Abstract
Charcot-Marie-Tooth disease (CMT) is a hereditary peripheral neuropathy characterized by progressive atrophy of distal muscles. Respiratory complications are rare. We present a case of a 49-year-old male with childhood-onset CMT bearing a genetic mutation of MFN2. He had difficulty breathing when he was 46. Imaging examination revealed complications of phrenic nerve paralysis and pneumothorax with a funnel chest. Respiratory function test demonstrated severe restrictive ventilatory impairment. Polysomnography supported the diagnosis of mild sleep apnea syndrome. Noninvasive positive pressure ventilation successfully reduced respiratory symptoms. To our knowledge, this is the first demonstration of multiple respiratory complications in a CMT patient.
Keywords: Charcot-Marie-Tooth disease, Diaphragmatic dysfunction, Thoracic cage deformity, Pneumothorax, Restrictive pulmonary impairment, Sleep apnea syndrome
Abbreviations: CMT, Charcot-Marie-Tooth disease; NPPV, non-invasive positive pressure ventilation; OSAS, obstructive sleep apnea syndrome
Highlights
-
•
Respiratory complications in Charcot-Marie-Tooth disease (CMT) are rare.
-
•
This is the first report of multiple respiratory complications in a CMT patient.
-
•
Non-invasive ventilation was safely introduced and effective in this case.
1. Introduction
Charcot-Marie-Tooth disease (CMT) is a hereditary peripheral neuropathy characterized by gradually progressive bilateral atrophy, weakness of the lower extremity muscles, and a predominant radial nerve involvement. Patients with CMT also present with foot deformities and sensory disturbances of the lower extremity. CMT is classified into four types based on the type of inheritance, age of onset, peripheral nerve conduction findings, histological analysis of sural nerve, and genetic abnormality of myelin proteins [1,2].
Numerous genetic anomalies have been identified for abnormal myelin proteins. Currently, a diagnostic approach using comprehensive genetic assessment is available for CMT. Genetic abnormality of MFN2, a protein present in the outer mitochondrial membrane, accounts for about 25% of type 2 CMT [3]. It is involved in mitochondrial axonal transport, where it serves as an adapter protein for the prevention of degradation of other proteins necessary for the maintenance of nerve axons. These functions synergistically prevent skeletal muscle atrophy [4].
The frequency of respiratory complications in patients with CMT is considered to be low; the occurrence of respiratory failure as the cause of death in patients with CMT is also rare. However, previous reports have reported instances of phrenic nerve palsy, thoracic abnormalities, and/or sleep apnea syndrome in patients with CMT, suggesting the importance of these conditions as prognostic factors [5,6].
We report a difficult-to-treat CMT patient with an MFN2 gene abnormality who experienced multiple respiratory disorders, including pneumothorax, funnel chest, phrenic nerve palsy, and sleep apnea syndrome. To our knowledge, this is the first demonstration of a CMT patient carrying an MFN2 gene abnormality and presenting with multiple respiratory complications.
2. Case presentation
A 49-year-old male reported gait abnormalities and bilateral, peripheral motor and sensory neuropathy with absent deep tendon reflexes at the age of three. He had also had gradually progressive muscular atrophy, predominantly in the distal musculature. Characteristic limb deformations had also been observed. Based on these findings, a diagnosed of CMT was made. Genetic screening test had revealed a genetic mutation of MFN2, the most common cause of axonal CMT. Lower extremity muscle weakness had continued to progress, and the patient had been unable to walk when he was 18. He had become wheelchair-dependent when he was 30. When he was 46, he felt exertional dyspnea and experienced nocturnal awakening and sweating.
He presented to us with extreme weight loss (height 165 cm, weight 38 kg, BMI 14.0). On physical examination, funnel chest was observed as a thoracic cage deformity. He reported a history of smoking for a brief period. He did not have any pedal edema indicative of right ventricular failure.
