Abstract
Purpose
Does existing scientific literature suggest an impact of oocyte dysmorphisms on biological or clinical outcomes of assisted reproduction treatments?
Methods
Studies of interest were selected from an initial cohort of 6651 potentially relevant records retrieved. PubMed was systematically searched for peer-reviewed original papers and reviews identified by keywords and medical subject heading (MeSH) terms. The most relevant publications were critically evaluated to identify criteria for oocyte morphological evaluation and IVF outcomes. For each morphological abnormality, we generated an oocyte literature score (OLS) through the following procedure: (a) papers showing a negative, absence of, or positive correlation between a given abnormality and IVF outcome were scored 1, 0, and − 1, respectively; (b) the sum of these scores was expressed as a fraction of all analyzed papers; (c) the obtained fraction was multiplied by 10 and converted into decimal number.
Result
We identified eleven different dysmorphisms, of which six were extracytoplasmic (COC, zona pellucida, perivitelline space, polar body 1, shape, giant size) and five intracytoplasmic (vacuoles, refractile bodies, SER clusters, granularity, color). Among the extracytoplasmic dysmorphisms, abnormal morphology of the COC generated an OLS of 8.33, indicating a large prevalence (5/6) of studies associated with a negative outcome. Three intracytoplasmic dysmorphisms (vacuoles, SER clusters, and granularity) produced OLS of 7.14, 7.78, and 6.25, respectively, suggestive of a majority of studies reporting a negative outcome.
Conclusion
COC morphology, vacuoles, SER clusters, and granularity produced OLS suggestive of a prevalence of studies reporting a negative outcome.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02370-3.
Keywords: Oocyte, Morphology, Fertilization, Embryo development, Zona pellucida
Introduction
The success of assisted reproductive technologies (ART) depends on each individual step in the procedure. An adequate evaluation of the oocyte morphology can represent a measure complementary to embryonic evaluation to achieve embryo selection and maximize efficiency.
Routine embryo assessment is well codified by internationally recognized criteria for semi-quantitative characterization [1]. On the contrary, the assessment of the oocyte morphology is still performed more arbitrarily. In standard IVF, the cumulus-oocyte complex may be roughly assessed using a stereomicroscope, while in ICSI, case after cumulus cell removal, the oocyte is subjectively evaluated based on the stage of maturation and appearance of the perivitelline space and cytoplasm.
The question of oocyte dysmorphisms is highly prevalent, following cumulus cell removal, approximately 60–70% of oocytes show abnormal morphological characteristics [2]. Since dysmorphisms may be associated with cytoskeletal and other cellular defects [3], indiscriminate use of abnormal oocytes might imply the risk to generate developmentally incompetent embryos. In particular, cortical actin and spindle abnormalities associated with specific dysmorphisms could affect cell cleavage and chromosome segregation during early development [3]. This could affect pregnancy rates or result in further negative consequences, such as miscarriages or neonatal malformations.
Numerous studies have explored the relationship between oocyte morphological abnormalities and IVF/ICSI outcomes [4–7]. Nevertheless, to date, results are conflicting and the impact of oocyte morphology remains an open question.
In consideration of the aforementioned gaps in the literature, the purpose of this systematic review was to evaluate studies on non-invasive assessment of oocyte morphology to identify predictors of embryo developmental competence. In addition, we elaborated a methodology to generate an oocyte literature score (OLS) able to express semi-quantitatively the cumulative outcome of evidence available for specific dysmorphisms.
Materials and methods
Literature search methodology
This systematic review was performed following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [8]. The review protocol was recorded a priori on the international prospective register of systematic reviews PROSPERO (Registration Number: CRD42021257409) and it is available online (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021257409). No institutional review board approval was needed because only published, de-identified data were analyzed.
PubMed was systematically searched for peer-reviewed original and reviews identified by relevant keywords and medical subject heading (MeSH) terms, such as ‘oocyte’, ‘human’, ‘ooplasm’, ‘zona pellucida’, ‘perivitelline space’, ‘morphology, ‘quality’, ‘outcome’, and ‘pregnancy’. Keywords and Mesh terms were used in multiple and overlapping combinations in order to identify those publications strictly relevant to predictive value of oocyte morphology. Additional studies were identified by thorough analysis of reference lists from relevant publications. Full manuscripts were obtained for all selected papers and the decision for the final inclusion was made after detailed evaluation of the articles.
The most relevant publications, i.e., those concerning the most recurrent correlations between oocyte morphology and outcome in IVF, were critically evaluated and discussed to identify oocyte morphological selection criteria and relevant clinical evidence.
Study selection
Two reviewers (AB and GI) assessed independently all studies for inclusion or exclusion. Disagreements were solved in discussion with a third author (GC).
During the first screening, titles and abstracts were investigated and studies with lack of any relevance were excluded; review articles were also excluded (Fig. 1). The remaining articles were retrieved full-length and assessed according to the eligibility criteria. The following information of such studies was collected: first author’s last name, year of publication, research objective, design of the study, outcomes investigated, and conclusions. No time restrictions were applied. Full-length articles were considered eligible if written in English. Studies referring to animal models were excluded. Data extraction was performed in 76 papers. A summary of the extraction results is shown in Table 1.
Fig. 1.
PRISMA flow diagram of study selection for systematic review
Table 1.
Summary of the results from 76 papers identified in a systematic review of the literature
| Outcome | COC | ZP | PVS | PB | SHAPE | GIANT | VAC | RB | SER | GR | DARK | MULTIPLE | Remark | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akarsu et al. (2009) [9] | OP | – | – | – | – | – | – | – | – | YES* | – | – | – | *Fetal anomalies |
| Alikani et al. (1995) [10] | F,CL | – | – | – | – | – | – | NO | – | – | – | – | – | |
| Ashrafi et al. 2015 [11] | F,EQ,IM,CP | – | – | NO | – | – | – | – | – | – | – | – | – | |
| Balaban et al. (1998) [2] | F, EQ, CP, IM | – | NO | NO | – | NO | – | – | NO | – | – | NO | – | |
| Balaban et al. (2006) [12] | IM | – | – | – | – | – | – | YES* | – | – | – | – | – | *For IM |
| Balaban et al. (2008) [13] | CSE, BL, HA | – | NO | NO | – | – | – | YES* | – | – | YES* | NO | – | * For CSE |
| Balakier et al. (2002) [14] | ED, PL | – | – | – | – | – | YES* | – | – | – | – | – | – | * Giant oocytes for aneuploidy |
| Bassil et al. (2021) [15] | BL, BLQ | – | – | – | – | YES* | – | – | – | – | – | – | – | *For BLQ |
| Bertrand et al. (1995) [16] | F | – | YES* | – | – | – | – | – | – | – | – | – | – | *Thickness |
| Braga et al. (2013) [17] | BL,HA,CP | – | – | – | – | YES* | – | – | YES* | – | – | – | *For BLQ, CP and AB | |
| Chamayou et al. (2006) [18] | EQ, CP, IM | – | – | YES* | YES* | NO | – | – | – | – | – | – | YES** | *For EQ; **cumulative ooplasm assessment |
| Ciotti et al. (2004) [19] | F, CL, CP, IM | – | – | – | NO | – | – | – | – | – | – | – | – | |
| Dal Canto et al. (2012) [20] | F,CP,IM | YES* | – | – | – | – | – | – | – | – | – | – | – | *For CP and IM |
| Dal Canto et al. (2017) [3] | MS,AC | – | – | – | – | – | – | – | – | YES* | YES* | – | – | *Meiotic spindle and actin cytoskeleton |
