Abstract
Three strains of a gram-positive catalase-negative, facultatively anaerobic coccus-shaped organism originating from human clinical samples were characterized by phenotypic and molecular taxonomic methods. Sequencing of genes encoding 16S rRNA showed that the strains are phylogenetically closely related (99.9 to 100% sequence similarity) and represent a new subline within the genus Facklamia. The unknown bacterium was readily distinguished from all currently described species of the genus Facklamia (viz., Facklamia hominis, Facklamia ignava, and Facklamia sourekii) by biochemical tests and electrophoretic analysis of whole-cell proteins. Based on phylogenetic and phenotypic evidence, it is proposed that the unknown bacterium be classified as Facklamia languida sp. nov. The type strain of F. languida is CCUG 37842.
Over the past few years there has been a considerable expansion in the number of genera and species of aerobic or facultatively anaerobic catalase-negative gram-positive cocci identified from human clinical sources. Much of this change has stemmed from the increased use of molecular genetic (e.g., 16S rRNA gene sequencing) and molecular chemical (e.g., whole-cell protein analysis) methodologies for the identification of these organisms from clinical samples. In particular, 16S rRNA gene sequencing has resulted in phylogenetically based descriptive frameworks, which, together with rapid sequencing technology and readily accessible sequence libraries, are providing diagnostic laboratories with immensely powerful technology for identifying not only atypical or problematic members of existing taxa but also a wealth of new and diverse organisms. Examples of new catalase-negative gram-positive cocci from human sources include Abiotrophia elegans (16), Aerococcus urinae (1), Alloiococcus otitis (2), Dolosigranulum pigrum (3), Facklamia hominis (5), Facklamia ignava (10), Gemella bergeri (9), Gemella sanguinis (8), Globicatella sanguinis (4), Helcococcus kunzii (6), and Ignavigranum ruoffiae (11). During an investigation of atypical gram-positive catalase-negative chain-forming cocci from human sources, we have encountered three Facklamia-like isolates from clinical materials. Preliminary biochemical studies indicated that these strains did not conform to any of the currently recognized species of this genus, viz., F. hominis (5), F. ignava (10), and F. sourekii (7). In this paper, we report the phenotypic characteristics of these clinical isolates and the results of a molecular genetic and molecular chemical analysis. Based on the findings presented here, we consider the unknown chain-forming coccus to represent a new species of the genus Facklamia, for which the name F. languida sp. nov. is described.
Two strains (1144-97 and 1664-95) from human sources were referred to the Centers for Disease Control and Prevention (Atlanta, Ga.) for identification. Strain 1144-97 (Culture Collection of the University of Göteborg, CCUG 37842T) was recovered from a blood culture of a 5-year-old female child with bacteremia in Ohio. Strain 1664-95 (=CCUG 37420) was received from Marguerite Lovegren (Edmonton, Canada) and originated from blood cultures of a 74-year-old woman with a urinary tract infection. Strain CCUG 30940 was submitted by the Public Health Laboratory Service in Göteborg (Sweden) to the Culture Collection of the University of Göteborg for identification and originated from cerebrospinal fluid in a 40-year-old woman. No additional clinical information is available on the three strains. The unidentified organisms were cultured on Columbia agar (Difco, Detroit, Mich.) supplemented with 5% defibrinated horse blood (Oxoid, Unipath Ltd., Basingstoke, United Kingdom) at 37°C, in air plus 5% CO2. The strains were biochemically characterized by using the API rapid ID32S and API ZYM systems according to the manufacturer’s instructions (API bioMérieux, Marcy l’Etoile, France). Conventional physiological tests were also conducted as described by Facklam and Elliott (13). Polyacrylamide gel electrophoresis (PAGE) analysis of whole-cell proteins was performed as described by Pot et al. (15). For densitometric analysis, normalization, and interpretation of protein patterns the GelCompar GCW 3.0 software package (Applied Maths, Kortrijk, Belgium) was used. The similarity between all pairs of traces was expressed by the Pearson product moment correlation coefficient converted for convenience to a percentage similarity value. Phylogenetic analysis was conducted by 16S rRNA gene sequencing. A large fragment of the 16S rRNA gene was amplified by PCR using conserved primers close to the 3′ and 5′ ends of the gene and directly sequenced with a Taq Dye-Deoxy terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.) and an automatic DNA sequencer (model 373A; Applied Biosystems). The closest known relatives of the new isolate were determined by performing database searches. These sequences and those of other known related strains were retrieved from the GenBank or Ribosomal Database Project libraries and aligned with the newly determined sequence by using the program PILEUP (12). The resulting multiple-sequence alignment was corrected manually, and a distance matrix was calculated by using the programs PRETTY and DNADIST (using the Kimura-2 correction parameter) (14). A phylogenetic tree was constructed according to the neighbor-joining method with the program NEIGHBOR, and the stability of the groupings was estimated by bootstrap analysis (500 replications) by using the programs DNABOOT, DNADIST, NEIGHBOR, and CONSENSE (14).
