Abstract
Introduction
Blood biomarkers represent a major advance for improving the management, diagnosis, and monitoring of Alzheimer's disease (AD). However, their context of use in relation to routine cerebrospinal fluid (CSF) analysis for the quantification of amyloid peptides and tau proteins remains to be determined.
Methods
We studied in two independent cohorts, the performance of blood biomarkers in detecting “nonpathological” (A−/T−/N−), amyloid (A+) or neurodegenerative (T+ /N+) CSF profiles.
Results
Plasma Aβ1–42/Aβ1–40 ratio and phosphorylated tau (p-tau(181)) were independent and complementary predictors of the different CSF profile and in particular of the nonpathological (A−/T−/N−) profile with a sensitivity and specificity close to 85%. These performances and the corresponding biomarker thresholds were significantly different from those related to AD detection.
Conclusion
The use of blood biomarkers to identify patients who may benefit from secondary CSF testing represents an attractive stratification strategy in the clinical management of patients visiting memory clinics. This could reduce the need for lumbar puncture and foreshadow the use of blood testing on larger populations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00702-022-02474-9.
Keywords: Blood, Biomarkers, Clinical management, Lumbar puncture, CSF
Introduction
Detection of Alzheimer's disease (AD) with high sensitivity and specificity is key for the management of patients. Diagnosis can include detection of amyloid and tau biomarkers in the cerebrospinal fluid (CSF), which is one of international guidelines’ criteria (Dubois et al. 2014; McKhann et al. 2011). Thus, the identification of AD processes years before the onset of symptoms recently triggered a paradigm shift in which AD could be viewed as a biological rather than clinical entity (Jack et al. 2018). The importance of biomarkers was also emphasized when defining the unbiased "A/T/N" classification system (Jack et al. 2016). These evolutions are coupled with the prospect of introducing treatments that would modify the trajectory of the disease by delaying its clinical expression. However, the use of CSF to detect AD at an early stage in a large population remains difficult because of the invasive nature of lumbar puncture (LP). Blood biomarkers are in this context, of particular interest. The possibility of detecting amyloid peptides and tau proteins in plasma has recently shaken the field of neurodegenerative diseases detection. Many research groups, including ours, are evaluating the diagnostic value of these biomarkers for the accurate detection of AD, through cross sectional or longitudinal studies using retrospective samples (Alcolea et al. 2021; Lewczuk et al. 2018; Brickman et al. 2021).
However, one context of use (COU) that has not yet been directly addressed is in relation to CSF testing performed in a routine clinical setting. The question is whether blood biomarkers can be used to decide the need for further CSF analysis. Thus, the objective here is to evaluate the performance of blood biomarkers in detecting a “nonpathological” (A−/T−/N−) CSF profile, rather than a specific pathological profile as seen in AD or brain injury. Note that the notion of “nonpathological” does not refer here to the globality of the CSF analysis which includes many other biochemical, immunological or microbiological analyses, but is restricted to the results of the amyloid and tau biomarkers. The results of this study suggest that blood markers can predict the presence of nonpathological CSF profile and could thus be decisive in whether or not to perform a LP.
Methods
Participants
The Barcelona cohort included 150 participants from the Sant Pau Initiative on Neurodegeneration (SPIN cohort) (Alcolea et al. 2019) evaluated at the Sant Pau Memory Unit (Barcelona, Spain) between November 2013 and October 2019. Participants in this cohort mostly were patients with a diagnosis of AD, Dementia with Lewy bodies (DLB), frontotemporal lobar degeneration-related syndromes (FTLD), and mild cognitive impairment (MCI), or cognitively normal controls. The two cohorts differ in the distribution of A/T/N profiles based on CSF biomarkers, with the Barcelona cohort having a much higher percentage of isolated amyloid-positive patients (A+/N−/T−) than the Montpellier cohort (Table 1). All participants had received neurological and neuropsychological evaluation and provided CSF and plasma samples. The Montpellier cohort included 161 patients recruited from September 2009 to June 2017 (Lehmann et al. 2020). All patients underwent a thorough clinical examination including biological laboratory tests, neuropsychological assessments, and brain imaging. In addition to AD, DLB, FTLD and MCI, patient from this cohort had also mixed dementia, normal pressure hydrocephalus and Parkinson disease with cognitive signs or subjective cognitive impairment.
Table 1.