| Daya et al. (1990) [21] | CL | YES* | – | – | – | – | – | – | – | – | – | – | – | *Blood clots in cum |
| De cassia et al. (2010) [22] | F | – | – | – | – | – | – | YES | – | – | – | – | – | |
| De Santis et al. (2005) [23] | F, EQ | – | – | – | NO | – | – | – | – | – | – | – | – | |
| De Sutter et al. (1996) [6] | F, EQ | – | NO | NO | – | NO | – | NO | NO | – | – | NO | – | |
| Ebner et al. (2000) [24] | F, EQ | – | – | – | YES* | – | – | – | – | – | – | – | – | |
| Ebner et al. (2005) [25] | F,BL | – | – | – | – | – | – | YES | – | – | – | – | – | |
| Ebner et al. (2006) [26] | F, CL, BL, IM | – | – | – | – | – | – | YES* | – | – | – | – | – | *For FR |
| Ebner et al. (2008a) [4] | F, BL | YES* | – | – | – | – | – | – | – | – | YES | – | – | *Blood clots in cum |
| Ebner et al. (2008b) [27] | CP, AB, THB, M | – | – | – | – | – | – | – | – | YES* | – | – | – | *For AB and THB |
| Ebner et al. (2008c) | F, CL, CM, BL | – | – | – | – | YES* | – | – | – | – | – | – | – | *Ovoid: delay |
| Ebner et al. (2010) [28] | ED, IM, CP | – | YES* | – | – | – | – | – | – | – | – | – | – | *Birefringence of the inner zona layer |
| Esfandiari et al. (2006) [29] | F, ED, CP | – | NO* | – | – | – | – | – | – | – | – | NO** | – | * Thick zona; **brown eggs |
| Fancsovits et al. (2006) [30] | F, EQ | – | – | – | YES* | – | – | – | – | – | – | – | – | *For FR |
| Fancsovits et al. (2012) [31] | F, CL, ED, EQ | – | – | – | – | – | – | – | – | – | NO | – | – | |
| Faramarzi et al. (2017) [32] | EM | – | YES* | – | – | – | – | – | – | – | – | – | – | *t2,t5 timings delayed |
| Faramarzi et al. (2019) [33] | EM | – | – | NO | – | YES* | – | – | – | – | – | – | – | *tPB2, t5 and t8 timings delayed |
| Farhi et al. (2002) [34] | F, CP, IM | – | – | YES* | – | – | – | – | – | – | – | – | – | *Coarse granules |
| Ferreux et al. (2019) [35] | F, CP, THB, M | – | – | – | – | – | – | – | – | NO | – | – | – | |
| Gonzalez-Ortega et al. (2016) [36] | F, EQ, CC | – | YES* | – | – | – | – | – | – | – | – | – | – | *Birefringence values of zona pellucida |
| Hassa et al. (2014) [37] | ED | – | – | YES | – | – | – | – | – | – | – | – | – | |
| Hassan-Ali et al. (1998) [38] | F, CL, CP, IM | – | – | NO* | – | – | – | – | – | – | – | – | – | *Coarse dark granules |
| Hattori et al. (2014) [39] | F, BL, IM, CP, AB, THB, M | – | – | – | – | – | – | – | – | NO | – | – | – | *For FR and IM |
| Host et al. (2002) [40] | EQ | – | YES* | – | – | – | – | – | – | – | – | – | – | *Thickness |
| Kahraman et al. (2000) [41] | F, EQ, CP, OP | – | – | – | – | – | – | – | – | – | YES* | – | – | *For OP |
| La Sala et al. (2009) [42] | F, PR, THB, MB | – | – | – | – | – | – | – | – | – | – | – | NO | |
| Lehner et al. (2015) [43] | F, EQ, CP | – | – | – | – | – | YES* | – | – | – | – | – | – | *Fertilization rate in giant oocyte group contained |
| Lin et al. (2003) [44] | F, CL, EQ, BL | YES | – | – | – | – | – | – | – | – | – | – | – | |
| Loutradis et al. (1999) [45] | F, CL, EQ, CP | – | – | – | – | – | – | – | – | – | – | – | YES* | *For EQ and CP |
| Machtinger et al. (2011) [46] | CL, EQ, IM, CP | – | – | – | – | – | YES* | – | – | – | – | – | – | *Associated with abnormal cleavage |
| Madaschi et al. (2009) [47] | F, IM, CP, OP | – | YES* | – | – | – | – | – | – | – | – | – | – | *For clinical outcome |
| Mateizel et a. (2013) [48] | F, ED, THB, M | – | – | – | – | – | – | – | – | NO | – | – | – | |
| Meriano et al. (2001) [49] | F, EQ, IM, CP | – | – | YES* | – | – | – | – | – | – | – | – | – | *Debris in the PVS |
| Montag et al. (2008) [50] | ED, IM, CP, THB | – | YES* | – | – | – | – | – | – | – | – | – | – | *Inner layer retardation |
| Navarro et al. (2009) [51] | F, CL, EQ | – | – | – | YES | – | – | – | – | – | – | – | – | |
| Ng et al. (1999) [52] | F, CL, CP, IM | YES* | – | – | – | – | – | – | – | – | – | – | – | *For FR and CP |
| Otsuki et al. (2004) [53] | F, CP | – | – | – | – | – | – | – | – | YES* | – | – | – | *For CP |
| Otsuki et al. (2007) [54] | F, BL | – | – | – | – | – | – | – | YES* | – | – | – | – | *For FR and BL |
| Rama Rayu et al. (2007) [55] | F, BL | – | YES | – | – | – | – | – | – | – | – | – | – | |
| Rattanachaiyanont et al. (1999) [56] | F, CL, CP | NO | – | – | – | – | – | – | – | – | – | – | – | |
| Rienzi et al. (2008) [5] | PNM, F, EQ, CP | – | NO | YES | YES | NO | – | YES | – | – | YES | YES | YES | |
| Rosenbusch et al. (2002) [57] | PL | – | – | – | – | – | YES* | – | – | – | – | – | – | *Giant oocyte |
| Sa et al. (2011) [58] | F, ED,IM, CP, AB, THB, M | – | – | – | – | – | – | – | – | YES* | – | – | – | *For ED, IM and M |
| Sauerbrun-Cutler et al. (2015) [59] | F, BL, IM, CP, THB, AB | – | YES* | YES* | – | – | – | – | – | – | – | – | – | *For IM and CP |
| Sheral et al. (1997) [60] | CL, EQ, IM, CP | – | – | – | – | – | – | NO | – | – | – | – | YES* | *For IM and CP |
| Setti et al. (2011) [61] | F, EQ | – | – | – | – | – | – | YES* | – | – | NO | – | – | *For FR |
| Setti et al. (2016) [62] | F, EQ, BL, BLQ, IM, CP, AB | – | – | – | – | – | – | – | – | YES* | – | – | – | *For IM |
| Shaw-Jackson et al. (2016) [63] | F, EQ, BL, BLQ, IM, CP, AB, THB, M | – | – | – | – | – | – | – | – | NO | – | – | – | |
| Shen et al. (2005) [64] | F, ED, CP | – | YES* | – | – | – | – | – | – | – | – | – | – | *For ED and CP |
| Shi et al. (2014) [65] | F, CL, EQ, IM, CP, AB, THB | – | YES* | – | – | – | – | – | – | – | – | – | – | *For FR, IM and CP |
| Sousa et al. (2015) [66] | F, CL, BL, IM, CP, THB | – | YES* | – | – | – | – | – | – | – | – | – | – | *For CP and THB |
| Sousa et al. (2016) [67] | F, CL, EQ, BL, IM, CP, AB, THB | – | – | – | – | – | – | YES | – | – | – | – | – | |
| Tabibnejad et al. (2018) [68] | EM | – | NO | – | – | – | – | – | – | – | – | – | – | |
| Takahashi et al. (2020) [69] | F, BL, BLQ, IM, AB, THB, M | – | – | – | – | – | – | – | YES* | – | – | – | – | *For BL and IM |
| Ten et al. (2007) [7] | F, EQ | – | NO | NO | NO | NO | – | NO | – | – | – | YES* | NO | *For EQ |
| Verlinsky et al. (2003) [70] | F, EQ, BL, CP, PL | – | – | – | NO | – | – | – | – | – | – | – | – | |
| Wallbutton et al. (2010) [71] | CP | – | – | – | – | – | – | YES | – | – | – | – | – | Case report |
| Weghofer et al. (2019) [72] | F, EQ, | – | – | YES* | – | – | – | - | – | – | – | – | – | *Oocytes yeld |
| Wilding et al. (2007) [73] | F, ED, CP | – | – | – | – | - | - | - | – | – | NO | – | YES | |
| Xia et al. (1997) [74] | F, EQ | – | – | – | – | - | - | YES | – | – | – | – | YES | |
| Yakin et al. (2007) [75] | F, CL, BL, PL | – | – | – | – | NO | – | – | – | – | – | – | YES* | *For BL |
| Yi et al. (2019) [76] | F, CL, EQ, CP, AB, THB | – | – | – | – | – | – | – | – | – | NO | – | – | |
| Zhou et al. (2016) [77] | F, EQ, BL, BLQ, IM, CP | – | – | – | YES* | – | – | – | – | – | – | – | – | *For EQ and BLQ |
COC, cumulus-oocyte complex; ZP, zona pellucida; PVS, perivitelline space; VAC, vacuolization; RB, refractile bodies; SER, smooth endoplasmic reticulum aggregates; GR, granularity; PNM, pronuclear morphology; F, fertilization; CL, cleavage; ED, embryo development; EQ, embryo quality; CSE, cryosurvival of embryo; CM, compaction; BL, blastulation; BLQ, blastocyst quality; HA, hatching; MS, meiotic spindle; AC, actin cytoskeleton; EM, embryo-morphokinetics; IM, implantation; CP, clinical pregnancy; OP, ongoing pregnancy; AB, abortion; M, malformation; THB, take home baby; MB, multiple birth; CC, conception cycle; PL, aneuploidy; –, not investigated; NO, investigated but no correlation found; YES, investigated and correlation found
In the following step, we attributed an oocyte literature score (OLS) to each individual anomaly (Fig. 2, Table 2). This was done by assigning a score calculated from the percentage of papers reporting a correlation between a specific abnormality and at least one IVF outcome (biological and/or clinical outcome). Specifically, 0 means that no study found a negative correlation between such abnormality and IVF outcome, while 10 indicates that all studies converged towards a unanimous decision of negative correlation.
