Summary
Dispersal is a fundamental community assembly process that maintains soil microbial biodiversity across spatial and temporal scales, yet the impact of dispersal on ecosystem function is largely unpredictable. Dispersal is unique in that it contributes to both ecological and evolutionary processes and is shaped by both deterministic and stochastic forces. The ecosystem-level ramifications of dispersal outcomes are further compounded by microbial dormancy dynamics and environmental selection. Here we review the knowledge gaps and challenges that remain in defining how dispersal, environmental filtering, and microbial dormancy interact to influence the relationship between microbial community structure and function in soils. We propose the classification of microbial dispersal into three categories, through vegetative or active cells, through dormant cells, and through acellular dispersal, each with unique spatiotemporal dynamics and microbial trait associations. This conceptual framework should improve the integration of dispersal in defining soil microbial community structure-function relationships.
Subject areas: Soil science, Microbiology, Soil ecology
Graphical abstract
Soil science; Microbiology; Soil ecology;
Introduction
The interplay between microbial dispersal, environmental filtering, and microbial dormancy introduces ecoevolutionary dynamics to soil ecosystems that limit our ability to decipher, much less predict, community structure-function relationships. Despite rapid development of high throughput molecular methods, decreasing sequencing costs, and accelerating generation of large ecological data, soils remain messy. This is because soils are massively complex superorganisms with emergent functions that are not yet easily predicted. To inform our predictions of amassed scale effects that determine soil behavior, we need a better understanding of how microbial processes, such as dormancy and environmental filtering, compound dispersal outcomes that transpire to whole ecosystems.
We suggest an adaptable framework for thinking about how microbial dispersal across space and time influences soil biodiversity, and ultimately, ecosystem function. Modes of microbial dispersal are categorized as cellular (i.e., vegetative or dormant cells) and acellular (i.e., genetic material associated with viruses and/or gene flow independent of cellular life), where dispersal outcomes for organisms and/or their genes happen across different spatiotemporal scales (Figure 1). Here we discuss the mechanistic constraints of microbial dispersal modes and the interplay between dispersal, environmental filtering, and dormancy. Finally, we propose a traits-based approach for quantifying dispersal outcomes, and suggest how this framework can be used to evaluate soil microbial structure-function relationships.
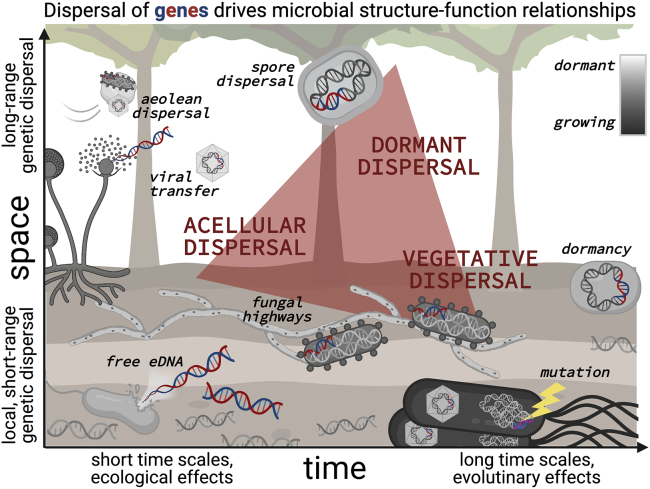
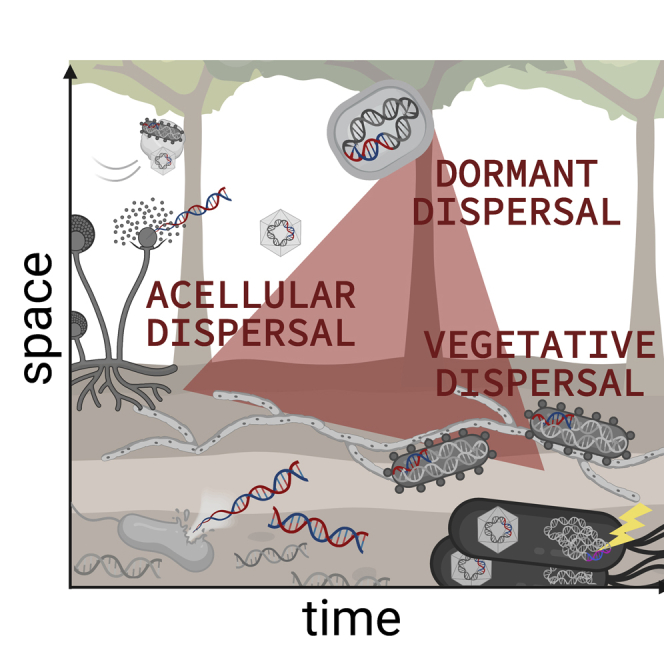
Figure 1.
