Abstract
Coronavirus disease 2019 (COVID-19) is a new and severe infectious disease and new global disaster and is spreading rapidly worldwide. Natural products have a long history and have been widely used to treat various acute, chronic, and even life-threatening diseases worldwide. However, the natural products have reduced bioavailability and availability as they have poor kinetic properties, such as large molecular weight, inability to cross lipid membranes, and weak absorption ability. With the rapid development of nanotechnology, using novel nanotechnology in conjunction with natural products can effectively eliminate the molecular restriction of the entry of nanoproducts into the body and can be used to diagnose and treat various diseases, including COVID-19, bringing new strategies and directions for medicine. This article reviews the role and implementation of natural products against COVID-19 based on nanotechnology.
Keywords: COVID-19, nanotechnology, natural products, kinetic properties, bioavailability
Introduction
It has been longer than 2 years since the coronavirus disease 2019 (COVID-19) outbreak, which continues to affect human social and economic life in more than 200 countries and regions around the world (Li et al., 2020; Abu-Raddad et al., 2021; Chen B. et al., 2021; Berwanger, 2021). There have been more than 246 million diagnosed cases and 5 million deaths due to COVID-19 worldwide until October 31, 2021, and thousands continue to die daily. The most affected countries include the United States and India, where more than 1.1 million people have died from COVID-19 (Sinha et al., 2021).
The development of vaccines and plasma therapy has impacted the prevention and treatment of COVID-19 profoundly (Benner et al., 2020; Li C. et al., 2021; Tang P. et al., 2021; Barda et al., 2021; Kunze et al., 2021; Macchia et al., 2021; Tartof et al., 2021; Tedder and Semple, 2021); however, a shortage of medical capacity remains in all countries. The application of traditional medicines including nano-loaded natural products, has also been listed as a major treatment strategy to aid the recovery of patients with COVID-19 and combat the global pandemic (Sinha et al., 2021). Our purpose of this review focus on using novel nanotechnology in conjunction with natural products that used to diagnose and treat COVID-19.
Natural Products
Natural products have a long history and are widely used around the world (Devi et al., 2010; Al-Zahrani et al., 2021; Kaur et al., 2021). At present, nearly 200,000 natural compounds are extracted for medicinal purposes from higher plants, animals, fungi, and marine organisms, mostly from medicinal and aromatic plants (Atanasov et al., 2015; Ahmed, 2016).
Currently, different natural products reportedly exert anti-cancer, anti-oxidation, anti-malarial, anti-anxiety, and anti-organ effects, and treat various cardiovascular diseases effectively, and facilitate the effective treatment of various cardiovascular diseases (Weng et al., 2019; Li L. et al., 2021). Researchers have found that herbs can directly inhibit pathogens associated with common diseases that infect the respiratory tract or coordinate the activities of the immune system to prevent or alleviate infection (Yan et al., 2021). The application and research of natural products has undergone a long development process from the initial drug compound formulation to the isolation and extraction of highly effective compounds with therapeutic potential from plants and animals (Devi et al., 2010). Even during the COVID-19 pandemic, scholars worldwide found an effective drug to prevent and treat COVID-19. Mohammad et al. tried to prevent COVID-19 by studying the pharmacological effects of black cumin seeds and extracted their bioactive compounds (Islam et al., 2021), Salim et al. conducted a molecular link-based study to explore nigellidine and α-hederin in N. sativa compounds as novel severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) inhibitors (Duru et al., 2021). In addition, several high-quality peer-reviewed herbal clinical trials are ongoing (Jotz et al., 2020; Singh et al., 2021).
Compared with synthetic drugs, natural products are easy to obtain, exert several pharmacological effects on different diseases, and their side effects are relatively weak. However, natural products exhibit poor kinetic performance due to characteristics such as their large molecular weight, inability to cross lipid membranes, and weak absorption capacity, which result in reduced bioavailability (Figure 1).
FIGURE 1.
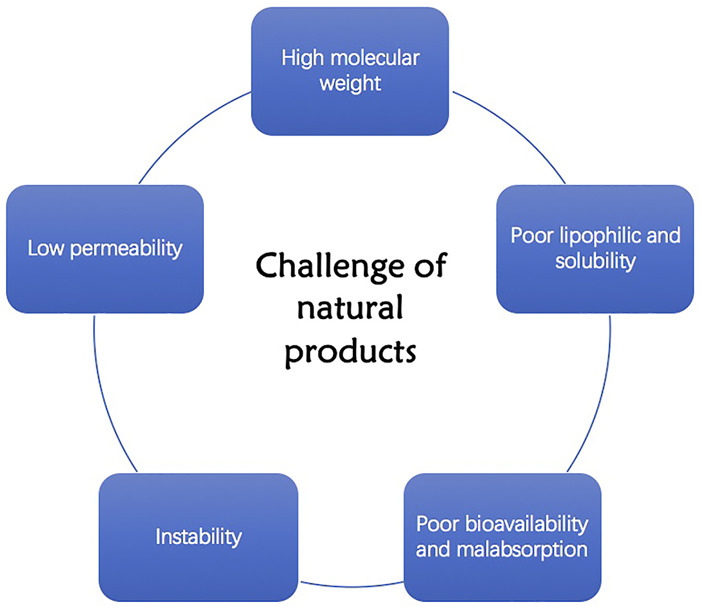
Challenges of natural products.
Up to now, there are several natural products (polyphenols, alkaloids, terpenoids etc.) used in COVID 2019 treatment and prevention. Mhatre et al. investigated two tea polyphenols: epigallocatechin-3-gallate and theaflavins and found that they exhibited antiviral activities against positive-sense single-stranded RNA viruses (Mhatre et al., 2021). Typically alkaloids carry one or more nitrogen atom within a heterocyclic ring. In Huang’s study, berbamine (one of alkaloids) was found to inhibit genome replication, and reduced infectious virus production using Vero E6 cells (He et al., 2021). In Pratibha Mishra’s description, Terpenoids also have potential to be developed as a treatment for COVID-19 (Mishra et al., 2021).