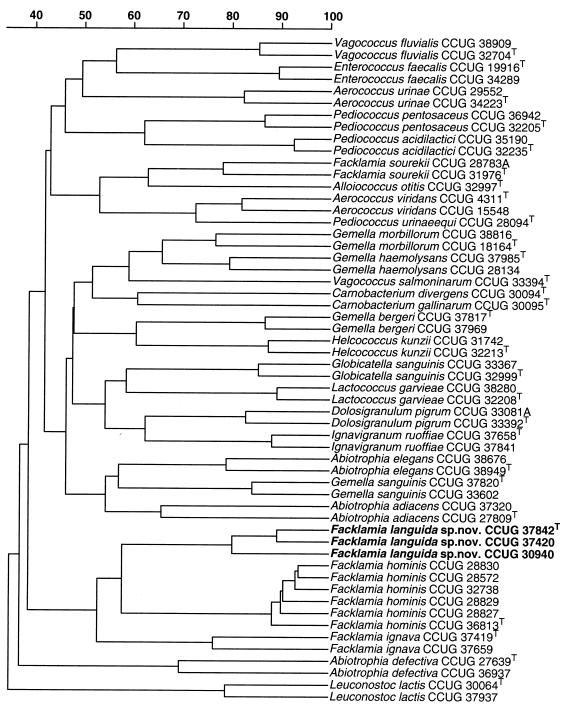
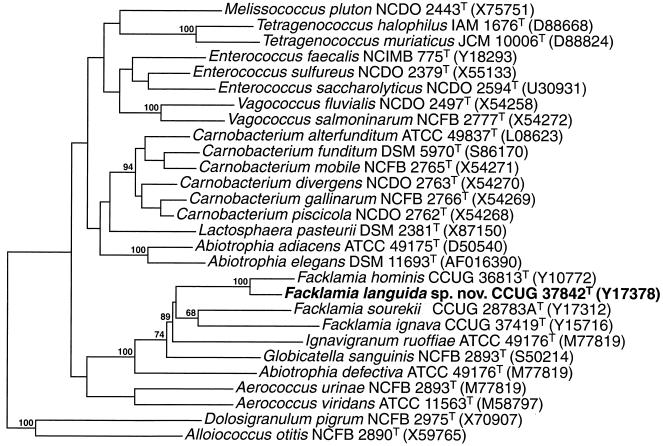
On Trypticase soy agar containing 5% sheep blood (TSA-SB) (Becton Dickinson Co., Cockeysville, Md.), after 24 h of incubation in an atmosphere of increased CO2, the isolates formed small gray to colorless colonies with little hemolytic activity on the sheep blood cells. After 48 h of incubation the colonies were surrounded by a small zone of alpha hemolysis. All three isolates were ovoid and most commonly formed pairs, but single cells and short chains were also observed. They were gram positive, non-spore forming, catalase negative, oxidase negative, and facultatively anaerobic. The strains grew in 6.5% NaCl at 37°C but not at all at 10 or 45°C and were pyrrolidonyl arylamidase and leucine aminopeptidase positive in conventional tests (13). The three isolates gave the profile 00020500000 with the API rapid ID32S strip and corresponded to a doubtful profile. Significant taxa were Gemella morbillorum (four tests against) and Gemella haemolysans (three tests against). When commercial API systems were used, the isolates produced acid from trehalose but failed to produce acid from d-arabitol, l-arabinose, cyclodextrin, glycogen, lactose, maltose, mannitol, melibiose, melezitose, pullulan, raffinose, ribose, sorbitol, sucrose, tagatose, or methyl-β-d-glucopyranoside. Alkaline phosphatase, pyroglutamic acid arylamidase, leucine arylamidase, and glycyl-tryptophan arylamidase activities were detected. No activity was detected for arginine dihydrolase, acid phosphatase, alanine-phenylalanine-proline arylamidase, β-galacturonidase, α-galactosidase, β-galactosidase, β-glucuronidase, α-glucosidase, β-glucosidase, lipase C14, α-fucosidase, α-mannosidase, β-mannosidase, N-acetylglucosaminidase, cysteine arylamidase, chymotrypsin, trypsin, or urease. Esterase C4 and ester lipase C8 activity was found to be variable. The strains did not hydrolyze hippurate and were Voges-Proskauer negative. A numerical analysis of the whole-cell protein patterns of the three clinical strains along with a comprehensive range of other gram-positive catalase-negative reference organisms is shown in Fig. 1. The three strains formed a distinct cluster grouping at a correlation level of approximately 80%. The PAGE groupings nearest to the unknown bacterium were formed by strains of F. hominis and F. ignava. It is very evident from the protein analysis that the