Abstract
The human hippocampus is involved in forming new memories: damage impairs memory. The dual stream model suggests that object “what” representations from ventral stream temporal cortex project to the hippocampus via the perirhinal and then lateral entorhinal cortex, and spatial “where” representations from the dorsal parietal stream via the parahippocampal gyrus and then medial entorhinal cortex. The hippocampus can then associate these inputs to form episodic memories of what happened where. Diffusion tractography was used to reveal the direct connections of hippocampal system areas in humans. This provides evidence that the human hippocampus has extensive direct cortical connections, with connections that bypass the entorhinal cortex to connect with the perirhinal and parahippocampal cortex, with the temporal pole, with the posterior and retrosplenial cingulate cortex, and even with early sensory cortical areas. The connections are less hierarchical and segregated than in the dual stream model. This provides a foundation for a conceptualization for how the hippocampal memory system connects with the cerebral cortex and operates in humans. One implication is that prehippocampal cortical areas such as the parahippocampal TF and TH subregions and perirhinal cortices may implement specialized computations that can benefit from inputs from the dorsal and ventral streams.
Keywords: diffusion tractography, entorhinal cortex, hippocampal connections, hippocampus, memory, parahippocampal cortex
Introduction
The human hippocampus is essential for memory function, with major disorders in at least forming new episodic and semantic memory produced by damage to it (Corkin 2002; Maguire et al. 2016; Clark et al. 2019). To understand how the hippocampus is involved in memory, and its disorders, we need to know its connections to other brain areas, and especially to the cerebral cortex (Aggleton 2012; Rolls 2018, 2021b). Research in animals has led to the dual stream model, in which ventral cortical processing streams concerned with representations of “what” object or person is present reach the hippocampus by the perirhinal and then lateral entorhinal cortex; and the dorsal cortical stream concerned with “where” representations of spatial locations is connected via the parahippocampal gyrus and medial entorhinal cortex with the hippocampus (Van Hoesen 1982; Suzuki and Amaral 1994; Burwell et al. 1995; Burwell 2000; Knierim et al. 2014; Doan et al. 2019) (shown in schematic and simplified form in Fig. 1). Then, in the CA3 part of the hippocampus, where there is a single network of neurons, what and where streams can be combined to form a memory of what happened where (Kesner and Rolls 2015; Rolls 2018, 2021b). That memory can then be recalled using a partial retrieval cue to the neocortex by corresponding hierarchically organized and segregated backprojection pathways from the hippocampus (Treves and Rolls 1994; Rolls 2018, 2021b).
Figure 1 .
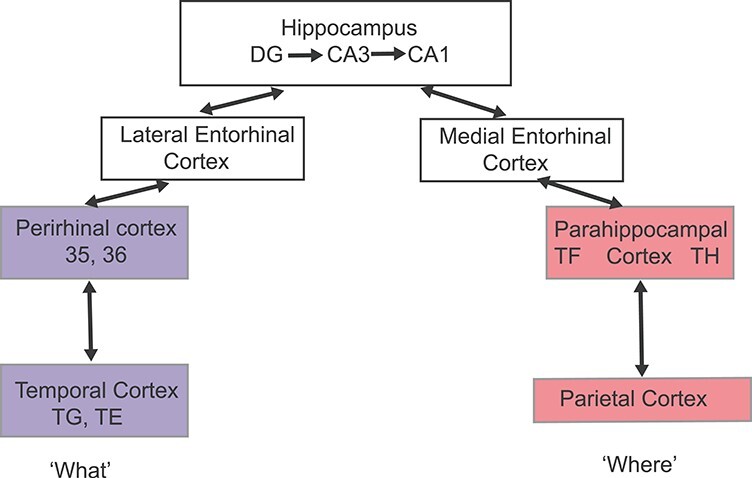
A simplified, schematic, conceptual diagram of the dual stream model of hippocampal connectivity. Neuroanatomical tract-tracing in animals suggests that spatial, “where,” representations from the dorsal stream parietal cortex project via the parahippocampal gyrus and then medial entorhinal cortex to the hippocampus, and that “what” object representations from the ventral stream temporal cortex project via the perirhinal cortex and lateral entorhinal cortex to the hippocampus (Van Hoesen and Pandya 1975; Van Hoesen 1982; Amaral et al. 1983; Burwell et al. 1995; Saleem and Tanaka 1996; Burwell 2000; Aggleton 2012; Knierim et al. 2014; Nilssen et al. 2019). It is suggested that the CA3 neurons of the hippocampus with their highly developed associative recurrent collateral connections can then associate the inputs to form object-location episodic memories of what happened where on a particular occasion (Kesner and Rolls 2015; Rolls 2018). In macaques, the connections are known to be more extensive than shown here, as described in the Introduction.
In macaques, some hippocampal connections have been described that are not included in the conceptual dual stream model shown in Figure 1. For example, in macaques, the hippocampal CA1 region has some direct connections to the cortex in the anterior part of the superior temporal sulcus (Zhong et al. 2005) and to the orbitofrontal and anterior cingulate cortex (Cavada et al. 2000; Zhong et al. 2006; Morecraft et al. 2012). It has further been reported in macaques that CA1 neurons have direct connections to a number of temporal cortical areas, including the posterior parahippocampal (areas TF and TH), perirhinal (areas 35 and 36) (Insausti and Munoz 2001), and ventral inferotemporal areas (areas TEav and TEpv) (Yukie 2000), with connections from CA1 also to the temporal pole (TG) and subiculum (Blatt and Rosene 1998), to the pregenual cingulate cortex (Insausti and Munoz 2001), and to anteroventral TE (Zhong and Rockland 2004; Ichinohe and Rockland 2005) and the orbitofrontal cortex (Zhong et al. 2006). Moreover, direct projections to CA1 in macaques have been reported from areas 7a and 7b, area TF, and a region in the occipitotemporal sulcus (Rockland and Van Hoesen 1999; Ding et al. 2000); and from superior temporal sulcus, the rostral and retrosplenial portions of the cingulate cortex, the agranular insular cortex, and the caudal orbitofrontal cortex (Suzuki and Amaral 1990); and also from anteroventral TE (Zhong and Rockland 2004). The presubiculum has connections to the cortex in the superior temporal sulcus, the lateral orbitofrontal cortex, and the temporal pole (Insausti and Munoz 2001). [In rodents, CA1 projects to the retrosplenial cingulate cortex (Haugland et al. 2019) and hypothalamus as well as the midline thalamic nuclei (Cenquizca and Swanson 2006).] In rodents, division of the entorhinal cortex into medial and lateral components as illustrated in Figure 1 is usual, but in at least primates and perhaps rodents too, there may be less segregation than is implied by Figure 1 (Insausti et al. 2017; Nilssen et al. 2019). Thus, reality is more complex at least in primates than the simple dual stream model, as has been recognized (Kravitz et al. 2011, 2013; Knierim et al. 2014; Milner 2017).
A key aim of the present investigation is to obtain evidence on the direct connections between the human hippocampal system and other cortical areas, as this is fundamental to understanding how the human hippocampus operates in health and disease (Rolls 2021b).
Key questions we asked in humans were as follows. Is the hippocampal system mainly hierarchical, with the entorhinal cortex a gateway to and from the hippocampus, and direct connections mainly between each of the series of stages, as shown in Figure 1? Do the what (temporal lobe) and where (parietal lobe) pathways (Ungerleider and Haxby 1994; Ungerleider 1995) remain segregated through the entorhinal cortex until they reach the hippocampus, as in the simple dual stream model? What are the connections of each of the hippocampal system-related areas in humans? What are the anatomical connections of the hippocampal system with the very highly developed ventral and dorsal visual stream processing areas in humans? These questions are essential for understanding the mechanisms of operation of the hippocampal memory system in humans. The nine areas we investigated were the hippocampus, subiculum, presubiculum, entorhinal cortex, perirhinal cortex, and parahippocampal gyrus [area TF, and TH in terms of three subregions of parahippocampal area (PHA1–3)] (Fig. 2).
Figure 2 .
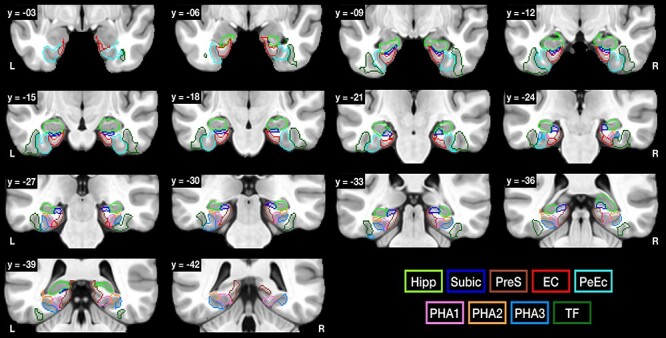
The hippocampal, parahippocampal, and related regions of interest as defined in the HCP atlas (Glasser et al. 2016a) that were used as seed regions of interest (ROIs) for the diffusion tractography. EC, entorhinal cortex; Hipp, hippocampus; PeEc, perirhinal cortex; PHA1–3, parahippocampal gyrus areas 1–3; TF, parahippocampal area TF; PreS, presubiculum; Subic, subiculum. For the hippocampus and subiculum, the templates were from Winterburn et al. (2013). The y values of these coronal slices are in MNI coordinates. R indicates right hemisphere.
Here, we describe a large-scale new investigation of human hippocampal system connections using diffusion tractography imaging in >170 participants in the Human Connectome Project (HCP) between nine hippocampal system regions and 360 cortical areas defined in the HCP atlas (Glasser et al. 2016a; Huang et al. 2021). The use of this atlas was important for the research described here, because it utilizes a multimodal approach to the parcellation of the cerebral cortex and defines a large number of anatomical areas in visual and related cortex, many of which in addition have been functionally defined (Glasser et al. 2016a). We show that the hippocampus has direct connections with many cortical areas beyond the entorhinal (and even perirhinal and parahippocampal) cortex, consistent with the hypothesis that in humans these areas are not the exclusive gateway with the hippocampus. Instead, the hippocampus communicates directly with some what (temporal cortex) and where (parietal and posterior cingulate) cortical areas, and even early sensory cortical areas, which may facilitate the recall of memories with sensory detail. We show that lateral parahippocampal gyrus area TF is, with the perirhinal cortex, strongly connected with the hippocampus and with what ventral stream/temporal lobe cortical areas, leading to a reevaluation of the functions of the parahippocampal gyrus. We show that medial parahippocampal gyrus area TH is strongly linked with the hippocampus and dorsal visual stream, parietal, and posterior cingulate cortical areas in the where visual system. These visual streams are so highly developed in primates compared with rodents (Rolls 2021b) that a key aim of the investigation described here was to examine the connections of the hippocampus with both these visual streams in humans. We also wished to investigate connections between these perirhinal what/parahippocampal where stages of processing found in humans, and this also led to an evaluation for humans of the dual stream hierarchical and segregated processing streams to and from the hippocampus. These investigations, and the strong connections with human anterior temporal lobe areas involved in semantic memory, lead to new concepts about how the hippocampal system operates in memory in humans.