Natural Products Based on Nanotechnology
"Nano" refers to a billionth of a meter in length. Nanomaterials can be manipulated, controlled and modified physically or chemically to acquire specific functional properties. Nanostructures are derived from microscopic structure, changes in size can bring variations in properties, which also makes the application of nanotechnology have great potential (Bustos Cruz et al., 2017; Bayda et al., 2019).
Nanotechnology has developed rapidly in recent years, and natural products, including herbal therapy based on nanotechnology, are becoming increasingly popular and are showing very broad application prospects (Liu et al., 2021; Zhang et al., 2021; Zhu et al., 2021). Nanotechnology usually refers to the use of nanomaterials with sizes between 1 and 100 nm, which can be classified as organic and inorganic nanoparticles. Nanoparticles can be designed in different shapes and sizes, loaded with drugs, and modified functionally and physiochemically according to the characteristics of the active substances. Chemical functionalization and identification of unique physical and chemical properties have been applied in various fields based on the synthesis and characterization of rich engineering materials (Zhang and Tang, 2016; Weiss et al., 2020; Ahmed et al., 2021; Manikandan et al., 2021; Venturi, 2021).
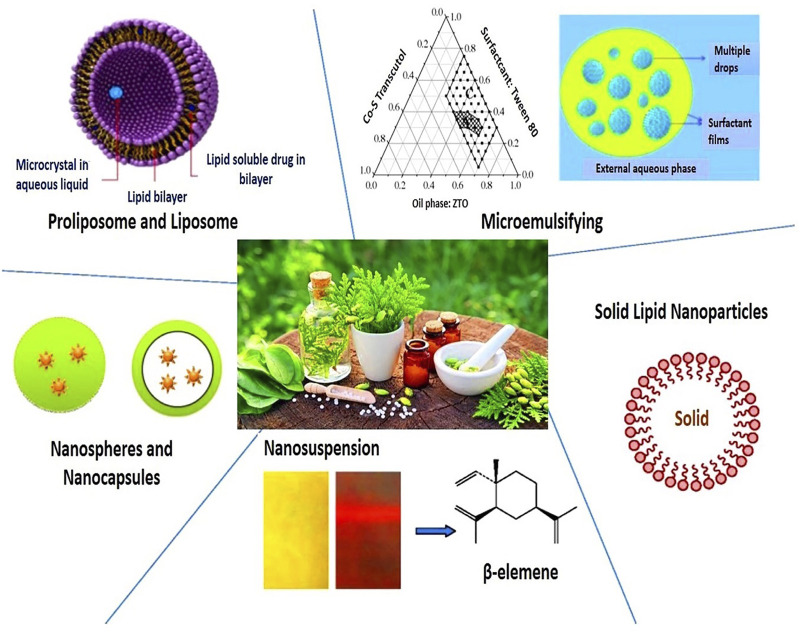
The amalgamation of novel drug delivery nanocarriers with drug complexes can effectively eliminate the molecular limitations of their entry into the body (Nabi et al., 2019). By using nanocarriers such as nanoparticles, nanoliposomes, and alcohol solutes, natural products can be loaded and smoothly inserted into the appropriate parts of the body (Allaw et al., 2020; Raja et al., 2021; Salarbashi et al., 2021). On the one hand, nanotechnology increases the solubility of a low soluble compound and improves its stability, however, nanotechnology can be combined to enhance the effectiveness of the therapeutic drug and reduce its side effects (Kaur et al., 2021) (Figures 2 and 3). To date, natural products based on nanotechnology have been used to diagnose and treat various diseases, bringing new strategies and directions to medicine (Alfuraydi et al., 2019; Valsalam et al., 2019; Hashemi et al., 2021).
FIGURE 2.
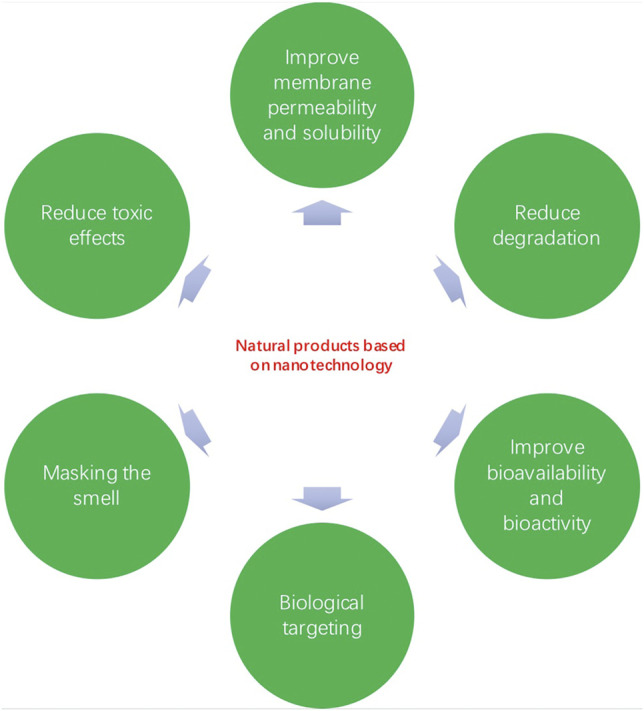
Natural products based on nanotechnology.
FIGURE 3.
Nanoparticle drug delivery systems for herbal drug formulations (Ahmed et al., 2021).
Natural Products for COVID-19 Treatment Based on Nanotechnology
Diagnostic and therapeutic techniques based on nanotechnology, such as nutritional nanotechnology, have been a concern for researchers since the start of the COVID-19 pandemic, or the development of highly sensitive and specific antigens for COVID-19 detection tests (Layqah and Eissa, 2019). Palmieri et al. conjugated the COVID-19 anti-S protein antibody to a graphene sheet as a sensitive area, preventing antigenic cross-reactivity with MERS-CoV, and successfully detected the virus in clinical samples with high sensitivity and no sample pretreatment (Palmieri and Papi, 2020; Dacrory, 2021). Ahmed et al. developed Au NP-quantum dot nanocomposites that could contain viral lipid tails and promote envelope aggregation and rupture. Layqah et al. also used an Au NP-modified carbon electrode recombinant spike protein SI as a biomarker (Layqah and Eissa, 2019; Lobo-Galo et al., 2021).
