The complex interaction between rain, vegetation, soil, and climate can only be understood by studying form–function relationships of leaves in a meaningful ecological context.
Keywords: Drip tips, drop impact, epicuticular wax, leaf inclination angle, leaf movement, leaf trait adaptation, splash erosion, trichomes, water repellency, water shedding
Abstract
Water shedding from leaves is a complex process depending on multiple leaf traits interacting with rain, wind, and air humidity, and with the entire plant and surrounding vegetation. Here, we synthesize current knowledge of the physics of water shedding with implications for plant physiology and ecology. We argue that the drop retention angle is a more meaningful parameter to characterize the water-shedding capacity of leaves than the commonly measured static contact angle. The understanding of the mechanics of water shedding is largely derived from laboratory experiments on artificial rather than natural surfaces, often on individual aspects such as surface wettability or drop impacts. In contrast, field studies attempting to identify the adaptive value of leaf traits linked to water shedding are largely correlative in nature, with inconclusive results. We make a strong case for taking the hypothesis-driven experimental approach of biomechanical laboratory studies into a real-world field setting to gain a comprehensive understanding of leaf water shedding in a whole-plant ecological and evolutionary context.
Introduction
In times of global change, with increasing frequency of high-intensity rainfall events (Allan, 2011) aggravating the loss of arable soil through erosion while a growing world population demands ever more food (Pimentel et al., 1995), the interaction of vegetation with atmospheric water has never been a hotter topic in plant science. Plants, and their leaves in particular, intercept rainfall and act as condensation surfaces for fog and dew, and thereby completely change how this precipitation reaches and impacts the ground below (Dunkerley, 2020). The retention of surface water on leaves not only alters the hydrological cycle by increasing evaporation, it also has diverse impacts on the plant itself (summarized in Dawson and Goldsmith, 2018).
The effects of surface water retention on plants are, however, strongly context dependent. On the one hand, persistent wetness on leaves can impede transpiration and photosynthesis (Aparecido et al., 2016, 2017; Berry and Goldsmith, 2020); however, on the other hand, foliar water uptake (Berry et al., 2019; Schreel et al., 2020) can boost photosynthesis and growth (Eller et al., 2013; Carmichael et al., 2020). Depending on the relative solute content of the interstitial fluid and the surface water, nutrients can leach from the leaf (Tukey, 1970) or be taken up (Templer et al., 2015). In epiphytic bromeliads, wettable leaves have gained an important function for water and nutrient uptake (Zambrano et al., 2019), and many species store rain water in tightly sealed leaf ‘tanks’ (Freschi et al., 2010; Ladino et al., 2019). Leaf surface wetness has been shown to promote epiphyll and pathogen growth (Huber and Gillespie, 1992), but mutualistic fungi benefit too (Arnold et al., 2003).
In general, it appears that temporary water cover on leaf surfaces can have benefits, but long-term wetness tends to be disadvantageous. Therefore, plants have ubiquitously evolved adaptations to promote water shedding from their leaves. These can be simplified into two general mechanisms: (i) increased water repellency of the leaf surface and (ii) steeper leaf inclination angle. In the following, we will explore both strategies in detail and discuss their interaction with each other as well as trade-offs with other leaf functions, and implications for leaf ecology and evolution. We will first consider the (simpler) case of a droplet or water layer on a static leaf, as might occur after rain or as a result of condensation, before exploring the more complex effects of drop impacts during rain. Finally, we will take a look at some specialized adaptations such as the ‘drip tips’ on the leaves of a diversity of tropical species, and anisotropic surface structures promoting directional water transport.
Wetting versus water shedding
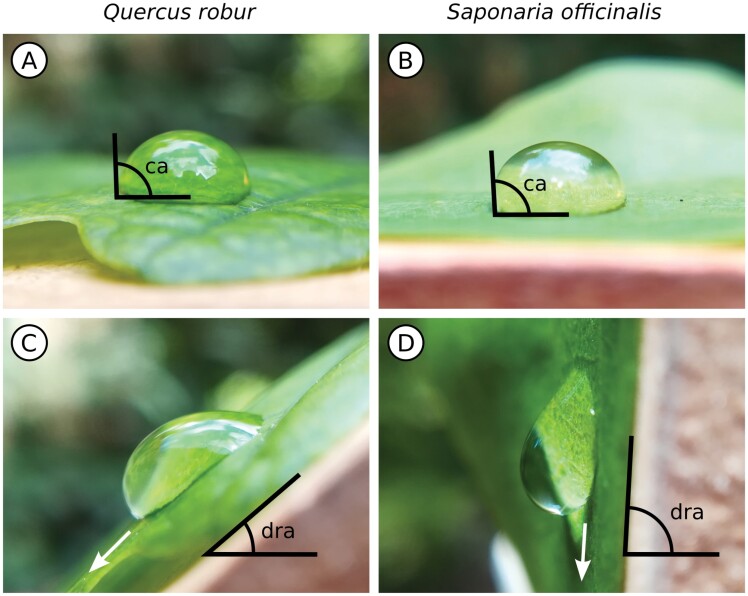
Two factors dictate how easily water is shed from a leaf: (i) the water repellency and (ii) the inclination of the surface. In brief, the more easily a drop can move and the steeper the surface, the more easily water will be shed. Both factors are intuitively captured in the drop retention angle—the angle at which a drop starts to roll off a surface when the surface is gradually tilted (Fig. 1C, D). Note that we use ‘water repellency’ to describe the ease of drop movement across the surface. As we will show below, this is not synonymous with ‘non-wettability’ or hydrophobicity (Callies and Quéré, 2005), which is characterized by the contact angle of a sessile drop on the horizontal surface (Kung et al., 2019; Fig. 1A, B). On hydrophilic surfaces, contact angles are small and drops spread; on hydrophobic surfaces, contact angles are large and drops are increasingly spherical in shape (Fig. 2A–D). The exact boundary between hydrophilic and hydrophobic has been disputed repeatedly (Guo et al., 2008; Law, 2015). The maximum contact angle of water measured on a flat surface is 119° (Nishino et al., 1999). Higher apparent contact angles are measured on rough surfaces where microscopic roughness can effectively act as an enhancer of the inherent chemical surface properties (Bico et al., 2002; Bhushan and Jung, 2008). In extreme cases, contact angles of 180° (i.e. perfectly spherical drops) can be achieved (Herminghaus, 2000), most famously on the lotus leaf (Barthlott and Neinhuis, 1997).
Fig. 1.
Two parameters that are commonly measured to characterize the interaction of water with leaf surfaces are the static contact angle of a sessile drop (A, B) and the drop retention angle of a sliding drop (C, D). The drop retention angle is the angle of a tilting stage at which a drop begins to slide or roll off. It is the more meaningful parameter in the context of water shedding, as can be seen by comparing static contact angles (ca) and drop retention angles (dra) on oak (Quercus robur, A, C) and soapwort (Saponaria officinalis, B, D) leaves. While the static contact angles on both leaves are very similar, the drop retention angles differ drastically.
Fig. 2.
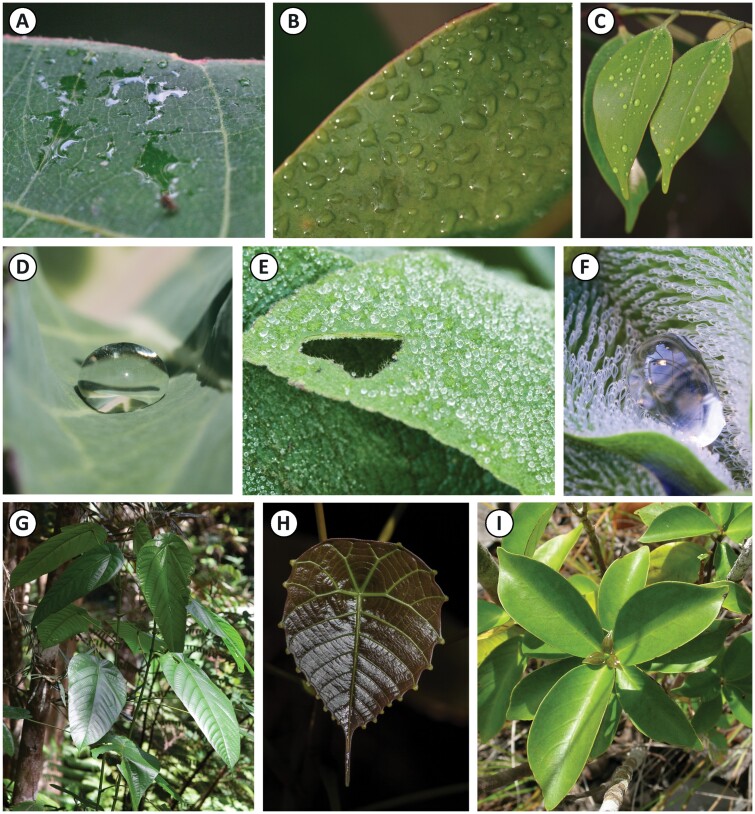
Leaf traits that affect water shedding. (A–C) Depending on the surface chemistry, leaves with relatively smooth surfaces can be either more (A, B) or less (C) wettable. (D–F) Three-dimensional surface features such as microscopic epicuticular wax crystals on glaucous leaves (D) or trichomes (E, F) act as enhancers of the intrinsic surface chemistry; in this case, they render the surfaces even more hydrophobic. (F) A special case is the so-called Salvinia effect, where hydrophobic trichomes with hydrophilic tips ‘pin’ a drop in place, away from the cuticular surface (Barthlott et al., 2010). (G) Leaves on the same plant can be held at vastly different inclination angles. (H) Elongated apical drip tips are commonly found on the leaves of tropical rainforest understorey plants; here in Brunei, Northern Borneo. (I) Drip tips are absent from the leaves in a nearby open, dry habitat in Brunei, Northern Borneo.