Microbial dispersal modes
Dispersal affects microbial structure-function relationships in soils by distributing genes (i.e., potential ecological functions, which are represented here by blue/red DNA fragments) in three primary ways: through active or vegetative cell dispersal, through dormant cell dispersal, and through acellular dispersal. Dispersal can occur at different scales over space and time, and can be independent of environmental filtering and ecological constraints that structure organism-level rules of microbial community assembly. Long-range spatial migration is likely dominated by dormant (i.e., spore) dispersal through aeolian deposition, though active cells and viruses also constitute the air microbiome. Local dispersal over short time scales includes viral-mediated genetic transfer as well as uptake of free environmental DNA (eDNA) from soil necromass pools. Cellular dispersal over intermediate spatial scales can occur via fungal highways or vectors including soil arthropods. Over longer time scales, dormancy shapes population genetics by effecting evolutionary diversification processes
Microbial community assembly
To predict ecosystem function from community composition, we first need to understand the community assembly processes that create and maintain patterns of microbial diversity. In both microbial ecology and the broader field of ecology, niche theory and selection-based models have classically explained patterns of community assembly, looking to environmental selection and biotic interactions to define niche space and determine what conditions a species will persist (Chase and Leibold, 2003; Holt, 2009). Alternatively, neutral theory relies on stochastic processes to explain community ecology patterns (Hubbell, 2001; Chave, 2004). Few elements of ecology are an absolute either-or, and mathematical frameworks unify both niche and neutral theory (Harshey, 2003; Mutshinda and O’Hara, 2011). In reality, comprehensive theory explains that variations in community assembly arise through both deterministic and stochastic processes, and that individual processes exist somewhere along a continuum between selection and neutrality (Chase and Myers, 2011).
A useful synthesis describes community assembly as a function of the four fundamental ecoevolutionary processes of dispersal, selection or environmental filtering, ecological drift, and diversification (Vellend, 2010). How these same mechanisms extend to microbial biogeography has been eloquently summarized previously (Nemergut et al., 2013; Hanson et al., 2012; Martiny et al., 2006). Much research in the last few decades has quantified the relative contributions of these community assembly processes in microbial systems, see (Stegen et al., 2013, 2015; Caruso et al., 2011; Ofiţeru et al., 2010; Liao et al., 2016). Of Vellend’s four fundamental processes, dispersal is the least understood in terrestrial microbial systems and often assumed to be negligible. Owing to small cell size, large populations, high potential for dispersal, and a bias for niche-based approaches, the influence of stochastic processes, including dispersal, is historically under-explored in microbial ecology (Zhou and Ning, 2017). We believe that a renewed focus of research efforts on understanding microbial dispersal will advance our understanding of microbial structure-function relationships considerably.
What is microbial dispersal?
Dispersal is predominantly defined as ”the movement of individuals or propagules with potential consequences for gene flow across space” (Ronce, 2007). Consequently, dispersal entails both dissemination and establishment or colonization, each with unique constraints. But dispersal remains poorly conceptualized for microbes. This is because theoretical frameworks in ecology were historically built on observations of plants and animals, yet it is crucial to apply, adapt, or develop theory that includes the microbial perspective (Prosser et al., 2007). Although unification of microecology and macroecology theories seem conceptually attainable, there remain unique aspects of microbial systems, including scaling, microbial species concepts, and gene flow dynamics that continue to impose challenges to reconciliation (Shade et al., 2018, Barberán et al., 2014a).
Current sampling methods limit our ability to accurately enumerate soil microbes and their dispersal, though sampling challenges are certainly not unique to microbes (Shade et al., 2018; Elphick, 2008). Perhaps the largest hurdle to quantifying microbial dispersal is counting individuals and species and identifying their presence-absence across sites. For census numbers, direct counts using microscopy has both low feasibility and little resolution beyond basic cell morphology. Alternatively, quantification using molecular approaches like qPCR of 16S rRNA genes (or other marker genes) is preferred, although imperfect given biases in nucleic acid extraction, amplification, and uneven distribution rRNA operon copy numbers. More importantly, marker genes lack sufficient resolution to address dispersal patterns of individual species (Choudoir et al., 2012).