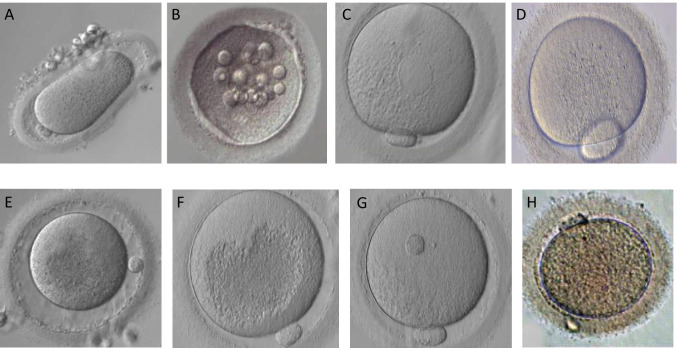
Fig. 2.
Different human oocyte morphological abnormalities (arrows) observed by light microscopy (400 × magnification): A, abnormal zona pellucida and cytoplasm shape; B, vacuoles; C, smooth endoplasmic reticulum clusters; D, giant polar body; E, large perivitelline space; F, centrally located cytoplasmic granulation; G, refractile body; H, brown oocyte
Table 2.
Oocyte literature score of individual abnormalities
| Studies analyzed | Studies with negative correlation on IVF outcome (value = 1) | Studies with no correlation on IVF outcome (value = 0) | Studies with positive correlation on IVF outcome (value = − 1) | OLS | |
|---|---|---|---|---|---|
| Extracytoplasmatic abnormalities | |||||
| Cumulus-oocyte complex | 6 | 5 | 1 | 0 | 8.33 |
| Zona pellucida | 17 | 10 | 6 | 1 | 5.29 |
| Perivitelline space | 12 | 6 | 6 | 0 | 5.00 |
| Polar body | 10 | 5 | 4 | 1 | 4.00 |
| Shape | 9 | 3 | 6 | 0 | 3.33 |
| Giant | 3 | 0 | 3 | 0 | N.A |
| Intracytoplasmic abnormalities | |||||
| Vacuolization | 14 | 10 | 4 | 0 | 7.14 |
| Refractile bodies | 4 | 2 | 2 | 0 | 5.00 |
| Ser cluster | 9 | 7 | 2 | 0 | 7.78 |
| Granularity | 8 | 5 | 3 | 0 | 6.25 |
| Color variation | 6 | 2 | 4 | 0 | 3.33 |
| Cumulative abnormalities | 10 | 6 | 4 | 0 | 6.00 |
OPS, oocyte predictive score; N.A., not applicable
Papers showing negative (a decrease in outcome), absence of (no change in outcome), or positive (an increase in outcome) correlations between a given abnormality and clinical outcome were assigned scores of 1, 0, and − 1, respectively. In the end, the number obtained allowed us to calculate a percentage (number calculated / number of papers analyzed for that abnormality), which was converted into a decimal number and attributed as a cumulative score to that specific abnormality. This was carried out to ensure that the scores were comparable and had the same weight despite having a different number of papers analyzed for each individual abnormality. Therefore, the higher the score attributed, the greater the ability of an oocyte abnormality to predict a decreased IVF outcome.
The major limitation of our OLS is its inability to take into consideration differences in size, and therefore relative weight, and endpoints among the studies relevant to a specific dysmorphism. Therefore, our OLS is not comparable to the results expressed by a meta-analysis; rather it represents an elaboration which complements the written information of the manuscript.
Studies reporting a correlation based on embryo morphokinetics alone were excluded from the calculation of the oocyte predictive score, but commented — if appropriate.
Results
Extracytoplasmic abnormalities
Cumulus-oocyte complex
MII oocytes and their cumulus cells (CCs) communicate with each other through gap junctions for the exchange of metabolites and regulatory molecules [78]. This suggests that the morphological evaluation of a cumulus cell oocyte complex (COC) is a potential parameter for determining oocyte quality. COC abnormalities concern the type and degree of expansion of the cumulus mass and/or the presence of blood clots. In most cases, COCs are recovered in an expanded form, i.e., the oocyte surrounded by an expanded corona radiata and an expanded and loose cumulus mass. Alternatively, the mass of the cumulus may show a varying degree of expansion. Non-invasive assessment of COC was investigated in 6 of the 76 papers.
In hCG-primed in vitro maturation cycles, Dal Canto et al. correlated the expansion of the COC with the maturation stage of the oocytes [20]. Specifically, expanded COCs were found to enclose mature oocytes, while compact COCs contained immature oocytes. Fertilization rate was comparable between the two groups, but positive correlations between cumulus expansion and implantation and pregnancy rates were observed. On other hand, in two studies [27, 56] found a negative correlation between the cellular density of the corona radiata and the rate of mature oocytes; in the same studies, no correlations between the COC morphology and fertilization, cleavage, and clinical pregnancy rates were observed. Using a scoring system (from 1 to 5) based on the morphology of the ooplasm, cumulus mass, corona radiata, and membrana granulosa cells, Lin et al. found positive correlations with fertilization rate and blastocyst quality [44]. Adopting a different scoring system, Ng and colleagues observed a correlation between COC quality and fertilization and pregnancy rates, but not cleavage rates [52].
Other studies indicate that blood clots embedded in the COC negatively affect fertilization and cleavage [21], blastulation [27] rates. Interestingly, removal of blood clots from COCs did not rescue the performance of affected embryos [27]. The adverse effects of blood clots are likely to originate from the production of reactive oxygen species (ROS), which are found at high levels in blood components [79]. Collectively, this evidence suggests that the oocytes from COC displaying blood clots may perform suboptimally. We analyzed 6 studies relevant to COC morphology, of which 5 showed a negative correlation with IVF outcome. This led us to attribute a score of 8.33. Such a score suggests that COC has a potential as an indicator of oocyte quality.
Zona pellucida
The ZP may display several morphological anomalies, including increased thickness, irregularities of the surface, or apparently increased density associated with reduced translucency (a feature commonly referred to as “dark ZP”). ZP anomalies may result from perturbations of folliculogenesis and therefore reflect an altered oocyte quality. The prognostic value of this feature was individually analyzed in 17 publications.
Several studies highlighted a positive correlation between high birefringence of the inner layer — measured by polarized light microscopy — and increased fertilization rate and embryo development [50, 55, 64], in contrast, Madaschi et al. observed similar fertilization rate among oocytes with high or low zona pellucida birefringence [47]. In other reports, implantation and pregnancy rates were significantly higher following transfer of embryos derived from oocytes wityh higher ZP birefringence [47, 55, 64], whereas lower ZP birefringence was correlated with higher miscarriage rates [47]. Similarly, Montag et al. reported that development, implantation, pregnancy, and live birth rates were higher in relation to embryos derived from oocyte with high zona birefringence [50].
Ebner et al. observed that the birefringence of the inner layer of the oocyte ZP was a good predictor of blastocyst formation, but it was not associated with embryo quality or pregnancy rate [80]. Furthermore, fertilization rate, embryo quality, and conception cycles were higher in cycles with oocytes displaying increased birefringence values [36]. A recent study showed that embryo morphokinetic parameters are not related to zona pellucida birefringence [68] in contrast to Faramarzi et al. that observed a correlation between morphokinetic parameters and ZP birefringence [32], in particular t5 (time at which the 5-cell stage was achieved) occurred earlier in high birefringent ZP compared with low birefringent oocytes. Although not entirely consistent, collectively, these studies suggest that embryo selection based on zona pellucida birefringence may lead to increased implantation and pregnancy rates. Translucency of the ZP has been actively investigated. In several studies, darkness (i.e., reduced translucency) of the zona was not found associated with fertilization rates, embryo quality and implantation rates [2, 6, 7, 29], embryo cryosurvival, and blastocyst and hatching rates [13]. In contrast, Shi et al. showed that oocytes with a dark ZP produced lower fertilization, implantation, and clinical pregnancy rates in IVF/ICSI cycles [65].
An association between dark ZP and diminished pregnancy, implantation, and live birth rates was also reported in another study [59]. Additional ZP dysmorphisms were investigated in other studies. Sousa et al. observed low maturation, implantation, pregnancy and live -birth rates in relation with oocytes with indented ZP [66]. Bertrand et al. found that the ZP of fertilized oocytes was significantly thinner (16.6 ± 3.2 μm) than that of unfertilized oocytes (18.9 ± 4.0 μm; P < 0.001) [16]. On the contrary, Høst et al. found a positive correlation between ZP thickness and embryo quality [40].
Finally, Rienzi et al. found no correlation between abnormal zona pellucida (thick and/or dark) and fertilization rate, pronuclear morphology, embryo development, and clinical pregnancy rate [5]. Non-invasive assessment of zona pellucida produced a low score — 5.29 —, therefore suggesting a low predictive value.