As natural leaves are never perfectly flat and smooth, their wettability is always based on a combination of surface chemistry and topography (Koch et al., 2008; Rosado and Holder, 2013). Surface chemistry is determined by the composition of the epicuticular wax layer that forms the major transpiration barrier on the epidermis of terrestrial plants and therefore tends to be hydrophobic (Jetter et al., 2006); however, some leaf surfaces have been shown to be extremely hydrophilic (Bohn and Federle, 2004; Koch et al., 2009). Papillae, trichomes, cuticular folds, and epicuticular wax crystals generally increase the surface roughness and may thereby influence the wettability (Wenzel, 1936; Koch et al., 2008; Fig. 2D–F). Depending on their density and inherent wettability, trichomes have been shown to either impede (Brewer et al., 1991) or enhance wetting (Bauer et al., 2013; Pina et al., 2016; Schreel et al., 2020). Similarly, the effect of surface roughness is not straightforward, as becomes obvious when comparing the ‘petal effect’ (Feng et al., 2008; Janairo et al., 2016) and the ‘lotus effect’ (Barthlott and Neinhuis, 1997; Schulte et al., 2011). In both cases, hierarchical surface structures result in apparent contact angles >150°, but depending on the aspect ratio, namely the height-to-distance ratio of the asperities, drops either stick or bead off (Bhushan and Jung, 2008; Lin and Chou, 2014; Gong et al., 2015).
Traditionally, most studies on biological surfaces report static contact angles; however, the above examples illustrate that this may not be the best variable to explain water shedding from leaves. This is further aggravated because biological surfaces tend to be inhomogeneous in texture and chemistry (Barthlott et al., 2010; Fig. 2F). Even on artificial surfaces, contact angles are poorly reproducible, with variation of up to 20° between repeated measurements (Gao and McCarthy, 2006). Empirical studies so far failed to show a consistent correlation between static contact angles and drop retention angles—the direct measure of how easily water sheds from a leaf (Brewer and Nuñez, 2007; Aryal and Neuner, 2010; Ginebra-Solanellas et al., 2020; Fig. 1). This may not be too surprising because the dynamic contact angles of a sliding or rolling drop are often vastly different from the static contact angle on the same surface. Therefore, it is increasingly acknowledged that the more meaningful parameter for the understanding of water shedding from leaves is the contact angle hysteresis, namely the difference between the advancing and receding contact angle of a sliding drop (Brewer and Nuñez, 2007; Aryal and Neuner, 2010; Rosado and Holder, 2013). The lower the contact angle hysteresis, the lower the drop retention angle and the more easily the drop is shed from the surface. Water will run off if the leaf inclination angle exceeds the drop retention angle (Konrad et al., 2012). A complicating factor is the drop size, especially for smaller drops on more hydrophilic leaves. As the drop rolls off, a thin water film will stay behind. This can eventually reduce the drop to a size where it stops to move (Oqielat et al., 2011). Barfield et al. (1973) found that the amount of water retained on lettuce, tomato, and cucumber leaves was dependent on the drop size.
Falling drops: bouncing, splashing, and impact wetting
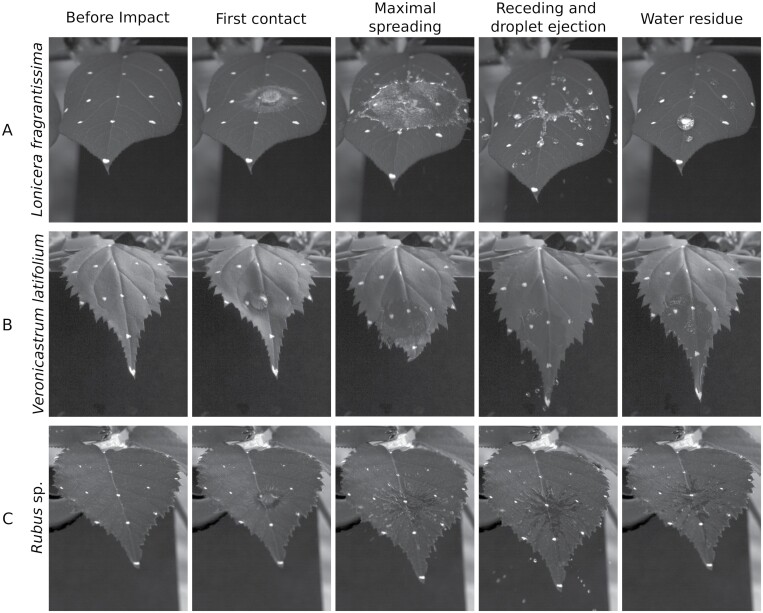
So far, we have considered the relatively simple case of a sessile drop on a static leaf. However, rain drops hit the leaf with impact forces of >1000 times their static mass (Soto et al., 2014). Upon impact, drops either adhere, bounce, or splash, depending on a complex interplay of leaf surface wettability, leaf inclination angle, and the rigidity of the leaf and petiole, as well as drop size and impact velocity (Fig. 3). Recent studies have tried to disentangle these effects to a certain degree, but much remains yet to be understood. On horizontal leaf surfaces, drops up to a critical size and impact velocity tend to adhere, while larger and faster drops bounce or splash (Bassette and Brussière, 2008; Kwon et al., 2014; Fig. 3A). The critical drop size and speed at which this transition occurs is 50–85% lower for hydrophobic than for hydrophilic leaves. Bouncing appears to be confined to hydrophobic leaves, where it occurs at intermediate drop sizes and impact velocities; larger and faster drops splash (Dorr et al., 2015). Studies on artificial surfaces suggest that in cases where hydrophobicity is based on surface roughness, drop impacts above a critical velocity can enforce wetting, if the kinetic energy of the drop exceeds the energetic barrier for the water to penetrate in between the surface asperities (Bartolo et al., 2006; Marengo et al., 2011). This means that on hydrophobic leaves, raindrops with a low impacting speed will most probably roll or bounce off the surface, while heavy rainfall could lead to complete wetting of the leaves; however, this has not yet been investigated with natural leaf surfaces.
Fig. 3.
Impact behaviour of a water drop (volume=50 µl, speed ~3ms–1) on three different leaves. (A) Leaf surface hydrophobic (winter honeysuckle, Lonicera fragrantissima). The drop splashes and breaks up into smaller droplets which bounce off the surface. Some drops are decelerated enough to adhere to the surface. (B) Leaf surface hydrophilic (Chinese veil, Veronicastrum latifolium). The drop spreads and partly runs off as the leaf is tilted downward following the impact. The water residue is visible as a continuous wet area. (C) Leaf surface with trichomes (bramble, Rubus sp.). The drop spreads and breaks up irregularly (‘fingering’).
We already showed for the static case that the inclination angle determines the transition between drop retention and run-off for a surface of a given water repellency. For the dynamic case, it has been shown that the wettability strongly influences the transition points between adhesion, bouncing, and splashing. For hydrophobic leaves, higher inclination angles broaden the range of drop sizes and impact velocities at which bouncing occurs; that is, the steeper the leaf inclination, the less likely the drop will stick or splash (Kwon et al., 2014; Dorr et al., 2015). A similar shift is observed for hydrophilic leaves, but instead of bouncing, drops spread and run off (Bassette and Brussière, 2008; Fig. 3B). While these are generally valid trends, the drop behaviour on natural leaves depends on many more factors (e.g. the rigidity of the leaf as well as macro-topographic features such as vascular bundles or trichomes, Fig. 3C), and can differ drastically from the theoretical predictions (Papierowska et al., 2019). Note that all of the above studies classify leaves by wettability (i.e. static contact angle) and not water retention. It is also worth noting that so far, studies have only considered individual drop impacts on previously dry leaves while, during natural rainfall, multiple drops will impact simultaneously or in short succession, and will interact with water already on the leaf surface. Interactions of multiple drops have so far mainly been explored theoretically (Rein, 1996; Damak et al., 2016; Wang and Bourouiba, 2018). Lejeune and Gilet (2019) investigated the interaction of an impacting with a sessile drop on a Perspex surface experimentally, and found that both drops splash off the surface as one, with the splashing distance depending on the inclination angle of the surface.