Microbial species concepts are well supported with theoretical and empirical data (Achtman and Wagner, 2008; Rosselló-Móra and Amann, 2015; Ward et al., 2008), but practical demarcations of microbial species remain challenging. Gene flow across space and time further obscures microbial population boundaries. Gene exchange dynamics vary greatly between macro and microorganisms, making it difficult to apply macroecology dispersal theory to microbial systems. Furthermore, recombination patterns differ between microbial taxa, with microbes ranging from strictly clonal to wildly promiscuous (Gogarten et al., 2002; Didelot and Maiden, 2010; Jain et al., 2002). This is not to say that quantifying microbial dispersal is unattainable, but it does require careful experimental design and appropriate cultivation-based and/or molecular methods.
Modes of microbial dispersal
We propose classifying microbial dispersal into three categories, each with unique microbial trait associations and spatiotemporal dynamics: vegetative or active cells, dormant cells, and acellular or genetic dispersal (Figure 1). This conceptual framework is intended to better integrate microbial dispersal outcomes into community structure-function relationships. We note that molecular ecology methods (e.g., 16S rRNA gene amplicon surveys, shotgun metagenomics, whole genome sequencing of isolates) are often exclusively used to infer patterns of cellular dispersal. Thus, we encourage moving away from a strictly cellular framework and toward thinking about dispersal and its consequences for ecosystem function in terms of genetic dispersal, because ultimately, genes underlie functional potential. Finally, we acknowledge that these categories are not mutually exclusive, sometimes overlap, and exist on a multidimensional continuum.
Vegetative dispersal
Vegetative dispersal is the movement of growing, physiologically-active microbial cells across space. Vegetative dispersal in soils can be passive or active and occurs at cellular, microhabitat, and local spatial scales. Sporadic wetting events that saturate soils can induce passive cell dispersal via Brownian motion (Mitchell and Kogure, 2006), but most of the time the soil is an unsaturated and irregular matrix of solid particles and liquids connected by gaseous pores (Or et al., 2007). From the perspective of a single microbial cell the soil is cavernous, and movement across this habitat requires some evolutionary ingenuity. Bacteria and archaea have evolved diverse methods of motility and active dispersal across surfaces including flagellar and non-flagellar swimming, twitching, or gliding mechanisms (Jarrell and McBride, 2008). Social microbes have evolved multicellular modes of dispersal like biofilm or fruiting body formation (Harshey, 2003). Hyphal growth in filamentous microorganisms, including some fungi and bacterial actinomycetes, is another form of dispersal that creates mycelial networks (Prosser and Tough, 1991). In addition to a filamentous developmental stage, some Streptomyces bacteria assume a newly discovered life stage termed ”exploratory growth” which allows cells to rapidly transverse surfaces in response to environmental or biotic signals (Jones and Elliot, 2017; Jones et al., 2017). Although similar in structure to filamentous bacterial hyphae, fungal hyphae are much larger, and in fact bacteria can migrate along these fungal highways (Kohlmeier et al., 2005; Warmink et al., 2011).
Dormant dispersal
Dormant dispersal is the movement of dormant microbial cells across space. Dormancy is an organism’s ability to reduce cell function to the minimum allowable energy expenditure, defined as maintenance energy (Pirt, 1987). Therefore, conduits of dormant dispersal are, by definition, passive. Dormancy is reversible, which permits survival during periods of unfavorable environmental conditions. In macroecology, ”the temporal storage effect” refers to a mechanism that contributes to species coexistence and depends on varying environmental conditions, competition, and a persistent long-lived state (Chesson and Warner, 1981; Warner and Chesson, 1985). In this sense, we can also conceptualize microbial dormancy as the dispersal of cells through time as well as space. Dormancy has recurrently evolved among microorganisms, manifesting in diverse physiologies which may include morphological differentiation and formation of spores, endospores, conidia, cysts, or akinetes (Lennon and Jones, 2011). Dormancy is also surmised to include ”resting states” in which minimal energy is invested only in stopping cell damage or decay, and is usually accompanied by a reduction in size, sometimes called viable-but-not-cultivable (Roszak and Cowell, 1987; Lennon and Jones, 2011). For fungi engaging in sexual reproduction, dispersal of both sexual and asexual spores may be crucial for successful establishment and range expansion.
