactivity [17]. A mild state of ketosis also contributes to a reduction in sympathetic tone; decreased sympathetic outflow from the brain to the kidney alters the pressure-natriuresis relationship with more renal excretion of sodium and water at a given pressure, thereby improving fluid retention [17]. SGLT-2is may also modulate the renal renin-angiotensin system and attenuate renal neprilysin activity [17].
This narrative review examines the role of SGLT-2is for the prevention and treatment of HF in individuals with T2D but also as a novel approach to HF treatment in patients without T2D. An assessment of important SGLT-2i CV outcome trials (CVOTs) will provide guidance on the use of SGLT-2is in routine clinical practice, specifically their placement in the T2D-specific and general HF treatment paradigm. This review also examines existing knowledge gaps and clinical ramifications of SGLT-2i therapy in clinical practice for the prevention and treatment of HF, with a focus on current clinical guidelines as well as physician perspectives.
Methods
The topic of this narrative review article is the use of SGLT-2is in the prevention and treatment of HF in patients with or without T2D. To identify key studies relevant to this topic, non-systematic literature searches were conducted in the PubMed database using search terms associated with SGLT-2is (i.e., “SGLT-2 inhibitor,” “sodium-glucose cotransporter 2 inhibitor,” “canagliflozin,” “dapagliflozin,” “empagliflozin,” “ertugliflozin,” “sotagliflozin”) and heart failure. The PubMed literature searches were limited to recent publications in English, published between July 2015 and October 2021, and to those reporting clinical trials and meta-analyses of SGLT-2is. Publications identified through the literature search results were reviewed, and studies reporting CV and/or renal outcomes for SGLT-2is were included in the review.
A potential limitation of the literature search is that only the PubMed database was searched. However, it is unlikely that studies meeting the selection criteria for this review were omitted, because the majority of journals are indexed on PubMed, including those in which CV and renal outcomes trials are likely to be published. Although applying a date range to the search excluded studies published before July 2015, it is unlikely to affect the content of this review because HF is a new indication for SGLT-2is.
Compliance with Ethics Guidelines
This article is based on the published literature and does not report any original data from human or animal studies conducted by the author.
SGLT-2i Effects on Cardiovascular Outcomes
Evidence of the cardioprotective as well as renoprotective effects of the SGLT-2is dapagliflozin, canagliflozin, empagliflozin, and ertugliflozin is based on several CVOTs (Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 [DECLARE-TIMI 58], Canagliflozin Cardiovascular Assessment Study [CANVAS], Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients [EMPA-REG OUTCOME], and Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants with Vascular Disease [VERTIS CV], respectively), which assessed their CV safety [9–14, 16, 18]. The DECLARE-TIMI 58 (dapagliflozin), CANVAS (canagliflozin), EMPA-REG OUTCOME (empagliflozin), and VERTIS CV (ertugliflozin) CVOTs enrolled patients with T2D and established CVD and/or a broad range of CVD risk factors (Table 1) [10, 13, 14, 18]. However, the patient populations of these studies did vary, with the proportion of patients having established CVD differing across the trials: 40.6% in DECLARE-TIMI 58, 65.6% in CANVAS, > 99% in EMPA-REG OUTCOME, and 100% in VERTIS CV. Considering the level of established CVD and range of CVD risk factors, the patient population of DECLARE-TIMI 58 was considered more indicative of the US population with T2D [10, 13, 14, 19].
Table 1.
Summary of SGLT-2i cardiovascular outcomes trials, including those in patients with HF (shown in italics)
| Trial name (date published) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EMPA-REG OUTCOME (2015) [14] | CANVAS (2017) [10] | DECLARE–TIMI 58 (2019) [13] | VERTIS CV (2020) [18] | DAPA-HF (2019) [29] | EMPEROR-Reduced (2020) [32] | SOLOIST-WHF (2021) [34] | EMPEROR-Preserved (2021) [36] | ||||
| Focus of trial | CV outcomes | CV outcomes | CV outcomes | CV outcomes | HF outcomes | HF outcomes | HF outcomes | HF outcomes | |||
| Interventions |
Empagliflozin Placebo |
Canagliflozin Placebo |
Dapagliflozin Placebo |
Ertugliflozin Placebo |
Dapagliflozin Placebo |
Empagliflozin Placebo |
Sotagliflozin Placebo |
Empagliflozin Placebo |
|||
| Population | Patients with T2D and CVD (n = 7020) | Patients with T2D and with or at risk of CVD (n = 10,142) | Patients with T2D and with or at risk of CVD (n = 17,160) | Patients with T2D and CVD (n = 8246) | Patients with NYHA class II–IV HFrEF (n = 4744)a | Patients with NYHA class II–IV HFrEF (n = 3730)b | Patients with T2D, hospitalized for signs/symptoms of HF and treated with IV diuretic (n = 1222)c | Patients with NYHA class II–IV HFpEF (n = 5988)d | |||
| Median follow-up | 3.1 yrs | 126.1 wks | 4.2 yrs | 3.0 yrs | 18.2 months | 16 months | 9 monthse,f | 26.2 months | |||
| History of CVD, n (%) | 6964 (99.2) | 6656 (65.6) | 6974 (40.6) | 8246 (100) | 2674 (56.3)g | 1929 (51.2)g | NR | 2117 (35.4)g | |||
| History of HF, n (%) | 706 (10.1 | 1461 (10.4) | 1742 (10) | 1958 (23.7) | 4744 (100) | 3730 (100) | 1222 (100) | 5987 (100)h | |||
| Outcomes (HR, 95% CI)i | |||||||||||
| MACE | 0.86 (0.74–0.99) | 0.86 (0.75–0.97) | 0.93 (0.84–1.03) | 0.97 (0.85–1.11) | NR | NR | NR | NR | |||
| CV death/HHF | 0.65 (0.50–0.85) | 0.78 (0.67–0.91) | 0.83 (0.73–0.95) | 0.88 (0.75–1.03) | 0.75 (0.65–0.85) | 0.75 (0.65–0.86) | NR | 0.79 (0.69–0.90) | |||
| HHF | 0.65 (0.50–0.85) | 0.67 (0.52–0.87) | 0.73 (0.61–0.88) | 0.70 (0.54–0.90) | 0.70 (0.59–0.83) | 0.69 (0.59–0.81) | NR | 0.71 (0.60–0.83) | |||
| CV death | 0.62 (0.49–0.77) | 0.87 (0.72–1.06) | 0.98 (0.82–1.17) | 0.92 (0.77–1.11) | 0.82 (0.69– 0.98) | 0.92 (0.75–1.12) | 0.84 (0.58–1.22) | 0.91 (0.76–1.09) | |||
| Worsening HF/CV death | NR | NR | NR | NR | 0.74 (0.65–0.85) | NR | NR | NR | |||
| Deaths from CV causes, HHF, and urgent visits for HF | NR | NR | NR | NR | NR | NR | 0.67 (0.52–0.85) | NR | |||
| Deaths from CV causes, HHF, and urgent visits for HF/HF events during hospitalization | NR | NR | NR | NR | NR | NR | 0.68 (0.54–0.86) | NR | |||
