Abstract
Objective:
Colorectal cancer (CRC) is a leading cause of cancer-related death worldwide. Obesity is a well-established risk factor for CRC, and fetal or developmental origins of obesity may underlie its effect on cancer in adulthood. We examined associations of maternal obesity, pregnancy weight gain, and birth weight and CRC in adult offspring.
Design:
The Child Health and Development Studies is a prospective cohort of women receiving prenatal care between 1959 and 1966 in Oakland, California (n=18,751 live births among 14,507 mothers). Clinical information was abstracted from mothers’ medical records six months prior to pregnancy through delivery. Diagnoses of CRC in adult (age ≥18 years) offspring were ascertained through 2019 by linkage with the California Cancer Registry. We used Cox proportional hazards models to estimate adjusted hazard ratios (aHR); we examined effect measure modification using single-referent models to estimate the relative excess risk due to interaction (RERI).
Results:
68 offspring were diagnosed with CRC over 738,048 person-years of follow-up, and half (48.5%) were diagnosed younger than age 50 years. Maternal obesity (≥30 kg/m2) increased risk of CRC in offspring (aHR 2.51, 95% CI 1.05, 6.02). Total weight gain modified the association of rate of early weight gain (RERI: −4.37, 95% CI: −9.49, 0.76), suggesting discordant growth from early to late pregnancy increases risk. There was an elevated association with birth weight (≥4,000g: aHR 1.95, 95% CI 0.86, 4.38).
Conclusion:
Our results suggest in utero events are important risk factors for CRC and may contribute to increasing incidence rates in younger adults.
Keywords: colorectal cancer, young adult, birth cohort, risk factor
Introduction
Colorectal cancer (CRC) is a leading cause of cancer-related morbidity and mortality worldwide.1 Incidence and mortality rates have evolved strikingly over the past several decades. For example, in many high-income countries, incidence and mortality rates have declined or stabilized in older adults but nearly doubled in younger adults.2 Low- and middle-income countries have experienced rapid increases in incidence and mortality rates across all ages.1 As a result of these temporal trends, by 2030, the global burden of CRC is expected to increase by 60% to more than 2.2 million new diagnoses and 1.1 million deaths.1
Obesity is a well-established risk factor of CRC,3–5 and several studies suggest fetal or developmental origins of obesity may underlie its effect on cancer in adulthood.6, 7 The concept that the intrauterine environment plays a major role in establishing a growth and health trajectory that extends over the life-course of the offspring is well-supported by epidemiologic and experimental studies.8–14 For example, maternal obesity predisposes infants to obesogenic growth patterns that persist across the life course;14, 15 pregnancy weight gain may have lasting effects on risks of obesity in offspring;9, 12, 13, 16–19 and birth size is consistently associated with measures of obesity in later life.20, 21 These factors are also independently associated with chronic diseases in adulthood, including cardiovascular disease and diabetes.22, 23
We examined associations of maternal obesity, pregnancy weight gain, and birth weight and CRC in adult offspring of the Child Health and Developments Studies (CHDS), a population-based cohort of more than 18,000 mother-child dyads receiving prenatal care in the Kaiser Foundation Health Plan (Oakland, CA) and followed for 60 years. We hypothesized that these factors may be markers of fetal growth and development and that increase risk of CRC in adulthood.
Materials and Methods
Study population
Established in 1959, the CHDS recruited nearly all (98%) pregnant women receiving prenatal care from the Kaiser Foundation Health Plan (Oakland, CA) between June 1959 and September 1966, with deliveries through June 1967 (n=18,751 live births excluding neonatal deaths among 14,507 mothers). At enrollment, mothers reported demographic and health-related information during in-person interviews. Clinical information, including prenatal visits, diagnosed conditions, and prescribed medications, were abstracted from mothers’ medical records beginning six months prior to pregnancy through labor and delivery. Additional details of the CHDS and methodology are available elsewhere.24–26
We monitor CHDS participants by annual linkage to the California Department of Motor Vehicles, California Department of Vital Statistics, and California Cancer Registry. Mothers and their families are matched to these sources using an accumulated name and address history, routinely identifying more than 80% of families.
Primary outcome
We ascertained incident diagnoses of CRC in adult (age ≥18 years) offspring through 2019 by linkage with the California Cancer Registry (International Classification of Disease in Oncology, 3rd edition, codes C18.0–1, C19.9, C20.9). The California Cancer Registry is one of the largest cancer registries in the U.S. and meets the highest quality data standards set by the National Program of Cancer Registries and U.S. Centers for Disease Control and Prevention.27, 28 We used a rigorous protocol to verify cases, comparing fixed (e.g., birth date, sex, race) and changeable (e.g., address) identifiers by manual review.
Exposures
Maternal body mass index.
We used height and weight reported by mothers during in-person interviews at enrollment or recorded at the first prenatal visit to measure body mass index (BMI, kg/m2). Weight was adjusted to compensate for variation in the timing of measurement by regressing weight on gestational age using the locally weighted scatterplot smoothing technique.29 Adjusted weight was then imputed as the fitted mean weight at day 104 of gestation (median value for day of interview) plus the residual from the regression procedure.30–32 We categorized BMI as underweight (<18.5 kg/m2) or healthy (18.5 – 24.9 kg/m2), overweight (25.0 – 29.9 kg/m2), and obese (≥30 kg/m2).
Pregnancy weight gain.
Using weight recorded at prenatal visits, we measured pregnancy weight gain as: 1) rate of early weight gain, or pounds gained per week through 32 weeks’ gestation; and 2) total weight gain, or the difference between the last pre-delivery weight and weight recorded at the first prenatal visit. Last pre-delivery weight was measured at a mean 272 days’ gestation (interquartile range [IQR] 265 – 282 days). We used these two measures of pregnancy weight gain based on evidence that the timing of weight gain has different consequences for fetal growth and development.33–36
Birth weight.
At the time of delivery, birth weight was measured using standardized scales maintained by CHDS research staff. We categorized birth weight using definitions of the U.S. Centers for Disease Control and Prevention and World Health Organization: low (<2,000g), average (2,000–3,999g), and high (≥4,000g or macrosomia). In sensitivity analyses, we used birth weight-for-gestation z-scores, calculated by subtracting individual birth weight from the mean for each gestational week and dividing the difference by the standard deviation of the mean.30 We examined z-scores continuously and at or above the 90th percentile.
