Abstract
We assessed the impact of the pediatric 13-valent pneumococcal conjugate vaccine (PCV13) on pneumococcal meningitis in adults in Japan in 2014–2018 by comparing epidemiological characteristics of adults with invasive pneumococcal disease with (n = 222) and without (n = 1258) meningitis. The annual incidence of pneumococcal meningitis in 2016–2018 was 0.20–0.26 cases/100,000 population. Age (p < 0.001) and case fatality rate (p = 0.003) were significantly lower in patients with meningitis than in those without meningitis. The odds of developing meningitis were higher in asplenic/hyposplenic or splenectomized patients (adjusted odds ratio [aOR] 2.29, 95% CI 1.27–4.14), for serotypes 10A (aOR 3.26, 95% CI 2.10–5.06) or 23A (aOR 3.91, 95% CI 2.47–6.19), but lower for those aged ≥ 65 years (aOR 0.59, 95% CI 0.44–0.81). PCV13 had an indirect effect on nonmeningitis, but its impact on meningitis was limited because of an increase in non-PCV13 serotypes. Of meningitis isolates, 78 (35.1%) and 3 (1.4%) were penicillin G- or ceftriaxone-resistant, respectively. We also confirmed an association of the pbp1bA641C mutation with meningitis (aOR 2.92, 95% CI 1.51–5.65).
Subject terms: Microbiology, Diseases, Medical research
Introduction
Streptococcus pneumoniae colonizes the nasopharynx asymptomatically, and often causes pneumococcal disease in children and adults1,2. Occasionally, it can enter the bloodstream and cause invasive pneumococcal diseases (IPD) such as meningitis and bacteremia3,4. S. pneumoniae is the most common cause of bacterial meningitis in children and adults, and causes serious sequelae and death5–7. In 2015, there were estimated to be 83,900 cases and 37,900 deaths in children caused by pneumococcal meningitis worldwide8. Of all deaths caused by pneumococcal infection, meningitis is estimated to account for 12%. Up to 30% of survivors have some type of neurological or neuro-behavioral sequelae9. These include seizures, hearing loss and vision loss, cognitive impairment, neuromotor disability and memory or behavior changes.
S. pneumoniae contains five high-molecular-mass penicillin-binding proteins (PBPs), named PBP1a, PBP1b, PBP2x, PBP2a, and PBP2b, as well as the low-molecular-weight PBP310. PBPs 2x, 2b, and 1a play a main role in β-lactam resistance. Mutations in PBP2x and PBP2b result in low levels of such resistance and are prerequisites for high-level β-lactam resistance mediated by an altered PBP1a protein. The relationship between mutations in PBP1b and β-lactam resistance is not yet clear. A recent genome-wide association study from the United States reported a significant association of the pbp1bA641C mutation with the clinical occurrence of meningitis in patients with IPD, but there is no evidence that this mutation results in β-lactam resistance11. However, it is unclear whether the pbp1bA641C mutation is associated with meningitis in adult patients with IPD in Japan.
The incorporation of pneumococcal conjugate vaccines (PCVs) into infant immunization programs worldwide resulted in significant reductions ranging from 41 to 97% in the vaccine-type IPD, including pneumococcal meningitis, in children and older age groups12. In Japan, PCV7 was introduced for children under 5 years old in November 2010, and subsequently included in the national immunization program in April 2013. It was then replaced by 13-valent PCV (PCV13) in November 2013, and the vaccination rate of PCV13 in children was approximately 90% in 2014. A significant reduction in the incidence of PCV7-type IPD in children under 5 was reported in Japan after the introduction of PCV713, although the incidence of IPD in children caused by nonvaccine serotypes increased. In 2014, a 23-valent pneumococcal polysaccharide vaccine (PPSV23) was included in the national immunization program for adults aged ≥ 65 years, while PCV13 was licensed for this group in 2014, and became available on a voluntary basis. We conducted an enhanced surveillance of IPD among adults in Japan from 2013 to 2015, and found an indirect effect of the use of the pediatric PCV7 on the epidemiology of adult IPD14. Although the epidemiological and bacteriological characteristics of pneumococcal meningitis have been recently reported from Israel, England and Wales15,16, the epidemiological features of meningitis have not been fully investigated in adult patients with IPD in Japan.
In this study, we characterized the epidemiological features of pneumococcal meningitis in adults in Japan between 2014 and 2018, and assessed the impact of pediatric PCV13 on this disease.
Results
IPD incidence and clinical characteristics of IPD
We enrolled 1480 cases of IPD during the study period. Of these cases, S. pneumoniae strain was isolated from a sterile site in 1477 cases, and serotyped with serotype-specific rabbit antiserum17. In the remained three cases, the lytA gene was detected using blood samples by polymerase chain reaction (PCR) amplification18, and the serotype was determined by multiplex serotyping PCR19.
The number of IPD cases per 100,000 population in adults gradually increased during 2014–2015, and plateaued during 2016–2018. Therefore, we consider that the number of IPD cases was underreported during 2014–2015, but we were able to estimate that the annual incidence of IPD and pneumococcal meningitis in adults was 1.40–1.98 cases and 0.20–0.26 cases/100,000 population during 2016–2018 (Table 1).
Table 1.
