Abstract
The human brain is a highly plastic ‘complex’ network—it is highly resilient to damage and capable of self-reorganisation after a large perturbation. Clinically, neurological deficits secondary to iatrogenic injury have very few active treatments. New imaging and stimulation technologies, though, offer promising therapeutic avenues to accelerate post-operative recovery trajectories. In this study, we sought to establish the safety profile for ‘interventional neurorehabilitation’: connectome-based therapeutic brain stimulation to drive cortical reorganisation and promote functional recovery post-craniotomy. In n = 34 glioma patients who experienced post-operative motor or language deficits, we used connectomics to construct single-subject cortical networks. Based on their clinical and connectivity deficit, patients underwent network-specific transcranial magnetic stimulation (TMS) sessions daily over five consecutive days. Patients were then assessed for TMS-related side effects and improvements. 31/34 (91%) patients were successfully recruited and enrolled for TMS treatment within two weeks of glioma surgery. No seizures or serious complications occurred during TMS rehabilitation and 1-week post-stimulation. Transient headaches were reported in 4/31 patients but improved after a single session. No neurological worsening was observed while a clinically and statistically significant benefit was noted in 28/31 patients post-TMS. We present two clinical vignettes and a video demonstration of interventional neurorehabilitation. For the first time, we demonstrate the safety profile and ability to recruit, enroll, and complete TMS acutely post-craniotomy in a high seizure risk population. Given the lack of randomisation and controls in this study, prospective randomised sham-controlled stimulation trials are now warranted to establish the efficacy of interventional neurorehabilitation following craniotomy.
Subject terms: Surgical oncology, CNS cancer
Introduction
The human brain is a highly plastic ‘complex’ network1,2: it self-organises without a hard blueprint, it adapts to evolving circumstances, and can withstand external insults. Our thoughts and behaviour are directly governed by how our brain networks handle, orchestrate, and execute various internal and external demands3. Nevertheless, similar to other naturally-occurring networks, brain networks can only endure a finite amount of damage before becoming maladaptive and fragmented4.
The practice of neurosurgery is based on therapeutically altering the brain’s global workspace to improve clinical outcomes with resective or stimulation surgery5,6. However, since the antiquity of neurosurgery, few strategies have been employed to directly address neurological deficits due to iatrogenic injury. In fact, the usual approach is to send patients to physiotherapy and hope they improve over time in a sufficiently stimulating environment. Moreover, rehabilitation is further complicated when surgical pathology implicates critical areas for motor initiation, alertness, motivation, and consciousness7. Furthermore, advanced neurocomputational models suggest the capacity for neuroplasticity greatly varies based on the type of cortical damage which has occurred8. Ideally, a fundamental goal of neuro-oncological surgery should be to drive cortical reorganisation and promote functional recovery in the immediate post-operative period. To advance this viewpoint, we coin a new concept called ‘interventional neurorehabilitation’: connectome-based therapeutic brain stimulation to promote network plasticity and functional recovery.
Over the past few years, monumental advancements have been made in neuroimaging and neurostimulation technologies. Today, state-of-the art connectome methods enable neuroscientists to make highly accurate single-subject predictions on cognition9–11. In addition, we are beginning to non-invasively stimulate focally at-depth without perturbing overlaying cortical structures12. However, before leveraging the most advanced technologies for interventional neurorehabilitation13, applying well-studied existing stimulation approaches is sensible. Repetitive transcranial magnetic stimulation (rTMS) is a Food and Drug Administration (FDA)-approved stimulation therapy routinely performed at hospitals across the world14. Given its relative ease and non-invasiveness, the field of TMS has flourished to treat a range of neurological and psychiatric illnesses. In acute and chronic stroke patients, rTMS facilitates cortical reorganization leading to functional preservation or compensation in motor and language abilities15. Unfortunately, prognosis is still poor in many these patients, which may be explained by the limited capacity for effective cerebral plasticity following some acute injuries compared to slow growing tumors8. While meta-analyses highlight the remarkable safety of rTMS in ischemic stroke patients with extremely low-risks for seizures16,17, there remains limited descriptions on the safety and efficacy of this treatment modality in tumor patients in the acute post-operative period. Given the striking advances in fields outside neuro-oncology, individualised TMS therapy merits investigation to accelerate recovery trajectories post-craniotomy.
In this proof-of-concept study, we sought to establish the safety profile and ability to recruit, enroll, and complete connectome-guided TMS to enhance network plasticity and promote functional recovery following glioma surgery.
Methods
This study was approved by the Human Research Ethics Committee of the South Eastern Sydney Local Health District (SESLHD). Patients provided written informed consent prior to enrolling in our study. All methods were performed in accordance with relevant guidelines and regulations Declaration of Helsinki.
Patient population
Patients with supratentorial gliomas who developed a significant post-operative neurological deficit related to motor or language function were invited to take part in an off-label treatment of FDA-approved rTMS. Subjects were only included in this study if TMS was initiated within two weeks post-surgery. Assessments for motor dysfunction were made using the standard Medical Research Council (MRC) 5-point scale18. To be eligible for rTMS therapy, weakness in an arm or leg needed to be 4−/5 or worse in the hand, proximal arm, foot or proximal leg at the time of treatment. Language dysfunction was defined using the Aphasia Rapid Test (ART) with a score greater than 3 considered evidence of significant language disturbance19.