The most common therapeutic strategies for resuing existing drugs to treat COVID-19 to reduce severe symptoms in infected patients have worked well, including those previously used safely as herbal therapy medicine, even if they were not originally intended as antivirals (Lobo-Galo et al., 2021). Some of the most promising herbal therapy drugs reduce viral load, length of hospital stay, disease severity, and mortality, and some have been tested in clinical trials (Riva et al., 2020; Tang W.-F. et al., 2021; Hashemi et al., 2021; Jia et al., 2021).
Chloroquine is a typical drug that is extracted from the original long-term application of natural products (Fitch, 1969; Mossaad et al., 2015), and is used to treat malaria after the identification and purification of active components, structural characterization, and understanding of the mechanism of drug action, as well as derivative modification of drugs. During the COVID-19 pandemic, the use of chloroquine to treat COVID-19 was initiated by the State Council of China. In a preliminary in vitro study, some trials conducted in patients reported reduced recovery times (Chen Z. et al., 2021; Jia et al., 2021; Roy et al., 2022). The Food Drug Administration approved chloroquine and hydroxychloroquine for emergency use in late March. The main mechanism of action is that entering lung cells can be activated by TMPRSS-2 (Lobo-Galo et al., 2021; Yao et al., 2021). Although some concerns regarding chloroquine remain due to the side effects caused by the drug dosage, the subsequent optimization of loading based on nanotechnology will likely result in improved functionality and efficacy of chloroquine or chloroquine in the treatment of COVID-19 [Table 1(Borba et al., 2020; Duvignaud et al., 2020; Group et al., 2020; Liu et al., 2020; Panda et al., 2020; Tirupakuzhi Vijayaraghavan et al., 2020; Weehuizen and Hoepelman, 2020; Abd-Elsalam et al., 2021; Gautret et al., 2021; Rea-Neto et al., 2021; Thakar et al., 2021)].
TABLE 1.
Main clinical trials of Chloroquine for Covid-19.
| Author | Design | Country | End point | References | |
|---|---|---|---|---|---|
| 1 | Philippe Gautret et al. | Open-label non-randomized clinical trial | France | Viral load | Gautret et al. (2021) |
| 2 | RECOVERY Collaborative Group et al. | Randomized, controlled, open-label platform trial | United Kingdom | Mortality | Group et al. (2020) |
| 3 | Duvignaud A. et al. | Phase III, multi-arm (5) and multi-stage (MAMS), randomized, open-label controlled superiority trial | France | Prevent hospitalisation or death | Duvignaud et al. (2020) |
| 4 | Prasan Kumar Panda et al. | Open label, Parallel arm design, stratified randomised controlled trial | India | Severe category | Panda et al. (2020) |
| 5 | Xi Liu et al. | Prospective, open-label, randomized controlled, multicenter clinical study | China | Clinical recovery time | Liu et al. (2020) |
| 6 | Mayla Gabriela Silva Borba et al. | Parallel, double-masked, randomized, phase IIb clinical trial | Brazil | Safety and lethality outcomes | Borba et al. (2020) |
| 7 | Bharath Kumar Tirupakuzhi Vijayaraghavan et al. | Multi-centre open-label parallel group randomized controlled trial | India et al. | The proportion of healthcare workers developing laboratory confirmed COVID-19 infection within 6 months of randomization | Tirupakuzhi Vijayaraghavan et al. (2020) |
| 8 | Alok Thakar et al. | Randomized clinical trial | India | Clinical progression and outcomes | Thakar et al. (2021) |
| 9 | Sherief Abd-Elsalam et al. | Randomized clinical trial | Egypt | Recovery within 28 days, the need for mechanical ventilation, and death | Abd-Elsalam et al. (2021) |
| 10 | Álvaro Réa-Neto et al. | Randomized, open-label, controlled, phase III trial | Brazil | Clinical status measured on day 14 | Rea-Neto et al. (2021) |
| 11 | Weehuizen JM et al. | Controlled, open label, cluster-randomized, superiority trial | Netherlands | Disease progression | Weehuizen and Hoepelman (2020) |
Active substances extracted and purified from various herbs have long been used as antiviral drugs. These active substances are usually designed based on natural composite structures. Homohar-ringtonine, isolated from Cephalotaxus, is another classic example of the use of herbal compounds against herpes viruses (Hassan, 2020). In addition, natural drug research strategies against COVID-19 include not only studies of one or more biomolecules in vivo, but also those conducted in silico derived from natural products with therapeutic or preventive potential to guide and assist in the successful treatment of COVID-19 (Ang et al., 2020).
Curcumin, a polyphenol extracted from the rhizome of turmeric (Kocaadam and Sanlier, 2017), is one of the most thoroughly studied molecules derived from dietary natural products. Its potential benign effects and safety of curcumin on inflammation, cancer, depression, and many other diseases in particular have been investigated (Gao et al., 2004; Hurley et al., 2013; Minassi et al., 2013; Thimmulappa et al., 2021). The related mechanisms include curcumin’s viral inhibition, regulation of inflammation, and immune response. Recently, Zahedipour et al. also preliminarily investigated curcumin’s potential to reverse brosis-associated pulmonary edema and pathways in COVID-19 infection and demonstrated its potential pharmacodynamic potential in the treatment of COVID-19 (Zahedipour et al., 2020). However, curcumin’s biopharmaceutical limitations and limitations on biological responses include physicochemical properties that reduce its bioavailability (Sanidad et al., 2019). Douglas et al. highlighted nanotechnology as a key way to overcome curcumin drug deficiencies and exert anti-COVID-19 efficacy (Dourado et al., 2021). Researchers have developed nanotechnological carriers such as nanoemulsions, liposomes, and nanogels to load curcumin, enhance its solubility, protect it from chemical and metabolic degradation, and modify it to be more easily transported through biofilms for its efficacy. In the current COVID-19 pandemic, there are three curcumin-based nanotechnology products available on the market in the form of polymer nanoparticles (NanocurcTM), liposomes (LipocurcTM), and nanoparticles (sinacurcu-min ®), which show initial benign therapeutic effects (Valizadeh et al., 2020; Saber-Moghaddam et al., 2021; Tahmasebi et al., 2021).