The effect of leaf shape
Narrow elongated leaves and extensions to the leaf apex—so-called ‘drip tips’ (Jungner, 1891; Fig. 2H)—are commonly interpreted as adaptations for improved surface water drainage. Because narrow elongated leaves also tend to have more pronounced drip tips, it is impossible to fully separate the effects of both. Numerous manipulative experiments on natural leaves (Dean and Smith, 1978; Williamson, 1981; Lightbody, 1985; Burd, 2007) and, more recently, observations on artificial leaves with and without drip tips (Lücking and Bernecker-Lücking, 2005; Wang et al., 2020) all indicate that drip tips promote water shedding from the apex. By funnelling the surface water onto a narrow protrusion, drip tips increase the frequency but decrease the size of the shed drops (Lightbody, 1985; Lücking and Bernecker-Lücking, 2005). However, it is less clear how drip tips affect the overall rate of water removal. Dean and Smith (1978) and Ivey and De Silva (2001) both report that experimental removal of drip tips slowed down the drainage of surface water from leaves; however, Lücking and Bernecker-Lücking (2005) found that drip tips mainly reduced the drying time for the leaf apex, but not the entire lamina. It appears likely that drip tips on their own, without further adaptations of leaf geometry, inclination angle, and surface topography promoting water flow across the lamina towards the tip, are insufficient to have a significant effect on water shedding and drying times.
Leaf movement
Up to here, we have ignored the fact that leaves can move. This includes both slow deformations as a result of loading, and rapid impact responses on a subsecond time scale. Upon impact, rain drops transfer momentum to the leaf, leading to a deformation of the leaf itself and/or the supporting petiole. The transfer of energy depends on the impact rate and location, the size and speed of the drop (which is influenced by wind and surrounding vegetation), and whether the drop bounces or spreads on the leaf. Drop behaviour on the surface in turn depends on drop size, speed, and impact angle, and on the leaf’s surface properties, size, rigidity, and angle of inclination, as well as presence or absence of water already on the surface. If part of the impacting water remains on the surface, the added mass will change the inclination angle, with the amount of change depending on the biomechanical properties of the leaf (Holder et al., 2020). It should be clear from these general arguments that leaf movement is a particularly complex issue and, apart from the trivial point that impact-induced deflection will result in a temporary increase of leaf inclination angle and thereby aid water shedding, obtaining general trends remains difficult.
In laboratory experiments, leaves that were fixed at the base of the petiole responded to a drop impact with characteristic damped oscillations (Ginebra-Solanellas et al., 2020; Holder et al., 2020). Several studies showed the amplitude of these oscillations to be directly proportional to the transfer of momentum. However, momentum transfer did not simply scale with drop size, but was dependent on the impact location (Bauer et al., 2015), material properties of the leaf (Ginebra-Solanellas et al., 2020), and the wettability of its surface (Gart et al., 2015). Using standardized beams of different surface properties and lengths, the latter study found not only higher impact transfer, but also a much stronger effect of the lever arm length for wettable compared with non-wettable surfaces. This is further complicated if the rigidity changes along the length of the leaf. Bhosale et al. (2020) found a ‘sweet spot’ around three-quarters along the length of Katsura sp. leaves, with more distal impacts leading to more bending of the leaf tip and less energy transfer to the whole leaf. In contrast to human-made materials, leaves are highly heterogeneous with a soft inner mesophyll encased in a layer of much stiffer epidermal cells (Onoda et al., 2015). A scaffold of lignified and sclerified veins provides further rigidity, and the density and spatial organization of these veins is a major determinant of the deformation characteristics of the leaf (Roth-Nebelsick et al., 2001).
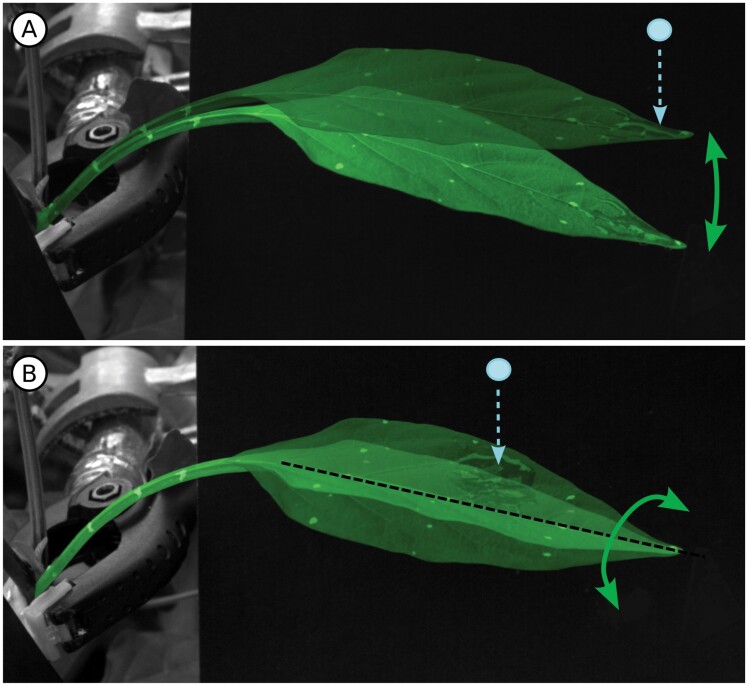
Apart from bending (Fig. 4A), leaves can also twist (Fig. 4B) or exhibit more complex movements such as undulating or flapping. Vogel (1992) defined the twist-to-bend ratio as the ratio of flexural rigidity to torsional rigidity, in order to categorize leaf movements due to petiole bending and twisting. Petioles with a non-circular cross-section favour twisting; however, the actual impact response is strongly dependent on the impact location (Fig. 4). Drops impacting along the midrib of superhydrophobic Katsura sp. leaves predominantly caused bending, while more lateral impacts increasingly caused twisting (Bhosale et al., 2020). Petioles are composite structures with variable proportions of soft central parenchyma and supporting peripheral collenchyma which may be reinforced by sclerenchyma. It is worth noting that fully hydrated plant tissue consists of up to 98% water, and leaf and petiole stiffness is therefore crucially dependent on the hydration status (Niklas and O’Rourke, 1987; Niklas, 1999). Leaf movement is also likely to be more complex during natural rainfall, but the effect of multiple simultaneous or consecutive drop impacts remains uninvestigated to date.
Fig. 4.
The drop impact response of a Capsicum sp. leaf is strongly dependent on the impact location. (A) An impact near the leaf tip leads to bending in the petiole and lamina. (B) A lateral impact mainly causes twisting in the petiole.
Towards a comprehensive understanding of water shedding
In order to understand water shedding in nature, we need to take into account that leaf shape, surface topography, inclination angle, and leaf movement all interact with each other, with rain characteristics such as drop size and frequency, with presence of water on the leaf surface, and with other factors such as wind. Few studies have looked at more than one or two of these factors in combination, and none so far has investigated the interaction of natural rainfall with leaves directly. A number of studies compared leaf inclination and drop retention angles, and found that natural leaf inclination angles mostly exceeded drop retention angles or, occasionally, were roughly equal. In the latter case, a temporary slight increase in inclination angle caused by a drop impact or added water load was sufficient to initiate water shedding (Holder, 2012; Ginebra-Solanellas et al., 2020). Dean and Smith (1978) and Ellenberg (1985) investigated the combined effects of leaf inclination angle and drip tips. Both concluded that the inclination angle was the more influential of the two factors. Nanko et al. (2013) investigated the combined effect of leaf inclination angle and surface properties, and found that the maximal size of drops shed from the leaves decreased with increasing inclination angle and hydrophobicity.
In the context of leaf inclination angle, it is particularly interesting to consider topographic features that promote directional water transport across the leaf surface. For example, longitudinal cuticular ridges on rice (Oryza sativa) leaves favour water movement along the leaf blade (Kwon et al., 2014), and unidirectionally angled trichomes on ryegrass (Lolium sp.) leaves result in a 3-fold lower drop retention angle in the proximal than in the distal direction along the leaf (Guo et al., 2012). Hierarchical groove structures and overlapping acute-angled arches on the hydrophilic trap rim of carnivorous Nepenthes pitcher plants cause highly effective water transport in the outward direction (Chen et al., 2016) and may play a role in preventing the dilution of the trap fluid with rain water during tropical downpours.
How can we predict leaf deformation due to surface water and movement in response to rain impact? Tadrist et al. (2014) modelled how leaf angles change under wind and static loading depending on various leaf traits. For static loading, they defined the elasto-gravity number which combines the relative lengths of petiole and lamina with the flexural rigidity of the petiole and the mass of the lamina. The higher the elasto-gravity number, the more easily the leaf will bend down and shed water. Under wind loading, the size of the lamina and the torsional rigidity of the petiole determine the amount of water shedding due to tilting. Vogel (1989) showed that the large, lobed leaves of the tulip tree (Liriodendron tulipifera) can curl up under wind load, thereby reducing drag and fluttering. This not only reduces the total exposed surface area but also mainly exposes the more hydrophobic abaxial surface (Goldsmith et al., 2017). On the other hand, fluttering and increased surface exposure to wind should promote water shedding and evaporation. Therefore, leaf curling might be counterproductive from a water shedding perspective and is more likely to be an adaptation for the prevention of damage. Further progress on understanding the complex interplay between leaves, rain, and wind requires a move away from laboratory studies to situations where leaves are held naturally and are subject to natural rainfall or condensation. The increasing availability of small, lightweight, and weatherproof measurement devices, affordable and powerful high-speed video cameras, and low-cost microcomputers for automated data collection make this approach more feasible than ever before (Chan et al., 2016; Kwok, 2017; Duke et al., 2019; Oellermann et al., 2021, Preprint).
