Abstract
Background: Porcine mesenchymal stromal cells (pMSCs) are considered a valuable research model for bone tissue engineering, which requires adequate amounts of viable cells with sufficient potential for osteogenic differentiation. For isolation and expansion of these cells through long-term culture, appropriate culture conditions are needed.
Objective: To study the effect of extended in vitro cultivation on pMSC proliferation and differentiation potential using different osteogenic and adipogenic induction media.
Methods: pMSCs were isolated from the bone marrow of adult Göttingen minipigs, cultured, expanded to passage 20 (~160 days) and characterized by their expression of cell surface markers (wCD44, CD45, CD90, SWC9, fibronectin), alkaline phosphatase (ALP), and osteocalcin and their potential for osteogenic and adipogenic differentiation using different induction media.
Results: pMSCs retained their capacity for proliferation and osteogenic differentiation, and the number of CD90-positive cells increased significantly over more than 60 population doublings. CD90 expression in uninduced cells correlated strongly with ALP expression following osteogenic induction. Medium enriched with calcium yielded a stronger osteogenic response.
Conclusion: The selection of CD90-positive MSCs and adequate levels of calcium seem to enhance the osteogenic phenotype for bone tissue engineering.
Keywords: Multilineage potential, Mesenchymal stromal cells, Culture conditions, CD90, calcium
Introduction
Adult mesenchymal stromal cells (MSCs) have a broad spectrum of biological properties, reflecting their usefulness for clinical applications, e.g., in hepatic[1] and cardiovascular disease[2] or for bone regeneration[3]. A positive impact of MSCs cultivated in vitro has been demonstrated on the suppression of (auto)immunological[4] and inflammatory[5] responses and on the regeneration of damaged tissues[6].
Regeneration of bone through tissue engineering (TE) can avoid autologous bone grafts[3,7], which are limited by availability, geometry, donor site morbidity, and cost[8]. TE combines progenitor or stem cells, growth factors and biocompatible, three-dimensional scaffolds (e.g., hydroxyapatite or β-tricalcium phosphate) to produce new bone when implanted into critical size defects[7,9]. While several clinical studies report beneficial effects when injecting “bone marrow concentrate aspirates” rich in MSCs in cases of osteoarthritis[10,11] or osteogenesis imperfecta[12], few clinical studies have focused on TE of bone[13], which is partially due to the isolation and expansion processes required to achieve adequately large numbers of suitable cells.
Currently, no consensus has been established on the effective cell dose (ECD), i.e., the minimum cell number needed to generate a significant therapeutic effect[13]. Choi et al. estimated the ECD to be approximately 1x107 MSCs/person[14]. According to Oryan et al., the mean ECD is either 1.86x108 MSCs/person or 6.64x106 MSCs/kg[13]. Unfortunately, such amounts of MSCs are impossible to directly harvest from a single patient[13]. Li et al., reported that one millilitre of bone marrow aspirate yields an average of 391 MSC colonies (clusters of at least 50 cells)[15]. Collecting multiple bone marrow aspirates from the same patient to harvest the ECD does not seem to be an option since the MSC yield decreases significantly with each aspiration[15]. Therefore, extended in vitro cultivation over multiple passages is needed to sufficiently expand the cell number. Furthermore, for TE of bone, the cells must maintain their osteogenic differentiation potential during in vitro expansion.
The effect of an extended cultivation period on the proliferation and differentiation potential of MSCs is not fully understood. While some studies report that MSCs lose their proliferative and osteogenic differentiation potential through prolonged cultivation by senescence[16–18], others demonstrate retention of osteogenic differentiation potential for late passages[19].
In this study, we used porcine MSCs (pMSCs), which compare well to human mesenchymal stromal cells (hMSCs): they are also plastic adherent, capable of differentiating into multiple mesenchymal tissues, express similar surface markers[20,21], and have similar immunomodulatory capabilities[22,23]. Noort et al., demonstrated that the in vitro osteogenic differentiation of pMSCs and hMSCs does not differ significantly when provided with the same induction media[22]. As pigs share many anatomical, physiological (e.g., bone turnover), and genetic features with humans[23], pMSCs are a suitable model for research on TE of bone.
First, this study aims to characterize bone marrow (BM)-derived pMSCs in long-term culture by their expression of CD90, CD44, CD45, and SWC9. CD90, a member of the immunoglobulin superfamily, is a common MSC marker[24] that has been linked to increased osteogenic differentiation potential[25]. The hyaluronic acid receptor CD44 is considered a reliable MSC marker when combined with the presence or lack of other surface markers[26,27]. The tyrosine phosphatase CD45 is exclusive to nucleated cells of the haematopoietic system[28] and absent on MSCs[24,29]. Only little CD45 expression can be detected in the early passages of MSC cultures[30]. SWC9 is expressed on mature porcine macrophages and weakly expressed on porcine thymocytes[31] and thus represents another negative MSC marker.
Due to the limited availability of antibodies directed towards porcine cell surface markers, the revised MSC criteria set by the ISCT in 2019 could not be completely met[32]. Therefore, the isolated cell populations were referred to as mesenchymal stromal cells instead of mesenchymal stem cells[32].
Second, this study examines the effect of extended in vitro cultivation on pMSC proliferation and differentiation potential using different osteogenic and adipogenic induction media by evaluating the expression of alkaline phosphatase (ALP), an early marker of osteogenic differentiation[33], and osteocalcin, a bone-specific protein[34].
Methods
Animal selection and care and the surgical protocol were approved by the Minister of Nature, Environment and Forestry of Schleswig- Holstein and conformed to the standards of the Animal Care and Use Committee of the University of Kiel.