Clinical assessment and definition of outcomes
Neurological assessments were performed immediately prior to treatment with rTMS and one week following the last rTMS session by a blinded team member. Improvement in motor function was defined as grade strength to at least 4+/5 in the affected limb, with either functional hand control or the ability to walk with assistance in the leg. In cases of hemiplegia, improvement in either hand or leg function was considered improvement. Finally, reduction in the patient’s pre-treatment ART score by 3 or more points was considered improvement in language.
Connectome-based TMS target selection in neurosurgical patients
Once recruited, participants underwent a T1-weighted MPRAGE and resting-state fMRI (rsfMRI) scan. The cortical target was selected based on the patient’s primary deficit (i.e. motor or language), our interpretation of any network fragmentation, and our experience with network topology from normative connectomes (i.e. Human Connectome Project [HCP] data)7,20,21.
Imaging acquisition and pre-processing parameters
The resting-state fMRI was performed on a Phillips 3 T Achieva which was acquired as a T2-star EPI sequence, with 3 × 3 × 3-mm voxels, 128 volumes/run, a TE = 27 ms, a TR = 2.8 s, a field of view = 256 mm, a flip angle = 90° and an 8 min total run time. Resting-state and diffusion pre-processing was performed using in-house custom machine learning algorithms in Python. Standard image processing steps included skull stripping, motion correction with a 6-dimensional rigid body registration, correcting for physiological noise (CompCor), slice time correction, spatial smoothing (6 FWHM Gaussian kernel), high-pass filtering, and co-registration to the patient’s structural space1. Of critical importance, we do not warp the brain into a standard space like Montreal Neurological Institute (MNI) or Talairach space at any stage of the processing. The patients then had a diffusion sequence acquired for subsequent connectivity analyses from patient-specific multi-modal imaging data.
Machine-learning aided parcellation for brain tumours
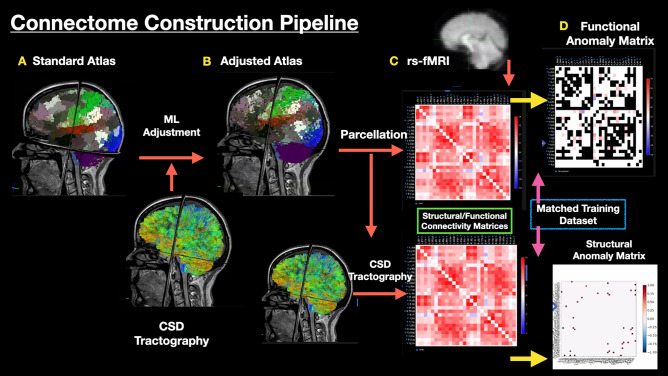
A fundamental challenge for interventional neurorehabilitation post-craniotomy is to apply a parcellation scheme to highly-distorted anatomical brains. The Glasser HCP parcellation scheme is a state-of-the art multi-modal neurobiological division of the cerebral cortex22. However, it was not designed to be applied to brains with large lesions and oedema. We aimed to directly address this challenge by determining new HCP parcellation locations by using a proprietary machine learning algorithm (Omniscient Technologies)—Fig. 1 is the connectome construction pipeline and Fig. 2 represents sample outputs. Using a supervised machine learning approach, we first trained our algorithms to identify each HCP parcel using network connectivity from a normative dataset. Then, we applied our machine to identify the most appropriate HCP parcels in brains after supratentorial tumour surgery based on the same input imaging data. To our knowledge, this approach is unique in that previous studies have resolved this issue by applying the HCP parcellation derived from healthy brains without any adjustment to cortical topology.
Figure 1.
The connectome construction pipeline used in this study. (A) A standard Glasser atlas was established using 300 healthy individuals from the Human Connectome Project (HCP). A supervised machine learning algorithm was employed to recognise connectivity patterns for each of the 360 HCP parcels in a healthy cohort. (B) Using diffusion sequences, we applied constrained spherical deconvolution (CSD) tractography to our patient cohort. Using these images, our algorithm was applied to recognise and adjust the locations of HCP parcels in highly atypical brains. (C) After establishing maximal likely structural connectivity, we used this data to inform and constrain functional connectivity using resting-state fMRI. (D) Finally, structural and functional anomaly matrices were generated to compare network connectivity differences (i.e. language) between our patient and a normative atlas. Adopted with permission from Reference23.
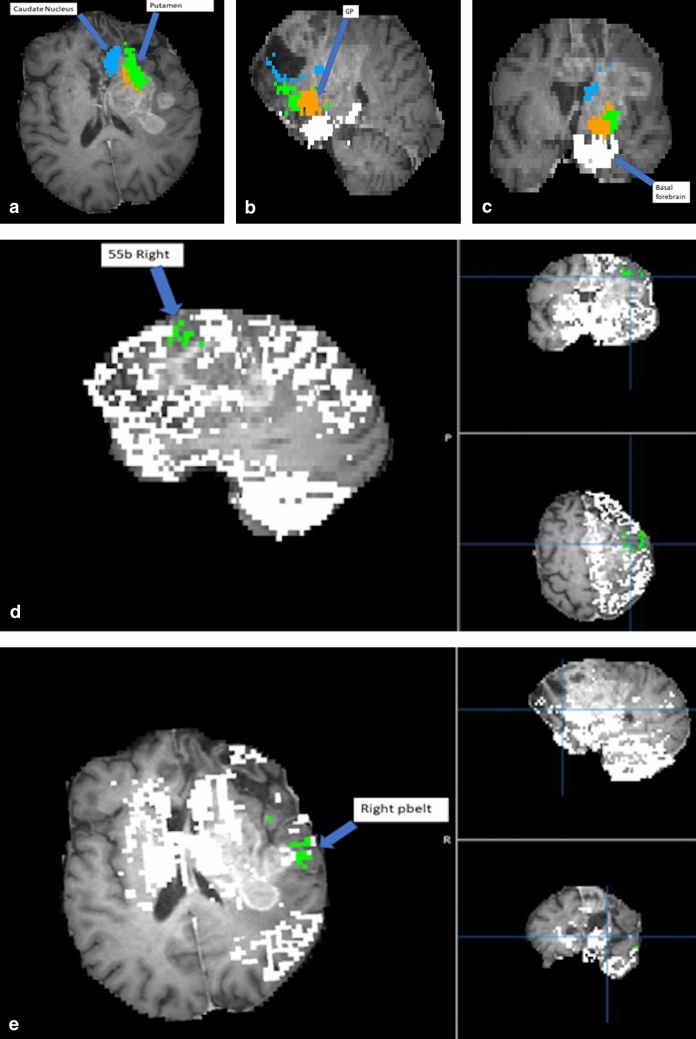
Figure 2.