Particles with different materials and structures are appear in different ways, such as lipid-based nanoparticles, polymer nanoparticles, and inorganic nanoparticles (Zazo et al., 2016). In addition to the advantages of nanoparticles in transporting large drug molecules, they also have the advantage of being easy to customize and functionalize (Gagliardi, 2017). The mechanism of action of nanoparticles to exert an anti-viral effect is realized through several pathways, such as 1) virus inactivation (direct or indirect), 2) virus action in host cells, 3) virus penetration, and 4) virus replication, depending on the nature and functionalization of the nanoparticles used (Chen and Liang, 2020). In this way, nanoparticles can physically or chemically block these steps and modify the structure of capsid proteins, thereby reducing the viral load (Gurunathan et al., 2020).
Conclusions and Perspectives
COVID-19 poses a huge challenge to and seriously threatens human health and economic development. In this context, many natural products have attracted great attention as promising anti-SARS-COV-2 drugs and have shown some degree of effectiveness. The present review focuses on recent progress in the application of some important natural products. However, some natural products may have disadvantages and limitations, such as poor water solubility and low bioavailability, which restrict their widespread clinical application. Therefore, there is still a long way to go before drug discovery and development based on natural products can be achieved.
In contrast, nanomaterials have unique physical and chemical properties, it has inherent resistance to pathogenicity and/or its ability to produce inactivated viruses through photothermal catalytic induction of reactive oxygen species (ROS). Secondly, nanoloading natural products can enhance the biocompatibility of natural products and minimize toxicity and side effects, thereby overcoming many obstacles such as adverse reactions. Nano-drug treatment strategies may therefore be a more effective therapeutic approach. In addition, the concept of "nano immunity" can help us design immunomodulatory materials, and has good prospects for COVID-19 development or against cytokine storm (Chauhan et al., 2020). The current study on the etiology of novel coronavirus and the pharmacological mechanism of natural products, in addition to the mature application of nano-drug delivery technology and the improvement of clinical trials of drugs such as chloroquine, natural products based on nanotechnology will provide a broad application prospect for the treatment of COVID-19.
Author Contributions
All authors contributed to the design of the study and writing of the manuscript. ZN and XC undertook the research, ZN and XC wrote the main manuscript text and prepared figures. ZL revised the article critically for important intellectual content and final approval of the version to be submitted. All authors reviewed the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
COVID-19, coronavirus disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; MERS-CoV, middle east respiratory syndrome coronavirus; TMPRSS-2, transmembrane serine protease 2.
References
- Abd-Elsalam S., Soliman S., Esmail E. S., Khalaf M., Mostafa E. F., Medhat M. A., et al. (2021). Do Zinc Supplements Enhance the Clinical Efficacy of Hydroxychloroquine?: a Randomized, Multicenter Trial. Biol. Trace Elem. Res. 199, 3642–3646. 10.1007/s12011-020-02512-1 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Abu-Raddad L. J., Chemaitelly H., Ayoub H. H., Yassine H. M., Benslimane F. M., Al Khatib H. A., et al. (2021). Association of Prior SARS-CoV-2 Infection with Risk of Breakthrough Infection Following mRNA Vaccination in Qatar. JAMA 326 (19), 1930–1939. 10.1001/jama.2021.19623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H. M. (2016). Ethnopharmacobotanical Study on the Medicinal Plants Used by Herbalists in Sulaymaniyah Province, Kurdistan, Iraq. J. Ethnobiol. Ethnomedicine 12, 8. 10.1186/s13002-016-0081-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H. M., Nabavi S., Behzad S. (2021). Herbal Drugs and Natural Products in the Light of Nanotechnology and Nanomedicine for Developing Drug Formulations. Mini Rev. Med. Chem. 21, 302–313. 10.2174/1389557520666200916143240 [DOI] [PubMed] [Google Scholar]
- Al-Zahrani S., Astudillo-Calderón S., Pintos B., Pérez-Urria E., Manzanera J. A., Martín L., et al. (2021). Role of Synthetic Plant Extracts on the Production of Silver-Derived Nanoparticles. Plants (Basel) 10 (8), 1671. 10.3390/plants10081671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfuraydi A. A., Devanesan S., Al-Ansari M., Alsalhi M. S., Ranjitsingh A. J. (2019). Eco-friendly green Synthesis of Silver Nanoparticles from the Sesame Oil Cake and its Potential Anticancer and Antimicrobial Activities. J. Photochem. Photobiol. B: Biology 192, 83–89. 10.1016/j.jphotobiol.2019.01.011 [DOI] [PubMed] [Google Scholar]
- Allaw M., Pleguezuelos-Villa M., Manca M. L., Caddeo C., Aroffu M., Nacher A., et al. (2020). Innovative Strategies to Treat Skin Wounds with Mangiferin: Fabrication of Transfersomes Modified with Glycols and Mucin. Nanomedicine 15, 1671–1685. 10.2217/nnm-2020-0116 [DOI] [PubMed] [Google Scholar]