MSC isolation and cultivation
Bone marrow was harvested from the iliac crests of 10 adult female Göttingen minipigs (age: 20.8+/-3.2 months, weight: 37.1+/-2.5 kg, subsequently listed as SI-SX, purchased from Ellegaard, Dalmose, Denmark), which were released after the procedure. The harvested specimens (2.5 ml per donor) were dissected into smaller pieces and pretreated with 0.1% collagenase for 2 hours (37 °C) to release BM cells. Cells were washed with phosphate-buffered saline (PBS) and seeded in T75 tissue culture flasks at a density of 50,000 cells/cm2. The culture medium (CM) consisted of DMEM supplemented with 10% FCS, 100 IE/ml penicillin, 100 μg/ml streptomycin, and 1 mM ascorbic-2-phosphate. The CM was changed first after 24 hours and twice per week thereafter in all experiments. Adherent cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. Primary cultures were subcultured after 14 days. Cultures were passaged at 70–80% confluence after treatment with Accutase™ (PAA, Cölbe, Germany) for 15 min at 37 °C. Detached cells were resuspended in CM and centrifuged at 200 g for 5 min. The supernatant was removed, and the pellet was resuspended in CM. Cell number and viability were determined after trypan blue staining using a haemocytometer, and the population doubling time was calculated. For expansion and isolation of pMSCs, cells were seeded at a density of 2,500 cells/cm2 in T75 culture flasks and subcultured up to passage 20 (~160 days).
In a series of preliminary tests, cells from two donors (SI and SII) were used to examine different methods for pMSC isolation and cultivation to determine the most efficient and practical method for our purposes (data not shown). The method described here yielded the best results and was subsequently used for all further donors (SIII-SX, n = 8). One cell line from the preliminary study that was isolated and cultivated using the exact conditions described above (SII3) was included in the analyses of cell proliferation, viability, and osteogenic differentiation under long-term cultivation (n = 9). The expression of cell surface markers was examined exclusively with cells derived from donors SIII-SX (n = 8).
Cell surface markers on pMSCs
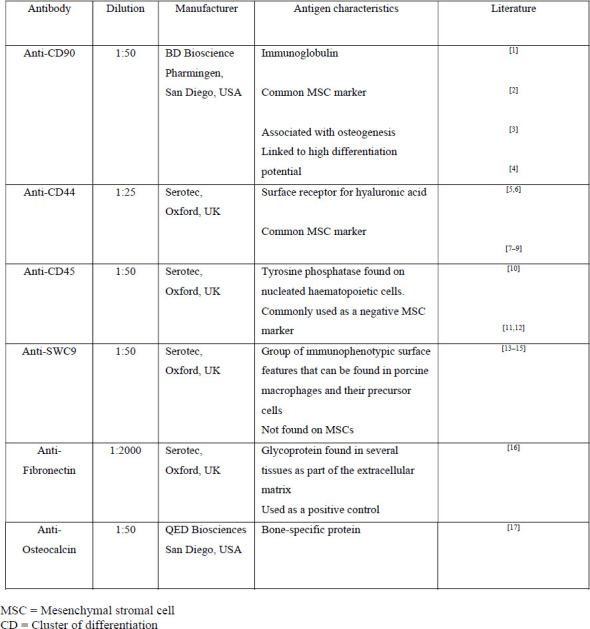
Cells from 8 donors (SIII-SX) were examined for their expression of CD90, CD44 CD45, SWC9, osteocalcin, and fibronectin (as a positive control) by immunocytology using the avidin-biotin- complex method (Supporting Table 1).
Cells at passages 1–5, 10, 15, and 20 were seeded at a density of 5,000 cells/cm2, cultivated for 3–4 days in CM, fixed with acetone, and incubated in methanol with 0.6% H2O2. Primary antibodies were purchased from Serotec, Oxford, UK (wCD44, CD45, fibronectin, SWC9), BD Bioscience Pharmingen, Franklin Lakes, USA (CD90), and QED Biosciences, San Diego, USA (osteocalcin), diluted in 1% bovine serum albumin TBS (anti-CD44 1:25; anti-fibronectin 1:2,000; the others: 1:50), and applied for 2 hours at room temperature (RT). Second, biotinylated antibodies were diluted in 10% porcine serum/TBS (1:500, 1:1,000, and 1:100) and applied for 60 min at RT. After washing with TBS, specimens were incubated in fresh ABC solution for 30 min, washed in TBS, stained for 3–5 min with diaminobenzidine, and then fixed in 4% paraformaldehyde for 10 min. After rinsing in TBS and distilled water, cell nuclei were stained with Meyer’s hemalum. The number of positively stained cells was determined for each surface marker by light microscopy and expressed as a percentage of all cells.
Alkaline phosphatase activity of pMSCs
Cells at passages 0–3 from 9 donors (SII3, SIII-SX) were seeded at a density of 5,000 cells/cm2, cultivated for 3–4 days in CM, and fixed with acetone. Alkaline phosphatase (ALP) activity was determined after cell staining with the BCIP/NBT assay (SigmaFAST™, Steinheim, Germany), which renders ALP-positive cells dark blue; ALP-negative cells remain uncoloured. For each donor, 500 cells were counted by light microscopy and judged as positive or negative. The number of ALP-positive cells was expressed as a percentage of counted cells.
Multilineage potential of pMSCs
To assess the multilineage potential of pMSCs, cells of passages 0–5, 10, 15, and 20 from each of the 9 donors (SII3, SIII-SX) were seeded in 6-well plates (5,000 cells/cm2) and cultivated in CM.
At subconfluence (70–80%), osteogenic or adipogenic differentiation was induced by cultivation over 14 days with commercially available osteogenic (OM1) or adipogenic induction/maintenance media (AIM1/AMM1) (Lonza, POPT 3002 or POPT 3004, Walkersville, USA). Additionally, in further separate assays, the effects of two different osteogenic media (OM2, OM3) and an adipogenic induction and maintenance medium (AIM2, AMM2) on differentiation according to Pittenger et al.[35] were tested and compared to those of the respective commercial medium. For OM2, which was previously used by Gassling et al.[36], CM was supplemented with 100 nM dexamethasone and 1 mM ascorbic-2-phosphate. For OM3, which was previously used by Thomsen et al.[37], 100 nM dexamethasone, 10 mM ß-glycerophosphate, 179 μM ascorbic-2-phosphate, and 1.56 mM CaCl2 were added to CM. The final calcium concentration in OM3 approximates the concentration of calcium in the serum of adult female Göttingen minipigs[38]. In addition to DMEM and 10% FCS, AMM2 contained 20 U/l insulin and 4 mM L-glutamine. For AIM2, AMM2 was supplemented with 0.5 mM 1-methyl-3-isobutylxanthine, 1 μM dexamethasone, and 60 μM indomethacine. Osteogenic or adipogenic differentiation of cells was evaluated by light microscopy after staining with Alizarin Red S or Oil Red O, respectively, and ALP expression in induced cultures was evaluated after BCIP/NBT staining by light microscopy. For all three stains, the portion of positively stained cells was expressed as a percentage of all cells.
