Abstract
Trichoderma longibrachiatum was recovered from stool surveillance cultures and a perirectal ulcer biopsy specimen from a 29-year-old male who had received an allogeneic bone marrow transplant for acute lymphoblastic leukemia. The amphotericin B (2.0 μg/ml) and itraconazole (1.0 μg/ml) MICs for the organism were elevated. Therapy with these agents was unsuccessful, and the patient died on day 58 posttransplantation. At autopsy, histologic sections from the lungs, liver, brain, and intestinal wall showed infiltration by branching septate hyphae. Cultures were positive for Trichoderma longibrachiatum. While Trichoderma species have been recognized to be pathogenic in profoundly immunosuppressed hosts with increasing frequency, this is the first report of probable acquisition through the gastrointestinal tract. Salient features regarding the identification of molds in the Trichoderma longibrachiatum species aggregate are presented.
The significant rise in profoundly immunocompromised patients in recent years has resulted in a concomitant escalation in opportunistic fungal infections (1, 6, 10, 22, 27, 30). In addition to the increased frequency of infection, the variety of species implicated has also broadened such that it is now apparent that the concept of nonpathogenic fungi has little meaning in the setting of the immunocompromised host. This report of a case of a fatal, disseminated Trichoderma longibrachiatum infection in an adult bone marrow transplant recipient is the fifth report documenting this species of Trichoderma as an etiologic agent of infection in an immunocompromised host and the first to suggest the gastrointestinal tract as a portal of entry. Given its potential pathogenicity and predilection for dissemination in profoundly neutropenic hosts, cultural characteristics useful for the presumptive identification in routine mycology laboratories of molds in the T. longibrachiatum species aggregate, and specifically T. longibrachiatum, will be addressed.
Case report.
A 29-year-old white male was diagnosed with acute lymphoblastic leukemia (ALL; L1 subtype, T-cell immunotype) in June 1993. He received treatment according to the Cancer and Leukemia Group B 9111 protocol for induction and maintenance. A bone marrow examination 28 days after induction showed remission. Five months after initial presentation, he had one central nervous system (CNS) relapse requiring intrathecal chemotherapy and whole-brain irradiation. One week prior to a planned admission for bone marrow transplantation, he experienced a second CNS relapse. A short course of irradiation and intrathecal chemotherapy resulted in a high level of partial remission.
The patient was admitted to the University of Iowa Hospitals and Clinics in May 1994 for a bone marrow transplant and was conditioned with total body irradiation, high-dose etoposide, and intrathecal 1-β-d-arabinofuranosylcytosine. At admission, he was placed on oral clotrimazole, oral nystatin, trimethoprim-sulfamethoxazole, amphotericin B nasal spray, and intravenous fluconazole (200 mg once a day [q.d.]) for antimicrobial prophylaxis. Ceftazidime and vancomycin were added 1 week later when the patient’s neutrophil count declined to 300/mm3. Ten days after admission, an unrelated allogeneic bone marrow transplant was performed. Acyclovir and metronidazole had been added to the antimicrobial regimen by day 3 posttransplantation. The patient’s initial course posttransplantation was uneventful except for the development of severe oral mucositis.
Stool surveillance studies were negative for fungi until days 8 and 10 posttransplantation, when a mold consistent with a Trichoderma species was identified. In response to the positive stool cultures and a small right upper lobe lung opacity identified on a chest computed tomography scan, amphotericin B (49 mg q.d.) was started on day 13. On day 15, methyl prednisolone sodium (Solu-Medrol; 70 mg every 12 h) was initiated for stage II skin graft-versus-host disease (GVHD). The amphotericin B was increased to 83 mg q.d. on day 18 due to slight enlargement of the nodular pulmonary infiltrate. On day 26, amphotericin B was discontinued because of deteriorating renal function, and itraconazole (600-mg loading dose, maintenance dosage of 400 mg q.d.) was introduced. A Trichoderma species was again isolated from a stool surveillance culture on day 27. The patient developed diarrhea, and a perianal ulcer was noted. A stool culture was positive for Clostridium difficile, and therapy with oral vancomycin was started on day 31. Itraconazole was discontinued because of the patient’s inability to take the oral medication, and amphotericin B (65 mg q.d.) was restarted on day 33.