Demonstration of proprietary machine learning algorithm (Omniscient) that assigns parcellations to very distorted brains. Patient with a frontal lobe GBM and resected regions resulting in total anterior brain shift. (a) Displays the modified location of the caudate nucleus and the putamen. (b) Displays the modified location of the GP. (c) Displays the modified location of the basal forebrain. (d) Displays the modified location of right 55b parcellation. (e) Displays the modified location of the right PBelt. This allows for the creation of a connectivity matrix of any brain despite.
Comparative connectome analyses
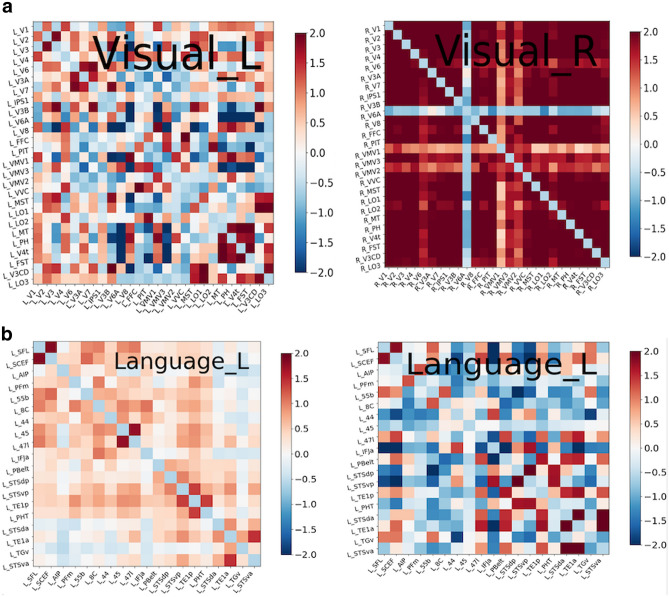
To gain additional insight into network connectivity, we processed n = 300 HCP connectomes to serve as a reference of healthy canonical brain network organisation. Using this normative data, we qualitatively compared healthy networks to those observed in patients with lesions in particular areas. For example, we compared the normative visual areas to a patient with hemianopia (Fig. 3a) or normative language network topology with that of a patient with aphasia (Fig. 3b). This intra-network analysis enabled us to perform a hypothesis-driven neuro-navigated rTMS target selection.
Figure 3.
(a) compares connectivity matrices of the left and right visual networks in a patient with hemianopia. The left visual network is dotted mostly blue, which means that areas of the visual system are not well synchronized to one another. By comparison, the right visual network displays strong intra-network connectivity. (b) compares the connectivity matrix of the language area of a healthy control on the left with the language area connectivity of an aphasic patient on the right. This aphasic matrix has the parcellations within the language system anticorrelated, therefore, predominantly blue, suggestion loss of connectivity within the language network. Note that columns 55b, 45 and STSdp are blue representing that they are isolated. We hypothesized that this is in part due to problems with the superior longitudinal fasciculus/ arcuate fasciculus system which links different components of the language system7. Conducting connectomics analysis by comparing connectivity matrices enables us to generate potential targets for TMS treatment.
rTMS treatment paradigm
The rTMS treatment was initiated within 1–2 weeks after standard awake glioma surgery. We utilized theta burst stimulation (TBS) protocols in all patients. Details of the TMS protocol and rationale available in the SI. We performed treatment five times per day over five consecutive days. In between TMS sessions, patients underwent rehabilitative therapy.
Complications and adverse events
All complications and side effects were systematically noted after each rTMS session and 1-week post-treatment. Seizures were defined as any observable seizure or possible seizure-like activity during the course of treatment. Neurological complications included any new or worsening of neurological dysfunction measured by the ART and MRC Motor scale.
Results
Recruitment data regarding rTMS treatment in neurosurgical patients
We successfully recruited 31/34 (91%) patients within two-weeks after glioma surgery and treated them with rTMS. The median participant age was 58 years with 20 females and 14 males. 30 patients had World Health Organization (WHO) grade II–IV gliomas, while four patients had low grade gliomas. Three patients had a history of seizures prior to surgery and were on anti-seizure medications. Of all the participants, n = 23 began rTMS therapy within a week of surgery, and n = 31 began within 2 weeks of surgery. The remaining 3 participants underwent treatment at 2 months, 4 months and 12 months and excluded in the recruitment rate citing logistic concerns. In total, 31 participants completed all planned treatment sessions with one participant missing one rTMS session due to a rehabilitation bed becoming available the day of their last scheduled treatment. No participant stopped therapy due to treatment intolerance. In 21 participants with a motor deficit, rTMS was applied to the sensorimotor network with an improvement noted in 19 patients after 1-week following the last TMS session. In 13 participants with a language deficit, rTMS was applied to the frontoparietal network with an improvement in 12 patients after 1-week of the final stimulation session.
