Highlights
-
•
To reduce onchocerciasis-associated morbidity, we need to focus on young children
-
•
Six-monthly ivermectin treatment may protect school children from developing epilepsy
-
•
School-based ivermectin distribution should be considered in addition to annual CDTI
-
•
Onchocerciasis during pregnancy may lead to “parasite tolerance” in the offspring
-
•
“Parasite tolerance” in children may lead to onchocerciasis-associated epilepsy
Key words: Onchocerciasis, epilepsy, nodding syndrome, elimination, ivermectin, school-age children
Abstract
Efforts are being directed toward the elimination of onchocerciasis transmission in endemic areas with community-directed treatment with ivermectin (CDTI) in Africa, which greatly reduces onchocerciasis-associated disease. However, onchocerciasis remains a major public health problem in areas of South Sudan, the Democratic Republic of the Congo, Cameroon, and the Central African Republic. Strengthening onchocerciasis elimination efforts in areas with a high prevalence of disease burden is crucial to decrease transmission, morbidity, and mortality. We argue that clinical trials are needed to investigate the safety and efficacy of ivermectin treatment of Onchocerca volvulus-infected pregnant women and children younger than 5 years. Crucially, 6-monthly administration of ivermectin in school-age children at risk of onchocerciasis-associated epilepsy could be achieved by supplementing annual CDTI with an extra round of ivermectin treatment during Child Health Days in schools and/or other distribution sites every year. These strategies would help achieve the elimination of onchocerciasis and its associated disease burden.
Introduction
Onchocerciasis is a neglected tropical disease (NTD) caused by the filarial nematode Onchocerca volvulus and transmitted by way of the bites from (Simulium) blackfly vectors (Brattig et al., 2021). It is estimated that 99% of the 21 million individuals currently infected with O. volvulus live in Africa (Brattig et al., 2021). Onchocerciasis has been typically considered a disease of the skin (itching, onchodermatitis, depigmentation) and the eyes (reduced visual acuity, blindness), mainly affecting adults, but there is increasing evidence that heavy O. volvulus infection in childhood can lead to a wide spectrum of severe developmental disorders in young children. Numerous epidemiological studies have shown a clear association between onchocerciasis and epilepsy, termed “onchocerciasis-associated epilepsy” (OAE) (Colebunders et al., 2021). OAE onset occurs in previously healthy children between the ages of 3–18 years (peaking between 5–12 years), mostly with generalized convulsive seizures (Colebunders et al., 2021). A subset of patients with OEA initially presents with a particular type of atonic head-nodding seizures, the hallmark feature of nodding syndrome (NS) (Idro et al., 2018). Nakalanga syndrome, a childhood disorder mainly characterized by stunting and other characteristic symptoms and signs shared with NS (Foger et al., 2017), is also found in onchocerciasis-endemic areas. Cognitive impairment of varying degrees as an indication of brain disease has been found in these onchocerciasis-related conditions (Colebunders et al., 2021, Foger et al., 2017, Idro et al., 2018). A recent study among 209 children in Cameroon suggested that O. volvulus infection may also lead to cognitive impairment even without manifest epileptic seizures(Siewe Fodjo et al., 2021).
Even if children were to acquire the infection at birth, newborn children would not harbor adult O. volvulus worms due to the 1–2-year incubation period. However, the worm's microfilarial progeny can be passed from infected mothers to the fetus by way of the connective tissue of the umbilical cord (Brinkmann et al., 1976) but disappear within several months. In the first year of life, children can become infected from bites of blackfly vectors harboring infective stage L3 larvae, e.g., when carried on the backs of their mothers/siblings as they go to the field. Following infection, they contribute to the perpetuation of the parasite's lifecycle as they become patent with microfilariae in the skin, the stage transmitted from humans to vectors (and mostly associated with clinical manifestations). In highly hyperendemic areas, the prevalence of patent infection can rise from zero in infancy to >60% in children aged 3–9 years, and >80% in those aged 10–14 years (Basáñez and Boussinesq, 1999). Since the pre-patent period is 2–3 years, this indicates that in these areas, children can be exposed from birth. Besides, children born to O. volvulus-infected mothers are more likely to be infected and to harbor higher microfilarial loads(Kirch et al., 2003). This results in a higher risk of developing OAE (Chesnais et al., 2018) and premature mortality (Kaiser et al., 2007).
