Abstract
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique, which has been increasingly used as an investigational tool in neuroscience. In social and affective neuroscience research, the prefrontal cortex has been primarily targeted, since this brain region is critically involved in complex psychobiological processes subserving both Șhotș and Școldș domains. Although several studies have suggested that prefrontal tDCS can enhance neuropsychological outcomes, meta-analyses have reported conflicting results. Therefore, we aimed to assess the available evidence by performing an umbrella review of meta-analyses. We evaluated the effects of prefrontal active vs sham tDCS on different domains of cognition among healthy and neuropsychiatric individuals. A MeaSurement Tool to Assess Systematic Reviews 2 was employed to evaluate the quality of meta-analyses, and the GRADE system was employed to grade the quality of evidence of every comparison from each meta-analysis. PubMed/MEDLINE, PsycINFO and the Cochrane Database of Systematic Reviews were searched, and 11 meta-analyses were included resulting in 55 comparisons. Only 16 comparisons reported significant effects favoring tDCS, but 13 of them had either very low or low quality of evidence. Of the remaining 39 comparisons which reported non-significant effects, 38 had either very low or low quality of evidence. Meta-analyses were rated as having critically low and low quality. Among several reasons to explain these findings, the lack of consensus and reproducibility in tDCS research is discussed.
Keywords: umbrella review, non-invasive brain stimulation, cognition, psychology, psychiatry, reproducibility
Introduction
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that consists on the application of weak, electric currents over the scalp (Brunoni et al., 2012). Since the seminal study of Nitsche and Paulus (2000), which showed that tDCS promoted polarity-dependent changes in motor cortical excitability according to the parameters of stimulation, the technique has been investigated as a clinical and research tool in neuropsychology (Shin et al., 2015) and neuropsychiatry (Moffa et al., 2018; Brunoni et al., 2019).
For these conditions, the prefrontal cortex (PFC) has been the preferential target of tDCS, since it is the brain region primarily involved in more complex psychological processes, including cognitive and emotional domains (Shin et al., 2015). In fact, several studies have investigated the effects of prefrontal tDCS on neuropsychological outcomes, such as working memory (Oliveira et al., 2013), cognitive control (Wolkenstein and Plewnia, 2013), vigilance to threat (Ironside et al., 2016) and rumination (Kuhn et al., 2012), mostly showing significant results. Nonetheless, non-significant results have also been found, with a recent meta-analysis suggesting that the net tDCS effects on cognition are null (Horvath et al., 2015). Several reasons could explain these heterogeneous findings, such as differences in tDCS montage, stimulation parameters and anatomical and functional individualities (Brunoni et al., 2012; Bikson et al., 2018; Chase et al., 2019). Issues in the design of tDCS studies also harm their internal validity, such as underpowered sample sizes (Medina and Cason, 2017) and methodological challenges in effective sham blinding (Fonteneau et al., 2019). To a broader extent, biases in cognitive sciences have been increasingly more common, with contradictory and non-replicable findings (Ioannidis et al., 2014). For instance, an attempt to reproduce the findings of 100 experimental and correlation studies in psychological science, using high-powered designs, was able to replicate approximately one-third of the them (Open Science Collaboration, 2015), which is suggestive of a Șreproducibility crisisș on the field.
Recently, umbrella reviews (URs) have been introduced as a new meta-analytical modality in evidence-based synthesis (Fusar-Poli and Radua, 2018). They are reviews of previous systematic reviews and meta-analyses that use standardized methods to assess and compare the evidence of included studies. Examples of these methods include performing a systematic review of the literature, using common effect sizes, assessing heterogeneity, grading the quality of evidence and presenting new research avenues based on the assessed evidence (Fusar-Poli and Radua, 2018). In fact, they represent a higher level of evidence than meta-analyses that can also present biases and reach discrepant conclusions (Ioannidis, 2009). For these reasons, URs are becoming increasingly in the biomedical field (Fusar-Poli and Radua, 2018) as a method to synthesize highest-quality evidence. Considering the discrepant findings of meta-analyses examining the effects of tDCS on Șhotș and Școldș cognition, an UR could be useful to critically assess the quality and availability of the evidence. Notwithstanding, no such study has been performed so far.
Therefore, our aim was to perform an UR of meta-analyses that examined the effects of prefrontal tDCS on cognition. Our study is important to provide critical, high-quality evidence of a commonly used tDCS application in neuropsychology, which can help better guiding and tailoring new studies according to our findings.
Methods
Search strategy and inclusion criteria for the UR
The protocol for this systematic review was pre-registered at PROSPERO (CRD42020140779). The electronic databases of PubMed, PsycINFO and the Cochrane Database of Systematic Reviews (CDSR) were searched in April 2019 for relevant references. Search strategies were tailored for each database, and detailed descriptions can be found on the supplementary data file. The search strategy was limited to meta-analyses in each one of the databases. The references section of review articles and meta-analyses were carefully read to look for additional references. No language restrictions were applied. No further efforts were made to search for unpublished research.
Titles and abstracts of references were screened by two independent reviewers (ARB, LCF) to identify those that were eligible for inclusion. Inclusion criteria were determined through the PICO (population, intervention, control and outcome) format; specifically, meta-analyses had to evaluate the comparative effects of prefrontal tDCS against sham tDCS on cognitive domains in healthy or neuropsychiatric individuals. No restrictions were made regarding age; diagnoses, i.e. any neuropsychiatric disorder was eligible for inclusion, e.g. depression, attention-deficit hyperactivity disorder, eating disorders, Parkinsonșs disease, etc.; polarity of tDCS, i.e. anodal tDCS (a-tDCS) and cathodal tDCS (c-tDCS) were eligible; number of treatment sessions; timing of outcome measurement, i.e. online and offline designs, when the study outcomes were measured during and after tDCS session, respectively, were eligible; and cognitive domains, i.e. any cognitive domain reported in the eligible meta-analysis were included in the UR. Only meta-analyses were eligible for our UR as we were interested on the effect sizes of tDCS interventions over the PFC.
Data extraction, methodological quality assessment and appraisal of the evidence
Data were extracted by two independent reviewers (ARB, LCF); any disagreement was solved through discussing and obtaining more information from study investigators. For each comparison from eligible meta-analyses, the following data were extracted: first author, year of publication, cognitive domain, cognitive tasks, number of studies included, pooled effect sizes‒either standardized mean difference (SMD) or Hedgesș g‒with their 95% confidence intervals (95% CIs) and I2 values. If the Q-statistic was provided, I2 was calculated as recommended by the Cochrane Handbook for Systematic Reviews of Interventions. Data were extracted in Summary of Finding (SoF) tables from the GRADEpro GDT (Grading of Recommendations, Assessment, Development and Evaluation Guideline Development Tool). The GRADEpro GDT can be accessed through the link www.gradepro.org and is a GRADE working group software for the production of SoF tables. SoF tables are tabular presentations of key information about relevant outcomes of health care interventions. For this UR, separate SoF tables were created for healthy and neuropsychiatric populations, as well as for a-tDCS, c-tDCS and tDCS.
The GRADE approach was employed (The GRADE Working Group, 2013) to rate the quality of evidence of every comparison from each eligible meta-analysis. The GRADE approach is a system for rating the quality of evidence in systematic reviews and/or meta-analysis, providing four grades depending on the certainty that the true effect is close to the effect size estimate: high, moderate, low and very low. It considers five reasons to possibly rate down, and three to possibly rate up, the quality of evidence. Factors that rate down quality of evidence are (a) risk of bias (RoB), (b) inconsistency of results, (c) indirectness of evidence, (d) imprecision and (e) publication bias. Factors that rate up the quality of evidence are (a) large magnitude of effect, (b) dose-response gradient and (c) effect of plausible residual confounding. A GRADE checklist to aid in the consistency and reproducibility of the GRADE approach was also employed (Meader et al., 2014). Quality of evidence was evaluated at the comparison level based on what was reported in each meta-analysis, and information from the trials included in each meta-analysis were not retrieved. A strict approach was employed to grade the quality of evidence to avoid validating results that were not of the highest possible quality. For instance, if statistical test for heterogeneity and/or I2 values were not reported, the quality of evidence was downgraded due to serious inconsistency regardless of the distribution of effect sizes and 95% CI‒those could only contribute to rate down further the quality of evidence due to very serious inconsistency. For RoB, a meta-analysis was considered to have not properly examined RoB if it did not perform a quality assessment of the included studies, i.e. if it did not check for methodological procedures that minimize biases, such as proper randomization, allocation concealment, blinding, attrition and incomplete or selective reporting, as described in the Cochrane guidelines for risk of bias assessment (Higgins et al., 2011; Sterne et al., 2019); quality of evidence was systematically rated down due to serious RoB if RoB was not properly examined. For publication bias, if meta-analyses did not evaluate publication bias either through funnel plot asymmetry or statistical criteria, quality of evidence was rated down due to strong suggestion of publication bias. Additionally, adjusted effect sizes, e.g. through the trim-and-fill method, were not considered a solution to the identified publication bias as these are simulations with issues of their own (Guyatt et al., 2011) and quality of evidence was downgraded regardless of whether such imputation approaches were employed or not; similarly, considering recommendations from the Cochrane Handbook for Systematic Reviews of Interventions, the fail-safe number was not considered an adequate assessment of publication bias when employed alone. For comparisons which included individuals with different neuropsychiatric disorders, quality of evidence was systematically rated down due to serious indirectness.
The methodological quality of each included meta-analysis was rated with the A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR-2) (Shea et al., 2017), a 16-item tool employed to help in the evaluation of the reporting quality of systematic reviews. A detailed description of the AMSTAR-2 methodology can be found at the supplementary data file. The online AMSTAR-2 checklist available at https://amstar.ca/Amstar_Checklist.php was employed to apply the AMSTAR-2 methodology to each meta-analysis included in the UR.
Results
Study selection and included meta-analysis
Figure 1 illustrates the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) flowchart representing the selection of studies for this UR. Of the 40 references excluded through screening of titles and abstracts, 16 were excluded as prefrontal tDCS was not the intervention studied 16 were excluded as the outcome evaluated was not a cognitive function and 8 studies were excluded as they were not meta-analyses. Of the nine references excluded through reading the full text, two reported results also presented in another paper which was already included in the UR (Dedoncker et al., 2016a; Hall et al., 2017), three did not report results from an independent comparison between active and sham prefrontal tDCS (Jansen et al., 2013; Brunoni and Vanderhasselt, 2014; Song et al., 2018), and four did not carry out meta-analyses (Khalighinejad et al., 2016; Lupi et al., 2017; Greenwood et al., 2018; Schluter et al., 2018).
Fig. 1 .
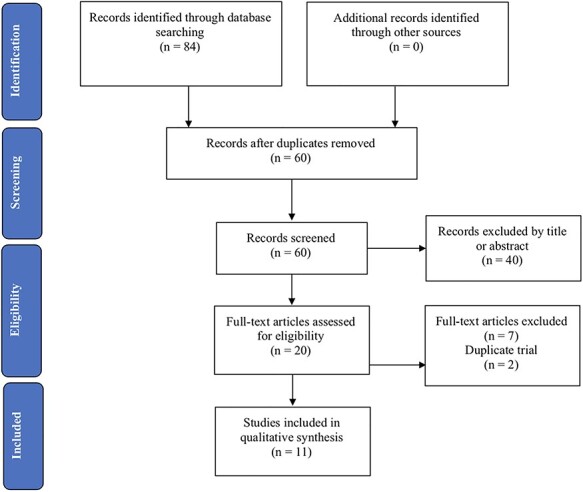
PRISMA flowchart depicting study selection results.
