Abstract
Introduction
Apremilast is approved for the treatment of psoriasis and psoriatic arthritis (PsA). Real-world evidence on the efficacy and safety of apremilast in clinical practice is limited. We assessed the use of apremilast in patients with PsA in Belgium clinical practice.
Methods
The multicentre, observational, prospective APOLO study enrolled patients with active PsA initiating apremilast in Belgium between April 2017 and December 2018. Primary outcome was PsA Response Criteria (PsARC) after 6 months of apremilast treatment. Secondary outcomes included PsA Impact of Disease 12 (PsAID12) and Health Assessment Questionnaire Disability Index (HAQ-DI). Disease-specific outcomes and patient-reported outcomes (PROs) were analysed for patients who received apremilast within 30 days prior to their study inclusion and completed at least 150 days of treatment (reference set [REF]).
Results
Of 107 patients enrolled in the study, 106 received at least one dose of apremilast and 69 were included in the REF. PsARC response was achieved by 43.5% of patients (30/69) in the REF at month 6; mean global and composite scores including 68-joint count for pain/tenderness (68-TJC) and 66-joint count for swelling (66-SJC) improved, and 27% and 42% of patients with 68-TJC and 66-SJC > 0 at baseline had complete joint count resolution, respectively. Mean global and composite PsAID12 and HAQ-DI scores decreased at 6 months, indicating improved quality of life. Apremilast was well tolerated and the reported adverse events were in line with the known safety profile.
Conclusion
Results from the APOLO study indicate that treatment with apremilast in Belgian clinical practice improves the signs and symptoms of PsA as well as patient quality of life.
Clinicaltrials.gov Identifier
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-02016-x.
Keywords: Apremilast, Psoriatic arthritis, Patient-reported outcome, Real-world evidence
Key Summary Points
| Why carry out this study? |
| Data on the real-world effectiveness and tolerability of apremilast for treatment of psoriatic arthritis are limited. |
| This prospective observational study assessed the effectiveness and safety of apremilast for the treatment of active psoriatic arthritis in Belgian clinical practice. |
| What was learned from the study? |
| Nearly half of patients achieved Psoriatic Arthritis Response Criteria after 6 months of treatment with apremilast. Apremilast also improved PROs and disease-specific measures including Health Assessment Questionnaire Disability Index, Psoriatic Arthritis Impact of Disease 12 Questionnaire and enthesitis and dactylitis scores. |
| Apremilast was well tolerated, and no new safety signals were identified. |
| Our results indicate that apremilast improves the signs and symptoms of psoriatic arthritis as well as patient quality of life in Belgian clinical practice. |
Introduction
Psoriatic arthritis (PsA) is a chronic, progressive, inflammatory joint disease, which affects 0.05–0.25% of the global population and an estimated 6–42% of patients with psoriasis [1–4]. PsA significantly impairs all aspects of patients’ life, including physical and emotional aspects, social activities and social participation [5]. While the pathogenesis of PsA is complex and multifactorial, and is still not fully understood, genetic, environmental and immunological factors are thought to play an important role [6, 7]. The articular and dermatological manifestations of PsA are heterogeneous and include peripheral arthritis, axial inflammation, enthesitis, dactylitis and skin and nail involvement/symptoms, which can be difficult to treat [8]. Moreover, progressive joint damage, increasing disability and accelerated atherosclerosis, increased cardiovascular morbidity and early mortality are associated with severe PsA [9–11].
PsA is a heterogeneous disease and several treatments are available to control the different disease manifestations [12]. Apremilast was approved by the US Food and Drug Administration in 2014 for the management of PsA in adults and for moderate or severe plaque psoriasis in patients who were candidates for phototherapy or systemic therapy [13–15]. It was then approved in Europe in 2015 for the treatment of active PsA, alone or in combination with conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) in adult patients presenting inadequate response or intolerant to prior csDMARD therapy. Apremilast is also approved for the treatment of moderate to severe chronic plaque psoriasis in adult patients (at least 18 years of age) who fail to respond to, who have a contraindication for, or are intolerant to other systemic therapy including cyclosporine, methotrexate or psoralen and ultraviolet A light [16].
In 2015, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) updated their recommendations for the management of PsA [17]. GRAPPA recognised the following six disease domains that should be addressed therapeutically for the treatment of PsA: (1) peripheral arthritis; (2) axial disease; (3) enthesitis; (4) dactylitis; (5) skin disease; (6) nail disease. Moreover, they developed treatment recommendations and evaluated the strength of the recommendations for each available therapy (‘strong’ or ‘conditional’), using the Grading of Recommendations, Assessment, Development and Evaluation. Apremilast received a strong recommendation by GRAPPA for patients with peripheral arthritis unresponsive to csDMARDs and a conditional recommendation for patients with peripheral arthritis who were DMARD-naïve [18]. In more recent guidelines, GRAPPA also strongly recommends apremilast for various PsA disease manifestations including plaque psoriasis, enthesitis, dactylitis, nail disease, and DMARD-naïve peripheral arthritis, and conditionally recommends apremilast for peripheral arthritis with insufficient response to DMARDs [19].