Although the extensive direct connections of the hippocampus in humans revealed here may seem surprising, there is supporting evidence from tract-tracing studies in nonhuman primates (Amaral et al. 1983; Suzuki and Amaral 1994; Saleem and Tanaka 1996; Yukie 2000; Zhong et al. 2006; Aggleton 2012; Nilssen et al. 2019), as described above. The support from the primate evidence is especially relevant because in primates there is also a highly developed visual system with ventral stream temporal lobe areas involved in object and face recognition, and dorsal stream parietal and posterior cingulate cortex areas involved in spatial function (Boussaoud et al. 1990; Ungerleider and Haxby 1994; Ungerleider 1995; Rolls 2021b), which both reach the hippocampus and are implicated in what and where episodic memory (Kesner and Rolls 2015; Rolls 2018; Rolls and Wirth 2018).
It is noted that diffusion tractography provides evidence on direct connections, but not on the direction of connections, and is measured by the streamlines described in this paper; that functional connectivity based on correlations between the BOLD signals in different brain areas implies an influence between the brain areas that need not be direct; and that effective connectivity based on delays between the BOLD signals in different brain areas may provide evidence on the direction of the influence of one brain area on another, but does not prove a direct connection (Cheng et al. 2016; Rolls et al. 2018; Rolls 2021b). These methods thus complement each other, and for this reason, cross-reference is made to findings on functional and effective connectivity in the same HCP participants in this paper.
Methods
The definition of regions in the HCP atlas is shown in Glasser et al. (2016a) (Glasser_2016_Table.xlsx). A list of these regions is provided in Supplementary Table S1, and in Supplementary Figure S1 we show coronal slices with labels for the regions defined in the HCP atlas.
In this investigation, the nine seed regions of interest (ROIs) for diffusion tractography were the Hippocampus (Hipp), Subiculum (Subic), Presubiculum (PreS), Entorhinal Cortex (EC), Perirhinal Cortex (PeEc), and four parts of the Parahippocampal gyrus (TF, and PHA1–3 which correspond to TH), as shown in Figure 1. The regions were as defined in the HCP atlas (Glasser et al. 2016a), unless otherwise stated.
Of the three parahippocampal areas defined in the HCP atlas that correspond approximately to area TH, PHA1 is medial, PHA2 is dorsolateral, and PHA3 is ventrolateral. Parahippocampal area TF is lateral to TH (Fig. 1 and Supplementary Fig. S1). The hippocampus was redefined in this investigation to include more of the hippocampus than is included in the HCP hippocampal RegionID and to produce separate regions for the hippocampus and subiculum, using the hippocampal template defined by Winterburn et al. (2013), as described in the Supplementary Material.
Participants
A total of all the 178 individuals who had completed diffusion and structural scans were included from the subject pool included in the publicly available Wu-Minn HCP 7 T dataset. [No participants were excluded. There are 69 males and 109 females in the 178 participants, with ages mainly in the range 22–36 years. The participants were almost all the same in two related investigations, of functional (Ma et al. 2021) and effective (Rolls et al. 2021) connectivity of the human hippocampal system.] The preprocessed diffusion (dMRI) and T1-weighted (T1w) images of the 178 subjects were obtained by ConnectomeDB (https://db.humanconnectome.org) and analyzed in this investigation.
MRI Acquisition
Details of the 7 T diffusion and T1w image acquisition protocols are provided in the HCP reference manual (https://humanconnectome.org/study/hcp-young-adult/document/1200-subjects-data-release). The dMRI scans used a monopolar scheme with single-shot 2D spin-echo multiband (factor = 2) EPI acquisition, with the main parameters as follows: 1.05 mm isotropic voxel [field of view (FOV): 210 × 210 mm2, matrix size: 200 × 200], 132 transversal slices acquired in interleaved order without a gap, phase encoding applied along the anterior–posterior direction, phase encoding acceleration (GRAPPA) factor 3, two shells with b-values = 1000 (142), 2000 s/mm2 (Δ = 34 ms and δ = 14.3 ms), repetition time/echo time = 7000/71 ms, 65 unique diffusion gradient directions and 6 b0 images were obtained for each phase encoding direction pair (AP and PA pairs). The total scanning time for the dMRI protocol was about 40 min.
Brain Parcellation
To reconstruct the whole-brain structural connectome that included all the ROIs in this study, we combined the following two atlases: HCP’s multimodal parcellation (HCP-MMP v1.0), consisting of 179 regions per hemisphere (i.e., 180 minus the hippocampus, which was defined separately, as described next) (Glasser et al. 2016a) and eight subcortical regions (per hemisphere) from the CIT168 reinforcement learning atlas (Pauli et al. 2018), including the hippocampus (which replaced the original hippocampus in the HCP atlas), thalamus, caudate nucleus, putamen, globus pallidus external segment, globus pallidus internal segment, amygdala, and nucleus accumbens. These two atlases were both defined in the asymmetric Montreal Neurological Institute (MNI) space of ICBM152 2009c (Fonov et al. 2011). To distinguish the subiculum from the hippocampus, we used the subiculum mask provided in the CoBrALab atlas (Winterburn et al. 2013). Thus, the atlas used here consisted of 376 parcels (including the subiculum) covering the cerebral cortex and some subcortical regions. Spatial normalization between MNI space and the T1w image was computed using Advanced Normalization Tools (Avants et al. 2009) with rigid, affine, and symmetric diffeomorphic registration approaches (three-stage Syn). Further details of this extended and reordered HCP atlas, and the HCPex atlas itself, are provided elsewhere (Huang et al. 2021). The brain parcellation used in this study was warped to each participant’s native space using nearest neighbor interpolation for further reconstruction of the tractography and pair-wise structural connection matrix.
Diffusion MRI Preprocessing and Tractography
The preprocessing was performed as described by Glasser et al. (2013), based on the updated diffusion pipeline (v3.19.0), including basic preprocessing, distortion correction, eddy current correction, motion correction, gradient nonlinearity correction, and registration of the mean b0 image to native T1w images with FLIRT BBR + bbregister and transformation of dMRI. The brain mask of each subject is based on FreeSurfer segmentation. To reconstruct white matter (WM) tracts by using tractography imaging, T1w images were first segmented into five tissue types using 5TT (MRtrix3 command: “5ttgen” with option: “-sgm_amyg_hipp,” which represents the amygdala and hippocampus as subcortical structures), including cortical gray matter (GM), subcortical GM, WM, cerebrospinal fluid (CSF), and pathological tissue, in order to anatomically constrain the tractography terminations in GM. Whole-brain tractography was reconstructed for each subject in native space. A Multi-Shell Multi-Tissue Constrained Spherical Deconvolution (MSMT-CSD) model with lmax = 8 and prior coregistered 5TT image was used on the preprocessed multi-shell DWI data to obtain the fiber orientation distribution (FOD) function (Smith 2002; Jeurissen et al. 2014). Based on the voxel-wise fiber orientation distribution, anatomically constrained tractography (ACT) using the iFOD2 (second-order integration based on FOD) with dynamic seeding algorithm was applied to generate the initial tractogram (1 million streamlines with maximum tract length = 250 mm and minimal tract length = 5 mm). To quantify the number of streamlines connecting pairs of regions, the updated version of the spherical-deconvolution informed filtering of tractograms (SIFT2) method was applied, which provides more biologically meaningful estimates of structural connection density (Smith et al. 2015). To enhance the validity of the reconstructed fibers, the aforementioned 5TT was utilized as prior information during the tracking, and six mandatory rules were applied as follows: (1) a streamline was terminated and accepted when it entered GM; (2) a streamline was rejected if it entered CSF; (3) a streamline was terminated and accepted if it left the FOV or user-defined brain mask (this is necessary to permit tracts to include the spinal column); (4) a streamline was terminated and rejected when it reached a voxel with a very low FOD amplitude or showed excessive curving angle in the WM (with default threshold: FOD amplitude 0.05 and curve angle 45°); (5) a streamline was accepted when rule (4) applied within subcortical regions; and (6) a streamline was not allowed to exit subcortical GM and was truncated when it reached a minimum FOD amplitude within voxels of the subcortical GM (Smith et al. 2012). Whole-brain tractography was used for connectome reconstruction. To show the connection pattern of a ROI based on the voxel-to-voxel-level connections, we included all voxel-to-voxel streamlines that terminated in the ROI.
Great care was taken to minimize any problems that might produce false positives for tracts due to following the incorrect route at fiber crossings. It has been difficult with diffusion tensor models to resolve fiber crossings (Tournier et al. 2012). Previous studies had reported false positive tract-tracing in regions with ambiguous fanning and bending fiber populations (Jbabdi and Johansen-Berg 2011). Accordingly, in the present investigation, we used a different approach, MSMT-CSD based, ACT using the iFOD2 (second-order integration over fiber orientation distributions) (Gutierrez et al. 2020) with SIFT2 (Smith et al. 2015) to control false positive biases in tractography (Smith et al. 2012). The SIFT2 method applied in this study corrects biases by weighting the tractogram based on the overall distribution of the fiber orientation distribution (FOD) function across voxels, which improves the accuracy of connectome reconstruction and permits the use of streamline counts as a relative biologically meaningful marker (Smith et al. 2013, 2015; Sinke et al. 2018). Evidence that false positives related to fiber crossings were not in practice a limitation of the findings described here is provided in the Discussion. False negatives in tracking long-distance connections are still a limitation for this current method (Donahue et al. 2016), so we may have underreported some long-distance connections.
To reduce potential false positive tracts generated by seeding in only a few specific regions, whole-brain tractography was performed and the connection profiles of the ROIs were then extracted. With the native space atlas, we used the tck2connectome command to generate the connection matrix for whole-brain tractography, in which a 4 mm radial search was applied to each streamline endpoint to locate the nearest node. The number of streamline fibers between pairs of brain regions was measured across the 376 brain regions. This enabled us to produce a 9 × 376 matrix of the connections (per hemisphere) between each of the nine hippocampal formation ROIs and every other region in the HCP-MMP v1.0 and subcortical regions.
To establish the termination map of streamline tracts throughout the brain for individual subjects, we extracted the coordinates of every fiber termination identified for each of the nine ROIs and recorded the number of connections to obtain the voxel-wise connection patterns in native space. The native termination maps were than warped into the MNI standard space for further representation and statistical analyses. To demonstrate tractography maps (as shown in Fig. 3), we used the same tractography method (ACT + iFOD2 with option “-sgm_amyg_hipp”) with random seeding in the hippocampal ROI (200 seeds per voxel) to reconstruct streamlines of the ROI in a selected representative participant.
Figure 5 .