Therefore, only 11 articles were included in this UR (Horvath et al., 2015; Price et al., 2015; Hill et al., 2016; Mancuso et al., 2016; Dedoncker et al., 2016b; Lowe et al., 2017; Nilsson et al., 2017; Bell and DeWall, 2018; Imburgio and Orr, 2018; Mostafavi et al., 2018; Salehinejad et al., 2019). These 11 meta-analyses yielded 55 comparisons distributed across the 12 cognitive domains; specifically, working memory, [long-term] memory, set shifting, response inhibition, language, aggression, overeating/food cravings, emotional and implicit bias, honesty, rumination, impulsivity and risk taking were the cognitive domains reported in these meta-analyses and were therefore included in our UR. The respective tasks for each one of these cognitive domains are depicted in Table 1. Of the 55 comparisons, 41 (~75%) were carried out among exclusively healthy individuals, and another 7 (~13%) were carried out among a mixed population of healthy and neuropsychiatric individuals; therefore, only 7 comparisons (~13%) were carried out among exclusively neuropsychiatric individuals. Table 2 illustrates the methodological quality assessment of each meta-analysis, while Table 3 describes the characteristics and quality of evidence assessment of each comparison among healthy (Table 3A), neuropsychiatric (Table 3B) and both healthy and neuropsychiatric (Table 3C) individuals.
Table 1.
Description of the outcomes and tasks included in the UR for each cognitive domain
| Cognitive domain | Outcomes and respective tasks | Meta-analysis investigating each outcome |
|---|---|---|
| Working memory | Accuracy, reaction time, dș values and working memory index in a multitude of working memory tasks such as 0-back, 1-back, 2-back, 3-back, n-back, Sternberg, Stroop, digit-span task, block-tapping task, paced auditory serial addition test, operation word span, working memory scale, Tower of London | Horvath et al., 2015, Dedoncker et al., 2016a, b, Hill et al., 2016, Mancuso et al., 2016, Nilsson et al., 2017, Imburgio and Orr, 2018, Salenijehad et al. 2019 |
| [Long-term] memory | Accuracy in the recognition memory task and the long-term verbal memory task | Horvath et al., 2015, Dedoncker et al., 2016a, b |
| Set shifting | Switch cost, resumption lag and errors in the task sequence learning, affective financial management, cognitive and motor set shifting task and the paced auditory serial addition test | Horvath et al., 2015, Dedoncker et al., 2016a, b, Imburgio and Orr, 2018, Salehinejad et al., 2019 |
| Response inhibition | Incongruent reaction time, flanker effect, accuracy and stop signal reaction time in the stroop, flanker, stop signal task, go/no-go task, simon | Horvath et al., 2015, Dedoncker et al., 2016a, b, Imburgio and Orr, 2018, Salehinejad et al., 2019 |
| Language | Accuracy and number of words in verbal fluency tasks | Horvath et al., 2015, Price et al., 2015 |
| Aggression | Aggression score in the Taylor aggression paradigm and negative affect state after a frustrating task | Bell and DeWall, 2018 |
| Overeating/food cravings | Subjective report of food and sweet cravings, visual analog scale scores, food craving questionnaire scores | Bell and DeWall, 2018/Mancuso et al., 2016, Lowe et al., 2017 |
| Emotional and implicit bias | Negativity rating after viewing both neutral and negative valence pictures, judgment score of a moral dilemma | Horvath et al., 2015, Bell and DeWall, 2018 |
| Honesty | Lying or reaction time in trust/truth games | Bell and DeWall, 2018 |
| Rumination | Rumination scores on the rumination response scale | Horvath et al., 2015 |
| Impulsivity | Error rate, errors in easy condition and errors on incorrect trials in the stroop, sentence completion task, cognitive reflection test | Bell and DeWall, 2018 |
| Risk taking | Number of pumps, high-risk choices, riskiness in gains, number of low-probability/high-reward choices in the balloon analog risk task, Columbia Card Task and gambling tasks (e.g. Iowa Gambling Task) | Horvath et al., 2015, Bell and DeWall, 2018 |
Table 2.
Methodological quality assessment of the included systematic reviews using the AMSTAR-2 tool
| Salehinejad et al., 2019 | Bell and DeWall, 2018 | Imburgio and Orr, 2018 | Mostafavi et al., 2018 | Lowe et al., 2017 | Nilsson et al., 2017 | Dedoncker et al., 2016b | Hill et al., 2016 | Mancuso et al., 2016 | Horvath et al., 2015 | Price et al., 2015 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PICO for research question | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Protocol determined a priori | No | No | No | Yes | No | No | No | Yes | No | No | No |
| 3. Inclusion criteria explained | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Comprehensive literature search | Yes | Yes | Partially yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| 5. Study selection in duplicate | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | No | Yes |
| 6. Data extraction in duplicate | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | No | Yes |
| 7. List of the excluded studies | No | No | No | No | No | No | Yes | No | No | No | No |
| 8. Thorough description of included trials | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 9. Risk of bias (RoB) assessed | Yes | No | No | No | Yes | No | Yes | Yes | No | No | No |
| 10. Included studiesș funding sources | No | No | No | No | No | No | No | No | No | No | No |
| 11. Meta-analysis statistics adequate | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12. Considered impact of RoB in studies effect sizes | No | - | - | - | No | - | No | No | - | - | - |
| 13. Discussed RoB | Yes | - | - | - | Yes | - | Yes | Yes | - | - | - |
| 14. Discussed heterogeneity findings | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No |
| 15. Examined publication bias? | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| 16. Disclosures | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
Bold indicates critical domains.
Table 3.
Characteristics of included comparisons of the effects of tDCS over the PFC on different domains of cognition among healthy individuals
| Identification | Intervention | Target | Task | Outcome | Effect size (95% CI) | P-value | N studies | n individuals (active vs sham) | Heterogeneity (I2 value) | Publication bias | GRADE certainty |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) | |||||||||||
| Working memory (WM) | |||||||||||
| Imburgio and Orr, 2018 | a-tDCS (offline and online) | DLPFC | 3-back, WAIS-WM, Sternberg, delayed-response working memory |
Accuracy, working memory index | 0.56 (0.19-0.93) | <0.01 | 10 | 354 (177 vs 177) | 0% | Not suggested | Low |
| Nilsson et al., 2017 | a-tDCS (offline and online) + cognitive training | DLPFC | n-back, Sternberg, digit-span task, Corsi block- tapping task, Stroop and others |
Accuracy, reaction time and dș values | 0.07 (−0.21-0.34) | 0.64 | 7 | 266 (131 vs 135) | 0% | Not suggested | Low |
| Mancuso et al., 2016 | a-tDCS + cognitive training | lDLPFC | n-back, PASAT, Sternberg, OSPAN, digit-span task, block-tapping task and others | Accuracy and reaction time | 0.29 (0.06-0.52) | Not reported | 10 | 285 (144 vs 141) | 0% | Funnel plot asymmetry identified | Very low |
| a-tDCS (offline and online) | 0.17 (0.03-0.30) | Not reported | 23 | 768 (385 vs 383) | Low | ||||||
| rDLPFC | 0.04 (−0.19-0.27) | Not reported | 8 | 283 (144 vs 139) | Very low | ||||||
| Hill et al., 2016 | a-tDCS (offline and online) | DLPFC | n-back, Sternberg and digit-span task | Accuracy | 0.15 (0.02-0.28) | 0.02 | 32 | 914 (459 vs 455) | 0% | Not suggested | Moderate |
| Reaction time | −0.15 (−0.29 - −0.01) | 0.003 | 28 | 814 (407 vs 407) | |||||||
| Horvath et al., 2015 | a-tDCS (offline) | lDLPFC | Digit-span task, Sternberg, 0-back, 1-back, 2-back, 3-back |
Accuracy | 0.17 (−0.06-0.40) | 0.151 | 13 | 293 (140 vs 153) | Not evaluated | Not evaluated | Very low |
| a-tDCS (online) | 0.29 (−0.04-0.62) | 0.089 | 8 | 167 (83 vs 84) | |||||||
| Reaction time | −0.10 (−0.37-0.17) | 0.474 | |||||||||
| [Long-term] memory | |||||||||||
| Horvath et al., 2015 | a-tDCS (online) | lDLPFC | Recognition memory task, long- term verbal memory |
Accuracy | 1.03 (−0.87-2.94) | 0.235 | 3 | 104 (52 vs 52) | Not evaluated | Not evaluated | Very low |
| c-tDCS (online) | −0.81 (−2.14-0.51) | 0.23 | |||||||||
| Set shifting | |||||||||||
| Horvath et al., 2015 | a-tDCS (offline) | lDLPFC | PASAT, cognitive and motor set shifting tasks | Errors | 0.03 (−0.27-0.32) | 0.836 | 3 | 212 (106 vs 106) | Not evaluated | Not evaluated | Very low |
| Imburgio and Orr, 2018 | a-tDCS (offline and online) | DLPFC | Task sequence learning,affective financial management | Switch cost, resumption lag | −0.04 (−0.68-0.59) | 0.552 | 13 | 648 (324 vs 324) | 69.92% | Not suggested | Very low |
| Response inhibition | |||||||||||
| Horvath et al., 2015 | a-tDCS (offline) | IFG | Stop signal task | Stop signal reaction time | −0.17 (−0.72-0.36) | 0.548 | 2 | 55 (21 vs 34) | Not evaluated | Not evaluated | Very low |
| −0.48 (−1.09-0.13) | 0.12 | ||||||||||
| rDLPFC | Stroop | Completion time | 0.49 (−0.06-0.98) | 0.082 | 3 | 60 (30 vs 30) | |||||
| lDLPFC | 0.56 (−1.60-0.48) | 0.291 | |||||||||
| Imburgio and Orr, 2018 | a-tDCS (offline and online) | DLPFC | Stroop, flanker, stop signal task, cognitive reflection test, go/no-go level 3, Simon | Incongruent reaction time, flanker effect, stop signal reaction time, accuracy | −0.10 (−0.45-0.24) | 0.55 | 13 | 616 (308 vs 308) | 11.49% | Not suggested | Low |
| Memory/attention/executive functioning | |||||||||||