Implications of water shedding for soil erosion
One interesting line of argument proposes that a major driver behind the evolution of leaf shape and surface properties might be to reduce the size of shed drops, and thereby mitigate splash erosion and nutrient loss in the immediate vicinity of the plant. As discussed before, smaller drops reach lower terminal velocity and therefore transfer less momentum upon impact. Interception by leaves has been shown to reduce average throughfall drop size by half in comparison with unfoliated trees (Nanko et al., 2016). Foot and Morgan (2005) looked at the effect of leaf inclination angles on the soil particles in a lab experiment. They found no difference between soil particle detachment below three different plant species and on bare soil, and concluded that the effects of leaf drips and shielding through the canopy probably cancel each other out. Nanko et al. (2013) compared the drop sizes shed from needles and broadleaves of different hydrophobicity. For broadleaves, drop size decreased with water repellency of the leaves. For needles, drop size was inconsistent and strongly depended on needle arrangement on the branch. Drops were frequently pinned between the tips of multiple needles, resulting in very large drops eventually being shed.
Drip tips also reduce the size of intercepted rain drops falling from leaves and thereby the impact energy transferred to the ground (Williamson, 1981; Lücking and Bernecker-Lücking, 2005; Burd, 2007). Williamson (1981) suggested testing this hypothesis by looking at the prevalence of drip tips in relation to soil type. If erosion was a major selection factor, drip tips should be less common in sites that are less prone to splash erosion, such as in swamps or on sandy soils. In contrast, there should be no such effect if the key selection pressure was the removal of water from the leaf surface. Williamson (1981) provides anecdotal evidence that drip tips are absent from mangrove forests, but a systematic survey of leaf shapes in regularly water-inundated habitats has not yet been done. In contradiction to the erosion hypothesis, Rebelo and Williamson (1996) found a higher prevalence of drip tips in two areas of Amazonian rainforest with sandy soil than in a nearby site with clay; however, this study is limited to a few individual sites and does not consider that in tropical rainforests, the forest is commonly covered in leaf litter.
Williamson et al. (1983) also investigated the height distribution of drip tips in lowland rainforest, arguing that absence of drip tips from foliage very close to the ground—at <50cm height—could also be interpreted as supportive of the erosion hypothesis because drops falling from such a low height have little kinetic energy. Conversely, water removal as a key selection factor should lead to higher prevalence of drip tips close to the ground where evaporation is hindered by high air humidity. In line with the erosion hypothesis, drip tips were less prominent closer to the ground (Williamson et al., 1983); however, the detailed relationship between leaf height on the one hand, and drop velocity and energy transfer to the substrate on the other hand, remains unstudied to date. While the erosion hypothesis is certainly intriguing, we feel that there is insufficient experimental evidence to draw evolutionary conclusions at present.
Trade-offs and synergies with other leaf functions
Effective water shedding is only one of many functional demands on leaves and, as an evolutionary driver, it probably plays a minor role behind other selection factors that determine photosynthetic efficiency, construction costs, and life span, the combination of which defines the carbon economics of a given leaf (Donovan et al., 2011; Fig. 5). Photosynthetic performance needs to balance water and light availability with gas exchange and temperature control. The selective power of this trade-off is well illustrated in the contrast between sun and shade leaves. Water on the surface can reduce water loss by transpiration and mitigate the risk of overheating; however, it also impedes light penetration and gas exchange, and the overall impact on photosynthesis tends to be negative (Aparecido et al., 2017; Berry and Goldsmith, 2020). Photosynthetic performance, nutrient availability, exposure to mechanical stress, and effective defence against herbivores and pathogens together define the trade-off between structural investment and life span. Again, rain water impacting and accumulating on the leaf surface is a contributing factor as it can cause nutrient leaching (Tukey, 1970), impose mechanical stress, and provide a suitable environment for pathogenic bacteria and fungal spores (Huber and Gillespie, 1992). Moreover, rain drops impacting on already wet leaves and the resulting splashes can effectively distribute pathogenic spores to other nearby leaves (Gilet and Bourouiba, 2014; Kim et al., 2019). In addition to physiological and ecological trade-offs, leaf size and shape are also subject to developmental constraints (Nicotra et al., 2011). The evolution of leaf traits that promote water shedding therefore has to be viewed in the greater context of leaf function and development.
Fig. 5.
An integrated view on water shedding in the greater ecological context. Leaves are multifunctional organs that need to balance photosynthesis, hydration, nutrient demand, and temperature control with resistance to mechanical stress, herbivores, and pathogens. Water shedding is further influenced by the source of leaf surface water (different types of rain, throughfall, drip water from other leaves, mist, and condensation), the position of the leaf on the plant and within the surrounding vegetation, the biomechanical properties of the entire plant, and additional weather factors such as wind. Water shed from leaves in turn impacts other leaves further down, or can cause splash erosion and nutrient loss upon impact on the soil.
Plant surface features that may render leaves water repellent, such as trichomes and epicuticular wax crystals, will also affect light penetration, gas exchange, and temperature control (Riederer and Müller, 2006); however, the interdependence of these physiological processes makes it nearly impossible to quantify individual trade-offs. Dense trichome cover can reduce light penetration and photosynthetic performance significantly (Ehleringer et al., 1976). A commonly cited adaptive function of epicuticular wax crystals is protection from harmful radiation by increasing UV reflectance (Reicosky and Hanover, 1978; Mauseth, 1988; Robinson and Osmond, 1994; Grant et al., 2003). Bukhanov et al. (2019) reported UV-induced fluorescence in the epicuticular wax crystal layer of wheat (Triticum sp.). This could provide a mechanism to shift harmful UV radiation into the visible spectrum, thereby increasing light availability for photosynthesis. Synergy effects are likely between water repellency and protective functions against herbivore and pathogen attacks. Epicuticular wax crystals not only impede drop adhesion but equally prevent insects from attaching to the surface (Eigenbrode and Espelie, 1995; Gorb et al., 2005). Water shedding from epicuticular waxes can help to remove debris and fungal spores from the surface (Barthlott and Neinhuis, 1997; Neinhuis and Barthlott, 1998). A similar effect has also been demonstrated for drip tips where increased water shedding prevented the accumulation of debris (Lücking and Bernecker-Lücking, 2005) and fungal spores on the leaf surface (Ivey and De Silva, 2001).
Leaf inclination angles undoubtedly affect water shedding; however, for leaves with water-repellent surfaces, a small deviation from the horizontal is sufficient. The large natural variation in leaf angles (Fig. 2G) is more convincingly explained by physiological demands such as optimized light capture, photoprotection during times of high light intensity, and temperature control (King, 1997; Falster and Westoby, 2003). Thus, the existence of traits that enhance water shedding from leaves does not imply that water shedding is an important aspect of the selective regime that has led to the expression of these traits.
An alternative approach to testing the adaptive value of traits is to look at the ecological context in which these traits prevail. Comparisons of contact angles on leaves across habitats failed to show an association of hydrophobicity with wetter climate (Holder, 2007; Goldsmith et al., 2017; Sikorska et al., 2017). Instead, low wettability was characteristic of a cold and dry climate (Aryal and Neuner, 2010; Goldsmith et al., 2017). Brewer and Nuñez (2007) proposed that frequent heavy rainfall might remove fragile epicuticular waxes from leaf surfaces and thus increase wettability. Experiments with simulated rain on a range of common crops provided some evidence for wax crystal erosion (Baker and Hunt, 1986). An alternative hypothesis is that low wettability is favoured in arid environments because it can help to channel any water falling or condensing on the leaves towards the soil (Holder, 2007; Masrahi, 2020). In this case, one would expect leaf surface topography, geometry, and inclination angles adapted to funnel surface water towards the stem, and thereby the root system of the plant.
A few studies provide direct evidence linking the prevalence of drip tips to wetter habitats (Lightbody, 1985; Schneider et al., 2003; Fig. 2H, I). Meng et al. (2014) argued that steep inclination angles and drip tips may be alternative strategies for water shedding. Low light conditions in the forest understorey should favour drip tips because a steep leaf angle will further reduce light interception. Indeed, drip tips were more common and leaves more horizontal in shade-adapted than in sun-adapted species in the same rainforest site. The majority of studies, however, examined species distributions more broadly and used statistical models to correlate the presence of drip tips, determined from images or herbarium specimens, with large-scale climate variables. This approach is problematic not only because many of the investigated climate variables are closely correlated with each other, but also because it ignores small-scale temporal and spatial variations of the microclimate that the plant experiences, as well as intraspecific variability and developmental plasticity of the leaf morphology. Consequently, the results are highly inconsistent: drip tip occurrence was correlated with total precipitation during the wettest season in Amazonian rainforest (Malhado et al., 2012), with annual temperature along an elevation gradient in Peru (Goldsmith et al., 2017), and with none of the tested parameters in a South American mixed savannah–woodland (Sullivan and Queenborough, 2020). Fritsch et al. (2018) slightly improved the study design by comparing herbarium specimens with climate data from their respective place of collection, thereby taking regional variation into account. Rainfall during both the wettest and the driest quarter best predicted the extent of drip tips on North American redbud (Cercis sp.) leaves; however, both climate parameters were also intercorrelated, making further interpretations difficult. The authors suggest that drip tips might be selected against in drier climates where retaining nightly accumulating dew on the leaves may reduce transpirational water loss in the morning.