Other covariates
Other covariates included race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Asian, other), age at pregnancy, parity at pregnancy (primiparous, multiparous), maternal education (less than high school, high school or trade school, some college or more), total family income (above or below the median, adjusted for 1960 dollars), and gestational age (<37 weeks, ≥37 weeks). Gestational age was calculated by subtracting the date of the last menstrual period from the date of delivery (range 20 – 42 weeks). We also measured family history of CRC, defined as a mother or father ever diagnosed with CRC, by linking maternal and paternal records to the California Cancer Registry.
Statistical analysis
We used Cox proportional hazards models to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) for associations of maternal BMI, pregnancy weight gain, and birth weight and CRC in offspring. To account for correlation between observations from siblings (n=4,244), we used robust sandwich estimators. Follow-up time was accrued from date of birth through date of CRC diagnosis, date of death, or date of last contact (range 6 months to 58.5 years). Because participants are regularly monitored for residence and vital status, we used year of last contact from all sources to create date of last contact. We assessed the proportional hazards assumption in all models by visually examining plots of the survival function vs. survival time, as well as log(−log(survival)) vs. log(survival time). The assumption was not violated in any model.
We explored non-linear relationships between pregnancy weight gain, including rate of early weight gain and total weight gain, and CRC in offspring using restricted cubic splines, with three knots at the 10th, 50th, and 90th percentiles.37 The relationship did not deviate from linearity, and model fit was similar when pregnancy weight gain was modeled as a continuous measure vs. restricted cubic spline (assessed via Akaike Information Criterion38). Therefore, we modeled rate of early weight gain and total weight gain as continuous measures, and to facilitate interpretation, we report HRs and their 95% CIs at the median of each quartile of the rate of early weight gain (0.40, 0.71, 0.93, and 1.25 pounds per week) and total weight gain (12, 18, 23, and 29 pounds).
We used a theory-based causal model39 (Figure 1) to guide selection of potential confounders and to identify a minimally sufficient adjustment set for each model.40 Adjustment sets included: race/ethnicity (maternal BMI); race/ethnicity, gestational age, and maternal BMI (rate of early weight gain); race/ethnicity, gestational age, maternal BMI, and rate of early weight gain (total weight gain); and race/ethnicity, gestational age, maternal BMI, rate of early weight gain, and total weight gain (birth weight).
Figure 1.
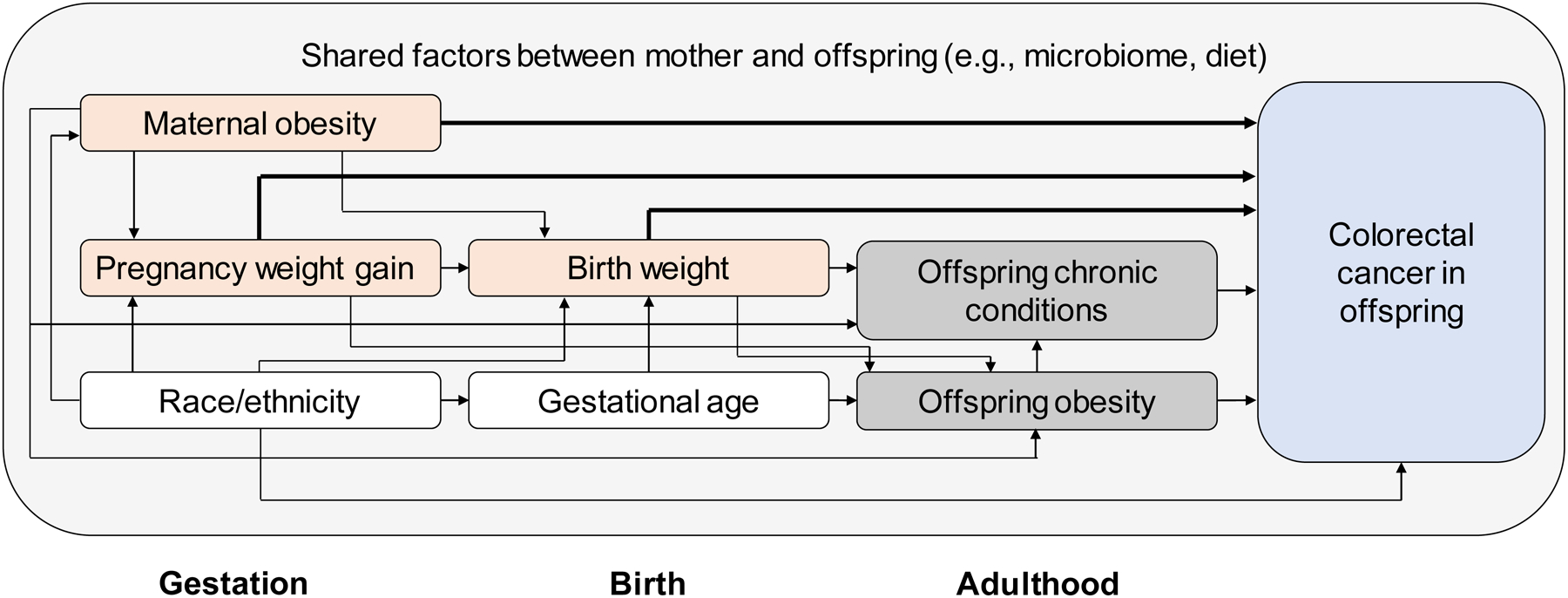
Theory-based causal model illustrating relationships among maternal body mass index, pregnancy weight gain, birth weight, and colorectal cancer in adult offspring.
Bold lines illustrate associations modeled in this study; shading denotes the following: orange: independent variable; blue: dependent variable; white: measured covariate; grey: unmeasured covariate.
We examined modification of high rate of early weight gain (quartile 4 vs. quartile 1) by total weight gain (below vs. at or above median) on both additive and multiplicative scales. First, we used single-referent models to estimate the relative excess risk due to interaction (RERI) and corresponding 95% CI.41 Second, we compared nested models with and without a high rate of early weight gain*total weight gain product term using a likelihood ratio test. We also estimated stratum-specific HRs to evaluate heterogeneity on a multiplicative scale.
Sensitivity Analyses
Missingness ranged from 0.0% (birth weight) to 15.6% (rate of early weight gain). In a sensitivity analysis, we used multiple imputation by fully conditional specification42 to estimate associations of maternal BMI, pregnancy weight gain, and birth weight and CRC in offspring. Fully conditional specification relaxes assumptions of joint multivariate normality and linearity and is well-suited for imputation of both categorical and continuous variables. Results in the imputed dataset did not materially differ from the complete case analysis and are reported in Supplementary Table 1.