| Dedoncker et al., 2016b | a-tDCS (offline and online) | DLPFC | Digit span from WAIS II, SART, detection task: picture and sentence, go/no-go, 2-back, 3-back, n-back, EAT, GDT, BART IAT, Sternberg, PASAT, CPT, TOL, RAT and others |
Accuracy | 0.04 (−0.02-0.11) | 0.19 | 131 | 4415 (2208 vs 2207) | 24% | Not suggested | Moderate |
| Reaction time | −0.10 (−0.16‒-0.04) | < 0.01 | 102 | 3470 (1735 vs 1735) | 0% | Not suggested | Moderate | ||||
| Language | |||||||||||
| Price et al., 2015 | a-tDCS (offline) | lPFC | Verbal fluency | Accuracy | 0.48 (0.04-0.92) | Not reported | 3 | 80 (40 vs 40) | Not evaluated | Not evaluated | Very low |
| a-tDCS (online) | lPFC | 0.33 (−0.06-0.73) | 100 (50 vs 50) | ||||||||
| Horvath et al., 2015 | a-tDCS (offline) | lDLPFC | Verbal fluency | Number of words | 0.23 (−0.09-0.55) | 0.16 | 7 | 208 (104 vs 104) | Not evaluated | Not evaluated | Very low |
| a-tDCS (online) | 0.35 (−0.22-0.91) | 0.226 | 3 | 100 (50 vs 50) | |||||||
| a-tDCS (offline) | Novel language learning | Accuracy | −0.01 (−0.50-0.48) | 0.416 | 2 | 58 (29 vs 29) | |||||
| Aggression | |||||||||||
| Bell and DeWall, 2018 | a-tDCS (offline and online) | DLPFC | Taylor aggression paradigm, frustrating task (PASAT), essay criticism paradigm | Total aggression, PANAS negative affect, anger and state rumination | −0.17 (−0.44-0.09) | 0.19 | 6 | 339 (170 vs 169) | 29.48% | Not suggested | Very low |
| Overeating | |||||||||||
| Bell and DeWall, 2018 | a-tDCS (offline and online) | DLPFC | - | Calories eaten, desire to eat high- calorie foods, food cravings, appetite for sweets, sweet craving |
−0.25 (−0.49‒-0.01) | 0.03 | 7 | 326 (163 vs 163) | 17.69% | Funnel plot asymmetry identified | Very low |
| Emotional and implicit bias | |||||||||||
| Horvath et al., 2015 | a-tDCS (online) | lDLPFC | Negative valence pictures | Negativity rating | −0.17 (−0.52-0.18) | 0.333 | 3 | 134 (59 vs 75) | Not evaluated | Not evaluated | Very low |
| Neutral valence pictures | 0.01 (−0.42-0.45) | 0.957 | 2 | 88 (36 vs 52) | |||||||
| rDLPFC | Negative valence pictures |
−0.19 (−1.20-0.81) | 0.709 | 88 (43 vs 45) | |||||||
| Neutral valence pictures | 0.12 (−0.30-0.54) | 0.575 | |||||||||
| c-tDCS (online) | lDLPFC | Negative valence pictures | 0.03 (−0.54-0.60) | 0.915 | 2 | 88 (36 vs 52) | |||||
| Neutral valence pictures | 0.37 (−0.06-0.81) | 0.094 | |||||||||
| Bell and DeWall, 2018 | a-tDCS (offline and online) | DLPFC | Moral dilemma value judgment score; affective biasing task with emotional go/no-go; race IAT |
−0.25 (−0.48‒-0.03) | 0.02 | 7 | 447 (224 vs 223) | 12.79% | Not suggested | Low | |
| Honesty | |||||||||||
| Bell and DeWall, 2018 | a-tDCS (offline and online) | DLPFC | Selection trials, trust game, truth response (personal information) |
Reaction time, lying | 0.06 (−0.16-0.28) | 0.57 | 4 | 322 (162 vs 160) | 0% | Not suggested | Low |
| Rumination | |||||||||||
| Horvath et al., 2015 | a-tDCS (offline) | DLPFC | - | Rumination Response Scale | −0.01 (−0.36-0.34) | 0.95 | 2 | 126 (61 vs 65) | Not evaluated | Not evaluated | Very low |
| Impulsivity | |||||||||||
| Bell and DeWall, 2018 | a-tDCS (offline and online) | DLPFC | Stroop, sentence completion task, CRT problem-solving task | Error rate, errors in easy condition, errors on incorrect trials |
−0.02 (−0.21-0.16) | 0.70 | 9 | 676 (338 vs 338) | 33.17% | Not suggested | Low |
| Risk taking | |||||||||||
| Horvath et al., 2015 | a-tDCS (online) | DLPFC | BART, risk task, gambling task | Number of low- probability/high-reward choices |
−0.67 (−2.39-1.06) | 0.451 | 3 | 76 (38 vs 38) | Not evaluated | Not evaluated | Very low |
| c-tDCS (online) | −1.70 (−3.61-0.22) | 0.082 | 126 (61 vs 65) | ||||||||
| Bell and DeWall, 2018 | a-tDCS (offline and online) | DLPFC | BART, Columbia Card Task, Iowa Gambling Task | Number of pumps, high-risk choice, riskiness in gains |
−0.36 (−0.65‒−0.07) | 0.01 | 13 | 676 (338 vs 338) | 65.50% | Funnel plot asymmetry identified | Very low |
| (B) | |||||||||||
| Working memory | |||||||||||
| Hill et al., 2016 | a-tDCS (offline and online) | DLPFC | n-back, Sternberg and digit-span task | Accuracy | 0.11 (−0.07-0.29) | 0.24 | 15 | 860 (430 vs 430) | 10% | Not suggested | Low |
| Reaction time | −0.14 (−0.39-0.11) | 0.26 | 8 | 232 (126 vs 126) | 0% | Very low | |||||
| Salehinejad et al., 2019 | a-tDCS (online and offline) | DLPFC | Digit span, Corsi cube, 1-back, 2-back, quantified behavior | Amount, accuracy, reaction time, quantified behavior score |
0.12 (−0.32-0.55) | 0.5996 | 16 | 382 (191 vs 191) | 0% | Not evaluated | Very low |
| Response inhibition | |||||||||||
| Salehinejad et al., 2019 | a-tDCS (offline and online) | DLPFC + rIFG | Go/no-go, stop signal task, CPT, flanker, Stroop, | Reaction time, accuracy, true-positive errors, false- positive errors, omission errors, commission errors, correct responses, etc. |
0.26 (0.07-0.44) | 0.0079 | 34 | 1404 (702 vs 702) | 0% | Not evaluated | Very low |
| c-tDCS (offline and online) | 0.09 (−0.19-0.37) | 0.53 | 13 | 468 (234 vs 234) | 0% | Not evaluated | Very low | ||||
| Memory/attention/executive functioning | |||||||||||
| Dedoncker et al., 2016b | a-tDCS (offline and online) | DLPFC | Digit span from WAIS II, SART, detection task: picture and sentence, go/no-go, 2-back, 3-back, n-back, EAT, GDT, BART, IAT, Sternberg, PASAT, CPT, TOL, RAT and others |
Accuracy | 0.22 (0.04-0.40) | < 0.05 | 30 | 944 (472 vs 472) | 42.5% | Not suggested | Very low |
| Reaction time | −0.15 (−0.30-0.01) | 0.065 | 22 | 660 (330 vs 330) | 0% | Not suggested | Low | ||||
| (C) | |||||||||||
| Memory/attention/executive functioning | |||||||||||
| Dedoncker et al., 2016b | a-tDCS (offline and online) | DLPFC | Digit span from WAIS II, SART, detection task: picture and sentence, go/no-go, 2-back, 3-back, n-back, EAT, GDT, BART, IAT, Sternberg, PASAT, CPT, TOL, RAT and others |
Accuracy | 0.18 (0.03-0.18) | < 0.01 | 165 | 5359 (2680 vs 2679) | 52.50% | Not suggested | Very low |
| Reaction time | −0.11 (−0.17 - -0.05) | < 0.01 | 124 | 4130 (2065 vs 2065) | 0% | Low | |||||
| c-tDCS (offline and online) | Accuracy | 0.03 (−0.13-0.19) | 0.70 | 28 | 942 (471 vs 471) | 33.8% | Not suggested | Very low | |||
| Reaction time | 0.18 (−0.07-0.44) | 0.16 | 36 | 1182 (591 vs 591) | 82.5% | Very low | |||||
| Food cravings | |||||||||||
| Lowe et al., 2017 | tDCS | DLPFC | - | Visual analog scale, Food Craving Questionnaire | −0.25 (−0.80-0.29) | 0.3647 | 4 | 114 (57 vs 57) | Not reported | Funnel plot asymmetry identified | Very low |
| Mostafavi et al., 2018 | tDCS | DLPFC | - | Food Craving Questionnaire | −0.54 (−0.85‒−0.24) | <0.001 | 4 | 145 (72 vs 73) | 0% | Not suggested | Very low |
| Visual analog scale | −0.78 (−1.12‒-0.44) | <0.001 | 13 | 416 (208 vs 208) | 71.4% | ||||||
a-tDCS, anodal tDCS; c-tDCS, cathodal tDCS; DLPFC, dorsolateral PFC; rDLPFC, right dorsolateral PFC; lDLPFC, lateral dorsolateral PFC; IFG, inferior frontal gyrus; lPFC, left prefrontal cortex; PASAT, paced auditory serial addition test; OSPAN, operation span; WAIS-WM, Wechsler Adult Intelligence Scale Working Memory; SART, sustained attention to respond task; TOL, Tower of London; RAT, Remote Associates Test; GDT, game of dice task; BART, balloon analog risk task; EAT, error awareness task; IAT, implicit association test; CRT, cognitive reflection test; PANAS, positive and negative affect schedule. Values in bold represent significant comparisons.
Anodal tDCS
Working memory. Six meta-analyses (Horvath et al., 2015, Hill et al., 2016, Mancuso et al., 2016, Nilsson et al., 2017, Imburgio and Orr, 2018, Salehinejad et al. 2019) resulting in 13 comparisons, 10 among healthy and 3 among neuropsychiatric individuals, evaluated the effects of prefrontal a-tDCS on working memory performance. AMSTAR-2 quality assessment indicated that these reviews varied from critically low to low quality.
Among healthy individuals, all 10 comparisons targeted the dorsolateral PFC (DLPFC); in 7 comparisons, both offline and online designs were included; besides, in 8 comparisons, only single-session design studies were included, whereas in the remaining 2 multiple-session designs with adjuvant working memory training studies were included.
The mean number of studies and individuals in the single-session design comparisons were 16.25 (range 8-32) and 457.5 (range 167-914), respectively. Of these eight comparisons, four reported a significant effect favoring a-tDCS (ES = 0.56, 95% CI [0.19, 0.93], P < 0.01; ES = 0.17, 95% CI [0.03, 0.30], P = not reported; ES = 0.15, 95% CI [0.02, 0.28], P = 0.02; ES = −0.15, 95% CI [−0.29, −0.01], P = 0.003), but GRADE assessment of quality of evidence indicated moderate and low certainty that the true effect is close to these estimates as quality of evidence was rated down to Șmoderateș due to serious RoB (RoB was either not assessed or there was evidence of significant unclear RoB regarding randomization, allocation concealment and blinding) for all four comparisons and to Șlowș due to the serious imprecision (relatively small number of trials [N = 10] and individuals [n = 354]) for one of these comparisons and due to the strong suggestion of publication bias (funnel plot asymmetry was identified) for another comparison. The remaining four comparisons yielded non-significant findings, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to these estimates as quality of evidence was rated down to Șmoderateș due to serious RoB (RoB was not assessed), to Șlowș due to the strong suggestion of publication bias (publication bias was either not assessed or funnel plot asymmetry was identified) and to Șvery lowș due to the serious imprecision (relatively small number of trials [N = 8] and individuals [n = 283/167]) for all five comparisons.