- Ang L., Lee H. W., Kim A., Lee M. S. (2020). Herbal Medicine for the Management of COVID-19 during the Medical Observation Period: A Review of Guidelines. Integr. Med. Res. 9, 100465. 10.1016/j.imr.2020.100465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasov A. G., Waltenberger B., Pferschy-Wenzig E.-M., Linder T., Wawrosch C., Uhrin P., et al. (2015). Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 33, 1582–1614. 10.1016/j.biotechadv.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barda N., Dagan N., Cohen C., Hernan M. A., Lipsitch M., Kohane I. S., et al. (2021). Effectiveness of a Third Dose of the BNT162b2 mRNA COVID-19 Vaccine for Preventing Severe Outcomes in Israel: an Observational Study. Lancet 398 (10316), 2093–2100. 10.1016/s0140-6736(21)02249-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayda S., Adeel M., Tuccinardi T., Cordani M., Rizzolio F. (2019). The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 25, 112. 10.3390/molecules25010112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner S. E., Patel E. U., Laeyendecker O., Pekosz A., Littlefield K., Eby Y., et al. (2020). SARS-CoV-2 Antibody Avidity Responses in COVID-19 Patients and Convalescent Plasma Donors. J. Infect. Dis. 222, 1974–1984. 10.1093/infdis/jiaa581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwanger O. (2021). Antithrombotic Therapy for Outpatients with COVID-19. JAMA 326, 1685–1686. 10.1001/jama.2021.17460 [DOI] [PubMed] [Google Scholar]
- Borba M. G. S., Val F. F. A., Sampaio V. S., Alexandre M. A. A., Melo G. C., Brito M., et al. (2020). Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. JAMA Netw. Open 3, e208857. 10.1001/jamanetworkopen.2020.8857 [DOI] [PubMed] [Google Scholar]
- Bustos Cruz R. H., Sanchez M. M., Dominguez-Sanchez M. A., Barreto G. E., Lancheros D., Reynolds J. (2017). Nanotechnology in Neurosciences: An Approach. Curr. Pharm. Des. 23, 4154–4169. 10.2174/1381612823666170816115452 [DOI] [PubMed] [Google Scholar]
- Chauhan G., Madou M. J., Kalra S., Chopra V., Ghosh D., Martinez-Chapa S. O. (2020). Nanotechnology for COVID-19: Therapeutics and Vaccine Research. ACS Nano 14, 7760–7782. 10.1021/acsnano.0c04006 [DOI] [PubMed] [Google Scholar]
- Chen B., Wang M., Huang X., Xie M., Pan L., Liu H., et al. (2021a). Changes in Incidence of Notifiable Infectious Diseases in China under the Prevention and Control Measures of COVID-19. Front. Public Health 9, 728768. 10.3389/fpubh.2021.728768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liang J. (2020). An Overview of Functional Nanoparticles as Novel Emerging Antiviral Therapeutic Agents. Mater. Sci. Eng. C 112, 110924. 10.1016/j.msec.2020.110924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Liu A., Cheng Y., Wang X., Xu X., Huang J., et al. (2021b). Hydroxychloroquine/chloroquine in Patients with COVID-19 in Wuhan, China: a Retrospective Cohort Study. BMC Infect. Dis. 21, 805. 10.1186/s12879-021-06477-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacrory S. (2021). Antimicrobial Activity, DFT Calculations, and Molecular Docking of Dialdehyde Cellulose/Graphene Oxide Film against Covid-19. J. Polym. Environ. Online ahead of print. 1–13. 10.1007/s10924-020-02039-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi V. K., Jain N., Valli K. S. (2010). Importance of Novel Drug Delivery Systems in Herbal Medicines. Pharmacogn Rev. 4, 27–31. 10.4103/0973-7847.65322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourado D., Freire D. T., Pereira D. T., Amaral-Machado L., N. Alencar É., De Barros A. L. B., et al. (2021). Will Curcumin Nanosystems Be the Next Promising Antiviral Alternatives in COVID-19 Treatment Trials. Biomed. Pharmacother. 139, 111578. 10.1016/j.biopha.2021.111578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duru C. E., Duru I. A., Adegboyega A. E. (2021). In Silico identification of Compounds from Nigella Sativa Seed Oil as Potential Inhibitors of SARS-CoV-2 Targets. Bull. Natl. Res. Cent. 45, 57. 10.1186/s42269-021-00517-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvignaud A., Lhomme E., Pistone T., Onaisi R., Sitta R., Journot V., et al. (2020). Home Treatment of Older People with Symptomatic SARS-CoV-2 Infection (COVID-19): A Structured Summary of a Study Protocol for a Multi-Arm Multi-Stage (MAMS) Randomized Trial to Evaluate the Efficacy and Tolerability of Several Experimental Treatments to Reduce the Risk of Hospitalisation or Death in Outpatients Aged 65 Years or Older (COVERAGE Trial). Trials 21, 846. 10.1186/s13063-020-04619-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D. (1969). Chloroquine Resistance in Malaria: a Deficiency of Chloroquine Binding. Proc. Natl. Acad. Sci. 64, 1181–1187. 10.1073/pnas.64.4.1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi M. (2017). Biomimetic and Bioinspired Nanoparticles for Targeted Drug Delivery. Ther. Deliv. 8, 289–299. 10.4155/tde-2017-0013 [DOI] [PubMed] [Google Scholar]
- Gao X., Kuo J., Jiang H., Deeb D., Liu Y., Divine G., et al. (2004). Immunomodulatory Activity of Curcumin: Suppression of Lymphocyte Proliferation, Development of Cell-Mediated Cytotoxicity, and Cytokine Production In Vitro . Biochem. Pharmacol. 68, 51–61. 10.1016/j.bcp.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Honoré S., Hoang V. T., Colson P., Raoult D. (2021). Hydroxychloroquine and Azithromycin as a Treatment of COVID-19: Results of an Open Label Non-randomized Clinical Trial Revisited. Int. J. Antimicrob. Agents 57, 106243. 10.1016/j.ijantimicag.2020.106243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group R. C., Horby P., Mafham M., Linsell L., Bell J. L., Staplin N., et al. (2020). Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 383, 2030–2040. 10.1056/NEJMoa2022926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S., Qasim M., Choi Y., Do J. T., Park C., Hong K., et al. (2020). Antiviral Potential of Nanoparticles-Can Nanoparticles Fight against Coronaviruses. Nanomaterials (Basel) 10, 1645. 