Abstract
Study design
Retrospective Case Series.
Objectives
Minimally invasive techniques have emerged as a useful tool in the treatment of neoplastic spine pathology due to decrease in surgical morbidity and earlier adjuvant treatment. The objective of this study was to analyze outcomes and complications in a cohort of unstable, symptomatic pathologic fractures treated with percutaneous pedicle screw fixation (PPSF).
Methods
A retrospective review was performed on consecutive patients with spinal stabilization for unstable pathologic neoplastic fractures between 2007 and 2017. Patients who underwent PPSF through a minimally invasive approach were included. Surgical indications included intractable pain, mechanical instability, and neurologic compromise with radiologic visualization of the lesion.
Results
20 patients with mean Tomita Score of 6.3 ± 2.1 points [95% CI, 5.3–7.2] were treated with constructs that spanned a mean of 4.7 ± 1.4 [95% CI, 4.0–5.3] instrumented levels. 10 (50%) patients were augmented with vertebroplasty. Majority of patients (65%) had no complications during their hospital stay and were discharged home (60%). Four patients received reoperation: two extracavitary corpectomies, one pathologic fracture at a different level, and one adjacent segment disease.
Conclusion
Minimally invasive PPSF is a safe and effective option when treating unstable neoplastic fractures and may be a viable alternative to the traditional open approach in select cases.
Level of evidence
4.
Keywords: Minimally invasive spine surgery, Pathologic fracture, Percutaneous pedicle screw fixation, Thoracolumbar spine, Spine metastasis
Abbreviations: MIS, Minimally Invasive Surgery; MISS, Minimally Invasive Spine Surgery; PPSF, Percutaneous Pedicle Screw Fixation
1. Introduction
Oncologic-related spinal instability is the loss of spinal integrity due to a neoplastic process that results in a symptomatic or progressive deformity, functional pain, or neural compromise.1,2 When adjunct treatments such as chemotherapy, radiotherapy, and immunotherapy fail in providing stability or when patients develop a neurologic deficit, surgical stabilization can facilitate mobilization and improve the patients quality of life.3,4 Within all options, open surgery for spinal metastases has been shown to have high complication rates up to 30%, which has lead the spine community to seek alternative methods of treatment.5,6
Minimally invasive surgery (MIS) has gained popularity and revolutionized spine surgery due to the improvement in techniques and equipment.7 In addition, MIS has become an attractive alternative to open surgery because of its reduced morbidity and palliative potential.7,8 Also, MIS has the potential for faster initiation of adjuvant treatment.9, 10, 11 Due to a decrease in trauma to the soft tissues caused by less exposure and dissection, MIS can also reduce the risk of wound related problems and infections which is paramount in individuals who are often already immunocompromised and with limited healing potential.12, 13, 14 MIS can also enable faster post-operative ambulation and has been shown to improve pain, providing a faster recovery and a boost to quality of life standards.15 As the management of spinal metastases is often palliative, this is an important consideration in the management of pathologic fractures.
However, due to the paucity of literature evaluating MIS for pathologic fractures of the spine, the outcomes and complications resulting from these MIS procedures remains unclear. Therefore, the goal of this study was to analyze outcomes and complications of patients with thoracic and lumbar pathologic neoplastic fractures who were treated with percutaneous pedicle screw fixation (PPSF).
2. Materials and methods
Following approval from our Institutional Review Board (HP-00082698), we retrospectively evaluated a consecutive series of patients from 2007 to 2017 presenting with thoracic or lumbar pathologic spine fractures treated operatively. We followed the Strengthening the Report of Observational Studies in Epidemiology (STROBE) Guidelines to assure high quality of our study.16 Patients were identified from a surgical billing database by querying for current procedural terminology (CPT) and International Statistical Classification of Diseases and Related Health Problems (ICD) codes. All procedures were performed by one of four fellowship-trained orthopedic spine surgeons at a tertiary academic trauma center. All patients underwent surgical intervention due to symptomatic mechanical instability - most commonly presenting with movement-related and positional pain - in the presence of radiological evidence of a pathologic fracture.
Patients who underwent PPSF through a minimally invasive approach were included in this study. Patients who received posterior open surgery were excluded. Patients who only underwent percutaneous vertebroplasty and kyphoplasty were also excluded, as well as those who underwent treatment for non-neoplastic etiologies such as infection or osteoporosis. Following eligibility criteria, a total of 20 patients were included for final analysis.