Annual incidence of pneumococcal meningitis and nonmeningitis cases among adults in Japan during 2016⎼2018.
| Year | Incidence by age, cases per 100,000 persons | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All IPD | Meningitis | Nonmeningitis | |||||||
| ≥ 15 y | 15–64 y | ≥ 65 y | ≥ 15 y | 15–64 y | ≥ 65 y | ≥ 15 y | 15–64 y | ≥ 65 y | |
| 2016 | 1.4 | 0.56 | 3.14 | 0.2 | 0.12 | 0.35 | 1.2 | 0.44 | 2.79 |
| 2017 | 1.98 | 0.99 | 3.97 | 0.26 | 0.22 | 0.34 | 1.72 | 0.78 | 3.63 |
| 2018 | 1.88 | 0.85 | 3.9 | 0.26 | 0.17 | 0.46 | 1.62 | 0.68 | 3.45 |
| Mean | 1.75 | 0.8 | 3.68 | 0.24 | 0.17 | 0.38 | 1.51 | 0.63 | 3.29 |
IPD invasive pneumococcal disease.
A total of 1480 IPD cases were classified as meningitis (n = 222) and nonmeningitis (1258). Of the 222 cases of meningitis, 174 (78.4%) were positive for bacterial culture in cerebrospinal fluid (CSF), 10 (4.5%) were both positive for bacterial culture and pneumococcal antigen in CSF20, and 38 (17.1%) were positive in blood cultures and were associated with typical meningeal signs. No case was identified in which the CSF was positive for pneumococcal antigen but negative for bacterial culture. Meningitis cases accounted for 15.0% of the total IPD cases (222/1480). The proportion of meningitis cases to total IPD cases (21.0%; 98/467) in patients aged 15–64 years was significantly higher than in patients ≥ 65 years (12.2%; 124/1013; p < 0.001).
The clinical characteristics of patients with meningitis and nonmeningitis IPD were compared (Table 2). The proportion of men was 59.7%. The median age (range) of patients with meningitis (66 years; age range; 15–100 years) was significantly lower than that of those with nonmeningitis (72 years; age range; 16–103 years; p < 0.001). The case fatality rate (CFR) was significantly lower for meningitis than for nonmeningitis (p = 0.004), although the difference between two groups was not significant when the patients were divided into two age groups. By contrast, the proportion of asplenic/hyposplenic or splenectomized patients was significantly higher for meningitis (9.5%) than for nonmeningitis (3.0%) (p < 0.001). Although the proportion of IPD patients with a history of PCV13 vaccination was only 0.3%, the proportion with a history of PPSV23 vaccination was approximately 10%. No difference was found in the rates of vaccination with PCV13 or PPSV23 between meningitis and nonmeningitis patients.
Table 2.
Comparison of clinical characteristics between meningitis and nonmeningitis cases among adults in Japan, 2014⎼2018.
| Variables | Total IPD (n = 1480) | Meningitis (n = 222) | Nonmeningitis (n = 1258) | p-value by χ2 test |
|---|---|---|---|---|
| Male | 883 (59.7%) | 133 (59.9%) | 750 (59.6%) | 0.935 |
| Median age (range) | 71 (15–103) | 66 (15–100) | 72 (16–103) | < 0.001* |
| 15–64 years | 467 (31.6%) | 98 (44.1%) | 369 (29.3%) | < 0.001 |
| ≥ 65 years | 1013 (68.4%) | 124 (55.9%) | 889 (70.7%) | |
| Smoking history | 643 (43.4%) | 82 (36.9%) | 561 (44.6%) | 0.034 |
| Alcohol abuse | 257 (17.4%) | 43 (19.4%) | 214 (17.0%) | 0.392 |
| Immunocompromised conditions | 400 (27.0%) | 54 (24.3%) | 346 (27.5%) | 0.325 |
| Asplenia or hyposplenia or splenectomy | 59 (4.1%) | 21 (9.5%) | 38 (3.0%) | < 0.001 |
| Autoimmune disease | 101 (6.8%) | 10 (4.5%) | 91 (7.2%) | 0.137 |
| Corticosteroid therapy | 108 (7.3%) | 13 (5.9%) | 95 (7.6%) | 0.370 |
| Solid organ cancer | 151 (10.2%) | 14 (6.3%) | 137 (10.9%) | 0.037 |
| Hematologic cancer | 60 (4.1%) | 5 (2.3%) | 55 (4.4%) | 0.140 |
| Anti-cancer agent | 106 (7.2%) | 11 (5.0%) | 95 (7.6%) | 0.167 |
| Fatal outcome | 244 (16.5%) | 22 (9.9%) | 222 (17.6%) | 0.004 |
| 15–64 years | 58/467 (12.4%) | 7/98 (7.1%) | 51/369 (13.8%) | 0.075 |
| ≥ 65 years | 186/1013 (18.4%) | 15/124 (12.1%) | 171/889 (19.2%) | 0.054 |
| Vaccination history of PCV13 | 4 (0.3%) | 0 (0%) | 4 (0.3%) | > 0.999** |
| Vaccination history of PPSV23 | 147 (9.9%) | 24 (10.8%) | 123 (9.8%) | 0.635 |
*Mann–Whitney nonparametric U test, **Fisher’s exact test.