APOLO was designed to provide real-world evidence on apremilast use among Belgian patients with PsA. The primary objective was to evaluate the effect of apremilast on the Psoriatic Arthritis Response Criteria (PsARC) after 6 months of treatment. Secondary objectives included evaluation of the effect of apremilast on established patient-reported outcomes (PROs), including the PsA Impact of Disease 12 (PsAID12) and the Health Assessment Questionnaire Disability Index (HAQ-DI), which are important complementary components for assessing the impact of the disease and response to therapy [20]. Additional disease-specific parameters, such as Leeds Enthesitis Index (LEI) and dactylitis, duration of treatment with apremilast and its tolerability were also assessed.
Methods
Patients
APOLO enrolled adults diagnosed with active PsA initiating apremilast (30 mg twice daily, after a period of up-titration for 5 days) between April 2017 and December 2018 in Belgium according to local label and reimbursement criteria. Patients with hypersensitivity to apremilast or its excipients, pregnant or lactating women and women of childbearing potential not under an acceptable method of contraception were excluded. The study protocol was approved by the medical ethics committee of UZ Leuven and local independent ethics committees and written informed consent was obtained from each eligible patient before study enrolment. The study was conducted in accordance with the principles stated in the Declaration of Helsinki and Good Clinical Practice Guideline (CPMP/ICH/135/95).
Study Design
APOLO was a multicentre, prospective, observational study (ClinicalTrials.gov Identifier NCT03096990). Patients were followed up for at least 6 months and at most 18 months after apremilast initiation, using data collected at routine visits to their treating rheumatologist up to 31 December 2018 (no mandatory visits). Per local reimbursement criteria [21], response to treatment was assessed 6 months after apremilast initiation, and treatment continued for an additional 12 months if patients met the required criteria. The study was terminated early, on 31 December 2018.
Study Outcomes
The primary outcome was PsARC response 6 months after apremilast initiation. Secondary disease-specific outcomes included the LEI and dactylitis. PROs included the PsAID12 questionnaire and HAQ-DI.
PsARC is a composite of four measures: (1) 68-joint count for pain/tenderness (68-TJC); (2) 66-joint count for swelling (66-SJC); (3) Patient Global Assessment (PtGA) of disease activity; and (4) Physician Global Assessment (PGA) of disease activity. PsARC response was defined as improvement in at least two of these four measures, one of which must be a joint score including 68-TJC and/or 66-SJC, and no worsening in the remaining measures. Improvement in 68-TJC and 66-SJC was defined as a decrease by at least 30% in the score. PtGA and PGA of disease activity were assessed using a Likert scale 1–5 (1 = no symptoms; 5 = very strong symptoms) and improvement was defined as a decrease by one category on the Likert scale [1].
LEI quantifies joint tenderness at six pre-defined sites [22] (bilateral Achilles tendon insertions, medial femoral condyles and lateral epicondyles of the humerus; 0 = no enthesitis; 6 = six enthesitis). Dactylitis was assessed through the dactylitis count (range 0–20 digits).
PsAID12 assesses the disease domains of pain, fatigue, skin problems, work and/or leisure activities, functional capacity, discomfort, sleep disturbance, coping, anxiety, embarrassment, social participation and depression from the patients’ perspective. Each domain has a different weight and uses a 0–10 numerical rating scale (10 = worst health score). Changes over time in PsAIDs were summarised for patients with a PsAID ≥ 4 at apremilast initiation (4 = cut-off value for patient-acceptable symptom state) [23].
HAQ measures health status and health-related quality of life and represents a generic, patient-centred measure of functioning and disability. HAQ consists of 20 items covering activities of daily living classified in eight domains (dressing and grooming, arising, eating, walking, hygiene, reach, grip, activities). Patients indicate whether they could perform each activity ‘without any difficulty’, with ‘some’ or ‘much difficulty’, or if they were ‘unable to do’ the activity [24]. The HAQ yields a disability index (HAQ-DI) between 0 and 3 (0 = no functional disability to 3 = severe functional disability).
The safety and tolerability of apremilast were assessed by the number of treatment-emergent adverse events (TEAEs; AEs that occurred or worsened within 6 days of the patient’s first dose of apremilast and up to 30 days after the last dose). TEAEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 22.0.