The selection of operative technique (MIS vs open) as well as construct length was made at the discretion of the operative surgeon. However, indications for MIS surgery included spinal cord compression to single unstable vertebra requiring short-segment fusions. The decision to directly decompress was determined by the presence and severity of neural compression, as well as the patient's symptoms. The technique for PPSF placement was also employed at the discretion of the surgeon, and either consisted of biplanar fluoroscopy or true antero-posterior (AP) placement, as described by Gomez and Ludwig.13
Post-operatively, patients were instructed to ambulate as tolerated after surgery and were evaluated by inpatient physical and occupational therapists prior to being discharged. Patients were not routinely braced postoperatively but were restricted from heavy lifting or strenuous activity for 3 months. Patients were seen at 2-week, 6-week, 3-month, and 6-month intervals, with yearly visits thereafter. Patients received AP and lateral radiographs at each postoperative clinic visit to evaluate for fixation failure and/or pseudarthrosis, defined by progression or new deformity, presence of radiolucency surrounding the hardware, implant failure, or loss of fixation. During the postoperative period, adjuvant treatment was subject to the patient's primary oncologist.
Data was collected from inpatient and outpatient medical records. In addition, perioperative variables including surgical approach, operative procedures, spine fracture level, construct length, estimated blood loss, intraoperative blood transfusions, and in-hospital postoperative complications were collected. We also retrieved basic demographic characteristics such as age, gender, and body mass index (BMI). In addition, further variables such as the neurological status at initial evaluation, Charlson comorbidity index (CCI), American Society of Anesthesiologist (ASA) score, Tomita neoplastic score,17 length of stay, and follow-up was obtained from chart review.
Primary outcomes were failure of fixation, pseudarthrosis, and reoperations in patients with symptomatic neoplastic fractures in the thoracic and lumbar spine managed with PPSF. Secondary outcomes included hospital length of stay, inpatient complications, estimated blood loss, and intraoperative blood transfusion.
Data was managed using Microsoft Excel (Microsoft, Redmond, WA, USA). Mean, standard deviation, median, and 95% confidence intervals (CI) were calculated for continuous variables. The Shapiro-Wilk test was used to assess for normality of continuous variables. Frequencies were calculated for ordinal and nominal variables. An a priori alpha was set at 0.05 for significance. All statistical analyses were performed using JMP Pro 14 statistical software (SAS Institute, Cary, NC, USA).
3. Results
A total of 20 patients met our inclusion and exclusion criteria and were included in the analysis. Overall, the patients were 45% female and had a mean age of 58 ± 12 years, BMI of 29.1 ± 7.1 kg/m2, and CCI of 8.2 ± 1.6 (Table 1). The mean Tomita score of 6.3 ± 2.1, with 14 (70%) having a Tomita score less than 8 points. The most common primary malignancies were lung (n = 5, 25%), breast (n = 5, 25%), and kidney (n = 3, 15%). Most patients received neoadjuvant chemotherapy (n = 14, 70%), while 4 patients (20%) received local neoadjuvant radiation to the spine.
Table 1.
Patient demographic and oncology characteristics.
| Number of Patients | 20 | |
|---|---|---|
| Age | 58.2 ± 12.3 [95% CI, 52.4–63.9] | |
| Sex | ||
| Female | 9 (45%) | |
| Male | 11 (55%) | |
| BMI | 28.9 ± 7.6 [95% CI, 25.1–32.6] | |
| CCI | 8.2 ± 1.5 [95% CI, 7.5–8.9] | |
| Tomita Scorea | 6.3 ± 2.1 [95% CI, 5.3–7.2] | |
| Primary Cancer | ||
| Lung | 5 (25%) | |
| Breast | 5 (25%) | |
| Kidney | 3 (15%) | |
| Multiple Myeloma | 2 (10%) | |
| Colorectal | 1 (5%) | |
| Liver | 1 (5%) | |
| Esophageal | 1 (5%) | |
| Chondrosarcoma | 1 (5%) | |
| T-Cell Lymphoma | 1 (5%) | |
| Preoperative Treatment | ||
| Chemotherapy | 14 (70%) | |
| Primary Tumor Excision | 9 (45%) | |
| Radiation | 4 (20%) | |
Tomita et al. Spine, 2001. BMI, body mass index; CCI, Charlson Comorbidity Index; IQR, interquartile range.