Association of variables with the odds of developing meningitis
We next analyzed variables including age ≥ 65 years, asplenia/hyposplenia or splenectomy, and major serotypes to determine whether they were significantly associated with meningitis after adjusting for confounders (Table 3). The two most common serotypes of meningitis cases were 10A (17.6%) and 23A (16.7%). While the odds of meningitis were higher for asplenic/hyposplenic or splenectomized patients (adjusted odds ratio [aOR] 2.29, 95% CI 1.27–4.14) and for serotypes 10A (aOR 3.26, 95% CI 2.10–5.06) or 23A (aOR 3.91, 95% CI 2.47–6.19), they were lower for those age ≥ 65 years (aOR 0.59, 95% CI 0.44–0.81) or having serotype 19A (aOR 0.20, 95% CI 0.07–0.56).
Table 3.
Association of serotype with meningitis or nonmeningitis cases among adults in Japan during 2014⎼2018.
| Variables | No. (%) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Total cases | Meningitis | Nonmeningitis | OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| (n = 1480) | (n = 222) | (n = 1258) | |||||
| Age years | 71 (62–82) | 66 (57–75) | 72 (63–83) | ||||
| ≧65 years | 1013 (68.4) | 124 (55.9) | 889 (70.7) | 0.53 (0.39–0.70) | < 0.001 | 0.59 (0.44–0.81) | < 0.001 |
| Asplenia/ hyposplenia, or splenectomy | 59 (4.1%) | 21 (9.5%) | 38 (3.0%) | 3.35 (1.93–5.83) | < 0.001 | 2.29 (1.27–4.14) | 0.006 |
| Serotype | |||||||
| 3 | 187 (12.6) | 18 (8.1) | 169 (13.4) | 0.57 (0.34–0.95) | 0.03 | 0.77 (0.46–1.31) | 0.334 |
| 12F | 154 (10.4) | 18 (8.1) | 136 (10.8) | 0.73 (0.44–1.22) | 0.226 | ||
| 19A | 138 (9.3) | 4 (1.8) | 134 (10.7) | 0.15 (0.06–0.42) | < 0.001 | 0.20 (0.07–0.56) | 0.002 |
| 10A | 112 (7.6) | 39 (17.6) | 73 (5.8) | 3.46 (2.28–5.26) | < 0.001 | 3.26 (2.10–5.06) | < 0.001 |
| 23A | 97 (6.6) | 37 (16.7) | 60 (4.8) | 3.99 (2.58–6.19) | < 0.001 | 3.91 (2.47–6.19) | < 0.001 |
| 22F | 89 (6.0) | 9 (4.1) | 80 (6.4) | 0.62 (0.31–1.26) | 0.187 | ||
| 35B | 81 (5.5) | 14 (6.3) | 67 (5.3) | 1.20 (0.66–2.17) | 0.554 | ||
| 15A | 75 (5.1) | 14 (6.3) | 61 (4.8) | 1.32 (0.73–2.41) | 0.363 | ||
| 6C | 74 (5.0) | 9 (4.1) | 65 (5.2) | 0.78 (0.38–1.58) | 0.484 | ||
| 11A/E | 56 (3.8) | 12 (5.4) | 44 (3.5) | 1.58 (0.82–3.04) | 0.173 | ||
| Other serotypes | 417 (28.2) | 48 (21.6) | 369 (29.3) | ||||
OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval. Serotypes responsible for > 50 cases of invasive pneumococcal disease were included for analysis.
Vaccine coverage
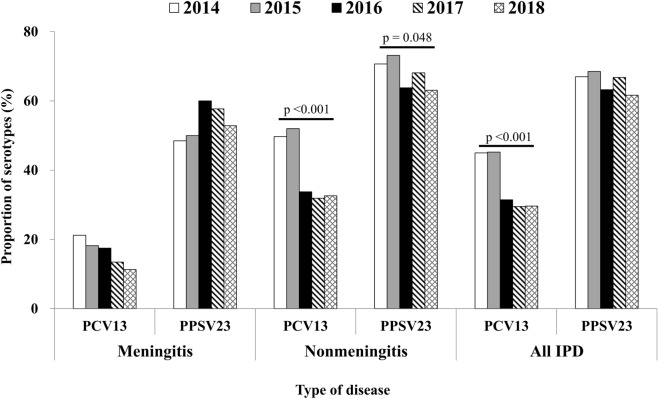
The percentages of PCV13 or PPSV23 serotypes within pneumococcal isolates, stratified by isolation year and disease type, are shown in Fig. 1. Over all cases of IPD, the percentages of PCV13 serotypes significantly decreased over the study period (p < 0.001), although no between-year differences were found in the percentage of PPSV23 serotypes. By contrast, no significant between-year differences were found in the percentages of PCV13 serotypes in the meningitis cases, even with the gradual decrease in the percentages of PCV13 serotypes during the study period. The percentages of PPSV23 serotypes in meningitis cases also did not differ over the study period. However, the percentages of PCV13 (p < 0.001) or PPSV23 serotypes (p = 0.048) significantly decreased during the study period in nonmeningitis cases.
Figure 1.