About 5–10% of CRC is due to underlying genetic predisposition (e.g., Lynch syndrome), and the proportion is increased in persons diagnosed at a younger age.43 We conducted an additional sensitivity analysis to estimate associations of maternal BMI, pregnancy weight gain, and birth weight and CRC in offspring with no family history of CRC (n=17,686).
Quantitative Bias Analysis
Associations of maternal BMI, pregnancy weight gain, and birth weight and CRC in offspring may be confounded by shared factors between mother and offspring (e.g., diet, microbiome), which were not measured in the CHDS. We conducted a probabilistic bias analysis44, 45 to model error from unmeasured confounding. Additional detail is provided in the Online Supplement.
Patient and Public Involvement
The CHDS routinely engages its own cohort members in community-based participatory research. We meet quarterly with our Participant Advisory Council (PAC), a racially and sex diverse representation of the cohort, to develop research questions, resolve ethical issues related to study participation, design innovative recruitment methods, and improve dissemination of findings. At the beginning of this study, we met with PAC members to provide an overview of CRC and to discuss study concepts, rationale, and approach; they asked questions and offered feedback. Future meetings will be scheduled for presenting and interpreting ongoing results, and for discussing plans to optimize communication of findings to the larger cohort. Results will be disseminated through the CHDS website, social media platforms, and email newsletter.
The Institutional Review Board at the Public Health Institute and the University of Texas Health Science Center at Houston approved this study. All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC).
Results
Table 1 shows characteristics of 18,751 offspring. Most (48.2%) were born in the early 1960s. About one-third were racial/ethnic minorities (23.5% Black, 3.3% Hispanic, 3.9% Asian, 2.9% Other), and half (52.1%) were in families with an annual income less than the median.
Table 1.
Characteristics of 18,751 offspring1 in the Child Health and Development Studies, 1959 – 1967
| Male (n=9,582) |
Female (n=9,169) |
Total (n=18,751) |
|
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Offspring characteristics | |||
| Year of birth | |||
| 1959–61 | 2887 (30.1) | 2716 (29.6) | 5603 (29.9) |
| 1962–64 | 4636 (48.4) | 4409 (48.1) | 9045 (48.2) |
| 1965–67 | 2059 (21.5) | 2044 (22.3) | 4103 (21.9) |
| Race/ethnicity | |||
| Non-Hispanic White | 6266 (66.4) | 5999 (66.4) | 12265 (66.4) |
| Non-Hispanic Black | 2224 (23.6) | 2108 (23.3) | 4332 (23.5) |
| Hispanic | 305 (3.2) | 308 (3.4) | 613 (3.3) |
| Asian | 375 (4.0) | 344 (3.8) | 719 (3.9) |
| Other | 262 (2.8) | 281 (3.1) | 543 (2.9) |
| Missing | 150 | 129 | 279 |
| Gestational age | |||
| < 37 weeks | 794 (8.4) | 666 (7.4) | 1460 7.9 |
| ≥ 37 weeks | 8622 (91.6) | 8371 (92.6) | 16993 92.1 |
| Missing | 166 | 132 | 298 |
| Maternal characteristics 2 | |||
| Maternal age at pregnancy (years) | |||
| < 20 | 830 (8.8) | 847 (9.3) | 1677 (9.0) |
| 20–24 | 2863 (30.2) | 2785 (30.7) | 5648 (30.4) |
| 25–29 | 2748 (29.0) | 2632 (29.0) | 5380 (29.0) |
| 30–34 | 1721 (18.1) | 1595 (17.6) | 3316 (17.9) |
| 35–39 | 1003 (10.6) | 921 (10.1) | 1924 (10.4) |
| ≥ 40 | 326 (3.4) | 306 (3.4) | 632 (3.4) |
| Missing | 91 | 83 | 174 |
| Parity | |||
| Primiparous | 2940 (30.9) | 2825 (31.0) | 5765 (31.0) |
| Multiparous | 6567 (69.1) | 6285 (69.0) | 12852 (69.0) |
| Missing | 75 | 59 | 134 |
| Maternal education | |||
| Less than high school | 1458 (17.9) | 1441 (18.4) | 2899 (18.1) |
| High school or trade school | 3132 (38.4) | 3071 (39.1) | 6203 (38.8) |
| Some college or college degree | 3558 (43.7) | 3335 (42.5) | 6893 (43.1) |
| Missing | 1434 | 1322 | 2756 |
| Annual family income3 | |||
| ≤ median | 3460 (51.4) | 3433 (52.8) | 6893 (52.1) |
| > median | 3267 (48.6) | 3063 (47.2) | 6330 (47.9) |
| Missing | 2855 | 2673 | 5528 |
Live births excluding neonatal deaths among 14,507 women
Because mothers may have had more than one live birth during the study period, maternal characteristics are reported at the level of offspring
Median income adjusted to 1960 dollars = $6,303
Over 738,048 person-years of follow-up, 68 offspring were diagnosed with CRC (Table 2). Offspring were diagnosed in 1986 – 2017, between ages 18 – 56 years, and about half (48.5%) were diagnosed before age 50 years. The majority were diagnosed with regional (44.1%) or distant (25.0%) stage disease and with tumors in the distal colon (42.4%) or rectum (28.8%). Nearly 20% had a family history of CRC.
Table 2.