Soil microbes continuously fluctuate between active and dormant physiological states (Stenström et al., 2001), and these varying stages of resting states is exemplified by the wide diversity of soil microbes that respond within minutes to the first season’s rain in a Mediterranean grassland (Placella et al., 2012). This is a demonstration of the taphonomic gradient (Lynch and Neufeld, 2015), an idea which suggests that cellular metabolic state is not a dichotomy of ”active” or ”dormant”, but that microbial activity falls along a gradient from active to dormant to fossilized. Measurements of soil microbes being dormant at any one time range from most (Lennon and Jones, 2011) to almost none (Papp et al., 2018). Modern estimates of dormancy in soils are largely based on the detection of rRNA, and in fact, many papers use the absence of rRNA as an indication of dormancy (Aanderud et al., 2016; Loeppmann et al., 2018; Kearns et al., 2016). The use of rRNA as a proxy for active populations is problematic, as not all taxa degrade their rRNA as they move into dormancy (Blazewicz et al., 2013). This means that certain taxa will retain rRNA even when dormant, which can create a stochastic, or worse, phylogenetically-conserved bias in discriminating between dormant and active microbes. In other words, because dormant cells can include rRNA, the use of rRNA as an indicator of an active state will underestimate the dormant population in natural systems.
Acellular dispersal
Genetic dispersal is the movement of genes across space that can be independent of cellular dispersal. Acellular dispersal can facilitate the expansion of functional capabilities with ecosystem-level ramifications. For example, genetic dispersal has long been observed for antibiotic resistance genes (Zhu et al., 2019) and microbial virulence factors (Wagner and Waldor, 2002). Viruses are ubiquitous with microbes and are a major source of genetic diversity in natural systems (Correa et al., 2021). Viral-mediated horizontal gene exchange creates a model of dispersal that, while dependent on cellular machinery for replication and transmission, possesses unique spatial and temporal dynamics. New research has demonstrated that viruses are agents of genetic diversity that shape biogeochemical cycling (Starr et al., 2019; Trubl et al., 2018). Viruses direct carbon flows in ecosystems through a top-down manner, in which viral cell lysis increases organic matter concentrations. Although the ’viral shunt’ as a source of fresh organic matter from viral predation has long been appreciated in marine systems, it is also important in terrestrial systems (Hungate et al., 2021). In a study of viral sequences from across a permafrost thaw gradient, authors found that many viruses encoded glycoside hydrolases, some with confirmed activity, targeted at degradation of pectin, hemicellulose, and starch. Further, modeling revealed that in almost every case viral abundance predicted pore water dissolved organic carbon, sometimes better than the host abundance (Emerson et al., 2018). Though evidence for viral-mediated genetic dispersal in soils remains somewhat limiting, there is clear precedent for viruses to act as agents of dispersal of genes that can shape the functional capacity of soil microbial communities.
Extracellular relic DNA is abundant and stable in soils (Carini et al., 2016; Lennon et al., 2018), representing a large reservoir of genetic diversity uncoupled from cellular identity. Furthermore, transformation of free environmental DNA (eDNA) by naturally competent soil bacteria (Paget and Simonet, 1994) may represent an underappreciated mechanism of gene flow and introduction of new heritable traits in soil populations. While acellular dispersal is not unique to microbes, it likely plays a much larger role in microbial ecology than it does in plant or animal ecology.
Long-distance dispersal
Aeolian deposition, or dispersal promoted by the action of wind, can cause dramatic changes in immigration rates of microbes in natural environments. Microbes from terrestrial, marine, and glacial origins were found in the Arctic air microbiome (Šantl-Temkiv et al., 2018), indicating that the atmosphere represents a potentially important channel connecting Earth’s biospheres. Aerial dispersal shapes fungal community structure at local scales (500 m) with strong seasonal trends (Adams et al., 2013). At continental scales, regional climatic and environmental variables shape the distribution of bacterial and fungal taxa associated with settled dust (Barberán et al., 2015). Wind and weather patterns have been connected to microbial migration at global scales (Kellogg and Griffin, 2006; Smith et al., 2013), and in particular, microbes on dust particles originating from seasonal desert storms are associated with transoceanic and intercontinental airborne dispersal routes (Kellogg and Griffin, 2006; Barberán et al., 2014b). Functional attributes related to dormancy are enriched in desert microbes (Fierer et al., 2012), supporting the hypothesis that airborne dispersal is dominated by dormant cells.