Percentage of vaccine-covered serotypes in pneumococcal isolates from 1480 adult patients with invasive pneumococcal disease in Japan between 2014 and 2018, stratified by year and disease type. PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Antimicrobial susceptibility
A total of 1476 strains were examined for antimicrobial susceptibility (Table 4). Of the meningitis cases (n = 222), a total of 78 strains (35.1%) were resistant to penicillin G (PCG). By contrast, of the nonmeningitis isolates (n = 1254), 14 strains (1.1%) showed intermediate resistance (n = 9) or resistance (n = 5) to PCG. All the meningitis strains with serotypes 23A (37 strains) and 15A (14 strains) were resistant to PCG, and strains resistant to PCG were detected for several other serotypes including 35B, and 6C (Appendix Figure). Three meningitis strains were ceftriaxone resistant, but no meropenem-resistant strain was found. All meningitis strains were susceptible to vancomycin. The minimum inhibitory concentration (MIC)50 and MIC90 values were similar for meningitis and nonmeningitis strains for all antimicrobial agents tested.
Table 4.
Susceptibility of pneumococcal isolates from meningitis and nonmeningitis cases against four antimicrobial agents.
| Antimicrobial agents | The pneumococcal strains isolated from; No of strain (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Meningitis (n = 222) | Nonmeningitis (n = 1254) | |||||||||
| Susceptible | Intermediate | Resistant | MIC50 (μg/mL) | MIC90 (μg/mL) | Susceptible | Intermediate | Resistant | MIC50 (μg/mL) | MIC90 (μg/mL) | |
| Penicillin G | 144 (64.9) | –* | 78 (35.1) | 0.03 | 1 | 1240 (98.9) | 9 (0.7) | 5 (0.4) | 0.03 | 1 |
| Ceftriaxone | 209 (94.1) | 10 (4.5) | 3 (1.4) | 0.25 | 0.5 | 1230 (98.1) | 12 (1.0) | 12 (1.0) | 0.25 | 0.5 |
| Meropenem | 208 (93.7) | 14 (6.3) | 0 (0) | 0.015 | 0.25 | 1157 (92.3) | 87 (6.9) | 10 (0.8) | 0.015 | 0.25 |
| Vancomycin | 222 (100) | –* | –* | 0.25 | 0.5 | 1254 (100) | –* | –* | 0.25 | 0.5 |
* Breakpoint undefined.
Association of the pbp1bA641C mutation with meningitis
We evaluated the effect of the pbp1bA641C mutation on the odds of developing meningitis using mixed-effects logistic regression explicitly controlled for patient age group, all pneumococcal serotypes, and susceptibility to three β-lactam antibiotics (Table 5). The odds of causing meningitis were higher for strains bearing the pbp1bA641C mutation (aOR 2.92, 95% CI 1.51–5.65), but lower for patients aged ≥ 65 years (aOR 0.55, 95% CI 0.40–0.74). We also evaluated whether the pbp1bA641C mutation was associated with the susceptibility to PCG of both meningitis and nonmeningitis isolates (Appendix Table 2). The presence of the pbp1bA641C mutation was significantly associated with PCG resistance for meningitis isolates (p < 0.001), but not for nonmeningitis isolates (p = 0.683).
Table 5.
Logistic regression analysis of isolates from meningitis and nonmeningitis cases in adults on the association between meningitis and pbp1bA641C mutation.
| Variables | Meningitis (n = 222) | Nonmeningitis (n = 1254) | Univariate logistic regression | Effects | Mixed-effects logistic regression** | ||||
|---|---|---|---|---|---|---|---|---|---|
| No | % | No | % | OR (95% CI) | p value | aOR (95% CI) | p value | ||
| pbp1bA641C mutation | Fixed | ||||||||
| No | 174 | 78.4 | 1152 | 91.9 | Reference | Reference | |||
| Yes | 48 | 21.6 | 102 | 8.1 | 3.12 (2.13–4.55) | < 0.001 | 2.92 (1.51–5.65) | 0.001 | |
| Patient age (years) | Fixed | ||||||||
| 15–64 | 98 | 44.1 | 369 | 29.4 | Reference | Reference | |||
| ≥ 65 | 124 | 55.9 | 885 | 70.6 | 0.53 (0.39–0.71) | < 0.001 | 0.55 (0.40–0.74) | < 0.001 | |
| Susceptibility to three β-lactam antibiotics* | Random | ||||||||
| Serotypes | Random | ||||||||
OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval. *Penicillin G, cefotaxime, and meropenem. **Mixed-effects logistic regression model with binary outcome (meningitis vs nonmeningitis) and the indicated explanatory variables. Calculated by Stata software version 16 (StataCorp LLC, College Station, TX, USA).
Discussion
In the present study, we determined the annual incidence of pneumococcal meningitis in adults in Japan during the period 2016–2018. The incidence rates of pneumococcal meningitis among patients aged 15–64 years and ≥ 65 years remained unchanged during this period. The incidence of pneumococcal meningitis (0.20 cases/100,000 population) in adults in 2016 was approximately one fourth of that (0.85) reported in Israel in 2014–2015, and similar to that (0.29) in England and Wales in 2015–201615,16. We also found that the CFR of adult patients with IPD was significantly lower for those with meningitis (9.9%) than for nonmeningitis cases (17.6%), consistent with a report from Israel15. The lower CFR of adult patients with meningitis in our study might be explained partially by the significantly lower age of adult patients with meningitis compared with those with nonmeningitis.