Statistical Analysis
APOLO was an observational study and therefore no formal sample size calculation was performed. A planned sample size of 150 patients was considered a meaningful cohort relative to the prescribing use of apremilast within Belgium. All analyses were descriptive in nature. Quantitative variables were reported as number of observed values, mean and standard deviation (SD). Qualitative variables were reported as absolute frequency, percentage and number of missing observations. Measurements not performed or recorded were treated as missing data and included in percentage calculations.
Disease-specific outcomes and PROs were analysed for patients who received apremilast within 30 days prior to their inclusion in the study and completed at least 150 days of treatment (reference set [REF]). TEAEs were summarised for all enrolled patients who received at least one dose of apremilast (safety analysis set [SAF]). All analyses were performed using the statistical analysis software version 9.4.
Results
Patient Disposition
Between 21 April 2017 and 31 December 2018, 107 eligible patients were enrolled in the study. Of these, 106 (99.1%) received at least one dose of apremilast and were included in the SAF. Sixty-nine (65.1%) patients received apremilast within 30 days prior to their inclusion in the study and completed at least 150 days of treatment and were included in the REF (Fig. 1). One-fifth (21/106 [19.8%]) of patients in the SAF discontinued the study before month 6, mostly due to TEAEs (12 patients) and investigator decision (9 patients); approximately half (49/106 [46.2%]) of the patients discontinued the study before month 18. Therefore, we report data up to 12 months after apremilast treatment initiation. On study termination, 58 (54.7%) patients in the SAF were still receiving apremilast treatment. Mean (SD) follow-up in the SAF and REF was 8.47 (4.69) and 10.70 (4.03) months, respectively.
Fig. 1.
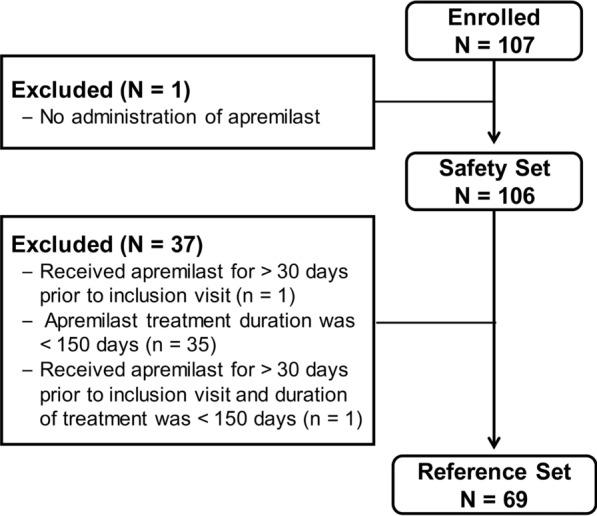
Patient disposition
Most patients in the SAF received apremilast for at least 6 months (64/106 [60.4%]; status unknown for 4 patients). Nearly half (47/106 [44.3%]) stopped apremilast within 12 months, mainly because of insufficient effectiveness (21 patients) and TEAEs (18 patients). Mean (SD) treatment duration in the SAF and REF was 4.56 (3.21) and 7.63 (2.68) months, respectively.
Patient Characteristics
Patient characteristics at apremilast initiation are summarised in Table 1. Mean (SD) age of patients in the SAF was 53 years and ranged from 24 to 79 years. The majority of patients had a concomitant diagnosis of psoriasis (82/106 [77.4%]). All except 1 patient had received previous treatment for PsA, with csDMARDs (95.3%) and non-steroidal anti-inflammatory drugs (NSAIDs; 40.6%) being the most common prior therapies. The majority of patients in the SAF were biologic-naïve, with less than 20% previously treated with biologic DMARDs. Approximately two-thirds (68/106 [64.2%]) of patient in the SAF were receiving concomitant PsA treatment at enrolment, and approximately one-quarter (29/106 [27.4%]) received concomitant PsA medications during the study (Table S1). Almost half (49/106 [46.7%]) of all patients were working. Few (12/56 [21.4%]) non-working patients reported their non-working status to have an established relationship with PsA. In general, patients included in the REF were similar to those included in the SAF (Table 1).
Table 1.