Plasticity of leaf traits
We already saw that inferring functional or evolutionary relationships from the presence or absence of certain leaf traits in different (micro-) habitats can be problematic for various reasons. Maybe the most imminent problem for such survey-based studies is that virtually all of the traits we considered in the context of water shedding are highly variable not only between individuals of the same species, but also between leaves of the same individual, and even within the same leaf over time. Epicuticular wax crystals can be reduced or absent in plants growing under low light conditions (Hallam, 1970) or under elevated air humidity (Koch et al., 2006). Seasonal changes of epicuticular wax load and composition are widespread and have been attributed to leaf ontogeny (Jetter and Schäffer, 2001), temperature and water availability (Ziv et al., 1982; Jordan et al., 1983), and erosion of wax crystals over time (Neinhuis and Barthlott, 1998; Kang et al., 2018). Multiple studies report an increase in leaf wettability for broad-leaved trees towards the later part of the growth season, namely with increasing leaf age (Neinhuis and Barthlott, 1998; Tranquada and Erb, 2014; Kang et al., 2018; Xiong et al., 2018).
Drip tips are both more common and more pronounced in saplings than in mature trees of the same species (Leigh, 1975; Zhu, 1997; Panditharathna et al., 2008). It remains unclear whether this is due to different physiological demands, developmental constraints, or differing height off the ground. The importance of relative leaf height is corroborated by multiple reports of drip tips being more widespread in understorey than in subcanopy species, and least common in canopy species (Rollet, 1990; Roth et al., 1990; Yáñez-Espinosa, 2003). How drip tip formation is regulated during leaf development remains unstudied to date, leaving the amount of trait plasticity in response to short-term environmental variation open to speculation. Transplant experiments with species that show a high variation of drip tip prevalence throughout their distributional range could provide first insights into this interesting question.
By far the most plastic of the leaf traits considered in this review is leaf inclination angle which can be highly variable depending on leaf and plant age (Liu et al., 2019) and position on the plant (Niinemets and Fleck, 2002; Fig. 2G). However, leaf inclination angles are not only variable in space—they can also change with the seasons (Raabe et al., 2015) and even with the time of day (Dean and Smith, 1978; Lovelock et al., 1994). Seasonal changes of leaf inclination angle have been most commonly linked to light intensity and angle of radiation (Falster and Westoby, 2003; Meng et al., 2014), but also to temperature control and drought resistance (Ryel and Beyschlag, 1995; Gratani and Bombelli, 2000; Raabe et al., 2015; Peguero-Pina et al., 2020). Furthermore, the influence of water load or drop impacts causes a temporary increase of leaf inclination angles as discussed above.
Plants can also actively change leaf angles in response to touch stimuli (reviewed by Braam, 2005) and to the time of day (reviewed by Minorsky, 2019). Circadian rhythms of leaf movements have mostly been attributed to changes of light incidence (Lovelock et al., 1994; Peguero-Pina et al., 2020), but also to patterns of the gravimetric tide (Barlow, 2015). Although there is currently no evidence that rhythmic temporal changes of leaf inclination angle are related to water shedding, it is conceivable that, especially in humid tropical environments, steeper nocturnal leaf angles facilitate drainage of rain water and condensation from the leaf surface. Dean and Smith (1978) even suggested that the leaves of some tropical plants change to a more vertical inclination in direct response to rainfall. However, they did not provide any data to support this assertion. Active changes in leaf angle in response to drop impacts have only been shown for Mimosa pudica touch-me-not plants (Applewhite, 1972; Wallace et al., 1987); however, the ecological relevance of this behaviour with regards to water shedding has not been investigated to date.
Conclusions and outlook
Leaves certainly vary in their water-shedding properties, and this variation is linked to a number of leaf traits. What has not been established is whether these leaf traits evolved in part because of the effect they have on water shedding. We discussed a number of studies that correlated leaf traits with site-specific climate and soil variables in the context of water shedding. This work has been generally equivocal and does not provide evidence for a direct impact of leaf traits associated with water shedding on fitness correlates such as plant survival, growth, or seed production. While the available field studies suffer from too much reliance on multifactor correlations and too few mechanistic experiments, our functional understanding of water-shedding processes from leaves is almost entirely based on laboratory experiments involving artificial rain and surfaces. These studies have been valuable in helping to develop a theoretical framework; however, in order to further advance our understanding of water shedding, experiments will have to move out of the lab and into the field.
One problem with lab studies is that surface and material properties of leaves start changing as soon as a leaf is separated from the plant. Laboratory air is typically very dry, and contact angle measurements can strongly depend on relative air humidity (Gledhill et al., 1977; Hołysz et al., 2008). Impact responses of leaves depend not only on the properties of the leaf itself, but also on the structural properties of the petiole, and the branch or stem that the leaf is attached to. Lastly, the nature of water shedding from a leaf will be affected by the nature of the rainfall (e.g. duration, drop size, and drop frequency), the effects of other climatic variables such as wind and air humidity, the stature and structural rigidity of the entire plant, and even the surrounding vegetation (influencing whether drops impacting on a particular leaf are direct rainfall or previously shed from higher vegetation). Water will impact not only on leaves but also on all other above-ground parts of a plant. Much of the water impacting on lower leaves of a plant will have been shed from further up the plant, and much of that water will ultimately end up in the substrate around the roots of the plant.
Future experiments should combine hypothesis-driven manipulative approaches (e.g. transplant experiments and targeted manipulations of leaf traits that affect water shedding) with biomechanical measurements (e.g. accelerometry or 3D motion analysis of leaf impact responses) and quantification of whole-plant fitness correlates (e.g. growth, survival, or seed set) in a natural field setting. While this approach is certainly challenging, we have now, for the first time, the necessary technology to take research on water shedding to the next level (Bauer et al., 2020). In order to really understand water shedding in nature, we have to consider the complex interplay of leaf and plant biomechanical properties within the greater framework of the natural environment.
Acknowledgements
The authors thank Michal R. Golos for fruitful discussions about wettability and wetting, and two anonymous reviewers for their constructive comments. Figures 1, 3, and 4 would not have been possible without the Bristol Botanic Garden granting us access to their collection during the pandemic.
Author contributions
All authors contributed equally to the conceptualization and writing of the paper. A-KL and UB compiled the figures.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
The authors acknowledge funding from The Royal Society (University Research Fellowship UF150138 and Enhancement Award RGF/EA/180059 to UB, with the latter providing funding for A-KL).
References
- Allan RP. 2011. Climate change: human influence on rainfall. Nature 470, 344–345. [DOI] [PubMed] [Google Scholar]
- Aparecido LMT, Miller GR, Cahill AT, Moore GW.. 2016. Comparison of tree transpiration under wet and dry canopy conditions in a Costa Rican premontane tropical forest. Hydrological Processes 30, 5000–5011. [Google Scholar]
- Aparecido LMT, Miller GR, Cahill AT, Moore GW.. 2017. Leaf surface traits and water storage retention affect photosynthetic responses to leaf surface wetness among wet tropical forest and semiarid savanna plants. Tree Physiology 37, 1285–1300. [DOI] [PubMed] [Google Scholar]
- Applewhite PB. 1972. Behavioral plasticity in the sensitive plant, Mimosa. Behavioral Biology 7, 47–53. [DOI] [PubMed] [Google Scholar]
- Arnold AE, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA.. 2003. Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Sciences, USA 100, 15649–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal B, Neuner G.. 2010. Leaf wettability decreases along an extreme altitudinal gradient. Oecologia 162, 1–9. [DOI] [PubMed] [Google Scholar]