Our study also demonstrated that the proportion of asplenic/hyposplenic or splenectomized adult patients with meningitis (9.5%) was significantly higher than for those with nonmeningitis (3.0%), as was the aOR for meningitis. Collectively, our data indicate that impaired splenic function may increase the odds of meningitis irrespective of the infecting serotype or the patient’s age. It is well known that asplenic or hyposplenic or splenectomized patients are at increased risk for fulminant infections with encapsulated bacteria; this is attributable to a lack of splenic filtering and decreased production of specific antibodies and memory B cells21,22. A recent study of 2435 adult patients with IPD demonstrated that the proportion of asplenic patients with meningitis (6/37; 21.0%) was significantly higher than in patients with a spleen (112/2398; 4.7%)23. The authors also reported that the proportions of asplenic patients requiring intensive care admission or mechanical ventilation use and suffering acute kidney injury were significantly higher than for patients with a spleen, although the difference in the CFR between the two groups was not significant. These findings confirmed that asplenic patients had more severe IPD than patients with a spleen.
We also found significantly higher odds of meningitis in patients infected with serotypes 10A or 23A, which are not included in PCV13. A recent study in England and Wales reported similar findings of significantly increased odds of meningitis with serotypes 10A, 23B, and 35B16. Another study from Israel also demonstrated that the percentage of adult patients with meningitis was significantly higher for IPD caused by serotypes 24F, 23F, 15B/C, 23B, or 23A15. These findings indicate that the serotypes that commonly cause meningitis in adults include both types contained in PPSV23 (such as 10A and 15B/C) and nonvaccine types (such as 23A, 23B, 24F), plus 23F, and support the idea that there is limited impact of pediatric PCV13 on meningitis in adults. A recent study from the Pneumococcal Serotype Replacement and Distribution Estimation project assessed the serotype distribution of the remaining serotypes involved in pneumococcal meningitis worldwide24. The study demonstrated the percentage of pneumococcal meningitis occurring after infection with serotypes included in the current PCV13 and upcoming PCV products including PCV20 or PCV24, for all cases of meningitis in locations using PCV1325–28. While the percentage of PCV13 serotypes was 14.8% for patients aged < 5 years and 25.2% for those aged ≥ 5 years, the percentages of PCV20 or PCV24 serotypes were 56.5%–57.3% and 61.4–63.4%, respectively, for patients of all ages24. The higher percentages of PCV20 or PCV24 serotypes in cases of meningitis indicate that the higher-valency PCVs have enhanced potential to prevent more cases of pneumococcal meningitis in children and adults.
Because the rate of vaccination with PCV13 in Japanese adults is currently negligible, the significant decrease of the percentage of PCV13 serotypes in all cases of IPD during the study period suggests an indirect effect of pediatric PCV13 vaccination. Although no difference was found in the percentage of PPSV23 serotypes for total cases of IPD, a slight, but significant decrease was found in the percentage of PPSV23 serotypes in nonmeningitis cases, but not in meningitis cases. This may be because the indirect effect of PCV13 is more evident in nonmeningitis than in meningitis: although the percentage of PCV13 serotypes was significantly decreased in nonmeningitis cases, no significant difference was found in meningitis cases. This finding indicates that the indirect effect of pediatric PCV13 vaccination on meningitis in adults in Japan has a limited impact, probably because of an increase in cases caused by the non-PCV13 serotypes, such as 10A or 23A. We also noted that there was a small, but consistent decease from 2014 to 2018 in meningitis cases. This could be attributable to our inability to detect a statistical decrease because of the relatively small number of cases caused by PCV13 serotypes. A study from Israel also reported that nonmeningitis IPD but not meningitis decreased after PCV13 implementation15. The authors suggested that this was because of an increase in cases caused by non-PCV13 serotypes. By contrast, a study in England and Wales reported that the replacement of PCV7 by PCV13 in 2010 decreased the incidence of pneumococcal meningitis, mainly those caused by the additional serotypes included in PCV13, without any increase in cases caused by non-PCV13 serotypes16.
In our study, 35.1% of pneumococcal isolates (n = 222) from patients with meningitis were PCG resistant. By contrast, only 0.7% and 0.4% of pneumococcal isolates (n = 1254) from nonmeningitis cases showed intermediate resistance or resistance to PCG. The difference in the MIC values for PCG between meningitis and nonmeningitis cases was responsible for the different MIC breakpoints for PCG, because the values of MIC50 and MIC90 for each antimicrobial agent were similar for the two groups. Based on this finding of reduced β-lactam susceptibility, it may be advisable for clinicians to administer ceftriaxone or meropenem plus vancomycin for adult patients suspected of having pneumococcal meningitis until susceptibility results are reported6,29. Japanese investigators recently reported that all patients with pneumococcal meningitis were treated with two or more antibiotics30. In that study, antimicrobial treatment was frequently initiated with ceftriaxone, followed by sulbactam/ampicillin, tazobactam/piperacillin, and vancomycin.