Due to community-directed treatment with ivermectin (CDTI), introduced by the African Program for Onchocerciasis Control (APOC) (Amazigo et al., 2021), onchocerciasis ceased to pose a public health problem in several African countries (Brattig et al., 2021). In 2010, and after evidence emerged that (annual or 6-monthly) mass drug administration (MDA) with ivermectin could lead to the elimination of transmission (EOT) (Diawara et al., 2009), APOC's focus shifted from morbidity control to EOT (Brattig, 2009). To control onchocerciasis as a public health problem, APOC recommended annual CDTI with a minimum therapeutic coverage (of the total population aged ≥5 years) of 65% (Brattig et al., 2021). However, therapeutic coverage of 80% and geographical coverage of 100% will be needed for EOT. APOC closed in December 2015, and the World Health Organization established the Expanded Special Project for the Elimination of NTDs (ESPEN) (Hopkins, 2016). ESPEN's aims include supporting endemic countries in the identification and delineation of hypoendemic areas, previously excluded from CDTI as deemed not to contribute to morbidity. This requires onchocerciasis elimination mapping and includes seroprevalence surveys in high-risk areas (World Health Organisation, 2016).
Following the implementation of EOT efforts, the number of new blind and visually impaired people due to onchocerciasis decreased substantially (although prevalent blindness cases still remain) (Brattig et al., 2021). However, annual CDTI may not be sufficient to eliminate the disease burden, particularly in areas where transmission is high and/or CDTI coverage is sub-optimal. Recent community-based surveys in several African countries found lower coverage in countries with annual CDTI compared with those with a 6-monthly strategy, with younger age groups being least likely to participate (Siewe Fodjo et al., 2020). Consequently, in villages with persistent onchocerciasis transmission, there likely remains a substantial burden of onchocerciasis-associated morbidity, including OAE (Colebunders et al., 2019).
The unrecognized disease burden caused by onchocerciasis
Onchocerciasis remains a major public health problem in communities where OAE is still highly prevalent, such as those in many parts of South Sudan(Colebunders et al., 2018, Raimon et al., 2021), the Democratic Republic of the Congo (Mandro et al., 2018), some areas in Cameroon (Siewe et al., 2019), and in the Central African Republic (Smet et al., 2020). Likely causes for this include low therapeutic coverage, less than the annual frequency of CDTI, poor treatment adherencek (Colebunders et al., 2019), or not receiving CDTI because of insecurity (Colebunders et al., 2019). Besides, annual CDTI may not reduce O. volvulus load sufficiently to protect against clinical disease because skin repopulation by microfilariae starts rising a few months after ivermectin treatment (Dusabimana et al., 2020; Opoku et al., 2018). It was estimated that, in 2015, there were about 381,000 cases of OAE across onchocerciasis foci in Africa (Vinkeles Melchers et al., 2018). OAE is responsible for approximately 13% of the total number of years lived with disability (YLDs) attributable to onchocerciasis and 10% of total YLDs attributable to epilepsy(Vinkeles Melchers et al., 2018).
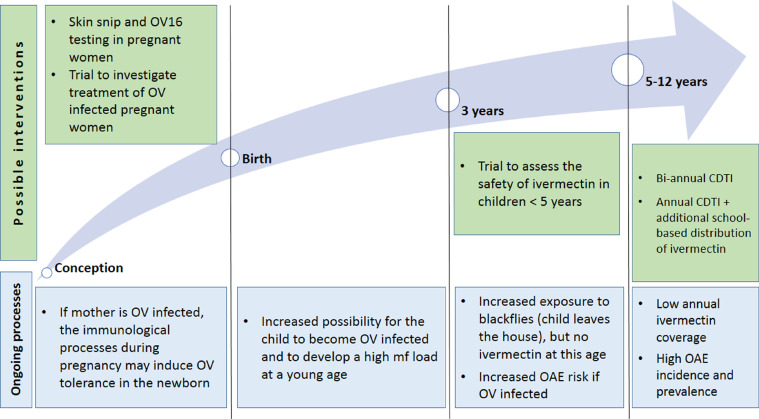
Proposed strategies to prevent onchocerciasis-associated morbidity (Figure 1)
Figure 1.
Factors influencing the development of onchocerciasis-associated epilepsy and proposed strategies to strengthen evidence-based interventions towards its prevention.
Two cohort studies in Cameroon showed that microfilarial load in young children determines their risk of developing epilepsy in later childhood (Chesnais et al., 2020). Six-monthly CDTI with high therapeutic coverage could substantially reduce microfilarial load for nearly a year (Turner et al., 2014). However, increased CDTI frequency may be considered costly and logistically challenging in most African countries, and it also requires high therapeutic coverage to achieve EOT. Modeling studies suggest that annual community-directed treatment with moxidectin (CDTM) could achieve a similar result, but the current regulatory approval for moxidectin does not include children under 12 years of age and it is not yet known whether the drug will be donated(Milton et al., 2020).