- Baker EA, Hunt GM.. 1986. Erosion of waxes from leaf surfaces by simulated rain. New Phytologist 102, 161–173. [DOI] [PubMed] [Google Scholar]
- Barfield B, Payne F, Walker J.. 1973. Surface water storage capacity of selected crop leaves under irrigation sprays. Agricultural Meteorology 12, 105–111. [Google Scholar]
- Barlow PW. 2015. Leaf movements and their relationship with the lunisolar gravitational force. Annals of Botany 116, 149–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthlott W, Neinhuis C.. 1997. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202, 1–8. [Google Scholar]
- Barthlott W, Schimmel T, Wiersch S, et al. 2010. The Salvinia paradox: superhydrophobic surfaces with hydrophilic pins for air retention under water. Advanced Materials 22, 2325–2328. [DOI] [PubMed] [Google Scholar]
- Bartolo D, Bouamrirene F, Verneuil É, Buguin A, Silberzan P, Moulinet S.. 2006. Bouncing or sticky droplets: impalement transitions on superhydrophobic micropatterned surfaces. Europhysics Letters 74, 299–305. [Google Scholar]
- Bassette C, Bussière F.. 2008. Partitioning of splash and storage during raindrop impacts on banana leaves. Agricultural and Forest Meteorology 148, 991–1004. [Google Scholar]
- Bauer U, Paulin M, Robert D, Sutton GP.. 2015. Mechanism for rapid passive–dynamic prey capture in a pitcher plant. Proceedings of the National Academy of Sciences, USA 112, 13384–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer U, Poppinga S, Müller UK.. 2020. Mechanical ecology—taking biomechanics to the field. Integrative and Comparative Biology 60, 820–828. [DOI] [PubMed] [Google Scholar]
- Bauer U, Scharmann M, Skepper J, Federle W.. 2013. ‘Insect aquaplaning’ on a superhydrophilic hairy surface: how Heliamphora nutans Benth. pitcher plants capture prey. Proceedings of the Royal Society B: Biological Sciences 280, 20122569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry ZC, Emery NC, Gotsch SG, Goldsmith GR.. 2019. Foliar water uptake: processes, pathways, and integration into plant water budgets. Plant, Cell & Environment 42, 410–423. [DOI] [PubMed] [Google Scholar]
- Berry ZC, Goldsmith GR.. 2020. Diffuse light and wetting differentially affect tropical tree leaf photosynthesis. New Phytologist 225, 143–153. [DOI] [PubMed] [Google Scholar]
- Bhosale Y, Esmaili E, Bhar K, Jung S.. 2020. Bending, twisting and flapping leaf upon raindrop impact. Bioinspiration & Biomimetics 15, 036007. [DOI] [PubMed] [Google Scholar]
- Bhushan B, Jung YC.. 2008. Wetting, adhesion and friction of superhydrophobic and hydrophilic leaves and fabricated micro/nanopatterned surfaces. Journal of Physics 20, 225010. [Google Scholar]
- Bico J, Thiele U, Quere D.. 2002. Wetting of textured surfaces. Colloid Surface A 206, 41–46. [Google Scholar]
- Bohn HF, Federle W.. 2004. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proceedings of the National Academy of Sciences, USA 101, 14138–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J. 2005. In touch: plant responses to mechanical stimuli. New Phytologist 165, 373–389. [DOI] [PubMed] [Google Scholar]
- Brewer CA, Nuñez CI.. 2007. Patterns of leaf wettability along an extreme moisture gradient in western Patagonia, Argentina. International Journal of Plant Sciences 168, 555–562. [Google Scholar]
- Brewer CA, Smith WK, Vogelmann TC.. 1991. Functional interaction between leaf trichomes, leaf wettability and the optical properties of water droplets. Plant, Cell & Environment 14, 955–962. [Google Scholar]
- Bukhanov ER, Gurevich YL, Shabanova KA.. 2019. A study of wheat wax optical properties. In: 2019 Photonics & Electromagnetics Research Symposium - Fall (PIERS - Fall), 2890–2897. [Google Scholar]
- Burd M. 2007. Adaptive function of drip-tips: a test of the epiphyll hypothesis in Psychotria marginata and Faramea occidentalis (Rubiaceae). Journal of Tropical Ecology 23, 449–455. [Google Scholar]
- Callies M, Quéré D.. 2005. On water repellency. Soft Matter 1, 55–61. [Google Scholar]
- Carmichael MJ, White JC, Cory ST, Berry ZC, Smith WK.. 2020. Foliar water uptake of fog confers ecophysiological benefits to four common tree species of southeastern freshwater forested wetlands. Ecohydrology 13, e2240. [Google Scholar]
- Chan BK, Lima FP, Williams GA, Seabra R, Wang HY.. 2016. A simplified biomimetic temperature logger for recording intertidal barnacle body temperatures. Limnology and Oceanography 14, 448–455. [Google Scholar]
- Chen H, Zhang P, Zhang L, Liu H, Jiang Y, Zhang D, Han Z, Jiang L.. 2016. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature 532, 85–89. [DOI] [PubMed] [Google Scholar]
- Damak M, Hyder MN, Varanasi KK.. 2016. Enhancing droplet deposition through in-situ precipitation. Nature Communications 7, 12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TE, Goldsmith GR.. 2018. The value of wet leaves. New Phytologist 219, 1156–1169. [DOI] [PubMed] [Google Scholar]
- Dean JM, Smith AP.. 1978. Behavioral and morphological adaptations of a tropical plant to high rainfall. Biotropica 10, 152–154. [Google Scholar]
- Donovan LA, Maherali H, Caruso CM, Huber H, de Kroon H.. 2011. The evolution of the worldwide leaf economics spectrum. Trends in Ecology & Evolution 26, 88–95. [DOI] [PubMed] [Google Scholar]
- Dorr GJ, Wang S, Mayo LC, McCue SW, Forster WA, Hanan J, He X.. 2015. Impaction of spray droplets on leaves: influence of formulation and leaf character on shatter, bounce and adhesion. Experiments in Fluids 56, 143. [Google Scholar]
- Duke DJ, Knast T, Thethy B, Gisler L, Edgington-Mitchell D.. 2019. A low-cost high-speed CMOS camera for scientific imaging. Measurement Science and Technology 30, 075403. [Google Scholar]
- Dunkerley D. 2020. A review of the effects of throughfall and stemflow on soil properties and soil erosion. In: Van StanJT, II, Gutmann E, Friesen J, eds. Precipitation partitioning by vegetation—a global synthesis. Cham: Springer, 183–214. [Google Scholar]
- Ehleringer J, Björkman O, Mooney HA.. 1976. Leaf pubescence: effects on absorptance and photosynthesis in a desert shrub. Science 192, 376–377. [DOI] [PubMed] [Google Scholar]
- Eigenbrode SD, Espelie KE.. 1995. Effect of plant epicuticular lipids on insect herbivores. Annual Review of Entomology 40, 171–194. [Google Scholar]
- Ellenberg H. 1985. Unter welchen Bedingungen haben Blätter sogenannte ‘Träufelspitzen’? Flora 176, 169–188. [Google Scholar]
- Eller CB, Lima AL, Oliveira RS.. 2013. Foliar uptake of fog water and transport belowground alleviates drought effects in the cloud forest tree species, Drimys brasiliensis (Winteraceae). New Phytologist 199, 151–162. [DOI] [PubMed] [Google Scholar]
- Falster DS, Westoby M.. 2003. Leaf size and angle vary widely across species: what consequences for light interception? New Phytologist 158, 509–525. [DOI] [PubMed] [Google Scholar]
- Feng L, Zhang Y, Xi J, Zhu Y, Wang N, Xia F, Jiang L.. 2008. Petal effect: a superhydrophobic state with high adhesive force. Langmuir 24, 4114–4119. [DOI] [PubMed] [Google Scholar]
- Foot K, Morgan R.. 2005. The role of leaf inclination, leaf orientation and plant canopy architecture in soil particle detachment by raindrops. Earth Surface Processes and Landforms 30, 1509–1520. [Google Scholar]
- Freschi L, Takahashi CA, Cambui CA, et al. 2010. Specific leaf areas of the tank bromeliad Guzmania monostachia perform distinct functions in response to water shortage. Journal of Plant Physiology 167, 526–533. [DOI] [PubMed] [Google Scholar]
- Fritsch PW, Nowell CF, Leatherman LST, Gong W, Cruz BC, Burge DO, Delgado-Salinas A.. 2018. Leaf adaptations and species boundaries in North American Cercis: implications for the evolution of dry floras. American Journal of Botany 105, 1577–1594. [DOI] [PubMed] [Google Scholar]
- Gao L, McCarthy TJ.. 2006. Contact angle hysteresis explained. Langmuir 22, 6234–6237. [DOI] [PubMed] [Google Scholar]
- Gart S, Mates JE, Megaridis CM, Jung S.. 2015. Droplet impacting a cantilever: a leaf–raindrop system. Physical Review Applied 3, 044019. [Google Scholar]
- Gilet T, Bourouiba L.. 2014. Rain-induced ejection of pathogens from leaves: revisiting the hypothesis of splash-on-film using high-speed visualization. Integrative and Comparative Biology 54, 974–984. [DOI] [PubMed] [Google Scholar]
- Ginebra-Solanellas RM, Holder CD, Lauderbaugh LK, Webb R.. 2020. The influence of changes in leaf inclination angle and leaf traits during the rainfall interception process. Agricultural and Forest Meteorology 285, 107924. [Google Scholar]
- Gledhill RA, Kinloch AJ, Shaw SJ.. 1977. Effect of relative humidity on the wettability of steel surfaces. The Journal of Adhesion 9, 81–85. [Google Scholar]
- Goldsmith GR, Bentley LP, Shenkin A, et al. 2017. Variation in leaf wettability traits along a tropical montane elevation gradient. New Phytologist 214, 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Wu J, Jin X, Jiang L.. 2015. Adhesion tuning at superhydrophobic states: from petal effect to lotus effect. Macromolecular Materials and Engineering 300, 1057–1062. [Google Scholar]