Here we found that the odds of meningitis were higher in the presence of the pbp1bA641C mutation (aOR 2.92, 95% CI 1.51–5.65), and lower for patients aged ≥ 65 years (aOR 0.55, 95% CI 0.40–0.74), although we also found a significant association between the pbp1bA641C mutation and PCG resistance. These data confirm the previously reported association of the pbp1bA641C mutation with meningitis11.
This study had several limitations. First, 33.1% of all 2213 cases reported to the National Epidemiological Surveillance of Infectious Disease from 10 prefectures in Japan during the study period were not enrolled in this study. Second, the reporting of some variables was incomplete for some enrolled cases. Therefore, the results of our study may not be fully representative of the population of interest. Third, abdominal computed tomography scans were not examined for all enrolled cases to detect asplenia or hyposplenia. The clinical information about splenectomy may be inadequate. Therefore, we might have underestimated the number of IPD patients with asplenia/hyposplenia or splenectomy.
In conclusion, the incidence of pneumococcal meningitis in adults remained unchanged during 2016–2018. Patient ages and the CFR were significantly lower in meningitis cases than in nonmeningitis cases. The odds of developing meningitis were higher for asplenic/hyposplenic or splenectomized patients and for infection with serotypes 10A or 23A. An indirect effect of pediatric PCV13 on nonmeningitis cases in adults in Japan was evident, but its impact on meningitis cases was limited because of an increase in cases caused by non-PCV13 serotypes.
Materials and methods
IPD surveillance, case definition, bacterial strains and serotyping
The Adult IPD Study Group (https://www.niid.go.jp/niid/ja/ibi.html) conducted population-based surveillance of IPD in Japan from January 2014 to December 2018. IPD occurring in people over the age of 15 who lived in 10 prefectures (Hokkaido, Miyagi, Yamagata, Niigata, Mie, Nara, Kochi, Fukuoka, Kagoshima, and Okinawa) was included in this surveillance. A case of IPD was defined as isolation of S. pneumoniae by bacterial culture or detection of S. pneumoniae-specific DNA targeting lytA by PCR amplification18 from a sterile site such as blood or CSF, or a positive test result of pneumococcal antigen in CSF. As the routine procedure, we isolated S. pneumoniae in samples from the sterile sites, and pneumococcal isolate was serotyped by a capsule Quellung reaction with serotype-specific rabbit antiserum (Statens Serum Institute, Copenhagen, Denmark), as described17. Detection of S. pneumoniae-specific DNA targeting lytA and the serotyping by multiplex serotyping PCR for the cases with a positive reaction from the initial PCR were carried out in response to the request from clinicians at the Department of Bacteriology I, National Institute of Infectious Diseases (NIID), although these were not done routinely. Pneumococcal antigen testing in CSF was performed as a supportive diagnosis for pneumococcal meningitis at a hospital laboratory.
Pneumococcal isolates were identified by commonly used methods such as colony morphology on sheep blood agar, etc., alpha-hemolysis, optochin-sensitivity, and bile solubility at a hospital laboratory19. As a general procedure, the pneumococcal strain isolated on 5% sheep blood agar, etc. at a hospital laboratory was collected by the public health center, and sent to the prefectural public health institute. The prefectural public health institute sent the isolate in transport medium to NIID by a special parcel delivery. When the bacterial isolate showed positive reaction with pneumococcal group antiserum 11 and factor antiserum 11c, but showed negative reaction with factor antisera 11b, 11f, and 11g using the Quellung reaction, the serotype of the strain was determined to be type 11A/E. When the bacterial isolate did not show a positive reaction with any antiserum, and no obvious capsule was detected by India ink staining, the serotype was determined to be nontypeable31.
All IPD cases were classified into two groups: meningitis or nonmeningitis. A meningitis case was defined as IPD with typical meningeal signs, and nonmeningitis as IPD other than meningitis. Pneumococcal meningitis in adults often appears rapidly with typical symptoms such as high fever, headache, altered mental status and neck stiffness32. The annual incidence rates of IPD and pneumococcal meningitis were calculated based on the population denominators of the 10 prefectures obtained from the Statistics Bureau of Japan33. When a case of IPD was reported to the Study Group, clinical information and the pneumococcal isolates were sent to NIID. The clinical information included sex, age, smoking history, alcohol use, history of pneumococcal vaccination, current underlying diseases, immunocompromised conditions, asplenia/splenic hypoplasia (hyposplenia) or splenectomy, and fatal outcome. One isolate per case was included. Neither the laboratory methods nor the procedures of specimen collection have changed during the study period.
Antimicrobial susceptibility test
Because one of the isolates did not grow under the conditions used for the antimicrobial susceptibility test, 1476 strains were examined by the microbroth dilution method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. The MIC breakpoints were determined for PCG, ceftriaxone, meropenem, and vancomycin according to the CLSI criteria34. These antimicrobial agents were included in antimicrobial susceptibility tests, because β-lactam antibiotics are commonly used to treat pneumococcal infections and vancomycin is the first-line drug for treatment of meningitis caused by penicillin-resistant S. pneumoniae. In addition, the CLSI has stated that penicillin, ceftriaxone, or meropenem should be tested by a reliable MIC method and reported routinely for pneumococcal isolates from CSF34. Such isolates can also be tested against vancomycin using the MIC or disk diffusion method.