Characteristics of 68 adult offspring diagnosed with colorectal cancer, by age at diagnosis
| Age 18 – 49 years (n=33) | Age 50 – 56 years (n=35) | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Sex | ||||
| Male | 14 | (42.4) | 17 | (48.6) |
| Female | 19 | (57.6) | 18 | (51.4) |
| Year of birth | ||||
| 1959–61 | 10 | (30.3) | 18 | (51.4) |
| 1962–64 | 19 | (57.6) | 14 | (40.0) |
| 1965–67 | 4 | (12.1) | 3 | (8.6) |
| Race/ethnicity | ||||
| Non-Hispanic White | 19 | (57.6) | 16 | (50.0) |
| Non-Hispanic Black | 13 | (39.4) | 6 | (18.8) |
| Hispanic | 0 | (0.0) | 5 | (15.6) |
| Asian | 0 | (0.0) | 3 | (9.4) |
| Other | 1 | (3.0) | 2 | (6.3) |
| Missing | 0 | 3 | ||
| Age at diagnosis (years) | ||||
| Median (IQR) | 44 | (40 – 48) | 52 | (51 – 53) |
| Year of diagnosis | ||||
| 1980–89 | 2 | (6.1) | 0 | (0.0) |
| 1990–99 | 3 | (9.1) | 0 | (0.0) |
| 2000–09 | 17 | (51.5) | 0 | (0.0) |
| 2010–16 | 11 | (33.3) | 35 | (100.0) |
| Stage at diagnosis | ||||
| Local | 6 | (18.2) | 13 | (39.4) |
| Regional | 19 | (57.6) | 11 | (33.3) |
| Distant | 8 | (24.2) | 9 | (27.3) |
| Missing | 0 | 2 | ||
| Tumor location | ||||
| Proximal colon | 7 | (21.9) | 12 | (35.3) |
| Distal colon | 19 | (59.4) | 9 | (26.5) |
| Rectum | 6 | (18.8) | 13 | (38.2) |
| Missing | 1 | 1 | ||
| Family history of CRC2 | ||||
| No | 27 | (81.8) | 29 | (82.9) |
| Yes | 6 | (18.2) | 6 | (17.1) |
Stage at diagnosis defined by SEER summary stage and includes: local (disease is confined to the large bowel), regional (disease is limited to nearby lymph nodes or other organs), and distant (systemic metastasis)
Family history of CRC defined as having a mother or father ever diagnosed with CRC and ascertained by linking maternal and paternal records to the California Cancer Registry
Table 3 illustrates relationships among maternal BMI, pregnancy weight gain, and birth weight. For example, a higher proportion of obese (16.2%) mothers had offspring weighing ≥4,000g at birth compared to underweight/healthy weight (7.4%) and overweight (11.0%) mothers. Rate of early weight gain and total weight gain also differed by maternal BMI.
Table 3.
Pregnancy weight gain and birth weight by maternal body mass index
| Maternal body mass index | |||
|---|---|---|---|
| Underweight/Healthy (n=12,223) | Overweight (n=3,005) | Obese (n=1,023) | |
| Rate of early weight gain1 | |||
| Quartile 1 | 2119 (19.2%) | 821 (34.6%) | 407 (54.1%) |
| Quartile 2 | 2897 (26.2%) | 479 (20.2%) | 114 (15.2%) |
| Quartile 3 | 3079 (27.9%) | 493 (20.8%) | 106 (14.1%) |
| Quartile 4 | 2960 (26.8%) | 583 (24.5%) | 125 (16.6%) |
| Missing | 1168 | 629 | 271 |
| Total weight gain2 | |||
| Quartile 1 | 2204 (18.3%) | 816 (27.7%) | 447 (44.8%) |
| Quartile 2 | 3148 (26.2%) | 661 (22.4%) | 192 (19.2%) |
| Quartile 3 | 3345 (27.8%) | 689 (23.4%) | 168 (16.8%) |
| Quartile 4 | 3339 (27.7%) | 779 (26.5%) | 191 (19.1%) |
| Missing | 187 | 60 | 25 |
| Birth weight | |||
| <2,500g | 734 (6.0%) | 147 (4.9%) | 54 (5.3%) |
| 2,500g – 3,999g | 10586 (86.6%) | 2527 (84.1%) | 803 (78.5%) |
| ≥4,000g | 903 (7.4%) | 331 (11.0%) | 166 (16.2%) |
| Maternal race | |||
| Non-Black | 9859 (81.2%) | 1919 (64.3%) | 536 (52.8%) |
| Black | 2287 (18.8%) | 1068 (35.8%) | 479 (47.2%) |
| Missing | 77 | 18 | 8 |
| Gestational age | |||
| <37 weeks | 886 (7.3%) | 257 (8.6%) | 113 (11.1%) |
| ≥37 weeks | 11337 (92.8%) | 2748 (91.5%) | 910 (89.0%) |
NOTE: 2,500 missing maternal body mass index
Rate of early weight gain (pounds per week) quartiles: <0.58, 0.58 – <0.81, 0.81 – <1.05, ≥1.05
Total weight gain (total pounds) quartiles: <15, 15 – <20, 20 – <25, ≥25
Adjusted HRs from main effects models estimating associations of maternal BMI, pregnancy weight gain, and birth weight and CRC in offspring are reported in Table 4.
Table 4.
Adjusted hazard ratios for maternal body mass index, rate of early pregnancy weight gain, total pregnancy weight gain, and birth weight and colorectal cancer in adult offspring
| Person-years | n | aHR1 | 95% CI | p-value | |
|---|---|---|---|---|---|
| Maternal body mass index | 0.02 | ||||
| Underweight/healthy | 477,430 | 32 | 1.00 | ||
| Overweight | 121,235 | 18 | 2.12 | 1.18, 3.82 | |
| Obese | 43,117 | 7 | 2.51 | 1.05, 6.02 | |
| Rate of early weight gain2 | 625,752 | 50 | 1.17 | 0.71, 1.95 | 0.54 |
| Quartile 1 | -- | -- | 1.07 | 0.87, 1.31 | |
| Quartile 2 | -- | -- | 1.12 | 0.78, 1.61 | |
| Quartile 3 | -- | -- | 1.16 | 0.72, 1.86 | |
| Quartile 4 | -- | -- | 1.22 | 0.65, 2.31 | |
| Total weight gain2 | 689,740 | 60 | 1.03 | 0.99, 1.08 | 0.15 |
| Quartile 1 | -- | -- | 1.47 | 0.88, 2.47 | |
| Quartile 2 | -- | -- | 1.78 | 0.82, 3.88 | |
| Quartile 3 | -- | -- | 2.09 | 0.77, 5.65 | |
| Quartile 4 | -- | -- | 2.54 | 0.72, 8.88 | |
| Birth weight | 0.17 | ||||
| < 2,500 g | 42,973 | 3 | 0.44 | 0.06, 3.15 | |
| 2,500g – 3,999g | 631,307 | 56 | 1.00 | ||
| ≥ 4,000g | 63,768 | 9 | 1.95 | 0.86, 4.38 | |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval
Adjusted hazard ratios derived from main effects models; maternal BMI adjusted for race/ethnicity; rate of early weight gain adjusted for race/ethnicity, maternal BMI, and gestational age; total weight gain adjusted for race/ethnicity, maternal BMI, rate of early weight gain, and gestational age; birth weight adjusted for race/ethnicity, maternal BMI, rate of early weight gain, and total weight gain
aHRs and their 95% CIs at the median of each quartile of the rate of early weight gain (0.40, 0.71, 0.93, and 1.25 pounds per week) and total weight gain (12, 18, 23, and 29 pounds)
Maternal overweight (aHR 2.12, 95% CI 1.18, 3.82) and obesity (aHR 2.51, 95% CI 1.05, 6.02) were associated with CRC in offspring compared to maternal underweight/healthy weight (Table 4). Incidence rates of CRC were 16.2 per 100,000 (95% CI 6.5, 33.5), 14.8 (95% CI 8.8, 23.5), and 6.7 per 100,000 (95% CI 4.6, 9.5) in offspring of obese, overweight and underweight/healthy weight mothers, respectively.