Most fractures occurred in the thoracolumbar spine (n = 10, 45%) and lower thoracic spine (n = 6, 30%); Table 2. Only two patients had incomplete neurological deficits (10%). The median time from initial cancer diagnosis to spine surgery was 236 days. PPSF surgery typically involved 4.7 ± 1.4 instrumented segments and a median estimated blood loss of 100 mL (IQR 88–275 mL). Intraoperative transfusion was given to 4 patients (20%). Vertebroplasty was additional performed for 10 patients (50%) and instrumented fusions for 3 patients (15%).
Table 2.
Surgical characteristics.
| Number of Patients | 20 |
|---|---|
| Pathologic Fracture Location | |
| High Thoracic (T1-T5) | 1 (5%) |
| Low Thoracic (T6-T9) | 6 (30%) |
| Thoracolumbar (T10-L2) | 10 (50%) |
| Lumbar (L3-L5) | 3 (15%) |
| Neurological Status | |
| Intact | 18 (90%) |
| Incomplete | 2 (10%) |
| ASA Classification | |
| 2 | 4 (20%) |
| 3 | 16 (80%) |
| Cancer Diagnosis to Spine Surgery (Days) | 236 [IQR, 62–665] |
| Estimated Blood Loss (mL) | 100 [IQR, 88–275] |
| Number of Instrumented Levels | 4.7 ± 1.4 [95% CI, 4.0–5.3] |
| Vertebroplasty | 10 (50%) |
| Fusion (Allograft) | 3 (15%) |
| Intraoperative blood transfusion | 4 (20%) |
American Society of Anesthesiologists physical status classification system.
EBL, estimated blood loss; IQR, interquartile range; CI, confidence interval.
The median length of stay was 7.6 days (IQR 4.4–10.7 days). In our cohort, 3 patients (15%) developed respiratory complications, 2 (10%) developed infection, and 2 (10%) required intraoperative transfusion (Table 3). Discharge to home was achieved in 12 patients (60%) while the remaining 8 patients (40%) were discharged to a sub-acute rehabilitation facility based on recommendations from the physical therapy team. For adjuvant treatment, 4 patients (20%) had both adjuvant chemotherapy and radiotherapy, while 4 patients (20%) had adjuvant chemotherapy only and 5 patients (20%) had local adjuvant radiotherapy only. Patients followed up in clinic for median 98 days (IQR 37–273 days).
Table 3.
Postoperative characteristics and complications.
| Length of Stay (Days) | 7.6 ± 6.8 [95% CI, 4.4–10.7] |
|---|---|
| Hospital Complications | |
| Respiratory Complications | 3 (15%) |
| Infection | 2 (10%) |
| Postoperative Transfusion | 2 (10%) |
| Discharge Disposition | |
| Home | 12 (60%) |
| Sub-acute Rehabilitation | 8 (40%) |
| Adjuvant Treatment | |
| Chemotherapy | 4 (20%) |
| Radiation | 5 (25%) |
| Chemotherapy & Radiation | 6 (30%) |
| None | 5 (25%) |
| Postoperative Complications | |
| Reoperation | 4 (20%) |
| Disease Progression | 4 (20%) |
| Mortality within 30 days | 1 (5%) |
| Length of Follow-up (Days) | 98 [IQR, 37–273] |
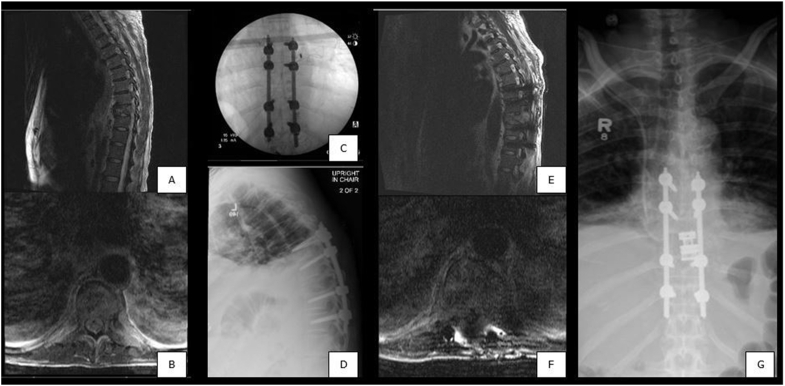
There were four reoperations, with the most acute reoperation occurring at 37 days postoperatively. This patient initially underwent percutaneous fixation of T7 to T11 for metastatic hepatocellular carcinoma, but subsequently required extracavitary corpectomy with cage reconstruction and revision of posterior instrumentation due to tumor progression and onset of new neurologic symptoms (Fig. 1). The second patient who required reoperation also underwent extracavitary corpectomy and interbody fusion without removal of posterior hardware 273 days after the index procedure due to disease progression. The last two patients required surgery at sites distant from the index procedure and after more than 5 years postoperatively. One patient underwent L1 and L2 kyphoplasty for symptomatic relief of new metastatic pathologic fractures and the other patient elected for extension of their decompression for adjacent segment disease.