Atmospheric viral transmission of genetic material is possible considering estimates of viral particles in the air microbiome. By one account, viral-like particles and bacterial-like particles exist at concentrations of about 10 × 105 per cubic meter of air, with similar concentrations inside and outside, and a viral to bacterial ratio of about 1.4–1 (Prussin et al., 2015). The enumeration of bacterial and viral particles based on size may have resulted in overestimating their abundances, but reliably quantifying airborne biotic particles is notoriously difficult (Judith et al., 2020). Further, it is unclear whether the viral constituents of the air microbiome are mostly human-derived, or whether the focus on human health has biased this estimation (Prussin and Marr, 2015). Most of the work on the viral component of air microbiomes is focused on the built (i.e., indoor) environment with an effort to quantify pathogens, so the natural ecology of outdoor particles and their dispersal constraints remain under-explored.
Vector-mediated dispersal
Finally, microbes can disperse through animal vectors across varying spatial scales. Across intermediate to long-range distances, small mammals and birds are dispersers of arbuscular mycorrhizal (AM) spores (Correia et al., 2019; Mangan and Adler, 2000). At local scales, it’s long been appreciated that soil arthropods assist fungal and bacterial dispersal (Ruddick and Williams, 1972; Lussenhop, 1992). A recent study demonstrates that geosmin, a volatile compound emitted by sporulating actinomycetes that smells like fresh soil after the rain, recruited arthropods and facilitated spore dispersal (Becher et al., 2020). Soil arthropods Collembolans accelerated the dispersal of antibiotic resistance genes in a controlled experiment, likely indirectly as a result of altered bacterial community structure in Collembolan-inhabited soils (Zhu et al., 2019).
Consequences of microbial dispersal
Dispersal is a key ingredient for spatial structuring of genetic diversity and population structure. Dispersal is also a unique mechanism as it impacts both ecological (Stegen et al., 2015) and evolutionary (Thompson and Fronhofer, 2019) processes. Dispersal connects local populations with regional pools, and thus dispersal is the important glue connecting metacommunities and facilitating metacommunity dynamics (e.g., patch dynamics, species-sorting, and mass effects) (Leibold et al., 2004). For instance, cellular dispersal can influence community ecology by altering local abundance and distribution patterns of community members. As an evolutionary force related to gene flow, dispersal can increase local diversity through the introduction of novel genetic material or can homogenize genetic diversity at high dispersal rates because of mass effects.
The prevalence of nonrandom distributions of bacterial species supports the idea that dispersal limitation is an important factor shaping community assembly (Martiny et al., 2006). Dispersal limitation refers to geographic or ecological constraints of dispersal and in some cases can create distance-decay relationships. Distance-decay relationships are observed in patterns of soil microbial community composition and structure across geographic distances ranging from micro to local to global scales (Albright and Martiny, 2018; Peay et al., 2007; Martiny et al., 2006). The taxa-area relationship is another illustration of dispersal limitation (Horner-Devine et al., 2004; Green and Bohannan, 2006). For instance, isolation by distance (IBD) describes a linear relationship between genetic variation and geographic distance (Wright, 1943), and this pattern is observed in the population structure of the soil microbe Myxococcus xanthus (Vos and Velicer, 2008). Biogeography studies in other microbial systems highlight the importance of dispersal limitation on spatial structuring of genetic and genomic diversity (Reno et al., 2009; Peay et al., 2010; Andam et al., 2016; Bottos et al., 2018).
Dispersal and its dependencies
The outcomes of dispersal on community function are interdependent on environmental filtering and dormancy dynamics acting at dispersal locations. Stronger environmental filtering reduces perceived rates of dispersal and shifts dispersal outcomes from more stochastic to more deterministic. Dormancy can mitigate environmental selection in heterogeneous or changing habitats, effectively increasing perceived rates of dispersal. In this way, environmental filtering and dormancy are opposing constraints related to dispersal in community assembly processes. However, the variables that dictate dispersal outcomes on soil community composition are still not mapped out to an extent that will facilitate prediction of structure-function relationships in soil.