Statistical evaluation
Data from the 8 or 9 donors were pooled and evaluated using “RStudio” for Windows (version 3.6.1) by descriptive statistics, the Mann–Whitney U-test, and Pearson regression analysis.
Results
Cell proliferation, cell viability, and population doubling time
Each subculture yielded an average of 1.8x106 cells per T75 tissue culture flask, which is a 9.6-fold increase in cell numbers, indicating that by passage 20, expansion from one single cell to 4.4x1019 cells can theoretically be achieved (Figure 1A).
Figure 1A, B: Cell proliferation data from 9 donors (thin lines). (A) Extrapolated cell numbers for passages 0–20 and (B) individual numbers of population doublings in passages 0–20. Thick black lines indicate the means.
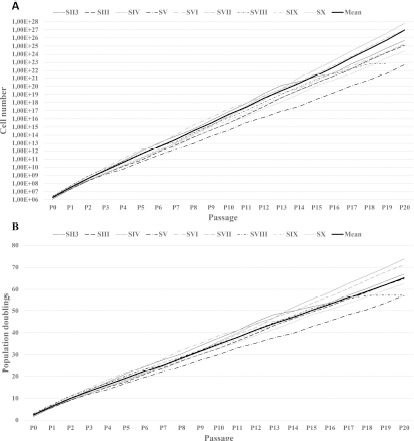
The average number of population doublings for every donor during ~160 days of culture until P20 was 65 (Figure 1B).
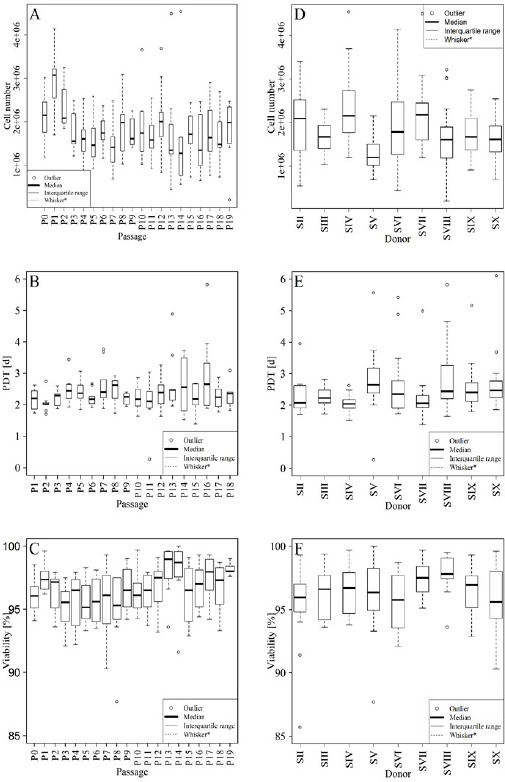
The average population doubling time was 2.5 days (range 2.0–3.0), and the average cell viability was 96.3%. These parameters were similar among donors and constant over the entire culture period (Figure 2A-F).
Figure 2 A-F: Cell numbers (A and D), population doubling time (B and E), and cell viability (C and F). Values of passages 0 – 19 (A – C) and donors SII3 – SX (D – F). The boxplots show the median (horizontal, center line), the interquartile range (box limits) and the outermost values within the interval of 1.5 times the interquartile range above and below the box (represented by the length of the whiskers). If no values are within this interval, then the interval itself is taken. Each measure outside of this range is considered an outlier and marked as a circle (○).S (II-X) = Donor (II-X); P (0–19) = Passage (0–19); PDT = Population doubling time.
Expression of cell surface markers
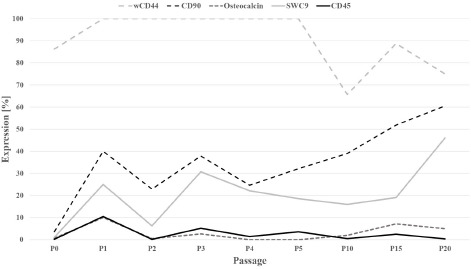
All BM-derived cells from P1 through P20 were positive for fibronectin, which confirms the validity of the immunostaining protocol. Initially, wCD44 expression reached 100% but decreased after P5 to approximately 65.5%.
The expression levels of the haematopoietic stem cell marker CD45 and osteocalcin, a late marker of osteogenesis, at all times amounted to 10.5% and ≤10%, respectively. In P0, the average expression levels of CD90 and SWC9 were 3.6% and 1.1%, respectively. With increasing culture time, the expression of both markers increased significantly (p < 0.005) to 60.5% and 46.0% at P20, respectively (Figure 3).
Figure 3: Expression of the cell surface markers wCD44, CD45, CD90, SWC9, and osteocalcin. P (0–5, 10, 15, 20) = Passage (0–5, 10, 15, 20).
Alkaline phosphatase activity
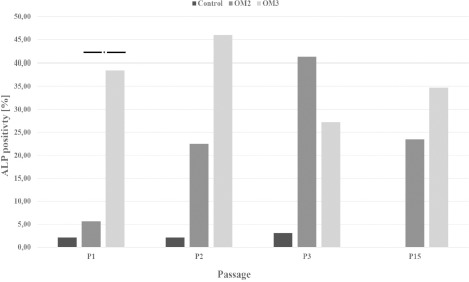
Porcine BM-derived cells of P0 through P3 showed very little ALP activity (0.2%–9.8%, mean: 2.4%). In the osteogenic induction assay (P1-P3 and P15), a more than 10-fold increase in ALP activity (5.6%– 45.9%, mean: 29.9%) compared to that in uninduced control cultures was noted. Except for P3, ALP activity was higher in cultures induced with OM3 than in cultures induced with OM2 or OM1, although this difference was statistically significant only for P1 (Figure 4).