Demography, CSF biomarker values and AT(N) classification of the cohort of Montpellier and Barcelona
| Variable | The Montpellier cohort | The Barcelona cohort | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 68.3 | 10.5 | 67.6 | 12.3 |
| Sex (M%)* | 60.3 | – | 39.4 | – |
| CSF biomarkers | ||||
| Aβ1–40 (pg/mL) | 15,940 | 6875 | 12,078 | 3838 |
| Aβ1–42 (pg/mL) | 826 | 377 | 906 | 446 |
| Tau (pg/mL) | 461 | 320 | 467 | 326 |
| p-tau(181) (pg/mL) | 66 | 43 | 73 | 63 |
| ATN* | ||||
| A−/T−/N− | 38.1% | – | 31.8% | – |
| A−/T−/N+ | 4.8% | – | 3.9% | – |
| A−/T+/N− | 1.6% | – | 0.6% | – |
| A−/T+/N+ | 4.8% | – | 2.6% | – |
| A+/T−/N− | 4.8% | – | 25.8% | – |
| A+/T−/N+ | 3.2% | – | 0,6% | – |
| A+/T+/N− | 4.8% | – | 3.9% | – |
| A+/T+/N+ | 38.1% | – | 31.1% | – |
SD standard deviation
*Significant difference
All participants gave their written informed consent to participating in clinical research on CSF and plasma biomarkers, and protocols at both centers were approved by the respective Ethics Committees.
Primary outcomes
CSF Aβ1–42, Aβ1–40, total tau and phosphorylated tau 181 [p-tau(181)] were measured using Fujirebio Lumipulse or Innotest assay as described (Lehmann et al. 2020). The cutoff values were initially obtained from groups of patients clinically diagnosed with AD (whose clinical diagnoses were made blind to biomarker results) and, for the Barcelona cohort, from amyloid-PET positive and amyloid-PET negative participants (Alcolea et al. 2019, PMID 31464088) or, for Montpellier, from control population of the memory clinic with various etiology (Lehmann et al. 2013, 2018). Based on these data, a nonpathological CSF profile corresponding the (A−/T−/N−) situation is defined as having a value of the Aβ1–42/Aβ1–40 ratio (A) above the cutoff and values of tau (N) and p-tau(181) (T) below the pathological cutoffs. We also identified amyloid (A+/A−) and tau-neurodegeneration (N+T+/N−T−) CSF profiles.
Three different approaches were used to measure plasma levels of Aβ1–42 and Aβ1–40: “Neurology 3-Plex A” (Q3, both cohorts) and “Neurology 4-plex E Advantage kit” (Q4, Montpellier cohort) in the Simoa platform (Quanterix) and an IP-MS approach from Shimadzu (Nakamura et al. 2018) (both cohorts) implemented in Montpellier’s laboratory and slightly modified from the original protocol (Alcolea et al. 2021). Levels of p-tau(181) were measured in the Simoa platform (Quanterix).
Statistical analysis
Statistical analyses were completed with Medcalc (v19.8). The accuracy of the blood-based assays to discriminate nonpathological amyloid and tau CSF profile was evaluated using receiver operating characteristic (ROC) curve analysis and calculation, using the area under the curve (AUC) as a measure of diagnostic accuracy. Comparison of ROC curves to test the statistical significance between assay values derived from the method of DeLong et al. (1988) for the calculation of the standard error of the AUCs. Multiple regression was used to examine the relationship between CSF and blood assays allowing to combine them and evaluate if they were independent or not. Logistic regression used to combine independent factors was employed using different ways of introducing the factors into the algorithm (Enter/Forward/Backward/Stepwise) if their p values were < 0.05 and removed if p > 0.1.
Results
We first tested the performance (AUC) of blood biomarkers to distinguish a nonpathological (A−/T−/N−) from a pathological CSF profile represented by the other A/T/N situations (Table 1). Plasma Aβ1–40 and Aβ1–42, individually, show variable but low accuracy for nonpathological profiles detection (Table 2 and Supp Table 1). In contrast, the Aβ1–42/Aβ1–40 ratio showed much higher performance regardless of the analytical method used (Q3, Q4 or IP-MS).
Table 2.