Fig. 1.
Patient with known metastatic hepatocellular carcinoma. A-B) Preoperative sagittal and axial MRI showing pathologic T9 vertebral fracture. C-D) Intraoperative AP fluoroscopy and postoperative lateral radiograph showing T7 to T11 percutaneous fixation. E-F) Sagittal and axial MRI prior to reoperation for tumor progression and new neurologic deficits. G) Postoperative AP radiograph showing revision of posterior instrumentation with extracavitary corpectomy and cage reconstruction.
4. Discussion
The goals of surgical treatment of unstable spinal metastases are to restore stability, enable neurologic recovery, provide palliation, and facilitate mobilization. MIS techniques have become a feasible option for pathologic neoplastic fractures of the spine due to potential improvement in wound healing, the ability for enabling faster initiation of systemic therapies, earlier post-operative ambulation, and decreased pain. Our study cohort had overall favorable results, with few reoperations and minimal post-operative complications.
Although we found positive outcomes when utilizing MIS techniques, the indications for surgical intervention and the type of intervention remain a challenge that requires a multi-disciplinary approach and long discussion with the patient.3,8 One of the biggest challenges in medical and surgical oncology is having a prognostic measure that can help guide clinical decision-making.1,5,6 We utilized the Tomita score, which has been studied and correlated with predicting survival in such patients and takes factors into account such as the rate of primary tumor growth, and number of bony and visceral metastases.4 Surgical treatment is recommended for a score of less than or equal to 8.4,17 Nearly all patients in our series had a Tomita score less than 8, with all patients reporting intractable, progressive mechanical pain refractory to conservative treatment.
After identifying patients that are indicated for intervention, options for surgical approach and technique depend on a variety of factors. Percutaneous treatments such as kyphoplasty and vertebroplasty are well described in the management of symptomatic pathologic vertebral compression fractures.8,9 In a systematic review, Mendel et al. strongly recommended vertebroplasty or kyphoplasty for providing pain relief and improving functional outcomes in patients with vertebral body fractures and axial pain due to metastatic disease.18 However, the presence of extensive epidural involvement, painful deformity, or invasion of the posterior elements often renders stand-alone vertebroplasty or kyphoplasty ineffective and mandates more robust stabilization, with direct decompression in some cases.19 The use of PPSF in the treatment of other spine disease such as trauma, deformity, and degenerative conditions has gained recent popularity for reducing blood loss, operative times, and postoperative pain scores when compared to traditional open surgery.12,20,21 Thus, the use of PPSF techniques provides an option to reduce approach-related morbidity and physiological burden in an already frail patient.
Recent studies have begun to describe using PPSF in treating spinal instability secondary to metastatic disease. Schwab et al. reports reliable pain relief and restoration of ambulatory status after MIS stabilization in 24 patients with plasmacytomas or spinal metastases.15 Moussazedeh et al. described using cement augmented PPSF in 44 patients with lesions extending into the posterior elements resulting in good pain relief, but reported fracture, screw pullout, and several subsequent decompressions.11 Other techniques such as mini-open laminectomy or transpedicular decompression in conjunction with PPSF have been described in level V studies with similar results.3,14 In the largest case series to date, Hamad et al. analyzed 51 patients with symptomatic spinal metastases treated with MIS stabilization and reported improvement in performance and pain scores, with 2 patients revised for aseptic loosening.10
In our series of patients, there were no hardware failures and no wound complications, even in patients who were irradiated postoperatively within several weeks of surgery. While no implant or surgery-related complications were reported, due to our suboptimal follow-up we were not able to assess survival and long-term functionality. Further prospective trials, with a greater number of patients, would potentially increase our ability to assess the true benefits of PPSF for the treatment of symptomatic metastatic pathologic fractures of the spine.