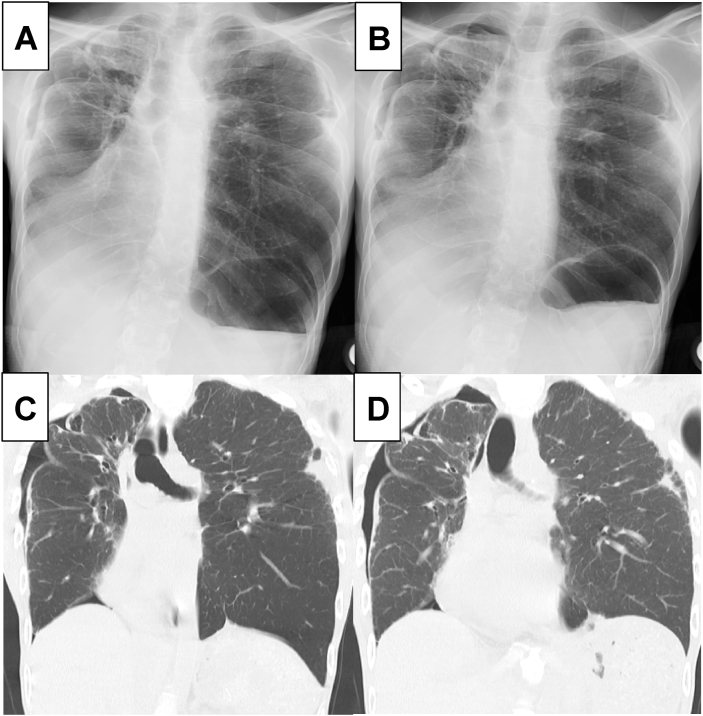
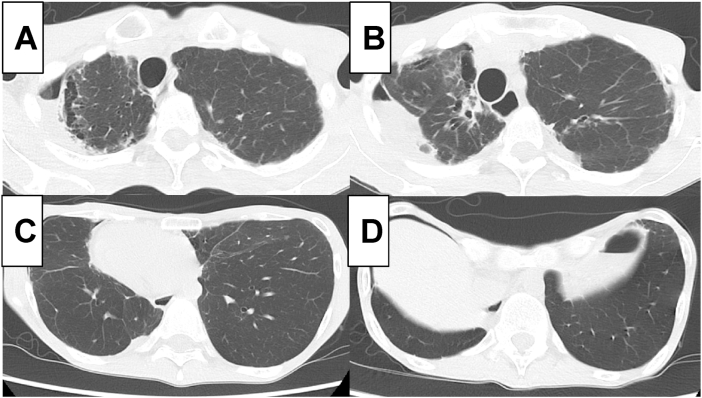
Further examinations were performed to uncover the cause of these symptoms, suspected to be related to respiratory disorders. Chest radiograph showed bilateral diaphragmatic elevation (Fig. 1A and B). Chest radiograph and chest computed tomography demonstrated diaphragmatic dysfunction, in that the diaphragm was motionlessness during inspiration and expiration (Fig. 1A–D). This abnormality was dominant on the right side. In addition, bilateral pneumothorax and subpleural cysts at the apex of the lung were also observed (Fig. 2A–D).
Fig. 1.
Inspiration-expiration study of chest radiography and computed tomography.
Fig. 2.
Axial images of computed tomography.
Chest radiography during the inspiratory phase (A) and expiratory phase (B), and computed tomography during the inspiratory phase (C) and expiratory phase (D) demonstrated a loss of respiratory diaphragmatic movement, indicative of its dysfunction in this patient.
Axial images of computed tomography demonstrated pneumothorax and bullae in bilateral upper lobes with thickened pleura (A, B). A thoracic cage deformity of a funnel chest was evident in CT images; bilateral lower lobes showed no interstitial fibrotic changes (C, D).
Electrophysiological tests were performed to assess phrenic nerve function. Phrenic nerve stimulation did not induce diaphragmatic movements and/or hiccups, suggesting that the diagnosis of phrenic nerve paralysis was appropriate. However, magnetic resonance imaging showed no presence of cervical cord lesions causative of phrenic nerve paralysis.
Respiratory function tests demonstrated marked restrictive ventilation disturbance (vital capacity, 1.32 L; predicted rate of vital capacity, 32.6%; forced expiratory volume % in 1 s, 98.21%).
Polysomnography was performed to identify the cause of nocturnal dyspnea. Calculated apnea-hypopnea index was 5.5 times/hour. The lowest oxygen concentration in his sleep was 79%. Thus, the patient was diagnosed with mild sleep apnea syndrome.