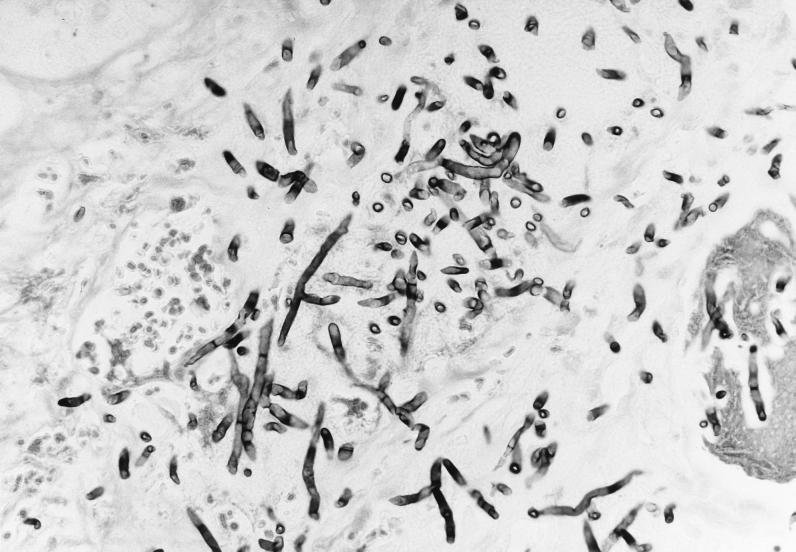
By day 34, the leukocyte count had not recovered and it became apparent that the engraftment had failed. Plans were made for a second transplant. The perianal ulcer was enlarging, and a punch biopsy on day 35 showed a necrotic lesion infiltrated with branching septate hyphae (Fig. 1). A culture was positive for Trichoderma species. Daily granulocyte infusions were initiated, and the amphotericin B was increased to 100 mg q.d. on day 37. Ablation therapy was initiated on day 43. Due to declining renal function, liposomal amphotericin B (335 mg q.d.) was introduced on day 47. A second unrelated allogeneic bone marrow transplant was performed 49 days after the initial graft. The patient began to have spiking temperatures of up to 40°C 2 days later. The perianal ulcer continued to enlarge, and abdominal distention was noted. Progression of the pulmonary infiltrate was demonstrated radiographically, with multiple nodules involving both lungs. An abdominal film showed small-bowel distention with thickening of the intestinal wall. The patient’s condition continued to deteriorate, and he died 9 days after the second transplant.
FIG. 1.
Methenamine silver stain of the perianal ulcer biopsy specimen shows necrosis and infiltration by branching septate hyphal forms. Magnification, ×100.
An autopsy revealed a disseminated fungal infection. Multiple areas of infarction with infiltration by branching septate hyphae were identified on Gomori methenamine silver-stained sections of the liver and both lungs. Sections of exophytic ulcerations of the sigmoid colon and the ileocecal valve showed transmural necrosis with invasion by septate branching fungal forms. The heaped ileocecal valve lesion was partially blocking the lumen and probably caused the small-bowel obstruction that had been observed clinically. Focal cerebritis with invasive septate hyphae was identified in the right parietal cortex. Postmortem cultures of liver, lung, and sigmoid colon specimens were positive for a Trichoderma species. Complications of sepsis were identified and included intravascular thrombi suggesting disseminated intravascular coagulopathy, massive pulmonary edema consistent with extensive capillary leakage, and acute tubular necrosis. The possibility of residual leukemia was suggested by a few clusters of atypical mononuclear cells in the spleen.
Mycology.
All specimens for fungal culture were cultured on Sabouraud dextrose agar (SDA; Becton Dickinson, Cockeysville, Md.) and Mycosel agar (Baltimore Biological Laboratory, Cockeysville, Md.) at 30°C. Isolates were identified as Trichoderma species on the basis of their macroscopic morphology (rapidly growing flat green colonies) and microscopic characteristics (branching divergent tufts of conidiophores). Isolates were submitted to the Fungus Testing Laboratory, Department of Pathology, University of Texas Health Science Center, San Antonio (accession no. 94-1620), for species identification.