Our study had several limitations. The small sample size is due to the rare occurrence of patients with operative metastatic spine and PPSF. Given that PPSF is a relatively new technique for metastatic disease, traditional posterior open approach was more commonly used. Furthermore, long-term follow-up is difficult with this patient population, which may have resulted in under-reporting of post-operative complications. Finally. The retrospective nature of this study limits gathering of more detailed information that is not usually documented. Nonetheless, this case series of 20 patients provides to the growing evidence that PPSF may be an option for treating these patients, however further research is needed.
5. Conclusion
Minimally invasive surgery continues to grow as an attractive alternative to open surgery by accomplishing the same operative goals while minimizing injury to surrounding soft tissues. This case series further supports the use of percutaneous pedicle screw fixation as a viable alternative to more traditional approaches, especially in the metastatic patient with poor prognosis.
IRB approval
Approval was obtained from our institutional review board, HP-00082698.
CRediT authorship contribution statement
Matthew Chin: Methodology, Investigation, Formal analysis, Data curation, Writing – original draft. Jael E. Camacho: Methodology, Investigation, Software, Formal analysis, Data curation, Writing – original draft. Ivan B. Ye: Software, Formal analysis, Writing – review & editing. Jacob J. Bruckner: Software, Data curation, Writing – original draft. Alexandra E. Thomson: Writing – review & editing. Julio J. Jauregui: Methodology, Writing – review & editing. Kendall Buraimoh: Conceptualization, Resources, Writing – review & editing. Daniel L. Cavanaugh: Conceptualization, Resources, Writing – review & editing. Eugene Y. Koh: Conceptualization, Resources, Writing – review & editing. Daniel E. Gelb: Conceptualization, Resources, Writing – review & editing. Steven C. Ludwig: Conceptualization, Resources, Writing – review & editing, Supervision.
Declaration of competing interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MC, JEC, IBY, JJB, AET, JJJ, KB, DLC: No disclosures. SL: American Board of Orthopaedic Surgery, Inc: Board or committee member; American Orthopedic Association: Board or committee member; AO Spine North America Spine Fellowship Support: Research support; American Society for Investigative Pathology, Innovative Surgical Designs: Stock or stock options; Cervical Spine Research Society: Board or committee member; DePuy, A Johnson & Johnson Company: IP royalties; Paid consultant; Paid presenter or speaker; Globus Medical: Paid consultant; Research support; Journal of Spinal Disorders and Techniques: Editorial or governing board; K2M spine: Research support; K2Medical: Paid consultant; OMEGA: Research support; Pacira: Research support; SMISS: Board or committee member; Synthes: Paid consultant; Paid presenter or speaker; Thieme, QMP: Publishing royalties, financial, or material support. DG: Advanced Spinal Intellectual Property: Stock or stock options; Depuy-Synthes Spine: IP royalties; Paid presenter or speaker; Globus Medical: IP royalties. EK: Biomet: Paid consultant; DePuy, A Johnson & Johnson Company: Paid presenter or speaker.