Safety and preliminary efficacy of rTMS in neurosurgical patients
No participants reported any general or partial seizures or seizure-like events during the course of treatment and follow-up. Four patients reported transient headaches which resolved at the end of each individual session. Light headedness (n = 1) and nausea (n = 1) was also reported but resolved before the start of the next session. Transient tingling was reported at the site of stimulation during stimulation onset, but also resolved immediately. These results are consistent with well-documented side-effects during rTMS of non-craniotomy patients13,17,24. We noted no worsening of neurological deficits and no other obvious side effects. While our study was not designed to answer timing questions, there were no differences in safety outcomes between the groups who started rTMS therapy one week versus two weeks post-surgery. The Supplemental Digital Content is a video of a typical procedure; the participants consented to publication of his/her image. Two brief clinical vignettes are presented below.
Statistical analyses of neurological scores following rTMS
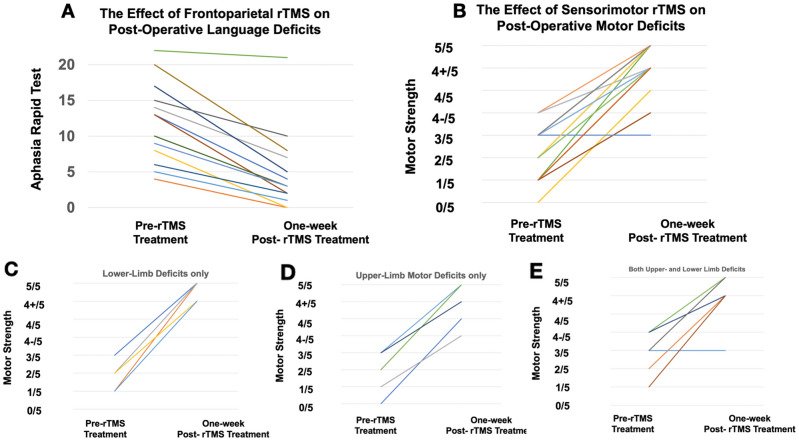
Neurological scores from patients in this study who experienced a post-operative language or motor deficit is presented in Fig. 4. Statistical analysis was performed using the non-parametric Wilcoxon signed ranked test given that the observations were paired ordinal data. Figure 4A demonstrates a significant decrease in the ART 1-week following the last TMS session (p = 1.48 × 10–3); these patients experienced post-operative language deficits and underwent frontoparietal TMS stimulation. Figure 4B demonstrates a significant increase in the MRC motor strength scale 1-week following the last TMS session (p = 8.0 × 10–5); these patients experience post-operative motor deficits and underwent sensorimotor TMS stimulation. Finally, Fig. 4C-E represents the data with patients with only lower-limb (Fig. 4C) or upper-limb (Fig. 4D), or both (Fig. 4E) limb deficits.
Figure 4.
Neurological data (both language and motor) pre-rTMS treatment and 1-week post-rTMS treatment. (A) demonstrates a significant decrease in the ART 1-week following frontoparietal rTMS (p = 1.48 × 10–3. (B) demonstrates a significant increase in the MRC motor strength scale 1-week following sensorimotor rTMS (p = 8.0 × 10–5). (C–E) represents the data with patients with only lower-limb (C) or upper-limb (D), or both (E) limb deficits.
Clinical vignettes
Case 1
A 63-year-old woman with a left parietal glioblastoma presented with preoperative aphasia and near complete hemiplegia. Following resection, she developed complete expressive aphasia and right hemiplegia. Connectome analyses revealed that her sensorimotor networks were fragmented as two independent parcellations, likely due to the destruction of the callosal fibers. Specifically, the injured side demonstrated satellite areas anterior to the dysfunctional sensorimotor networks (Fig. 5). Additionally, the left frontoparietal network revealed a clear component of Broca’s area, area 55b, and an Supplementary Motor Area (SMA) component. However, the temporal component appeared to be less organized, appearing abnormal compared to normative data. Thus, to potentially enhance functional recovery and address both delocalised networks, we sought to select a stimulation target that would lead to enhanced network recruitment.
Figure 5.