Detection of a point mutation in the pbp1b gene
Primer sequences, PCR amplification, and sequencing for detection of the pbp1bA641C mutation in 1477 pneumococcal strains were performed as previously described 11.
Statistical analysis
The demographic data and clinical characteristics of patients with meningitis and nonmeningitis IPD were compared using the χ2 test, Fisher’s exact test, or the Mann–Whitney nonparametric U test. The odds for serotypes of strains of developing meningitis were estimated using multivariate logistic regression model, adjusted by the variables higher for meningitis cases in univariate analysis. Categorical variables are presented as the number and frequency, and continuous variables as means (± standard deviations) or median (range). The Mantel–Haenszel test for trend was used to evaluate the trend in vaccine coverage from 2014 to 2018. The effect of the pbp1bA641C mutation on meningitis was estimated using a mixed-effects logistic regression model as described11. All statistical analyses were performed using IBM SPSS Statistics version 24 (IBM Corp., Armonk, NY, USA) or Stata software version 16 (StataCorp LLC, College Station, TX, USA). Statistical significance was set at p < 0.05.
Ethics statement
This study was reviewed and approved by the Medical Research Ethics Committee of National Institute of Infectious Diseases for the Use of Medical Subjects (approval no. 707), and was conducted according to the principles expressed in the Declaration of Helsinki. The requirement for informed consent was waived because the data do not contain any patient identifiers and samples were taken as part of standard patient care.
Supplementary Information
Acknowledgements
This work was supported by the Ministry of Health, Labour and Welfare of Japan HA Program Grant Number JPMH19HA1005.
Abbreviations
- IPD
Invasive pneumococcal disease
- PBPs
Penicillin-binding proteins
- PCV
Pneumococcal conjugate vaccine
- PPSV23
23-Valent pneumococcal polysaccharide vaccine
- PCR
Polymerase chain reaction
- CFR
Case fatality rate
- PCG
Penicillin G
- MIC
Minimum inhibitory concentration
- CSF
Cerebrospinal fluid
- CLSI
Clinical and Laboratory Standards Institute
- NIID
National Institute of Infectious Diseases
Author contributions
B.C., K.T., H.F., and K.O., conceived and designed the study, and prepared the manuscript. B.C. and Y.K. generated bacteriological data. H.W., Y.T., K. K., J.F., K.O., T.M., S.A., K.K, J.N., T.K., Y.S., R.S., M.F., T.S., and M.S. collected epidemiological data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Bin Chang, Kosuke Tamura and Hiroyuki Fujikura.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Kazunori Oishi, Email: toyamaeiken1@chic.ocn.ne.jp.
the Adult IPD Study Group:
Bin Chang, Kosuke Tamura, Hiroyuki Fujikura, Hiroshi Watanabe, Yoshinari Tanabe, Koji Kuronuma, Jiro Fujita, Kengo Oshima, Takaya Maruyama, Shuichi Abe, Kei Kasahara, Junichiro Nishi, Tetsuya Kubota, Yuki Kinjo, Yusuke Serizawa, Reiko Shimbashi, Munehisa Fukusumi, Tomoe Shimada, Tomimasa Sunagawa, Motoi Suzuki, Kazunori Oishi, Kenji Gotoh, Chikako Tsubata, Hiroki Takahashi, Tetsuji Aoyagi, Masashi Nakamatsu, Naoko Imuta, Akihito Yokoyama, Hiroaki Takeda, and Masayuki Ishida
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06950-w.
References
- 1.Gray BM, Converse GM, III, Dillon HC., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: Acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 1980;142:923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 2.Yasuda I, et al. The low carriage prevalence of pneumococcus among community-dwelling older people: A cross-sectional study in Japan. Vaccine. 2020;38:3752–3758. doi: 10.1016/j.vaccine.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 4.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018;16:355–367. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thigpen MC, et al. Emerging infections programs network. Bacterial meningitis in the United States, 1998–2007. N. Engl. J. Med. 2011;364:2016–2025. doi: 10.1056/NEJMoa1005384. [DOI] [PubMed] [Google Scholar]
- 6.Janoff EN, Musher DM. Streptococcus pneumoniae. In: Bennet JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennet’s Principles and Practice of Infectious Diseases. 9. Elsevier Press; 2020. pp. 2473–2491. [Google Scholar]
- 7.Centers for Disease Control and Prevention. Pneumococcal disease.https://www.cdc.gov/pneumococcal/index.html (Accessed 31 December 2020).