- Gorb E, Haas K, Henrich A, Enders S, Barbakadze N, Gorb S.. 2005. Composite structure of the crystalline epicuticular wax layer of the slippery zone in the pitchers of the carnivorous plant Nepenthes alata and its effect on insect attachment. Journal of Experimental Biology 208, 4651–4662. [DOI] [PubMed] [Google Scholar]
- Grant RH, Heisler GM, Gao W, Jenks M.. 2003. Ultraviolet leaf reflectance of common urban trees and the prediction of reflectance from leaf surface characteristics. Agricultural and Forest Meteorology 120, 127–139. [Google Scholar]
- Gratani L, Bombelli A.. 2000. Correlation between leaf age and other leaf traits in three Mediterranean maquis shrub species: Quercus ilex, Phillyrea latifolia and Cistus incanus. Environmental and Experimental Botany 43, 141–153. [Google Scholar]
- Guo C, Wang S, Liu H, Feng L, Song Y, Jiang L.. 2008. Wettability alteration of polymer surfaces produced by scraping. Journal of Adhesion Science and Technology 22, 395–402. [Google Scholar]
- Guo P, Zheng Y, Liu C, Ju J, Jiang L.. 2012. Directional shedding-off of water on natural/bio-mimetic taper-ratchet array surfaces. Soft Matter 8, 1770–1775. [Google Scholar]
- Hallam ND. 1970. Growth and regeneration of waxes on the leaves of Eucalyptus. Planta 93, 257–268. [DOI] [PubMed] [Google Scholar]
- Herminghaus S. 2000. Roughness-induced non-wetting. Europhysics Letters 52, 165. [Google Scholar]
- Holder CD. 2007. Leaf water repellency as an adaptation to tropical montane cloud forest environments. Biotropica 39, 767–770. [Google Scholar]
- Holder CD. 2012. The relationship between leaf hydrophobicity, water droplet retention, and leaf angle of common species in a semi-arid region of the western United States. Agricultural and Forest Meteorology 152, 11–16. [Google Scholar]
- Holder CD, Lauderbaugh LK, Ginebra-Solanellas RM, Webb R.. 2020. Changes in leaf inclination angle as an indicator of progression toward leaf surface storage during the rainfall interception process. Journal of Hydrology 588, 125070. [Google Scholar]
- Hołysz L, Mirosław M, Terpiłowski K, Szcześ A.. 2008. Influence of relative humidity on the wettability of silicon wafer surfaces. Annales Universitatis Mariae Curie-Sklodowska 63, 223–239. [Google Scholar]
- Huber L, Gillespie TJ.. 1992. Modeling leaf wetness in relation to plant disease epidemiology. Annual Review of Phytopathology 30, 553–577. [Google Scholar]
- Ivey CT, DeSilva N.. 2001. A test of the function of drip-tips. Biotropica 33, 188–191. [Google Scholar]
- Janairo JI, Degaños SM, Lim RA, San Pedro JK, Toriaga FJ, Haygood KJ, Promentilla MA.. 2016. Occurrence of near-petal effect on the leaf surface of Annona squamosa. BioNanoScience 6, 272–275. [Google Scholar]
- Jetter R, Kunst L, Samuels AL.. 2006. Composition of plant cuticular waxes. In: Riederer M, Müller C eds. Biology of the plant cuticle. Oxford: Blackwell Publishing, 145–181. [Google Scholar]
- Jetter R, Schäffer S.. 2001. Chemical composition of the Prunus laurocerasus leaf surface. Dynamic changes of the epicuticular wax film during leaf development. Plant Physiology 126, 1725–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan WR, Monk RL, Miller FR, Rosenow DT, Clark LE, Shouse PJ.. 1983. Environmental physiology of Sorghum. I. Environmental and genetic control of epicuticular wax load. Crop Science 23, 552–558. [Google Scholar]
- Jungner JR. 1891. Anpassungen der pflanzen an das klima in den gegenden der regenreichen kamerungebirge. Botanisches Zentralblatt 47, 353–360. [Google Scholar]
- Kang H, Graybill PM, Fleetwood S, Boreyko JB, Jung S.. 2018. Seasonal changes in morphology govern wettability of Katsura leaves. PLoS One 13, e0202900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Park H, Gruszewski HA, Schmale DG 3rd, Jung S.. 2019. Vortex-induced dispersal of a plant pathogen by raindrop impact. Proceedings of the National Academy of Sciences, USA 116, 4917–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DA. 1997. The functional significance of leaf angle in Eucalyptus. Australian Journal of Botany 45, 619–639. [Google Scholar]
- Koch K, Bhushan B, Barthlott W.. 2008. Diversity of structure, morphology and wetting of plant surfaces. Soft Matter 4, 1943–1963. [Google Scholar]
- Koch K, Blecher IC, König G, Kehraus S, Barthlott W.. 2009. The superhydrophilic and superoleophilic leaf surface of Ruellia devosiana (Acanthaceae): a biological model for spreading of water and oil on surfaces. Functional Plant Biology 36, 339–350. [DOI] [PubMed] [Google Scholar]
- Koch K, Hartmann KD, Schreiber L, Barthlott W, Neinhuis C.. 2006. Influences of air humidity during the cultivation of plants on wax chemical composition, morphology and leaf surface wettability. Environmental and Experimental Botany 56, 1–9. [Google Scholar]
- Konrad W, Ebner M, Traiser C, Roth-Nebelsick A.. 2012. Leaf surface wettability and implications for drop shedding and evaporation from forest canopies. Pure and Applied Geophysics 169, 835–845. [Google Scholar]
- Kung CH, Sow PK, Zahiri B, Mérida W.. 2019. Assessment and interpretation of surface wettability based on sessile droplet contact angle measurement: challenges and opportunities. Advanced Materials Interfaces 6, 1900839. [Google Scholar]
- Kwok R. 2017. Field instruments: build it yourself. Nature 545, 253–255. [Google Scholar]
- Kwon DH, Huh HK, Lee SJ.. 2014. Wettability and impact dynamics of water droplets on rice (Oryza sativa L.) leaves. Experiments in Fluids 55, 1691. [Google Scholar]
- Ladino G, Ospina-Bautista F, Estévez Varón J, Jerabkova L, Kratina P.. 2019. Ecosystem services provided by bromeliad plants: a systematic review. Ecology and Evolution 9, 7360–7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law KY. 2015. Water–surface interactions and definitions for hydrophilicity, hydrophobicity and superhydrophobicity. Pure and Applied Chemistry 87, 759– 765. [DOI] [PubMed] [Google Scholar]
- Leigh EG Jr. 1975. Structure and climate in tropical rain forest. Annual Review of Ecology and Systematics 6, 67–86. [Google Scholar]
- Lejeune S, Gilet T.. 2019. Drop impact close to the edge of an inclined substrate: liquid sheet formation and breakup. Physical Review Fluids 4, 053601. [Google Scholar]
- Lightbody JP. 1985. Distribution of leaf shapes of Piper sp. in a tropical cloud forest: evidence for the role of drip-tips. Biotropica 17, 339–342. [Google Scholar]
- Lin Y-T, Chou J-H.. 2014. A low-cost filler-dissolved process for fabricating super-hydrophobic poly (dimethylsiloxane) surfaces with either lotus or petal effect. Journal of Micromechanics and Microengineering 24, 055021. [Google Scholar]
- Liu J, Skidmore AK, Wang T, Zhu X, Premier J, Heurich M, Beudert B, Jones S.. 2019. Variation of leaf angle distribution quantified by terrestrial LiDAR in natural European beech forest. ISPRS Journal of Photogrammetry and Remote Sensing 148, 208–220. [Google Scholar]
- Lovelock CE, Osmond CB, Jebb M.. 1994. Photoinhibition and recovery in tropical plant species: response to disturbance. Oecologia 97, 297–307. [DOI] [PubMed] [Google Scholar]
- Lücking R, Bernecker-Lücking A.. 2005. Drip-tips do not impair the development of epiphyllous rain-forest lichen communities. Journal of Tropical Ecology 21, 171–177. [Google Scholar]
- Malhado AC, Malhi Y, Whittaker RJ, et al. 2012. Drip-tips are associated with intensity of precipitation in the Amazon rain forest. Biotropica 44, 728–737. [Google Scholar]
- Marengo M, Antonini C, Roisman IV, Tropea C.. 2011. Drop collisions with simple and complex surfaces. Current Opinion in Colloid & Interface Science 16, 292–302. [Google Scholar]
- Masrahi YS. 2020. Ecological significance of leaf surface micromorphology and wettability in Tragus berteronianus schult. Asian Journal of Plant Sciences 19, 372–382. [Google Scholar]
- Mauseth JD. 1988. Plant anatomy. Menlo Park, CA: Benjamin/Cummings. [Google Scholar]
- Meng F, Cao R, Yang D, Niklas KJ, Sun S.. 2014. Trade-offs between light interception and leaf water shedding: a comparison of shade- and sun-adapted species in a subtropical rainforest. Oecologia 174, 13–22. [DOI] [PubMed] [Google Scholar]
- Minorsky PV. 2019. The functions of foliar nyctinasty: a review and hypothesis. Biological Reviews 94, 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanko K, Hudson SA, Levia DF.. 2016. Differences in throughfall drop size distributions in the presence and absence of foliage. Hydrological Sciences Journal 61, 620–627. [Google Scholar]
- Nanko K, Watanabe A, Hotta N, Suzuki M.. 2013. Physical interpretation of the difference in drop size distributions of leaf drips among tree species. Agricultural and Forest Meteorology 169, 74–84. [Google Scholar]
- Neinhuis C, Barthlott W.. 1998. Seasonal changes of leaf surface contamination in beech, oak and gingko in relation to leaf micromorphology and wettability. New Phytologist 138, 91–98. [Google Scholar]