Perivitelline space
The perivitelline space (PVS) is the volume delimited by the ZP and not occupied by the oocyte. Generally, normal MII oocytes have a small PVS including the single polar bodies (PBs) and no granulated dispersed material [81]. PVS morphology in relation to embryo development was investigated in 12 papers.
Large PVS are associated with markedly diminished pregnancy and implantation rates [59]. Rienzi et al. also showed a decreased fertilization rate and compromised pronuclear morphology, but not reduced embryo quality, in oocytes with large PVS [5]. Chamayou et al. found a correlation between PVS size and presence of granulated material and embryo quality, but not implantation and other clinical outcomes [18]. No correlations between increased PVS and developmental characteristics were reported in other studies [2, 6, 11, 13]. No relationship was found between PVS size and the following morphokinetic values: second PB extrusion, pronuclear morphology, time of pronuclei breakdown, formation of two to eight cells (t2 to t8), and irregular cleavage events [33] as well as oocyte yield [72].
Farhi et al. found that the presence of coarse granules in the PVS correlated with low implantation and pregnancy rates in IVF-ICSI cycles [34]. Similarly, Hassa et al. showed that prominent PVS abnormalities (large perivitelline space with or without granules) might affect embryo development [37].
Several authors also reported that debris in the PVS negatively impact on fertilization and embryo quality [2, 6, 49]. In sharp contrast, no correlation between the presence of PVS granularity and embryo development was found by two studies [7, 38]. We analyzed 12 studies relevant to PVS, of which only 6 showed a negative correlation with IVF outcome. This led us to attribute a low score — 5.0 —, suggesting a low predictive value.
First polar body morphology
The 1st polar body (PB1) is extruded to eliminate one of the two copies of each homologous chromosome at the first meiotic division; therefore, correct extrusion of the PB1 indicates successful meiotic maturation of the oocytes [82]. For this reason, the morphology of the polar body could represent a marker of quality. Non-invasive assessment of the PB1 was performed in 10 investigations.
Ebner et al. found a strong correlation between morphological features of the PB1 (smooth surface, rough surface, fragmented and huge) and fertilization rate and embryo quality [24]. A study by Fancsovits et al. reported that fragmentation or degeneration of the PB1 was related with higher fertilization rates and lower rates of embryo fragmentation [30]; embryos from oocytes with a large PB1 showed instead reduced viability.
PB1 degeneration or increased size was associated with reduced fertilization rate, but not pronuclear morphology or embryo quality [5]. Navarro et al. found an association between an increase in PB1 size and decreased rates of fertilization, cleavage, and embryo quality [51]. Zhou et al. reported that an intact PB1 was more strongly associated with rates of good quality day 3 and day 5 embryos, as well as overall embryo yield [77]. In contrast, Verlinsky et al. did not observe any relationship between PB1 morphology and ploidy status, embryo quality, and outcome of embryos transfer [70].
The aforementioned features, including also PB1 size, were investigated by Ciotti et al. [19], who did not detect correlations with fertilization rate, cleavage rate, embryo quality, implantation, and pregnancy rates. De Santis et al. did not find associations between surface characteristics and fragmentation of the PB1 and fertilization rate, embryo quality, and blastocyst formation [23].
The report of Chamayou et al. [18] investigated surface, fragmentation, and size of the PB1; such characteristics resulted moderately associated with embryo quality, but not implantation or pregnancy rates. Fertilization rates and embryo quality were not related to PB1 shape (normal, fragmented, or irregular) in the study of Ten and colleagues [7].
Collectively, although some of the above cited studies showed a correlation between polar body anomalies and diverse parameters of preimplantation development [5, 24, 51], no correlations with clinical outcomes (implantation or clinical pregnancy) were found.
We analyzed 10 PB1 studies; only 5 showed a negative correlation with IVF outcome and 1 a positive correlation. This led us to attribute a score of 4.00, suggesting a low predictive value.
Shape
Good-quality mature human oocytes have a round and clear zona pellucida. Oocytes with extremely abnormal shape are regularly reported (Fig. 2), with rates ranging around 5.9% rate [2]. Oocyte shape was considered in 9 studies.
Braga et al. [17] showed that oocyte shape anomalies, i.e., non-spherical shapes, were correlated with abnormalities in cleavage, delayed compaction, and decrease blastocyst development, confirming previous reports [28]. Faramarzi et al. showed that morphokinetic parameters tPB2, t5 and t8 (time of second PB extrusion and development to the 5-cell and 8-cell stage, respectively), were related to the ooplasm diameter [33], according to Bassil et al. [15] that reported a correlation between oocyte diameter and good-quality blastulation rates. In contrast, several studies did not find correlations between shape anomalies and developmental or viability outcomes, such as fertilization rate, embryo quality [2, 6], aneuploidy [75], cryosurvival [5, 7], implantation, and pregnancy rates [18].
Overall, oocyte shape does not appear to affect the fertilization rate, embryo development, and implantation or pregnancy rates except for the ovoid oocytes.
We analyzed 9 studies focusing on oocyte shape, of which only 3 showed a negative correlation with IVF outcome. The relevant score was 3.33 suggesting that have a low predictive value.
Giant oocyte
A giant oocyte has about twice the volume of a normal oocyte [14]; it is presumably derived from fusion of two oogonia and therefore tetraploid [83]. Generally, the incidence of giant oocytes is around 0.26% among recovered oocytes [57, 84].
Women producing giant oocytes possess higher levels of estradiol, although the significance, if any, of such an association is unclear [14, 43].
Embryo quality and clinical pregnancy rate are not affected in cycles in which a giant oocyte is collected [43], although the rate of abnormal cleavage may be increased [46]. Finally, embryos developing from giant oocytes are a higher risk of digynic triploidy [57].
Based on this evidence, giant oocytes should not be used for clinical practice.
Intracytoplasmic abnormalities
Vacuolization
Vacuolization is a dynamic cytoplasmic dysmorphism. Vacuoles are membrane-bound cytoplasmic inclusions filled with fluid. It is assumed that vacuoles arise either as independent formations [85] or from fusion of preexisting vesicles derived from the SER and/or Golgi apparatus [86]. In 2010, the alpha scientists in reproductive medicine and the ESHRE special interest group of embryology agreed that large vacuoles (> 14 mm in diameter) must be taken into account in the morphological evaluation of the oocytes [1]. The presence of vacuoles was investigated in 14 publications.
Vacuole size may be directly proportional to oocyte damage. Ebner et al. found a significant correlation between vacuole diameter and fertilization, since no fertilization was achieved in the presence of a vacuole diameter > 14 μm [25]. According to Wallbutton et al. [71], very large vacuoles (> 25 μ m diameter) distort the oocyte cytoskeletal structure, impairing sperm–oocyte signaling, sperm binding, meiotic resumption, and embryonic cleavage. According to a results from a meta-analysis [61] also indicate a significant reduction in the probability of fertilization in the presence of large inclusions, such as vacuoles and refractile bodies. The negative influence of vacuoles and refractile bodies on fertilization is also confirmed by previous publications [5, 22, 26].
In another report, although fertilization rates and embryo quality were not affected [7], the oocytes with vacuolated cytoplasm exhibited declined cryosurvival and were unable to develop into good quality blastocysts able to reach hatching stage [13]. Consistent with these findings, the presence of vacuoles was associated with compromised embryo development [12] and impaired blastocyst formation [25, 67].
Xia et al. also reported decreased fertilization and embryo development in vacuolated oocytes [74]. Nevertheless, previous studies observed comparable fertilization rates in morphologically normal and vacuolated oocytes [6, 10, 60].
We analyzed 14 studies onvacuoles, of which 10 showed a negative correlation with IVF outcome. This led us to attribute a score of 7.14. Such a score suggests that vacuoles are a potential indicator of oocyte quality.
Refractile bodies
Refractile bodies (RFs) consist of a mixture of lipids and dense granular materials. They display yellow autofluorescence, consistent with the typical autofluorescence of lipofuscin. The presence of RFs was investigated in 4 publications.
The presence of refractile bodies correlates with a decreased fertilization rate and defective blastocyst formation [54]. A reduced blastocyst development was also described by Takahashi and colleagues [69]. In their study, the chances of implantation of high-quality blastocysts developed from oocytes with refractile bodies were also compromised. On the contrary, other investigators [2, 6] observed that refractile bodies in oocytes do not impact on embryological (fertilization and embryo quality) and clinical (implantation and pregnancy). We analyzed 4 studies on RFs, of which only 2 showed a negative correlation with IVF outcome. This led us to attribute a score of 5.00, suggesting a low predictive value.
Smooth endoplasmic reticulum clusters
Aggregates of SER appear as flat disks in the oocyte cytoplasm corresponding to large tubular SER clusters surrounded by mitochondria [58].
One of the key roles of the SER is calcium storage and release, which are needed for oocyte activation at fertilization. Moreover, complexes of SER in association with mitochondria play a crucial role in energy accumulation, protein and lipid production, and synthesis of nuclear membranes throughout early embryo development [58]. The presence of SER was investigated in 9 publications.