| Death from CV causes/HHF/nonfatal MI/nonfatal strokes | NR | NR | NR | NR | NR | NR | 0.72 (0.56–0.92) | NR | |||
| HHF and urgent visits for HF | NR | NR | NR | NR | NR | NR | 0.64 (0.49–0.83) | NR | |||
CANVAS Canagliflozin Cardiovascular Assessment Study, CI confidence interval, CV cardiovascular, CVD cardiovascular disease, DAPA-HF Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure, DECLARE-TIMI 58 Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58, EMPA-REG OUTCOME Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients, EMPEROR-Preserved Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction, EMPEROR-Reduced Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction, HF heart failure, HFpEF heart failure with preserved ejection fraction, HFrEF heart failure with reduced ejection fraction, HHF hospitalization for heart failure, HR hazard ratio, IV intravenous, MACE major adverse cardiovascular events, MI myocardial infarction, NR not reported, NYHA New York Heart Association, SGLT-2i sodium-glucose cotransporter-2 inhibitor, SOLOIST-WHF Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure, T2D type 2 diabetes, VERTIS CV Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants with Vascular Disease
a41.8% of patients had T2D
b49.8% of patients had T2D
c79.1% of patients had left ventricular ejection fraction of < 50%
d49.1% of patients had T2D
e8.9 months in the placebo group and 9.2 months in the sotagliflozin group
fTrial enrolment was terminated early due to loss of funding from sponsor
gIschemic HF
hIschemic or nonischemic HF category not reported for one patient
iPrimary endpoint is shown in bold
In DECLARE-TIMI 58, dapagliflozin was associated with a significant 17% reduction in the composite endpoint of CV death/hospitalization for HF (HHF; P = 0.005) and was non-inferior to placebo for the composite endpoint of major adverse CV event rate (MACE; composite outcome of CV death, nonfatal myocardial infarction [MI], or nonfatal ischemic stroke; Table 1) [13]. The reduction in the risk of the composite outcome of CV death/HHF was similar among patients with established CVD or multiple CV risk factors and was primarily driven by a lower rate of HHF in the dapagliflozin group (hazard ratio [HR], 0.73 [95% CI 0.61–0.88]) [13]. Reduction in the risk of CV death or HHF with dapagliflozin was observed irrespective of prior MI, with a greater absolute benefit in those with versus without prior MI [16]. Regarding canagliflozin (CANVAS) [10] and empagliflozin (EMPA-REG OUTCOME) [14], the relative risk of the rate of the 3-point MACE (i.e., death from CV causes, nonfatal MI, or nonfatal stroke) was significantly reduced by 14% for both agents [10, 14] versus placebo. In addition, the risk of HHF was reduced by 33% with canagliflozin, with the greatest benefit observed in patients with a prior history of HF at baseline (Table 1) [10, 11]. The risk of HHF was reduced by 35% with empagliflozin versus placebo, with similar benefits observed irrespective of HF status at baseline (Table 1) [9, 14]. For the SGLT-2i ertugliflozin (VERTIS CV), MACE (a composite of death from CV causes, nonfatal MI, or nonfatal stroke) occurred in a similar proportion of patients in the ertugliflozin and placebo groups (11.9% versus 11.9%; P < 0.001 for noninferiority) [18]. The outcomes of CV death/HHF or CV death alone were not significantly affected by ertugliflozin; however, HHF alone was positively impacted by ertugliflozin (30% risk reduction; Table 1) [18].
A meta-analysis of three of these CVOTs (DECLARE-TIMI 58, CANVAS, and EMPA-REG OUTCOME) showed significant reductions in the risk of CV death or HHF by 23% and the risk of HHF by 31% with SGLT-2is across patients, regardless of the presence of CVD or HF at baseline [20]. The benefits for HHF were greatest among patients with the lowest estimated glomerular filtration rate (eGFR; < 60 ml/min/1.73 m2), with a significant 40% reduction in risk (P < 0.0001) [20]. The results of these CVOTs are supported by the findings of several large-scale, real-world studies that compared SGLT-2is with other glucose-lowering therapies [21–25].
Renoprotective outcomes were the focus of the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) and Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) studies, which investigated the effects of SGLT-2is (canagliflozin and dapagliflozin, respectively) on renal failure and CV outcomes in patients with T2D (DAPA-CKD also included patients without T2D) and albuminuric chronic kidney disease (CKD) [15, 26]. DAPA-CKD demonstrated a significantly reduced risk of the primary composite outcome (i.e., sustained decline in eGFR ≥ 50%, end-stage renal disease [ESRD], or death from renal or CV causes; HR, 0.61; P < 0.001) with dapagliflozin versus placebo [26]. A benefit with dapagliflozin versus placebo was also demonstrated on the secondary composite CV outcome (HHF or death from CV causes; HR, 0.71; P = 0.009) and on the secondary composite renal outcome (≥ 50% decline in eGFR, ESRD, or renal death; HR, 0.56; P < 0.001) [26]. Moreover, DAPA-CKD reported a significant reduction in the risk of all-cause mortality with dapagliflozin versus placebo (HR, 0.69; P = 0.004), which is the first report of a SGLT-2i reducing the risk of all-cause mortality in patients with CKD [26]. In the CREDENCE study with a patient population at high risk of kidney failure, canagliflozin was associated with significant reductions in the risk of HHF (39%; P < 0.001), the risk of CV death or HHF (31%; P < 0.001), and the risk of the composite renal outcome (ESRD, doubling of serum creatinine level, or death from renal or CV causes; 30%; P = 0.00001) [15]. Similarly, the Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED) trial assessed the effect of sotagliflozin and placebo in patients with T2D and CKD who had risks for CVD [27]. Despite being terminated early because of the loss of funding from the study sponsor, the study found that sotagliflozin reduced the risk of the composite primary endpoint of the number of deaths from CV causes, HHF, and urgent visits for HF by 26% (HR, 0.74 [95% CI 0.63–0.88]; P < 0.001). A significant effect was also seen on the secondary endpoint of the number of HHF and urgent visits for HF (0.67 [0.55–0.82]; P < 0.001) but not on other secondary endpoints, including those related to renal function.