Patient characteristics
| Characteristic | SAF (N = 106) | REF (N = 69) | |
|---|---|---|---|
| Age (years) | N | 106 | 69 |
| Mean (SD) | 52.5 (11.1) | 52.7 (10.2) | |
| Gender | N | 106 | 69 |
| Male | n (%) | 47 (44.3) | 29 (42.0) |
| Female | n (%) | 59 (55.7) | 40 (58.0) |
| Weight (kg) | N | 103 | 66 |
| Mean (SD) | 83.2 (16.4) | 82.0 (14.9) | |
| BMI (kg/m2) | N | 102 | 65 |
| Mean (SD) | 28.5 (4.9) | 27.9 (4.5) | |
| Diagnosis of psoriasis | N | 106 | 69 |
| Yes | n (%) | 82 (77.4) | 55 (79.7) |
| No | n (%) | 24 (22.6) | 14 (20.3) |
| Time since initial PsA diagnosis (months) | N | 106 | 69 |
| Mean (SD) | 87.1 (92.8) | 88.6 (94.8) | |
| ≤ 2 years | n | 35 | 23 |
| Mean (SD) | 10.0 (5.5) | 10.1 (5.0) | |
| > 2 years | N | 71 | 46 |
| Mean (SD) | 125.1 (92.0) | 127.8 (94.1) | |
| PsA prior treatment | N | 106 | 69 |
| Yes | n (%) | 105 (99.1) | 68 (98.6) |
| No | n (%) | 1 (0.9) | 1 (1.4) |
| Type of prior treatment (at least one) | |||
| csDMARDs | n (%) | 101 (95.3) | 66 (95.7) |
| NSAIDs | n (%) | 43 (40.6) | 25 (36.2) |
| Corticosteroids | n (%) | 34 (32.1) | 18 (26.1) |
| bDMARDs | n (%) | 17 (16.0) | 13 (18.8) |
| Other(s) | n (%) | 8 (7.5) | 4 (5.8) |
| Professional status | N | 105 | 68 |
| Working | n (%) | 49 (46.7) | 32 (47.1) |
| Number of sick leave daysa | N | 48 | 32 |
| 0 days | n (%) | 41 (85.4) | 28 (87.5) |
| 3 days | n (%) | 2 (4.2) | 0 (0.0) |
| 6 days | n (%) | 1 (2.1) | 1 (3.1) |
| 7 days | n (%) | 1 (2.1) | 1 (3.1) |
| 15 days | n (%) | 1 (2.1) | 0 (0.0) |
| 20 days | n (%) | 1 (2.1) | 1 (3.1) |
| 21 days | n (%) | 1 (2.1) | 1 (3.1) |
| Not workingb | n (%) | 56 (53.3) | 36 (52.9) |
| No established relationship to PsA | n (%) | 44 (78.6) | 27 (75.0) |
| Established relationship to PsA | n (%) | 12 (21.4) | 9 (25.0) |
Unless otherwise stated, percentages or mean (SD) are calculated from the number of patients with non-missing data
n number of subjects with non-missing data, BMI body mass index, bDMARD biologic disease-modifying anti-rheumatic drug, csDMARDs conventional synthetic disease-modifying anti-rheumatic drug, NSAID non-steroidal anti-inflammatory drug, PsA psoriatic arthritis, REF reference set, SAF safety analysis set, SD standard deviation
aSick leave days due to PsA within the last 3 months before apremilast initiation
bPercentages calculated from the number of patients not working
PsARC Response
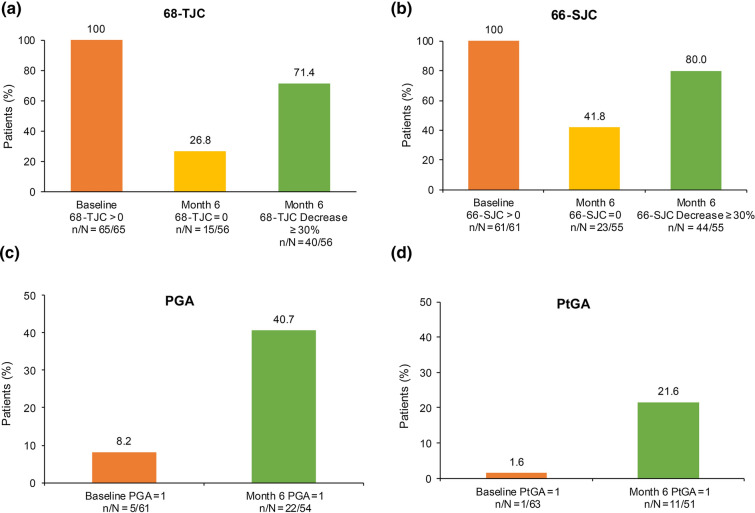
At 6 months after apremilast initiation, 30 (43.5%) patients in the REF achieved PsARC response, 16 (23.2%) were non-responders and data were missing for 23 (33.3%) patients. Of the patients with non-missing data, 65.2% (30/46) were PsARC responders. In the SAF, 32 (30.2%) patients achieved PsARC response at 6 months, 17 (16.0%) were non-responders and data were missing for 57 (53.8%) patients (Table S2a). The composite measures for PsARC response are provided in Table S3. Over half of all patients had improvements in 68-TJC and 66-SJC scores (at least 30% decrease in score: 40/56 [71.4%] and 44/55 [80.0%], respectively) at month 6. Among patients with 68-TJC > 0 and 66-SJC > 0 at apremilast initiation, 26.8% (15/56) and 41.8% (23/55) had complete resolution at month 6 (Fig. 2). PGA and PtGA scores were lower at month 6 compared with at apremilast initiation, indicating an overall improvement (Table S3). The percentage of patients with no symptoms (score = 1) increased from 8.2% (5/61) and 1.6% (1/63) at baseline to 40.7% (22/54) and 21.6% (11/51) at month 6 for PGA and PtGA, respectively (Fig. 2). Similar trends were observed in the SAF (Table S2b; Fig. S1).