Rate of early weight gain was not associated with CRC in offspring (aHR 1.17, 95% CI 0.71, 1.95; Table 4), although the association was elevated in the highest quartile (1.25 pounds per week: aHR 1.22, 95% CI 0.64, 2.31). Total weight gain was associated with CRC in offspring (aHR 1.03, 95% CI 0.99, 1.08). For example, risk was increased at the median of the third and fourth quartiles: 23 (aHR 2.09, 95% CI 0.77, 5.65) and 29 (aHR 2.54, 95% CI 0.72, 8.88) pounds, respectively.
There was an elevated association of high (≥4,000g) birth weight (aHR 1.95, 95% CI 0.86, 4.38) compared to average (2,000–3,999g) birth weight (Table 4). Results were similar when birth weight was modeled using z-scores (continuous: aHR 1.32, 95% CI 0.99, 1.78, p-value 0.06; ≥90th percentile: aHR 1.46, 95% CI 0.62, 3.45, p-value 0.39).
Associations of maternal BMI, pregnancy weight gain, and birth weight and CRC were similar in the subgroup of offspring with no family history of CRC (Supplementary Table 2).
Total weight gain modified the association between high rate of early weight gain (Q4 vs. Q1) and CRC in offspring, and there was evidence of both additive and multiplicative interaction (Table 5). Specifically, a high rate of early weight gain was associated with CRC in offspring in the strata of low total weight gain (aHR 4.78, 95% CI 1.45, 15.74) but had the inverse association in the strata of high total weight gain (aHR 0.41, 95% CI 0.14, 1.20). We observed a similar pattern of incidence rates by rate of early weight gain and total weight gain (Table 5), suggesting discordant growth from early to late pregnancy increased risk.
Table 5.
Additive and multiplicative interaction between high rate of early pregnancy weight gain (quartile 4 vs. quartile 1) and total pregnancy weight gain (at or above vs. below median)
| Total weight gain1 | Rate of early weight gain2 | Person-years | n | Incidence rate (95% CI) per 100,0003 | Single-referent aHR4 (95% CI) | Stratified aHR4 (95% CI) |
|---|---|---|---|---|---|---|
| Below median | Q1 | 127,875 | 8 | 6.2 (2.7, 12.2) | 1.00 | 1.00 |
| Q4 | 17,407 | 4 | 21.3 (5.8, 54.7) | 4.09 (1.34, 12.48) | 4.78 (1.45, 15.74) | |
| Above median | Q1 | 20,602 | 5 | 26.2 (8.5, 61.3) | 2.77 (1.19, 6.45) | 1.00 |
| Q4 | 137,509 | 12 | 8.2 (4.1, 14.6) | 1.49 (0.62, 3.60) | 0.41 (0.14, 1.20) |
NOTE: Additive interaction evaluated by estimating the relative excess risk due to interaction (RERI: −4.37, 95% CI: −9.49, 0.76); multiplicative interaction evaluated by comparing nested models with and without a high rate of early weight gain*total weight gain product term with the likelihood ratio test (p-value: 0.01).
Abbreviations: Q, quartile; CI, confidence interval; aHR, adjusted hazard ratio
Total weight gain median: 20 pounds
Rate of early weight gain quartile 1: <0.58 pounds per week, and quartile 4: ≥1.05 pounds per week
Incidence rates and 95% confidence intervals were calculated based on the discrete probability distribution for a binomial parameter
Adjusted for race/ethnicity, maternal BMI, and gestational age
Discussion
In a population-based cohort of more than 18,000 mother-child dyads, maternal obesity increased risk of CRC in adult offspring. Trajectories of pregnancy weight gain, which may be markers of fetal growth and development, similarly increased risk. Half of cases were diagnosed younger than age 50 years, and these findings suggest in utero events may contribute to increasing incidence rates of CRC in younger adults.
Maternal obesity more than doubled risk of CRC in offspring, suggesting the well-established relationship between obesity and CRC4, 46, 47 may have origins in periods that begin before birth. This process may occur through fetal programming,6 the concept that the maternal environment determines risk of disease in later stages via developmental, genetic, and epigenetic changes. For example, nutrients received in utero may lead to persistent adaptations in the structure and function of adipose tissue, appetite regulation, and metabolism.48, 49 Excess exposure to insulin and growth hormone in utero may effect insulin sensitivity,50, 51 and high levels of maternal glucose are often accompanied by episodes of fetal hyperinsulinemia.52 Epigenetic processes may also play a role;53 several studies have now identified links between maternal obesity and methylation in placenta, cord blood, and child saliva at several genes involved energy metabolism (e.g., PPARG).54–56
Trajectories of pregnancy weight gain were also associated with CRC in offspring, implicating discordant fetal growth as a possible risk factor. Incidence rates of CRC in offspring were highest when the rate of early weight gain was discordant from total weight gain, and the same pattern held regardless of whether the rate of early weight gain was higher or lower than total weight gain. Like maternal obesity, pregnancy weight gain may increase risk of obesity in offspring,9, 12, 13, 16–19 but the modification of rate of early weight gain by total weight gain suggests the timing of weight gain may be most important. Specifically, and independent of maternal obesity, early vs. late weight gain may have different consequences for placental and fetal development,33, 35, 36 which may translate into different long-term risks of chronic disease and cancer.34 Weight gain in early pregnancy reflects an increase in maternal fuels (e.g., glucose, insulin),57 whereas weight gain in late pregnancy reflects fetal growth and fluid expansion.58 The hormonal milieu of mother and fetus also differs substantially in early and late pregnancy.59 For example, hormone levels in cord blood differ by timing of pregnancy weight gain. Early weight gain is strongly associated with glucose and insulin whereas weight gain in late pregnancy correlates with hormones related to fetal adiposity.57
Another possibility is that the timing of pregnancy weight gain reflects other in utero exposures that increase risk of CRC in offspring. For example, in the Helsinki Birth Cohort,60 risk of CRC in offspring increased as the placental surface became longer and more oval.61 Placental shape and surface area are determined by events and exposures in very early pregnancy, such as maternal smoking.62–64 A longer and more oval placental surface may increase risk of oxidative damage to the fetus65 during early pregnancy, corresponding to the time when the colon differentiates from the rectum.66 Collectively, these finding suggests discordant weight gain over the course of pregnancy may contribute to CRC in offspring via two mechanisms: 1) establish obesogenic growth patterns that persist into adulthood; or 2) program the sensitivity of developing fetal tissue in the gastrointestinal tract that impacts pathology later in life.