unknown coccus is distinct from the aforementioned species and all the other reference species examined, including Abiotrophia spp., Aerococcus spp., Alloiococcus otitis, Carnobacterium spp., D. pigrum, Gemella spp., and Globicatella sanguinis (Fig. 1). To assess the phylogenetic relations of the unknown clinical isolates, their 16S rRNA gene sequences were determined and subjected to comparative analysis. Over 1,440 bases were determined, and pairwise analysis revealed that the 16S rRNA genes of the three strains were 99.9 to 100% similar (corresponding to 0 or 1 base difference). Sequence searches of GenBank and Ribosomal Database Project libraries revealed that the unknown coccus was phylogenetically most closely associated with the lactic acid group of bacteria. Closely related sequences were retrieved, and a tree constructed by neighbor-joining depicting the phylogenetic affinity of the unknown coccus as exemplified by strain CCUG 37842T is shown in Fig. 2. The unknown bacterium clustered within the Facklamia clade, and from the branching pattern it is evident that F. hominis is its closest phylogenetic relative. Indeed, the clustering of these organisms occurred in 100% of 500 tree replications. Despite the statistically significant association between the unknown bacterium and F. hominis, pairwise comparisons showed that the 16S rRNA of the unknown organism possessed 44 (strains CCUG 37842T and CCUG 37420) or 43 (strain CCUG 30940) base differences (based on 1,440 positions) with F. hominis (equivalent to 3% 16S rRNA sequence divergence).
FIG. 1.
Similarity dendrogram based on whole-cell protein pattern of F. languida sp. nov. and related species. Levels of correlation are expressed as percentages for convenience.
FIG. 2.
Unrooted tree showing the phylogenetic relationships of F. languida sp. nov. and some other low-G+C gram-positive bacteria. The tree was constructed by the neighbor-joining method and was based on a comparison of approximately 1,320 nucleotides. Bootstrap values, expressed as percentages of 500 replications, are given at branching points. Abbreviations: ATCC, American Type Culture Collection; DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH; IAM, Institute of Applied Microbiology; JCM, Japanese Collection of Microorganisms; NCDO, National Collection of Dairy Organisms; NCFB, National Collection of Food Bacteria; NCMIB, National Collection of Industrial and Marine Bacteria.