The presence of SER aggregates has been found associated with lower rates of oocyte maturation [62], fertilization rates [58], embryo quality [4, 17, 58], implantation, and pregnancy[53, 62], while miscarriage rates appear increased [4, 17, 53]. Higher rates of perinatal complications, birth defects, and imprinting disorders have also been reported [4, 9, 53, 58]. However some studies have reported the birth of healthy babies derived from cycles including oocytes with SER aggregates (with at least one oocyte displaying SER aggregates in the cohort), as well as from oocytes with SER aggregates [48, 63].
We analyzed 9 SER studies, of which 7 showed a negative correlation with IVF outcome. This led us to attribute a score of 7.78. Such a score suggests that SER are a potential indicator of oocyte quality.
Granularity and color variation of the cytoplasm
In general, the cytoplasm of normal oocytes exhibits uniform fine granulation due to the presence of aggregates of various organelles in the cytoplasm. However, dense opaque granularity at the center of the cytoplasm is considered abnormal [60].
Serhal et al. [60] firstly defined centrally located cytoplasmic granulation (CLCG): the center of the oocyte cytoplasm appeared to be more dense than other regions and considered to have an adverse effect on embryonic development. Cytoplasmic granularity can be scattered or centrally located, with different degree of severity. Its origin and constitution is unclear. The correlation between CLCG of oocytes and developmental competence was discussed in 8 papers.
Fancsovits and colleagues reported that the occurrence of CLCG might be related to the patient's age and gonadotropin stimulation [31]. In another study, oocytes presenting with central granulation were associated with defective pronuclear morphology and reduced embryo quality [5, 27].
Decreased survival and impaired in vitro development after cryopreservation of embryos derived from oocytes with central granulation was reported by Balaban and colleagues [13]. Conversely, Kahraman et al. did not found any correlation with fertilization rates, embryo development; however, ongoing pregnancy rates were significantly affected compromised when embryos from centrally granulated oocytes were transferred [41]. In contrast, in another study, oocytes with centrally located granulation had similar fertilizing and in vitro developmental ability compared with a control group with normal morphology [73]. Consistent with a previous meta-analysis [61], a recent study of 2019 suggests centrally located cytoplasmic granulation (CLCG) might be a normal manifestation of oocyte morphology. This hypothesis is based on the observation that oocytes with or without CLCG had comparable developmental potential and that CLCG embryos generated healthy babies. The developmental potential of oocytes with different intensity of CLCG is comparable [76]. We analyzed 8 CLCG studies, of which 5 showed a negative correlation with IVF outcome. This led us to attribute a score of 6.25.
The color variation of the ooplasm is rarely observed isolated from other morphological characteristics. In fact, it is often described as ‘dark cytoplasm–granular cytoplasm’ [2] ‘dark cytoplasm with slight granulation’ [13], ‘brown oocytes’ [29], and ‘diffused cytoplasmic granularity’ [5].
The correlation between oocyte color variation and developmental competence was discussed in 6 papers. Ooplasm darkness was associated to reduced embryo quality [7, 45], in contrast to other studies that showed no negative correlation on IVF outcome [2, 6, 13, 29]. Assessment of oocyte color variation produced a score 3.33, corresponding to a low predictive value.
Cumulative effect of multiple abnormalities
Ten studies have examined possible cumulative effects of diverse oocyte morphological abnormalities on embryo development and IVF outcome.
An MII oocyte morphologic score (MOMS) was described by Rienzi et al. [5] based on the impact of different oocyte morphological abnormalities (vacuoles, abnormal PB1, large perivitelline space, diffuse cytoplasmic granularity and/or centrally located granular area) on fertilization rate, pronuclear pattern, and embryo morphology. A significant relationship was found between MOMS and female age, female basal FSH, and clinical outcome. Yakin et al. showed that oocyte dysmorphisms of the meiotic spindle, ZP, PVS and PB, as well as the presence of vacuoles or granulations, were not associated with a higher risk of aneuploidy but adversely affected blastocyst formation [75].
The oocyte grading system of Xia et al., based on the assessment of the perivitelline space, PB morphology, and cytoplasmic inclusions, suggests that multiple anomalies of the PB1, PVS size, and cytoplasmic inclusions affect fertilization rate and embryo quality after ICSI [74].
In a study of Wilding et al. [73], top quality oocytes, identified on the basis of cytoplasmic granularity, presence of vacuoles, and mechanical response of the oolemma to microinjection, achieved fertilization at much higher rate (96%) compared with low scoring oocytes (25.6%).
Balaban et al. [2] showed no difference in terms of pregnancy and implantation rates between embryos derived from morphologically normal or abnormal oocytes (the latter affected by extracytoplasmic, cytoplasmic, shape, and multiple defects). Similar results were obtained in the study of La Sala et al., which showed similar rates of implantation, total pregnancies/ET, clinical intrauterine pregnancy, singleton and multiple births, and ‘‘take-home’’ baby rates between oocyte with morphologically abnormalities (COC, ZP, PVS, PB, vacuolization, or granulation of the oocytes) and control group [42].
Likewise, Ten et al. did not observe associations between oocyte dysmorphisms (PVS content, shape, PB, cytoplasmic granularity, cytoplasmic vacuoles) and fertilization rate and embryo quality [7].
Chamayou et al. reported no associations between implantation, clinical pregnancy rates, and oocyte morphological abnormalities such as texture, inclusions, vacuoles, central granulation, PVS, PB, and shape [18].
In another study, oocytes with poor morphology (dark cytoplasm; many vacuoles in cytoplasm) developed into poor-quality embryos and produced lower pregnancy rate (5.5 versus 29.4%, affected and normal oocytes, respectively) [45].
In the study of Serhal et al. [60] were oocytes with abnormal cytoplasm (excessive granularity, cytoplasmic inclusions, SER clustering and refractile bodies) normal fertilization and early embryo development, but the resulting embryos had a lower implantation potential compared with controls.
With regard to cumulative effect of multiple abnormalities, 6 out of 10 papers indicated negative correlations with IVF outcome, generating an OLS of 6.00.
Discussion
The purpose of this systematic review was to evaluate studies focusing on assessment of oocyte morphology, with the aim to identify features able to predict developmental competence. From relevant studies selected for this analysis, we did not find unanimity for correlations between 11 specific dysmorphisms and IVF outcome. Meta-analyses are normally performed to extract collective information from multiple studies.
To elaborate this review, we opted for an alternative methodology, due to high inconsistency in experimental design outcome parameters of selected studies regardless, for each dysmorphism, we produced a semi-quantitative outcome — the OLS — which may be used a as tool for oocyte assessment in the daily IVF routine and considered as a starting point for future more focused research. Therefore, the higher the score, the greater the predictive value of an abnormality and the likelihood that IVF outcome is affected.
Among extracytoplasmic abnormalities, we analyzed six studies relevant to the COC morphology (i.e., degree of compaction), of which five showed a negative correlation with IVF outcome. This led us to attribute a score of 8.33. Such a score suggests that COC has a potential as indicator of oocyte quality.
Other extracytoplasmic abnormalities (ZP, PVS, PB1, and cell shape) produced a low score (5.29, 5.00, 4.00, and 3.33 respectively) and therefore have a low predictive value. Only if analyzed separately, ZP birefringence generates a top literature score [20]. Birefringence of the ZP could then assist oocyte/embryo selection procedures and contribute to improve the efficiency of IVF treatments.
Cytoplasmic abnormalities include vacuolization, presence of refractile bodies, SER clusters, and variation in granularity and color of the cytoplasm (dark colored cytoplasm, slightly, or excessive whole/centrally located granulation).
Vacuoles are associated with a relatively high OLS (7.14). Unfortunately, in most papers, vacuole size was rarely reported. Future studies could then focus on quantitative vacuoles assessment (size) and outcome.
The presence of SER clusters was also associated with a high score (7.78). SER clusters therefore represent a strong candidate to predict oocyte quality and ultimately IVF outcome. Higher rates of perinatal complications, birth defects, and imprinting disorders have been reported in association with this dysmorphism [4, 9, 48, 53, 58]. Based on these data, reporting a higher risk of malformations in babies originating from oocyte, the Istanbul Consensus Workshop in 2011, recommended de-selection of SER-positive oocytes [1]. However, this question remains controversial also because some studies reported the birth of healthy babies derived from cycles with oocytes affected by SER aggregates (with at least one SER oocyte in the cohort) and even from oocytes showing SER aggregates [39, 48].
The conflicting data on SER aggregates of the last decade led to the conclusion of the Vienna Consensus [87] that a case by case approach should be adopted. However, personalized approaches are not reproducible, by definition. Therefore, to prevent oocyte wastage in the absence of a final evidence against SEr aggregates, the use of oocytes with SER aggregates and the prioritization for transfer of embryos derived from SER negative oocytes may be an option [35].
Cytoplasmic granularity produced an OLS of 6.25. The assessment of other cytoplasmic abnormalities (refractile bodies and dark cytoplasm) generated less consiste results and therefore low scores.