The mean number of studies and individuals in the two multiple-session design comparisons with adjuvant working memory training were 8.5 (range 7-10) and 275.5 (range 266-285), respectively. Only one comparison reported a small significant effect favoring a-tDCS (ES = 0.29, 95% CI [0.06, 0.52], P not reported), but GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to the strong suggestion of publication bias (funnel plot asymmetry was identified), to Șlowș due to serious RoB (RoB was not assessed) and to Șvery lowș due to serious imprecision (relatively small number of trials [N = 10] and individuals [n = 285]). The remaining comparison yielded a non-significant effect, and GRADE assessment of quality of evidence indicated low certainty that the true effect is close to this estimate; quality of evidence was rated down to Șmoderateș due to serious RoB (RoB was not assessed) and to Șlowș due to serious imprecision (relatively small number of trials [N = 7] and individuals [n = 266] included).
Among neuropsychiatric individuals, all three comparisons targeted the DLPFC in a single-session design and included both an offline and online design. The mean number of studies and individuals included in these comparisons were 12 (range 8-16) and 491 (range 232-860), respectively. None of the three comparisons yielded significant effects, and GRADE assessment of quality of evidence indicated low and very low certainty that the true effect is close to these estimates. Quality of evidence was rated down to Șmoderateș due to serious RoB (there was evidence of significant unclear RoB regarding randomization, allocation concealment and/or blinding) for the three comparisons and to Șlowș due to serious indirectness (data included were from a sample with diverse neuropsychiatric individuals) for two comparisons and due to the strong suggestion of publication bias (publication bias was not evaluated) for the other comparison. Quality of evidence was rated down further to Șvery lowș due to serious imprecision (relatively small number of trials [N = 8] and individuals [n = 232]) for one of these comparisons and due to serious inconsistency (effect size estimates from individual studies varied considerably with relatively little overlap of CIs) for another of these comparisons.
[Long-term] memory. One meta-analysis (Horvath et al., 2015) resulting in one comparison among healthy individuals evaluated the effects of prefrontal a-tDCS on [long-term] memory. AMSTAR-2 quality assessment indicated that this review was of critically low quality. The comparison targeted the DLPFC and only included an online design. A small number of trials (N = 3) and individuals (n = 104) were included. No significant effects were reported, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șlowș due to very serious imprecision (relatively small number of trials [N = 3] and individuals [n = 104]; extremely large 95% CI [−0.87, 2.94]) and to Șvery lowș due to the strong suggestion of publication bias (publication bias was not evaluated).
Set shifting and response inhibition. Three meta-analyses (Horvath et al., 2015; Imburgio and Orr, 2018; Salehinejad et al., 2019) resulting in eight comparisons, seven among healthy and one among neuropsychiatric individuals, evaluated the effects of prefrontal a-tDCS on set shifting and response inhibition. AMSTAR-2 quality assessment indicated that these reviews were of critically low quality. Among healthy individuals, two comparisons evaluated set shifting, whereas the remaining five evaluated response inhibition; among neuropsychiatric individuals, only response inhibition was evaluated.
For set shifting, both comparisons among healthy individuals targeted the DLPFC; one included offline and online designs, whereas the other only included offline designs. The mean number of studies and individuals in both comparisons were 8 (range 3-13) and 430 (range 212-648). None of the two comparisons yielded significant effects, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to these estimates as quality of evidence was rated down to Șmoderateș due to serious RoB (RoB was not assessed) and to Șlowș due to serious inconsistency (either heterogeneity was not assessed statistically or was considerable [I2 = 69.92%]) for both comparisons; quality of evidence was rated down further to Șvery lowș due to the strong suggestion of publication bias (publication bias was not assessed) for one comparison and due to serious imprecision (effect size estimate with wide 95% CI [−0.68, 0.59]) for the other comparison. For response inhibition, of the five comparisons among healthy individuals, three targeted the DLPFC and two the IFG; one included offline and online designs, whereas four only included offline designs. The mean number of studies and individuals in these five comparisons were 3.83 (range 2-13) and 141 (range 55-616). None of these five comparisons yielded significant effects, and GRADE assessment of quality of evidence indicated low and very low certainty that the true effect is close to these estimates. Quality of evidence was rated down to Șmoderateș due to serious RoB (RoB was not assessed) and to Șlowș due to serious inconsistency (either heterogeneity was not assessed statistically or effect size estimates from individual studies varied considerably with relatively little overlap of CIs) for all five comparisons; quality of evidence was rated down further to Șvery lowș due to strong suggestion of publication bias (publication bias was not assessed) for four comparisons.
For response inhibition, the comparison among neuropsychiatric individuals targeted the DLPFC and IFG and included offline and online designs. The number of trials and individuals included were 34 and 1404, respectively; only individuals with attention-deficit hyperactivity disorder were included. This comparison reported a small significant effect favoring a-tDCS (ES = 0.23, 95% CI [0.07-0.40], P = 0.0065), and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to serious RoB (there was evidence of significant unclear RoB in blinding outcome assessment), to Șlowș due to serious inconsistency (effect size estimates from individual studies varied considerably) and to Șvery lowș due to the strong suggestion of publication bias (publication bias was not assessed).
Memory/attention/executive functioning. One meta-analysis (Dedoncker et al., 2016a, b) resulting in six comparisons, two among healthy, two among neuropsychiatric and two among both healthy and neuropsychiatric individuals, evaluated the effects of prefrontal a-tDCS on memory/attention/executive functioning. AMSTAR-2 quality assessment indicated that this review was of low quality.
Among healthy individuals, both comparisons targeted the DLPFC and included offline and online designs. The mean number of trials and individuals included were 116.5 (range 102-131) and 3942.5 (range 3470-4415), respectively. One of the two comparisons reported a small significant effect favoring a-tDCS (ES = − 0.10, 95% CI [−0.16, −0.04], P < 0.01), and GRADE assessment of quality of evidence indicated moderate certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to serious RoB (only 6/61 studies included in the Dedoncker et al., 2016a, b meta-analysis had a low risk of allocation concealment bias). The remaining comparison reported a non-significant effect, and GRADE assessment of quality of evidence also indicated moderate certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to serious RoB (only 6/61 studies included in the Dedoncker et al., 2016a, b meta-analysis had a low risk of allocation concealment bias).
Among neuropsychiatric individuals, both comparisons targeted the DLPFC and included offline and online designs. The mean number of trials and individuals included were 26 (range 22-30) and 802 (range 660-944), respectively. One of these comparisons reported a small significant effect favoring a-tDCS (ES = 0.22, 95% CI [0.04, 0.40], P < 0.05), but GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to serious RoB (only 6/61 studies included in the Dedoncker et al., 2016a, b meta-analysis had a low risk of allocation concealment bias), to Șlowș due to serious indirectness (data included were from a sample with diverse neuropsychiatric individuals) and to Șvery lowș due to serious inconsistency (I2 = 42.5%). The remaining comparison reported a non-significant effect, and GRADE assessment of quality of evidence indicated low certainty that the true effect is close to its estimate as quality of evidence was rated down to Șmoderateș due to serious RoB (only 6/61 studies included in the Dedoncker et al., 2016a, b meta-analysis had a low risk of allocation concealment bias) and to Șlowș due to serious indirectness (data included were from a sample with diverse neuropsychiatric individuals).
Among both healthy and neuropsychiatric individuals, both comparisons targeted the DLPFC and included both offline and online designs. The mean number of trials and individuals included were 144.5 (range 124-165) and 4744.5 (range 4130-5359), respectively. The two comparisons reported small significant effects favoring a-tDCS (ES = 0.18, 95% CI [0.03, 0.18], P < 0.01; ES = − 0.11, 95% CI [−0.17, −0.05], P < 0.01), and GRADE assessment of quality of evidence indicated very low and low certainty that the true effect is close to these estimates as quality of evidence was rated down to Șmoderateș due to serious RoB (only 6/61 studies included in the Dedoncker et al., 2016a, b meta-analysis had a low risk of allocation concealment bias) and to Șlowș due to serious indirectness for both comparisons (data included were from a sample with diverse neuropsychiatric individuals); quality of evidence was rated down further to Șvery lowș due to serious inconsistency (I2 = 52.5%) for one comparison.
Language. Two meta-analyses (Horvath et al., 2015;Price et al., 2015) resulting in five comparisons, all among healthy individuals, evaluated the effects of prefrontal a-tDCS on language. AMSTAR-2 quality assessment indicated that these reviews were of critically low quality. All five comparisons targeted the lPFC/DLPFC; three included only offline designs, whereas the remaining two included only online designs.
For the 3 offline comparisons, the mean number of studies and individuals included were 4 (range 3-7) and 115.3 (range 58-208). Only one comparison yielded a small-to-moderate significant effect favoring a-tDCS (ES = 0.48, 95% CI [0.35, 0.92], P not reported), but GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to serious imprecision (small number of studies [N = 3] and individuals [n = 80] included), to Șlowș due to strong suggestion of publication bias (publication bias was not assessed) and to Șvery lowș due to serious inconsistency (heterogeneity was not assessed statistically). The remaining two offline comparisons did not yield significant effects, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to these estimates as quality of evidence was rated down due to serious imprecision (small number of studies [N = 2, 7] and individuals [n = 58, 208] included), to Șlowș due to strong suggestion of publication bias (publication bias was not assessed) and to Șvery lowș due to serious inconsistency (heterogeneity was not assessed statistically) for both comparisons.
For the two online comparisons, the mean number of studies and individuals included were 3 (range 3-3) and 100 (range 100-100). None of the two comparisons yielded significant findings, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to these estimates as quality of evidence was rated down due to significant imprecision (small number of studies and individuals included), to Șlowș due to strong suggestion of publication bias (publication bias was not assessed) and to Șvery lowș due to significant inconsistency (heterogeneity was not assessed statistically).
Aggression. One meta-analysis (Bell and DeWall, 2018) resulting in one comparison among healthy individuals evaluated the effects of prefrontal a-tDCS on aggression. AMSTAR-2 quality assessment indicated that this review was of critically low quality. The comparison evaluated a-tDCS, targeted the DLPFC and included both an offline and an online design. The number of studies and individuals included was 6 and 339, respectively. The comparison yielded a non-significant finding, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to serious RoB (RoB was not assessed), to Șlowș due to serious imprecision (relatively small number of studies and individuals included) and to Șvery lowș due to serious inconsistency (effect size estimates from individual studies varied considerably with relatively little overlap of CIs).
Overeating. One meta-analysis (Bell and DeWall, 2018) resulting in one comparison among healthy individuals evaluated the effects of prefrontal a-tDCS on overeating. AMSTAR-2 quality assessment indicated that this review was of critically low quality. The comparison targeted the DLPFC and included both offline and online designs. The number of trials and individuals included were 6 and 339, respectively. This comparison reported a small treatment effect favoring a-tDCS (ES = −0.25, 95% CI [−0.49, −0.01], P = 0.03), and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate, as quality of evidence was rated down to Șmoderateș due to serious RoB (RoB was not assessed), to Șlowș due to serious imprecision (relatively small number of trials and individuals included) and to Șvery lowș due to strong suggestion of publication (funnel plot asymmetry was identified).