SGLT-2is and HF Outcomes
There is evidence to indicate that SGLT-2is are effective in the management of HFrEF. A prespecified subgroup analysis of the DECLARE-TIMI 58 trial data showed that dapagliflozin, when administered concomitantly with standard CV therapeutics, reduced the risk of CV death or HHF to a greater extent in patients with HFrEF than in those without HFrEF; these results were found to be largely driven by large reductions in CV death and all-cause mortality in patients with HFrEF [28]. Ertugliflozin has demonstrated effects on HF-related outcomes based on a subgroup analysis of the VERTIS CV study [19]. Ertugliflozin treatment was associated with significant reductions in the risk of first HHF (HR, 0.07; P = 0.006), the total number of HHF (first + recurrent HHF rate ratio [RR], 0.70; P = 0.001), and total CV death or HHF events (RR, 0.83; P = 0.011) [19].
The effects of SGLT-2is in the management of HFrEF have been observed irrespective of the presence of T2D (Table 1). In one of the first SGLT-2i CVOTs (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure [DAPA-HF]) to enroll patients specifically diagnosed with HFrEF (≤ 40% EF; New York Heart Association [NYHA] class II–IV HF symptoms), with or without T2D [29], dapagliflozin significantly reduced the risk of the composite outcome of worsening HF (i.e., hospitalization or an urgent visit due to HF) or CV death by 26% (P < 0.001; Table 1). Reductions were also observed in both of the components of the primary composite outcome, CV death (HR, 0.82 [95% CI 0.69–0.98]) and HHF (0.70 [95% CI 0.59–0.83]) [29]. Similar effectiveness was observed in patients with or without T2D [29], and the observations in patients without T2D were not affected by glycated hemoglobin level [30]. These findings from the DAPA-HF study led to the approval by the US Food and Drug Administration (FDA) of dapagliflozin for adults with HFrEF (NYHA class II–IV HF) to reduce the risk of CV death and HHF [31].
Additional findings pertaining to the beneficial effects of an SGLT-2i in patients with HFrEF were reported by the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced) study [32]. This study enrolled adults with chronic HFrEF (≤ 40% left EF; NYHA class II–IV HF symptoms), with or without T2D, treated with empagliflozin or placebo in addition to the patient’s usual therapy for HF. Empagliflozin reduced the risk of the composite outcome, adjudicated CV death or HHF, by 25% (P < 0.001) and the risk of first HHF by 31% (HR, 0.69 [95% CI 0.59–0.81]; Table 1). This effect was observed regardless of the presence of T2D. Furthermore, the total number of HHF was significantly lower with empagliflozin (HR, 0.70; P < 0.001), and the rate of decline in eGFR was significantly reduced (absolute difference, 1.73; P < 0.001) [32]. On the basis of these results from the EMPEROR-Reduced study, the US FDA approved empagliflozin as a treatment to reduce the risk of CV death and HHF in adults with HFrEF [33].
The Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trial assessed the effect of sotagliflozin versus placebo in patients with T2D and HF with reduced or preserved left EF who had been recently hospitalized for worsening HF and treated with an intravenous diuretic [34]. Patients were randomized to receive sotagliflozin or placebo. Trial enrollment was closed early due to loss of funding from the sponsor, which resulted in a change to the primary and secondary endpoints. Sotagliflozin reduced the risk of the revised primary endpoint of total number of deaths from CV causes and HHF and urgent visits for HF by 33% (HR, 0.67 [95% CI 0.52–0.85]; P < 0.001). Importantly, this effect was seen in patients with reduced (HR, 0.72 [95% CI 0.56–0.94]) and preserved (HR, 0.48 [95% CI 0.27–0.86]) left EF. A significant reduction in the first hierarchical secondary outcome of the number of HHF/urgent visits for HF was also seen (HR, 0.64; P < 0.001), but there were no significant effects on other secondary endpoints, including all-cause deaths. A prespecified analysis of the SOLOIST-WHF trial compared the number of days alive and out of hospital (DAOH) and percent DAOH in the two treatment groups [35]. Sotagliflozin significantly increased the number of DAOH compared with placebo (risk ratio 1.03 [95% CI 1.00–1.06]; P = 0.027) but had no significant effect on the percent DAOH.
Additional clinical evidence about the benefit of SGLT-2is in patients with HFpEF has been reported from the Empagliflozin Outcome Trial in Patients With Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) trial [36]. Patients with and without T2D who had NYHA class II–IV chronic HFpEF (left ventricular EF > 40%; median 54%) were enrolled and treated with empagliflozin (n = 2997) or placebo (n = 2991) in addition to standard therapy. The primary composite endpoint of death from CV causes or HHF was significantly reduced with empagliflozin compared with placebo by 21% (HR, 0.79 [95% CI 0.69–0.90]; P < 0.001) [36]. There was also a significant reduction in the total number of HHF (secondary endpoint) with empagliflozin by 27% (HR, 0.73 [95% CI 0.61–0.88]; P < 0.001). The patient response to empagliflozin was similar in patients with and without T2D at baseline.
Further studies are needed to ascertain the mechanisms responsible for improved HF outcomes with SGLT-2is.