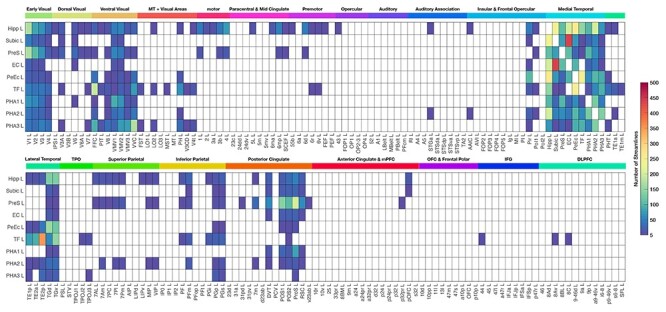
Streamline terminations of hippocampal formation regions with tractography for the left (L) hemisphere. The rows show the seed region of interest, and the columns show the regions in the HCP atlas, ordered and with the abbreviations shown in Supplementary Table S1. The brain regions are labeled on coronal slices of the brain in Supplementary Figure S1. The numbers of streamlines are shown without normalization in this figure, in order to allow assessment of the absolute magnitude of the numbers of streamlines. An average number of streamlines of <5 between any two regions is shown as blank in the connection matrix. Hipp, hippocampus; Subic, subiculum; PreS, presubiculum; EC, entorhinal cortex; PeEc, perirhinal cortex; TF, parahippocampal gyrus region TF; PHA1–3, parahippocampal gyrus TH subregions 1–3.
We note that diffusion tractography as a method for following connections and measuring their strength is supported by comparisons made in macaques between diffusion tractography and conventional anatomical methods with tracers (van den Heuvel et al. 2015; Donahue et al. 2016).
Statistics
To investigate the connection pattern of fiber projections from nine hippocampal formation seed ROI regions to each HCP-MMP atlas regions throughout the brain, we obtained the averaged map of fiber endpoints across 178 participants at the voxel level to demonstrate the possible fiber terminations from each seed ROI. Moreover, the nine seed ROIs’ connection patterns were also demonstrated with a connection matrix showing the average number of the streamlines across the 178 participants captured by the nine ROIs to any other of the 376 cortical and subcortical regions. The connection matrices in Figure 5 and Supplementary Figure S11 show entries where the average across participants is five or more streamlines between two regions. This produces a reasonably low sparseness of the connection matrix of 13.5% between the 360 cortical areas in the HCP-MMP atlas, which is broadly in the range of what has been found in previous diffusion tractography investigations (Hagmann et al. 2008; Gong et al. 2009). (The sparseness measure here is the proportion of possible connections across the whole brain with more than five streamlines.) For example, for the 78 areas in the AAL atlas (Tzourio-Mazoyer et al. 2002), the sparseness was 11% (Gong et al. 2009), and as expected with 998 areas was somewhat lower at ~3% (Hagmann et al. 2008). The voxel-level connections shown in Figure 4 and Supplementary Figures S2–S9 in coronal slices provides further evidence that the threshold used for Figure 5 is reasonable to avoid false positives.
Figure 4 .
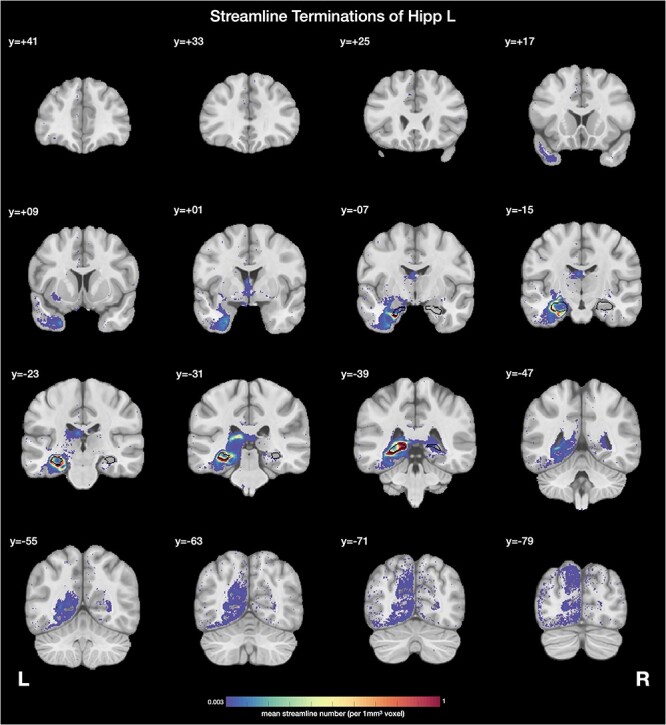
Examples of streamline terminations, shown in this case for the left hippocampus. The seed region of interest (ROI), the left hippocampus, is indicated with black and is also indicated for clarity in the right hemisphere. The mean numbers of streamlines averaged across the 178 participants between the seed ROI, the left hippocampus, and each voxel in all other brain areas are shown. The threshold was selected at 0.003 streamlines per 1 mm3 voxel with the hippocampus in order to reveal the weaker as well as the stronger connections and to be approximately consistent with what is shown in Figure 3. The y value is in MNI coordinates.
Results
The questions raised above were addressed with diffusion tractography imaging to analyze the hippocampal system connections in humans, with the neuroimaging data acquired at 7 T from 178 participants in the HCP (Glasser et al. 2016a, 2016b). We used the HCP atlas for this research, because it has been defined with great care using multimodal data 180 cortical regions in each hemisphere that are not only anatomically defined, but also about which there is considerable knowledge of the functions performed in its 360 different regions (Glasser et al. 2016a). We wished to analyze the connections of many of the cortical areas related to the human hippocampal formation, so we performed the tractography for the nine separate regions, as shown in Figure 2, which we termed as seed regions of interest (“seed ROIs”). Most of these regions were as defined in the HCP atlas. Full details of the methods are provided in the Methods section.
Streamlines Showing Connection Trajectories of the Human Hippocampus
First, we asked what the streamlines, each of which reflects a bundle of nerve fibers visualized with tractography, can reveal about the connections of the human hippocampal system. Figure 3 provides an answer using the hippocampus as the seed ROI. Streamlines can be seen connecting not only with local cortical areas such as the entorhinal cortex, but also with distant areas including the anterior temporal lobe, the parietal cortex, early visual cortical areas, the orbitofrontal cortex and anterior cingulate cortex, and also the anterior thalamus via the fornix.
Figure 3 .
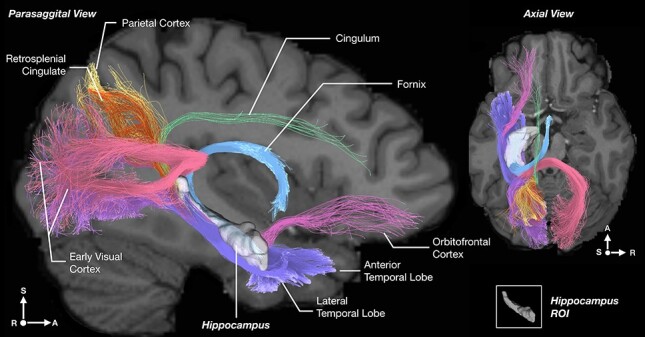
Example of the streamlines showing the hippocampal connections using diffusion tractography imaging for a single participant from the Human Connectome Project dataset. The tractography is from a transparent view through the brain, with parasagittal and axial slices of the human brain overlaid to show the trajectory of the pathways in the context of the brain. Voxels in the left hippocampus were seeded for the case illustrated. Streamlines from the hippocampus reach local areas such as the entorhinal and perirhinal cortex, and the parahippocampal gyrus (violet). However, in addition, streamlines reach more distant areas, including early visual cortical areas bilaterally via the (dorsal) hippocampal commissure (purple, pink), and the parietal cortex (yellow), the orbitofrontal cortex (magenta), the anterior cingulate cortex via the cingulum just above the corpus callosum (light green), and the anterior thalamus and mammillary bodies via the fornix (light blue). The hippocampus is shown in white with slight opacity and appears small posteriorly just because it rotates laterally (S, superior/inferior; L, left/right; A, anterior/posterior).
The streamlines in Figure 3 are shown transparently through the whole brain, and for a single participant. To analyze the exact brain areas between which there were connections, we next analyzed the brain regions in which the streamlines from each of the nine seed ROIs terminated. This was done by showing the sites and numbers of the terminations of the streamlines on coronal slices of the brain, as described next, and moreover doing this across the whole set of 178 participants.
Streamline Terminations Showing Connections of the Human Hippocampal System
The streamline terminations of the hippocampus using this quantitative approach are shown on coronal slices of the brain in Figure 4. The seed region (in this case Hipp) is outlined in black. The color bar represents the mean number of streamlines from the whole hippocampal seed region with each voxel throughout the brain, averaged across the 178 participants. This shows that the brain regions with which the human hippocampus has connections include the entorhinal cortex, and also parts of the ventral stream areas including the anterior temporal lobe, parts of the dorsal stream areas including parts of the parietal cortex, and even early visual cortical areas. Supplementary Figure S1 provides a labeled version of the HCP atlas (Supplementary Table S1) to help the reader identify brain regions in these coronal slices of the human brain.
We designed this method of presenting the structural connections assessed with tractography between one region (the seed ROI) with the termination region in coronal slices (Figure 4 and Supplementary Figures S2–S9), because it is the clearest way to show the connections of each brain region and is consistent with the way that has been developed in classical neuroanatomy for presenting experimental tract-tracing data using anterograde and retrograde axonal transport methods.
Similar coronal slices for each of the other eight seed ROIs (entorhinal, perirhinal, parahippocampal, etc.) are provided in Supplementary Figures S2–S9. We describe what is found for all the nine seed ROIs below. However, for this systematic description, we next needed and asked how we could put together all of the information from this tractography into a form where the connections of all nine hippocampal system seed ROIs (Fig. 2) with every brain region of the HCP atlas could be put together for quantitative assessment.
The Connections of Each Hippocampal System Region with Many Other Cortical Regions Shown Quantitatively
To address how we could make the description of the connectivity quantitative, we next measured the number of streamlines between each seed ROI and all of the 180 brain regions defined on each side of the brain in the HCP atlas (Glasser et al. 2016a) (Supplementary Table S1 and Figure S1), and then averaged this number across all 178 participants. This produced a matrix of the connections, as reflected in the number of streamlines, as shown in Figure 5 for the left hemisphere. The numbers are not normalized, so that they reflect the absolute magnitude (number) of the connections between each pair of brain regions, which is likely to reflect the amount of information transmitted between the regions (Rolls 2021b).
We designed the method of presenting diffusion tractography data in the form of a matrix of streamline connection numbers in Figure 5 and Supplementary Figure S11 in order to make explicit quantitative aspects of the connectome, and in a way that is analogous to the ways in which functional connectivity data are frequently presented.
The connections between each of the nine hippocampal system seed ROIs (Fig. 2) and every brain region in the HCP atlas based on the matrix shown in Figure 5 and on the coronal slices shown in Figure 4 and Supplementary Figures S2–S9 are described in the next sections. Figure 5 and Supplementary Figure S11 show the connections parcellated by the HCP atlas, so that the brain areas between which there are connections can be named and the number of streamlines can be indicated. Supplementary Figures S2–S9 present the results at the voxel level, so that the exact brain regions between which there are connections can be shown. The results will be consistent between these methods of presentation, and in the descriptions that follow points that are highlighted from these two types of presentation do not imply that the underlying connections are different.