Figure 4: ALP expression of cells at passages 1–3 and 15 after cultivation with OM2 and OM3. P (1–3, 15) = Passage (1–3, 15), OM = Osteogenic medium; ALP = Alkaline phosphatase;* = p-value < 0.05.
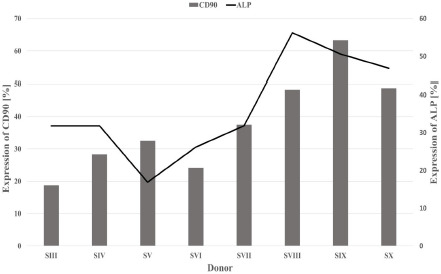
A positive correlation was identified between CD90 expression by uninduced pMSCs and ALP activity after osteogenic induction with OM3 (Pearson r = 0.76) (Figure 5).
Figure 5: CD90 expression by uninduced cells (bars) and ALP expression after osteogenic induction with OM3 (line) were positively correlated (Pearson r = 0.76). S (III – X) = Donor (III – X); ALP = Alkaline phosphatase.
Multilineage potential
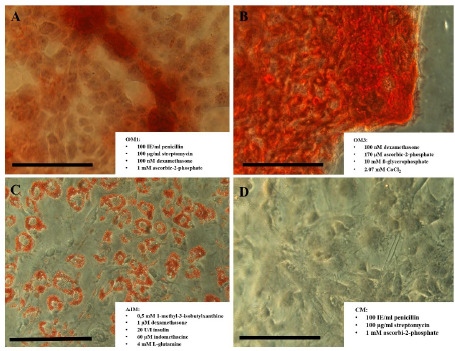
pMSCs showed multilineage potential after 14 days of osteogenic or adipogenic induction, as demonstrated by the presence of extracellular calcium deposits or intracellular lipid vesicles, respectively (Figure 6).
Figure 6: Representative light microscopic images (original magnification x32) of Alizarin Red S-stained calcium aggregates (A, B) and Oil Red O- stained intracellular lipid vesicles (C) produced by pMSCs after 14 days of induction in OM2 (A), OM3 (B), or AIM2/AMM2 (C). Cells cultivated in culture media without induction factors (D). All media contained DMEM supplemented with 10% FCS. The black bar represents 100 μm. OM2/3 = Osteogenic medium 2/3; AIM2/AMM2 = Adipogenic induction medium 2/adipogenic maintenance medium 2; FCS = Foetal calf serum; DMEM = Dulbecco’s modified Eagle’s medium.
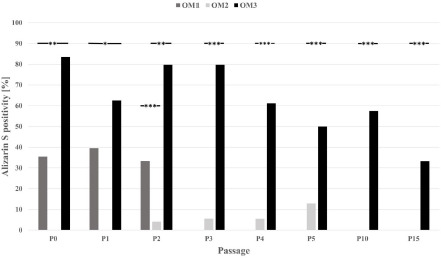
Cells cultured with culture medium only and without induction factors (controls) did not form calcium complexes or intracellular lipid vesicles. Osteogenic induction with OM3 yielded a significantly stronger response than that with OM1 and OM2 (Mann–Whitney-U- Test; p < 0.05), which decreased significantly during the cultivation period but was present up to P15 (Figure 7).
Figure 7: Results of Alizarin Red S staining after cultivation of pMSCs with the three different osteogenic media. In all passages, osteogenic induction with OM3 resulted in a significantly stronger response. The osteogenic phenotype was also elicited in high passages.* = p-value < 0.05; ** = p-value < 0.01; *** = p-value < 0.001 P (0–5, 10, 15) = Passage (0–5, 10, 15); OM = Osteogenic medium.
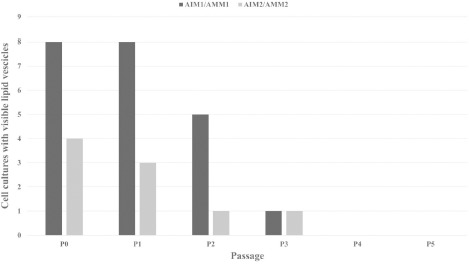
Adipogenic differentiation decreased significantly with increasing cultivation time in P2 and was absent after P3 (Figure 8).
Figure 8: Cell cultures (SIII - SX) with identifiable Oil Red O-positive intracellular lipid vesicles after culturing in AIM1/AMM1 or AIM2/AMM2. AIM = Adipogenic induction medium; AMM = Adipogenic maintenance medium. P (0–5) = Passage (0–5).
Discussion
Porcine MSCs were characterized according to established protocols[24] by their plastic adherence and fibroblast-like morphology, their ability to differentiate into at least two mesenchymal phenotypes (e.g., the osteogenic and adipogenic phenotypes) and their immunohistochemical phenotypes.
Cells did not express the haematopoietic stem cell marker CD45 but expressed wCD44, a widely used marker for MSCs[26,39]. In the primary cultures, the number of CD90-positive cells was very low and increased during the cultivation period, reflecting the initial heterogeneity of the cell populations with probably small numbers of stem cells and an increase in number and/or in osteogenic potential during culture expansion. While CD90 expression increased, wCD44 expression showed a decline after passage 5. Early passages have been suggested to contain stem cells and progenitor cells that fulfil many, if not all, MSC criteria; however, through expansion by subcultivation, they lose some of their multipotency[32].
We therefore hypothesize that the simultaneous increase in CD90 expression and decline in wCD44 expression may result from the commitment of initially multipotent BM-MSCs to the osteogenic lineage with increasing in vitro cultivation time, thus losing stem cell characteristics associated with the expression of wCD44[27]. This hypothesis is supported by the loss of adipogenic differentiation capacity observed in induced cell cultures after passage 3.