Emotional and implicit bias. Two meta-analyses (Horvath et al., 2015; Bell and DeWall, 2018) resulting in five comparisons among healthy individuals evaluated the effects of prefrontal a-tDCS on emotional and implicit bias; AMSTAR-2 quality assessment indicated that these reviews were of critically low quality. All comparisons targeted the DLPFC; four only included an online design and one included both an offline and an online design. Four of them only evaluated emotional bias, whereas one of them included both emotional and implicit bias measures. The mean number of studies and individuals included were 3.2 (range 2-7) and 169 (range 88-447), respectively. One of the comparisons, the one which included mixed outcomes, reported a small significant effect favoring a-tDCS (ES = −0.25, 95% CI [−0.48, −0.03], P = 0.02). GRADE assessment of quality of evidence indicated low certainty that the true effect is close to this estimate; quality of evidence was rated down to Șmoderateș due to serious RoB (RoB was not assessed) and to Șlowș due to serious imprecision (relatively small number of trials [N = 7] and individuals [n = 447] included). The remaining four comparisons yielded non-significant findings, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to these estimates as quality of evidence was rated down to Șmoderateș due to serious inconsistency (heterogeneity was not assessed statistically), to Șlowș due to serious RoB (RoB was not assessed) and to Șvery lowș due to strong suggestion of publication bias (publication bias was not assessed).
Honesty. One meta-analysis (Bell and DeWall, 2018) resulting in one comparison among healthy individuals evaluated the effects of prefrontal a-tDCS on honesty. AMSTAR-2 quality assessment indicated that this review was of critically low quality. The comparison evaluated a-tDCS, targeted the DLPFC and included both an online and an offline design. The number of studies and individuals included were 4 and 322, respectively. The comparison yielded a non-significant finding, and GRADE assessment of quality of evidence indicated low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to serious imprecision (relatively small number of trials and individuals included) and to Șlowș due to serious RoB (RoB was not assessed).
Rumination. One meta-analysis (Horvath et al., 2015) resulting in one comparison among healthy individuals evaluated the effects of prefrontal a-tDCS on rumination. AMSTAR-2 quality assessment indicated that this review was of critically low quality. The comparison targeted the DLPFC and included only online designs. The number of studies and individuals included were 2 and 126, respectively. The comparison yielded a non-significant finding, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to serious inconsistency (heterogeneity was not assessed statistically), to Șlowș due to serious RoB (RoB was not assessed) and to Șvery lowș due to strong suggestion of publication bias (publication bias was not assessed).
Impulsivity. One meta-analysis (Bell and DeWall, 2018) resulting in one comparison among healthy individuals evaluated the effects of prefrontal a-tDCS on impulsivity. AMSTAR-2 quality assessment indicated that this review was of critically low quality. The comparison evaluated a-tDCS, targeted the DLPFC and included both an offline and an online design. The number of studies and individuals included were 9 and 676, respectively. The comparison yielded a non-significant finding and GRADE assessment of quality of evidence indicated low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to serious RoB (RoB was not assessed) and to Șlowș due to serious inconsistency (effect size estimates from individual studies varying considerable with relatively little overlap of CIs).
Risk taking. Two meta-analyses (Horvath et al., 2015; Bell and DeWall, 2018) resulting in two comparisons, all among healthy individuals, evaluated the effects of prefrontal a-tDCS on risk taking. AMSTAR-2 quality assessment indicated that these reviews were of critically low quality. Both comparisons targeted the DLPFC, and while one only included online designs, the other one included both offline and online designs. The number of studies and individuals included were 8 (range 3-13) and 376 (range 76-676), respectively. One of the comparisons yielded a small, significant effect in favor of a-tDCS (ES = −0.36, 95% CI [−0.65, −0.07], P = 0.01), but GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to due to strong suggestion of publication bias (funnel plot asymmetry was identified), to Șlowș due to serious RoB (RoB was not assessed) and to Șvery lowș due to serious inconsistency (I2 = 65.5%). The remaining comparison yielded non-significant findings, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to serious inconsistency (heterogeneity was not assessed), to Șlowș due to serious RoB (RoB was not assessed) and to Șvery lowș due to strong suggestion of publication bias (publication bias was not assessed).
Cathodal tDCS
[Long-term] memory. One meta-analysis (Horvath et al., 2015) resulting in one comparison among healthy individuals evaluated the effects of prefrontal c-tDCS on [long-term] memory. AMSTAR-2 quality assessment indicated that this review was of critically low quality. The comparison targeted the DLPFC and only included online designs. A small number of trials (N = 3) and individuals (n = 103) were included. No significant effects were reported, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to strong suggestion of publication bias (publication bias was not assessed), to Șlowș due to the serious heterogeneity (heterogeneity was not accessed statistically) and to Șvery lowș due to serious RoB (RoB was not assessed).
Response inhibition. One meta-analysis (Salehinejad et al., 2019) resulting in one comparison among neuropsychiatric individuals evaluated the effects of prefrontal c-tDCS on response inhibition. AMSTAR-2 quality assessment indicated that this review was of critically low quality. The comparison targeted the DLPFC and included both offline and online designs. A small number of trials (N = 13) and individuals (N = 468) were included. No significant effects were reported, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate, as quality of evidence was rated down to Șmoderateș due to serious RoB (there was evidence of significant unclear RoB in blinding outcome assessment), to Șlowș due to serious inconsistency (effect size estimates from individual studies varied considerably with relatively little overlap of CIs) and to Șvery lowș due to the strong suggestion of publication bias (publication bias was not assessed).
Memory/attention/executive functioning. One meta-analysis (Dedoncker et al., 2016a, b) resulting in two comparisons among both healthy and neuropsychiatric individuals evaluated the effects of prefrontal c-tDCS on memory/attention/executive functioning. AMSTAR-2 quality assessment indicated that this review was of low quality. The two comparisons included a mean number of studies and individuals of 32 (range 28-36) and 1062 (range 942-1182), respectively. None of them yielded significant effects, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to these estimates as quality of evidence was rated down to Șmoderateș due to serious RoB (only 6/61 studies included in the Dedoncker et al., 2016a, b meta-analysis had a low risk of allocation concealment bias) and to Șvery lowș due to very serious inconsistency (I2 = 82.5% or I2 = 33.8% with effect size estimates from individual studies varying considerable with relatively little overlap of CIs) for both comparisons.
Emotional bias. One meta-analysis (Horvath et al., 2015) resulting in two comparison among healthy individuals evaluated the effects of prefrontal c-tDCS on emotional bias. AMSTAR-2 quality assessment indicated that this review was of critically low quality. Both comparisons targeted the DLPFC and included online designs. The mean number of studies and individuals included were 2 (range 2-2) and 88 (range 88-88), respectively. None of the comparisons reported significant effects, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to these estimates; quality of evidence was rated down to Șmoderateș due to serious inconsistency (heterogeneity was not assessed statistically), to Șlowș due to serious RoB (RoB was not assessed) and to Șvery lowș due to strong suggestion of publication bias (publication bias was not assessed).
Risk taking. One meta-analysis (Horvath et al., 2015) resulting in one comparison among healthy individuals evaluated the effects of prefrontal c-tDCS on risk taking. AMSTAR-2 quality assessment indicated that this review was of critically low quality. The comparison targeted the DLPFC and only included online designs. A small number of trials (N = 3) and individuals (n = 126) were included. No significant effects were reported, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to strong suggestion of publication bias (publication bias was not assessed), to Șlowș due to the serious heterogeneity (heterogeneity was not accessed statistically) and to Șvery lowș due to serious RoB (RoB was not assessed).
tDCS
Three comparisons from two meta-analyses (Lowe et al., 2017; Mostafavi et al., 2018) did not report separate effect sizes for c-tDCS and a-tDCS, but rather analyzed results from both c-tDCS and a-tDCS together. All of these three comparisons evaluated the effects of prefrontal tDCS on food cravings, targeted the DLPFC and included a mixed population of healthy and neuropsychiatric individuals. The mean number of studies and individuals included were 7.66 (range 4-13) and 264 (range 145-416), respectively. Two comparisons yielded moderate-to-large significant effects (ES = − 0.54, 95% CI [−0.85, −0.24], P < 0.001; ES = −0.78, 95% CI [−1.12, −0.44], P < 0.001), but GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to these estimates as quality of evidence was rated down to Șmoderateș due to serious RoB (RoB was not assessed), to Șlowș due to serious indirectness (data included were from a sample with diverse neuropsychiatric individuals) and to Șvery lowș due to serious imprecision (small number of studies [N = 4] and individuals [n = 145] included) for one comparison and due to serious inconsistency (I2 = 71.4%) for the other comparison. The remaining comparison yielded non-significant findings, and GRADE assessment of quality of evidence indicated very low certainty that the true effect is close to this estimate as quality of evidence was rated down to Șmoderateș due to strong suggestion of publication bias (funnel plot asymmetry was identified) and to Șvery lowș due to the very serious imprecision (effect size estimates with wide 95% CI [−0.80, 0.29] and a small number of trials and individuals included).
Discussion
This study provides a critical assessment of the available evidence regarding the effects of prefrontal tDCS on cognition. We identified previously published meta-analyses on the topic through a systematic literature search and rated the quality of included meta-analyses as well as of each included comparison evaluating the effects of prefrontal a-tDCS, c-tDCS or tDCS on several cognitive domains among either healthy or neuropsychiatric individuals. Our UR included 11 meta-analyses which were of either low (n = 2) or critically low (n = 9) quality. Our UR also included 55 comparisons, of which 41 (~75%) were exclusively among healthy individuals; therefore, only 25% of the included comparisons also involved individuals with different neuropsychiatric disorders, which were frequently collapsed together without abiding by diagnostic boundaries. Of the 55 available comparisons between active tDCS and sham tDCS, only 16 reported significant findings. Table 4 highlights the main findings for each cognitive domain reported in this UR. Among healthy individuals, a significant effect favoring a-tDCS was reported for working memory (ES = 0.56, 0.29, 0.17, 0.15 [accuracy], −0.15 [reaction time]), memory/attention/executive functioning (ES = −0.10), language (ES = 0.48), overeating (ES = −0.25), emotional and implicit bias (ES = −0.25) and risk taking (ES = −0.36). Among neuropsychiatric individuals, a significant effect favoring a-tDCS was reported for response inhibition (ES = 0.26) and memory/attention/executive functioning (ES = 0.22). Among both healthy and neuropsychiatric individuals, a significant effect favoring tDCS was reported for memory/attention/executive functioning (ES = 0.18 [accuracy], −0.11 [reaction time]) and food cravings (ES = −0.54, −0.78). However, of these 16 significant effects, GRADE assessment of quality of evidence indicated that only 3 of them had moderate quality, while the remaining 13 had either low (n = 4) or very low (n = 9) quality. Of the 39 non-significant effects, GRADE assessment of quality of evidence indicated that only 1 of them had moderate quality, while the remaining 38 had either low (n = 7) or very low (n = 31) quality. When taken together, these findings highlight that, although several meta-analyses evaluating the effects of prefrontal tDCS on cognition have been carried out, they do not provide any definitive conclusion due to the low certainty of evidence.
Table 4.