Reference to the summary diagram Figure 6 may be helpful, though it is considered later. Reference to Supplementary Table S1 and Figure S1 may be useful in the rest of the Results and Discussion, for they show the exact brain regions that are referred to with connections with the human hippocampal system.
Figure 6 .
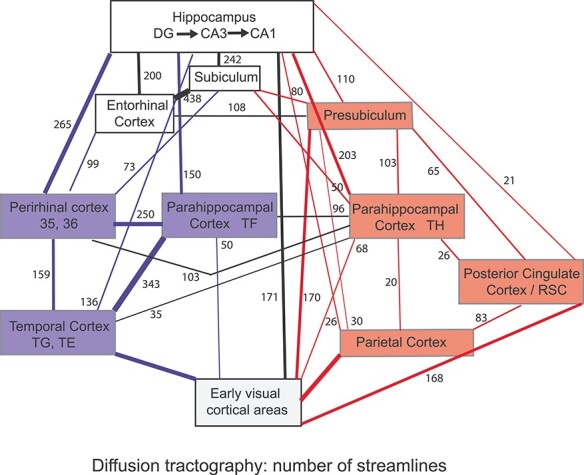
Summary of connections within the human hippocampal system, as shown by diffusion tractography. The numbers of streamlines between areas are indicated by the width of the arrows and the numbers, using the data shown in Figure 5. Blue indicates ventral cortical stream areas and connections that are primarily between them, and red indicates dorsal cortical stream areas and connections that are primarily between them. Of the parahippocampal areas, TF has higher connections with ventral stream (temporal lobe) areas, and the TH areas PHA1–3 have higher connections with parietal cortex areas, as shown in the diagram. Where several regions are involved, for example, the early visual cortical areas, the number represents the maximal value of the number of streamlines to any region. Connections with <20 streamlines are not shown in this figure. In this diagram, early visual cortical areas refer to V1–V4t in the HCP atlas, the parietal areas refer to PSL to PGs, and the temporal areas refer to TE1a to TGv, using the nomenclature and order in Supplementary Table S1.
Hippocampus: The Human Hippocampus has Many Connections that Bypass the Entorhinal Cortex and Even Reach Some Early Sensory Cortical Areas
As shown in Figure 5, the hippocampus has strong to moderate direct connections with the perirhinal cortex (PeEc), entorhinal cortex, and subiculum. (In this case, the hippocampus was the seed region and streamlines were followed from it, but the diffusion tractography does not provide evidence on the direction of the connection.) The hippocampus has moderate connections with the parahippocampal gyrus including TF, TH (which is labeled as PHA1–PHA3 in the HCP atlas), and presubiculum. The hippocampus also has moderate connections with some temporal lobe cortical regions including TG (temporal pole) and TE (inferior temporal visual cortex); with the parietal cortex including area 7 (medially), LIP, MIP and VIP, and PF and PG (laterally); with the posterior cingulate cortex [e.g., in the parieto-occipital sulcus, prestriate prostriate area, and retrosplenial cingulate cortex (RSC)]; with some early sensory cortical areas including V1, V2, V3, and V4, the somatosensory cortex, and the piriform (olfactory) cortex; with the ventromedial visual areas (VMV), which together with TH form the parahippocampal place areas (PPAs) that respond to parts of scenes (Sulpizio et al. 2020); and with the orbitofrontal cortex (pOFC).
Figure 4 emphasizes the connections of the hippocampus with parts of the human anterior temporal lobe (including the temporal pole TG and the parahippocampal gyrus TH and TF), medial parietal cortex (e.g., at Y = −63) and lateral parietal cortex (PG at Y = −79), posterior cingulate/retrosplenial cortex (RSC) (e.g., at Y = −47), and occipital visual cortical areas, with the magnitude of the connections with the different HCP regions shown in Figure 5. Connections with an anterior ventral part of the insula are also shown in Figure 4. (Note that the streamlines are shown terminating just where WM enters the insula, because the streamlines are not usually followed far into the GM because they spread out and disperse in the GM.) Connections with parts of the anterior thalamus via the fornix are also shown in Figure 4. Light label with the supracallosal anterior cingulate cortex and the lateral orbitofrontal cortex is also evident in Figure 4 as well as in Figure 3. In addition, the hippocampus has streamlines with the amygdala and ventral striatum (Fig. 4).
Most of these hippocampal connections demonstrated that bypass the entorhinal cortex to connect with neocortical areas directly are reasonable in humans in the sense that they are consistent with the literature in macaques described in the Introduction. The connections evident in the diffusion tractography with some early sensory including visual cortical areas were less expected. For this reason, further analyses were performed. The direct connections between the hippocampus and V1 and the parietal cortex were also evident when V1 and the parietal cortex were seed regions, when streamlines terminating at the posterior hippocampus were found (Supplementary Fig. S10). The reliability of these hippocampal connections with early sensory cortical areas is considered in the Discussion, including evidence from the same cohort that the human hippocampus does have functional connectivity with these same cortical areas.
Subiculum and Presubiculum: Connections not only with the Hippocampus and Entorhinal Cortex, but also with Parahippocampal and Perirhinal Cortex, Posterior and Retrosplenial Cingulate Cortex, and Parietal Cortex
Figure 2 shows the subiculum as a relatively small region close to CA1, with the presubiculum more prominent, especially posteriorly. With the subiculum (Subic) as the seed region (Fig. 5), the strongest connections are with the entorhinal cortex and moderate with the hippocampus. The subiculum also has moderate connections with the TH parahippocampal cortex, presubiculum, perirhinal, and posterior cingulate cortex. Supplementary Figure S2 emphasizes that the subiculum has strong connections especially with the entorhinal cortex, some connections with perirhinal and PHAs, the posterior cingulate cortex, with medial parietal areas and the RSC. Figure 6 provides evidence that the subiculum acts as a pathway with many connections that link the hippocampus with the entorhinal cortex. The subiculum is connected with many fewer cortical areas than the hippocampus (Fig. 5).
The presubiculum (Ding 2013) is notable for its strong connections with the hippocampus, entorhinal cortex, parahippocampal gyrus TH and VMV areas where the PPA (scene) is located, and early visual cortical areas (Fig. 5). It also has many connections with the posterior cingulate cortex including the retrosplenial cortex (Fig. 5). The presubiculum also has many connections with medial and lateral parietal cortex (Fig. 5). The presubiculum is notable among the nine hippocampal system regions in having connections with the anterior cingulate cortex (p32pr which is supracallosal; Fig. 5). Supplementary Figure S3 emphasizes connections with entorhinal and perirhinal cortex, with the anterior cingulate cortex, with medial parietal areas and the posterior cingulate cortex (24) and RSC, with insula (PoI1), and with medial early visual cortical areas (V1–V8, etc.).
Entorhinal Cortex: In Humans not the Main Gateway Between the Hippocampus and Cortical Areas
With the entorhinal cortex (EC) as the seed region (Figs 5 and 6 and Supplementary Fig. S4), the strongest connections are with the hippocampus, and subiculum, which in turn has strong connections with the hippocampus (Fig. 6). There are also moderate connections with the perirhinal cortex and the presubiculum. Supplementary Figure S4 emphasizes that the EC has strong connections especially with the hippocampus/subiculum and perirhinal cortex, and much more limited cortical connections than the hippocampus.
Perirhinal Cortex: In Humans, Connections with the Hippocampus and with Parahippocampal Region TF as well as Ventral Visual Stream What Areas
With the perirhinal cortex (PeEc) as the seed region (Figs 5 and 6 and Supplementary Fig. S5), the strongest connections are with the hippocampus, parahippocampal gyrus TF, and inferior temporal cortical visual areas and especially the temporal pole (TG); with moderate connections with the entorhinal cortex, subiculum, and the pyriform (olfactory) cortex; and some connections with parahippocampal TH. Supplementary Figure S5 emphasizes that the perirhinal cortex has streamlines with the entorhinal cortex and inferior temporal cortical areas and the temporal pole. The cortical areas with which there are connections are more limited than those of the hippocampus.
Parahippocampal Cortex: TF Laterally has Connections with the Hippocampus, Perirhinal Cortex, and Ventral Stream What Areas; TH Medially has Connections with the Hippocampus, Presubiculum, and the Dorsal Stream Parietal and Posterior Cingulate Where Areas
Figures 5 and 6 and Supplementary Figure S6 emphasize strong connections of TF (the lateral parahippocampal cortex) with the hippocampus, perirhinal cortex, inferior temporal TE cortical areas and the fusiform face complex, and temporal pole TG cortical areas. It has some connections with early visual cortical areas and the pyriform (olfactory) cortex. Its numerous connections with the perirhinal cortex are noticeable (Figs 5 and 6).
The parahippocampal TH areas (PHA1–PHA3 in the HCP atlas) have many connections with the hippocampus, moderate connections with the presubiculum and early visual cortical areas, and some connections with parietal and posterior cingulate cortical areas (Figs 5 and 6). There is some gradient within PHA1–3, with the more lateral PHA3 and PHA2 having some connections with inferior temporal (TE and TG) areas, and PHA1 medially has connections with posterior cingulate cortex (Fig. 5 and Supplementary Figs S7–S9).
The Right Versus Left Hippocampal System Connections Revealed are Largely Ipsilateral, Apart from the Hippocampus and Presubiculum
We next asked the question about whether the connections of the human hippocampal system are mainly unilateral or bilateral. The full anatomical connection matrix for each of the left and right ROIs (the rows) with all HCP atlas areas is shown in Supplementary Figure S11. The top matrix shows the connections with the right hemisphere, and the bottom matrix indicates the connections of the ROIs with the left hemisphere. The number of streamlines between the HCP regions is shown without normalization in Supplementary Figure S11, in order to allow assessment of the absolute magnitude of the connections between each pair of HCP regions.
What is most evident in Supplementary Figure S11, 4, and Supplementary Figures S2–S9 is that most of the hippocampal formation connections revealed by diffusion tractography are ipsilateral, with the hippocampus showing some contralateral connections followed by the presubiculum with the contralateral hippocampus, presubiculum, posterior and retrosplenial cortex, medial parietal area 7, and early visual cortical areas.
The numbers of streamlines between corresponding regions within the left and right hemispheres were compared by using a paired t-test across the 178 participants with false discovery rate (FDR) correction, considering only cortical regions with connections, as shown in Figure 5. This showed that there are significantly (P < 0.05 FDR) more streamlines of the presubiculum with VMV1–3, VVC, and PHA1–3 (i.e., TH) in the right than the left hemisphere. These areas comprise the PPA (Sulpizio et al. 2020). The hippocampus and posterior including retrosplenial cingulate cortex followed the same pattern of strong connectivity with the PPA in the right hemisphere. The implication is that the presubiculum provides for more connectivity of the PPAs with the hippocampal system in the right than the left hemisphere. In the left hemisphere, there were significantly more streamlines between the hippocampus and perirhinal cortex, TF, TE1a, TE2p, TGv, and TGd. These left anterior temporal lobe areas are probably involved in semantic processing (Bonner and Price 2013; DeWitt and Rauschecker 2013, 2016), and a further link with language is that area TF has more streamlines with area 44, part of Broca’s area, in the left hemisphere. The implication is that the human left hippocampus is strongly connected to brain systems involved in semantic processing and language.