The increase in SWC9 expression in late passages was puzzling. As freshly isolated SWC9-negative blood monocytes express SWC9 during culturing[31], this may also apply to leukocyte subpopulations in this study, which demonstrated plastic adherence[40] and are therefore not removed during the first steps of MSC isolation. These cells must be highly proliferative to expand during subculturing. Conceivably, these cells accounted for an increasing share of the entire cell population during late passages after MSCs lost some of their stemness, as described above.
To the best of our knowledge, this is the first study to examine the proliferation and differentiation potential of BM-derived porcine MSCs over an extended in vitro cultivation period of ~ 160 days up to passage 20, with an average of 65 population doublings. Overall cell proliferation remained constant throughout the study for all donors with only a few intra- and interindividual exceptions. These results cannot be explained by donor age but rather by the presence of MSC subpopulations with variably well-preserved telomerase activity, as suggested by Baxter et al.[16], reflecting different developmental stages of MSCs[41]. ALP expression is an early marker of osteogenic differentiation and can be detected as early as two days after osteogenic induction[33]. The low expression of ALP in BM-derived cells of the proliferation assays and in uninduced controls of the osteogenic induction assays was not surprising, as it reflects the presence of osteoprogenitor cells and the absence of differentiating osteogenic cells in pMSC cultures. The marked increase in ALP activity after 14 days of osteogenic induction indicates osteogenic differentiation in the presence of preosteoblasts[33]. While ALP expression was not consistently different between the induction media, except for P1, Alizarin S staining was significantly stronger in calcium-rich assays in all examined passages. Alizarin S visualizes the formation of extracellular calcium deposits, i.e., mineralization of extracellular matrix, by differentiating cells during the late stages of osteogenic differentiation[33].
Differentiation assays were conducted for 14 days. At this time, cells cultured in either OM2 or OM3 had entered osteogenic differentiation, expressing elevated levels of ALP. Cells cultured in calcium-rich induction media (OM3) seemed to differentiate along the osteogenic cascade faster (data not shown) and more efficiently, as reflected by stronger Alizarin S staining.
MSCs have been suggested to lose not only their multilineage potential after extended in vitro cultivation[19] but also their capacity for osteogenic differentiation[17]. In this study, the potential for adipogenic differentiation was lost in passage 3. However, the osteogenic phenotype, as reflected by increased ALP activity and extracellular calcium aggregation, could be induced up to passage 15, particularly when an induction medium with a high calcium concentration was used. Although a positive influence of high extracellular calcium concentrations on osteogenic differentiation has been demonstrated[42], the selective addition of calcium to culture media is not a routine technique. Apart from calcium, OM3 was also supplemented with β-glycerophosphate, a well-established substance in osteogenic media[43–45], which was lacking in OM2. The significantly stronger effect of OM3 compared to OM2 could be due to the elevated calcium concentration or the addition of β- glycerophosphate in OM3. However, OM3 also yielded significantly better results than OM1, which contained comparable amounts of β- glycerophosphate. The important differences between OM1 and OM3 were the elevated calcium concentration and a higher dose of ascorbic-2-phosphate in the latter. A beneficial effect of higher ascorbic-2-phosphate concentrations in osteogenic media has been suggested[46]. This, however, only seems to be the case for adipose tissue-derived MSCs. Ascorbic-2-phosphate concentrations in osteogenic media that exceed the concentration of 50 μM recommended by Pittenger et al.[43] and Jaiswal et al.[45] do not support or even impede extracellular calcium deposition by BM- MSCs[47]. We therefore conclude that the significantly stronger deposition of extracellular calcium by cells cultured in OM3 must result from the elevated calcium concentration.
Calcium is a component of extracellular minerals as well as a signalling molecule. Calcium-binding proteins are expressed during the differentiation of human BM-derived MSCs into osteoblasts[48]. Elevated calcium concentrations are osteoinductive by regulating osteoblast proliferation, aggregation, differentiation and activity[49] and by enhancing collagen type I synthesis[50].
In view of bone TE, identifying pMSC subpopulations with high osteogenic potential is desirable. CD90 expression has been linked to the ability of MSCs to form bone[25]. The strong positive correlation between CD90 expression by uninduced cells and ALP activity in osteogenically induced pMSCs observed in this study supports this association. Thus, we hypothesize that tissue engineering of bone might be enhanced by isolating and employing subpopulations rich in CD90-positive cells.
Conclusion
Long-term in vitro cultivation of BM-derived pMSCs up to passage 20 leads to high numbers of viable cells with maintained proliferation capacity. Adipogenic differentiation potential may be lost in higher passages. However, pMSCs retain their potential for osteogenic differentiation, which can be elicited using adequate induction media enriched with calcium. Selection of CD90-positive MSCs may enhance osteogenic capacities for bone TE.
Acknowledgments
The authors wish to acknowledge Gisela Otto and Arne Döhring for technical assistance and Natalie Duerkopp and Dr. Andreas Thomsen for critical input.
Glossary
List of Abbreviations
- AIM
Adipogenic Induction Medium
- ALP
Alkaline Phosphatase
- AMM
Adipogenic Maintenance Medium
- BM
Bone Marrow
- CM
Culture Medium
- MSC
Mesenchymal Stromal Cell
- pMSC
Porcine Mesenchymal Stromal Cell
- OM
Osteogenic Medium
Funding
This study was funded by a grant from the Federal Ministry of Economics and Labour, Grant # KF 0007001 UL7. The grant was awarded to establish a porcine model for bone tissue engineering.
Supplementary Information
Supplementary Table 1: Antibodies used to characterize cell populations.