Diagnostic accuracy of plasma biomarkers to discriminate non pathological CSF (A−/T−/N−) profiles in the cohort of Montpellier and Barcelona
| Non-pathological CSF (A−/T−/N−) | The Montpellier cohort | The Barcelona cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Blood biomarkers | AUC | SE | 95% CI | p | AUC | SE | 95% CI | p |
| Aβ1–40(Q3) | 0.661 | 0.069 | 0.530–0.777 | 0.020 | 0.638 | 0.080 | 0.490–0.769 | 0.085 |
| Aβ1–40(Q4) | 0.672 | 0.068 | 0.542–0.785 | 0.011 | – | – | – | – |
| Aβ1–40(IP-MS) | 0.526 | 0.074 | 0.396–0.653 | 0.728 | 0.549 | 0.051 | 0.463–0.633 | 0.329 |
| Aβ1–42(Q3) | 0.548 | 0.077 | 0.417–0.675 | 0.534 | 0.715 | 0.076 | 0.571–0.834 | 0.044 |
| Aβ1–42(Q4) | 0.542 | 0.076 | 0.410–0.669 | 0.601 | – | – | – | – |
| Aβ1–42(IP-MS) | 0.638 | 0.073 | 0.507–0.755 | 0.060 | 0.656 | 0.048 | 0.571–0.733 | 0.001 |
| Tau | 0.612 | 0.078 | 0.480–0.733 | 0.149 | 0.618 | 0.081 | 0.470–0.752 | 0.495 |
| p-tau(181) | 0.865 | 0.049 | 0.756––0.938 | < 0.0001 | 0.773 | 0.039 | 0.697–0.837 | < 0.0001 |
| Aβ1–42/Aβ40(Q3) | 0.709 | 0.068 | 0.580–0.818 | 0.002 | 0.848 | 0.062 | 0.719–0.934 | < 0.0001 |
| Aβ1–42/Aβ40(Q4) | 0.753 | 0.065 | 0.627–0.854 | < 0.0001 | – | – | – | – |
| Aβ1–42/Aβ40(IP-MS) | 0.715 | 0.066 | 0.587–0.822 | 0.001 | 0.661 | 0.048 | 0.577–0.739 | 0.0008 |
| Logistic regression Aβ1–40, Aβ1–42, p-tau(181) | 0.904 | 0.040 | 0.804–0.964 | < 0.0001 | 0.882 | 0.050 | 0.759–0.956 | < 0.0001 |
Biomarkers were quantified using either Quanterix technology (Q3 and Q4) or Shimadzu approach (IP-MS). Significant differences are indicated by bolded p (threshold 0.05)
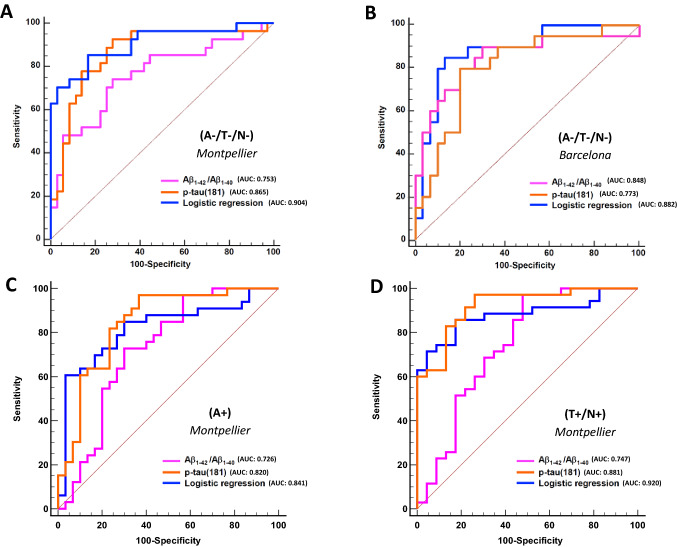
In both cohorts, plasma levels of total tau were comparable between nonpathological and pathological CSF profiles, while elevated plasma p-tau(181) was associated with a pathological CSF profile (Supp Table 1). Plasma p-tau(181) showed an AUC of 0.865 in Montpellier and 0.773 in Barcelona to discriminate a nonpathological (A−/T−/N−) CSF profile (Table 1). Pairwise comparison of AUCs confirmed the high performance of p-tau(181) when compared to Aβ1–42 and total tau (p < 0.05) in Montpellier cohort. In Barcelona cohort, AUCs obtained for p-tau(181), Aβ1–42(Q3) and Aβ1–42(IP-MS) were similar (Table 2) and AUC of p-tau(181) was significantly higher than AUC of total tau (p = 0.022).To assess the value of combining biomarkers, we first tested the correlation between the different factors and observed that Aβ1–42, Aβ1–40, Aβ1–42/Aβ1–40 on the one hand, and tau, p-tau(181) on the other hand, were correlated together (Pearson correlation; p < 0.001). We therefore selected as independent variable Aβ1–42/Aβ1–40 and p-tau(181) (with amyloid peptides measured with Q4 and Q3 Quanterix in Montpellier and Barcelona, respectively) that had the best AUCs. Combining biomarkers requires a stepwise approach (Mamtani et al. 2006), however in our case we only have two factors and we tested different logistic regression approaches (see “Methods”) which all resulted in the same algorithm confirming the independence and statistical relevance of the two selected biomarkers. As illustrated (Table 2 and Fig. 1A), AUCs obtained for Aβ1–42/Aβ1–40 (both measured with Quanterix Q4), p-tau(181) and logistic regression combining these three parameters were very close and pairwise comparison of the different AUCs was significant only in Montpellier cohort between Aβ1–42/Aβ1–40 and logistic regression (Supp Table 3).