The concomitant presence of multiple abnormalities was associated with an OLS of 6.00. This result has some degree of uncertainty, as it does not clarify which dysmorphism has larger impact on IVF outcomes.
This review recapitulates and expresses in semi-quantitative form the relevance of dysmorphisms to oocyte quality, offering a guidance for oocyte assessment and embryo selection phenotypic deviations. However, the use of dysmorphic oocytes remains an open question. Some categories of such oocytes may have a reduced developmental potential and thereby impact on clinical outcome.
Regardless, they could contribute to maximize the cumulative outcome, i.e., the overall chances to achieve a live birth per treatment cycle. While in the short and medium term this proposition suggests the use of all dysmorphic oocytes — although prioritizing the use of embryos derived from normal oocytes — in the longer term, large studies based of national birth registries are needed to investigate the hypothesis that some dysmorphisms conceal a health risk to the newborn.
Conclusions
The proposed OLS methodology offers semi-quantitative information on studies investigating the possible relationships between oocyte dysmorphisms and biological or clinical outcomes of assisted reproduction treatments.
Overall, we identified six extracytoplasmic (COC, zona pellucida, perivitelline space, polar body 1, shape, giant size) and five intracytoplasmic (vacuoles, refractile bodies, SER clusters, granularity, color) oocyte dysmorphisms amenable to analysis. COC, vacuoles, SER clusters, and granularity produced OLS suggestive of a prevalence of studies reporting a negative outcome.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
The study was funded by the authors’ institution.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 2.Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod. 1998;13(12):3431–3433. doi: 10.1093/humrep/13.12.3431. [DOI] [PubMed] [Google Scholar]
- 3.Dal Canto M, Guglielmo MC, Mignini Renzini M, Fadini R, Moutier C, Merola M, et al. Dysmorphic patterns are associated with cytoskeletal alterations in human oocytes. Hum Reprod. 2017;32(4):750–757. doi: 10.1093/humrep/dex041. [DOI] [PubMed] [Google Scholar]
- 4.Ebner T, Moser M, Shebl O, Sommerguber M, Tews G. Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. Reprod Biomed Online. 2008;16(1):113–118. doi: 10.1016/s1472-6483(10)60563-9. [DOI] [PubMed] [Google Scholar]
- 5.Rienzi L, Ubaldi FM, Iacobelli M, Minasi MG, Romano S, Ferrero S, et al. Significance of metaphase II human oocyte morphology on ICSI outcome. Fertil Steril. 2008;90(5):1692–1700. doi: 10.1016/j.fertnstert.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 6.De Sutter P, Dozortsev D, Qian C, Dhont M. Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1996;11(3):595–597. doi: 10.1093/humrep/11.3.595. [DOI] [PubMed] [Google Scholar]
- 7.Ten J, Mendiola J, Vioque J, de Juan J, Bernabeu R. Donor oocyte dysmorphisms and their influence on fertilization and embryo quality. Reprod Biomed Online. 2007;14(1):40–48. doi: 10.1016/s1472-6483(10)60762-6. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akarsu C, Çağlar G, Vicdan K, Sözen E, Biberoğlu K. Smooth endoplasmic reticulum aggregations in all retrieved oocytes causing recurrent multiple anomalies: case report. Fertil Steril. 2009;92(4):1496.e1–1496.e3. doi: 10.1016/j.fertnstert.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 10.Alikani M, Palermo G, Adler A, Bertoli M, Blake M, Cohen J. Intracytoplasmic sperm injection in dysmorphic human oocytes. Zygote. 1995;3(4):283–288. doi: 10.1017/s0967199400002707. [DOI] [PubMed] [Google Scholar]
- 11.Ashrafi M, Karimian L, Eftekhari-Yazdi P, Hasani F, Arabipoor A, Bahmanabadi A, et al. Effect of oocyte dysmorphisms on intracytoplasmic sperm injection cycle outcomes in normal ovarian responders. J Obstet Gynaecol Res. 2015;41(12):1912–1920. doi: 10.1111/jog.12818. [DOI] [PubMed] [Google Scholar]
- 12.Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod Biomed Online. 2006;12(5):608–615. doi: 10.1016/s1472-6483(10)61187-x. [DOI] [PubMed] [Google Scholar]
- 13.Balaban B, Ata B, Isiklar A, Yakin K, Urman B. Severe cytoplasmic abnormalities of the oocyte decrease cryosurvival and subsequent embryonic development of cryopreserved embryos. Hum Reprod. 2008;23(8):1778–1785. doi: 10.1093/humrep/den127. [DOI] [PubMed] [Google Scholar]
- 14.Balakier H, Bouman D, Sojecki A, Librach C, Squire JA. Morphological and cytogenetic analysis of human giant oocytes and giant embryos. Hum Reprod. 2002;17(9):2394–2401. doi: 10.1093/humrep/17.9.2394. [DOI] [PubMed] [Google Scholar]
- 15.Bassil R, Casper RF, Meriano J, Smith R, Haas J, Mehta C, et al. Can oocyte diameter predict embryo quality? Reprod Sci. 2021;28(3):904–908. doi: 10.1007/s43032-020-00306-3. [DOI] [PubMed] [Google Scholar]
- 16.Bertrand E, Van den Bergh M, Englert Y. Does zona pellucida thickness influence the fertilization rate? Hum Reprod. 1995;10(5):1189–1193. doi: 10.1093/oxfordjournals.humrep.a136116. [DOI] [PubMed] [Google Scholar]
- 17.Braga DPAF, Setti AS, de Figueira RCS, Machado RB, Laconelli A, Borges E. Influence of oocyte dysmorphisms on blastocyst formation and quality. Fertil Steril. 2013;100(3):748–54. doi: 10.1016/j.fertnstert.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Chamayou S, Ragolia C, Alecci C, Storaci G, Maglia E, Russo E, et al. Meiotic spindle presence and oocyte morphology do not predict clinical ICSI outcomes: a study of 967 transferred embryos. Reprod Biomed Online. 2006;13(5):661–667. doi: 10.1016/s1472-6483(10)60656-6. [DOI] [PubMed] [Google Scholar]
- 19.Ciotti PM, Notarangelo L, Morselli-Labate AM, Felletti V, Porcu E, Venturoli S. First polar body morphology before ICSI is not related to embryo quality or pregnancy rate. Hum Reprod. 2004;19(10):2334–2339. doi: 10.1093/humrep/deh433. [DOI] [PubMed] [Google Scholar]
- 20.Dal Canto M, Brambillasca F, Mignini Renzini M, Coticchio G, Merola M, Lain M, et al. Cumulus cell-oocyte complexes retrieved from antral follicles in IVM cycles: relationship between COCs morphology, gonadotropin priming and clinical outcome. J Assist Reprod Genet. 2012;29(6):513–519. doi: 10.1007/s10815-012-9766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daya S, Kohut J, Gunby J, Younglai E. Influence of blood clots in the cumulus complex on oocyte fertilization and cleavage. Hum Reprod. 1990;5(6):744–746. doi: 10.1093/oxfordjournals.humrep.a137179. [DOI] [PubMed] [Google Scholar]
- 22.de Cássia Figueira RS, de Almeida Ferreira Braga DP, Semião-Francisco L, Madaschi C, Iaconelli A, Borges E. Metaphase II human oocyte morphology: contributing factors and effects on fertilization potential and embryo developmental ability in ICSI cycles. Fertil Steril. 2010;94(3):1115–7. doi: 10.1016/j.fertnstert.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 23.De Santis L, Cino I, Rabellotti E, Calzi F, Persico P, Borini A, et al. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod Biomed Online. 2005;11(1):36–42. doi: 10.1016/s1472-6483(10)61296-5. [DOI] [PubMed] [Google Scholar]
- 24.Ebner T, Yaman C, Moser M, Sommergruber M, Feichtinger O, Tews G. Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum Reprod. 2000;15(2):427–430. doi: 10.1093/humrep/15.2.427. [DOI] [PubMed] [Google Scholar]
- 25.Ebner T, Moser M, Sommergruber M, Gaiswinkler U, Shebl O, Jesacher K, et al. Occurrence and developmental consequences of vacuoles throughout preimplantation development. Fertil Steril. 2005;83(6):1635–1640. doi: 10.1016/j.fertnstert.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Ebner T, Moser M, Tews G. Is oocyte morphology prognostic of embryo developmental potential after ICSI? Reprod Biomed Online. 2006;12(4):507–512. doi: 10.1016/s1472-6483(10)62006-8. [DOI] [PubMed] [Google Scholar]
- 27.Ebner T, Moser M, Shebl O, Sommergruber M, Yaman C, Tews G. Blood clots in the cumulus-oocyte complex predict poor oocyte quality and post-fertilization development. Reprod Biomed Online. 2008;16(6):801–807. doi: 10.1016/s1472-6483(10)60145-9. [DOI] [PubMed] [Google Scholar]