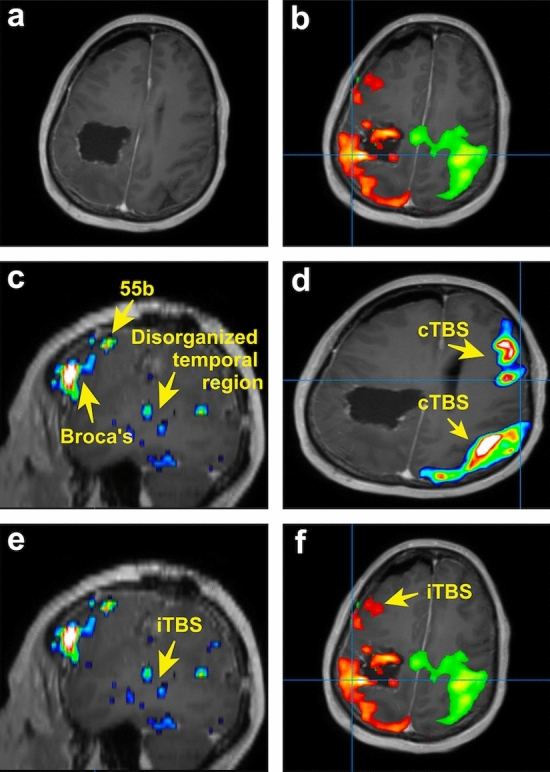
TMS strategy for patient presenting with aphasia and near complete hemiplegia secondary to glioblastoma. (a) Postoperative MRI of patient demonstrating resection cavity. (b) Independent right sided (green) and left sided (orange) sensorimotor networks. Although presented on the same image, these networks appeared as separate networks on connectomic analysis. The anterior satellite areas in the left (orange) dysfunctional sensorimotor network. (c) Left frontoparietal network demonstrating clear Broca’s area and area 55b. The temporal component of the network is disorganized. (d) cTBS was administered to both the middle of the sensorimotor network and the right posterior frontal component of the right frontoparietal network. (e) iTBS was administered to the disorganized temporal component of the left frontoparietal network and the (f) anterior areas of the pathological left sensorimotor network.
Beginning on post-operative day five, we performed five days of daily continuous TBS (cTBS) to both the middle of the right sensorimotor network and the posterior frontal component of the right frontoparietal network (both targets treated once per day). We then performed intermittent TBS (iTBS) to the areas of scattered activation in the posterior left temporal lobe and the areas near the abnormal sensorimotor regions. This treatment was well tolerated, and by the end of the treatment, the patient was able to ambulate with a cane and speak in full sentences. There were no serious complications, however, she had some persistent arm weakness.
Case 2
A 71-year-old man with a posterior left insular glioblastoma had moderate pre-operative expressive aphasia that persisted post-surgery. Connectome analyses demonstrated that his posterior temporal region was appropriately organized but did not co-activate within the same network as Broca’s area (Fig. 6)6. Thus, we hypothesized that this was the result of inactivation of the arcuate fasciculus fibers by the tumor or related to oedema. We also noted that he was recruiting the right analog of Broca’s area, as both regions were functionally co-activated. As a result, we chose to perform accelerated (spaced-delivery of stimulation25) iTBS to the left posterior temporal site to enhance the recruitment of additional connections for speech improvement. This treatment began on post-operative day four. At the end day five of rTMS, his speech markedly improved with no complications to report. Nevertheless, he persisted with residual paraphasia after his therapy.
Figure 6.
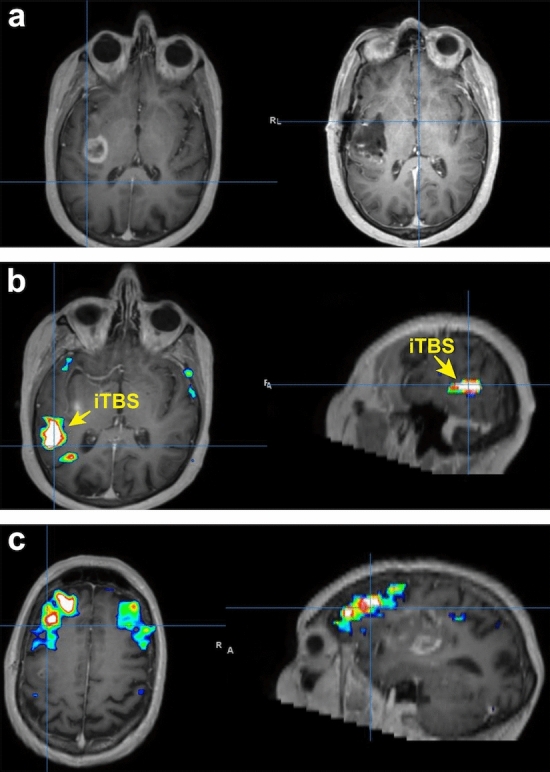
TMS strategy for patient presenting with moderate expressive aphasia secondary to glioblastoma. (a) Preoperative MRI (left) demonstrating left insula glioblastoma and postoperative MRI (right) demonstrating complete resection. (b) Network analysis demonstrating a strongly organized posterior temporal region that is not in in the same network as Broca’s area. This was the area that was selected for treatment with iTBS. (c). Further network analysis demonstrating Broca’s area with bilateral representation that is not in the same network as the posterior temporal region.
Discussion
In this study, we demonstrate the safety of rTMS post-craniotomy with the goal of promoting functional recovery. Specifically, we demonstrate that no seizures were induced in 31 patients post-craniotomy and transient side effects were reported in 6 patients. This work complements safety data from dozens of rTMS studies completed in non-craniotomy individuals26,27. Despite the uncontrolled and open-label nature of the study, we cautiously interpret that rTMS can potentially facilitate functional recovery post-craniotomy.
Similar results have been illustrated in acute and chronic stroke patients suggesting the possible role of TMS as a therapeutic modality for a variety of clinical conditions to facilitate motor and language improvement15. Given the widely demonstrated safety profile of TMS, it would be a disservice not to further investigate the efficacy of technology to optimize post-surgical clinical outcomes. To fully harness interventional neurorehabilitation’s potential for neuro-oncological care, additional research is required in two areas: target engagement and simulation protocol. Here, we elucidate the role of individualized TMS in standard inpatient rehabilitation and discuss implications for future study on rTMS to optimize clinical outcomes.