Fig. 1.
Receiver operating characteristic (ROC) curves for plasma biomarkers to discriminate non pathological (A−/T−/N−) (A and B), amyloid (A+) (C) or neurodegenerative (T+/N+) (D) CSF profiles. Lines indicate areas under the curve (AUC) for individual biomarker (orange) or ratios (pink) to discriminate CSF profiles. Blue line corresponds to the ROC curve yielded by a logistic regression that included all three plasma markers and ratios
The highest Youden index in this context was obtained when performing the logistic regression, reaching a sensitivity of 85.2% and a specificity of 83.6% for the detection of nonpathological amyloid and tau CSF profiles. In the Barcelona cohort (Table 1 and Fig. 1B), pairwise comparison was not significant between p-tau(181) and Aβ1–42/Aβ1–40 (measured with Quanterix Q3). However, the AUC of logistic regression was higher than that of p-tau(181) alone (p = 0.002). In this cohort, the highest Youden index was obtained when performing the logistic regression, reaching a sensitivity of 85.0% and a specificity of 86.7% for the detection of nonpathological amyloid and tau CSF profiles.
We also studied the performance of biomarkers to identify two specific pathological situations with amyloid (A+, Sup Table 1, Fig. 1C) or tau-neurodegeneration (T+/N+, Sup Table 2, Fig. 1D) profiles. p-tau(181) and logistic regression were again the most discriminant. The performance of the Aβ1–42/Aβ1–40 ratio was comparatively lower, especially for the T+/N+ profile.
Discussion
An important stage in the management of patients consulting for cognitive complaints is the decision to perform or not a LP, which likely provides early indicators of neurodegenerative diseases. Indeed, CSF analysis provides indirect signs to a broader range of diagnoses than AD since amyloid and tau biomarkers might also be altered in different pathological situations such as non-Alzheimer's neurodegenerative diseases like DLB, FTLD and Creutzfeldt-Jakob disease (Gabelle et al. 2011; Bousiges et al. 2018; Bibl et al. 2008; Lehmann et al. 2019) as well as brain damage (Alosco et al. 2018), normal pressure hydrocephalus (Manniche et al. 2020) and cerebral amyloid angiopathy (Renard et al. 2012) 19. This is illustrated in our cohorts by the fact that non-AD patients represent 40–50% of the CSF pathological profiles.
In this study, we evaluated the performance of blood biomarkers for the detection of “nonpathological” (A−/T−/N−), amyloid (A+) or neurodegenerative (T+/N+) CSF profiles. The main interest of the detection of a nonpathological (A−/T−/N−) profile lies in the fact that this information can be taken into account in the decision to perform a LP or not. Using different analytical approaches and in two independent cohorts, we show that plasma p-tau(181) and Aβ1–42/Aβ1–40 ratio achieve the best performance to detect nonpathological CSF profiles. Interestingly, the amyloid ratio performed better in the Barcelona cohort, which may be explained by the fact that this cohort has a significantly higher percentage of positive amyloid profiles (Table 1). These plasma biomarkers are also those identified as the best predictors of AD (Palmqvist et al. 2021), but here, the context of use is different, and their performance are event higher than for discriminating AD from non-AD. Other differences are noted such as the fact that Aβ1–42 detection by IP-MS outperformed other Aβ1–42 detections, but this was not the case when considering the Aβ1–42/Aβ1–40 ratio, thus differently than when AD is the performance criterion (Janelidze et al. 2021). As mentioned above, this could be partly explained by the fact that diseases other than AD showed pathological amyloid or tau profiles. Thus, combining plasma amyloid and p-tau(181) slightly increased the performance, therefore suggesting their complementarity. This was confirmed when the blood biomarker performance criteria were based on the detection of amyloid (A+) or neurodegenerative (T+/N+) CSF profiles (Fig. 1). Strikingly, blood p-tau(181) outperforms the amyloid ratio for the detection of an amyloid profile in CSF. This confirms the value of detecting phosphorylated tau proteins in blood. In this work we quantified blood p-tau(181) but other phosphorylated isoforms, such as p-tau(217) or p-tau(231) which have shown better diagnostic performance (Brickman et al. 2021; Barthelemy et al. 2020; Bayoumy et al. 2021), can be expected to be even more effective.