We observed an elevated risk of CRC in offspring associated with birth weight. Birth weight is correlated but not redundant with maternal obesity and pregnancy weight gain and therefore may have an independent association with CRC. European studies linking birth records with cancer registries report no or only modest associations between birth weight and CRC.61, 67–69 Only one of these studies reported an association between birth weight and CRC, and in that study, low birth weight increased risk in men only.70 Studies relying on self-reported birth weight conflict: some show high birth weight is associated with CRC,71, 72 one shows no association,73 and yet another shows a J-shaped relationship.74 Finally, Mendelian randomization studies report no association between birth weight and CRC.75, 76 These prior studies were conducted across multiple generations and countries, and differences in maternal characteristics, such as obesity, in these studies may explain inconsistent results.
Half of offspring diagnosed with CRC in our study were diagnosed younger than age 50 years, and our findings suggest maternal obesity and pregnancy weight gain may contribute to increasing incidence rates of CRC in younger (age <50 years) adults. Incidence rates of early-onset CRC have increased across successive generations,77 implicating exposures increasingly prevalent in early life – critical periods of growth and development, such as gestation, infancy, and childhood.78 This is consistent with a well-established literature on the consequences of early life exposures for several adult cancers.79, 80 Given population trends in maternal obesity, which has multiplied in prevalence by nearly six since the 1960s,81–83 we may see a growing burden of early-onset CRC for decades to come. Other factors in early life, including environmental toxins, medications, chronic conditions, and microbiome, and that are likely related to maternal obesity, may also contribute to early-onset cancers.84, 85
A strength of our study is the multi-generational cohort, allowing us to examine associations between maternal characteristics and CRC in offspring in a large, prospective sample over 60 years. Studying exposures in the earliest periods of life is challenging and requires detailed information on both mothers and offspring, collected prospectively over generations, and the ability to systematically ascertain cancer diagnoses. The CHDS is one of the few studies in the U.S. to prospectively collect health information across multiple generations, avoiding pitfalls of recall bias and measurement error.
There were some limitations of our study. Associations of maternal obesity and pregnancy weight gain and CRC in offspring may be confounded by factors shared between mother and child, such as diet and microbiome, and that were not measured in the CHDS. Similarly, we did not measure BMI of offspring through their adulthood. We addressed unmeasured confounding by conducting a probabilistic bias analysis; for maternal BMI, the median bias corrected association from all simulations was slightly attenuated but similar to the observed association. These results suggest an unmeasured confounder could only explain the entire observed association if the confounder was a strong predictor of CRC and its distribution substantially differed between exposed and unexposed offspring, scenarios that are very unlikely. Several studies18, 19 also suggest associations of maternal obesity and/or pregnancy weight gain persist in sibling-controlled analyses, supporting results of our bias analysis. Finally, gestational diabetes, likely related to both maternal obesity and pregnancy weight gain, may also contribute to risk of CRC in offspring. Screening for gestational diabetes was not part of routine clinical practice in the 1950s and 60s, and very few (<1.0%) mothers enrolled in the CHDS had pre-existing diabetes. This precluded meaningful analyses of its association with CRC; ongoing and future studies of recent birth cohorts may examine gestational diabetes as a risk factor because it can now be ascertained from several sources, including birth certificates.86
In summary, our results provide compelling evidence that in utero events are important risk factors of CRC and may contribute to increasing incidence rates in younger adults. There may also be other as yet unknown exposures during gestation and early life that give rise to this disease and warrant further study.
Supplementary Material
Short Summary.
What is already known about this subject?
Obesity is a well-established risk factor of colorectal cancer, and several studies suggest fetal or developmental origins of obesity may underlie its effect on cancer in adulthood.
What are the new findings?
In a population-based cohort of more than 18,000 mother-child dyads, maternal obesity increased risk of CRC in adult offspring. Trajectories of pregnancy weight gain, which may be markers of fetal growth and development, similarly increased risk.
How might it impact clinical practice in the foreseeable future?
Given increasing population prevalence of maternal obesity and pregnancy weight gain, the burden of CRC is likely to continue increasing in the future.
Acknowledgements
Research reported in this publication was supported by the National Cancer Institute at the National Institutes of Health under award number R01CA242558 (CC Murphy). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mention herein of trade names, commercial products, or organizations does not imply endorsement by the U.S. government.
Funding statement:
This work was supported by the National Cancer Institute at the National Institutes of Health under award number R01CA242558 (CC Murphy). The sponsor had no role in: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations:
- BMI
body mass index
- CHDS
Child Health and Development Studies
- CI
confidence interval
- CRC
colorectal cancer
- HR
hazard ratio
Footnotes
Competing of Interests:
CCM reports consulting for Freenome; AGS reports consulting/advisory boards for Bayer, Eisai, Genentech, BMS, Exelixis, Exact Sciences, and GRAIL; PMC, NYK, ML, TZ, EB, and BAC have no financial disclosures or competing interest.
Ethics Approval: The Institutional Review Board at the Public Health Institute and the University of Texas Health Science Center at Houston approved this study.
Data Availability Statement
De-identified (anonymized) data are available upon request from Barbara A. Cohn, PhD, Director of the Child Health and Development Studies. Requests will be reviewed by Dr. Cohn, research staff, and the Institutional Review Board at the Public Health Institute. Approval of requests for de-identified (anonymized) data requires execution of a data use agreement.