- 8.Wahl B, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–15. Lancet Global Health. 2018;6:e744–e757. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiess N, Groce NE, Dua T. The impact and burden of neurological sequela following bacterial meningitis: A narrative review. Microorganisms. 2021;9:900. doi: 10.3390/microorganisms9050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakenbeck R, Grebe T, Zahner D, Stock JB. β-Lactam Resistance in Streptococcus pneumoniae: Penicillin-binding proteins and non-penicillin-binding proteins. Mol. Microbiol. 1999;33:673–678. doi: 10.1046/j.1365-2958.1999.01521.x. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, et al. Genome-wide association analyses of invasive pneumococcal isolates identify a missense bacterial mutation associated with meningitis. Nat. Commun. 2019;10:178. doi: 10.1038/s41467-018-07997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feikin DR, et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: A pooled analysis of multiple surveillance sites. PLoS Med. 2013;10:e1001517. doi: 10.1371/journal.pmed.1001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suga S, et al. Nationwide population-based surveillance of invasive pneumococcal disease in Japanese children: Effects of the seven-valent pneumococcal conjugate vaccine. Vaccine. 2015;33:6054–6060. doi: 10.1016/j.vaccine.2015.07.069. [DOI] [PubMed] [Google Scholar]
- 14.Fukusumi M, et al. Invasive pneumococcal disease among adults in Japan, April 2013 to March 2015: Disease characteristics and serotype distribution. BMC Infect. Dis. 2017;17:2. doi: 10.1186/s12879-016-2113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regev-Yochay G, et al. Pneumococcal meningitis in adults after introduction of PCV7 and PCV13, Israel, July 2009–June 2015. Emerg. Infect. Dis. 2018;24:1275–1284. doi: 10.3201/eid2407.170721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oligbu G, et al. Effect of pneumococcal conjugate vaccines on pneumococcal meningitis, England and Wales, July 1, 2000–June 30, 2016. Emerg. Infect. Dis. 2019;25:1708–1718. doi: 10.3201/eid2509.180747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang B, et al. Capsule switching and antimicrobial resistance acquired during repeated Streptococcus pneumoniae pneumonia episodes. J. Clin. Microbiol. 2015;53:3318–3324. doi: 10.1128/JCM.01222-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ubukata K, Asahi Y, Yamane A, Konno M. Combinational detection of autolysin and penicillin-binding protein 2B genes of Streptococcus pneumoniae by PCR. J. Clin. Microbiol. 1996;34:592–596. doi: 10.1128/jcm.34.3.592-596.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Gloria Carvalho M, et al. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J. Clin. Microbiol. 2010;48:1611–1618. doi: 10.1128/JCM.02243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moisi JC, et al. Enhanced diagnosis of pneumococcal meningitis with use of the Bianx Now immunochromatographic test of Streptococcus pneumoniae antigen: A multisite study. Clin. Infect. Dis. 2009;48:S49–56. doi: 10.1086/596481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruetzmann S, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.William BM, Corazza GR. Hyposplenism: A comprehensive review. Part I: Basic concepts and causes. Hematology. 2007;12:1–13. doi: 10.1080/10245330600938422. [DOI] [PubMed] [Google Scholar]
- 23.Marrie TJ, Tyrrell GJ, Majumdar SR, Eurich DT. Asplenic patients and invasive pneumococcal disease-how bad is it these days? Int. J. Infect. Dis. 2016;51:27–30. doi: 10.1016/j.ijid.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Garcia QM, et al. Serotype distribution of remaining pneumococcal meningitis in the mature PCV10/13 period: Findings from the PSERENADE Project. Microorganisms. 2021;9:738. doi: 10.3390/microorganisms9040738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitz-Patrick D, et al. A randomized phase 1 study of the safety and immunogenicity of 2 novel pneumococcal conjugate vaccines in healthy Japanese adults in the United States. Hum. Vaccin. Immunother. 2021;5:1–8. doi: 10.1080/21645515.2020.1863177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurley D, et al. Safety, tolerability, and immunogenicity of a 20-valent pneumococcal conjugate vaccine (PCV20) in adults 60 to 64 years of age. Clin. Infect. Dis. 2020;73:e1489–e1497. doi: 10.1093/cid/ciaa1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson A, et al. Phase 1 trial of a 20-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2019;37:6201–6207. doi: 10.1016/j.vaccine.2019.08.048. [DOI] [PubMed] [Google Scholar]
- 28.Fairman J, et al. Non-clinical immunological comparison of a next-generation 24-valent pneumococcal conjugate vaccine (VAX-24) using site-specific carrier protein conjugation to the current standard of care (PCV13 and PPV23) Vaccine. 2021;39:3917–3206. doi: 10.1016/j.vaccine.2021.03.070. [DOI] [PubMed] [Google Scholar]
- 29.van Ettekoven CN, van de Beek D, Brouwer MC. Update on community-acquired bacterial meningitis: Guidance and challenges. Clin. Microbiol. Infect. 2017;23:601–606. doi: 10.1016/j.cmi.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Yanagihara K, et al. Serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae associated with invasive pneumococcal disease among adults in Japan. Int. J. Infect. Dis. 2021;102:260–268. doi: 10.1016/j.ijid.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Park IH, et al. Nontypable pneumococci can be divided into multiple cps types, including one type expressing the novel gene pspK. MBio. 2012;3:e00035-00112. doi: 10.1128/mBio.00035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huwerndiek S, Steiner T, Tonshoff B. When should you suspect meningitis? MMW Fortschr. Med. 2007;149(Suppl 2):15–18. [PubMed] [Google Scholar]
- 33.Statistics of Bureau of Japan. Population Estimates. (Accessed on 8 September 2021) (https://www.stat.go.jp/english/data/jinsui/).
- 34.CLSI. Performance Standards for Antimicrobial Susceptibility Testing; CLSI supplement M100, 29th ed. Clinical and Laboratory Standards Institute, Wayne, PA
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.