Description of the main findings in the UR for each cognitive domain
| Cognitive domain | Findings from the umbrella review |
|---|---|
| Working memory | 5 comparisons among healthy individuals indicated significant benefit of a-tDCS for working memory, but there was at best moderate certainty that the true effect is close to the estimates from the meta-analyses |
| 5 comparisons among healthy individuals indicated no benefit of a-tDCS for working memory | |
| 3 comparisons among neuropsychiatric individuals indicated no benefit of a-tDCS for working memory | |
| [Long-term] memory | 2 comparisons among healthy individuals indicated no benefit of either a-tDCS or c-tDCS for [long-term] memory improvement |
| Set shifting | 2 comparisons among healthy individuals indicated no benefit of a-tDCS for set shifting |
| Response inhibition | 5 comparisons among healthy individuals indicated no benefit of a-tDCS for response inhibition |
| 1 comparison among neuropsychiatric individuals indicated a significant benefit of a-tDCS for response inhibition. But there was very low certainty that the true effect is close to the estimate from the meta-analysis | |
| 1 comparison among neuropsychiatric individuals indicated no benefit of c-tDCS for response inhibition | |
| Language | 1 comparison among healthy individuals indicated a significant benefit of a-tDCS for language performance, but there was very low certainty that the true effect is close to the estimate from the meta-analysis |
| 4 comparisons among healthy individuals indicated no benefit of a-tDCS for language performance | |
| Aggression | 1 comparison among healthy individuals indicated no benefit of a-tDCS for aggression |
| Overeating/food cravings | 1 comparison among healthy individuals indicated significant benefit of a-tDCS for overeating, but there was very low certainty that the true effect is close to the estimate from the meta-analysis |
| 2 comparisons among mixed samples of healthy and neuropsychiatric individuals indicated a significant benefit of tDCS for food cravings, but there was very low certainty that the true effect is close to the estimates from meta-analyses | |
| 1 comparison among mixed samples of healthy and neuropsychiatric individuals indicated no benefit of tDCS for food cravings | |
| Emotional and implicit bias | 1 comparison among healthy individuals indicated a significant benefit of a-tDCS for emotional bias and implicit bias, but there was low certainty that the true effect is close to the estimate from the meta-analysis |
| 6 comparisons among healthy individuals indicated no benefit of a-tDCS or c-tDCS for emotional bias | |
| Honesty | 1 comparison among healthy individuals indicated no benefit of a-tDCS for honesty |
| Rumination | 1 comparison among healthy individuals indicated no benefit of a-tDCS for rumination |
| Impulsivity | 1 comparison among healthy individuals indicated no benefit of a-tDCS for impulsivity |
| Risk taking | 1 comparison among healthy individuals indicated significant benefit of a-tDCS for risk taking, but there was very low certainty that the true effect is close to the estimate from the meta-analysis |
| 2 comparisons among healthy individuals indicated no benefit of either a-tDCS or c-tDCS for risk taking |
In our UR, most of the included meta-analyses were rated as of low or critically low quality according to AMSTAR-2 methodology. Some of the identified weaknesses of the included meta-analyses were likely the result of continuing changes in the best practices for reporting meta-analysis. For instance, PRISMA (Moher et al., 2009) guidelines and the first international registry for systematic reviews (PROSPERO) (Page et al., 2018) were made available only in 2009 and 2011, respectively. It is reasonable to suppose that these innovations take some time to be implemented; in this context, the lack of a priori publication of a protocol might be, to some extent, understandable for the meta-analyses, especially the oldest ones. Editorial policies might also contribute to explain why lists of excluded studies were not frequently reported given most journals usually determine word limits that might be considered relatively stringent for meta-analyses. Nevertheless, other weaknesses likely cannot be better explained by external factors. Among these, perhaps most importantly, is the fact that few meta-analyses evaluated the RoB of the trials included in their analyses. RoB assessments are necessary to evaluate the internal validity of randomized trials, i.e. whether it answers the proposed research question Școrrectlyș. Without a proper assessment of RoB, meta-analyses have limited capability to draw accurate conclusions from their findings.
Additionally, although we have considered meta-analyses statistics as adequate for the 11 included studies, it should be noted that some meta-analyses frequently included, in the same model, data from two different experiments carried out with the same participants, which might be troubling as these data are likely not independent from one another (Peters and Mengersen, 2008). For instance, Hoy et al., (2013) developed a crossover trial to evaluate the effects of a-tDCS on working memory as measured by 10-minute blocks of n-back, 5 min each of 2-back and 3-back. In this trial, participants underwent 20-min a-tDCS sessions, either 1 mA, 2 mA or sham, 1 week apart from one another to avoid carryover effects. Participants also underwent the 10-min blocks of n-back immediately, 20 and 40 min after stimulation, totaling six trials of n-back, three 2-back and three 3-back, per week of stimulation. Although it seems reasonable to consider data from this trial as non-independent, particularly data from the same stimulation strength, which were all collected within 1 hour after the a-tDCS session, the meta-analysis by Hill et al. (2016) included data of each stimulation strength from Hoy et al. (2013) as six independent trials. Similarly, Dedoncker et al. (2016a, b) included data of each stimulation strength from Hoy et al. (2013) as three independent trials, as it did not consider differences in 2-back and 3-back. Although the 1-week difference between active and sham tDCS might be enough to avoid carryover effects, it likely is not an adequate time period to consider their data as independent.
Additionally, methodological issues with the trials included in the meta-analyses also have to be taken in consideration. For instance, some of the meta-analyses in our UR (Dedoncker et al., 2016a, b; Hill et al., 2016) included mostly crossover single-session within-subject design trials in which active and sham tDCS were administered to the same individual separated by a period of time. This approach has several advantages, such as the relatively smaller cost and number of individuals required to complete the study. Yet, there are also important caveats to consider; importantly, the order of administration of active and sham tDCS can likely influence the outcomes of the study if an appropriate washout is not carefully respected. Although a large number of trials employed a large washout period, others have employed smaller periods ,e.g. 30 minutes, Gladwin et al., 2012) which could have had carryover effects.
Besides, many trials from meta-analyses in our UR frequently did not acknowledge how investigators involved with recruitment of subjects and treatment administration were blinded to the order of treatments of each subject. In fact, some trials even acknowledged employing a single-blind design, in which only the subjects who were receiving the treatment were unaware of the order of treatment administration. A recent report reinforced the need to improve blinding procedures in tDCS research, particularly when employing a single-session design (Bikson et al., 2018). Double-blinding in tDCS studies can be achieved by using specific tDCS research devices in which active/sham stimulation is delivered according to a 6-digit code inputted in a keypad, guaranteeing that neither subjects nor researchers are aware of the allocation group. This method was employed in recent tDCS trials (Brunoni et al., 2017; Sampaio-Junior et al., 2018). Although this would be the preferable method, in some scenarios specific research-tailored tDCS devices are not available. In such contexts, it is advisable that tDCS operators are instructed to not interact further with the subjects and critically not when assessing study outcomes. Such approach has also been employed successfully in previous tDCS studies (Brunoni et al., 2013).
Additionally, sample sizes included in each individual trial were usually critically small, limiting the statistical power of comparison, and active and sham tDCS methodology varied considerably. Variation in the position and size of electrodes might influence how much current passes through different brain regions (Chase et al., 2019); the use of different sham parameters for stimulation likely adds more variability and makes it increasingly difficult to compare results across different trials (Fonteneau et al., 2019). Recently, several guidelines establishing adequate procedures for tDCS research have been published (Brunoni et al., 2011; Woods et al., 2016). The understanding of the effects of prefrontal tDCS on cognition is likely going to improve when trials adopt these procedures.
Future trials designed to evaluate the cognitive effects of prefrontal tDCS could also benefit from including a bigger number of individuals with neuropsychiatric disorders besides only healthy volunteers. In this UR, most of the comparisons evaluated (~75%) were generated with data from trials which only included healthy volunteers; given healthy individuals are more likely to have normal cognitive functioning, studies which only included such participants might have been unable to detect a treatment effect in favor of tDCS due to the fact that there was a small room for improvement in cognitive functioning among healthy volunteers. Cognitive impairment is widely recognized in several neuropsychiatric disorders such as depression, bipolar disorder and schizophrenia (Millan et al., 2012; Bortolato et al., 2015; Bortolato et al., 2016), and it is likely that by giving preference to such populations, future studies would be better equipped to detect treatment effects of tDCS regarding cognitive functioning, although it should be noted that effect sizes were mostly similar between comparisons performed among healthy and neuropsychiatric individuals, as well as among mixed samples.
Future meta-analysis evaluating the cognitive effects of prefrontal tDCS could also benefit from establishing separate comparison for individuals with different neuropsychiatric disorders. By collapsing individuals with different neuropsychiatric conditions in the same group, such approach includes subjects with distinct conditions which might limit the external validity of the findings identified in the meta-analysis. Although transdiagnostic approaches are useful under the Research Domain Criteria (RDoC) framework (Insel et al., 2010) that sustains that dysfunctions in cognitive domains occurring in psychiatric disorders should be investigated together in order to develop interventions specifically tailored for such dysfunctions, and not to the disorders per se, at the current moment it would likely be more clinically informative to have separate effect sizes for each neuropsychiatric disorder.
Our UR has several important strengths. A comprehensive search for eligible references was carried out. Considerable efforts were made to collect as much data as possible, and emails were sent to several authors asking for additional information. We were also able to include the vast majority‒if not all‒cognitive outcomes evaluated in prefrontal tDCS trials. Yet, this UR also has some limitations. We did not include reviews which did not report a separate comparison between active tDCS and sham tDCS, which might have limited‒although to a little extent‒the outcomes included. We did not carry out a quantitative analysis. We applied the GRADE criteria to meta-analyses without considering the information reported in individual trials; different results could have been obtained if we had done a careful examination of each trial included in each comparison from each meta-analysis, e.g. we could have assessed RoB instead of immediately downgrading for serious RoB if authors did not report RoB in their meta-analyses. However, we chose to apply the quality assessment criteria at the meta-analysis level as meta-analysis constitute one of the highest levels of evidence and are frequently employed by researchers and clinicians as a guide to future research/intervention; by highlighting the issues with available evidence from meta-analyses in this field, we clearly indicate there is actually an evidence of absence, and no definitive conclusions regarding whether a-tDCS, c-tDCS or tDCS are either effective or ineffective in improving cognitive functioning can be made. Additionally, we did not examine unpublished meta-analyses, which could have added new information to our findings. However, in contrast with pre-registration of clinical trials, pre-registration of systematic reviews and meta-analyses is still on its infancy and, in most journals, optional; therefore, there is no standard procedure for checking for unpublished meta-analyses yet. Lastly, since most meta-analyses did not perform sensitivity analyses on the range of parameters that possibly influence the effects of tDCS such as single vs multi-session, online vs offline stimulation and others, we could not report separate findings in our UR, and future research should look further into this when data are available.
In conclusion, although a significant volume of trials and meta-analyses have been performed to provide an assessment of the effects of prefrontal tDCS on cognition, poor quality of trials and meta-analyses does not allow to take definitive conclusion as to whether tDCS is effective in improving cognitive function among healthy and neuropsychiatric individuals. At the moment, trials employing better methodology are warranted. Researchers aiming at developing future trials to evaluate the effects of tDCS on cognition should abide by the increasing recommendations from guidelines to enable the reproducibility of their experiments and the comparison of their findings with those from other researchers. Researchers aiming at synthetizing data of the cognitive effects of tDCS on cognition should also abide by the recommendations currently available in guiding formularies such as the PRISMA statement to ensure transparency and to provide more reliable estimates of effect.
Supplementary Material
Contributor Information
Luis C Farhat, Departamento de Psiquiatria da Faculdade de Medicina FMUSP, Universidade de São Paulo, São Paulo, Brazil.