References
- 1.Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–691. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019;68:2179–2185. [DOI] [PubMed] [Google Scholar]
- 3.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 2007;16:2533–47. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556–65. [DOI] [PubMed] [Google Scholar]
- 5.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut 2013;62:933–947. [DOI] [PubMed] [Google Scholar]
- 6.Godfrey KM, Barker DJ. Fetal programming and adult health. Public health nutrition 2001;4:611–624. [DOI] [PubMed] [Google Scholar]
- 7.Shankar K, Harrell A, Liu X, et al. Maternal obesity at conception programs obesity in the offspring. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2008;294:R528–R538. [DOI] [PubMed] [Google Scholar]
- 8.Rath SR, Marsh JA, Newnham JP, et al. Parental pre-pregnancy BMI is a dominant early-life risk factor influencing BMI of offspring in adulthood. Obes Sci Pract 2016;2:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olson CM, Demment MM, Carling SJ, et al. Associations Between Mothers’ and Their Children’s Weights at 4 Years of Age. Child Obes 2010;6:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oostvogels A, Hof MHP, Gademan MGJ, et al. Does maternal pre-pregnancy overweight or obesity influence offspring’s growth patterns from birth up to 7years? The ABCD-study. Early Hum Dev 2017;113:62–70. [DOI] [PubMed] [Google Scholar]
- 11.Zalbahar N, Najman J, McIntyre HD, et al. Parental pre-pregnancy obesity and the risk of offspring weight and body mass index change from childhood to adulthood. Clin Obes 2017;7:206–215. [DOI] [PubMed] [Google Scholar]
- 12.Voerman E, Santos S, Patro Golab B, et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med 2019;16:e1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuebe AM, Forman MR, Michels KB. Maternal-recalled gestational weight gain, pre-pregnancy body mass index, and obesity in the daughter. Int J Obes (Lond) 2009;33:743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drake AJ, Reynolds RM. Focus on obesity: Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction 2010;140:387–398. [DOI] [PubMed] [Google Scholar]
- 15.Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long-term health of offspring. The lancet Diabetes & endocrinology 2017;5:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sridhar SB, Darbinian J, Ehrlich SF, et al. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obstet Gynecol 2014;211:259.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nehring I, Lehmann S, von Kries R. Gestational weight gain in accordance to the IOM/NRC criteria and the risk for childhood overweight: a meta-analysis. Pediatr Obes 2013;8:218–24. [DOI] [PubMed] [Google Scholar]
- 18.Houghton LC, Ester WA, Lumey LH, et al. Maternal weight gain in excess of pregnancy guidelines is related to daughters being overweight 40 years later. Am J Obstet Gynecol 2016;215:246.e1–246.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ester WA, Houghton LC, Lumey LH, et al. Maternal and Early Childhood Determinants of Women’s Body Size in Midlife: Overall Cohort and Sibling Analyses. Am J Epidemiol 2017;185:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Wang SF, Mu M, et al. Birth weight and overweight/obesity in adults: a meta-analysis. Eur J Pediatr 2012;171:1737–46. [DOI] [PubMed] [Google Scholar]
- 21.Boney CM, Verma A, Tucker R, et al. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290–e296. [DOI] [PubMed] [Google Scholar]
- 22.Curhan GC, Chertow GM, Willett WC, et al. Birth weight and adult hypertension and obesity in women. Circulation 1996;94:1310–1315. [DOI] [PubMed] [Google Scholar]
- 23.Armitage JA, Poston L, Taylor PD. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Obesity and metabolism 2008;36:73–84. [DOI] [PubMed] [Google Scholar]
- 24.Van den Berg B The California child health and development studies. Handbook of longitudinal research 1984;1:166–179. [Google Scholar]
- 25.van den Berg BJ, Christianson RE, Oechsli FW. The California child health and development studies of the School of Public Health, University of California at Berkeley. Paediatric and perinatal epidemiology 1988;2:265–282. [DOI] [PubMed] [Google Scholar]
- 26.Susser E, Buka S, Schaefer C, et al. The early determinants of adult health study. Journal of developmental origins of health and disease 2011;2:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Killion JA, Giddings BM, Chen Y, et al. Cancer in California, 1998–2015. Sacramento, CA: California Department of Public Health, Chronic Disease Surveillance and Research Branch, 2018. [Google Scholar]
- 28.Cancer Reporting in California. In: Brant MK, Moody CJ, Hansen DM, eds. California Cancer Reporting System Standards, Volume I: Abstracting and Coding Procedures. Sacramento, CA: California Department of Public Health, 2021. [Google Scholar]
- 29.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. Journal of the American statistical association 1979;74:829–836. [Google Scholar]
- 30.Cirillo PM, Cohn BA. Gestational biomarkers of daughter’s breast cancer in the Child Health and Development Studies. Reprod Toxicol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang ET, Cirillo PM, Kao CN, et al. Birth weight and childhood growth in daughters of women with irregular menstrual cycles. Gynecol Endocrinol 2013;29:615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang ET, Cirillo PM, Vittinghoff E, et al. Menstrual irregularity and cardiovascular mortality. J Clin Endocrinol Metab 2011;96:E114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Retnakaran R, Wen SW, Tan H, et al. Association of Timing of Weight Gain in Pregnancy With Infant Birth Weight. JAMA Pediatr 2018;172:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaillard R, Steegers EA, Franco OH, et al. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int J Obes (Lond) 2015;39:677–85. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Wan S, Gu S, et al. Gestational weight gain and adverse pregnancy outcomes: a prospective cohort study. BMJ Open 2020;10:e038187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davenport MH, Ruchat SM, Giroux I, et al. Timing of excessive pregnancy-related weight gain and offspring adiposity at birth. Obstet Gynecol 2013;122:255–261. [DOI] [PubMed] [Google Scholar]
- 37.Hall CA, Meyer WW. Optimal error bounds for cubic spline interpolation. Journal of Approximation Theory 1976;16:105–122. [Google Scholar]
- 38.Pan W Akaike’s information criterion in generalized estimating equations. Biometrics 2001;57:120–5. [DOI] [PubMed] [Google Scholar]
- 39.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology 2011;22:745. [DOI] [PubMed] [Google Scholar]
- 41.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol 2007;17:227–36. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, De A. Multiple Imputation by Fully Conditional Specification for Dealing with Missing Data in a Large Epidemiologic Study. Int J Stat Med Res 2015;4:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoffel EM, Koeppe E, Everett J, et al. Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology 2018;154:897–905.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lash TL, Fox MP, Fink AK. Applying quantitative bias analysis to epidemiologic data: Springer Science & Business Media, 2011.