Knowledge Gaps Regarding the Use of SGLT-2is for HF in Clinical Practice
Despite mounting evidence of the cardioprotective effects of SGLT-2is in patients with and without T2D, there are several knowledge gaps associated with their use in clinical practice for the prevention and treatment of HF [37]. For example, the differential effects of SGLT-2is on HF outcomes in patients with HFpEF versus HFrEF are only just being clarified. In clinical practice, patients with HF are categorized by the presence of symptoms and EF status, including those with reduced (< 40%; HFrEF), mid-range (40–49%), and preserved EF (> 50%; HFpEF), and treated accordingly [4]. Historically, treatments that have shown efficacy in patients with HFrEF are typically not effective in those with HFpEF [38]. Furthermore, there are multiple potential pathways involved in the mechanism of disease for HFrEF and HFpEF. Until these various mechanisms are clarified, it will be difficult to develop treatment or dosing strategies that are relevant to each HF variant. Thus, there is a clinical unmet need for effective treatments in patients with HFpEF, a distinct clinical entity typically with an increase in left ventricular wall thickness and/or increased left atrial size as a sign of increased filling pressures as well as diastolic dysfunction [4, 5]. This is especially important, as patients with HFpEF and T2D have been demonstrated to have an increased risk of mortality, hospitalizations, and other adverse CV outcomes compared with that of those with HFpEF without T2D [39–42]. The CVOTs of SGLT-2is did not prospectively stratify patients by HF category [9, 11, 13]. Subgroup analyses of the DECLARE-TIMI 58 and CANVAS trials suggest that SGLT-2is reduced the risk of CV death or HHF to a greater extent in patients with HFrEF than in those without HFrEF [28] and that they reduced the overall risk of HF events but with no clear differences between patients with HFrEF and those with HFpEF [43]. However, a meta-analysis of eight studies concluded that SGLT-2is are associated with a “strong trend” toward lowering the risk of CVD/HHF in patients with HFpEF [44], driven mainly by data from the SOLOIST-WHF trial of sotagliflozin, the first to provide robust evidence that SGLT-2is may be beneficial in patients with HFpEF [34].
Several ongoing randomized trials aim to further guide the use of SGLT-2is in clinical practice (see Table S1 in the Electronic Supplementary Material) [45]. The Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) study is investigating the effects of SGLT-2is on CV and HF outcomes in patients with HFpEF [46]. Several studies (Dapagliflozin Effects on Biomarkers, Symptoms and Functional Status in Patients with HF with Reduced Ejection Fraction [DEFINE-HF], Dapagliflozin Effect on Exercise Capacity Using a 6-min Walk Test in Patients With Heart Failure With Reduced Ejection Fraction [DETERMINE-Reduced], Dapagliflozin Effect on Exercise Capacity Using a 6-min Walk Test in Patients With Heart Failure With Preserved Ejection Fraction [DETERMINE-Preserved], Impact of Empagliflozin on Cardiac Function and Biomarkers of Heart Failure in Patients With Acute Myocardial Infarction [EMMY], and Dapagliflozin in PRESERVED Ejection Fraction Heart Failure [PRESERVED-HF]) have been evaluating other valuable CV-related outcomes. In addition, similar to DAPA-CKD [26], another study (The Study of Heart and Kidney Protection With Empagliflozin [EMPA-KIDNEY]) aims to evaluate renal outcomes and CV mortality with SGLT-2is in patients with CKD.
Clinicians need evidence-based options when considering the use of SGLT-2is as part of a patient’s treatment plan for HF. Although SGLT-2is appear to be well tolerated in patients with HF, their longer-term safety when used to manage HF does need further confirmation. Thus far, clinical trials that focused specifically on patients with HF (EMPEROR-Reduced [32], DAPA-HF [29], SOLOIST-WHF [34], and EMPEROR-Preserved [36]) have been of relatively shorter duration (median follow-up of 9–26 months) compared with the trials in patients with T2D (median follow-up of up to 4.2 years). It is hoped that data from ongoing and future studies will provide additional evidence regarding the beneficial effects of SGLT-2is on outcomes in patients with HF irrespective of T2D status as well as demonstrating that SGLT-2is are safe and effective in both patients with HFpEF and HFrEF and those with acute HF.
Clinical Ramifications of SGLT-2is for HF Prevention and Treatment
The majority of SGLT-2is available in the US (i.e., canagliflozin, dapagliflozin, and empagliflozin) have indications to reduce adverse CV outcomes in adult patients with T2D and established CVD or multiple CV risk factors [31, 33, 47, 48]. As previously mentioned, dapagliflozin and empagliflozin have received US FDA approval for the treatment of adults with HFrEF that includes those without T2D, specifically to reduce the risk of CV death and HHF [31, 33].
Given the poor prognosis of HF, an effective therapy targeted for prevention of HF remains an unmet need [46]. In clinical practice, SGLT-2is have been recognized as a “new HF remedy” for the management of patients with T2D and HF [17]. Moreover, there is speculation that because of their wide-ranging therapeutic effects, SGLT-2is may also prove useful for reducing medication burden, which is an important consideration in patients with multiple CV-related comorbidities requiring several therapeutic interventions [49].
The American Diabetes Association and the European Association for the Study of Diabetes 2019 consensus report recommends the use of SGLT-2is in patients with T2D and associated CVD or at high risk of CVD [50–54], whereas cardiology guidelines have proceeded more cautiously. In 2019, the European Society of Cardiology (ESC) guidelines recommended SGLT-2is (canagliflozin, dapagliflozin, and empagliflozin) to reduce the risk of HHF in patients with T2D and risk of CKD progression [8]. This was followed by two position papers in 2020 by the Heart Failure Association (HFA) of the ESC recommending the use of SGLT-2is (empagliflozin, canagliflozin, dapagliflozin, and ertugliflozin) to reduce the risk of HHF in patients with T2D with either established CVD or high CV risk [55, 56]. Additionally, based on a review of the existing evidence for dapagliflozin and empagliflozin, the HFA-ESC recommended these drugs as a treatment for HFrEF in patients with and without T2D [55, 56]. With the American College of Cardiology’s release of the 2021 update to the expert consensus decision pathway for HF optimization, updates were included about the use of new therapies for HFrEF including SGLT-2is [57]. Specifically, in patients with chronic HFrEF already receiving beta blockers, an ARNi/ACEi/ARB, and aldosterone antagonists, SGLT-2is (dapagliflozin or empagliflozin) are an add-on to the aforementioned treatment regimen [57]. Subsequently, the 2021 ESC guidelines recommended SGLT-2is (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, and sotagliflozin) for the reduction of HHF, major CV events, end-stage renal dysfunction, and CV death in patients with T2D at risk of CV events [58]. Moreover, these aforementioned SGLT-2is are recommended for chronic HF prevention in patients with T2D at high risk of CVD or with CVD [58]. In patients with HFrEF and T2D, the SGLT-2is dapagliflozin, empagliflozin, and sotagliflozin were recommended to reduce HHF and CV death by the 2021 ESC guidelines [59]. These guidelines will be quite valuable for providing broader and more accessible guidance for the use of SGLT-2is in clinical practice in patients with or without T2D at risk of CVD and HF [59].