Treatment of O. volvulus-infected pregnant women
Treatment of O. volvulus-infected pregnant women with ivermectin may reduce the risk of their children developing OAE. The underlying mechanism is that of “parasite tolerance” in the child, whereby intra-uterine exposure to filarial antigens reduces cellular responsiveness to parasite antigens in the offspring(Soboslay et al., 1999). Therefore, children born to O. volvulus-infected pregnant women may be at risk of acquiring O. volvulus infection earlier and of developing a higher microfilarial load that would put them at risk of developing OAE (Chesnais et al., 2018). Given the fact that, so far, about 3•7 billion doses of ivermectin have been distributed in mass drug administration campaigns globally and that no pregnancy tests were performed during these campaigns, it is likely that a very large number of pregnant women have taken ivermectin inadvertently (Nicolas et al., 2020). Ivermectin treatment of pregnant women is contraindicated even if they are infected with O. volvulus. However, there is no evidence that ivermectin is teratogenic. A randomized placebo-controlled double-blind clinical trial would be justified to determine the safety and efficacy of ivermectin treatment in O. volvulus-infected pregnant women given the potential benefit to their children.
Treatment of children younger than five years
A proportion of children develop OAE before the age of 5. Therefore, it would be worthwhile to consider lowering the age of ivermectin treatment in children, e.g., starting at the age of 3 or below 15 kg of body weight, and integrating the distribution of ivermectin in the childhood vaccination program. This is not currently possible because of a lack of safety and pharmacokinetic data in younger children. A registered double-blind, placebo-controlled trial to assess the safety, pharmacokinetics, and efficacy of ascending doses of oral ivermectin in scabies-infected children weighing 5 to fewer than 15 kg is expected to provide valuable information (Lorenz von Seidlein and Kobylinski, 2021).
Targeting school-age children (SAC) for ivermectin distribution
As current onchocerciasis-associated morbidity is mainly related to children with a high microfilarial load developing epilepsy, (Chesnais et al., 2018), it is crucial to keep young children free of O. volvulus microfilariae for as long as possible, which may not be achieved through annual CTDI. An additional round of ivermectin to school-age children, targeting children between the ages of 5‒6 and 12‒14 years, 6 months after a CDTI round, could help achieve 6-monthly ivermectin administration to this high-risk age group. Such targeted distribution would be a low-cost intervention, particularly if delivered during Child Health Days and school-based programs together with other NTD treatment campaigns, e.g., for schistosomiasis and/or soil-transmitted helminthiasis (STH). UNICEF and other partners in sub-Saharan Africancountries have implemented Child Health Days since 2000, achieving high coverage of vitamin A supplementation and deworming (Oliphant et al., 2010). Recent studies have shown that it is clinically and programmatically safe to co-administer ivermectin, albendazole, and praziquantel (ivermectin also being efficacious against STH). This approach could prove effective in countries like Cameroon, where the rate of primary school enrolment exceeds 70% in rural areas (Institut National de la Statistique du Cameroun 2018). In other countries with lower rates such as South Sudan, many children in onchocerciasis-endemic villages do not attend school. Also, school dropout rates have worsened as a result of the COVID-19 pandemic. In such situations, Child Health days outreach in communities could provide a viable ivermectin distribution option for non-schooled children.
Conclusion
National onchocerciasis programs face the dual challenge of reaching and protecting onchocerciasis elimination in areas with minimal disease burden (implementing stop-MDA surveys and post-treatment surveillance), while also dealing with persisting health burden in hard-to-reach, high-transmission areas. OAE is presently a major contributor to the remaining health consequences of onchocerciasis. We propose several avenues to address this problem. Conducting safety and efficacy studies of ivermectin treatment in O. volvulus-infected pregnant women and children under 5 years of age could pave the way for earlier interventions to prevent OAE and sustainably reduce onchocerciasis-associated disease burden. Treating school-aged children with an additional dose of ivermectin every year as part of existing school-based programs and Child Health Days would ensure their 6-monthly treatment to keep their microfilarial loads to a minimum. We conclude that investing in these intensified interventions would help decrease both onchocerciasis transmission and onchocerciasis-associated morbidity and mortality.
Contributors
RC and JNSF conceived the paper; CK, MGB, PO, and TL contributed intellectual input; RC wrote the first draft; CK and MGB reviewed/edited the draft; all authors read and approved the final manuscript.
Conflict of interests
We declare no competing interests.