10.3390/nano10091645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi B., Akram F. A., Amirazad H., Dadashpour M., Sheervalilou M., Nasrabadi D., et al. (2021). Emerging Importance of Nanotechnology-Based Approaches to Control the COVID-19 Pandemic; Focus on Nanomedicine Iterance in Diagnosis and Treatment of COVID-19 Patients. J. Drug Deliv. Sci. Technol. 67, 102967. 10.1016/j.jddst.2021.102967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S. T. S. (2020). Shedding Light on the Effect of Natural Anti-herpesvirus Alkaloids on SARS-CoV-2: A Treatment Option for COVID-19. Viruses 12, 476. 10.3390/v12040476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.-L., Huang L.-Y., Wang K., Gu C.-J., Hu J., Zhang G.-J., et al. (2021). Identification of Bis-Benzylisoquinoline Alkaloids as SARS-CoV-2 Entry Inhibitors from a Library of Natural Products. Sig Transduct Target. Ther. 6, 131. 10.1038/s41392-021-00531-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley L. L., Akinfiresoye L., Nwulia E., Kamiya A., Kulkarni A. A., Tizabi Y. (2013). Antidepressant-like Effects of Curcumin in WKY Rat Model of Depression Is Associated with an Increase in Hippocampal BDNF. Behav. Brain Res. 239, 27–30. 10.1016/j.bbr.2012.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. N., Hossain K. S., Sarker P. P., Ferdous J., Hannan M. A., Rahman M. M., et al. (2021). Revisiting Pharmacological Potentials of Nigella Sativa Seed: A Promising Option for COVID ‐19 Prevention and Cure. Phytotherapy Res. 35, 1329–1344. 10.1002/ptr.6895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q., Fu J., Liang P., Wang S., Wang Y., Zhang X., et al. (2021). Investigating of Interactions between Chloroquine/hydroxychloroquine and Their Single Enantiomers and Angiotensin-Converting Enzyme 2 by a Cell Membrane Chromatography Method. J. Sep. Sci. 45(2):456-467. 10.1002/jssc.202100570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jotz G. P., Stein A., Sirena S., Barros E., Baldisserotto J., Figueiredo J. A. P. d., et al. (2020). The COVID-19 Pandemic and Planetary Health. A Critical Review of Epidemiology, Prevention, Clinical Characteristics and Treatments for Oral, Head and Neck Health Professionals. Do We Have a Roadmap. Int. Arch. Otorhinolaryngol. 24, e351–e358. 10.1055/s-0040-1714143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A., Kaur L., Singh G., Dhawan R. K., Mahajan A. (2021). Nanotechnology Based Herbal Formulations: A Survey of Recent Patents, Advancements and Transformative Headways. Recent Pat Nanotechnol. Online ahead of print. 10.2174/1872210515666210428135343 [DOI] [PubMed] [Google Scholar]
- Kocaadam B., Şanlier N. (2017). Curcumin, an Active Component of Turmeric (Curcuma Longa), and its Effects on Health. Crit. Rev. Food Sci. Nutr. 57, 2889–2895. 10.1080/10408398.2015.1077195 [DOI] [PubMed] [Google Scholar]
- Kunze K. L., Johnson P. W., Van Helmond N., Senefeld J. W., Petersen M. M., Klassen S. A., et al. (2021). Mortality in Individuals Treated with COVID-19 Convalescent Plasma Varies with the Geographic Provenance of Donors. Nat. Commun. 12, 4864. 10.1038/s41467-021-25113-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layqah L. A., Eissa S. (2019). An Electrochemical Immunosensor for the corona Virus Associated with the Middle East Respiratory Syndrome Using an Array of Gold Nanoparticle-Modified Carbon Electrodes. Microchim Acta 186, 224. 10.1007/s00604-019-3345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Yu D., Wu X., Liang H., Zhou Z., Xie Y., et al. (2021a). Twelve-month Specific IgG Response to SARS-CoV-2 Receptor-Binding Domain Among COVID-19 Convalescent Plasma Donors in Wuhan. Nat. Commun. 12, 4144. 10.1038/s41467-021-24230-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Meng Y., Wang J., Zhang Y., Zeng Y., Xiao H., et al. (2021b). Effect of Knowledge/Practice of COVID-19 Prevention Measures on Return-To-Work Concerns; Attitudes about the Efficacy of Traditional Chinese Medicine: Survey on Supermarket Staff in Huanggang, China. Front. Public Health 9, 722604. 10.3389/fpubh.2021.722604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Cheng B., Zeng W., Chen S., Tu M., Wu M., et al. (2020). Analysis of the Risk Factors for Mortality in Adult COVID-19 Patients in Wuhan: A Multicenter Study. Front. Med. 7, 545. 10.3389/fmed.2020.00545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chen H., Shang Y., Zhu H., Chen G., Chen Y., et al. (2020). Efficacy of Chloroquine versus Lopinavir/ritonavir in Mild/general COVID-19 Infection: a Prospective, Open-Label, Multicenter, Randomized Controlled Clinical Study. Trials 21, 622. 10.1186/s13063-020-04478-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Hong H., Xue J., Luo J., Liu Q., Chen X., et al. (2021). Near-Infrared Radiation-Assisted Drug Delivery Nanoplatform to Realize Blood-Brain Barrier Crossing and Protection for Parkinsonian Therapy. ACS Appl. Mater. Inter. 13, 37746–37760. 10.1021/acsami.1c12675 [DOI] [PubMed] [Google Scholar]
- Lobo-Galo N., Gálvez-Ruíz J.-C., Balderrama-Carmona A. P., Silva-Beltrán N. P., Ruiz-Bustos E. (2021). Recent Biotechnological Advances as Potential Intervention Strategies against COVID-19. 3 Biotech. 11, 41. 10.1007/s13205-020-02619-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchia A., Ferrante D., Angeleri P., Biscayart C., Mariani J., Esteban S., et al. (2021). Evaluation of a COVID-19 Vaccine Campaign and SARS-CoV-2 Infection and Mortality Among Adults Aged 60 Years and Older in a Middle-Income Country. JAMA Netw. Open 4, e2130800. 10.1001/jamanetworkopen.2021.30800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikandan S., Subbaiya R., Saravanan M., Ponraj M., Selvam M., Pugazhendhi A. (2022). A Critical Review of Advanced Nanotechnology and Hybrid Membrane Based Water Recycling, Reuse, and Wastewater Treatment Processes. Chemosphere 289, 132867. 10.1016/j.chemosphere.2021.132867 [DOI] [PubMed] [Google Scholar]