Although treatment guidelines recommend SGLT-2is in patients with T2D and established CVD or at high CV risk, real-world data suggest that < 6% of eligible patients receive SGLT-2is in clinical practice, and < 5% of current prescriptions of SGLT-2is appear to be initiated by cardiologists [60, 61]. Improving cardiologists’ familiarity with and utilization of SGLT-2is is an unmet need for HF management in this high-risk population and will be relevant to expanding the use of SGLT-2is in the general HF population [60, 61].
Physicians’ Perspectives Regarding the Role of SGLT-2is in Treating HF
There is a lack of information available to clinicians about the use of SGLT-2is in a HF treatment paradigm. Moreover, with a large proportion of patients having concomitant T2D and HF, routine clinical practice should consider a holistic, individualized patient approach, considering both of these interrelated risk factors as a means to improve outcomes [59]. Effective integration of SGLT-2i therapy into the treatment management of HF will require examination of the existing HF care models in collaboration across the patient’s healthcare providers [62, 63]. The involvement of a well-coordinated team of healthcare providers, including cardiologists and primary care providers, will likely result in improved patient outcomes and increased satisfaction with care.
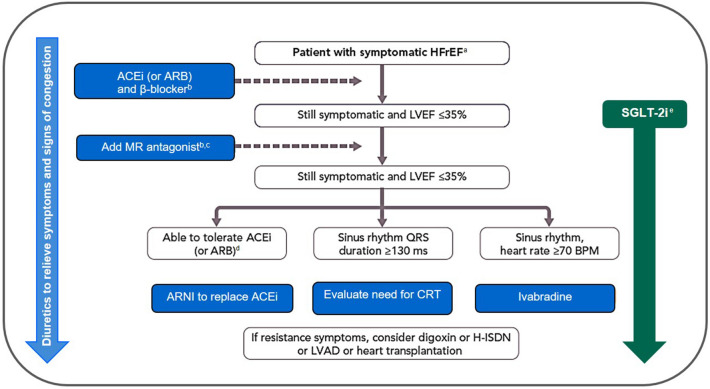
To optimize CV outcomes, an important consideration may be the use of SGLT-2i therapy as part of the initial therapeutic approach in patients with T2D at risk of CVD [64]. Early identification of patients at risk of HF would ensure targeting of SGLT-2is to those who may achieve maximal benefits, irrespective of whether the patient needs additional glycemic control [65]. A therapeutic framework considering diabetes- and CV-specific risk factors would be an effective tool to guide therapy with SGLT-2is [65]. Such a therapeutic framework would include referral of certain patients to clinicians with expertise in managing CV complications of diabetes, including treatment optimization to reduce CV risk [65]. Cardiologists are likely to encounter patients with CVD and HF who may benefit from SGLT-2i therapy; consequently, they are well positioned to initiate SGLT-2is in appropriately selected patients [62]. A disease management approach has been proposed as a framework for guiding cardiologists through patient selection, initiation of SGLT-2i therapy, and long-term monitoring of patients (Fig. 1) [4, 62, 66].
Fig. 1.
Proposed modification to the therapeutic algorithm for a patient with symptomatic HFrEF following results from DAPA-HF and EMPEROR-Reduced [4, 66]. Reprinted with permission from Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. https://doi.org/10.1093/eurheartj/ehw128. ©Oxford University Press. aNYHA class II–IV, LVEF < 40%. bUp-titrate to maximum tolerated evidence-based dose. cWith a hospital admission for HF within the last 6 months or with elevated natriuretic peptides (BNP > 250 pg/ml or NT-proBNP > 500 pg/ml in men and 750 pg/ml in women). dWith an elevated plasma natriuretic peptide level (BNP ≥ 150 pg/ml or plasma NT-proBNP ≥ 600 pg/ml, or if HF hospitalization within 12 months, plasma BNP ≥ 100 pg/ml or plasma NT-proBNP ≥ 400 pg/ml). eDapagliflozin and empagliflozin are the only SGLT-2is that have demonstrated significant and clinically meaningful reductions in both the CV deaths and worsening HF components of the primary composite endpoint in patients with HFrEF, both with and without T2D. ACEi angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, ARNI angiotensin receptor-neprilysin inhibitor, BNP B-type natriuretic peptide, CRT cardiac resynchronization therapy, CV cardiovascular, DAPA-HF Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure, EMPEROR-Reduced Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction, H-ISDN hydralazine and isosorbide dinitrate, HF heart failure, HFrEF heart failure with reduced ejection fraction, LVAD left ventricular assist device, LVEF left ventricular ejection fraction, MR mineralocorticoid receptor, NT-proBNP N-terminal pro-B-type natriuretic peptide, NYHA New York Heart Association, SGLT-2i sodium-glucose cotransporter 2 inhibitor, T2D type 2 diabetes
Another practical consideration for introducing SGLT-2i therapy in the HF patient population is the potential for SGLT-2i-related adverse events (AEs) [62]. Genitourinary infections, mild in severity and easily treated, are the most common AEs. The following serious AEs, although rare, have been associated with SGLT-2i therapy and should be taken into consideration in patients with underlying comorbidities and concomitant medications: diabetic ketoacidosis (DKA), renal injury, volume depletion, and lower-limb amputation [62]. Indeed, in the recently published SCORED trial of sotagliflozin, some significant AEs (primarily diarrhea, genital mycotic infections, volume depletion, and DKA) were noted [27]. The use of SGLT-2is as a monotherapy is not associated with an increased risk of hypoglycemia among patients with T2D; however, the risk of hypoglycemia is increased with concomitant use of a SGLT-2i and insulin or insulin secretagogues (e.g., sulfonylurea) [54]. Moreover, the evidence available thus far suggests that the hypoglycemia risk is low among nondiabetic patients with HF. There has been concern about hypoglycemia in patients without T2D receiving SGLT-2i therapy, but an exploratory analysis of DAPA-HF demonstrated no major hypoglycemia in the patients without T2D [30]. DKA is not seen in patients without T2D, whereas in patients with T2D, DKA is a rare AE [67]. The following factors contribute to an increased risk of DKA in patients with T2D: insulin/pancreatic deficiency, low carbohydrate intake, postoperative stress, and excessive alcohol intake [31, 47, 67]. Additionally, in patients with T2D, physicians should be aware of euglycemic DKA, which is diagnosed on the basis of the presence of serum or urinary ketones irrespective of normal serum glucose levels [67]. In general, based on an assessment of overall effectiveness and safety and tolerability, the benefit-risk profile of SGLT-2is is considered favorable (Table 2) [68]. Healthcare professionals can reduce the potential for serious or severe AEs among their patients through risk assessment and identification of patients who are at a lower risk of AEs before initiating SGLT-2i therapy, ongoing monitoring through regular clinic visits, and by being aware of and educating their patients to recognize early signs and symptoms associated with possible development of complications and the need to seek medical attention should these occur.