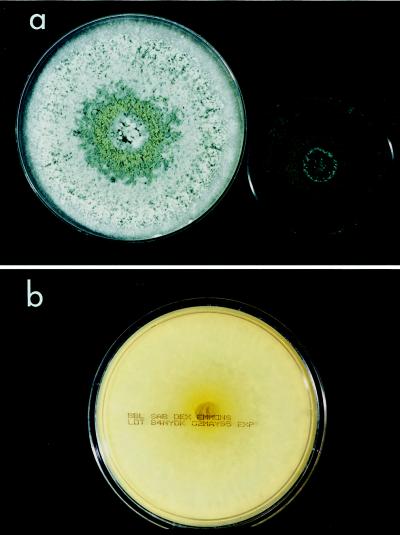
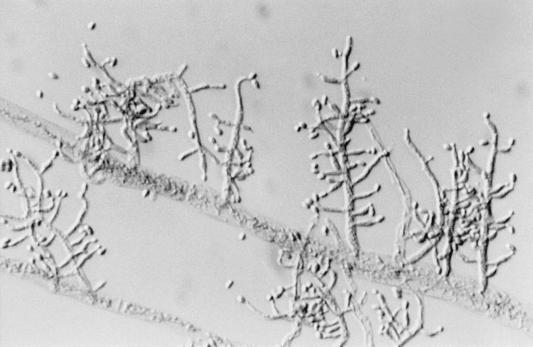
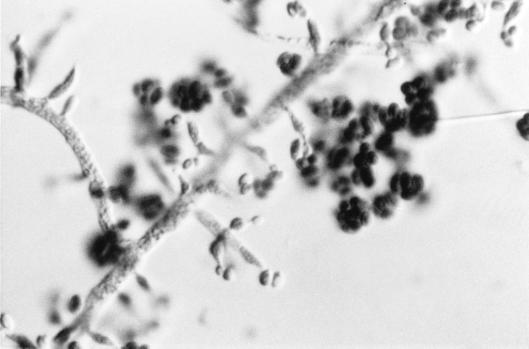
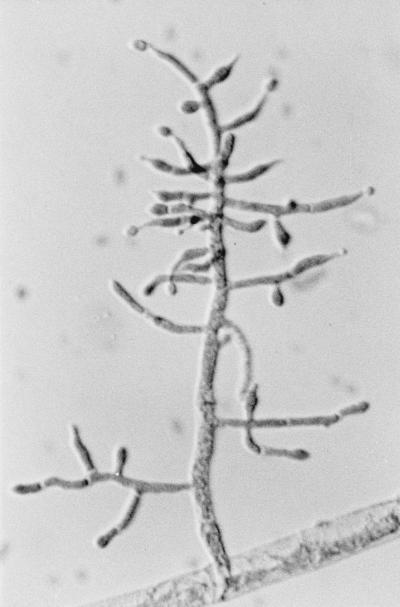
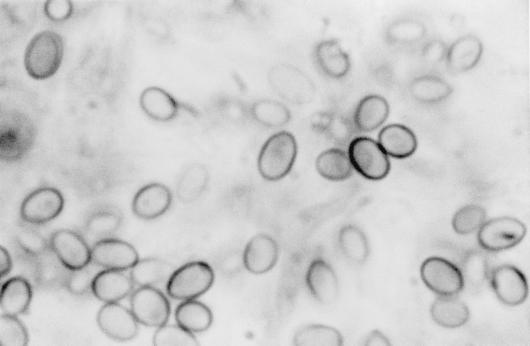
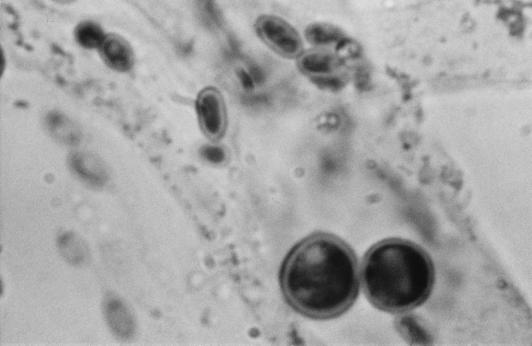
There the isolate was subcultured onto potato flakes agar (PFA), which was prepared in house, and SDA at 25, 35, and 42°C, with good growth occurring at all temperatures. Colonies were initially smooth, somewhat glabrous, translucent, and a watery white but very rapidly became light green to olivaceous green and woolly with distinct tufts of conidiophores in a zonal radial pattern (Fig. 2a). A striking lemon yellow diffusible pigment was evident throughout the agar (Fig. 2b). Microscopically, the hyphae were smooth, septate, and ramified (branched) and bore conidiophores with long main branches (Fig. 3). The main branches in turn produced relatively few, short, often slightly curved side branches at right angles, each terminating in a phialide. Phialides were bottle shaped, mostly solitary, often somewhat inflated in the middle and bent at the apex, and slightly constricted at the base and measured mostly 5 to 11 by 2 to 3 μm (Fig. 4 and 5). Terminal phialides were more elongate, were not constricted at the base, and were up to 14 μm long (Fig. 5). Phialoconidia were smooth walled, ellipsoidal to cylindrical, and green and measured 3.4 to 6.4 by 2.4 to 3.0 μm (Fig. 6). Occasional smooth, thick-walled, subglobose to ellipsoidal chlamydoconidia that measured from 5 to 10 μm in diameter were also observed (Fig. 7). On the basis of the characteristics described above and our experience with similar Trichoderma isolates, this organism was placed in the section Longibrachiatum (2) and was tentatively identified as T. longibrachiatum. Species confirmation was provided by Gary Samuels (Agricultural Research Service, U.S. Department of Agriculture, Beltsville, Md.) on the basis of analysis of ribosomal DNA internal transcribed spacer ITS-1 and ITS-2 sequences.