- Mhatre S., Srivastava T., Naik S., Patravale V. (2021). Antiviral Activity of green tea and Black tea Polyphenols in Prophylaxis and Treatment of COVID-19: A Review. Phytomedicine 85, 153286. 10.1016/j.phymed.2020.153286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassi A., Sánchez-Duffhues G., Collado J. A., Muñoz E., Appendino G. (2013). Dissecting the Pharmacophore of Curcumin. Which Structural Element Is Critical for Which Action. J. Nat. Prod. 76, 1105–1112. 10.1021/np400148e [DOI] [PubMed] [Google Scholar]
- Mishra P., Sohrab S., Mishra S. K. (2021). A Review on the Phytochemical and Pharmacological Properties of Hyptis Suaveolens (L.) Poit. Futur J. Pharm. Sci. 7, 65. 10.1186/s43094-021-00219-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossaad E., Furuyama W., Enomoto M., Kawai S., Mikoshiba K., Kawazu S.-i. (2015). Simultaneous Administration of 2-aminoethyl Diphenylborinate and Chloroquine Reverses Chloroquine Resistance in Malaria Parasites. Antimicrob. Agents Chemother. 59, 2890–2892. 10.1128/aac.04805-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi B., Rehman S., Baboota S., Ali J. (2019). Insights on Oral Drug Delivery of Lipid Nanocarriers: a Win-Win Solution for Augmenting Bioavailability of Antiretroviral Drugs. AAPS PharmSciTech 20, 60. 10.1208/s12249-018-1284-9 [DOI] [PubMed] [Google Scholar]
- Palmieri V., Papi M. (2020). Can Graphene Take Part in the Fight against COVID-19. Nano Today 33, 100883. 10.1016/j.nantod.2020.100883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda P. K., Bandyopadhyay A., Singh B. C., Moirangthem B., Chikara G., Saha S., et al. (2020). Safety and Efficacy of Antiviral Combination Therapy in Symptomatic Patients of Covid-19 Infection - a Randomised Controlled Trial (SEV-COVID Trial): A Structured Summary of a Study Protocol for a Randomized Controlled Trial. Trials 21, 866. 10.1186/s13063-020-04774-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja M. A., Maldonado M., Chen J., Zhong Y., Gu J. (2021). Development and Evaluation of Curcumin Encapsulated Self-Assembled Nanoparticles as Potential Remedial Treatment for PCOS in a Female Rat Model. Int. J. Nanomedicine 16, 6231–6247. 10.2147/ijn.s302161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réa-Neto Á., Bernardelli R. S., Câmara B. M. D., Reese F. B., Queiroga M. V. O., Oliveira M. C. (2021). An Open-Label Randomized Controlled Trial Evaluating the Efficacy of Chloroquine/hydroxychloroquine in Severe COVID-19 Patients. Sci. Rep. 11, 9023. 10.1038/s41598-021-88509-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva L., Yuan S., Yin X., Martin-Sancho L., Matsunaga N., Burgstaller-Muehlbacher S., et al. (2020). A Large-Scale Drug Repositioning Survey for SARS-CoV-2 Antivirals. bioRxiv. Online ahead of print. 10.1101/2020.04.16.044016 [DOI] [Google Scholar]
- Roy A., Das R., Roy D., Saha S., Ghosh N. N., Bhattacharyya S., et al. (2022). Encapsulated Hydroxychloroquine and Chloroquine into Cyclic Oligosaccharides Are the Potential Therapeutics for COVID-19: Insights from First-Principles Calculations. J. Mol. Struct. 1247, 131371. 10.1016/j.molstruc.2021.131371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber-Moghaddam N., Salari S., Hejazi S., Amini M., Taherzadeh Z., Eslami S., et al. (2021). Oral Nano-Curcumin Formulation Efficacy in Management of Mild to Moderate Hospitalized Coronavirus Disease-19 Patients: An Open Label Nonrandomized Clinical Trial. Phytother Res. Online ahead of print. 10.1002/ptr.7004 [DOI] [PubMed] [Google Scholar]
- Salarbashi D., Tafaghodi M., Fathi M., Aboutorabzade S. M., Sabbagh F. (2021). Development of Curcumin‐loaded Prunus Armeniaca Gum Nanoparticles: Synthesis, Characterization, Control Release Behavior, and Evaluation of Anticancer and Antimicrobial Properties. Food Sci. Nutr. 9, 6109–6119. 10.1002/fsn3.2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanidad K. Z., Sukamtoh E., Xiao H., Mcclements D. J., Zhang G. (2019). Curcumin: Recent Advances in the Development of Strategies to Improve Oral Bioavailability. Annu. Rev. Food Sci. Technol. 10, 597–617. 10.1146/annurev-food-032818-121738 [DOI] [PubMed] [Google Scholar]
- Singh M., Trivedi D., Mohapatra R., Bagchi T., Durthi C. P., Kuppam C. (2021). Phytotherapic Drugs for COVID-19 Treatment: A Scoping Review. Curr. Pharm. Des. 27, 3389–3398. 10.2174/1381612827666210705163807 [DOI] [PubMed] [Google Scholar]
- Sinha K., Som Chaudhury S., Sharma P., Ruidas B. (2021). COVID-19 Rhapsody: Rage towards Advanced Diagnostics and Therapeutic Strategy. J. Pharm. Anal. 11, 529–540. 10.1016/j.jpha.2021.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebi S., El‐Esawi M. A., Mahmoud Z. H., Timoshin A., Valizadeh H., Roshangar L., et al. (2021). Immunomodulatory Effects of Nanocurcumin on Th17 Cell Responses in Mild and Severe COVID‐19 Patients. J. Cel Physiol 236, 5325–5338. 10.1002/jcp.30233 [DOI] [PubMed] [Google Scholar]
- Tang P., Hasan M. R., Chemaitelly H., Yassine H. M., Benslimane F. M., Al Khatib H. A., et al. (2021a). BNT162b2 and mRNA-1273 COVID-19 Vaccine Effectiveness against the SARS-CoV-2 Delta Variant in Qatar. Nat. Med. 27 (12), 2136–2143. 10.1038/s41591-021-01583-4 [DOI] [PubMed] [Google Scholar]