Laboratory investigations demonstrated an elevated eosinophil count in peripheral blood while concentrations of total IgE and brain natriuretic peptide were within the normal range (Table 1). Cardiac ultrasonography showed no valvular diseases, and ejection fraction was normal. These results ruled out the possibility of asthma and heart failure. Arterial blood gas test showed a slightly elevated level of carbon dioxide (PaCO2, 51.7 mm Hg) with normal oxide concentrations (PaO2, 75.9 mm Hg) (Table 1).
Table 1.
Laboratory findings of patients at the first visit.
| Hematological parameters | Serological and biochemical parameters | Arterial blood gas analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| White blood cells | 5,700 | /uL | T-Bil | 0.52 | mg/dL | CRP | <0.3 | mg/dL | pH | 7.393 | |
| Neutrophil | 67.8 | % | AST | 23 | IU/L | KL‐6 | 642 | U/mL | PaCO2 | 51.7 | Torr |
| Lymphocyte | 19.0 | % | ALT | 14 | IU/L | SP-A | 47.4 | ng/mL | PaO2 | 75.9 | Torr |
| Basophil | 0.9 | % | LDH | 241 | IU/L | SP-D | 120 | ng/mL | HCO3− | 30.8 | mmol/L |
| Eosinophil | 9.5 | % | Alb | 3.8 | g/dL | IgE | 71 | IU/mL | BE | 5.2 | mmol/L |
| Monocyte | 2.8 | % | BUN | 16 | mg/dL | BNP | 9.1 | pg/mL | SaO2 | 95.5 | % |
| Red blood cells | 441 | × 104/μL | Cr | 0.41 | mg/dL | sIL-2R | 445 | U/mL | A-aDO2 | 9.2 | Torr |
| Hemoglobin | 12.9 | g/dL | Na | 142 | mEq/L | α1-antitrypsin | 142 | mg/dL | |||
| Hematocrit | 41 | % | K | 4.4 | mEq/L | ANA | <40 | times | |||
| Platelets | 20.8 | × 104/μL | Cl | 102 | mEq/L | Rheumatoid Factor | <3.0 | U/mL | |||
| HbA1c (NGSP) | 5.5 | % | T-SPOT. TB | (−) | |||||||
Abbreviation: KL-6, Krebs von den Lungen‐6; SP-A, surfactant protein-A; SP-D, surfactant protein-D.
BNP, brain natriuretic peptide; sIL-2R, soluble interleukin-2 receptor; ANA, anti nuclear antibody.
Based on these findings, we speculated that nocturnal dyspnea was induced by a combination of respiratory disorders including phrenic nerve paralysis, pneumothorax, funnel chest, and sleep apnea syndrome with respiratory muscle weakness. Non-invasive positive pressure ventilation therapy (NPPV) was introduced to improve symptoms with attention to pneumothorax. Dyspnea was improved with NPPV, which was administered at the following setting: inspiratory positive airway pressure (IPAP): 5.8 cm H2O and expiratory positive airway pressure (EPAP): 3.0 cm H2O.
3. Discussion
Unlike other neuromuscular disorders such as myasthenia gravis and amyotrophic lateral sclerosis, patients with CMT rarely present with respiratory complications. Among the few cases of CMT reported with respiratory disorders [5], phrenic nerve paralysis and/or diaphragmatic dysfunction are common [[7], [8], [9], [10], [11]]. Autopsy in one case with phrenic nerve paralysis revealed that the pathological changes in the phrenic nerve were essentially the same as those in somatic nerves, indicative of a common pathophysiology [12]. This also suggested a positive relationship between phrenic nerve involvement and the severity of motion disability in CMT [13]. Consistent with this report, in our case, CMT was severely progressive because of the severity of the muscular atrophy which extended to the proximal muscles of the upper limbs.
Thoracic cage abnormalities in patients with CMT have been also reported [5], commonly due to spinal deformities possibly induced by neuropathic spinal arthropathy [14,15]. However, there are no reports of funnel chest or scoliosis in CMT patients – other thoracic cage abnormalities that reduce the lung volume. Diaphragmatic paralysis and thoracic abnormalities can synergistically worsen restrictive ventilatory impairment. Although surgical treatment may be provided for these abnormalities, it was unsuitable for this patient because of severe restrictive ventilatory insufficiency with pneumothorax.