Dispersal and environmental filtering
The Baas Becking hypothesis, ”Everything is everywhere, but the environment selects” (Translated from the original Dutch: ”Alles is overal: maar het milieu selecteert”) (O’Malley, 2007) has persisted since its publication in the 1930s because of our continued and growing appreciation for microbial biodiversity and the rare biosphere, with modern high throughput methods still not plumbing the depths of the microbial species catalog (Lynch and Neufeld, 2015). This hypothesis has been rejected (Papke et al., 2003; Telford et al., 2006) and accepted (Finlay, 2002; Finlay and Fenchel, 2004) for various ecosystems, scales, and populations. At its heart, the Baas Becking hypothesis is a direct test of the relative contributions of dispersal and environmental selection in determining patterns of biogeography. Spatial scale plays an important role, with niche selection functioning at smaller scales and dispersal at broader scales (Wisnoski et al., 2019). The hypothesis of cosmopolitan dispersal has been recently evaluated for genes, and authors found that gene pools show stronger evidence of environmental filtering and lower geographic constraints compared to whole organisms (Fodelianakis et al., 2019).
In a study modeling the interaction between dispersal rates and environmental filtering on microbial communities assembled on different litter qualities, dispersal limitation (defined as less than 25 percent turnover) resulted in high within-group and between-group distances, suggesting a prevalence of stochastic processes (Evans et al., 2017). Community distance decreased in simulations with higher dispersal rates, yet stochastic assembly was more prevalent under conditions of stronger selection, highlighting an important relationship between selection and dispersal. Conversely, drought stress shifted microbial community assembly to more deterministic processes (Chase, 2007). Under scenarios of environmental stress, we can imagine how the consequences of dispersal will also depend on what microbes and their associated traits are dispersing, their relative fitness, and their adaptive potentials.
Dispersal and dormancy
An accurate estimate of microbial dormancy in soils is critical to understanding how community assembly processes shape soil biodiversity and to extrapolating the impact of dispersal on community function. Seed banks constructed of dormant microorganisms, many of which are members of the rare biosphere, are important contributors to generating and maintaining soil microbial diversity (Jones and Lennon, 2010; Lennon and Jones, 2011; Aanderud et al., 2015). Furthermore, ecosystem models indicate dormancy dynamics are important for predicting biogeochemical nutrient cycling (Stolpovsky et al., 2011; Wang et al., 2015). Dormancy also has the potential to shape population genetics and fundamental evolutionary processes (Shoemaker and Lennon, 2018).
There is a strong theoretical grounding for the hypothesis that dormancy shapes patterns of microbial biogeography by enhancing dispersal, but empirical evidence has been harder to come by (Epstein, 2009). Mestre and Höfer (Mestre and Höfer, 2020) outline a compelling conceptual framework, the Microbial Conveyor Belt, for surmising how dormancy, dispersal, and resuscitation interact to shape marine microbial community structure and function at the global scale. The Theory of Island Biogeography likewise indirectly supports the link between dormancy and dispersal, where modeling exercises show that increasing the dormancy rate (expressed as a dampening of extinction rates over time) increases community richness (Lennon and Jones, 2011). Some of the first direct evidence linking microbial dormancy and dispersal limitation to microbial biogeography shows that dormancy dampens environmental and spatial distance-decay relationships for microbes in forested ponds (Locey et al., 2020). Another recent study found that both resuscitation of local dormant cells and regional dispersal of active cells contribute to soil community resilience following a period of thermal stress (Sorensen and Shade, 2020). This study poses the question, what are the long-term outcomes of dormant versus active cellular dispersal in natural systems?
Dispersal outcomes on community function
The outcomes of dispersal on community function depend on the interplay between microbial traits associated with dispersers and the strength of local environmental filtering. Dispersal-colonization tradeoffs may structure microbial trait distributions across the spatial and environmental landscape (Smith et al., 2018). In a recent wood decomposition study spanning sites along a forest/non-forest ecotone, dispersal limitation of traits associated with rapid wood-degradation shaped community composition and function such that fungal communities farther from forests decomposed wood blocks more slowly (Smith and Peay, 2021). Independent of forest proximity, there was also a significant negative relationship between alpha-diversity (shaped by stochastic dispersal) and decomposition because of interspecific competition, linking dispersal to independent drivers of community function in this system (Smith and Peay, 2021).