FIG. 2.
(a) T. longibrachiatum on SDA (left) and PFA (right), 5 days, 25°C. (b) Yellow diffusable pigment produced by T. longibrachiatum on reverse of SDA, 5 days, 25°C.
FIG. 3.
Long main branch of T. longibrachiatum producing shorter side branches terminating in phialides. Magnification, ×320.
FIG. 4.
Side branches, phialides, and phialoconidia of T. longibrachiatum. Magnification, ×920.
FIG. 5.
Terminal phialides (arrows) of T. longibrachiatum. Magnification, ×920.
FIG. 6.
Phialoconidia of T. longibrachiatum. Magnification, ×2,300.
FIG. 7.
Smooth, thick-walled chlamydoconidia of T. longibrachiatum. Magnification, ×2,300.
Antifungal susceptibility testing.
Broth microdilution testing was performed in the Department of Pathology at the University of Iowa Hospitals and Clinics according to National Committee for Clinical Laboratory Standards (NCCLS) proposed standard guidelines as described previously (21, 23). An inoculum concentration of 0.4 × 104 to 5 × 104 CFU/ml and RPMI 1640 medium buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid buffer (Sigma, St. Louis, Mo.) were used (21, 23). Antifungal agents were obtained from their respective manufacturers and included amphotericin B, flucytosine (5FC), fluconazole, and itraconazole. Fungal inocula (100 μl) were added to each well of the microdilution tray, and each well contained 100 μl of drug solution (2× final concentration). The tray was incubated in air at 35°C and was read after 48 h of incubation. The MIC endpoints were read visually. For 5FC, fluconazole, and itraconazole the MIC endpoints were defined as the lowest concentration in which a prominent decrease in turbidity (approximately 50% inhibition) was observed, and for amphotericin B the MIC endpoint was defined as the lowest concentration that produced complete inhibition of growth (21, 23). Drug-free and fungus-free controls were included, and quality control was ensured by testing Candida parapsilosis ATCC 22019, a strain recommended for this purpose (21, 24).
The MICs of amphotericin B (2.0 μg/ml), itraconazole (1.0 μg/ml), fluconazole (16 μg/ml), and 5FC (>256 μg/ml) for the organism were elevated (23). These values were comparable to those previously reported in the literature for this species (Table 1).
TABLE 1.
Antifungal susceptibilities of T. longibrachiatum isolates
| Reference | Source | Antifungal agent | MIC (μg/ml) | Treatment | Outcome |
|---|---|---|---|---|---|
| 8 | Ethmoidal and maxillary sinuses | Amphotericin B | 1.16 | Amphotericin B, surgery | Survival |
| 5 FC | >322.75 | ||||
| Fluconazole | 80 | ||||
| Itraconazole | 0.3 | ||||
| 20 | Skin biopsy | Amphotericin B | 2.0 | Amphotericin B, amphotericin B lipid complex | Survival |
| 5 FC | >64 | ||||
| Fluconazole | >64 | ||||
| Itraconazole | 2.0 | ||||
| 32 | Brain | Amphotericin B | 2.5 | Amphotericin B plus 5 FC | Survival |
| 5 FC | 50 | Amphotericin B plus ketoconazole | |||
| Fluconazole | 12.5 | Amphotericin B plus itraconazole | |||
| Itraconazole | 1.25 | Surgery | |||
| 34 | Peritonitis | Amphotericin B | 5.0 | Amphotericin B | Death |
| Present study | Disseminated infection | Amphotericin B | 2.0 | Amphotericin B, itraconazole, liposomal amphotericin B | Death |
| 5 FC | >256 | ||||
| Fluconazole | 16 | ||||
| Itraconazole | 1.0 |
Discussion.