- 28.Ebner T, Shebl O, Moser M, Sommergruber M, Tews G. Developmental fate of ovoid oocytes. Hum Reprod. 2008;23(1):62–66. doi: 10.1093/humrep/dem280. [DOI] [PubMed] [Google Scholar]
- 29.Esfandiari N, Burjaq H, Gotlieb L, Casper RF. Brown oocytes: implications for assisted reproductive technology. Fertil Steril. 2006;86(5):1522–1525. doi: 10.1016/j.fertnstert.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 30.Fancsovits P, Tóthné ZG, Murber A, Takács FZ, Papp Z, Urbancsek J. Correlation between first polar body morphology and further embryo development. Acta Biol Hung. 2006;57(3):331–338. doi: 10.1556/ABiol.57.2006.3.7. [DOI] [PubMed] [Google Scholar]
- 31.Fancsovits P, Tóthné ZG, Murber Á, Rigó J, Urbancsek J. Importance of cytoplasmic granularity of human oocytes in in vitro fertilization treatments. Acta Biol Hung. 2012;63(2):189–201. doi: 10.1556/ABiol.63.2012.2.3. [DOI] [PubMed] [Google Scholar]
- 32.Faramarzi A, Khalili MA, Agha-Rahimi A, Omidi M. Is there any correlation between oocyte polarization microscopy findings with embryo time lapse monitoring in ICSI program? Arch Gynecol Obstet. 2017;295(6):1515–1522. doi: 10.1007/s00404-017-4387-8. [DOI] [PubMed] [Google Scholar]
- 33.Faramarzi A, Khalili MA, Omidi M. Morphometric analysis of human oocytes using time lapse: does it predict embryo developmental outcomes? Hum Fertil (Camb) 2019;22(3):171–176. doi: 10.1080/14647273.2017.1406670. [DOI] [PubMed] [Google Scholar]
- 34.Farhi J, Nahum H, Weissman A, Zahalka N, Glezerman M, Levran D. Coarse granulation in the perivitelline space and IVF-ICSI outcome. J Assist Reprod Genet. 2002;19(12):545–549. doi: 10.1023/A:1021243530358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreux L, Sallem A, Chargui A, Gille A-S, Bourdon M, Maignien C, et al. Is it time to reconsider how to manage oocytes affected by smooth endoplasmic reticulum aggregates? Hum Reprod. 2019;34(4):591–600. doi: 10.1093/humrep/dez010. [DOI] [PubMed] [Google Scholar]
- 36.González-Ortega C, Cancino-Villarreal P, Alonzo-Torres VE, Martínez-Robles I, Pérez-Peña E, Gutiérrez-Gutiérrez AM. Polarized light microscopy for evaluation of oocytes as a prognostic factor in the evolution of a cycle in assisted reproduction. Ginecol Obstet Mex. 2016;84(4):217–227. [PubMed] [Google Scholar]
- 37.Hassa H, Aydın Y, Taplamacıoğlu F. The role of perivitelline space abnormalities of oocytes in the developmental potential of embryos. J Turk Ger Gynecol Assoc. 2014;15(3):161–163. doi: 10.5152/jtgga.2014.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassan-Ali H, Hisham-Saleh A, El-Gezeiry D, Baghdady I, Ismaeil I, Mandelbaum J. Perivitelline space granularity: a sign of human menopausal gonadotrophin overdose in intracytoplasmic sperm injection. Hum Reprod. 1998;13(12):3425–3430. doi: 10.1093/humrep/13.12.3425. [DOI] [PubMed] [Google Scholar]
- 39.Hattori H, Nakamura Y, Nakajo Y, Araki Y, Kyono K. Deliveries of babies with normal health derived from oocytes with smooth endoplasmic reticulum clusters. J Assist Reprod Genet. 2014;31(11):1461–1467. doi: 10.1007/s10815-014-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Høst E, Gabrielsen A, Lindenberg S, Smidt-Jensen S. Apoptosis in human cumulus cells in relation to zona pellucida thickness variation, maturation stage, and cleavage of the corresponding oocyte after intracytoplasmic sperm injection. Fertil Steril. 2002;77(3):511–515. doi: 10.1016/s0015-0282(01)03006-0. [DOI] [PubMed] [Google Scholar]
- 41.Kahraman S, Yakin K, Dönmez E, Samli H, Bahçe M, Cengiz G, et al. Relationship between granular cytoplasm of oocytes and pregnancy outcome following intracytoplasmic sperm injection. Hum Reprod. 2000;15(11):2390–2393. doi: 10.1093/humrep/15.11.2390. [DOI] [PubMed] [Google Scholar]
- 42.La Sala GB, Nicoli A, Villani MT, Di Girolamo R, Capodanno F, Blickstein I. The effect of selecting oocytes for insemination and transferring all resultant embryos without selection on outcomes of assisted reproduction. Fertil Steril. 2009;91(1):96–100. doi: 10.1016/j.fertnstert.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Lehner A, Kaszas Z, Murber A, Rigo J, Urbancsek J, Fancsovits P. Giant oocytes in human in vitro fertilization treatments. Arch Gynecol Obstet. 2015;292(3):697–703. doi: 10.1007/s00404-015-3679-0. [DOI] [PubMed] [Google Scholar]
- 44.Lin Y-C, Chang S-Y, Lan K-C, Huang H-W, Chang C-Y, Tsai M-Y, et al. Human oocyte maturity in vivo determines the outcome of blastocyst development in vitro. J Assist Reprod Genet. 2003;20(12):506–512. doi: 10.1023/B:JARG.0000013651.37866.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loutradis D, Drakakis P, Kallianidis K, Milingos S, Dendrinos S, Michalas S. Oocyte morphology correlates with embryo quality and pregnancy rate after intracytoplasmic sperm injection. Fertil Steril. 1999;72(2):240–244. doi: 10.1016/s0015-0282(99)00233-2. [DOI] [PubMed] [Google Scholar]
- 46.Machtinger R, Politch JA, Hornstein MD, Ginsburg ES, Racowsky C. A giant oocyte in a cohort of retrieved oocytes: does it have any effect on the in vitro fertilization cycle outcome? Fertil Steril. 2011;95(2):573–576. doi: 10.1016/j.fertnstert.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 47.Madaschi C, Aoki T, de Almeida Ferreira Braga DP, de Cássia Sávio Figueira R, Semião Francisco L, Iaconelli A, et al. Zona pellucida birefringence score and meiotic spindle visualization in relation to embryo development and ICSI outcomes. Reprod Biomed Online. 2009;18(5):681–6. doi: 10.1016/s1472-6483(10)60014-4. [DOI] [PubMed] [Google Scholar]
- 48.Mateizel I, Van Landuyt L, Tournaye H, Verheyen G. Deliveries of normal healthy babies from embryos originating from oocytes showing the presence of smooth endoplasmic reticulum aggregates. Hum Reprod. 2013;28(8):2111–2117. doi: 10.1093/humrep/det241. [DOI] [PubMed] [Google Scholar]
- 49.Meriano JS, Alexis J, Visram-Zaver S, Cruz M, Casper RF. Tracking of oocyte dysmorphisms for ICSI patients may prove relevant to the outcome in subsequent patient cycles. Hum Reprod. 2001;16(10):2118–2123. doi: 10.1093/humrep/16.10.2118. [DOI] [PubMed] [Google Scholar]
- 50.Montag M, Schimming T, Köster M, Zhou C, Dorn C, Rösing B, et al. Oocyte zona birefringence intensity is associated with embryonic implantation potential in ICSI cycles. Reprod Biomed Online. 2008;16(2):239–244. doi: 10.1016/s1472-6483(10)60580-9. [DOI] [PubMed] [Google Scholar]
- 51.Navarro PA, de Araújo MM, de Araújo CM, Rocha M, dos Reis R, Martins W. Relationship between first polar body morphology before intracytoplasmic sperm injection and fertilization rate, cleavage rate, and embryo quality. Int J Gynaecol Obstet. 2009;104(3):226–229. doi: 10.1016/j.ijgo.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Ng ST, Chang TH, Wu TC. Prediction of the rates of fertilization, cleavage, and pregnancy success by cumulus-coronal morphology in an in vitro fertilization program. Fertil Steril. 1999;72(3):412–417. doi: 10.1016/s0015-0282(99)00290-3. [DOI] [PubMed] [Google Scholar]
- 53.Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19(7):1591–1597. doi: 10.1093/humrep/deh258. [DOI] [PubMed] [Google Scholar]
- 54.Otsuki J, Nagai Y, Chiba K. Lipofuscin bodies in human oocytes as an indicator of oocyte quality. J Assist Reprod Genet. 2007;24(7):263–270. doi: 10.1007/s10815-007-9130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rama Raju GA, Prakash GJ, Krishna KM, Madan K. Meiotic spindle and zona pellucida characteristics as predictors of embryonic development: a preliminary study using PolScope imaging. Reprod Biomed Online. 2007;14(2):166–174. doi: 10.1016/s1472-6483(10)60784-5. [DOI] [PubMed] [Google Scholar]
- 56.Rattanachaiyanont M. Lack of correlation between oocyte- corona-cumulus complex morphology and nuclear maturity of oocytes collected in stimulated cycles for intracytoplasmic sperm injection. Fertil Steril. 1999;71(5):937–940. doi: 10.1016/s0015-0282(99)00100-4. [DOI] [PubMed] [Google Scholar]