- 45.Fox MP, Lash TL, Greenland S. A method to automate probabilistic sensitivity analyses of misclassified binary variables. Int J Epidemiol 2005;34:1370–6. [DOI] [PubMed] [Google Scholar]
- 46.Hong S, Cai Q, Chen D, et al. Abdominal obesity and the risk of colorectal adenoma: a meta-analysis of observational studies. Eur J Cancer Prev 2012;21:523–31. [DOI] [PubMed] [Google Scholar]
- 47.Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 2013;8:e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. Bmj 2017;356:j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alfaradhi M, Ozanne S. Developmental programming in response to maternal overnutrition. Frontiers in genetics 2011;2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tam WH, Ma RCW, Ozaki R, et al. In Utero Exposure to Maternal Hyperglycemia Increases Childhood Cardiometabolic Risk in Offspring. Diabetes Care 2017;40:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catalano PM, Presley L, Minium J, et al. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009;32:1076–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehnen H, Zechner U, Haaf T. Epigenetics of gestational diabetes mellitus and offspring health: the time for action is in early stages of life. Molecular Human Reproduction 2013;19:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevenson K, Lillycrop KA, Silver MJ. Fetal programming and epigenetics. Current Opinion in Endocrine and Metabolic Research 2020;13:1–6. [Google Scholar]
- 54.Nogues P, Dos Santos E, Jammes H, et al. Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta. Clinical epigenetics 2019;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gemma C, Sookoian S, Alvariñas J, et al. Maternal pregestational BMI is associated with methylation of the PPARGC1A promoter in newborns. Obesity 2009;17:1032–1039. [DOI] [PubMed] [Google Scholar]
- 56.Oelsner KT, Guo Y, To SB-C, et al. Maternal BMI as a predictor of methylation of obesity-related genes in saliva samples from preschool-age Hispanic children at-risk for obesity. BMC genomics 2017;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rifas-Shiman SL, Fleisch A, Hivert MF, et al. First and second trimester gestational weight gains are most strongly associated with cord blood levels of hormones at delivery important for glycemic control and somatic growth. Metabolism 2017;69:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hytten FE, Leitch I. The physiology of human pregnancy. The physiology of human pregnancy. 1964. [Google Scholar]
- 59.Kaijser M, Granath F, Jacobsen G, et al. Maternal pregnancy estriol levels in relation to anamnestic and fetal anthropometric data. Epidemiology 2000;11:315–319. [DOI] [PubMed] [Google Scholar]
- 60.Barker DJ, Osmond C, Kajantie E, et al. Growth and chronic disease: findings in the Helsinki Birth Cohort. Annals of human biology 2009;36:445–458. [DOI] [PubMed] [Google Scholar]
- 61.Barker DJ, Osmond C, Thornburg KL, et al. The shape of the placental surface at birth and colorectal cancer in later life. American Journal of Human Biology 2013;25:566–568. [DOI] [PubMed] [Google Scholar]
- 62.Burton GJ, Fowden AL, Thornburg KL. Placental Origins of Chronic Disease. Physiol Rev 2016;96:1509–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zdravkovic T, Genbacev O, McMaster MT, et al. The adverse effects of maternal smoking on the human placenta: a review. Placenta 2005;26 Suppl A:S81–6. [DOI] [PubMed] [Google Scholar]
- 64.Christianson RE. Gross differences observed in the placentas of smokers and nonsmokers. Am J Epidemiol 1979;110:178–87. [DOI] [PubMed] [Google Scholar]
- 65.Burton GJ, Jauniaux E. Oxidative stress. Best practice & research Clinical obstetrics & gynaecology 2011;25:287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamauchi M, Lochhead P, Morikawa T, et al. Colorectal cancer: a tale of two sides or a continuum? Gut 2012;61:794–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cnattingius S, Lundberg F, Iliadou A. Birth characteristics and risk of colorectal cancer: a study among Swedish twins. Br J Cancer 2009;100:803–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahlgren M, Wohlfahrt J, Olsen LW, et al. Birth weight and risk of cancer. Cancer 2007;110:412–9. [DOI] [PubMed] [Google Scholar]
- 69.McCormack VA, dos Santos Silva I, Koupil I, et al. Birth characteristics and adult cancer incidence: Swedish cohort of over 11,000 men and women. Int J Cancer 2005;115:611–7. [DOI] [PubMed] [Google Scholar]
- 70.Nilsen TI, Romundstad PR, Troisi R, et al. Birth size and colorectal cancer risk: a prospective population based study. Gut 2005;54:1728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith NR, Jensen BW, Zimmermann E, et al. Associations between birth weight and colon and rectal cancer risk in adulthood. Cancer Epidemiol 2016;42:181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spracklen CN, Wallace RB, Sealy-Jefferson S, et al. Birth weight and subsequent risk of cancer. Cancer Epidemiol 2014;38:538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang TO, Reeves GK, Green J, et al. Birth weight and adult cancer incidence: large prospective study and meta-analysis. Ann Oncol 2014;25:1836–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sandhu MS, Luben R, Day NE, et al. Self-reported birth weight and subsequent risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2002;11:935–8. [PubMed] [Google Scholar]
- 75.Jarvis D, Mitchell JS, Law PJ, et al. Mendelian randomisation analysis strongly implicates adiposity with risk of developing colorectal cancer. Br J Cancer 2016;115:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gao C, Patel CJ, Michailidou K, et al. Mendelian randomization study of adiposity-related traits and risk of breast, ovarian, prostate, lung and colorectal cancer. Int J Epidemiol 2016;45:896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murphy CC, Singal AG, Baron JA, et al. Decrease in incidence of young-onset colorectal cancer before recent increase. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Y, Land KC. Age-period-cohort analysis: new models, methods, and empirical applications: CRC Press, 2013. [Google Scholar]
- 79.Wild CP. How much of a contribution do exposures experienced between conception and adolescence make to the burden of cancer in adults? Cancer Epidemiol Biomarkers Prev 2011;20:580–1. [DOI] [PubMed] [Google Scholar]
- 80.Perera FP. Cancer: the big questions to address in coming years. Cancer Epidemiol Biomarkers Prev 2011;20:571–3. [DOI] [PubMed] [Google Scholar]
- 81.Troiano RP, Flegal KM, Kuczmarski RJ, et al. Overweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991. Arch Pediatr Adolesc Med 1995;149:1085–91. [DOI] [PubMed] [Google Scholar]
- 82.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ogden CL, Carroll MD, Lawman HG, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. Jama 2016;315:2292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hofseth LJ, Hebert JR, Chanda A, et al. Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol 2020;17:352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akimoto N, Ugai T, Zhong R, et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol 2021;18:230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hosler AS, Nayak SG, Radigan AM. Agreement between self-report and birth certificate for gestational diabetes mellitus: New York State PRAMS. Matern Child Health J 2010;14:786–789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified (anonymized) data are available upon request from Barbara A. Cohn, PhD, Director of the Child Health and Development Studies. Requests will be reviewed by Dr. Cohn, research staff, and the Institutional Review Board at the Public Health Institute. Approval of requests for de-identified (anonymized) data requires execution of a data use agreement.