References
- 1.Chung MT, Liu C, Hyun JS, Lo DD, Montoro DT, Hasegawa M, Li S, Sorkin M, Rennert R, Keeney M, Yang F, Quarto N, Longaker MT, Wan DC. CD90 (Thy-1)-Positive Selection Enhances Osteogenic Capacity of Human Adipose-Derived Stromal Cells. Tissue Eng Part A. 2013;19((7-8)):989–97. doi: 10.1089/ten.tea.2012.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8((4)):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 3.Chen XD, Qian HY, Neff L, Satomura K, Horowitz MC. Thy-1 antigen expression by cells in the osteoblast lineage. J Bone Miner Res. 1999;14((3)):362–75. doi: 10.1359/jbmr.1999.14.3.362. [DOI] [PubMed] [Google Scholar]
- 4.Yu G, Wu X, Dietrich MA, Polk P, Scott LK, Ptitsyn AA, Gimble JM. Yield and characterization of subcutaneous human adipose-derived stem cells by flow cytometric and adipogenic mRNA analyses. Cytotherapy. 2010;12((4)):538–46. doi: 10.3109/14653241003649528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tienthai P. The porcine sperm reservoir in relation to the function of hyaluronan. J Reprod Dev. 2015;61((4)):245–50. doi: 10.1262/jrd.2015-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woods A, Wang G, Beier F. Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J Cell Physiol. 2007;213((1)):1–8. doi: 10.1002/jcp.21110. [DOI] [PubMed] [Google Scholar]
- 7.Morath I, Hartmann TN, Orian-Rousseau V. CD44: More than a mere stem cell marker. Int J Biochem Cell Biol. 2016;81((Pt A)):166–73. doi: 10.1016/j.biocel.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 8.L Ramos T, Sánchez-Abarca LI, Muntión S, Preciado S, Puig N, López-Ruano G, Hernández-Hernández Á, Redondo A, Ortega R, Rodríguez C, Sánchez-Guijo F, del Cañizo C. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal. 2016;142 doi: 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khatri M, O’Brien TD, Chattha KS, Saif LJ. Porcine lung mesenchymal stromal cells possess differentiation and immunoregulatory properties. Stem Cell Res Ther. 2015;6 doi: 10.1186/s13287-015-0220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rheinländer A, Schraven B, Bommhardt U. CD45 in human physiology and clinical medicine. Immunol Lett. 2018. pp. 9622–32. [DOI] [PubMed]
- 11.Alvarez-Viejo M, Menendez-Menendez Y, Blanco-Gelaz MA, Ferrero-Gutierrez A, Fernandez-Rodriguez MA, Gala J, Otero-Hernandez J. Quantifying mesenchymal stem cells in the mononuclear cell fraction of bone marrow samples obtained for cell therapy. Transplant Proc. 2013;45((1)):434–9. doi: 10.1016/j.transproceed.2012.05.091. [DOI] [PubMed] [Google Scholar]
- 12.Brückner S, Tautenhahn H-M, Winkler S, Stock P, Jonas S, Dollinger M, Christ B. Isolation and hepatocyte differentiation of mesenchymal stem cells from porcine bone marrow--“surgical waste” as a novel MSC source. Transplant Proc. 2013;45((5)):2056–8. doi: 10.1016/j.transproceed.2013.01.101. [DOI] [PubMed] [Google Scholar]
- 13.McCullough KC, Basta S, Knötig S, Gerber H, Schaffner R, Kim YB, Saalmüller A, Summerfield A. Intermediate stages in monocyte– macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology. 1999;98((2)):203–12. doi: 10.1046/j.1365-2567.1999.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saalmüller A, Pauly T, Lunney J, Boyd P, Aasted B, Sachs D, Arn S, Bianchi A, Binns R, Licence S, Whyte A, Blecha F, Chen Z, Chu R, Davis W, Denham S, Yang H, Whittall T, Parkhouse R, Dominguez J, Ezquerra A, Alonso F, Horstick G, Howard C, Sopp P, Kim Y, Lipp J, Mackay C, Magyar A, McCullough K, Arriens A, Summerfield A, Murtaugh M, Nielsen J, Novikov B, Pescovitz M, Schuberth H, Leibold W, Schütt C, Shimizu M, Stokes C, Haverson K, Bailey M, Tlaskalova H, Trebichavsky I, Valpotic I, Walker J, Lee R, Zuckermann F. Overview of the Second International Workshop to define swine cluster of differentiation (CD) antigens. Vet Immunol Immunopathol. 1998;60((3-4)):207–28. doi: 10.1016/s0165-2427(97)00098-6. doi:10.1016/s0165-2427(97)00098-6. [DOI] [PubMed] [Google Scholar]
- 15.McCullough KC, Schaffner R, Natale V, Kim YB, Summerfield A. Phenotype of porcine monocytic cells: modulation of surface molecule expression upon monocyte differentiation into macrophages. Vet Immunol Immunopathol. 1997;58((3-4)):265–75. doi: 10.1016/s0165-2427(97)00045-7. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Ni H. Fibronectin maintains the balance between hemostasis and thrombosis. Cell Mol Life Sci. 2016;73((17)):3265–77. doi: 10.1007/s00018-016-2225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura A, Dohi Y, Akahane M, Ohgushi H, Nakajima H, Funaoka H, Takakura Y. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng Part C Methods. 2009;15((2)):169–80. doi: 10.1089/ten.tec.2007.0334. [DOI] [PubMed] [Google Scholar]
References
- 1.Hu C, Zhao L, Wu Z, Li L. Transplantation of mesenchymal stem cells and their derivatives effectively promotes liver regeneration to attenuate acetaminophen-induced liver injury. Stem Cell Res Ther. 2020;11((1)):88. doi: 10.1186/s13287-020-01596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, Li X, Zhu W, Zhang Y, Hong Y, Liang X, Fan B, Zhao H, He H, Zhang F. Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. J Cell Physiol. 2020;235((11)):8010–22. doi: 10.1002/jcp.29456. [DOI] [PubMed] [Google Scholar]
- 3.Probst FA, Fliefel R, Burian E, Probst M, Eddicks M, Cornelsen M, Riedl C, Seitz H, Aszódi A, Schieker M, Otto S. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci Rep. 2020;10((1)):2062. doi: 10.1038/s41598-020-59038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujii S, Miura Y, Fujishiro A, Shindo T, Shimazu Y, Hirai H, Tahara H, Takaori-Kondo A, Ichinohe T, Maekawa T. Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Is Associated with Peripheral Preservation of Naive T Cell Populations. Stem Cells. 2018;36((3)):434–45. doi: 10.1002/stem.2759. [DOI] [PubMed] [Google Scholar]