An intuitive hypothesis is that dispersal can mitigate microbial responses to environmental stress by introducing stress-tolerant microbes, but this prediction depends on the regional pool of microbial traits, their adaptive potential, and the extent of functional redundancy. In an experimental evolution experiment, dispersal elevated community growth under ambient conditions but hindered growth in a warming treatment (Lawrence et al., 2016), suggesting that dispersal may dampen the ability of microbial communities to adapt to environmental change by introducing maladapted individuals. In another study looking at the interaction between dispersal and drought, dispersal altered the community composition to a greater extent under drought conditions but also resulted in loss of community function, which was contrary to the hypothesis that dispersal could mitigate drought stress by introducing tolerant microbes (Evans et al., 2020). In a common garden experiment across a natural precipitation gradient, enhanced dispersal had no effect on community composition, which was the strongest predictor of functional responses to changes in moisture (Waring and Hawkes, 2018). However, under certain conditions, dispersal can enhance community stability in the face of environmental change for both acute and more gradual disturbances (Evans et al., 2020; Sorensen and Shade, 2020).
The order and timing of dispersal events can also influence dispersal outcomes. For instance, the release of fungal spores during day versus night influences dispersal longevity and survival (Oneto et al., 2020). Historical contingencies are past biological interactions or environmental conditions, whose order and timing impact the trajectory of a community response. Priority effects are a specific example of a biotic historical contingency where the early or late arrival of a species determines community assembly outcomes (Fukami, 2015). The importance of historical contingencies (Hawkes and Keitt, 2015) and priority effects (Sprockett et al., 2018; Hiscox et al., 2015; Svoboda et al., 2018) on microbial community assembly has been demonstrated across a range of habitats.
Traits-based approach for predicting dispersal outcomes
Traits are increasingly invoked as the key parameters to understand ecosystem function. Traits include the physiological, life history, and behavioral characteristics of organisms that underlie ecosystem function (Martiny et al., 2015). Because traits more directly relate to ecosystem function, and most traits are phylogenetically conserved to some degree, traits are a valuable tool in linking microbial biogeography to ecosystem function (Green et al., 2008; Nelson et al., 2016; Fierer et al., 2012). Quantification of traits related to dormancy and dispersal should also be valuable to understanding their interaction, but current attempts are hampered by the breadth of traits that contribute to these processes.
For example, range size correlates with genomic and phenotypic attributes of dust-associated microbes, suggesting that these traits may be related to dispersal capabilities (Choudoir et al., 2018). For AM fungi, a recent study showed that small spore size was positively associated with aerial dispersal (Chaudhary et al., 2020), whereas another study found spore size to be a poor predictor of AM fungal range size (Kivlin, 2020). It’s clear we are far from understanding physiological traits that determine dispersal outcomes. Because atmospheric dispersal is important to both plants and microbes, looking to decades of studies in plant ecology for inspiration about traits related to dispersal will likely yield fruitful insights (Thomson et al., 2010; Tamme et al., 2014). Once traits are identified, analyses developed for genome-wide association studies (GWAS) (Eriksson et al., 2010; San et al., 2020) may offer useful insights for identifying genetic variation related to common traits associated with dormancy and/or dispersal.
Ultimately, we need to develop a predictive framework for implementing dispersal traits into changes in ecosystem function. One suggestion is implementation of the response-effect framework (Lavorel and Garnier, 2002), where response traits determine community structure (indirect drivers) and effect traits influence ecosystem function (direct drivers). This framework has been previously applied to fungal systems (Crowther et al., 2014; Koide et al., 2014), and although it can be challenging to parse indirect versus direct effects on ecosystem function, there is predictive power when response and effect traits are correlated. Using this framework, microbial dormancy and dispersal traits are response traits which control microbial community structure directly (and ecosystem function indirectly), as separate from effect traits that govern ecosystem function directly. Another approach could implement a tradeoff framework, such as the yield-resource acquisition-stress (Y-A-S) traits framework developed as a microbial analog to Grime’s competitor-stress tolerator-ruderal (C-S-R) framework (Grime, 1977; Malik et al., 2020). For example, dormancy could be invoked as a measure of community stress response. A third approach could implement dormancy or dispersal as a performance filter along an ecological gradient overlaying other system traits (Webb et al., 2010). These frameworks could be high-level conceptualizations to be combined with statistical modeling. For example, the relative contribution of dispersal to ecosystem function can be estimated using generalized nonlinear models, with microbial traits as potential fixed effects screened in model selection. Structural equation modeling (SEM) can be employed downstream to define direct and indirect drivers of ecosystem function.