With performances close to 85% sensitivity and specificity for the detection of nonpathological (A−/T−/N−) CSF profiles, one can really consider the results of blood biomarkers, which would then condition the subsequent need for a LP. Importantly, the biomarker cutoff decision points will likely be different than for AD detection. Such an approach could help clinicians in the decision to add other diagnostic tests (such as imaging), depending on the clinical evaluation of the patient. Depending on the prevalence of AD as well as that of other diseases modifying CSF amyloid and tau concentrations in a cohort, the reduction in the need for LP could be well over 50%, significantly reducing the cost of management of these patients and limiting invasive and unnecessary medical procedures. In conclusion, blood amyloid and tau biomarkers perform well in detecting nonpathological amyloid and tau CSF patterns. The importance and value of this “prediction” are linked to the exclusion of pathologies that vary these biomarkers (AD but not only) and to the decision to perform a LP or not. Blood biomarkers can therefore represent the first step in the patient's management strategy, to determine whether or not other diagnostic examinations by more invasive or more expensive means are necessary.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
SL and CD had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: SL, CD, AL, DA. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: SL and CD. Critical revision of the manuscript for important intellectual content: all authors.
Funding
S. Lehmann is supported by the “Claude Pompidou Foundation”, H2020 Marie Skłodowska-Curie Actions MIRIADE project and the European Metrology Programme for Innovation and Research Neuromet2 project. H. Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the H2020 European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21–831381-C and #ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation (Richard and Susan Smith Family Foundation), Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), and the UK Dementia Research Institute at UCL.
Declarations
Conflict of interest
Dr. Lleó has served as a consultant or at advisory boards for Fujirebio-Europe, Roche, Biogen, Zambon and Nutricia. In addition, Dr. Lleó has a patent WO2019175379 A1 Markers of synaptopathy in neurodegenerative disease issued. Dr. Alcolea participated in advisory boards from Fujirebio-Europe and Roche Diagnostics and received speaker honoraria from Fujirebio-Europe, Roche Diagnostics, Nutricia, Krka Farmacéutica S.L., Zambon S.A.U. and Esteve Pharmaceuticals S.A. In addition, Dr. Alcolea has a patent WO2019175379 A1 Markers of synaptopathy in neurodegenerative disease issued. Dr. Lehmann has served as a consultant or at advisory boards for Fujirebio-Europe, Roche Diagnostics, Shimadzu and Euroimmun. Dr. Zetterberg has served at scientific advisory boards and/or as a consultant for Alector, Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies, CogRx and Red Abbey Labs, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. All other authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Constance Delaby and Daniel Alcolea share first authorship.