- 5.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29((12)):747–54. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barczewska M, Jezierska-Wozniak K, Habich A, Lipinski S, Holak P, Maksymowicz W, Wojtkiewicz J. Evaluation of regenerative processes in the pig model of intervertebral disc degeneration after transplantation of bone marrow-derived mesenchymal stem cells. Folia Neuropathol. 2018;56((2)):124–32. doi: 10.5114/fn.2018.76616. [DOI] [PubMed] [Google Scholar]
- 7.Tevlin R, Walmsley GG, Marecic O, Hu MS, Wan DC, Longaker MT. Stem and progenitor cells: advancing bone tissue engineering. Drug Deliv Transl Res. 2016;6((2)):159–73. doi: 10.1007/s13346-015-0235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernigou P, Dubory A, Pariat J, Potage D, Roubineau F, Jammal S, Flouzat Lachaniette CH. Beta-tricalcium phosphate for orthopedic reconstructions as an alternative to autogenous bone graft. Morphologie. 2017;101((334)):173–9. doi: 10.1016/j.morpho.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Agacayak S, Gulsun B, Ucan MC, Karaoz E, Nergiz Y. Effects of mesenchymal stem cells in critical size bone defect. Eur Rev Med Pharmacol Sci. 2012;16((5)):679–86. [PubMed] [Google Scholar]
- 10.Shapiro SA, Kazmerchak SE, Heckman MG, Zubair AC, O'Connor MI. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am J Sports Med. 2017;45((1)):82–90. doi: 10.1177/0363546516662455. [DOI] [PubMed] [Google Scholar]
- 11.Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32((5)):1254–66. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 12.Götherström C, Westgren M, Shaw SWS, Aström E, Biswas A, Byers PH, Mattar CNZ, Graham GE, Taslimi J, Ewald U, Fisk NM, Yeoh AEJ, Lin J-L, Cheng P-J, Choolani M, Le Blanc K, Chan JKY. Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: a two-center experience. Stem Cells Transl Med. 2014;3((2)):255–64. doi: 10.5966/sctm.2013-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oryan A, Kamali A, Moshiri A, Baghaban Eslaminejad M. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells, tissues, organs. 2017;204((2)):59–83. doi: 10.1159/000469704. [DOI] [PubMed] [Google Scholar]
- 14.Choi Y, Yoon DS, Lee K-M, Choi SM, Lee M-H, Park KH, Han SH, Lee JW. Enhancement of Mesenchymal Stem Cell-Driven Bone Regeneration by Resveratrol-Mediated SOX2 Regulation. Aging and disease. 2019;10((4)):818–33. doi: 10.14336/AD.2018.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Wong WH-S, Chan S, Chim JC-S, Cheung KM-C, Lee T-L, Au W-Y, Ha S-Y, Lie AK-W, Lau Y-L, Liang RH-S, Chan GC-F. Factors affecting mesenchymal stromal cells yield from bone marrow aspiration. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2011;23((1)):43–8. doi: 10.1007/s11670-011-0043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22((5)):675–82. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 17.Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005;205((2)):194–201. doi: 10.1002/jcp.20376. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y-HK, Ogando CR, Wang See C, Chang T-Y, Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9((1)):131. doi: 10.1186/s13287-018-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banfi A, Bianchi G, Notaro R, Luzzatto L, Cancedda R, Quarto R. Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng. 2002;8((6)):901–10. doi: 10.1089/107632702320934001. [DOI] [PubMed] [Google Scholar]
- 20.Brückner S, Tautenhahn H-M, Winkler S, Stock P, Dollinger M, Christ B. A fat option for the pig: hepatocytic differentiated mesenchymal stem cells for translational research. Exp Cell Res. 2014;321((2)):267–75. doi: 10.1016/j.yexcr.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Groth A, Ottinger S, Kleist C, Mohr E, Golriz M, Schultze D, Bruns H, Mehrabi A, Schemmer P, Büchler MW, Herr I. Evaluation of porcine mesenchymal stem cells for therapeutic use in human liver cancer. Int J Oncol. 2012;40((2)):391–401. doi: 10.3892/ijo.2011.1217. [DOI] [PubMed] [Google Scholar]
- 22.Noort WA, Oerlemans MIFJ, Rozemuller H, Feyen D, Jaksani S, Stecher D, Naaijkens B, Martens AC, Bühring HJ, Doevendans PA, Sluijter JPG. Human versus porcine mesenchymal stromal cells: phenotype, differentiation potential, immunomodulation and cardiac improvement after transplantation. J Cell Mol Med. 2012;16((8)):1827–39. doi: 10.1111/j.1582-4934.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatri M, O’Brien TD, Chattha KS, Saif LJ. Porcine lung mesenchymal stromal cells possess differentiation and immunoregulatory properties. Stem Cell Res Ther. 2015;6 doi: 10.1186/s13287-015-0220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8((4)):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 25.Chung MT, Liu C, Hyun JS, Lo DD, Montoro DT, Hasegawa M, Li S, Sorkin M, Rennert R, Keeney M, Yang F, Quarto N, Longaker MT, Wan DC. CD90 (Thy-1)-Positive Selection Enhances Osteogenic Capacity of Human Adipose-Derived Stromal Cells. Tissue Eng Part A. 2013;19((7-8)):989–97. doi: 10.1089/ten.tea.2012.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R, Jia W, Zou H, Wang X, Ren Y, Zhao J, Wang L, Li M, Qi Y, Shen Y, Liang W, Jiang J, Sun Z, Pang L, Li F. Expression of CD44 and CD29 by PEComa cells suggests their possible origin of mesenchymal stem cells. Int J Clin Exp Pathol. 2015;8((10)):13023–33. [PMC free article] [PubMed] [Google Scholar]