Similarly, there have been no reports of pneumothorax and/or pulmonary cyst in patients with CMT. This male patient had weight loss with decreased appetite, which are risk factors for idiopathic pneumothorax. Secondary causes for pneumothorax including chronic obstructive pulmonary disease, interstitial pneumonia, or lung tumor were not identified. A pulmonary cyst was found bilaterally at the apex of the lung that possibly caused pneumothorax. It is also possible that smoking-related cyst formation induced secondary pneumothorax in this case, though the short duration and slight smoking in the patient was probably insufficient.
Complicated obstructive sleep apnea syndrome (OSAS) has been reported in CMT [[16], [17], [18]]. OSAS can cause nocturnal dyspnea with hypopnea. In the present case, the severity of OSAS was mild, which might be insufficient to fully explain dyspnea at night. Instead, a combination of OSAS and other causes of pulmonary impairment could be responsible for the deterioration of the patient's respiratory symptoms.
CMT disease with MFN2 gene abnormality is classified as CMT type 2A. A previous review article summarized the relationship between respiratory complications and specific genetic abnormalities [5]. Diaphragmatic paralysis and/or diaphragmatic dysfunction is relatively common in CMT 1, CMT 2 (especially type C, not type A), and CMT 4. Thoracic abnormalities have been reported frequently in CMT 1 and CMT 4 type C in a female-dominated patient population. Both disorders are atypical for CMT 2 type A, indicative of the unique phenotype of this patient.
NPPV is an effective treatment for restrictive ventilation insufficiency due to phrenic nerve palsy, diaphragmatic dysfunction, thoracic abnormalities, and obstructive ventilation impairment caused by sleep apnea syndrome [19]. However, positive pressure ventilation might possibly worsen pneumothorax. Thus, the therapeutic strategy needs careful consideration. Additionally, high perspiration levels in this patient would pose difficulty in wearing a breathing mask for long durations.
4. Conclusion
To our knowledge, this is the first demonstration of a CMT patient carrying an MFN2 gene abnormality with multiple respiratory complications, thus raising a possibility that MFN2 dysfunction might cause several types of respiratory disorders. However, another unknown genetic mutation might exist in parallel with MFN2 abnormality in this patient. It would be helpful for pulmonologists and neurologists to be aware of these facts in patients with CMT, because respiratory complications in patients with CMT might be more frequent than expected. A careful examination should be performed if respiratory symptoms including dyspnea are observed in CMT patients in clinical settings.
Funding
None declared.
Declaration of competing interest
The authors have no conflict of interest to report.
References
- 1.Barbullushi K., Abati E., Rizzo F., Bresolin N., Comi G.P., Corti S. Disease modeling and therapeutic strategies in CMT2A: state of the art. Mol. Neurobiol. 2019;56:6460–6471. doi: 10.1007/s12035-019-1533-2. [DOI] [PubMed] [Google Scholar]
- 2.Bombelli F., Stojkovic T., Dubourg O., Echaniz-Laguna A., Tardieu S., Larcher K., et al. Charcot-Marie-Tooth disease type 2A: from typical to rare phenotypic and genotypic features. JAMA Neurol. 2014;71:1036–1042. doi: 10.1001/jamaneurol.2014.629. [DOI] [PubMed] [Google Scholar]
- 3.Tan C.A., Rabideau M., Blevins A., Westbrook M.J., Ekstein T., Nykamp K., et al. Autosomal recessive MFN2-related Charcot-Marie-Tooth disease with diaphragmatic weakness: case report and literature review. Am. J. Med. Genet. 2016;170:1580–1584. doi: 10.1002/ajmg.a.37611. [DOI] [PubMed] [Google Scholar]