It is evident from comparative 16S rRNA gene sequencing that the unidentified organism from clinical material represents a new subline within the genus Facklamia, close to, but distinct from, F. hominis. 16S rRNA sequence divergence values of 3% or more between the unknown bacterium and F. hominis, F. ignava, and F. sourekii unequivocally demonstrate that the bacterium represents a hitherto-unknown Facklamia species. It is pertinent that the three currently known Facklamia species have all been isolated from human clinical material. F. hominis, the type species of the genus, was described by Collins et al. (5) for some strains of chain-forming cocci isolated from a variety of specimens, including blood, urine, abscesses, and vaginal swabs. Similarly, F. ignava and F. sourekii also originated from human clinical sources (7, 10). The strains characterized in the present study clearly represent yet another member of this new genus recovered from clinical specimens. The three human isolates of this novel species were biochemically closely related to each other, and PAGE analysis of whole-cell proteins further demonstrated their high overall phenotypic homogeneity. Although displaying a close phylogenetic affinity with F. hominis, the unknown coccus could be readily distinguished from this species, in producing acid from trehalose, failing to hydrolyze hippurate, and not displaying arginine dihydrolase, alanine-phenylalanine-proline arylamidase, α-galactosidase, or β-galactosidase activity. Similarly, the unknown bacterium biochemically differs from F. ignava in producing glycyl-tryptophan arylamidase but not alanine-phenylalanine-proline arylamidase and from F. sourekii in failing to produce acid from a wide range of carbohydrates, viz., d-arabitol, maltose, mannitol, sorbitol, and sucrose, and by not hydrolyzing hippurate. Based on the results of the comparative 16S rRNA analysis and the distinct electrophoretic protein patterns and biochemical reactions of the unknown coccus, we consider that the bacterium merits classification as a new species, for which the name Facklamia languida (languida, pertaining to the organism’s lack of activity in the biochemical tests used) is proposed. Tests which distinguish F. languida sp. nov. from other members of the genus Facklamia are summarized in Table 1.
TABLE 1.
Characteristics differentiating F. languida sp. nov. from other Facklamia speciesa
| Test | F. sourekii | F. ignava | F. hominis | F. languida sp. nov. |
|---|---|---|---|---|
| Acid from: | ||||
| d-Arabitol | + | − | − | − |
| Maltose | + | − | − | − |
| Mannitol | + | − | − | − |
| Sorbitol | + | − | − | − |
| Sucrose | + | − | − | − |
| Trehalose | + | V | − | + |
| Production of: | ||||
| Arginine dihydrolase | − | − | + | − |
| Alanine-phenylalanine-proline arylamidase | − | + | + | − |
| α-Galactosidase | − | − | + | − |
| β-Galactosidase | − | − | + | − |
| Glycyl-tryptophan aryl-amidase | − | − | V | + |
| Hydrolysis of hippurate | + | + | + | − |
V, variable.
Description of Facklamia languida sp. nov.
On TSA-SB after 24 h of incubation in air plus CO2, small grey to colorless colonies which show little hemolytic activity are formed. After 48 h of incubation, colonies are surrounded by a small zone of alpha hemolysis. Cells are ovoid and usually form pairs, but single cells and short chains may also occur. Facultatively anaerobic and catalase and oxidase negative. Grows in 6.5% NaCl at 37°C. Does not grow at 10 or 45°C. Pyrrolidonyl arylamidase and leucine aminopeptidase positive in conventional tests. In commercial API systems acid is produced from trehalose. Acid is not produced from d-arabitol, l-arabinose, cyclodextrin, glycogen, lactose, melibiose, mannitol, maltose, melezitose, methyl-β-d-glucopyranoside, pullulan, raffinose, ribose, sorbitol, sucrose, or tagatose. Alkaline phosphatase, pyroglutamic acid arylamidase, leucine arylamidase, and glycyl-tryptophan arylamidase activities are detected. Acid phosphatase, alanine-phenylalanine-proline arylamidase, arginine dihydrolase, N-acetyl-β-glucosaminidase, chymotrypsin, trypsin, cysteine arylamidase, α-fucosidase, α-galactosidase, β-galactosidase, β-galacturonidase, α-glucosidase, β-glucosidase, β-glucuronidase, lipase C14, α-mannosidase, β-mannosidase, and urease activities are not detected. Esterase C4 and ester lipase C8 may or may not be detected. Acetoin is not produced. Hippurate is not hydrolyzed. Isolated from human clinical specimens. Habitat is not known. The type strain is CCUG 37842T.
Nucleotide sequence accession number.
The 16S rRNA gene sequence of strain CCUG 37842T has been deposited in GenBank under accession no. Y18053.
Acknowledgments
We are grateful to Hans Trüper (University of Bonn, Bonn, Germany) for coining the species epithet, and we also thank the depositors of the strains. The excellent technical assistance of Lena Dahl is acknowledged.