Acknowledgments
Funding Source
RC received a European Research Council (ERC) grant (671055) and a Flemish Interuniversity Council (VLIR-UOS) grant. MGB acknowledges funding from the Medical Research Council (MRC) Centre for Global Infectious Disease Analysis (MR/R015600/1), jointly funded by the UK MRC and the UK Foreign, Commonwealth & Development Office (FCDO), under the MRC/FCDO Concordat agreement and is also part of the European and Developing Countries Clinical Trials Partnership (EDCTP2) program supported by the European Union.
Ethical approval
Not applicable.
References
- Amazigo UV, Leak SGA, Zoure HGM, Okoronkwo C, Diop Ly M, Isiyaku S, et al. Community-directed distributors-The "foot soldiers" in the fight to control and eliminate neglected tropical diseases. PLoS Negl Trop Dis. 2021;15(3) doi: 10.1371/journal.pntd.0009088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basáñez MG, Boussinesq M. Population biology of human onchocerciasis. Philos Trans R Soc Lond B Biol Sci. 1999;354(1384):809–826. doi: 10.1098/rstb.1999.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattig NW, Cheke RA, Garms R. Onchocerciasis (river blindness) - more than a century of research and control. Acta Trop. 2021;218 doi: 10.1016/j.actatropica.2020.105677. [DOI] [PubMed] [Google Scholar]
- Brinkmann UK, Kramer P, Presthus GT, Sawadogo B. Transmission in utero of microfilariae of Onchocerca volvulus. Bull World Health Organ. 1976;54(6):708–709. [PMC free article] [PubMed] [Google Scholar]
- Chesnais CB, Bizet C, Campillo JT, Njamnshi WY, Bopda J, Nwane P, et al. A Second Population-Based Cohort Study in Cameroon Confirms the Temporal Relationship Between Onchocerciasis and Epilepsy. Open Forum Infect Dis. 2020;7(6):ofaa206. doi: 10.1093/ofid/ofaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebunders R, YC J, Olore PC, Puok K, Bhattacharyya S, Menon S, et al. High prevalence of onchocerciasis-associated epilepsy in villages in Maridi County, Republic of South Sudan: A community-based survey. Seizure. 2018;63:93–101. doi: 10.1016/j.seizure.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebunders R, Siewe Fodjo JN, Hopkins A, et al. From river blindness to river epilepsy: implications for onchocerciasis elimination programmes. PLoS Negl Trop Dis. 2019;13(7) doi: 10.1371/journal.pntd.0007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebunders R, Njamnshi AK, Menon S, Newton CR, Hotterbeekx A, Preux PM, et al. Onchocerca volvulus and epilepsy: A comprehensive review using the Bradford Hill criteria for causation. PLoS Negl Trop Dis. 2021;15(1) doi: 10.1371/journal.pntd.0008965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diawara L, Traoré MO, Badji A, et al. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: first evidence from studies in Mali and Senegal. PLoS Negl Trop Dis. 2009;3(7):e497. doi: 10.1371/journal.pntd.0000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusabimana A, Bhwana D, Raimon S, Mmbando BP, Hotterbeekx A, Tepage F, et al. Ivermectin Treatment Response in Onchocerca Volvulus Infected Persons with Epilepsy: A Three-Country Short Cohort Study. Pathogens. 2020;9(8) doi: 10.3390/pathogens9080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foger K, Gora-Stahlberg G, Sejvar J, Ovuga E, Jilek-Aall L, Schmutzhard E, et al. Nakalanga Syndrome: Clinical Characteristics, Potential Causes, and Its Relationship with Recently Described Nodding Syndrome. PLoS Negl Trop Dis. 2017;11(2) doi: 10.1371/journal.pntd.0005201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AD. Neglected tropical diseases in Africa: a new paradigm. Int Health. 2016;8(Suppl 1):i28–i33. doi: 10.1093/inthealth/ihv077. [DOI] [PubMed] [Google Scholar]
- Idro R, Ogwang R, Kayongo E, Gumisiriza N, Lanyero A, Kakooza-Mwesige A, et al. The natural history of nodding syndrome. Epileptic Disord. 2018;20(6):508–516. doi: 10.1684/epd.2018.1012. [DOI] [PubMed] [Google Scholar]
- Institut National de la Statistique du Cameroun. The DHS Program. Enquête Démographique et de Santé 2018. Available at https://dhsprogram.com/pubs/pdf/FR360/FR360.pdf. Accessed 21 December 2021.