A Quantitative Map of the Connections of the Human Hippocampal System: Beyond the Hierarchical and Segregated Dual Stream Model
Some of the key findings are brought together in Figure 6, which shows the number of streamline connections found between different parts of the hippocampal system and different brain regions, in a way that facilitates comparison with the simplified and schematic dual stream model illustrated in Figure 1. The many differences, and their implications for understanding the operation of the human hippocampal system, are considered in the Discussion.
Discussion
A view from classical studies incorporated into the dual stream model (with a simplified schematic form in Fig. 1) is that the hippocampal connections with the cerebral cortex are predominantly via the entorhinal cortex, “the gateway to the hippocampus.” The medial entorhinal cortex then connects with dorsal stream where areas via the parahippocampal gyrus with parietal and retrosplenial cingulate cortex areas; and the lateral entorhinal cortex connects with ventral stream what areas via the perirhinal cortex with the inferior temporal cortical areas (Van Hoesen 1982; Suzuki and Amaral 1994; Burwell et al. 1995; Burwell 2000; Knierim et al. 2014; Doan et al. 2019). The connections of the hippocampus in humans revealed from the present diffusion tractography study are with more widespread cortical areas than primarily the entorhinal cortex and indicate a much less purely hierarchical (stage to stage), and segregated what versus where, organization than in the schematic dual stream model of Figure 1. We consider next these connections in humans, which are summarized in Figure 6, and their potential implications, taking the different areas in turn. We note at the outset that there is support for most of the connections in humans, shown in Figure 6, from studies in nonhuman primates and refer to the research on nonhuman primates referred to in the Introduction. We consider the hippocampal system connections with early sensory cortical areas that appear with the diffusion tractography later below, but note for now that in any case a functional connectivity investigation (Ma et al. 2021) in the same HCP participants shows connectivity of the hippocampus with some of these early sensory cortical areas including V1–V3 and somatosensory/motor areas (Fig. 2 of Ma et al. 2021), so that functionally this connectivity is present in the human brain.
Hippocampus: Extensive Cortical Connections, Even with Some Early Sensory Cortical Areas
The cortical connections of the hippocampus in humans are far more widespread than with mainly the entorhinal cortex, as shown in Figures 4–6. The hippocampus has direct connections also with the perirhinal cortex, subiculum, presubiculum, and parahippocampal gyrus including both TF and TH, which with the VMV areas form the PPAs that respond to parts of scenes (Sulpizio et al. 2020). The hippocampus also has moderate direct connections with some temporal lobe cortical regions, including the temporal pole TGd and TGv, with the parietal cortex, the posterior including retrosplenial cingulate cortex, and with some early visual cortical areas including V1, V2, V3, and V4.
Taken together, the new evidence from humans, and the findings described in the Introduction in macaques, suggests that we should think beyond the hippocampus as mainly receiving input from the entorhinal cortex, passing this for pattern separation in the dentate gyrus/mossy fiber system, then for pattern completion in CA3 to retrieve a whole memory, and then via CA1 to entorhinal cortex to implement memory retrieval back to the neocortex (Treves and Rolls 1992, 1994; Rolls 2013; Bennett and Stark 2015; Rolls 2016b, 2018, 2021b). The evidence suggests that a number of cortical areas, including parts of the temporal, parietal, posterior cingulate, insular, and orbitofrontal cortex, have direct connections with the hippocampus, including CA1. What might be their function? It is suggested in a reconceptualization based on the new findings described here that direct cortical connections to CA1 neurons [with the directionality evident from the macaque literature described in the Introduction (Suzuki and Amaral 1990; Rockland and Van Hoesen 1999; Ding et al. 2000; Zhong and Rockland 2004)] could be associated with the other inputs to CA1 neurons from CA3; and that with the projections from CA1 back to the neocortical sites just referred to, this could help to recall those types of information back to the neocortex (Rolls 2021b). On this proposal, the trisynaptic circuit through the hippocampus that includes CA3 would still be important in setting up and retrieving episodic memories, but extra information could be associated with those memories in CA1 (Rolls 2021b).
However, what is more surprising is that the diffusion tractography indicated that the human hippocampus has direct connections with early visual cortical areas including V1–V4 and lighter connections with somatosensory/motor cortical areas and the piriform olfactory cortex (Pir). The connections between early visual cortical areas were validated by using V1 as a seed region for tractography and identifying streamlines terminating in the hippocampus (Supplementary Fig. S10). These connections are considered further in the section below entitled “Evaluation of the tractography.”
What might these direct connections of the hippocampus with a considerable number of cortical areas in humans signify? Or to put it in computational terms (Rolls 2021b), what computation may these connections be useful for? A clue comes from the following, in a further reconceptualization. The connections from some hippocampus-related areas in the macaque such as perirhinal cortex area 36 and TF are primarily directed to the superficial layers of earlier neocortical areas such as the temporal lobe, suggesting that they are backprojections (Lavenex et al. 2002) in the hierarchy shown in Figure 6. Consistent with this, the effective connectivity in humans is weaker in this direction from perirhinal and TF to temporal cortical areas, providing evidence that they are backprojections (Rolls et al. 2021). In addition, it is known that the amygdala has extensive backprojections to early visual cortical areas (including V1) that end in the superficial layers (Amaral and Price 1984). This leads to the new hypothesis that these backprojections from the perirhinal cortex and parahippocampal gyrus, and it appears from the hippocampus in humans, help to retrieve visual and other sensory details being recalled by the hippocampus. Memory recall is a key function proposed for backprojections to layer 1 of the neocortex (Rolls 1989, 2016a, 2021b; Treves and Rolls 1994). The hippocampal connections with early sensory processing areas for different sensory modalities, including vision, olfaction, somatosensation, and spatial representations in TH, which includes the parahippocampal spatial scene area (PPA) (Sulpizio et al. 2020), may be useful for storing and later recalling sensory details of memories. In terms of computational theory, the hippocampal backprojections, which some of these are likely to be, would become associated by pattern association learning with the low-level details represented by the neural activity present during the episode of what was present in the environment when the episodic memory was being formed (Rolls 1989, 2016a, 2021b; Treves and Rolls 1994). That would allow recalled hippocampal activity (involving completion in CA3 from a partial retrieval cue) to recall via the backprojections some of the low-level details of the whole episodic scene that subjectively seem to be part of episodic memories. Another possibility is that some of the connections between early sensory cortical areas and much higher cortical systems such as the hippocampus/hippocampo-cortical system are directed towards these higher systems. The proposal for any such connections is that they could provide access to low-level details of sensory processing for high-level systems for not only memory but also for the raw sensory details of conscious experience (Rolls 2020, 2021c). The low-level raw sensory details may not be well represented explicitly (i.e., easily decodable from the firing rates) at the higher stages of sensory hierarchies (Rolls 2021b).
Entorhinal Cortex: An Important but not the Sole Gateway for the Hippocampus in Humans
The entorhinal cortex has very many streamlines with the subiculum, many with the hippocampus, and a moderate number with the perirhinal cortex and presubiculum. However, a major feature evident in Figure 6 is that it is not the sole gateway with the hippocampus, with direct connections of the hippocampus that bypass it to make connections directly with the perirhinal, parahippocampal TF, and temporal cortex for the ventral stream, and with the presubiculum, parahippocampal TH, parietal, and posterior cingulate cortex for the dorsal stream. It is also shown in Figure 6 that in addition to direct connections with the hippocampus, the entorhinal cortex has connections via the subiculum with the hippocampus.
Perirhinal Cortex: Direct Connections with the Hippocampus in Humans as well as Ventral Stream What Areas
The perirhinal cortex has strong direct connections with the hippocampus, which at least in terms of the number of streamlines are more numerous than the connections via the entorhinal cortex (Fig. 6). The perirhinal cortex also, as expected, has many streamlines with ventral stream visual cortical areas, including the inferior temporal visual cortex (TE) and the temporal pole (TG) (Figs 5 and 6). These findings are in fact supported by reports in macaques (Lavenex et al. 2002; Blatt et al. 2003). Interestingly, the perirhinal cortex has strong connections with area TF in the parahippocampal gyrus, providing an indication that TF has close relations with ventral stream cortical areas. Interestingly, the perirhinal cortex also has some connections with parahippocampal TH, providing some connections with dorsal stream processing.
Parahippocampal Gyrus: In Humans, the Lateral Part, TF, has Connections with the Ventral Visual Stream What Areas, and the Medial Part, TH, is Related to the Spatial Scene and Parietal Where System
Of the PHAs, TF (which is lateral and isocortical) has relatively strong connections with temporal lobe ventral stream visual cortical areas and weaker connections with parietal areas (Fig. 5 and Supplementary Fig. S6). As a region strongly linked to the ventral visual stream, and which is just anterior to the fusiform face area (Supplementary Fig. S1), TF is probably linked in with face and object systems, and TF with its strong connections with the hippocampus probably interfaces between the hippocampus and ventral visual stream.
The emphasis of parahippocampal connections for TF has been different so far in macaques, in which TF has relatively strong connections with the posterior parietal cortex (Lavenex et al. 2002; Blatt et al. 2003), whereas in humans, TF has strong connections with ventral stream visual cortical areas (Figs 5 and 6), providing a further reconceptualization for humans. A reassessment is underway in the rat, in that the rat equivalent of the parahippocampal gyrus has now been shown to have connections with the what, lateral, part of the entorhinal cortex (Doan et al. 2019). This helps to provide support for the new finding identified here and reconceptualization in humans that TF, the more lateral part of the parahippocampal gyrus with an isocortical structure, does have connections with the what ventral visual system in the temporal lobe TE areas (Fig. 6).
In contrast, TH (which is medial as shown in Fig. 2 and Supplementary Fig. S1) has a proisocortical structure (Blatt et al. 2003) and has relatively stronger connections with the areas such as the VMV areas (VMV 1–3), which together with TH are the parietal place areas (PPA) which respond to spatial scenes (Sulpizio et al. 2020). TH probably thus provides a route for scene information to connect with the hippocampus, in which there are spatial view cells that respond to parts of scenes (Kesner and Rolls 2015;Rolls and Wirth 2018 ; Rolls 2021a). TH is closely linked to parietal cortical areas as shown by functional connectivity (Ma et al. 2021) and effective connectivity (Rolls et al. 2021), and is thereby implicated in dorsal stream processing. There is probably a similar arrangement in macaques of scene areas in the medial parahippocampal gyrus (Nasr et al. 2011; Kornblith et al. 2013). The direct connections of the parahippocampal cortex areas with the hippocampus reported here for humans are supported by the anatomical connections described in macaques (Yukie 2000).