Fig. 2.
Effect of apremilast on PsARC subscores at month 6 (REF). n umber of patients with desired outcome of interest, N number of patients with non-missing data available at that time point, 66-SJC 66-joint count for swelling, 68-TJC 68-joint count for pain/tenderness, PGA Physician Global Assessment, PsARC Psoriatic Arthritis Response Criteria, PtGA Patient Global Assessment, REF reference set
Enthesitis and Dactylitis
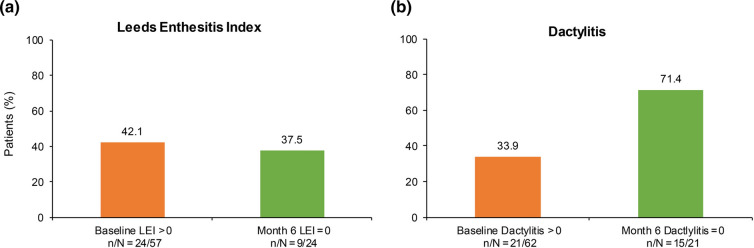
Improvements in enthesitis and dactylitis were observed in the REF at 6 months. Among patients with enthesitis (LEI > 0) at apremilast initiation, over one-third (9/24 [37.5%]) achieved complete resolution at month 6. Among patients with dactylitis at baseline, approximately three-quarters (15/21 [71.4%]) achieved complete resolution at month 6 (Fig. 3). The findings observed in the SAF were similar to those of the REF (Fig. S2).
Fig. 3.
Effect of apremilast on enthesitis and dactylitis at 6 months of treatment. n number of patients with desired outcome of interest at specific time point, N number of patients with non-missing data at that time point, LEI Leeds Enthesitis Index
Patient-Reported Outcomes
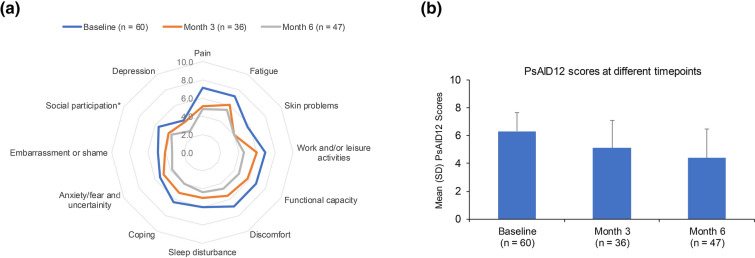
Most patients in the REF (60/69 [87.0%]) had a PsAID12 > 4 at apremilast initiation, the cut-off value for patient-acceptable symptom state. In these patients, mean overall PsAID12 score and the mean scores of all 12 domains were lower at months 3 and 6 (Fig. 4 and Table S4). Of patients with PsAID12 > 4 at baseline (n = 60), fewer than half (27/60 [45.0%]) had a PsAID12 > 4 at month 6, and the mean (SD) global score decreased to 4.4 (2.1), compared with 6.3 (1.4) at apremilast initiation.
Fig. 4.
Change in PsAID12 scores among patients with global score > 4 at apremilast initiation: a individual scores and b overall score. *Data were available for only 46 patients at 6 months for social participation domain. n number of subjects with non-missing data at each time point. PsAID12 ranges from 0 to 10, 10 = worst health score. PsAID12 Psoriatic Arthritis Impact of Disease 12, REF reference set, SD standard deviation
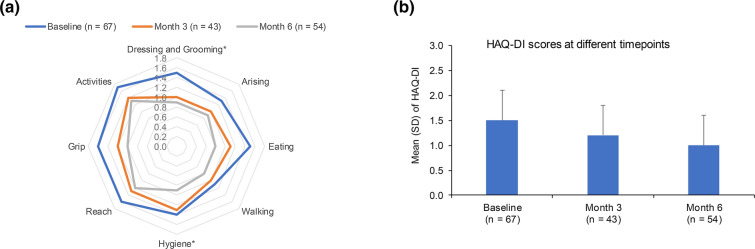
Similar reductions in mean global HAQ-DI were observed after apremilast initiation, indicating an improvement in patient’s health status and their level of functional activity. Of 67 patients in the REF with an HAQ-DI recorded at apremilast initiation, 4.5% (3/67) had HAQ-DI < 0.5 at apremilast initiation compared with 16.4% (11/67) at month 6; approximately half of all patients (35/67 [52.2%]) achieved a reduction of at least 0.35 at month 6. The mean (SD) HAQ-DI global score decreased to 1.0 (0.6) at month 6 from 1.5 (0.6) at apremilast initiation. Furthermore, a reduction was observed for all HAQ-DI domains, suggesting a gradual and sustained improvement of functional activities and daily living (Fig. 5 and Table S4). For both PsAID12 and HAQ-DI, the trends observed in the SAF were similar to that of REF (Figs. S3 and S4).