- Nicotra AB, Leigh A, Boyce CK, Jones CS, Niklas KJ, Royer DL, Tsukaya H.. 2011. The evolution and functional significance of leaf shape in angiosperms. Functional Plant Biology 38, 535–552. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Fleck S.. 2002. Petiole mechanics, leaf inclination, morphology, and investment in support in relation to light availability in the canopy of Liriodendron tulipifera. Oecologia 132, 21–33. [DOI] [PubMed] [Google Scholar]
- Niklas KJ. 1999. A mechanical perspective on foliage leaf form and function. New Phytologist 143, 19–31. [Google Scholar]
- Niklas KJ, O’Rourke TD.. 1987. Flexural rigidity of chive and its response to water potential. American Journal of Botany 74, 1033–1044. [Google Scholar]
- Nishino T, Meguro M, Nakamae K, Matsushita M, Ueda Y.. 1999. The lowest surface free energy based on −CF3 alignment. Langmuir 13, 4321–4323. [Google Scholar]
- Oellermann M, Jolles JW, Ortiz D, Seabra R, Wenzel T, Wilson H, Tanner R.. 2021. Harnessing the benefits of open electronics in science. arXiv 2106.15852. [Preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda Y, Schieving F, Anten NP.. 2015. A novel method of measuring leaf epidermis and mesophyll stiffness shows the ubiquitous nature of the sandwich structure of leaf laminas in broad-leaved angiosperm species. Journal of Experimental Botany 66, 2487–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oqielat MN, Turner IW, Belward JA, McCue SW.. 2011. Modelling water droplet movement on a leaf surface. Mathematics and Computers in Simulation 81, 1553–1571. [Google Scholar]
- Panditharathna PAKAK, Singhakumara BMP, Griscom HP, Ashton MS.. 2008. Change in leaf structure in relation to crown position and size class for tree species within a Sri Lankan tropical rain forest. Botany 86, 633–640. [Google Scholar]
- Papierowska E, Mazur R, Stańczyk T, Beczek M, Szewińska J, Sochan A, Ryżak M, Szatyłowicz J, Bieganowski A.. 2019. Influence of leaf surface wettability on the drop splash phenomenon. Agricultural and Forest Meteorology 279, 107762. [Google Scholar]
- Peguero-Pina JJ, Vilagrosa A, Alonso-Forn D, Ferrio JP, Sancho-Knapik D, Gil-Pelegrin E.. 2020. Living in drylands: functional adaptations of trees and shrubs to cope with high temperatures and water scarcity. Forests 11, 1028. [Google Scholar]
- Pimentel D, Harvey C, Resosudarmo P, et al. 1995. Environmental and economic costs of soil erosion and conservation benefits. Science 267, 1117–1123. [DOI] [PubMed] [Google Scholar]
- Pina ALCB, Zandavalli RB, Oliveira RS, Martins FR, Soares AA.. 2016. Dew absorption by the leaf trichomes of Combretum leprosum in the Brazilian semiarid region. Functional Plant Biology 43, 851–861. [DOI] [PubMed] [Google Scholar]
- Raabe K, Pisek J, Sonnentag O, Annuk K.. 2015. Variations of leaf inclination angle distribution with height over the growing season and light exposure for eight broadleaf tree species. Agricultural and Forest Meteorology 214, 2–11. [Google Scholar]
- Rebelo CF, Williamson GB.. 1996. Drip-tips vis-à-vis soil types in central Amazônia. Biotropica 28, 159–163. [Google Scholar]
- Reicosky DA, Hanover JW.. 1978. Physiological effects of surface waxes: I. Light reflectance for glaucous and nonglaucous Picea pungens. Plant Physiology 62, 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein M. 1996. The transitional regime between coalescing and splashing drops. Journal of Fluid Mechanics 306, 145–165. [Google Scholar]
- Riederer M, Müller C.. 2006. Biology of the plant cuticle. Oxford: Blackwell Publishing. [Google Scholar]
- Robinson SA, Osmond CB.. 1994. Internal gradients of chlorophyll and carotenoid pigments in relation to photoprotection in thick leaves of plants with crassulacean acid metabolism. Functional Plant Biology 21, 497–506. [Google Scholar]
- Rollet B. 1990. Leaf morphology. In: Roth I, ed. Stratification of tropical forests as seen in leaf structure. Dordrecht: Springer, 1–75. [Google Scholar]
- Rosado BH, Holder CD.. 2013. The significance of leaf water repellency in ecohydrological research: a review. Ecohydrology 6, 150–161. [Google Scholar]
- Roth I, Braun HJ, Carlquist C, Ozenda P.. 1990. Leaf structure of a Venezuelan cloud forest in relation to the microclimate. Stuttgart: Borntraeger. [Google Scholar]
- Roth-Nebelsick A, Uhl D, Mosbrugger V, Kerp H.. 2001. Evolution and function of leaf venation architecture: a review. Annals of Botany 87, 553–566. [Google Scholar]
- Ryel RJ, Beyschlag W.. 1995. Benefits associated with steep foliage orientation in two tussock grasses of the American Intermountain West. A look at water-use-efficiency and photoinhibition. Flora 190, 251–260. [Google Scholar]
- Schneider JV, Zipp D, Gaviria J, Zizka G.. 2003. Successional and mature stands in an upper Andean rain forest transect of Venezuela: do leaf characteristics of woody species differ? Journal of Tropical Ecology 19, 251–259. [Google Scholar]
- Schreel JDM, Leroux O, Goossens W, Brodersen C, Rubinstein A, Steppe K.. 2020. Identifying the pathways for foliar water uptake in beech (Fagus sylvatica L.): a major role for trichomes. The Plant Journal 103, 769–780. [DOI] [PubMed] [Google Scholar]
- Schulte AJ, Droste DM, Koch K, Barthlott W.. 2011. Hierarchically structured superhydrophobic flowers with low hysteresis of the wild pansy (Viola tricolor)—new design principles for biomimetic materials. Beilstein Journal of Nanotechnology 2, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorska D, Papierowska E, Szatyłowicz J, Sikorski P, Suprun K, Hopkins RJ.. 2017. Variation in leaf surface hydrophobicity of wetland plants: the role of plant traits in water retention. Wetlands 37, 997–1002. [Google Scholar]
- Soto D, De Larivière AB, Boutillon X, Clanet C, Quéré D.. 2014. The force of impacting rain. Soft Matter 10, 4929–4934. [DOI] [PubMed] [Google Scholar]
- Sullivan MK, Queenborough SA.. 2020. Precipitation gradients, plant biogeography, and the incidence of drip-tips in Cerrado plant species. Biotropica 52, 583–589. [Google Scholar]
- Tadrist L, Saudreau M, de Langre E.. 2014. Wind and gravity mechanical effects on leaf inclination angles. Journal of Theoretical Biology 341, 9–16. [DOI] [PubMed] [Google Scholar]
- Templer PH, Weathers KC, Ewing HA, Dawson TE, Mambelli S, Lindsey AM, Webb J, Boukili VK, Firestone MK.. 2015. Fog as a source of nitrogen for redwood trees: evidence from fluxes and stable isotopes. Journal of Ecology 103, 1397–1407. [Google Scholar]
- Tranquada GC, Erb U.. 2014. Morphological development and environmental degradation of superhydrophobic aspen and black locust leaf surfaces. Ecohydrology 7, 1421–1436. [Google Scholar]
- Tukey HB Jr. 1970. The leaching of substances from plants. Annual Review of Plant Physiology 21, 305–324. [Google Scholar]
- Vogel S. 1989. Drag and reconfiguration of broad leaves in high winds. Journal of Experimental Botany 40, 941–948. [Google Scholar]
- Vogel S. 1992. Twist-to-bend ratio and cross-sectional shapes of petioles and stems. Journal of Experimental Botany 43, 1527–1532. [Google Scholar]
- Wallace LL, Timpano P, Durgin P.. 1987. Leaf folding in Mimosa pudica (Fabaceae): a nutrient conservation mechanism? American Journal of Botany 74, 132–135. [Google Scholar]
- Wang T, Si Y, Dai H, Li C, Gao C, Dong Z, Jiang L.. 2020. Apex structures enhance water drainage on leaves. Proceedings of the National Academy of Sciences, USA 117, 1890–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bourouiba L.. 2018. Non-isolated drop impact on surfaces. Journal of Fluid Mechanics 835, 24–44. [Google Scholar]
- Wenzel RN. 1936. Resistance of solid surfaces to wetting by water. Industrial & Engineering Chemistry 28, 988–994. [Google Scholar]
- Williamson GB. 1981. Drip-tips and splash erosion. Biotropica 13, 228–231. [Google Scholar]
- Williamson GB, Romero A, Armstrong JK, Gush TJ, Hruska AJ, Klass PE, Thompson JT.. 1983. Drip-tips, drop size and leaf drying. Biotropica 15, 232–234. [Google Scholar]
- Xiong P, Chen Z, Jia Z, Wang Z, Palta JA, Xu B.. 2018. Variability in leaf wettability and surface water retention of main species in semiarid Loess Plateau of China. Ecohydrology 11, e2021. [Google Scholar]
- Yáñez-Espinosa L, Terrazas T, López-Mata L, Valdez-Hernández JI.. 2003. Leaf trait variation in three species through canopy strata in a semi-evergreen Neotropical forest. Canadian Journal of Botany 81, 398–404. [Google Scholar]
- Zambrano ARC, Linis VC, Nepacina MRJ, Silvestre MLT, Foronda JRF, Janairo JIB.. 2019. Wetting properties and foliar water uptake of Tillandsia L. Biotribology 19, 100103. [Google Scholar]
- Zhu H. 1997. Ecological and biogeographical studies on the tropical rain forest of south Yunnan, SW China with a special reference to its relation with rain forests of tropical Asia. Journal of Biogeography 24, 647–662. [Google Scholar]
- Ziv M, Meir G, Halevy AH.. 1982. Factors influencing the production of hardened glaucous carnation plantlets in vitro. The Plant Cell 2, 55–65. [Google Scholar]