The Human Hippocampal System has Connections Far Beyond the Entorhinal Cortex and is Less Hierarchical and Segregated than in the Dual Stream Model; This has Implications for How Memory Works in Humans
Some of the key findings brought together in Figure 6 are now considered. The striking point made is that the classical ventral and dorsal stream areas (Ungerleider and Mishkin 1982; Ungerleider and Haxby 1994; Ungerleider 1995; Rolls 2021b) are less segregated as they connect with the hippocampal system than the classical dual stream view illustrated in Figure 1. In a classical view, the ventral visual stream connects with the hippocampus via the perirhinal and lateral entorhinal cortex, and the dorsal visual system connects with the hippocampus via the parahippocampal gyrus and medial entorhinal cortex (Van Hoesen and Pandya 1975; Van Hoesen 1982; Amaral et al. 1983; Burwell et al. 1995; Saleem and Tanaka 1996; Burwell 2000; Knierim et al. 2014; Nilssen et al. 2019) (with a simplified form to make the concept clear in Fig. 1). What is shown in Figure 5 and summarized in Figure 6 is first that the hippocampus has connections far beyond the entorhinal cortex. There are strong connections (as indicated by the number of streamlines between regions shown in Figs 5 and 6) that bypass the entorhinal cortex and reach the perirhinal cortex and parahippocampal cortex directly. Second, it is also striking that the hippocampus has some direct connections with some temporal lobe areas in the ventral visual stream, and parietal areas and posterior cingulate cortex areas in the dorsal processing stream. Although surprising, there is evidence to substantiate this finding at least for the temporal lobes, from studies in macaques (Yukie 2000; Zhong et al. 2005) (see Introduction for more details). The remarkable connections between the hippocampus and early sensory cortical areas including the pyriform cortex and antero-ventral insula (PoI1) (Figs 5 and 6) are also very interesting, and a function in the recall of low-level sensory details of memories has been proposed above for these connections. In the mouse, there is some evidence for hippocampal influences on neuronal responses in V1 (Fournier et al. 2020). Here, we go beyond that and provide diffusion tractography evidence for direct connections of the human hippocampus with V1 as well as with other early visual cortical areas, and with early cortical areas for olfaction and touch.
It is also striking in Figure 6 that there are moderate connections between the what and where steams with the hippocampus that are present before the hippocampus is reached, so that segregation of these streams does not appear to be complete before the hippocampus is reached. For example, there are cross-connections of ventral stream perirhinal cortex, TF and temporal TE/TG, with dorsal stream TH.
Although there are some cross-connections between the perirhinal and parahippocampal connections with the hippocampus, the quantitative evidence in Figure 5 and reflected in the width of the connecting lines in Figure 6 does indicate that there is some segregation of the ventral/perirhinal and dorsal/parietal processing streams and the hippocampus in humans. This leaves the hippocampus with a key function to perform, to make sparse relatively uncorrelated representations suitable for associative memory from the ventral and dorsal stream inputs, and then to allow them to be associated together in a single network, the CA3 autoassociation or attractor network, in the hippocampus. The human hippocampus can therefore make a key contribution to episodic memory (Rolls 1989, 2018, 2021b; Treves and Rolls 1994; Kesner and Rolls 2015). Consistent with this, when there is a reduction of functional connectivity of in particular the hippocampus with other brain areas in hypertension, then impairments of episodic memory are found (Feng et al. 2020). The discoveries described here enrich our understanding of the hippocampal system by showing that the human hippocampus has many direct connections with temporal, parietal, and early sensory cortical areas; and that the segregation of connections between the temporal and parietal systems with the hippocampus is less distinct, at least in humans, than was previously known in humans. The computational implications are that while the hippocampal trisynaptic circuit from the dentate to CA3–CA1 may be especially important in enabling arbitrary associations between what (ventral stream) and where/action (dorsal stream) representations that occur at a particular time for episodic memory (Rolls 1989, 2018, 2021b; Treves and Rolls 1994; Kesner and Rolls 2015), there is the opportunity for cross-connections between the two streams before the hippocampus to build useful multimodal representations based in medial temporal lobe areas such as the parahippocampal and perirhinal cortices because of the convergence between the two streams in these areas.
Another key point evident in Figure 6 (and Fig. 5) is that the entorhinal cortex is in humans far from a main gateway into and out of the hippocampus. The entorhinal cortex is bypassed not only by direct connections of the hippocampus with perirhinal and parahippocampal gyrus, but also even with the temporal and parietal cortex and even with sensory cortical areas in all modalities. An implication and reconceptualization, it is proposed, is that because the entorhinal cortex, and for that matter the parahippocampal and perirhinal cortex, do not need to be devoted mainly to relaying inputs to and from the hippocampus, some specialized computations could be performed in many of the components of the human hippocampal system in humans. For example, the parahippocampal gyrus (as well as the hippocampus) contains spatial view cells implicated in memory for where objects are in scenes (Rolls et al. 1997, 1998, 2005b; Robertson et al. 1998; Georges-François et al. 1999; Rolls and Xiang 2006; Kesner and Rolls 2015; Rolls and Wirth 2018) and in navigation (Rolls and Wirth 2018; Rolls 2021a), and a corresponding parahippocampal place/scene area (PPA) is found in TH in humans (Epstein and Kanwisher 1998;Epstein and Baker 2019 ; Sulpizio et al. 2020) and macaques (Nasr et al. 2011; Kornblith et al. 2013). Another possible specialization is that the entorhinal cortex may be especially involved with the hippocampus in the generation of time cells in the hippocampus (Tsao et al. 2018; Rolls and Mills 2019). Another example is that the perirhinal cortex may include specialization for long-term familiarity memory (Holscher et al. 2003; Rolls et al. 2005a).
The direct connections of the hippocampus with the anterior temporal lobe including the temporal pole are striking and of interest in relation to how memory is organized, for the anterior temporal lobes are implicated in semantic memory (Bonner and Price 2013; DeWitt and Rauschecker 2013; Rolls 2021b). In macaques, the temporal pole area TG is a multimodal region that could provide a foundation for semantic memory, given the great development of this system in humans (Pandya et al. 2015). Moreover, in humans, the anterior temporal lobe has connections with Broca’s area (BA45 and 44) via the uncinate fasciculus, consistent with the hypothesis that the anterior temporal lobe in humans is involved in semantic memory that is relevant to language processing (Petrides 2014; Du et al. 2020; Rolls 2021b). This provides a foundation for new concepts about how the human hippocampus is involved in forming new semantic (as well as episodic) memories, and to how semantic information may be incorporated into episodic memory. This is potentially of great interest, for hippocampal damage impairs the learning of new semantic as well as episodic memory (Corkin 2002; Maguire et al. 2016; Clark et al. 2019). Furthermore, as shown in the Results, the left hippocampus has significantly more streamlines with anterior temporal lobe areas involved in semantic processing, and the right hippocampus has significantly more connections with the presubiculum and PPAs, providing fascinating evidence on the connectional asymmetry of the left and right human hippocampal systems.
Another point of interest is that the posterior cingulate and retrosplenial cingulate cortex (RSC) areas have direction connections with the hippocampus and with several parts of the dorsal visual pathway to the hippocampus, as shown in Figure 6. This brings the hippocampal system into close communication with the parietal/posterior cingulate cortex system, which is involved not just in where representations but also in actions, and this it is proposed is important in how navigational systems operate in humans and other primates, for it enables actions to be incorporated into navigational strategies, not just spatial locations (Rolls 2019a, 2021a, 2021b). However, in addition to actions, the posterior cingulate cortex/retrosplenial cingulate cortex areas do have spatial scene representations (Epstein and Baker 2019; Sulpizio et al. 2020), and these are probably part of the route to the hippocampus, for spatial scene representations do reach the hippocampus where spatial view cells are found and where they may be used for episodic memory and navigation (Kesner and Rolls 2015; Rolls and Wirth 2018; Rolls 2021a, 2021b).
Evaluation of the Tractography
Diffusion tractography neuroimaging can have limitations, but we used modern methods to minimize any possible problems with false positives that might arise with fiber crossings, as set out in detail in the Methods. Further arguments that fiber-crossing problems are not a major limitation with the findings reported here are as follows. First, the nine hippocampal system regions investigated here all have significantly different connections to each other, yet are close together in the medial temporal lobe with no clear possible differences in fiber crossings that might account for the different connections of the nine different regions. Second the functional connectivity based on the BOLD signal and completely different methodologically, and performed on the same HCP participants as in the present study, provides important supporting evidence that the pathways of the hippocampal system identified here with diffusion tractography are not false positives, in that the tractography connections are reflected by the functional connectivity, which would not occur if the tractography had followed pathways incorrectly (Ma et al. 2021). Third, in new observations, we are making with a further methodologically completely different method for identifying connectivity between brain regions, effective connectivity measured with a Hopf algorithm that uses the functional magnetic resonance imaging BOLD signal (Deco et al. 2019), we found in the same HCP participants reported here that most of the brain regions identified here as having streamlines with the hippocampal system regions have effective connectivity with the same regions, but not with most other cortical areas (Rolls et al. 2021). The functional connectivity and effective connectivity measures thus show that there is an influence between the brain regions shown here as having direct connections and that provides evidence that the results presented here are not due to fiber crossings, for in that case, no functional or effective connectivity would be measured between the brain regions identified here as having direct connections. Fourth, most of the connections described here are likely to be valid is that they are consistent which the connections described in macaques, as set out in the Introduction. [Of course, many of the cortical areas described here are very different in rodents, in which there may be, for example, no posterior cingulate cortex (Vogt 2009), no granular orbitofrontal cortex (Rolls 2019b, 2021b), and poorly developed visual cortical areas compared with those defined in the human HCP-MMP atlas (Rolls 2021b) and illustrated in Supplementary Figure S1 and Figure 5. For that reason, we refer to comparisons of the connections in the human hippocampal system with those in macaques.]
A technical issue is that it is possible to follow streamlines starting with a seed region such as the hippocampus, but it is harder to follow streamlines from other seed regions into the depths of the GM of the hippocampus, for the orientation of the fibers in GM becomes less consistent and the streamlines are then difficult to follow. What does often occur in a case such as this is that the streamlines reach close to the hippocampus and are then counted as terminations, as they cannot be followed deep into the GM. A strength of the approach taken here is that the connections shown in Figures 3 and 4 can be assessed quantitatively as shown in Figure 5 and Supplementary Figure S11, at least in so far as they are reflected by the number of streamlines between regions or groups of regions. Such quantitative measures are not usual in investigations of the corresponding systems in macaques and are an important step forward in the research described here. It will be interesting in future studies to compare these anatomical connection strengths with those found using functional connectivity in the same dataset.