References
- 1.Chen Y., He Y., Zhao C., Li X., Zhou C., Hirsch F.R. Treatment of spine metastases in cancer: a review. J Int Med Res. 2019 doi: 10.1177/0300060519888107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher C.G., DiPaola C.P., Ryken T.C., et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine. 2010;35(22):E1221–E1229. doi: 10.1097/BRS.0b013e3181e16ae2. [DOI] [PubMed] [Google Scholar]
- 3.Sundaresan N., Rothman A., Manhart K., Kelliher K. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine. 2002;27(16):1802–1806. doi: 10.1097/00007632-200208150-00021. [DOI] [PubMed] [Google Scholar]
- 4.Tomita K., Kawahara N., Kobayashi T., Yoshida A., Murakami H., Akamaru T. Surgical strategy for spinal metastases. Spine. 2001;26(3):298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein J., Zaveri G., Wai E., Vidmar M., Kreder H., Chow E. A population-based study of surgery for spinal metastases: survival rates and complications. J Bone Joint Surg Br. 2003;85(7):1045–1050. doi: 10.1302/0301-620x.85b7.14201. [DOI] [PubMed] [Google Scholar]
- 6.Jansson K.-Å., Bauer H.C. Survival, complications and outcome in 282 patients operated for neurological deficit due to thoracic or lumbar spinal metastases. Eur Spine J. 2006;15(2):196–202. doi: 10.1007/s00586-004-0870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barzilai O., Robin A.M., O'Toole J.E., Laufer I. Minimally invasive surgery strategies: changing the treatment of spine tumors. Neurosurg Clin. 2020;31(2):201–209. doi: 10.1016/j.nec.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuckerman S.L., Laufer I., Sahgal A., et al. When less is more: the indications for MIS techniques and separation surgery in metastatic spine disease. Spine. 2016;41(Suppl 20):S246. doi: 10.1097/BRS.0000000000001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fourney D.R., Schomer D.F., Nader R., et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg Spine. 2003;98(1):21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 10.Hamad A., Vachtsevanos L., Cattell A., Ockendon M., Balain B. Minimally invasive spinal surgery for the management of symptomatic spinal metastasis. Br J Neurosurg. 2017;31(5):526–530. doi: 10.1080/02688697.2017.1297374. [DOI] [PubMed] [Google Scholar]
- 11.Moussazadeh N., Rubin D.G., McLaughlin L., Lis E., Bilsky M.H., Laufer I. Short-segment percutaneous pedicle screw fixation with cement augmentation for tumor-induced spinal instability. Spine J. 2015;15(7):1609–1617. doi: 10.1016/j.spinee.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Camacho J.E., Usmani M.F., Strickland A.R., Banagan K.E., Ludwig S.C. The use of minimally invasive surgery in spine trauma: a review of concepts. J Spine Surg. 2019;5(Suppl 1):S91–S100. doi: 10.21037/jss.2019.04.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez J.A., Ludwig S.C. Minimally invasive techniques for thoracolumbar spine trauma. Contemp Spine Surg. 2012;13(5):1–7. [Google Scholar]
- 14.Hansen-Algenstaedt N., Knight R., Beyerlein J., Gessler R., Wiesner L., Schaefer C. Minimal-invasive stabilization and circumferential spinal cord decompression in metastatic epidural spinal cord compression (MESCC) Eur Spine J. 2013;22(9):2142–2144. doi: 10.1007/s00586-013-2959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwab J.H., Gasbarrini A., Cappuccio M., et al. Minimally invasive posterior stabilization improved ambulation and pain scores in patients with plasmacytomas and/or metastases of the spine. Int J Surg Oncol. 2011;2011:239230. doi: 10.1155/2011/239230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 17.Kim J., Lee S.H., Park S.J., et al. Analysis of the predictive role and new proposal for surgical strategies based on the modified Tomita and Tokuhashi scoring systems for spinal metastasis. World J Surg Oncol. 2014;12:245. doi: 10.1186/1477-7819-12-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendel E., Bourekas E., Gerszten P., Golan J.D. Percutaneous techniques in the treatment of spine tumors: what are the diagnostic and therapeutic indications and outcomes? Spine. 2009;34(22 Suppl):S93–S100. doi: 10.1097/BRS.0b013e3181b77895. Phila Pa 1976. [DOI] [PubMed] [Google Scholar]
- 19.Masala S., Fiori R., Massari F., Simonetti G. Kyphoplasty: indications, contraindications and technique. La Radiol Medica. 2005;110(1-2):97–105. [PubMed] [Google Scholar]
- 20.Afolabi A., Weir T.B., Usmani M.F., et al. Comparison of percutaneous minimally invasive versus open posterior spine surgery for fixation of thoracolumbar fractures: a retrospective matched cohort analysis. J Orthop. 2020;18:185–190. doi: 10.1016/j.jor.2019.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mobbs R.J., Sivabalan P., Li J. Minimally invasive surgery compared to open spinal fusion for the treatment of degenerative lumbar spine pathologies. J Clin Neurosci. 2012;19(6):829–835. doi: 10.1016/j.jocn.2011.10.004. [DOI] [PubMed] [Google Scholar]