- 4.Chandhok G., Lazarou M., Neumann B. Structure, function, and regulation of mitofusin-2 in health and disease. Biol. Rev. Camb. Phil. Soc. 2018;93:933–949. doi: 10.1111/brv.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aboussouan L.S., Lewis R.A., Shy M.E. Disorders of pulmonary function, sleep, and the upper airway in Charcot-Marie-Tooth disease. Lung. 2007;185:1–7. doi: 10.1007/s00408-006-0053-9. [DOI] [PubMed] [Google Scholar]
- 6.de Carvalho Alcantara M., Nogueira-Barbosa M.H., Fernandes R.M., da Silva G.A., Lourenco C.M., Sander H.H., et al. Respiratory dysfunction in Charcot-Marie-Tooth disease type 1A. J. Neurol. 2015;262:1164–1171. doi: 10.1007/s00415-015-7677-8. [DOI] [PubMed] [Google Scholar]
- 7.Abboud L., El S.F., Takubo T., Byrd R.P., Jr., Roy T.M. Phrenic nerve involvement in Charcot-Marie-Tooth disease. Tenn. Med. 2005;98:495–497. [PubMed] [Google Scholar]
- 8.Chan C.K., Mohsenin V., Loke J., Virgulto J., Sipski M.L., Ferranti R. Diaphragmatic dysfunction in siblings with hereditary motor and sensory neuropathy (Charcot-Marie-Tooth disease) Chest. 1987;91:567–570. doi: 10.1378/chest.91.4.567. [DOI] [PubMed] [Google Scholar]
- 9.Laroche C.M., Carroll N., Moxham J., Stanley N.N., Evans R.J., Green M. Diaphragm weakness in Charcot-Marie-Tooth disease. Thorax. 1988;43:478–479. doi: 10.1136/thx.43.6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osanai S., Akiba Y., Nakano H., Matsumoto H., Yahara O., Onodera S. Charcot-Marie-Tooth disease with diaphragmatic weakness. Intern. Med. 1992;31:1267–1270. doi: 10.2169/internalmedicine.31.1267. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava V., Pasha T., Knowles A., Boothman B., Paracha M., Kalkat M., et al. Dramatic improvement after bilateral diaphragmatic plication in Charcot-Marie-Tooth disease. Ann. Thorac. Surg. 2014;97:2177–2179. doi: 10.1016/j.athoracsur.2013.04.143. [DOI] [PubMed] [Google Scholar]
- 12.Gilchrist D., Chan C.K., Deck J.H. Phrenic involvement in Charcot-Marie-Tooth disease. A pathologic documentation. Chest. 1989;96:1197–1199. doi: 10.1378/chest.96.5.1197. [DOI] [PubMed] [Google Scholar]
- 13.Spiesshoefer J., Henke C., Kabitz H.J., Akova-Oeztuerk E., Draeger B., Herkenrath S., et al. Phrenic nerve involvement and respiratory muscle weakness in patients with Charcot-Marie-Tooth disease 1A. J. Peripher. Nerv. Syst. 2019;24:283–293. doi: 10.1111/jns.12341. [DOI] [PubMed] [Google Scholar]
- 14.Hensinger R.N., MacEwen G.D. Spinal deformity associated with heritable neurological conditions: spinal muscular atrophy, Friedreich's ataxia, familial dysautonomia, and Charcot-Marie-Tooth disease. J. Bone Joint Surg. Am. 1976;58:13–24. [PubMed] [Google Scholar]
- 15.Walker J.L., Nelson K.R., Stevens D.B., Lubicky J.P., Ogden J.A., VandenBrink K.D. Spinal deformity in Charcot-Marie-Tooth disease. Spine. 1994;19:1044–1047. doi: 10.1097/00007632-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Darquennes K., De Jonghe P., Daems D., De Backer W., Verbraecken J. Intermittent positive airway pressure by nasal mask as a treatment for respiratory insufficiency in a patient with Charcot-Marie-Tooth disease. Acta Clin. Belg. 2006;61:176–181. doi: 10.1179/acb.2006.030. [DOI] [PubMed] [Google Scholar]
- 17.Dematteis M., Pepin J.L., Jeanmart M., Deschaux C., Labarre-Vila A., Levy P. Charcot-Marie-Tooth disease and sleep apnoea syndrome: a family study. Lancet. 2001;357:267–272. doi: 10.1016/S0140-6736(00)03614-X. [DOI] [PubMed] [Google Scholar]
- 18.Dziewas R., Waldmann N., Bontert M., Hor H., Muller T., Okegwo A., et al. Increased prevalence of obstructive sleep apnoea in patients with Charcot-Marie-Tooth disease: a case control study. J. Neurol. Neurosurg. Psychiatry. 2008;79:829–831. doi: 10.1136/jnnp.2007.137679. [DOI] [PubMed] [Google Scholar]
- 19.Misuri G., Lanini B., Gigliotti F., Iandelli I., Pizzi A., Bertolini M.G., et al. Mechanism of CO(2) retention in patients with neuromuscular disease. Chest. 2000;117:447–453. doi: 10.1378/chest.117.2.447. [DOI] [PubMed] [Google Scholar]