- 57.Rosenbusch B, Schneider M, Gläser B, Brucker C. Cytogenetic analysis of giant oocytes and zygotes to assess their relevance for the development of digynic triploidy. Hum Reprod. 2002;17(9):2388–2393. doi: 10.1093/humrep/17.9.2388. [DOI] [PubMed] [Google Scholar]
- 58.Sá R, Cunha M, Silva J, Luís A, Oliveira C, Teixeira da Silva J, et al. Ultrastructure of tubular smooth endoplasmic reticulum aggregates in human metaphase II oocytes and clinical implications. Fertil Steril. 2011;96(1):143–149.e7. doi: 10.1016/j.fertnstert.2011.04.088. [DOI] [PubMed] [Google Scholar]
- 59.Sauerbrun-Cutler M-T, Vega M, Breborowicz A, Gonzales E, Stein D, Lederman M, et al. Oocyte zona pellucida dysmorphology is associated with diminished in-vitro fertilization success. J Ovarian Res. 2015;27(8):5. doi: 10.1186/s13048-014-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12(6):1267–1270. doi: 10.1093/humrep/12.6.1267. [DOI] [PubMed] [Google Scholar]
- 61.Setti AS, Figueira RCS, Braga DPAF, Colturato SS, Iaconelli A, Borges E. Relationship between oocyte abnormal morphology and intracytoplasmic sperm injection outcomes: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):364–370. doi: 10.1016/j.ejogrb.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 62.Setti AS, Figueira RCS, de Almeida Ferreira Braga DP, de Azevedo MC, Iaconelli A, Borges E. Oocytes with smooth endoplasmic reticulum clusters originate blastocysts with impaired implantation potential. Fertil Steril. 2016;106(7):1718–24. doi: 10.1016/j.fertnstert.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Shaw-Jackson C, Thomas A-L, Van Beirs N, Ameye L, Colin J, Bertrand E, et al. Oocytes affected by smooth endoplasmic reticulum aggregates: to discard or not to discard? Arch Gynecol Obstet. 2016;294(1):175–184. doi: 10.1007/s00404-016-4066-1. [DOI] [PubMed] [Google Scholar]
- 64.Shen Y, Stalf T, Mehnert C, Eichenlaub-Ritter U, Tinneberg HR. High magnitude of light retardation by the zona pellucida is associated with conception cycles. Hum Reprod. 2005;20(6):1596–1606. doi: 10.1093/humrep/deh811. [DOI] [PubMed] [Google Scholar]
- 65.Shi W, Xu B, Wu L-M, Jin R-T, Luan H-B, Luo L-H, et al. Oocytes with a dark zona pellucida demonstrate lower fertilization, implantation and clinical pregnancy rates in IVF/ICSI cycles. PLoS ONE. 2014;9(2):e89409. doi: 10.1371/journal.pone.0089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sousa M, Teixeira da Silva J, Silva J, Cunha M, Viana P, Oliveira E, et al. Embryological, clinical and ultrastructural study of human oocytes presenting indented zona pellucida. Zygote. 2015;23(1):145–57. doi: 10.1017/S0967199413000403. [DOI] [PubMed] [Google Scholar]
- 67.Sousa M, Cunha M, Silva J, Oliveira E, Pinho MJ, Almeida C, et al. Ultrastructural and cytogenetic analyses of mature human oocyte dysmorphisms with respect to clinical outcomes. J Assist Reprod Genet. 2016;33(8):1041–1057. doi: 10.1007/s10815-016-0739-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tabibnejad N, Soleimani M, Aflatoonian A. Zona pellucida birefringence and meiotic spindle visualization are not related to the time-lapse detected embryo morphokinetics in women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2018;230:96–102. doi: 10.1016/j.ejogrb.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi H, Otsuki J, Yamamoto M, Saito H, Hirata R, Habara T, et al. Clinical outcomes of MII oocytes with refractile bodies in patients undergoing ICSI and single frozen embryo transfer. Reprod Med Biol. 2020;19(1):75–81. doi: 10.1002/rmb2.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verlinsky Y, Lerner S, Illkevitch N, Kuznetsov V, Kuznetsov I, Cieslak J, et al. Is there any predictive value of first polar body morphology for embryo genotype or developmental potential? Reprod Biomed Online. 2003;7(3):336–341. doi: 10.1016/s1472-6483(10)61874-3. [DOI] [PubMed] [Google Scholar]
- 71.Wallbutton S, Kasraie J. Vacuolated oocytes: fertilization and embryonic arrest following intra-cytoplasmic sperm injection in a patient exhibiting persistent oocyte macro vacuolization–case report. J Assist Reprod Genet. 2010;27(4):183–188. doi: 10.1007/s10815-010-9399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weghofer A, Kushnir VA, Darmon SK, Jafri H, Lazzaroni-Tealdi E, Zhang L, et al. Age, body weight and ovarian function affect oocyte size and morphology in non-PCOS patients undergoing intracytoplasmic sperm injection (ICSI) PLoS ONE. 2019;14(10):e0222390. doi: 10.1371/journal.pone.0222390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilding M, Di Matteo L, D’Andretti S, Montanaro N, Capobianco C, Dale B. An oocyte score for use in assisted reproduction. J Assist Reprod Genet. 2007;24(8):350–358. doi: 10.1007/s10815-007-9143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xia P. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997;12(8):1750–1755. doi: 10.1093/humrep/12.8.1750. [DOI] [PubMed] [Google Scholar]
- 75.Yakin K, Balaban B, Isiklar A, Urman B. Oocyte dysmorphism is not associated with aneuploidy in the developing embryo. Fertil Steril. 2007;88(4):811–816. doi: 10.1016/j.fertnstert.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 76.Yi X-F, Xi H-L, Zhang S-L, Yang J. Relationship between the positions of cytoplasmic granulation and the oocytes developmental potential in human. Sci Rep. 2019;9(1):7215. doi: 10.1038/s41598-019-43757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou W, Fu L, Sha W, Chu D, Li Y. Relationship of polar bodies morphology to embryo quality and pregnancy outcome. Zygote. 2016;24(3):401–407. doi: 10.1017/S0967199415000325. [DOI] [PubMed] [Google Scholar]
- 78.Richani D, Dunning KR, Thompson JG, Gilchrist RB. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update. 2021;27(1):27–47. doi: 10.1093/humupd/dmaa043. [DOI] [PubMed] [Google Scholar]
- 79.Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, et al. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. 2000;45(5):314–320. [PubMed] [Google Scholar]
- 80.Ebner T, Balaban B, Moser M, Shebl O, Urman B, Ata B, et al. Automatic user-independent zona pellucida imaging at the oocyte stage allows for the prediction of preimplantation development. Fertil Steril. 2010;94(3):913–920. doi: 10.1016/j.fertnstert.2009.03.106. [DOI] [PubMed] [Google Scholar]
- 81.Ubaldi F, Rienzi L. Morphological selection of gametes. Placenta. 2008;29(Suppl B):115–20. doi: 10.1016/j.placenta.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 82.Eichenlaub-Ritter U, Schmiady H, Kentenich H, Soewarto D. Recurrent failure in polar body formation and premature chromosome condensation in oocytes from a human patient: indicators of asynchrony in nuclear and cytoplasmic maturation. Hum Reprod. 1995;10(9):2343–2349. doi: 10.1093/oxfordjournals.humrep.a136297. [DOI] [PubMed] [Google Scholar]
- 83.Austin CR. Anomalies of fertilization leading to triploidy. J Cell Comp Physiol. 1960;56(Suppl 1):1–15. doi: 10.1002/jcp.1030560404. [DOI] [PubMed] [Google Scholar]
- 84.Rosenbusch B. The potential significance of binovular follicles and binucleate giant oocytes for the development of genetic abnormalities. J Genet. 2012;91(3):397–404. doi: 10.1007/s12041-012-0195-x. [DOI] [PubMed] [Google Scholar]
- 85.Van Blerkom J. Occurrence and developmental consequences of aberrant cellular organization in meiotically mature human oocytes after exogenous ovarian hyperstimulation. J Electron Microsc Tech. 1990;16(4):324–346. doi: 10.1002/jemt.1060160405. [DOI] [PubMed] [Google Scholar]
- 86.Veeck L, El Shafie M, Sousa M, Windt M-L, Kruger TF. An Atlas of the Ultrastructure of Human Oocytes: A Guide for Assisted Reproduction, First Edition. New York: The Parthenon Publishing Group, 2000. Fertil Steril. 2001;75(4):838–9.
- 87.ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine. Electronic address: coticchio.biogenesi@grupposandonato.it. The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod Biomed Online. 2017; 35(5):494–510 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.