Table 2.
| Benefits | Risks |
|---|---|
|
Approved indication/guideline recommended (dapagliflozin and empagliflozin) Prevention of HF Reduced rates of MACE, CV mortality, and HHF Reduction in blood pressure Safe in patients with eGFR ≥ 30 ml/min/1.73 m2; preserves renal function in patients with T2D Improved glycemia in patients with comorbid T2D (but contraindicated in T1D); no risk of hypoglycemia in those without T2D Weight loss Few drug-drug interactions (most important are canagliflozin-digoxin interaction [may result in an increase in digoxin concentrations], and group interaction with diuretics [may result in volume depletion]) |
Genitourinary infections (particularly mycotic infections in women and uncircumcised men; may lead to increased urinary frequency but not directly associated with UTIs) Hypotension Diabetic ketoacidosis (euglycemic) in patients with comorbid T2D (low risk); diagnosis is based on presence of serum or urinary ketones irrespective of normal serum glucose levels Limb amputation (low risk, more apparent with canagliflozin and increased risk in those with previous amputation or PAD) Hypoglycemia in patients with comorbid T2D (low risk but more pronounced with concomitant use of insulin or sulfonylurea therapy) Fractures (very low risk) |
CV cardiovascular, eGFR estimated glomerular filtration rate, HF heart failure, HHF hospitalization for heart failure, MACE major adverse cardiovascular events, PAD peripheral artery disease, SGLT-2is sodium-glucose cotransporter-2 inhibitors, T1D type 1 diabetes, T2D type 2 diabetes, UTIs urinary tract infections
Therapeutic management of patients with multiple underlying comorbidities will likely involve co-administration of SGLT-2is with other CV therapies. Reported drug-drug interactions with SGLT-2is are minimal [62, 68]; moreover, it is noteworthy that many patients in the SGLT-2i CVOTs were receiving concomitant CV medications [9–11, 13, 14]. However, because of differences in the SGLT-2i CVOT patient populations, the proportion of patients who received concomitant SGLT-2is and CV medications varied [9–11, 13, 14]. For example, in the DECLARE-TIMI 58 study, 81.3% of patients received ACEis/ARBs, 52.4% received beta blockers, 74.9% received a statin or ezetimibe, and 40.6% received a diuretic [13]. Nevertheless, because of concomitant effects on diuresis, caution should be exercised when prescribing SGLT-2is in combination with ARNis or diuretics [37, 68]. Dose adjustments of SGLT-2is and/or loop diuretics may be necessary [8, 69]. In cases of clinical hypovolemia or ketoacidosis, temporary withdrawal of SGLT-2is and diuretics may be necessary, along with administration of fluids and sodium [69]. For canagliflozin, a P-glycoprotein substrate, its co-administration with digoxin may result in increased plasma levels of digoxin [62].
Sub-analyses of data from the CVOTs and HF trials indicate that the positive effects of SGLT-2is on HF outcomes are not affected by patient characteristics such as anemia status at initiation of treatment (dapagliflozin) [70], HF etiology (dapagliflozin) [71], presence of chronic obstructive pulmonary disease (dapagliflozin) [72], treatment initiation-associated “dip” in eGFR (empagliflozin) [73], or concomitant use of ARNis (empagliflozin) [74]. However, sub-analyses of the CANVAS trial indicated that patients receiving diuretics at baseline derived greater benefit from canagliflozin than patients not receiving diuretics [75], and that absolute, although not relative, risk reductions for CV and HF were greater in individuals with higher Kidney Disease: Improving Global Outcomes (KDIGO) risk categories [76].
The addition of an SGLT-2i to background CV therapy, as a component of a standard-of-care treatment plan, will require additional evidence-based assessments. It is important to bear in mind that existing HF-related guidelines are not necessarily a good fit for all patients, and SGLT-2i therapy may be a potentially beneficial complementary therapy to consider.
Conclusions
SGLT-2is have been clearly demonstrated as an effective therapeutic intervention for the treatment and prevention of HFrEF based on the findings of CVOTs in patients with HFrEF with and without T2D. Although guidelines for the treatment of T2D have recommended SGLT-2is as a therapy for T2D and CV protection for several years, cardiology guidelines are now beginning to integrate SGLT-2is into a standard-of-care treatment regimen for nondiabetic patients with HFrEF. Recently completed trials in patients with HFrEF have led to the approval of dapagliflozin and empagliflozin for the treatment of HFrEF in patients with or without T2D, and other similar approvals are likely to follow shortly. Additional studies are providing clinical evidence on the role of SGLT-2is in the treatment of patients with HFpEF with or without T2D. The EMPEROR-Preserved study results of empagliflozin in HFpEF are encouraging, and the ongoing DELIVER study will also provide data on dapagliflozin use in HFpEF. The increasing amount of evidence for SGLT-2i therapy in patients with HF who do not have T2D will likely lead to increased use in cardiology practice. Therefore, it is important that cardiologists become familiar with this drug class and its benefits and risks for their patients. Given their favorable benefit-risk profile and wide-ranging effects, it is hoped that SGLT-2is will provide a useful treatment option to help reduce the significant disease burden associated with HF in patients without T2D as well as in those with T2D.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
The development of this manuscript and the journal’s Rapid Service Fee were funded by AstraZeneca.
Authorship
Shaline Rao meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, takes responsibility for the integrity of the work as a whole, and has given her approval for the manuscript to be published.