A strength of the tractography described here is that it provides an estimate, the number of streamlines, that is likely to reflect (though not measure) the number of connections between two brain areas (Smith et al. 2013, 2015). This is helpful, because measures such as functional connectivity or effective connectivity may reflect how much the signal in one brain area is related to that in another brain area, but does not necessarily reflect the number of connections that there may be, nor the size of each brain area, both of which are helpful in understanding brain computations (Rolls 2021b). We note that the number of streamlines may not be linearly related to the number of axons that are being traced, for the number of streamlines depends on factors such as the axon diameter and probably with the distance followed.
Conclusions
In summary, this is the first quantitative assessment we know of the direct cortical connections of the human hippocampal system. Indeed, the type of quantitative analysis presented here has not been performed in most tract-tracing neuroanatomical studies in animals and is likely to be useful in future, given that the number of connections between brain areas is likely to be important in how much information can be transmitted (Rolls and Treves 2011; Rolls 2021b). The connections of the human hippocampus that are revealed are with more cortical areas than was previously assumed and lead to new concepts about specializations in different parts of the cortical system that connect with the hippocampus. A highlight of the findings is that they are in the framework provided by the HCP-MMP atlas, which with its 180 cortical areas in each hemisphere, many functionally identified, allows interpretation of some of the functionality of the different connections described here. Moreover, the interesting connections described here do not appear to be related to problems with the diffusion tractography methodology, in that the functional connectivity (Ma et al. 2021) and effective connectivity (Rolls et al. 2021) of the human hippocampal system in the same HCP participants is similar, providing evidence that the pathways described here have been correctly followed. The unique feature of course about the diffusion tractography is that it shows which of the connections are direct. Another highlight is that these results extend considerably what is known from rodents, because humans have highly developed cortical areas, including the posterior cingulate cortex and dorsal parietal and ventral visual stream areas, so important in human hippocampal function, and also connections with systems in the anterior temporal lobe implicated in semantics in humans (Rolls 2021b). Another highlight is that we show that important advances can be made, in this case about the connections of the human memory system, based on the large investments in studies designed to collect data on the human connectome such as the HCP.
Notes
Professor Menno P. Witter (Kavli Institute for Systems Neuroscience and Centre for Neural Computation, Trondheim, Norway) and Professor Kathleen S. Rockland (Department of Anatomy and Neurobiology, Boston University School of Medicine, Boston, MA 02118, USA) are warmly thanked for helpful discussions. The corresponding author Edmund Rolls wishes to acknowledge the important contributions of Dr Leslie Ungerleider to our understanding of the dorsal and ventral visual systems, and dedicates this paper to her. Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. Conflict of Interest: None declared.
Supplementary Material
Contributor Information
Chu-Chung Huang, Key Laboratory of Brain Functional Genomics (MOE & STCSM), Affiliated Mental Health Center (ECNU), School of Psychology and Cognitive Science, East China Normal University, Shanghai 200062, China; Shanghai Changning Mental Health Center, Shanghai 200335, China.
Edmund T Rolls, Institute of Science and Technology for Brain Inspired Intelligence, Fudan University, Shanghai 200433, China; Department of Computer Science, University of Warwick, Coventry CV4 7AL, UK; Oxford Centre for Computational Neuroscience, Oxford, UK.
Chih-Chin Heather Hsu, Center for Geriatrics and Gerontology, Taipei Veterans General Hospital, Taipei 11217, Taiwan; Institute of Neuroscience, National Yang-Ming Chiao Tung University, Taipei 11217, Taiwan.
Jianfeng Feng, Institute of Science and Technology for Brain Inspired Intelligence, Fudan University, Shanghai 200433, China; Department of Computer Science, University of Warwick, Coventry CV4 7AL, UK.
Ching-Po Lin, Institute of Science and Technology for Brain Inspired Intelligence, Fudan University, Shanghai 200433, China; Center for Geriatrics and Gerontology, Taipei Veterans General Hospital, Taipei 11217, Taiwan; Brain Research Center, National Yang-Ming Chiao Tung University, Taipei 11217, Taiwan.
Funding
Grant to Professor C.-P.L. that included research with Professor E.T.R.: Ministry of Science and Technology (MOST) of Taiwan (MOST 110-2321-B-010-004, MOST 110-2634-F-010-001, MOST 108-2321-B-010-013-MY2, MOST 108-2321-B-010-010-MY2). National Key R&D Program of China (No. 2019YFA0709502); 111 Project (No. B18015); Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), ZJLab, and Shanghai Center for Brain Science and Brain-Inspired Technology; National Key R&D Program of China (No. 2018YFC1312904 to Professor J.F.).
Ethical Permissions
No data were collected as part of the research described here. The data were from the Human Connectome Project, and the WU-Minn HCP Consortium obtained full informed consent from all participants; and research procedures and ethical guidelines were followed in accordance with the Institutional Review Boards, with details at the HCP website (http://www.humanconnectome.org/).
Authors’ Contributions
E.T.R. designed the investigation, performed the analyses, made some of the figures, and wrote the paper. A.H. made the modified version of the HCP-MMI v1.0 atlas used here, performed the tractography, and made most of the figures with help from H.H. All authors read and approved the paper.
References
- Aggleton JP. 2012. Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci Biobehav Rev. 36(7):1579–1596. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, Cowan WM. 1983. Evidence for a direct projection from the superior temporal gyrus to the entorhinal cortex in the monkey. Brain Res. 275(2):263–277. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. 1984. Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol. 230(4):465–496. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G. 2009. Advanced Normalization Tools (ANTS). Insight J. 2:1–35. [Google Scholar]
- Bennett IJ, Stark CE. 2015. Mnemonic discrimination relates to perforant path integrity: an ultra-high resolution diffusion tensor imaging study. Neurobiol Learn Mem. 129:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt GJ, Pandya DN, Rosene DL. 2003. Parcellation of cortical afferents to three distinct sectors in the parahippocampal gyrus of the rhesus monkey: an anatomical and neurophysiological study. J Comp Neurol. 466(2):161–179. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Rosene DL. 1998. Organization of direct hippocampal efferent projections to the cerebral cortex of the rhesus monkey: projections from CA1, prosubiculum, and subiculum to the temporal lobe. J Comp Neurol. 392(1):92–114. [DOI] [PubMed] [Google Scholar]
- Bonner MF, Price AR. 2013. Where is the anterior temporal lobe and what does it do? J Neurosci. 33(10):4213–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D, Ungerleider LG, Desimone R. 1990. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 296(3):462–495. [DOI] [PubMed] [Google Scholar]
- Burwell RD. 2000. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 911:25–42. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, Amaral DG. 1995. Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. 5(5):390–408. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. 2000. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 10(3):220–242. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. 2006. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol. 497(1):101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Qiu J, Liu W, Tang Y, Huang CC, Wang X, Zhang J, Lin W, Zheng L, et al. 2016. Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain. 139(12):3296–3309. [DOI] [PubMed] [Google Scholar]
- Clark IA, Hotchin V, Monk A, Pizzamiglio G, Liefgreen A, Maguire EA. 2019. Identifying the cognitive processes underpinning hippocampal-dependent tasks. J Exp Psychol Gen. 148(11):1861–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S. 2002. What’s new with the amnesic patient H.M.? Nat Rev Neurosci. 3(2):153–160. [DOI] [PubMed] [Google Scholar]
- Deco G, Cruzat J, Cabral J, Tagliazucchi E, Laufs H, Logothetis NK, Kringelbach ML. 2019. Awakening: predicting external stimulation to force transitions between different brain states. Proc Natl Acad Sci. 116(36):18088–18097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt I, Rauschecker JP. 2013. Wernicke’s area revisited: parallel streams and word processing. Brain Lang. 127(2):181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt, Rauschecker JP. 2016. Convergent evidence for the causal involvement of anterior superior temporal gyrus in auditory single-word comprehension. Cortex. 77:164–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding SL. 2013. Comparative anatomy of the prosubiculum, subiculum, presubiculum, postsubiculum, and parasubiculum in human, monkey, and rodent. J Comp Neurol. 521(18):4145–4162. [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen G, Rockland KS. 2000. Inferior parietal lobule projections to the presubiculum and neighboring ventromedial temporal cortical areas. J Comp Neurol. 425(4):510–530. [DOI] [PubMed] [Google Scholar]
- Doan TP, Lagartos-Donate MJ, Nilssen ES, Ohara S, Witter MP. 2019. Convergent projections from perirhinal and postrhinal cortices suggest a multisensory nature of lateral, but not medial, entorhinal cortex. Cell Rep. 29:617–627.e7. [DOI] [PubMed] [Google Scholar]
- Donahue CJ, Sotiropoulos SN, Jbabdi S, Hernandez-Fernandez M, Behrens TE, Dyrby TB, Coalson T, Kennedy H, Knoblauch K, Van Essen DC, et al. 2016. Using diffusion tractography to predict cortical connection strength and distance: a quantitative comparison with tracers in the monkey. J Neurosci. 36(25):6758–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Rolls ET, Cheng W, Li Y, Gong W, Qiu J, Feng J. 2020. Functional connectivity of the orbitofrontal cortex, anterior cingulate cortex, and inferior frontal gyrus in humans. Cortex. 123:185–199. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Baker CI. 2019. Scene perception in the human brain. Annu Rev Vis Sci. 5(1):373–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. 1998. A cortical representation of the local visual environment. Nature. 392(6676):598–601. [DOI] [PubMed] [Google Scholar]
- Feng R, Rolls ET, Cheng W, Feng J. 2020. Hypertension is associated with reduced hippocampal connectivity and impaired memory. EBioMedicine. 61:103082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL, Brain Development Cooperative Group . 2011. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 54(1):313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J, Saleem AB, Diamanti EM, Wells MJ, Harris KD, Carandini M. 2020. Mouse visual cortex is modulated by distance traveled and by theta oscillations. Curr Biol. 30:3811–3817.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-François P, Rolls ET, Robertson RG. 1999. Spatial view cells in the primate hippocampus: allocentric view not head direction or eye position or place. Cereb Cortex. 9(3):197–212. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, et al. 2016a. A multi-modal parcellation of human cerebral cortex. Nature. 536(7615):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Smith SM, Marcus DS, Andersson JL, Auerbach EJ, Behrens TE, Coalson TS, Harms MP, Jenkinson M, Moeller S, et al. 2016b. The Human Connectome Project’s neuroimaging approach. Nat Neurosci. 19:1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, et al. 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C. 2009. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 19(3):524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez CE, Skibbe H, Nakae K, Tsukada H, Lienard J, Watakabe A, Hata J, Reisert M, Woodward A, Yamaguchi Y, et al. 2020. Optimization and validation of diffusion MRI-based fiber tracking with neural tracer data as a reference. Sci Rep. 10(1):21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. 2008. Mapping the structural core of human cerebral cortex. PLoS Biol. 6(7):e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland KG, Sugar J, Witter MP. 2019. Development and topographical organization of projections from the hippocampus and parahippocampus to the retrosplenial cortex. Eur J Neurosci. 50(1):1799–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C, Rolls ET, Xiang J-Z. 2003. Perirhinal cortex neuronal activity related to long-term familiarity memory in the macaque. Eur J Neurosci. 18(7):2037–2046. [DOI] [PubMed] [Google Scholar]