Importance of target engagement and stimulation protocol
There is a growing body of evidence suggesting that effective TMS targeting is critical for success. For example, using image guidance to target rTMS improves efficacy28. Furthermore, targeting brain networks affected by disease-related processes is crucial for functional improvement. Recently, Momi and colleagues delivered TMS pulses to two frontoparietal nodes (prefrontal and parietal) to enhance fluid intelligence tasks24—adding another research consideration on multi-nodal, rather than uni-nodal, stimulation. In addition, it is likely that different patients with the same clinical deficit may need different target(s)29. Hence, there are many ways to interpret these observations, however, we advocate establishing “the right target for the right patient” as being critical to successful interventional rehabilitation.
Similar to target engagement, stimulation protocol is another important variable to consider. There are numerous different TMS protocols available for use. However, TBS protocols are better suited for neurosurgical patients. First, the lower stimulus intensities used in TBS likely have a lower seizure risk30. Second, TBS protocols achieve similar effects with shorter treatment times (typically 8 min per session) compared to standard 30 min with 10 Hz TMS protocols. This enables the use of accelerated protocols (spaced-delivery of stimulation sessions25) which are useful in treating patients in a subacute paradigm. Finally, the stimulation effects of TBS is believed to last 45–60 min which may fit better when coordinating inter-session rehabilitation31,32. Our view is that while seizures are a concern, given our clinical experience in managing this problem concomitant with the low occurrence rate of this complication, there is now sufficient evidence to justify offering neurosurgical patients rTMS.
Connectome-based stimulation for cognitive rehabilitation
In this study, we primarily focused on ameliorating motor and language deficits post-surgery in glioma patients. However, many patients experience cognitive deficits post-surgery and there are no clear guidelines on how to help these patients. The multiple demand (MD) system is a domain-general cognitive control network that acts as a skeleton for executing cognitive tasks3,7. Systematically studying this system and the implications of its removal during surgery would be useful for predicting post-operative cognitive trajectories33. More broadly, despite motor or language deficits in our cohort, the qualitative fundamental motivation to rehab greatly varied between our individuals34. An increasing line of evidence suggests that increasing the motivation to expend cognitive effort, rather than enhancing cognitive networks themselves, would be more effective in bolstering goal-directed behaviour35. Thus, if frontostriatal circuitry can be mapped and effectively modulated post-craniotomy, this would be a significant advancement and become important for other areas of neurosurgery, such as psychiatric surgery36,37.
More broadly, accelerating cognitive rehabilitation with connectome-based stimulation requires precision mapping and timely intervention38. This is particularly challenging in the context of neurosurgery given the highly atypical anatomy routinely encountered. Traditional approaches to this problem have typically involved parcellating the anatomically distorted brain with templates from standard space. While this approach is a reasonable to probe the connectome at the group-level, it is of insufficient accuracy for surgical planning or interventional neurorehabilitation at the single-subject level. In this study, we utilized a machine learning algorithm our group recently developed to parcellate the anatomically distorted cortex39,40. In brief, exploiting the principle that the functional relevance of a brain area is dependent on its connectivity with neighbouring brain areas1,2, we developed a connectivity-based ‘re-parcellation’ scheme that first learned voxel-to-parcel affiliation using diffusion data from a large normative dataset. This algorithm was then applied to classify voxel-to-parcel affiliation in anatomically distorted brains (i.e. due to pathology or surgery) which yielded clinically- and surgically-relevant results40. Ultimately, we envision that machine learning guided re-parcellation of anatomically distorted brains will increase the accuracy of interventional neurorehabilitation and clinical outcomes.
Insights from stroke rTMS therapy for neurosurgical rehabilitation
The literature on the role of TMS in motor and language functional recovery is well-established in stroke patients, providing most of our insight into the current benefits and limitations of this therapeutic modality. Thus, certain themes from stroke neurology may cautiously applied to neuro-oncological patients to guide therapeutic stimulation. For example, cerebral inflammation and angiogenesis are two of multiple overlapping processes between glioma surgery and cerebral ischemic stroke pathways41. A recent meta-analysis on 841 patients across 20 randomized controlled trials (RCTs) demonstrates that rTMS is beneficial to the treatment of post-stroke hemiplegia, especially in: lower limb functioning, grip strength, and attenuating stroke severity16. Interestingly, cortical reorganization can be observed between primary motor and secondary motor cortices in stroke patients to facilitate improved motor functioning, leading authors to suggest the need for future customized TMS applications based on the newly activated cortical pathways in these stroke patients42. Similar cortical re-organization has also been demonstrated to facilitate language functioning following ischemic stroke43. Thus, following iatrogenic injury due to tumor resection, it is likely that similar cortical pathways responsible for motor functioning and motor learning can also be strengthened with rTMS given they too demonstrate plasticity following tumor growth44,45. Indeed, our study shows that intra-network analyses can safely enable hypothesis-driven neuro-navigated rTMS target selections in perioperative environments46,47.
Study limitations
This study, however, is not without key limitations. First, the uncontrolled and early-nature of our intervention raises the possibility whether these patients would have improved without treatment. Now that we have established the TMS safety profile and recruitment rate for this complex patient population, future trials should employ prospective, randomised, double-blinded active- and sham-controlled TMS to determine the efficacy of improving recovery trajectories. While glioma patients are highly heterogenous given different tumors, different degrees of cortical reorganisation, and different resections completed, strict inclusion criteria and multi-site collaborations can overcome such limitations. An alternative approach would be to conduct large-scale international prospective registry-based studies. Second, to be included in our trial, patients must have had an MRC score of 4-/5 or worse in the arm or leg while an improvement was noted as 4+/5. While this difference may be subtle, majority of our patients were not antigravity pre-treatment and hence improvement was not subtle in most cases. Finally, we did not acquire long-term outcome data in these patients. Nevertheless, qualitatively, all patients reported they would undergo TMS therapy again and found that it assisted with their rehabilitative efforts. Despite these limitations, our primary aim was to establish the safety profile and recruitment rate for TMS post-craniotomy.