Disseminated fungal infections continue to cause significant morbidity and mortality in bone marrow transplant recipients, despite advances in patient management (33, 37). Risk factors for invasive fungal infections in this population include neutropenia, administration of broad-spectrum antimicrobial agents, steroid therapy for GVHD, mucosal barrier disruption, an allogeneic transplant, and an unrelated donor (5, 13, 19). All of these risk factors were present in the patient described here. Aspergillus and Candida species are the most common invasive fungal pathogens identified in bone marrow transplant recipients; however, disseminated Fusarium and Trichosporon infections are being reported with increasing frequency (4, 27, 37).
Five species of the genus Trichoderma (T. harzianum, T. koningii, T. longibrachiatum, T. pseudokoningii, and T. viride) have been identified as etiologic agents of infections in immunocompromised hosts. The lack of pathogenicity of Trichoderma species for the immunocompetent host is well illustrated by a report of the inadvertent infusion of T. viride via contaminated intravenous fluid (31). The patient was treated with amphotericin B for 24 h and remained well except for transient bacteremia.
Ten cases of Trichoderma species infections have been reported in immunocompromised hosts. The report of the initial case from 1976 described the isolation of T. viride from a pulmonary mycetoma in a patient with chronic lung disease (7). Four cases of peritonitis have been reported in continuous ambulatory peritoneal dialysis patients, with T. koningii (25), T. harzianum (11), T. viride (16), and T. longibrachiatum (34) identified as the etiologic agents. Only the patient with T. koningii peritonitis survived. T. viride infection of a perihepatic hematoma in a liver transplant recipient has also been described (12). Although the fungal infection was not the principal cause of death, T. viride was present at autopsy, despite surgical drainage and amphotericin B therapy. An erythroleukemia patient died from a disseminated T. pseudokoningii infection 43 days after bone marrow transplantation (9). A T. longibrachiatum brain abscess in a 17-year-old with ALL was successfully treated with antifungal therapy after surgical resection (32). An 11-year-old patient with aplastic anemia recovered from a T. longibrachiatum skin infection after receiving 3 months of antifungal therapy and a bone marrow transplant (20). Invasive T. longibrachiatum sinusitis in a liver and small-bowel transplant recipient was successfully treated with debridement and antifungal agents (8). The present report is the fifth report of T. longibrachiatum as a cause of infection in an immunocompromised host and the first to suggest the gastrointestinal tract as the portal of entry.
The recovery of T. longibrachiatum from stool surveillance cultures early in the course of the posttransplantation period (day 8) suggests that acquisition of the fungus was through the gastrointestinal tract. Prolonged therapy with fluconazole in addition to antibiotics may have selectively favored the occurrence of this filamentous organism. The progression of disease despite therapy with amphotericin B, itraconazole, and liposomal amphotericin B in appropriate dosage regimens demonstrates the inadequacy of current approaches to therapy of hyalohyphomycosis in this group of patients. In vitro antifungal susceptibility data for this patient correlated with the outcome and lend further credence to the usefulness of antifungal susceptibility testing in the determination of resistance to currently available antifungal agents. Unfortunately, no other therapeutic options were available at the time of this patient’s infection. Previous reports of T. longibrachiatum infections have also documented elevated MICs for most antifungal agents (2, 20, 32, 34), although a favorable outcome was obtained when the administration of amphotericin B was coupled with surgical resection (Table 1).