Andre F Carvalho, Center for Addiction and Mental Health, Toronto, ON, Canada; Department of Psychiatry, University of Toronto, Toronto, ON, Canada; IMPACT Strategic Research Centre (Innovation in Mental and Physical Health and Clinical Treatment), Deakin University, Geelong Vic, Australia.
Marco Solmi, Padua Neuroscience Center, University of Padua, Padua, Italy; Department of Neuroscience, University of Padua, Padua, Italy.
Andre R Brunoni, Departamento de Psiquiatria da Faculdade de Medicina FMUSP, Universidade de São Paulo, São Paulo, Brazil; Laboratory of Neurosciences (LIM-27), Department of Psychiatry, Faculdade de Medicina da Universidade de São Paulo, São Paulo, SP, Brazil; Department of Internal Medicine, Faculdade de Medicina da Universidade de São Paulo, São Paulo, SP, Brazil.
Supplementary data
Supplementary data are available at SCAN online.
Funding
There are no funders to report for this submission.
Declaration of interest
The authors declare no conflicts of interest.
References
- Aleman, A., Enriquez-Geppert, S., Knegtering, H., Dlabac-de Lange, J.J. (2018). Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: meta-analysis of controlled trials. Neuroscience and Biobehavioral Reviews, 89, 111–8. [DOI] [PubMed] [Google Scholar]
- Baldo, P., Doree, C., Molin, P., McFerran, D., Cecco, S. (2012). Antidepressants for patients with tinnitus. Cochrane Database of Systematic Reviews, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, S.B., DeWall, N. (2018). Does transcranial direct current stimulation to the prefrontal cortex affect social behavior? A meta-analysis. Social Cognitive and Affective Neuroscience, 13(9), 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieck, S.M., Artemenko, C., Moeller, K., Klein, E. (2018). Low to no effect: application of tRNS during two-digit addition. Frontiers in Neuroscience, 12, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson, M., Brunoni, A.R., Charvet, L.E., et al. (2018). Rigor and reproducibility in research with transcranial electrical stimulation: an NIMH-sponsored workshop. Brain Stimulation, 11(3), 465–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boayue, N.M., Csifcsak, G., Aslaksen, P., et al. (2019). Increasing propensity to mind-wander by transcranial direct current stimulation? A registered report. The European Journal of Neuroscience. [DOI] [PubMed] [Google Scholar]
- Bortolato, B., Miskowiak, K.W., Kohler, C.A., Vieta, E., Carvalho, A.F. (2015). Cognitive dysfunction in bipolar disorder and schizophrenia: a systematic review of meta-analyses. Neuropsychiatric Disease and Treatment, 11, 3111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato, B., Miskowiak, K.W., Kohler, C.A., et al. (2016). Cognitive remission: a novel objective for the treatment of major depression? BMC Medicine, 14, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni, A.R., Vanderhasselt, M.A. (2014). Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain and Cognition, 86, 1–9. [DOI] [PubMed] [Google Scholar]
- Brunoni, A.R., Amadera, J., Berbel, B., Volz, M.S., Rizzerio, B.G., Fregni, F. (2011). A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. The International Journal of Neuropsychopharmacology, 14(8), 1133–45. [DOI] [PubMed] [Google Scholar]
- Brunoni, A.R., Nitsche, M.A., Bolognini, N., et al. (2012). Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimulation, 5(3), 175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni, A.R., Valiengo, L., Baccaro, A., et al. (2013). The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry, 70(4), 383–91. [DOI] [PubMed] [Google Scholar]
- Brunoni, A.R., Shiozawa, P., Truong, D., et al. (2014). Understanding tDCS effects in schizophrenia: a systematic review of clinical data and an integrated computation modeling analysis. Expert Review of Medical Devices, 11(4), 383–94. [DOI] [PubMed] [Google Scholar]
- Brunoni, A.R., Baeken, C., Machado-Vieira, R., Gattaz, W.F., Vanderhasselt, M.A. (2015). BDNF blood levels after non-invasive brain stimulation interventions in major depressive disorder: a systematic review and meta-analysis. The World Journal of Biological Psychiatry, 16(2), 114–22. [DOI] [PubMed] [Google Scholar]
- Brunoni, A.R., Moffa, A.H., Sampaio-Junior, B., et al. (2017). Trial of electrical direct-current therapy versus escitalopram for depression. The New England Journal of Medicine, 376(26), 2523–33. [DOI] [PubMed] [Google Scholar]
- Brunoni, A.R., Sampaio-Junior, B., Moffa, A.H., et al. (2019). Noninvasive brain stimulation in psychiatric disorders: a primer. Braz J Psychiatry, 41(1), 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase, H.W., Boudewyn, M.A., Carter, C.S., Phillips, M.L. (2019). Transcranial direct current stimulation: a roadmap for research, from mechanism of action to clinical implementation. Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruccu, G., Garcia-Larrea, L., Hansson, P., et al. (2016). EAN guidelines on central neurostimulation therapy in chronic pain conditions. European Journal of Neurology, 23(10), 1489–99. [DOI] [PubMed] [Google Scholar]
- Dedoncker, J., Brunoni, A.R., Baeken, C., Erhasselt, M.-A. (2016a). The effect of the interval-between-sessions on prefrontal transcranial direct current stimulation (tDCS) on cognitive outcomes: a systematic review and meta-analysis. Journal of Neural Transmission, 123(10), 1159–72. [DOI] [PubMed] [Google Scholar]
- Dedoncker, J., Brunoni, A.R., Baeken, C., Erhasselt, M.-A. (2016b). A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tdcs) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: influence of stimulation parameters. Brain Stimulation, 9(4), 501–17. [DOI] [PubMed] [Google Scholar]
- Dougall, N., Maayan, N., Soares-Weiser, K., McDermott, L.M., McIntosh, A. (2015). Transcranial magnetic stimulation (TMS) for schizophrenia. Cochrane Database of Systematic Reviews, (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner, B., Kugler, J., Pohl, M., Mehrholz, J. (2016). Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database of Systematic Reviews, (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteneau, C., Mondino, M., Arns, M., et al. (2019). Sham tDCS: a hidden source of variability? Reflections for further blinded, controlled trials. Brain Stimulation, 12(3), 668–73. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli, P., Radua, J. (2018). Ten simple rules for conducting umbrella reviews. Evidence-Based Mental Health, 21(3), 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin, T.E., den Uyl, T.E., Fregni, F.F., Wiers, R.W. (2012) Enhancement of selective attention by tDCS: Interacttion with interference in a Sternberg task. Neuroscience letters. 512 (1), 33-37. [DOI] [PubMed] [Google Scholar]
- Greenwood, P.M., Blumberg, E.J., Scheldrup, M.R. (2018). Hypothesis for cognitive effects of transcranial direct current stimulation: externally- and internally-directed cognition. Neuroscience and Biobehavioral Reviews, 86, 226–38. [DOI] [PubMed] [Google Scholar]
- Guyatt, G.H., Oxman, A.D., Montori, V., et al. (2011). GRADE Guidelines: 5. Rating the Quality of Evidence-Publication Bias. Journal of Clinical Epidemiology, 64(12): 1277-82. [DOI] [PubMed] [Google Scholar]
- Hall, P.A., Lowe, C., Vincent, C. (2017). Brain stimulation effects on food cravings and consumption: an update on Lowe et al. (2017) and a response to Generoso et al. (2017). Psychosomatic Medicine, 79(7), 839–42. [DOI] [PubMed] [Google Scholar]
- Higgins, J.P., Altman, D.G., Gotzsche, P.C., et al. (2011). The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ, 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, A.T., Fitzgerald, P.B., Hoy, K.E. (2016). Effects of anodal transcranial direct current stimulation on working memory: a systematic review and meta-analysis of findings from healthy and neuropsychiatric populations. Brain Stimulation, 9(2), 197–208. [DOI] [PubMed] [Google Scholar]
- Hilton, M.P., Zimmermann, E.F., Hunt, W.T. (2013). Ginkgo biloba for tinnitus. Cochrane Database of Systematic Reviews, 3. [DOI] [PubMed] [Google Scholar]
- Hobson, J., Chisholm, E., El Refaie, A. (2012). Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database of Systematic Reviews, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra, C.E.L., Rynja, S.P., van Zanten, G.A., Rovers, M.M. (2011). Anticonvulsants for tinnitus. Cochrane Database of Systematic Reviews, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, J.C., Forte, J.D., Carter, O. (2015). Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial direct current stimulation (tDCS). Brain Stimulation, 8(3), 535–50. [DOI] [PubMed] [Google Scholar]
- Hou, W.H., Wang, T.Y., Kang, J.H. (2016). The effects of add-on non-invasive brain stimulation in fibromyalgia: a meta-analysis and meta-regression of randomized controlled trials. Rheumatology (Oxford), 55(8), 1507–17. [DOI] [PubMed] [Google Scholar]
- Hoy, K.E., Emonson, M.R.L., Arnold, S.L., Thomson, R.H., Daskalakis, Z.J., Fitzgerald, PB. (2013) Testing the limits: investigating the effects of tDCS dose on working memory enhancement in healthy controls. Neuropsychologia. 51(9), 1777-1784. [DOI] [PubMed] [Google Scholar]
- Hsu, J.H., Daskalakis, Z.J., Blumberger, D.M. (2018). An update on repetitive transcranial magnetic stimulation for the treatment of co-morbid pain and depressive symptoms. Current Pain and Headache Reports, 22(7), 51. [DOI] [PubMed] [Google Scholar]
- Imburgio, M.J., Orr, J.M. (2018). Effects of prefrontal tDCS on executive function: methodological considerations revealed by meta-analysis. Neuropsychologia, 117, 156–66. [DOI] [PubMed] [Google Scholar]
- Insel, T., Cuthbert, B., Garvey, M., et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American Journal of Psychiatry, 167(7), 748–51. [DOI] [PubMed] [Google Scholar]
- Ioannidis, J.P. (2009). Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ, 181(8), 488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis, J.P., Munafo, M.R., Fusar-Poli, P., Nosek, B.A., David, S.P. (2014). Publication and other reporting biases in cognitive sciences: detection, prevalence, and prevention. Trends in Cognitive Sciences, 18(5), 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside, M., O'Shea, J., Cowen, P.J., Harmer, C.J. (2016). Frontal cortex stimulation reduces vigilance to threat: implications for the treatment of depression and anxiety. Biological Psychiatry, 79(10), 823–30. [DOI] [PubMed] [Google Scholar]
- Jansen, J.M., Daams, J.G., Koeter, M.W.J., Veltman, D.J., van denBrink, W., Goudriaan, A.E. (2013). Effects of non-invasive neurostimulation on craving: a meta-analysis. Neuroscience and Biobehavioral Reviews, 37(10), 2472–80. [DOI] [PubMed] [Google Scholar]
- Katz, B., Au, J., Buschkuehl, M., et al. (2017). Individual differences and long-term consequences of tDCS-augmented cognitive training. Journal of Cognitive Neuroscience, 29(9), 1498–508. [DOI] [PubMed] [Google Scholar]
- Kennedy, N.I., Lee, W.H., Frangou, S. (2018). Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: a meta-analysis of randomized controlled trials. European Psychiatry, 49, 69–77. [DOI] [PubMed] [Google Scholar]
- Khalighinejad, N., Di Costa, S., Haggard, P. (2016). Endogenous action selection processes in dorsolateral prefrontal cortex contribute to sense of agency: a meta-analysis of tDCS studies of 'Intentional binding. Brain Stimulation, 9(3), 372–9. [DOI] [PubMed] [Google Scholar]