Conclusion
In conclusion, we present a proof-of-concept of ‘interventional neurorehabilitation’ for neuro-oncological clinicians to take charge in driving cortical reorganisation and functional recovery. Specifically, we demonstrate the safety profile and recruitment rate for connectome-based TMS acutely post-surgery for glioma patients. Given the clear enthusiasm from our patients, we believe that TMS treatment is of low-risk, well-tolerated, and could be of immense therapeutic benefit.
Supplementary Information
Acknowledgements
AP is funded through a Turing Doctoral Scholarship from the Alan Turing Institute and the National Science and Engineering Research Council of Canada. RRG is supported by a Guarantors of Brain Fellowship.
Author contributions
Conceptualization: A.P., I.M.Y. and M.E.S. Data curation: C.P., I.M.Y. Formal analysis: A.P., I.M.Y. Methodology, A.P., R.G.B., M.E.S.; Project administration, I.M.Y., C.T. and M.E.S.; Software S.A.A., K.C., R.R.G.; Writing—original draft, A.P.; Writing—review and editing, N.B.D., J.S., M.E.S.
Competing interests
IMY is an employee of Cingulum Health. MES is the chief medical officer of Omniscient and a shareholder of Cingulum Health. CT is a consultant for Aesculap. All other authors report no conflict of interest related to this study.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06766-8.
References
- 1.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 2.van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn. Sci. 2013;17(12):683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Duncan J. The multiple-demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends Cogn. Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 4.van den Heuvel MP, Sporns O. A cross-disorder connectome landscape of brain dysconnectivity. Nat. Rev. Neurosci. 2019;20:435–446. doi: 10.1038/s41583-019-0177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart MG, Romero-Garcia R, Price SJ, Suckling J. Global effects of focal brain tumors on functional complexity and network robustness: A prospective Cohort study. Neurosurgery. 2019;84:1201–1213. doi: 10.1093/neuros/nyy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivanishvili Z, Poologaindran A, Honey CR. Cyclization of motor cortex stimulation for neuropathic pain: A prospective, randomized, blinded trial. Neuromodulation. 2017;20(5):497–503. doi: 10.1111/ner.12610. [DOI] [PubMed] [Google Scholar]
- 7.Poologaindran A, Lowe SR, Sughrue ME. The cortical organization of language: Distilling human connectome insights for supratentorial neurosurgery. J. Neurosurg. 2020;134(6):1959–1966. doi: 10.3171/2020.5.JNS191281. [DOI] [PubMed] [Google Scholar]
- 8.Keidel JL, Welbourne SR, Lambon Ralph MA. Solving the paradox of the equipotential and modular brain: A neurocomputational model of stroke vs. slow-growing glioma. Neuropsychologia. 2010;48:1716–1724. doi: 10.1016/j.neuropsychologia.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Cui Z, et al. Individual variation in functional topography of association networks in youth. Neuron. 2020;106:340–353 e348. doi: 10.1016/j.neuron.2020.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, Constable RT, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015;18(11):1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaty RE, et al. Robust prediction of individual creative ability from brain functional connectivity. Proc. Natl. Acad. Sci. U. S. A. 2018;115:1087–1092. doi: 10.1073/pnas.1713532115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grossman N, et al. Noninvasive deep brain stimulation via temporally interfering electric fields. Cell. 2017;169:1029–1041 e1016. doi: 10.1016/j.cell.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honey CR, Krüger MT, Almeida T, Rammage LA, Tamber MS, Morrison MD, Hu A. Thalamic deep brain stimulation for spasmodic dysphonia: A phase I prospective randomized double-blind crossover trial. Neurosurgery. 2021;89(1):45–52. doi: 10.1093/neuros/nyab095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunoni AR, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: A systematic review with network meta-analysis. JAMA Psychiat. 2017;74:143–152. doi: 10.1001/jamapsychiatry.2016.3644. [DOI] [PubMed] [Google Scholar]
- 15.Hoyer EH, Celnik PA. Understanding and enhancing motor recovery after stroke using transcranial magnetic stimulation. Restor. Neurol. Neurosci. 2011;29:395–409. doi: 10.3233/RNN-2011-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, Li K, Chen Q, Yin J, Bai D. Understanding and enhancing motor recovery after stroke using transcranial magnetic stimulation. Am J Phys Med Rehabil. 2020;99(2):99–108. doi: 10.1097/PHM.0000000000001277. [DOI] [PubMed] [Google Scholar]
- 17.Lefaucheur, J. P. et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). [DOI] [PubMed]
- 18.Brain, M. R. C. G. O. Aids to the Examiantion of the Peripheral Nervous System. (Balliere Tindall, 1986).