Fig. 5.
Change in HAQ-DI: a individual scores and b overall score. *Data were available for only 42 patients at month 3 for dressing and grooming and hygiene domains. n number of subjects with non-missing data at each time point. HAQ-DI ranges from 0 to 3: 0 = no functional disability, 3 = severe functional disability. HAQ-DI Health Assessment Questionnaire Disability Index, REF reference set, SD standard deviation
Safety
Overall, 48 (45.3%) patients in the SAF experienced at least one treatment-related TEAE; the most frequent were diarrhoea (20 [18.9%] patients), nausea and headache (10 [9.4%] patients each), followed by affective disorder, pruritus and weight loss (4 [3.8%] patients each) (Table 2). All treatment-related TEAEs were assessed as non-serious and mild or moderate in severity, except for an event of tension headache that was assessed as severe. Overall, the observed treatment-related TEAEs aligned with the current apremilast safety profile [16].
Table 2.
Treatment-related TEAEs reported in more than 2% of patients
| SOC PT |
SAF N = 106 |
|---|---|
| Patient with at least 1 treatment-related TEAE, n (%) | 48 (45.3) |
| Gastrointestinal disorders, n (%) | 31 (29.2) |
| Diarrhoea | 20 (18.9) |
| Nausea | 10 (9.4) |
| Nervous system disorders, n (%) | 17 (16.0) |
| Headache | 10 (9.4) |
| Migraine | 3 (2.8) |
| Psychiatric disorders, n (%) | 11 (10.4) |
| Affective disorder | 4 (3.8) |
| Depressed mood | 3 (2.8) |
| Skin and subcutaneous tissue disorders, n (%) | 5 (4.7) |
| Pruritus | 4 (3.8) |
| Investigations, n (%) | 4 (3.8) |
| Weight decreased | 4 (3.8) |
| General disorders and administration site conditions, n (%) | 3 (2.8) |
PT preferred term, SAF safety analysis set, SOC system organ class, TEAE treatment-emergent adverse event
Discussion
While the efficacy and safety of apremilast have been reported in long-term clinical trials, few studies have assessed the effectiveness and safety of apremilast in the routine treatment of patients with active PsA. The observational APOLO study was designed to appraise the real-world effectiveness and tolerability of apremilast in patients with active PsA treated in Belgium, from a patient and rheumatologist perspective.
The effectiveness of apremilast was assessed using the PsARC, which is considered an acceptable primary outcome by the European Medicines Agency (EMA) [25]. After 6 months of treatment, the PsARC response indicated reduced inflammation, improvements in pain/tenderness and swelling in joints and an improvement in disease symptoms. This was accompanied by improvements in enthesitis and dactylitis, two important hallmarks of PsA.
PsA has a significant impact on the patient’s physical function, energy level, social participation, mood and quality of life. Therefore, a key element in the management of PsA is the patient’s assessment of their disease status and the effectiveness of their treatment. In the APOLO study, PROs were used to capture the patients’ global assessment, physical function and health-related quality of life, in addition to the physician’s assessments.
Our data show that apremilast treatment had a positive impact on the patient’s physical and psychological well-being after 6 months, as assessed either by the patient or by the physician. Specifically, using the PsAID questionnaire, patients reported an improvement in pain, skin problems and fatigue and also in functional capacity, social participation and discomfort. Furthermore, the physical impairment commonly faced by patients with PsA and assessed through the HAQ-DI appeared less pronounced following apremilast treatment. The results of the APOLO study are aligned with the efficacy of apremilast demonstrated in phase 3 PALACE 2–4 clinical trials [26–28] and in the observational, multicentre, prospective LAPIS-PsA study [29]. As assessed by the physician, over half (56%) of patients in LAPIS-PsA had no or minimal PsA symptoms after approximately 4 months of apremilast treatment compared with 41% of patients in APOLO after 6 months of apremilast treatment. The proportion of patients achieving complete resolution of dactylitis was also similar in LAPIS-PsA and APOLO (28–67% at month 4 in LAPIS-PsA and 71% at month 6 in APOLO), indicating a comparable, positive effect on joint diseases in the two studies. The proportion of patients achieving complete resolution of enthesitis was lower in APOLO (at 6 months) than in LAPIS-PsA (at approximately 4 months); however, the sample size for this outcome was notably smaller in APOLO. During the study, apremilast was well tolerated and the reported treatment-related TEAEs were in line with the most commonly reported AEs [16].