- Kuhn, S., Vanderhasselt, M.A., De Raedt, R., Gallinat, J. (2012). Why ruminators won't stop: the structural and resting state correlates of rumination and its relation to depression. Journal of Affective Disorders, 141(2-3), 352–60. [DOI] [PubMed] [Google Scholar]
- Lee, W.H., Kennedy, N.I., Bikson, M., Frangou, S. (2018). A computational assessment of target engagement in the treatment of auditory hallucinations with transcranial direct current stimulation. Frontiers in Psychiatry, 9, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Wang, J., Li, C., Xiao, Z. (2014). Repetitive transcranial magnetic stimulation (rTMS) for panic disorder in adults. Cochrane Database of Systematic Reviews, (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, C.J., Vincent, C., Hall, P.A. (2017). Effects of noninvasive brain stimulation on food cravings and consumption: a meta-analytic review. Psychosomatic Medicine, 79(1), 2–13. [DOI] [PubMed] [Google Scholar]
- Luedtke, K., Rushton, A., Wright, C., Geiss, B., Juergens, T.P., May, A. (2012). Transcranial direct current stimulation for the reduction of clinical and experimentally induced pain: a systematic review and meta-analysis. The Clinical Journal of Pain, 28(5), 452–61. [DOI] [PubMed] [Google Scholar]
- Lupi, M., Martinotti, G., Santacroce, R., et al. (2017). Transcranial direct current stimulation in substance use disorders: a systematic review of scientific literature. The Journal of ECT, 33(3), 203–9. [DOI] [PubMed] [Google Scholar]
- Luvizutto, G. J., Bazan, R., Braga, G. P., Resende, L., Bazan, S. G. Z.El Dib, R. (2015). Pharmacological interventions for unilateral spatial neglect after stroke. Cochrane Database of Systematic Reviews (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, D.G.d.S., Unal, G., Andrade, S.M., et al. (2018). Effect of transcranial direct current stimulation on exercise performance: a systematic review and meta-analysis. Brain Stimulation, pp. No Pagination Specified-No Pagination Specified. [DOI] [PubMed] [Google Scholar]
- Mancuso, L.E., Ilieva, I.P., Hamilton, R.H., Farah, M.J. (2016). Does transcranial direct current stimulation improve healthy working memory? A meta-analytic review. Journal of Cognitive Neuroscience, 28(8), 1063–89. [DOI] [PubMed] [Google Scholar]
- Martin, D.M., Yeung, K., Loo, C.K. (2016). Pre-treatment Letter Fluency Performance Predicts Antidepressant Response to Transcranial Direct Current Stimulation, Netherlands: Elsevier Science. [DOI] [PubMed] [Google Scholar]
- McMillan, R., Forssell, H., Buchanan, J.A.G., Glenny, A.M., Weldon, J.C., Zakrzewska, J.M. (2016). Interventions for treating burning mouth syndrome. Cochrane Database of Systematic Reviews, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meader, N., King, K., Llewellyn, A., et al. (2014). A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews, 3, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina, J., Cason, S. (2017). No evidential value in samples of transcranial direct current stimulation (tDCS) studies of cognition and working memory in healthy populations. Cortex, 94, 131–41. [DOI] [PubMed] [Google Scholar]
- Meng, Z., Liu, S., Zheng, Y., Phillips, J.S. (2011). Repetitive transcranial magnetic stimulation for tinnitus. Cochrane Database of Systematic Reviews, 10. [DOI] [PubMed] [Google Scholar]
- Micoulaud Franchi, J.A., Quiles, C., Belzeaux, R., Adida, M., Azorin, J.M. (2015). Negative symptoms of schizophrenia: from electrophysiology to electrotherapy. Encephale, 41(6 Suppl 1), 6s50–6. [DOI] [PubMed] [Google Scholar]
- Millan, M.J., Agid, Y., Brune, M., et al. (2012). Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nature Reviews. Drug Discovery, 11(2), 141–68. [DOI] [PubMed] [Google Scholar]
- Moffa, A.H., Brunoni, A.R., Nikolin, S., Loo, C.K. (2018). Transcranial direct current stimulation in psychiatric disorders. A Comprehensive Review. Psychiatr Clin North Am, 41(3), 447–63. [DOI] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D.G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine, 6(7), e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi, S.A., Khaleghi, A., Mohammadi, M.R., Akhondzadeh, S. (2018). Is transcranial direct current stimulation an effective modality in reducing food craving? A systematic review and meta-analysis. Nutritional Neuroscience, 1–13. [DOI] [PubMed] [Google Scholar]
- Mutz, J., Edgcumbe, D.R., Brunoni, A.R., Fu, C.H.Y. (2018). Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: a systematic review and meta-analysis of randomised sham-controlled trials. Neuroscience and Biobehavioral Reviews, 92, 291–303. [DOI] [PubMed] [Google Scholar]
- Nilsson, J., Lebedev, A.V.E., RydstrÖm, A., LÖvdèn, M. (2017). Direct-current stimulation does little to improve the outcome of working memory training in older adults. Psychological Science, 28(7), 907–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche, M.A., Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology, 527(Pt 3), 633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell, N.E., Wand, B.M., Marston, L., Spencer, S., Desouza, L.H. (2010). Non-invasive brain stimulation techniques for chronic pain. Cochrane Database of Systematic Reviews, (9), Cd008208. [DOI] [PubMed] [Google Scholar]
- Oliveira, J.F., Zanao, T.A., Valiengo, L., et al. (2013). Acute working memory improvement after tDCS in antidepressant-free patients with major depressive disorder. Neuroscience Letters, 537, 60–4. [DOI] [PubMed] [Google Scholar]
- Open Science Collaboration (2015). Psychology. Estimating the reproducibility of psychological science. Science, 349(6251), aac4716. [DOI] [PubMed] [Google Scholar]
- Page, M.J., Shamseer, L., Tricco, A.C. (2018). Registration of systematic reviews in PROSPERO: 30,000 records and counting. Systematic Reviews, 7(1), 32–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm, U., Ayache, S.S., Padberg, F., Lefaucheur, J.P. (2016). Transcranial direct current stimulation (tDCS) for depression: results of nearly a decade of clinical research. Encephale, 42(1), 39–47. [DOI] [PubMed] [Google Scholar]
- Peters, J.L., Mengersen, K.L. (2008). Meta-analysis of repeated measures study designs. Journal of Evaluation in Clinical Practice, 14(5), 941–50. [DOI] [PubMed] [Google Scholar]
- Phillips, J.S., McFerran, D. (2010). Tinnitus retraining therapy (TRT) for tinnitus. Cochrane Database of Systematic Reviews, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pievani, M., Pini, L., Ferrari, C., et al. (2017). Coordinate-based meta-analysis of the default mode and salience network for target identification in non-invasive brain stimulation of Alzheimer's disease and behavioral variant frontotemporal dementia networks. Journal of Alzheimer's Disease, 57(3), 825–43. [DOI] [PubMed] [Google Scholar]
- Poulet, E., Haesebaert, F., Saoud, M., Suaud-Chagny, M.F., Brunelin, J. (2010). Treatment of shizophrenic patients and rTMS. Psychiatria Danubina, 22(Suppl 1), S143–6. [PubMed] [Google Scholar]
- Price, A.R., McAdams, H., Grossman, M., Hamilton, R.H. (2015). A meta-analysis of transcranial direct current stimulation studies examining the reliability of effects on language measures. Brain Stimulation, 8(6), 1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Martin, J.L., Barbanoj, J.M., Schlaepfer, T.E., et al. (2002). Transcranial magnetic stimulation for treating depression. Cochrane Database of Systematic Reviews, (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Martin, J.L., Barbanoj, J.M., Pérez, V., Sacristan, M. (2003). Transcranial magnetic stimulation for the treatment of obsessive-compulsive disorder. Cochrane Database of Systematic Reviews, (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia, K. (2018). Cognitive neuroscience of attention deficit hyperactivity disorder (ADHD) and its clinical translation. Frontiers in Human Neuroscience, 12, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehinejad, M.A., Wischnewski, M., Nejati, V., Vicario, C.M., Nitsche, M.A. (2019). Transcranial direct current stimulation in attention-deficit hyperactivity disorder: a meta-analysis of neuropsychological deficits. PLoS One, 14(4), e0215095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Junior, B., Tortella, G., Borrione, L., et al. (2018). Efficacy and safety of transcranial direct current stimulation as an add-on treatment for bipolar depression: a randomized clinical trial. JAMA Psychiatry, 75(2), 158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter, R.S., Daams, J.G., vanHolst, R.J., Goudriaan, A.E. (2018). Effects of non-invasive neuromodulation on executive and other cognitive functions in addictive disorders: a systematic review. Frontiers in Neuroscience, 12, 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Advisory Secretariat (2004). Repetitive transcranial magnetic stimulation for the treatment of major depressive disorder: an evidence-based analysis. Ont Health Technol Assess Ser, 4(7), 1–98. [PMC free article] [PubMed] [Google Scholar]
- Shea, B.J., Reeves, B.C., Wells, G., et al. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ, 358, j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, Y.I., Foerster, A., Nitsche, M.A. (2015). Transcranial direct current stimulation (tDCS) - application in neuropsychology. Neuropsychologia, 69, 154–75. [DOI] [PubMed] [Google Scholar]
- Soares-Weiser, K., Rathbone, J., Ogawa, Y., Shinohara, K., Bergman, H. (2018). Miscellaneous treatments for antipsychotic-induced tardive dyskinesia. Cochrane Database of Systematic Reviews, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S., Zilverst, A., Gui, W., Li, H.-J., Zhou, X. (2018). Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: a meta-analysis. Brain Stimulation, No Pagination Specified-No Pagination Specified. [DOI] [PubMed] [Google Scholar]
- Sterne, J.A.C., Savovic, J., Page, M.J., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ, 366, l4898. [DOI] [PubMed] [Google Scholar]
- Stevens, B., Yamada, J., Ohlsson, A., Haliburton, S., Shorkey, A. (2016). Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database of Systematic Reviews, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The GRADE Working Group (2013). GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations.
- Tsoi, D.T., Porwal, M., Webster, A.C. (2013). Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database of Systematic Reviews, (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseghi, B., Zoghi, M., Jaberzadeh, S. (2014). Does anodal transcranial direct current stimulation modulate sensory perception and pain? A meta-analysis study. Clinical Neurophysiology, 125(9), 1847–58. [DOI] [PubMed] [Google Scholar]
- Wolkenstein, L., Plewnia, C. (2013). Amelioration of cognitive control in depression by transcranial direct current stimulation. Biological Psychiatry, 73(7), 646–51. [DOI] [PubMed] [Google Scholar]
- Woods, A.J., Antal, A., Bikson, M., et al. (2016). A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical Neurophysiology, 127(2), 1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, C.E., Yu, B., Zhang, W., Chen, W.H., Qi, Q., Miao, Y. (2017). Effectiveness and safety of transcranial direct current stimulation in fibromyalgia: a systematic review and meta-analysis. Journal of Rehabilitation Medicine, 49(1), 2–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