Author contributions
Shaline Rao contributed to the development and review of this manuscript and approved the final version of the manuscript for submission.
Medical writing assistance
Medical writing assistance in the preparation of this article was provided by Andrea Bothwell and Kate Palmer, inScience Communications, Springer Healthcare (Auckland, New Zealand), in accordance with Good Publication Practice (GPP-3). Support for this assistance was funded by AstraZeneca.
Disclosures
Shaline Rao has received consultant fees from AstraZeneca and Boehringer Ingelheim.
Compliance with ethics guidelines
This article is based on the published literature and does not report any original data from human or animal studies conducted by the author.
Data availability
Data sharing is not applicable as there were no datasets generated or analyzed for this article.
References
- 1.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesyuk W, Kriza C, Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord. 2018;18:74. doi: 10.1186/s12872-018-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2017;23:628–651. doi: 10.1016/j.cardfail.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seferovic PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853–872. doi: 10.1002/ejhf.1170. [DOI] [PubMed] [Google Scholar]
- 8.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 9.Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 11.Rådholm K, Figtree G, Perkovic V, et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation. 2018;138:458–468. doi: 10.1161/CIRCULATIONAHA.118.034222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma S, Wanner C, Zwiener I, et al. Influence of microvascular disease on cardiovascular events in type 2 diabetes. J Am Coll Cardiol. 2019;73:2780–2782. doi: 10.1016/j.jacc.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 14.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 15.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 16.Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139:2516–2527. doi: 10.1161/CIRCULATIONAHA.119.039996. [DOI] [PubMed] [Google Scholar]
- 17.Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71:471–476. doi: 10.1016/j.jjcc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 19.Cosentino F, Cannon CP, Cherney DZI, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. 2020;142:2205–2215. doi: 10.1161/CIRCULATIONAHA.120.050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 21.Gautam S, Agiro A, Barron J, Power T, Weisman H, White J. Heart failure hospitalization risk associated with use of two classes of oral antidiabetic medications: an observational, real-world analysis. Cardiovasc Diabetol. 2017;16:93. doi: 10.1186/s12933-017-0575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YG, Han SJ, Kim DJ, Lee KW, Kim HJ. Association between sodium glucose co-transporter 2 inhibitors and a reduced risk of heart failure in patients with type 2 diabetes mellitus: a real-world nationwide population-based cohort study. Cardiovasc Diabetol. 2018;17:91. doi: 10.1186/s12933-018-0737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL Study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors) Circulation. 2017;136:249–259. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patorno E, Goldfine AB, Schneeweiss S, et al. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. BMJ. 2018;360:k119. doi: 10.1136/bmj.k119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patorno E, Pawar A, Franklin JM, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation. 2019;139:2822–2830. doi: 10.1161/CIRCULATIONAHA.118.039177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 27.Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384:129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 28.Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528–2536. doi: 10.1161/CIRCULATIONAHA.119.040130. [DOI] [PubMed] [Google Scholar]
- 29.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 30.Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353–1368. doi: 10.1001/jama.2020.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.AstraZeneca Pharmaceuticals LP. Farxiga (dapagliflozin) tablets, for oral use. 2021. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=202293. Accessed 26 Oct 2021.
- 32.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 33.Boehringer Ingelheim Pharmaceuticals Inc. Jardiance (empagliflozin) tablets, for oral use. 2021. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=204629. Accessed 26 Oct 2021.
- 34.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 35.Szarek M, Bhatt DL, Steg PG, et al. Effect of sotagliflozin on total hospitalizations in patients with type 2 diabetes and worsening heart failure: a randomized trial. Ann Intern Med. 2021;174:1065–1072. doi: 10.7326/M21-0651. [DOI] [PubMed] [Google Scholar]
- 36.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 37.O'Meara E, McDonald M, Chan M, et al. CCS/CHFS heart failure guidelines: clinical trial update on functional mitral regurgitation, SGLT2 inhibitors, ARNI in HFpEF, and tafamidis in amyloidosis. Can J Cardiol. 2020;36:159–169. doi: 10.1016/j.cjca.2019.11.036. [DOI] [PubMed] [Google Scholar]
- 38.Lam CSP, Chandramouli C, Ahooja V, Verma S. SGLT-2 inhibitors in heart failure: current management, unmet needs, and therapeutic prospects. J Am Heart Assoc. 2019;8:e013389. doi: 10.1161/JAHA.119.013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguilar D, Deswal A, Ramasubbu K, Mann DL, Bozkurt B. Comparison of patients with heart failure and preserved left ventricular ejection fraction among those with versus without diabetes mellitus. Am J Cardiol. 2010;105:373–377. doi: 10.1016/j.amjcard.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindman BR, Davila-Roman VG, Mann DL, et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol. 2014;64:541–549. doi: 10.1016/j.jacc.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacDonald MR, Petrie MC, Varyani F, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 42.Mohammed SF, Borlaug BA, Roger VL, et al. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail. 2012;5:710–719. doi: 10.1161/CIRCHEARTFAILURE.112.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Figtree GA, Rådholm K, Barrett TD, et al. Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus. Circulation. 2019;139:2591–2593. doi: 10.1161/CIRCULATIONAHA.119.040057. [DOI] [PubMed] [Google Scholar]
- 44.Lu Y, Li F, Fan Y, Yang Y, Chen M, Xi J. Effect of SGLT-2 inhibitors on cardiovascular outcomes in heart failure patients: a meta-analysis of randomized controlled trials. Eur J Intern Med. 2021;87:20–28. doi: 10.1016/j.ejim.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Wojcik C, Warden BA. Mechanisms and evidence for heart failure benefits from SGLT2 inhibitors. Curr Cardiol Rep. 2019;21:130. doi: 10.1007/s11886-019-1219-4. [DOI] [PubMed] [Google Scholar]
- 46.Butler J, Handelsman Y, Bakris G, Verma S. Use of sodium-glucose co-transporter-2 inhibitors in patients with and without type 2 diabetes: implications for incident and prevalent heart failure. Eur J Heart Fail. 2020;22:604–617. doi: 10.1002/ejhf.1708. [DOI] [PubMed] [Google Scholar]
- 47.Janssen Pharmaceuticals Inc. Invokana (canagliflozin) tablets, for oral use. 2020. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=204042. Accessed 26 Oct 2021.