- Kaiser C, Asaba G, Kasoro S, Rubaale T, Kabagambe G, Mbabazi M. Mortality from epilepsy in an onchocerciasis-endemic area in West Uganda. Trans R Soc Trop Med Hyg. 2007;101(1):48–55. doi: 10.1016/j.trstmh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kirch AK, Duerr HP, Boatin B, et al. Impact of parental onchocerciasis and intensity of transmission on development and persistence of Onchocerca volvulus infection in offspring: an 18 year follow-up study. Parasitology. 2003;127(4):327–335. doi: 10.1017/s0031182003003834. [DOI] [PubMed] [Google Scholar]
- Lorenz von Seidlein L, Kobylinski K. Ivermectin in small children. Available at https://clinicaltrials.gov/ct2/show/NCT04332068?term=children&cond=ivermectin&draw=2&rank=2. Accessed 22 December 2021.
- Mandro M, Suykerbuyk P, Tepage F, Rossy D, Ngave F, Hasan MN, et al. Onchocerca volvulus as a risk factor for developing epilepsy in onchocerciasis endemic regions in the Democratic Republic of Congo: a case control study. Infect Dis Poverty. 2018;7(1):79. doi: 10.1186/s40249-018-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton P, Hamley JID, Walker M, Basáñez MG. Moxidectin: an oral treatment for human onchocerciasis. Expert Rev Anti Infect Ther. 2020;18(11):1067–1081. doi: 10.1080/14787210.2020.1792772. [DOI] [PubMed] [Google Scholar]
- Nicolas P, Maia MF, Bassat Q, Kobylinski KC, Monteiro W, Rabinovich NR, et al. Safety of oral ivermectin during pregnancy: a systematic review and meta-analysis. Lancet Glob Health. 2020;8(1):e92–e100. doi: 10.1016/S2214-109X(19)30453-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant NP, Mason JB, Doherty T, Chopra M, Mann P, Tomlinson M, et al. The contribution of child health days to improving coverage of periodic interventions in six African countries. Food Nutr Bull. 2010;31(3 Suppl):S248–S263. doi: 10.1177/15648265100313S304. [DOI] [PubMed] [Google Scholar]
- Raimon S, Dusabimana A, Abd-Elfarag G, Okaro S, Carter JY, Newton CR, et al. High Prevalence of Epilepsy in an Onchocerciasis-Endemic Area in Mvolo County, South Sudan: A Door-To-Door Survey. Pathogens. 2021;10(5) doi: 10.3390/pathogens10050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewe Fodjo JN, Mubiru F, Ukaga C, Logora MY, Mmbando BP, Mandro M, et al. Low ivermectin use among 5- to 6-year-old children: observations from door-to-door surveys in onchocerciasis-endemic regions in Africa. Int Health. 2020;12(1):72–75. doi: 10.1093/inthealth/ihz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewe Fodjo JN, Ngarka L, Nfor LN., et al. Association Between Ov16 Seropositivity and Neurocognitive Performance Among Children in Rural Cameroon: a Pilot Study. J Pediatr Neuropsychol. 2021;7(4):192–202. doi: 10.1007/s40817-021-00111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewe JFN, Ngarka L, Tatah G, Mengnjo MK, Nfor LN, Chokote ES, et al. Clinical presentations of onchocerciasis-associated epilepsy (OAE) in Cameroon. Epilepsy Behav. 2019;90:70–78. doi: 10.1016/j.yebeh.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Smet E, Metanmo S, Mbelesso P, Kemata B, Fodjo JNS, Boumediene F, et al. Focus of Ongoing Onchocerciasis Transmission Close to Bangui. Central African Republic. Pathogens. 2020;9(5) doi: 10.3390/pathogens9050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboslay PT, Geiger SM, Drabner B, Banla M, Batchassi E, Kowu LA, et al. Prenatal immune priming in onchocerciasis-onchocerca volvulus-specific cellular responsiveness and cytokine production in newborns from infected mothers. Clin Exp Immunol. 1999;117(1):130–137. doi: 10.1046/j.1365-2249.1999.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner HC, Walker M, Churcher TS, et al. Reaching the London Declaration on Neglected Tropical Diseases goals for onchocerciasis: an economic evaluation of increasing the frequency of ivermectin treatment in Africa. Clin Infect Dis. 2014;59(7):923–932. doi: 10.1093/cid/ciu467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkeles Melchers NVS, Mollenkopf S, Colebunders R, Edlinger M, Coffeng LE, Irani J, et al. Burden of onchocerciasis-associated epilepsy: first estimates and research priorities. Infect Dis Poverty. 2018;7(1):101. doi: 10.1186/s40249-018-0481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . World Health Organization; Geneva, Switzerland: 2016. Guidelines for stopping mass drug administration and verifying elimination of human onchocerciasis: criteria and procedures.https://www.who.int/publications/i/item/9789240006638 Report No.: WHO/HTM/NTD/PCT/2016.1. Available at. Accessed 22 December 2021. [PubMed] [Google Scholar]