The main strength of APOLO is that it assesses the impact of apremilast treatment in patients with PsA in a real-world setting, from the perspectives of both the physician and the patient. The study has few limitations: (1) being an observational study, there was a relatively high number of missing data during the study; (2) the study was a single-arm study and was not designed to make comparisons or perform hypothesis testing; (3) PROs (PsAID12, HAQ-DI) are subjective and lead to large inter-individual variability in the observed data when analysing low numbers of patients.
Conclusions
The results from APOLO confirm that apremilast reduces PsA signs and symptoms across various domains and physical functions, improving patient’s quality of life. Improvements were observed after 3 months of treatment and maintained or improved further for up to 1 year. Safety was consistent with the known safety profile.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the patients who participated in the APOLO study, as well as the investigators from each study centre and their collaborators.
Funding
This study was funded by Celgene. Amgen acquired the worldwide rights to Otezla® (apremilast) on November 21, 2019. Amgen funded the journal’s rapid service and open access fees.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: ANT, FVdB, KdV, M-JK, and RL. Methodology: ANT, FVdB, KdV, M-JK, and RL. Formal analysis and investigation: ANT, FVdB, JV, KdV, M-JK, MVdB, PR, RL, and SDR. Writing—review and editing: ANT, FVdB, JV, KdV, M-JK, MVdB, PR, RL, and SDR.
Medical Writing and Editorial Assistance
Roberta Farina, Caroline Montagner, and Marie-Anne Thil from Keyrus Life Science, funded by Amgen, provided support for data management, statistical analysis, writing of study protocol, and interim and final reports as well as the writing of this manuscript. Claire Desborough, Amgen (Europe) GmbH, also provided medical writing support for this manuscript. We thank all the patients who participated in the APOLO study, as well as the investigators from each study centre and their collaborators.
Disclosures
Kurt de Vlam has received grant/research support and/or consultation fees from Celgene, Amgen, LEO Pharma, Eli Lilly, Galapagos, Novartis, Pfizer, and UCB; Adrien Nzeusseu Toukap has received speaker and consultancy fees and/or research grants from AbbVie, BMS, Celgene, Eli Lilly, Janssen, MSD, Novartis, Pfizer, and UCB; Silvana Di Romana has received research grants from Viatris; Filip Van den Bosch has received speaker and/or consultancy fees from AbbVie, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Merck, Novartis, Pfizer, and UCB; Rik Lories has received consultancy, speaker fees and research grants from AbbVie, Amgen, Boehringer-Ingelheim, Biosplice Therapeutics, Eli Lilly, Galapagos, Janssen, Kabi-Fresenius, MSD, Novartis, Pfizer, Sandoz, and UCB; Marie-Joëlle Kaiser, Johan Vanhoof, Philip Remans, and Marthe Van den Berghe have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol was approved by the medical ethics committee of UZ Leuven and was conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guideline for Good Clinical Practice, the guiding principles of the Declaration of Helsinki 1964, and its later amendments. Informed consent was obtained from all the subjects participating in this study.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://www.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request
References
- 1.European Medicines Agency. Committee for Medicinal Products for Human Use. Guideline on clinical investigation of medicinal products for the treatment of psoriatic arthritis. 2006:1–10. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-psoriatic-arthritis_en.pdf. Accessed Aug 2021.