- 48.Merck Sharp & Dohme Inc. Steglatro (ertugliflozin) tablets, for oral use. 2021. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=209803. Accessed 26 Oct 2021.
- 49.Silva-Cardoso J, Sheikh O, Nashawi M, et al. Cardiorenal protection with SGLT2: lessons from the cardiovascular outcome trials. J Diabetes. 2020;12:279–293. doi: 10.1111/1753-0407.13007. [DOI] [PubMed] [Google Scholar]
- 50.Atherton JJ, Sindone A, De Pasquale CG, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of heart failure 2018. Med J Aust. 2018;209:363–369. doi: 10.5694/mja18.00647. [DOI] [PubMed] [Google Scholar]
- 51.Bashier A, Bin Hussain A, Abdelgadir E, Alawadi F, Sabbour H, Chilton R. Consensus recommendations for management of patients with type 2 diabetes mellitus and cardiovascular diseases. Diabetol Metab Syndr. 2019;11:80. doi: 10.1186/s13098-019-0476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43:487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;2018(61):2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 54.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes–2021. Diabetes Care. 2021;44:S111–S124. doi: 10.2337/dc21-S009. [DOI] [PubMed] [Google Scholar]
- 55.Seferovic PM, Fragasso G, Petrie M, et al. Heart Failure Association of the European Society of Cardiology update on sodium-glucose co-transporter 2 inhibitors in heart failure. Eur J Heart Fail. 2020;22:1984–1986. doi: 10.1002/ejhf.2026. [DOI] [PubMed] [Google Scholar]
- 56.Seferovic PM, Fragasso G, Petrie M, et al. Sodium glucose co-transporter-2 inhibitors in heart failure: beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1495–1503. doi: 10.1002/ejhf.1954. [DOI] [PubMed] [Google Scholar]
- 57.Maddox TM, Januzzi JLJ, Allen LA, et al. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77:772–810. doi: 10.1016/j.jacc.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 58.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 59.Ali A, Bain S, Hicks D, et al. SGLT2 inhibitors: cardiovascular benefits beyond HbA1c-translating evidence into practice. Diabetes Ther. 2019;10:1595–1622. doi: 10.1007/s13300-019-0657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnold SV, Inzucchi SE, Tang F, et al. Real-world use and modeled impact of glucose-lowering therapies evaluated in recent cardiovascular outcomes trials: An NCDR® Research to Practice project. Eur J Prev Cardiol. 2017;24:1637–1645. doi: 10.1177/2047487317729252. [DOI] [PubMed] [Google Scholar]
- 61.Vaduganathan M, Sathiyakumar V, Singh A, et al. Prescriber patterns of SGLT2i after expansions of U.S. Food and Drug Administration labeling. J Am Coll Cardiol. 2018;72:3370–3372. doi: 10.1016/j.jacc.2018.08.2202. [DOI] [PubMed] [Google Scholar]
- 62.Vardeny O, Vaduganathan M. Practical guide to prescribing sodium-glucose cotransporter 2 inhibitors for cardiologists. JACC Heart Fail. 2019;7:169–172. doi: 10.1016/j.jchf.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 63.Scheen AJ. Series: Implications of the recent CVOTs in type 2 diabetes. Impact on guidelines: the endocrinologist point of view. Diabetes Res Clin Pract. 2020;159:107726. doi: 10.1016/j.diabres.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez DE, Foresto RD, Ribeiro AB. SGLT-2 inhibitors in diabetes: a focus on renoprotection. Rev Assoc Med Bras (1992) 2020;66(66):s17–s24. doi: 10.1590/1806-9282.66.S1.17. [DOI] [PubMed] [Google Scholar]
- 65.Verma S, Sharma A, Kanumilli N, Butler J. Predictors of heart failure development in type 2 diabetes: a practical approach. Curr Opin Cardiol. 2019;34:578–583. doi: 10.1097/HCO.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosano G, Quek D, Martinez F. Sodium-glucose co-transporter 2 inhibitors in heart failure: recent data and implications for practice. Card Fail Rev. 2020;6:e31. doi: 10.15420/cfr.2020.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mistry S, Eschler DC. Euglycemic diabetic ketoacidosis caused by SGLT2 inhibitors and a ketogenic diet: a case series and review of literature. AACE Clin Case Rep. 2021;7:17–19. doi: 10.1016/j.aace.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Opingari E, Partridge ACR, Verma S, Bajaj HS. SGLT2 inhibitors: practical considerations and recommendations for cardiologists. Curr Opin Cardiol. 2018;33:676–682. doi: 10.1097/HCO.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 69.Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:1169–1186. doi: 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]
- 70.Docherty KF, Curtain JP, Anand IS, et al. Effect of dapagliflozin on anaemia in DAPA-HF. Eur J Heart Fail. 2021;23:617–628. doi: 10.1002/ejhf.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Butt JH, Nicolau JC, Verma S, et al. Efficacy and safety of dapagliflozin according to aetiology in heart failure with reduced ejection fraction: insights from the DAPA-HF trial. Eur J Heart Fail. 2021;23:601–613. doi: 10.1002/ejhf.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dewan P, Docherty KF, Bengtsson O, et al. Effects of dapagliflozin in heart failure with reduced ejection fraction and chronic obstructive pulmonary disease: an analysis of DAPA-HF. Eur J Heart Fail. 2021;23:632–643. doi: 10.1002/ejhf.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kraus BJ, Weir MR, Bakris GL, et al. Characterization and implications of the initial estimated glomerular filtration rate 'dip' upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. 2021;99:750–762. doi: 10.1016/j.kint.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 74.Packer M, Anker SD, Butler J, et al. Influence of neprilysin inhibition on the efficacy and safety of empagliflozin in patients with chronic heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J. 2021;42:671–680. doi: 10.1093/eurheartj/ehaa968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu J, Arnott C, Neuen BL, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline diuretic use: a post hoc analysis from the CANVAS program. ESC Heart Fail. 2021;8:1482–1493. doi: 10.1002/ehf2.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neuen BL, Ohkuma T, Neal B, et al. Relative and absolute risk reductions in cardiovascular and kidney outcomes with canagliflozin across KDIGO risk categories: findings from the CANVAS program. Am J Kidney Dis. 2021;77:23–34 e1. doi: 10.1053/j.ajkd.2020.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable as there were no datasets generated or analyzed for this article.