- Tang W.-F., Tsai H.-P., Chang Y.-H., Chang T.-Y., Hsieh C.-F., Lin C.-Y., et al. (2021b). Perilla (Perilla Frutescens) Leaf Extract Inhibits SARS-CoV-2 via Direct Virus Inactivation. Biomed. J. 44, 293–303. 10.1016/j.bj.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof S. Y., Slezak J. M., Fischer H., Hong V., Ackerson B. K., Ranasinghe O. N., et al. (2021). Effectiveness of mRNA BNT162b2 COVID-19 Vaccine up to 6 Months in a Large Integrated Health System in the USA: a Retrospective Cohort Study. The Lancet 398, 1407–1416. 10.1016/s0140-6736(21)02183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder R. S., Semple M. G. (2021). Appropriate Selection of Convalescent Plasma Donors for COVID-19. Lancet Infect. Dis. 21, 168–169. 10.1016/s1473-3099(20)30470-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakar A., Panda S., Sakthivel P., Brijwal M., Dhakad S., Choudekar A., et al. (2021). Chloroquine Nasal Drops in Asymptomatic & Mild COVID-19: An Exploratory Randomized Clinical Trial. Indian J. Med. Res. 153, 151–158. 10.4103/ijmr.ijmr_3665_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa R. K., Mudnakudu-Nagaraju K. K., Shivamallu C., Subramaniam K. J. T., Radhakrishnan A., Bhojraj S., et al. (2021). Antiviral and Immunomodulatory Activity of Curcumin: A Case for Prophylactic Therapy for COVID-19. Heliyon 7, e06350. 10.1016/j.heliyon.2021.e06350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirupakuzhi Vijayaraghavan B. K., Jha V., Rajbhandari D., Myatra S. N., John O., Ghosh A., et al. (2020). Hydroxychloroquine Plus Personal Protective Equipment versus Standard Personal Protective Equipment Alone for the Prevention of COVID-19 Infections Among Frontline Healthcare Workers: the HydrOxychloroquine Prophylaxis Evaluation(HOPE) Trial: A Structured Summary of a Study Protocol for a Randomized Controlled Trial. Trials 21, 754. 10.1186/s13063-020-04679-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valizadeh H., Abdolmohammadi-Vahid S., Danshina S., Ziya Gencer M., Ammari A., Sadeghi A., et al. (2020). Nano-curcumin Therapy, a Promising Method in Modulating Inflammatory Cytokines in COVID-19 Patients. Int. Immunopharmacology 89, 107088. 10.1016/j.intimp.2020.107088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsalam S., Agastian P., Arasu M. V., Al-Dhabi N. A., Ghilan A.-K. M., Kaviyarasu K., et al. (2019). Rapid Biosynthesis and Characterization of Silver Nanoparticles from the Leaf Extract of Tropaeolum Majus L. And its Enhanced In-Vitro Antibacterial, Antifungal, Antioxidant and Anticancer Properties. J. Photochem. Photobiol. B: Biol. 191, 65–74. 10.1016/j.jphotobiol.2018.12.010 [DOI] [PubMed] [Google Scholar]
- Venturi M. (2021). From Photochemistry to Supramolecolar Chemistry, Molecular Nanotechnology, and Societal Commitment. Chemistry 27 (70), 17508–17509. 10.1002/chem.202103948 [DOI] [PubMed] [Google Scholar]
- Weehuizen J. M., Hoepelman A. I. M. (2020). An Open-Label Cluster-Randomized Controlled Trial of Chloroquine, Hydroxychloroquine or Only Supportive Care in Patients Admitted with Moderate to Severe COVID-19 (ARCHAIC)-Protocol Publication. Eur. J. Clin. Invest. 50, e13297. 10.1111/eci.13297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J. A., Pasquali M., et al. (2020). Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 14, 6383–6406. 10.1021/acsnano.0c03697 [DOI] [PubMed] [Google Scholar]
- Weng J.-R., Lin C.-S., Lai H.-C., Lin Y.-P., Wang C.-Y., Tsai Y.-C., et al. (2019). Antiviral Activity of Sambucus FormosanaNakai Ethanol Extract and Related Phenolic Acid Constituents against Human Coronavirus NL63. Virus. Res. 273, 197767. 10.1016/j.virusres.2019.197767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Jiang Z., Yuan J., Li M., Zeng J., Tang J., et al. (2021). Effects and Safety of Herbal Medicines Among Community-Dwelling Residents during COVID-19 Pandemic: A Large Prospective, Randomized Controlled Trial (RCT). Phytomedicine 85, 153403. 10.1016/j.phymed.2020.153403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q., Xing Y., Ma J., Wang C., Zang J., Zhao G. (2021). Binding of Chloroquine to Whey Protein Relieves its Cytotoxicity while Enhancing its Uptake by Cells. J. Agric. Food Chem. 69, 10669–10677. 10.1021/acs.jafc.1c04140 [DOI] [PubMed] [Google Scholar]
- Zahedipour F., Hosseini S. A., Sathyapalan T., Majeed M., Jamialahmadi T., Al‐Rasadi K., et al. (2020). Potential Effects of Curcumin in the Treatment of COVID ‐19 Infection. Phytotherapy Res. 34, 2911–2920. 10.1002/ptr.6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazo H., Colino C. I., Lanao J. M. (2016). Current Applications of Nanoparticles in Infectious Diseases. J. Controlled Release 224, 86–102. 10.1016/j.jconrel.2016.01.008 [DOI] [PubMed] [Google Scholar]
- Zhang L. Q., Tang S. J. (2016). Application Research and Progress of the Nanotechnology on Tuberculosis. Zhonghua Jie He He Hu Xi Za Zhi 39, 393–395. 10.3760/cma.j.issn.1001-0939.2016.05.014 [DOI] [PubMed] [Google Scholar]
- Zhang P., Meng J., Li Y., Yang C., Hou Y., Tang W., et al. (2021). Nanotechnology-enhanced Immunotherapy for Metastatic Cancer. The Innovation 2, 100174. 10.1016/j.xinn.2021.100174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Liu Z., Li Y., Huang Q., Xia L., Li K. (2021). Delivery of Manganese Carbonyl to the Tumor Microenvironment Using Tumor-Derived Exosomes for Cancer Gas Therapy and Low Dose Radiotherapy. Biomaterials 274, 120894. 10.1016/j.biomaterials.2021.120894 [DOI] [PubMed] [Google Scholar]