The genus Trichoderma, first discovered by Persoon in the early 1800s, is a ubiquitous, widely distributed component of the soil microflora. Until the revision of the genus Trichoderma by Rifai (28) in 1969, most species were referred to as either T. viride or T. koningii, depending upon whether they produced globose or oval conidia, respectively. Rifai described nine groups of similar species differentiated primarily by conidiophore branching patterns and conidium morphology. He termed these groups “species aggregates,” one of which was the Trichoderma longibrachiatum Rifai aggregate (28). In the 1980s and early 1990s, Bissett (2, 3) proposed an infrageneric classification of Trichoderma whereby the genus was divided into sections: Trichoderma section Trichoderma, Trichoderma section Pachybasium, Trichoderma section Saturnisporum, Trichoderma section Longibrachiatum, and Trichoderma section Hypocreanum. Key features of the section Longibrachiatum included the following: (i) colonies are rapidly growing and are 6 to 9 cm after 4 days at 20°C; (ii) the reverse of fresh isolates is conspicuously yellowish green; (iii) chlamydospores are present or absent; (iv) conidiophores are sparingly branched, primary branches are long, and secondary branches are usually short and rarely rebranched; (v) phialides are mostly solitary ampulliform to lageniform or cylindrical; and (vi) conidia are one celled, green, smooth walled, and ellipsoidal to obovoid. Species in the section Longibrachiatum included T. citrinoviride, T. pseudokoningii, T. longibrachiatum, and T. atroviride. While Bissett (2, 3) did define specific characteristics that could be used to separate these species, their use without the aid of more recent molecular techniques (14, 15, 17, 18, 35, 36) is fraught with uncertainty. Recent molecular characterization of species previously identified as T. pseudokoningii were in fact found to be T. longibrachiatum on the basis of analysis of ribosomal DNA internal transcribed spacer sequences (15). The same investigators also suggest that the etiologic agent of human mycoses reported to be T. pseudokoningii was most likely T. longibrachiatum because their study of geographic trends indicated that T. pseudokoningii is restricted to Australia and New Zealand as an anamorph of a Hypocrea species (15, 35). Many strains that they received as T. pseudokoningii were found, by their methods, to be either T. citrinoviride or T. longibrachiatum.
Although molecular fingerprinting is necessary for the unequivocal confirmation of an organism as T. longibrachiatum, several characteristics that may be observed in the routine laboratory are suggestive of this species. Useful macroscopic characteristics include its rapid growth and rapid change from white to green, its frequent occurrence in concentric ringlike zones (Fig. 2a), and its lemon yellow diffusing pigment on PFA, although all strains may not be so intense (Fig. 2b). This species is thermotolerant, with good growth observed at both 35 and 42°C. Growth at elevated temperatures is one of the well-known virulence factors of neurotropic fungi, and this organism’s potential neurotropism was documented by brain abscess formation in the patient with ALL described in 1995 (32), as well as focal cerebritis in the patient in the present study. Microscopically, this species is characterized by sparsely formed, often slightly curved conidiophores from the main branch, unlike the pyramidal arrangement in other species aggregates, such as those in T. viride. Unlike the globose to subglobose conidia of T. viride, those in T. longibrachiatum are distinctly oval (Fig. 6). Given the morbidity and mortality associated with this agent in immunocompromised hosts, a preliminary report of a Trichoderma species in the section Longibrachiatum would alert clinicians to its potential pathogenicity.
As the number of profoundly immunosuppressed patients in the hospital and community has escalated, so too has the diversity of molds recognized in association with human infection. It is increasingly clear that the concept of pathogenicity of molds has little meaning in this patient group and that a wide range of normally saprobic molds may cause life-threatening infections in these individuals. The efficacy of stool surveillance cultures for the identification of fungal pathogens in this population and the usefulness of antifungal susceptibility testing for the determination of resistance are illustrated by the case of infection described here. T. longibrachiatum should be added to the list of potentially lethal neurotropic organisms in immunocompromised hosts, and early aggressive therapy should be instituted in patients with infections whose suspected etiologic agents resemble those in the Trichoderma section Longibrachiatum.
Acknowledgments
We are indebted to Gary Samuels (Agricultural Research Service, U.S. Department of Agriculture) for DNA fingerprinting of the isolate and Mary Lindsey for her mycological expertise.