- 27.Morath I, Hartmann TN, Orian-Rousseau V. CD44: More than a mere stem cell marker. Int J Biochem Cell Biol. 2016;81((Pt A)):166–73. doi: 10.1016/j.biocel.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Rheinländer A, Schraven B, Bommhardt U. CD45 in human physiology and clinical medicine. Immunol Lett. 2018;196:22–32. doi: 10.1016/j.imlet.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Brückner S, Tautenhahn H-M, Winkler S, Stock P, Jonas S, Dollinger M, Christ B. Isolation and hepatocyte differentiation of mesenchymal stem cells from porcine bone marrow--“surgical waste” as a novel MSC source. Transplant Proc. 2013;45((5)):2056–8. doi: 10.1016/j.transproceed.2013.01.101. [DOI] [PubMed] [Google Scholar]
- 30.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32((5)):414–25. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 31.McCullough KC, Basta S, Knötig S, Gerber H, Schaffner R, Kim YB, Saalmüller A, Summerfield A. Intermediate stages in monocyte–macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology. 1999;98((2)):203–12. doi: 10.1046/j.1365-2567.1999.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, Nolta J, Phinney DG, Sensebe L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21((10)):1019–24. doi: 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Kannan S, Ghosh J, Dhara SK. Osteogenic differentiation potential of porcine bone marrow mesenchymal stem cell subpopulations selected in different basal media. Biol Open. 2020;9((10)) doi: 10.1242/bio.053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komori T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int J Mol Sci. 2020;21((20)) doi: 10.3390/ijms21207513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pittenger MF. Mesenchymal stem cells from adult bone marrow. Methods Mol Biol. 2008;449:27–44. doi: 10.1007/978-1-60327-169-1_2. [DOI] [PubMed] [Google Scholar]
- 36.Gassling V, Hedderich J, Açil Y, Purcz N, Wiltfang J, Douglas T. Comparison of platelet rich fibrin and collagen as osteoblast-seeded scaffolds for bone tissue engineering applications. Clin Oral Implants Res. 2013;24((3)):320–8. doi: 10.1111/j.1600-0501.2011.02333.x. [DOI] [PubMed] [Google Scholar]
- 37.Thomsen AR, Aldrian C, Bronsert P, Thomann Y, Nanko N, Melin N, Rücker G, Follo M, Grosu AL, Niedermann G, Layer PG, Heselich A, Lund PG. A deep conical agarose microwell array for adhesion independent three-dimensional cell culture and dynamic volume measurement. Lab Chip. 2017;18((1)):179–89. doi: 10.1039/c7lc00832e. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen HD, Mikkelsen LF. Göttingen Minipigs as Large Animal Model in Toxicology. In: Biomarkers in Toxicology: Elsevier; 2019. p. 75–89.
- 39.Mas A, Prusinski L, Yang Q, Diaz-Gimeno P, Stone L, Diamond MP, Simón C, Al-Hendy A. Role of Stro1+/CD44+ stem cells in myometrial physiology and uterine remodeling during pregnancy†. Biol Reprod. 2016;96((1)):70–80. doi: 10.1095/biolreprod.116.143461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nielsen MC, Andersen MN, Møller HJ. Monocyte isolation techniques significantly impact the phenotype of both isolated monocytes and derived macrophages in vitro. Immunology. 2020;159((1)):63–74. doi: 10.1111/imm.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pochampally R. Colony forming unit assays for MSCs. Methods Mol Biol. 2008;449:83–91. doi: 10.1007/978-1-60327-169-1_6. [DOI] [PubMed] [Google Scholar]
- 42.Viti F, Landini M, Mezzelani A, Petecchia L, Milanesi L, Scaglione S. Osteogenic Differentiation of MSC through Calcium Signaling Activation: Transcriptomics and Functional Analysis. PLoS ONE. 2016;11((2)) doi: 10.1371/journal.pone.0148173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284((5411)):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 44.Ringe J, Kaps C, Schmitt B, Büscher K, Bartel J, Smolian H, Schultz O, Burmester GR, Häupl T, Sittinger M. Porcine mesenchymal stem cells. Induction of distinct mesenchymal cell lineages. Cell Tissue Res. 2002;307((3)):321–7. doi: 10.1007/s00441-002-0525-z. [DOI] [PubMed] [Google Scholar]
- 45.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64((2)):295–312. [PubMed] [Google Scholar]
- 46.Kyllönen L, Haimi S, Mannerström B, Huhtala H, Rajala KM, Skottman H, Sándor GK, Miettinen S. Effects of different serum conditions on osteogenic differentiation of human adipose stem cells in vitro. Stem Cell Res Ther. 2013;4((1)):17. doi: 10.1186/scrt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi K-M, Seo Y-K, Yoon H-H, Song K-Y, Kwon S-Y, Lee H-S, Park J-K. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J Biosci Bioeng. 2008;105((6)):586–94. doi: 10.1263/jbb.105.586. [DOI] [PubMed] [Google Scholar]
- 48.Carlier A, Chai YC, Moesen M, Theys T, Schrooten J, van Oosterwyck H, Geris L. Designing optimal calcium phosphate scaffold-cell combinations using an integrative model-based approach. Acta Biomater. 2011;7((10)):3573–85. doi: 10.1016/j.actbio.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 49.Chai YC, Roberts SJ, Schrooten J, Luyten FP. Probing the osteoinductive effect of calcium phosphate by using an in vitro biomimetic model. Tissue Eng Part A. 2011;17((7-8)):1083–97. doi: 10.1089/ten.TEA.2010.0160. [DOI] [PubMed] [Google Scholar]
- 50.Eklou-Kalonji E, Denis I, Lieberherr M, Pointillart A. Effects of extracellular calcium on the proliferation and differentiation of porcine osteoblasts in vitro. Cell Tissue Res. 1998;292((1)):163–71. doi: 10.1007/s004410051046. [DOI] [PubMed] [Google Scholar]