- 19.Azuar C, et al. The aphasia rapid test: An NIHSS-like aphasia test. J Neurol. 2013;260:2110–2117. doi: 10.1007/s00415-013-6943-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Garcia R, et al. Practical application of networks in neurosurgery: Combined 3-dimensional printing, neuronavigation, and preoperative surgical planning. World Neurosurg. 2020;137:e126–e137. doi: 10.1016/j.wneu.2020.01.085. [DOI] [PubMed] [Google Scholar]
- 21.Briggs RG, et al. A connectomic atlas of the human cerebrum-chapter 18: The connectional anatomy of human brain networks. Oper. Neurosurg. (Hagerstown) 2018;15:S470–S480. doi: 10.1093/ons/opy272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glasser MF, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Huixia, Zhu Jin, Su Xiaolin, Chen Siyan, Zeng Silin, Lan Xiaoyong, Zou Liang-Yu, Sughrue Michael E., Guo Yi. Application of structural and functional connectome mismatch for classification and individualized therapy in alzheimer disease. Frontiers in Public Health. 2020;8:720. doi: 10.3389/fpubh.2020.584430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Momi D, et al. Cognitive enhancement via network-targeted cortico-cortical associative brain stimulation. Cereb. Cortex. 2020;30:1516–1527. doi: 10.1093/cercor/bhz182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole EJ, et al. Stanford Accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am. J. Psychiatry. 2020;177:716–726. doi: 10.1176/appi.ajp.2019.19070720. [DOI] [PubMed] [Google Scholar]
- 26.Di Iorio, R. & Rossini, P. M. In Navigated Transcranial Magnetic Stimulation in Neurosurgery (ed Sandro M. Krieg) 67–83 (Springer, 2017).
- 27.Rossi R, Hallett SS, Rossini P, Pascual-Leone C, Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahdab R, Ayache SS, Brugieres P, Goujon C, Lefaucheur JP. Comparison of "standard" and "navigated" procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiol. Clin. 2010;40:27–36. doi: 10.1016/j.neucli.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Drysdale AT, et al. Erratum: Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017;23:264. doi: 10.1038/nm0217-264d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oberman L, Edwards D, Eldaief M, Pascual-Leone A. Safety of theta burst transcranial magnetic stimulation: A systematic review of the literature. J. Clin. Neurophysiol. 2011;28:67–74. doi: 10.1097/WNP.0b013e318205135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wischnewski M, Schutter DJ. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimul. 2015;8:685–692. doi: 10.1016/j.brs.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Wilson MT, et al. Biophysical modeling of neural plasticity induced by transcranial magnetic stimulation. Clin. Neurophysiol. 2018;129:1230–1241. doi: 10.1016/j.clinph.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Briggs RG, et al. The frontal aslant tract and supplementary motor area syndrome: Moving towards a connectomic initiation axis. Cancers. 2021 doi: 10.3390/cancers13051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satterthwaite TD, Xia CH, Bassett DS. Personalized neuroscience: Common and individual-specific features in functional brain networks. Neuron. 2018;98:243–245. doi: 10.1016/j.neuron.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Westbrook A, et al. Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Science. 2020;367:1362–1366. doi: 10.1126/science.aaz5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurwitz TA, Honey CR, McLeod KR, Poologaindran A, Kuan AJ. Hypoactivity in the paraterminal gyrus following bilateral anterior capsulotomy. Can. J. Psychiatry. 2020;65:46–55. doi: 10.1177/0706743719874181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemmings W, Hariz M, Visser-Vandewalle V, Zrinzo L, Coenen VA, Sheth SA, Bervoets C, et al. Deep brain stimulation for refractory obsessive-compulsive disorder (OCD): Emerging or established therapy? Mol. Psychiatry. 2021;26(1):60–65. doi: 10.1038/s41380-020-00933-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volz LJ, et al. Shaping early reorganization of neural networks promotes motor function after stroke. Cereb. Cortex. 2016;26:2882–2894. doi: 10.1093/cercor/bhw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doyen, S. et al. Connectivity-based parcellation of normal and anatomically distorted human cerbral cortex. Hum. Brain Mapp. Published online November 26, 2021. [DOI] [PMC free article] [PubMed]
- 40.Yeung, J. et al. Using quicktome for intracerebral surgery: Early retrospective study and proof of concept. World Neurosurg. (2021) (Online ahead of Print) [DOI] [PubMed]
- 41.Ghosh MK, Chakraborty D, Sarkar S, Bhowmik A, Basu M. The interrelationship between cerebral ischemic stroke and glioma: A comprehensive study of recent reports. Signal Transduct. Target. Ther. 2019;4:42. doi: 10.1038/s41392-019-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma N, Baron J-C, Rowe JB. Motor imagery after stroke: Relating outcome to motor network connectivity. Ann. Neurol. 2009;66:604–616. doi: 10.1002/ana.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton R, Keenan JP, Catala M, Pascual-Leone A. Alexia for Braille following bilateral occipital stroke in an early blind woman. Neuroreport. 2000;11(2):237–240. doi: 10.1097/00001756-200002070-00003. [DOI] [PubMed] [Google Scholar]
- 44.Kong NW, Gibb WR, Tate MC. Neuroplasticity: Insights from patients harboring gliomas. Neural Plast. 2016;2016:2365063–2365063. doi: 10.1155/2016/2365063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawashima, A. et al. Plastic reshaping of cortical language areas evaluated by navigated transcranial magnetic stimulation in a surgical case of glioblastoma multiforme. (2013). [DOI] [PubMed]
- 46.Dadario, N. B., Brahimaj, B., Yeung, J. & Sughrue, M. E. Reducing the cognitive footprint of brain tumor surgery. Front. Neurol1342 (2021). [DOI] [PMC free article] [PubMed]
- 47.Samuel N, Vetkas A, Pancholi A, Sarica C, Loh A, Germann J, Lozano AM. A network-based approach to glioma surgery: Insights from functional neurosurgery. Cancers. 2021;13(23):6127. doi: 10.3390/cancers13236127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.