REFERENCES
- 1.Aguirre M, Collins M D. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138:401–405. doi: 10.1099/00221287-138-2-401. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre M, Collins M D. Phylogenetic analysis of Alloiococcus otitis gen. nov., sp. nov., an organism from human middle ear fluid. Int J Syst Bacteriol. 1992;42:79–83. doi: 10.1099/00207713-42-1-79. [DOI] [PubMed] [Google Scholar]
- 3.Aguirre M, Morrison D, Cookson B D, Gay F W, Collins M D. Phenotypic and phylogenetic characterization of some Gemella-like organisms from human infections: description of Dolosigranulum pigrum gen. nov., sp. nov. J Appl Bacteriol. 1993;75:608–612. doi: 10.1111/j.1365-2672.1993.tb01602.x. [DOI] [PubMed] [Google Scholar]
- 4.Collins M D, Aguirre M, Facklam R R, Shallcross J, Williams A M. Globicatella sanguis gen. nov., sp. nov., a new gram-positive catalase-negative bacterium from human sources. J Appl Bacteriol. 1992;73:422–437. doi: 10.1111/j.1365-2672.1992.tb05000.x. [DOI] [PubMed] [Google Scholar]
- 5.Collins M D, Falsen E, Lemozy J, Åkervall E, Sjödén B, Lawson P A. Phenotypic and phylogenetic characterization of some Globicatella-like organisms from human sources: description of Facklamia hominis gen. nov., sp. nov. Int J Syst Bacteriol. 1997;47:880–882. doi: 10.1099/00207713-47-3-880. [DOI] [PubMed] [Google Scholar]
- 6.Collins M D, Facklam R R, Rodrigues U M, Ruoff K L. Phylogenetic analysis of some Aerococcus-like organisms from clinical sources: description of Helcoccus kunzii gen. nov., sp. nov. Int J Syst Bacteriol. 1993;43:425–429. doi: 10.1099/00207713-43-3-425. [DOI] [PubMed] [Google Scholar]
- 7.Collins, M. D., R. A. Hutson, E. Falsen, and B. Sjöden. Facklamia sourekii sp. nov., isolated from human sources. Int. J. Syst. Bacteriol., in press. [DOI] [PubMed]
- 8.Collins M D, Hutson R A, Falsen E, Sjöden B, Facklam R R. Description of Gemella sanguinis sp. nov., isolated from human clinical specimens. J Clin Microbiol. 1998;36:3090–3093. doi: 10.1128/jcm.36.10.3090-3093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins M D, Hutson R A, Falsen E, Sjödén B, Facklam R R. Gemella bergeriae sp. nov., isolated from human clinical specimens. J Clin Microbiol. 1998;36:1290–1293. doi: 10.1128/jcm.36.5.1290-1293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins M D, Lawson P A, Monasterio R, Falsen E, Sjödén B, Facklam R R. Facklamia ignava sp. nov., isolated from human clinical specimens. J Clin Microbiol. 1998;36:2146–2148. doi: 10.1128/jcm.36.7.2146-2148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins M D, Lawson P A, Monasterio R, Falsen E, Sjoden B, Facklam R R. Ignavigranum ruoffiae sp. nov., isolated from human clinical specimens. Int J Syst Bacteriol. 1998;36:2146–2148. doi: 10.1099/00207713-49-1-97. [DOI] [PubMed] [Google Scholar]
- 12.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Facklam R, Elliott J A. Identification, classification, and clinical relevance of catalase-negative, gram-positive cocci, excluding the streptococci and enterococci. Clin Microbiol Rev. 1995;8:479–495. doi: 10.1128/cmr.8.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felsenstein J. PHYLIP—phylogeny inference package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 15.Pot B, Vandamme P, Kersters K. Analysis of electrophoretic whole-organism protein fingerprints. In: Goodfellow M, O’Donnell A G, editors. Modern microbial methods. Chemical; 1994. pp. 493–521. methods in prokaryotic systematics. J. Wiley and Sons Ltd., Chichester, United Kingdom. [Google Scholar]
- 16.Roggenkamp A, Abele-Horn M, Trebesius K-H, Tretter U, Autenrieth I B, Heesemann J. Abiotrophia elegans sp. nov., a possible pathogen in patients with culture-negative endocarditis. J Clin Microbiol. 1998;36:100–104. doi: 10.1128/jcm.36.1.100-104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]