REFERENCES
- 1.Beck-Sague C M, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 2.Bissett J. A revision of the genus Trichoderma. I. Section Longibrachiatum sect. nov. Can J Bot. 1984;62:924–931. [Google Scholar]
- 3.Bissett J. A revision of the genus Trichoderma. IV. Additional notes on section Longibrachiatum. Can J Bot. 1991;69:2418–2420. [Google Scholar]
- 4.Boutati E I, Anaissie E J. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood. 1997;90:999–1008. [PubMed] [Google Scholar]
- 5.Castagnola E, Bussi B, Viscoli C. Fungal infections in patients undergoing bone marrow transplantation: an approach to a rational management protocol. Bone Marrow Transplant. 1996;18(Suppl. 2):97–106. [PubMed] [Google Scholar]
- 6.Dixon D M, McNeil M M, Cohen M L, Gellin B G, La Montagne J R. Fungal infections: a growing threat. Public Health Rep. 1996;111:226–235. [PMC free article] [PubMed] [Google Scholar]
- 7.Escudero Gil M R, Pino Corral E, Munoz Munoz R. Micoma pulmonar causado por Trichoderma viride. Actas Dermo-Sifiliograficas. 1976;67:673–680. [PubMed] [Google Scholar]
- 8.Furukawa H, Kusne S, Sutton D A, Manez R, Carrau R, Nichols L, Abu-Elmagd K, Skedros D, Todo S, Rinaldi M G. Acute invasive sinusitis due to Trichoderma longibrachiatum in a liver and small bowel transplant recipient. Clin Infect Dis. 1998;26:487–489. doi: 10.1086/516317. [DOI] [PubMed] [Google Scholar]
- 9.Gautheret A, Dromer F, Bourhis J H, Andremont A. Trichoderma pseudokoningii as a cause of fatal infection in a bone marrow transplant recipient. Clin Infect Dis. 1995;20:1063–1064. doi: 10.1093/clinids/20.4.1063. [DOI] [PubMed] [Google Scholar]
- 10.Groll A H, Shah P M, Mentzel C, Schneider M, Just-Nuebling G, Huebner K. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect. 1996;33:23–32. doi: 10.1016/s0163-4453(96)92700-0. [DOI] [PubMed] [Google Scholar]
- 11.Guiserix J, Ramdane M, Finielz P, Michault A, Rajaonarivelo P. Trichoderma harzianum peritonitis in peritoneal dialysis. Nephron. 1996;74:473–474. doi: 10.1159/000189374. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs F, Byl B, Bourgeois N, Coremans-Pelseneer J, Florquin S, Depre G, Van de Stadt J, Adler M, Gelin M, Thys J P. Trichoderma viride infection in a liver transplant recipient. Mycoses. 1992;35:301–303. doi: 10.1111/j.1439-0507.1992.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 13.Jantunen E, Ruutu P, Niskanen L, Volin L, Parkkali T, Koukila-Kahkola P, Ruutu T. Incidence and risk factors for invasive fungal infections in allogeneic BMT recipients. Bone Marrow Transplant. 1997;19:801–808. doi: 10.1038/sj.bmt.1700737. [DOI] [PubMed] [Google Scholar]
- 14.Kuhls K, Lieckfeldt E, Borner T. PCR-fingerprinting used for comparison of ex-type strains of Trichoderma species deposited in different culture collections. Microbiol Res. 1995;150:1–9. doi: 10.1016/S0944-5013(11)80017-0. [DOI] [PubMed] [Google Scholar]
- 15.Kuhls K, Lieckfeldt E, Samuels G J, Meyer W, Kubicek C P, Borner T. Revision of Trichoderma sect. Longibrachiatum including related teleomorphs based on analysis of ribosomal DNA internal transcribed spacer sequences. Mycologia. 1997;89:442–460. [Google Scholar]
- 16.Loeppky C B, Sprouse R F, Carlson J V, Everett E D. Trichoderma viride peritonitis. South Med J. 1983;76:798–799. doi: 10.1097/00007611-198306000-00029. [DOI] [PubMed] [Google Scholar]
- 17.Meyer W, Lieckfeldt K, Kuhls K, Freezman Z, Borner T, Mitchell T G. DNA and PCR fingerprinting in fungi. Exp Suppl. 1993;67:311–320. doi: 10.1007/978-3-0348-8583-6_28. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell T G, Sandin R L, Bowman B H, Meyer W, Merz W G. Molecular mycology: DNA probes and application of PCR technology. J Med Vet Mycol. 1994;32(Suppl. 1):351–356. doi: 10.1080/02681219480000961. [DOI] [PubMed] [Google Scholar]