- Huang C-C, Rolls ET, Feng J, Lin C-P. 2021. An extended Human Connectome Project multimodal parcellation atlas of the human cortex and subcortical areas. [DOI] [PubMed]
- Ichinohe N, Rockland KS. 2005. Zinc-enriched amygdalo- and hippocampo-cortical connections to the inferotemporal cortices in macaque monkey. Neurosci Res. 53(1):57–68. [DOI] [PubMed] [Google Scholar]
- Insausti R, Munoz M. 2001. Cortical projections of the non-entorhinal hippocampal formation in the cynomolgus monkey (Macaca fascicularis). Eur J Neurosci. 14(3):435–451. [DOI] [PubMed] [Google Scholar]
- Insausti R, Munoz-Lopez M, Insausti AM, Artacho-Perula E. 2017. The human periallocortex: layer pattern in presubiculum, parasubiculum and entorhinal cortex. A review. Front Neuroanat. 11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi S, Johansen-Berg H. 2011. Tractography: where do we go from here? Brain Connect. 1(3):169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J. 2014. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage. 103:411–426. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Rolls ET. 2015. A computational theory of hippocampal function, and tests of the theory: new developments. Neurosci Biobehav Rev. 48:92–147. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP, Deshmukh SS. 2014. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philos Trans R Soc Lond B Biol Sci. 369:20130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblith S, Cheng X, Ohayon S, Tsao DY. 2013. A network for scene processing in the macaque temporal lobe. Neuron. 79:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M. 2011. A new neural framework for visuospatial processing. Nat Rev Neurosci. 12:217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M. 2013. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn Sci. 17:26–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. 2002. Perirhinal and parahippocampal cortices of the macaque monkey: projections to the neocortex. J Comp Neurol. 447:394–420. [DOI] [PubMed] [Google Scholar]
- Ma Q, Rolls ET, Huang C-C, Cheng W, Feng J. 2021. Extensive cortical functional connectivity of the human hippocampal memory system. [DOI] [PubMed]
- Maguire EA, Intraub H, Mullally SL. 2016. Scenes, spaces, and memory traces: what does the hippocampus do? Neuroscientist. 22:432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner AD. 2017. How do the two visual streams interact with each other? Exp Brain Res. 235:1297–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal DW, Pandya DN. 2012. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res Bull. 87:457–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr S, Liu N, Devaney KJ, Yue X, Rajimehr R, Ungerleider LG, Tootell RB. 2011. Scene-selective cortical regions in human and nonhuman primates. J Neurosci. 31:13771–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilssen, Doan TP, Nigro MJ, Ohara S, Witter MP. 2019. Neurons and networks in the entorhinal cortex: a reappraisal of the lateral and medial entorhinal subdivisions mediating parallel cortical pathways. Hippocampus. 29:1238–1254. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B, Petrides M, Cipolloni PB. 2015. Cerebral cortex: architecture, connections, and the dual origin concept. New York: Oxford University Press. [Google Scholar]
- Pauli WM, Nili AN, Tyszka JM. 2018. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci Data. 5:180063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. 2014. Neuroanatomy of language regions of the human brain. New York: Academic Press. [Google Scholar]
- Robertson RG, Rolls ET, Georges-François P. 1998. Spatial view cells in the primate hippocampus: effects of removal of view details. J Neurophysiol. 79:1145–1156. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Van Hoesen GW. 1999. Some temporal and parietal cortical connections converge in CA1 of the primate hippocampus. Cereb Cortex. 9:232–237. [DOI] [PubMed] [Google Scholar]
- Rolls ET. 1989. Functions of neuronal networks in the hippocampus and neocortex in memory. In: Byrne JH, Berry WO, editors. Neural models of plasticity: experimental and theoretical approaches. San Diego: Academic Press, pp. 240–265. [Google Scholar]
- Rolls ET. 2013. The mechanisms for pattern completion and pattern separation in the hippocampus. Front Syst Neurosci. 7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. 2016a. Cerebral cortex: principles of operation. Oxford: Oxford University Press. [Google Scholar]
- Rolls ET. 2016b. Pattern separation, completion, and categorisation in the hippocampus and neocortex. Neurobiol Learn Mem. 129:4–28. [DOI] [PubMed] [Google Scholar]
- Rolls ET. 2018. The storage and recall of memories in the hippocampo-cortical system. Cell Tissue Res. 373:577–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. 2019a. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct. 224(9):3001–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. 2019b. The orbitofrontal cortex. Oxford: Oxford University Press. [Google Scholar]
- Rolls ET. 2020. Neural computations underlying phenomenal consciousness: a higher order syntactic thought theory. Front Psychol. 11:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. 2021a. Neurons including hippocampal spatial view cells, and navigation in primates including humans. Hippocampus. doi: 10.1002/hipo.23324. [DOI] [PubMed] [Google Scholar]
- Rolls ET. 2021b. Brain computations: what and how. Oxford: Oxford University Press. [Google Scholar]
- Rolls ET. 2021c. A neuroscience levels of explanation approach to the mind and the brain. Front Comput Neurosci. 15:649679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Cheng W, Gilson M, Qiu J, Hu Z, Ruan H, Li Y, Huang CC, Yang AC, Tsai SJ, et al. 2018. Effective connectivity in depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 3:187–197. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Deco G, Huang CC, Feng J. 2021. The effective connectivity of the human hippocampal memory system. [DOI] [PubMed]
- Rolls ET, Franco L, Stringer SM. 2005a. The perirhinal cortex and long-term familiarity memory. Q J Exp Psychol B. 58:234–245. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Mills P. 2019. The generation of time in the hippocampal memory system. Cell Rep. 28:1649–1658.e6. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Robertson RG, Georges-François P. 1997. Spatial view cells in the primate hippocampus. Eur J Neurosci. 9:1789–1794. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Treves A. 2011. The neuronal encoding of information in the brain. Prog Neurobiol. 95:448–490. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Treves A, Robertson RG, Georges-François P, Panzeri S. 1998. Information about spatial view in an ensemble of primate hippocampal cells. J Neurophysiol. 79:1797–1813. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Wirth S. 2018. Spatial representations in the primate hippocampus, and their functions in memory and navigation. Prog Neurobiol. 171:90–113. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Xiang J-Z. 2006. Spatial view cells in the primate hippocampus, and memory recall. Rev Neurosci. 17:175–200. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Xiang J-Z, Franco L. 2005b. Object, space and object-space representations in the primate hippocampus. J Neurophysiol. 94:833–844. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Tanaka K. 1996. Divergent projections from the anterior inferotemporal area TE to the perirhinal and entorhinal cortices in the macaque monkey. J Neurosci. 16:4757–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinke MRT, Otte WM, Christiaens D, Schmitt O, Leemans A, van der Toorn A, Sarabdjitsingh RA, Joels M, Dijkhuizen RM. 2018. Diffusion MRI-based cortical connectome reconstruction: dependency on tractography procedures and neuroanatomical characteristics. Brain Struct Funct. 223:2269–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. 2002. Fast robust automated brain extraction. Hum Brain Mapp. 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Tournier JD, Calamante F, Connelly A. 2012. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. 62:1924–1938. [DOI] [PubMed] [Google Scholar]
- Smith RE, Tournier JD, Calamante F, Connelly A. 2013. SIFT: spherical-deconvolution informed filtering of tractograms. Neuroimage. 67:298–312. [DOI] [PubMed] [Google Scholar]
- Smith RE, Tournier JD, Calamante F, Connelly A. 2015. SIFT2: enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. Neuroimage. 119:338–351. [DOI] [PubMed] [Google Scholar]
- Sulpizio V, Galati G, Fattori P, Galletti C, Pitzalis S. 2020. A common neural substrate for processing scenes and egomotion-compatible visual motion. Brain Struct Funct. 225:2091–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. 1990. Cortical inputs to the CA1 field of the monkey hippocampus originate from the perirhinal and parahippocampal cortex but not from area TE. Neurosci Lett. 115:43–48. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. 1994. Perirhinal and parahippocampal cortices of the macaque monkey - cortical afferents. J Comp Neurol. 350:497–533. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Connelly A. 2012. MRtrix: diffusion tractography in crossing fiber regions. Int J Imag Syst Tech. 22:53–66. [Google Scholar]
- Treves A, Rolls ET. 1992. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 2:189–199. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. 1994. A computational analysis of the role of the hippocampus in memory. Hippocampus. 4:374–391. [DOI] [PubMed] [Google Scholar]
- Tsao A, Sugar J, Lu L, Wang C, Knierim JJ, Moser MB, Moser EI. 2018. Integrating time from experience in the lateral entorhinal cortex. Nature. 561:57–62. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15:273–289. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG. 1995. Functional brain imaging studies of cortical mechanisms for memory. Science. 270:769–775. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. 1994. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 4:157–165. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. 1982. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. Cambridge (MA): MIT Press, pp. 549–586. [Google Scholar]
- van den Heuvel MP, de Reus MA, Feldman Barrett L, Scholtens LH, Coopmans FM, Schmidt R, Preuss TM, Rilling JK, Li L. 2015. Comparison of diffusion tractography and tract-tracing measures of connectivity strength in rhesus macaque connectome. Hum Brain Mapp. 36:3064–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN. 1975. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res. 95:1–24. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW. 1982. The parahippocampal gyrus. New observations regarding its cortical connections in the monkey. Trends Neurosci. 5:345–350. [Google Scholar]
- Vogt BA, editor. 2009. Cingulate neurobiology and disease. Oxford University Press. [Google Scholar]
- Winterburn JL, Pruessner JC, Chavez S, Schira MM, Lobaugh NJ, Voineskos AN, Chakravarty MM. 2013. A novel in vivo atlas of human hippocampal subfields using high-resolution 3 T magnetic resonance imaging. Neuroimage. 74:254–265. [DOI] [PubMed] [Google Scholar]
- Yukie M. 2000. Connections between the medial temporal cortex and the CA1 subfield of the hippocampal formation in the Japanese monkey (Macaca fuscata). J Comp Neurol. 423:282–298. [DOI] [PubMed] [Google Scholar]
- Zhong YM, Rockland KS. 2004. Connections between the anterior inferotemporal cortex (area TE) and CA1 of the hippocampus in monkey. Exp Brain Res. 155:311–319. [DOI] [PubMed] [Google Scholar]
- Zhong YM, Yukie M, Rockland KS. 2005. Direct projections from CA1 to the superior temporal sulcus in the monkey, revealed by single axon analysis. Brain Res. 1035:211–214. [DOI] [PubMed] [Google Scholar]
- Zhong YM, Yukie M, Rockland KS. 2006. Distinctive morphology of hippocampal CA1 terminations in orbital and medial frontal cortex in macaque monkeys. Exp Brain Res. 169:549–553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