- 2.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–ii17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd P, Ryan C, Menter A. Psoriatic arthritis: an update. Arthritis. 2012;2012:176298. doi: 10.1155/2012/176298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin N Am. 2015;41(4):545–568. doi: 10.1016/j.rdc.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol. 2018;14(5):405–417. doi: 10.1080/1744666X.2018.1468252. [DOI] [PubMed] [Google Scholar]
- 6.Barnas JL, Ritchlin CT. Etiology and pathogenesis of psoriatic arthritis. Rheum Dis Clin N Am. 2015;41(4):643–663. doi: 10.1016/j.rdc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391(10136):2273–2284. doi: 10.1016/S0140-6736(18)30830-4. [DOI] [PubMed] [Google Scholar]
- 8.Mease PJ. Psoriatic arthritis—update on pathophysiology, assessment, and management. Bull NYU Hosp Jt Dis. 2010;68(3):191–198. [PubMed] [Google Scholar]
- 9.Belasco J, Wei N. Psoriatic arthritis: what is happening at the joint? Rheumatol Ther. 2019;6(3):305–315. doi: 10.1007/s40744-019-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gladman DD, Farewell VT, Wong K, Husted J. Mortality studies in psoriatic arthritis: results from a single outpatient center. II. Prognostic indicators for death. Arthritis Rheum. 1998;41(6):1103–1110. doi: 10.1002/1529-0131(199806)41:6<1103::AID-ART18>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Horreau C, Pouplard C, Brenaut E, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(Suppl 3):12–29. doi: 10.1111/jdv.12163. [DOI] [PubMed] [Google Scholar]
- 12.Martin BC, Thomas LW, Dann FJ. Apremilast for the treatment of psoriatic arthritis. Dermatol Online J. 2017;23(2):13030/qt36n2k4jw. doi: 10.5070/D3232033960. [DOI] [PubMed] [Google Scholar]
- 13.Mease PJ. Apremilast: a phosphodiesterase 4 inhibitor for the treatment of psoriatic arthritis. Rheumatol Ther. 2014;1(1):1–20. doi: 10.1007/s40744-014-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poole RM, Ballantyne AD. Apremilast: first global approval. Drugs. 2014;74(7):825–837. doi: 10.1007/s40265-014-0218-4. [DOI] [PubMed] [Google Scholar]
- 15.Schett G, Sloan VS, Stevens RM, Schafer P. Apremilast: a novel PDE4 inhibitor in the treatment of autoimmune and inflammatory diseases. Ther Adv Musculoskelet Dis. 2010;2(5):271–278. doi: 10.1177/1759720X10381432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Medicines Agency. Otezla: Summary of Product Characteristics. 2019:1–41. https://www.ema.europa.eu/en/documents/product-information/otezla-epar-product-information_en.pdf.
- 17.Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 18.Gossec L, Coates LC, de Wit M, et al. Management of psoriatic arthritis in 2016: a comparison of EULAR and GRAPPA recommendations. Nat Rev Rheumatol. 2016;12(12):743–750. doi: 10.1038/nrrheum.2016.183. [DOI] [PubMed] [Google Scholar]
- 19.Coates LC, Soriano E, Corp N, et al. OP0229 The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) treatment recommendations 2021. Ann Rheum Dis. 2021;80(Suppl 1):139–140. doi: 10.1136/annrheumdis-2021-eular.4091. [DOI] [Google Scholar]
- 20.Orbai AM, Ogdie A. Patient-reported outcomes in psoriatic arthritis. Rheum Dis Clin N Am. 2016;42(2):265–283. doi: 10.1016/j.rdc.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The reimbursement criteria for Otezla in psoriasis in Belgium. https://ondpanon.riziv.fgov.be/SSPWebApplicationPublic/fr/Public/ProductSearch. Accessed Aug 2021.
- 22.Wong PC, Leung YY, Li EK, Tam LS. Measuring disease activity in psoriatic arthritis. Int J Rheumatol. 2012;2012:839425. doi: 10.1155/2012/839425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73(6):1012–1019. doi: 10.1136/annrheumdis-2014-205207. [DOI] [PubMed] [Google Scholar]
- 24.Janssens X, Decuman S, De Keyser F, Belgian Rheumatoid Arthritis Disability Assessment Study Group Assessment of activity limitations with the health assessment questionnaire predicts the need for support measures in patients with rheumatoid arthritis: a multicenter observational study. PLoS ONE. 2014;9(9):e106749. doi: 10.1371/journal.pone.0106749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mease PJ. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S64–85. doi: 10.1002/acr.20577. [DOI] [PubMed] [Google Scholar]
- 26.Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43(9):1724–1734. doi: 10.3899/jrheum.151376. [DOI] [PubMed] [Google Scholar]
- 27.Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3) Ann Rheum Dis. 2016;75(6):1065–1073. doi: 10.1136/annrheumdis-2015-207963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford) 2018;57(7):1253–1263. doi: 10.1093/rheumatology/key032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wollenhaupt J, Bach C, Roemmler-Zehrer J. Effectiveness and safety of apremilast in biologic-naive versus biologic-experienced patients with psoriatic arthritis in real-world clinical practice settings in Germany: Interim analysis of an ongoing, multicenter, prospective, non-interventional study [abstract]. Arthritis Rheumatol. 2020;72(suppl 10). https://www.acrabstracts.org/abstract/effectiveness-and-safety-of-apremilast-in-biologic-naive-versus-biologic-experienced-patients-with-psoriatic-arthritis-in-real-world-clinical-practice-settings-in-germany-interim-analysis-of-an-ongoi/. Accessed 5 Aug 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://www.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request