- 19.Morrison V A, Haake R J, Weisdorf D J. Non-Candida fungal infections after bone marrow transplantation: risk factors and outcome. Am J Med. 1994;96:497–503. doi: 10.1016/0002-9343(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 20.Munoz F M, Demmler G J, Travis W R, Ogden A K, Rossmann S N, Rinaldi M G. Trichoderma longibrachiatum infection in a pediatric patient with aplastic anemia. J Clin Microbiol. 1997;35:499–503. doi: 10.1128/jcm.35.2.499-503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium forming filamentous fungi. Proposed standard M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 22.Pfaller M, Wenzel R. Impact of the changing epidemiology of fungal infections in the 1990s. Eur J Clin Microbiol Infect Dis. 1992;11:287–291. doi: 10.1007/BF01962067. [DOI] [PubMed] [Google Scholar]
- 23.Pfaller M A, Marco F, Messer S A, Jones R N. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn Microbiol Infect Dis. 1998;30:251–255. doi: 10.1016/s0732-8893(97)00246-0. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller M A, Bale M, Buschelman B, Lancaster M, Espinel-Ingroff A, Rex J H, Rinaldi M G. Selection of candidate quality control isolates and tentative quality control ranges for in vitro susceptibility testing of yeast isolates by National Committee for Clinical Laboratory Standards proposed standard methods. J Clin Microbiol. 1994;32:1650–1653. doi: 10.1128/jcm.32.7.1650-1653.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ragnaud J M, Marceau C, Roche-Bezian M C, Wone C. Infection peritoneale a Trichoderma koningii sur dialyse peritoneale continue ambulatoire. Med Malad Infect. 1984;7:402–405. [Google Scholar]
- 26.Rex, J. H., M. A. Pfaller, A. L. Barry, P. W. Nelson, and C. D. Webb. 1995. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole vs. amphotericin B as treatment of nonneutropenic patients with candidemia. 39:40–44. [DOI] [PMC free article] [PubMed]
- 27.Rex J H, Walsh T J, Anaissie E J. Fungal infections in iatrogenically compromised hosts. Adv Intern Med. 1998;43:321–371. [PubMed] [Google Scholar]
- 28.Rifai M S. A revision of the genus Trichoderma. Mycol Papers. 1969;116:1–56. [Google Scholar]
- 29.Rinaldi M G. Use of potato flakes agar in clinical mycology. J Clin Microbiol. 1982;15:1159–1160. doi: 10.1128/jcm.15.6.1159-1160.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinaldi M G. Emerging opportunists. Infect Dis Clin N Am. 1989;3:65–77. [PubMed] [Google Scholar]
- 31.Robertson M H. Fungi in fluids—a hazard of intravenous therapy. J Med Microbiol. 1970;3:99–102. doi: 10.1099/00222615-3-1-99. [DOI] [PubMed] [Google Scholar]
- 32.Seguin P, Degeilh B, Grulois I, Gacouin A, Maugendre S, Dufour T, Dupont B, Camus C. Successful treatment of a brain abscess due to Trichoderma longibrachiatum after surgical resection. Eur J Clin Microbiol Infect Dis. 1995;14:445–448. doi: 10.1007/BF02114902. [DOI] [PubMed] [Google Scholar]
- 33.Serody J S, Shea T C. Prevention of infections in bone marrow transplant recipients. Infect Dis Clin N Am. 1997;11:459–477. doi: 10.1016/s0891-5520(05)70365-2. [DOI] [PubMed] [Google Scholar]
- 34.Tanis B C, van der Pijl H, van Ogtrop M L, Kibbelaar R E, Chang P C. Fatal fungal peritonitis by Trichoderma longibrachiatum complicating peritoneal dialysis. Nephrol Dial Transplant. 1995;10:114–116. [PubMed] [Google Scholar]
- 35.Turner D, Kovcs W, Kuhls K, Lieckfeldt E, Peter B, Arisan-Atac I, Strauss J, Samuels G J, Borner T, Kubicek C P. Biogeography and phenotypic variation in Trichoderma sect. Longibrachiatum and associated Hypocrea species. Mycol Res. 1997;101:449–459. [Google Scholar]
- 36.van Belkum A. DNA fingerprinting of medically important microorganisms by use of PCR. Clin Microbiol Rev. 1994;7:174–184. doi: 10.1128/cmr.7.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walter E A, Bowden R A. Infection in the bone marrow transplant recipient. Infect Dis Clin N Am. 1995;9:823–847. [PubMed] [Google Scholar]