References
- Alcolea D, Clarimón J, Carmona-Iragui M, Illán-Gala I, Morenas-Rodríguez E, Barroeta I, Ribosa-Nogué R, Sala I, Sánchez-Saudinós MB, Videla L, Subirana A, Benejam B, Valldeneu S, Fernández S, Estellés T, Altuna M, Santos-Santos M, García-Losada L, Bejanin A, Pegueroles J, Montal V, Vilaplana E, Belbin O, Dols-Icardo O, Sirisi S, Querol-Vilaseca M, Cervera-Carles L, Muñoz L, Núñez R, Torres S, Camacho MV, Carrió I, Giménez S, Delaby C, Rojas-Garcia R, Turon-Sans J, Pagonabarraga J, Jiménez A, Blesa R, Fortea J, Lleó A. The Sant Pau Initiative on Neurodegeneration (SPIN) cohort: a data set for biomarker discovery and validation in neurodegenerative disorders. Alzheimers Dement (N Y) 2019;5:597–609. doi: 10.1016/j.trci.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcolea D, Delaby C, Munoz L, Torres S, Estelles T, Zhu N, Barroeta I, Carmona-Iragui M, Illan-Gala I, Santos-Santos MA, Altuna M, Sala I, Sanchez-Saudinos MB, Videla L, Valldeneu S, Subirana A, Pegueroles J, Hirtz C, Vialaret J, Lehmann S, Karikari TK, Ashton NJ, Blennow K, Zetterberg H, Belbin O, Blesa R, Clarimon J, Fortea J, Lleo A. Use of plasma biomarkers for AT(N) classification of neurodegenerative dementias. J Neurol Neurosurg Psychiatry. 2021 doi: 10.1136/jnnp-2021-326603. [DOI] [PubMed] [Google Scholar]
- Alosco ML, Tripodis Y, Fritts NG, Heslegrave A, Baugh CM, Conneely S, Mariani M, Martin BM, Frank S, Mez J, Stein TD, Cantu RC, McKee AC, Shaw LM, Trojanowski JQ, Blennow K, Zetterberg H, Stern RA. Cerebrospinal fluid tau, Aβ, and sTREM2 in Former National Football League Players: modeling the relationship between repetitive head impacts, microglial activation, and neurodegeneration. Alzheimers Dement. 2018;14(9):1159–1170. doi: 10.1016/j.jalz.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelemy NR, Bateman RJ, Hirtz C, Marin P, Becher F, Sato C, Gabelle A, Lehmann S. Cerebrospinal fluid phospho-tau T217 outperforms T181 as a biomarker for the differential diagnosis of Alzheimer's disease and PET amyloid-positive patient identification. Alzheimers Res Ther. 2020;12(1):26. doi: 10.1186/s13195-020-00596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoumy S, Verberk IMW, den Dulk B, Hussainali Z, Zwan M, van der Flier WM, Ashton NJ, Zetterberg H, Blennow K, Vanbrabant J, Stoops E, Vanmechelen E, Dage JL, Teunissen CE. Clinical and analytical comparison of six Simoa assays for plasma P-tau isoforms P-tau181, P-tau217, and P-tau231. Alzheimers Res Ther. 2021;13(1):198. doi: 10.1186/s13195-021-00939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibl M, Lewczuk P, Esselmann H, Mollenhauer B, Klafki HW, Welge V, Wolf S, Trenkwalder C, Otto M, Kornhuber J, Wiltfang J. CSF amyloid-beta 1–38 and 1–42 in FTD and AD: biomarker performance critically depends on the detergent accessible fraction. Proteom Clin Appl. 2008;2(10–11):1548–1556. doi: 10.1002/prca.200800006. [DOI] [PubMed] [Google Scholar]
- Bousiges O, Bombois S, Schraen S, Wallon D, Quillard MM, Gabelle A, Lehmann S, Paquet C, Amar-Bouaziz E, Magnin E, Miguet-Alfonsi C, Delbeuck X, Lavaux T, Anthony P, Philippi N, Blanc F. Cerebrospinal fluid Alzheimer biomarkers can be useful for discriminating dementia with Lewy bodies from Alzheimer's disease at the prodromal stage. J Neurol Neurosurg Psychiatry. 2018;89(5):467–475. doi: 10.1136/jnnp-2017-316385. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Manly JJ, Honig LS, Sanchez D, Reyes-Dumeyer D, Lantigua RA, Lao PJ, Stern Y, Vonsattel JP, Teich AF, Airey DC, Proctor NK, Dage JL, Mayeux R. Plasma p-tau181, p-tau217, and other blood-based Alzheimer's disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021 doi: 10.1002/alz.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Gabelle A, Roche S, Geny C, Bennys K, Labauge P, Tholance Y, Quadrio I, Tiers L, Gor B, Boulanghien J, Chaulet C, Vighetto A, Croisile B, Krolak-Salmon P, Perret-Liaudet A, Touchon J, Lehmann S. Decreased sAbetaPPbeta, Abeta38, and Abeta40 cerebrospinal fluid levels in frontotemporal dementia. J Alzheimers Dis JAD. 2011;26(3):553–563. doi: 10.3233/JAD-2011-110515. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, Petersen RC, Scheltens P, Sperling RA, Dubois B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S, Teunissen CE, Zetterberg H, Allue JA, Sarasa L, Eichenlaub U, Bittner T, Ovod V, Verberk IMW, Toba K, Nakamura A, Bateman RJ, Blennow K, Hansson O. Head-to-head comparison of 8 plasma amyloid-beta 42/40 assays in Alzheimer disease. JAMA Neurol. 2021 doi: 10.1001/jamaneurol.2021.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S, Schraen S, Quadrio I, Paquet C, Bombois S, Delaby C, Dorey A, Dumurgier J, Hirtz C, Krolak-Salmon P, Laplanche JL, Moreaud O, Peoc'h K, Rouaud O, Sablonniere B, Thouvenot E, Touchon J, Vercruysse O, Hugon J, Gabelle A, Pasquier F, Perret-Liaudet A. Impact of harmonization of collection tubes on Alzheimer's disease diagnosis. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Lehmann S, Delaby C, Boursier G, Catteau C, Ginestet N, Tiers L, Maceski A, Navucet S, Paquet C, Dumurgier J, Vanmechelen E, Vanderstichele H, Gabelle A. Relevance of Abeta42/40 ratio for detection of alzheimer disease pathology in clinical routine: the PLMR scale. Front Aging Neurosci. 2018;10:138. doi: 10.3389/fnagi.2018.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S, Dumurgier J, Ayrignac X, Marelli C, Alcolea D, Ormaechea JF, Thouvenot E, Delaby C, Hirtz C, Vialaret J, Ginestet N, Bouaziz-Amar E, Laplanche JL, Labauge P, Paquet C, Lleo A, Gabelle A, Alzheimer's Disease Neuroimaging I. Cerebrospinal fluid A beta 1–40 peptides increase in Alzheimer's disease and are highly correlated with phospho-tau in control individuals. Alzheimers Res Ther. 2020;12(1):123. doi: 10.1186/s13195-020-00696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S, Paquet C, Malaplate-Armand C, Magnin E, Schraen S, Quillard-Muraine M, Bousiges O, Delaby C, Dumurgier J, Hugon J, Sablonniere B, Blanc F, Wallon D, Gabelle A, Laplanche JL, Bouaziz-Amar E, Peoc’h K. Diagnosis associated with Tau higher than 1200pg/mL: insights from the clinical and laboratory practice. Clin Chim Acta Int J Clin Chem. 2019;495:451–456. doi: 10.1016/j.cca.2019.04.081. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, Spitzer P, Maler JM, Kornhuber J, Blennow K, Zetterberg H. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer's disease. Alzheimers Res Ther. 2018;10(1):71. doi: 10.1186/s13195-018-0404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamtani MR, Thakre TP, Kalkonde MY, Amin MA, Kalkonde YV, Amin AP, Kulkarni H. A simple method to combine multiple molecular biomarkers for dichotomous diagnostic classification. BMC Bioinform. 2006;7:442. doi: 10.1186/1471-2105-7-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manniche C, Simonsen AH, Hasselbalch SG, Andreasson U, Zetterberg H, Blennow K, Høgh P, Juhler M, Hejl AM. Cerebrospinal fluid biomarkers to differentiate idiopathic normal pressure hydrocephalus from subcortical ischemic vascular disease. J Alzheimers Dis JAD. 2020;75(3):937–947. doi: 10.3233/jad-200036. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, Fowler C, Li QX, Martins R, Rowe C, Tomita T, Matsuzaki K, Ishii K, Arahata Y, Iwamoto S, Ito K, Tanaka K, Masters CL, Yanagisawa K. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature. 2018;554(7691):249–254. doi: 10.1038/nature25456. [DOI] [PubMed] [Google Scholar]
- Palmqvist S, Tideman P, Cullen N, Zetterberg H, Blennow K, Alzheimer's Disease Neuroimaging I, Dage JL, Stomrud E, Janelidze S, Mattsson-Carlgren N, Hansson O. Prediction of future Alzheimer's disease dementia using plasma phospho-tau combined with other accessible measures. Nat Med. 2021;27(6):1034–1042. doi: 10.1038/s41591-021-01348-z. [DOI] [PubMed] [Google Scholar]
- Renard D, Castelnovo G, Wacongne A, Le Floch A, Thouvenot E, Mas J, Gabelle A, Labauge P, Lehmann S. Interest of CSF biomarker analysis in possible cerebral amyloid angiopathy cases defined by the modified Boston criteria. J Neurol. 2012;259(11):2429–2433. doi: 10.1007/s00415-012-6520-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.