Road map and research recommendations
To improve our predictions of structure-function relationships in soils, we need to apply and evaluate a more precise, yet adaptable conceptualization of microbial dispersal. We propose a reframing of microbial dispersal into active, dormant, and acellular modes. The ecological, spatial, and temporal restraints vary between cellular and acellular dispersal (Figure 1), with genetic dispersal potentially decoupled from environmental filtering and organismal identity. We are not the first to frame patterns of biogeography through the lens of genetic dispersal. For example, Baltrus (Baltrus, 2020) discusses the dynamics of genetic element dispersal and the contributions to the biogeography of microbial pathways. We also encourage researchers to contemplate the limits of this thinking. Can microbial dispersal always be sufficiently represented as genetic dispersal, or is it sometimes more important to consider the individual organisms harboring these genes?
To close current knowledge gaps, we recommend starting with these research directions. First, we need to develop a quantitative theoretical framework that integrates microbial dispersal, dormancy, and environmental filtering. A new model describes the interactions between dispersal and dormancy and outcomes on community diversity across scales (Wisnoski and Shoemaker, 2021). This model considers multiple dormancy traits (i.e., survival and germination rates) and how these processes interact with dispersal to create nonlinear effects on metacommunity diversity across local and regional spatial scales. Models that capture dispersal and dormancy dynamics will continue to improve as we better measure these phenomena, both as ecosystem processes and as microbial traits.
Second, we need to develop more accurate methods for quantifying, and accounting for, microbial dispersal and dormancy. Golan and Pringle (Golan and Pringle, 2017) provide a comprehensive framework for considering fungal long distance dispersal that entails mathematical models, genetic inference, and direct quantification based on spore capture. We also need improved tools to quantify genetic dispersal (Brito, 2021). We recommend incorporating dispersal and dormancy explicitly into soil structure-function studies. A typical structure-function analysis neglects the influence of dispersal from a regional pool, and also assumes that all recovered DNA sequences are representative of active (or potentially active) organisms. Incorporating dispersal might mean a no-dispersal or enhanced-dispersal treatment as part of the experimental design, or accounting for new taxa from the atmosphere at regional scales or along fungal highways at local scales. Incorporating dormancy might mean including a resuscitation treatment (e.g., bacterial resuscitation factor Rpf, see (Kuo et al., 2021)), or filtering taxa based on microbial activity. Although at present our methods for quantifying active versus non-active fractions are imperfect. If we knew how to differentiate active versus inactive rRNA, we’d be a lot closer to estimating true dormancy rates. Cell-resolved metabolomics might offer a promising solution (Walsh et al., 2018).
Finally, we need to better qualify and quantify traits related to dormancy and dispersal. This may require different strategies for different microbial lineages, such as identifying a taxon-specific sporulation gene, or an environmental signal that is associated with entering dormancy or the resuscitation of a particular microbe. New research on the homeostasis of ribosomes in Methanococcus during energy limitation underscores the need for alternative traits that accompany activity and dormancy (Müller et al., 2021). We as microbial ecologists need to support research that focuses on specific members of a microbial community (e.g., revitalization of microbial cultivation efforts (Carini, 2019)). In understanding the trees, we may finally be able to see the forest.
Acknowledgments
This project was supported in part by a grant from the National Science Foundation (No. DEB-1749206). The figures were created using BioRender.com.
Author contributions
M.J.C. and K.M.D. conceptualized and investigated the perspective. M.J.C. and K.M.D. wrote and revised the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
References
- Aanderud Z.T., Jones S.E., Fierer N., Lennon J.T. Resuscitation of the rare biosphere contributes to pulses of ecosystem activity. Front. Microbiol. 2015;6:24. doi: 10.3389/fmicb.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aanderud Z.T., Vert J.C., Lennon J.T., Magnusson T.W., Breakwell D.P., Harker A.R. Bacterial dormancy is more prevalent in freshwater than hypersaline lakes. Front. Microbiol. 2016;7:853. doi: 10.3389/fmicb.2016.00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Wagner M. Microbial diversity and the genetic nature of microbial species. Nat. Rev. Microbiol. 2008;6:431–440. doi: 10.1038/nrmicro1872. [DOI] [PubMed] [Google Scholar]
- Adams R.I., Miletto M., Taylor J.W., Bruns T.D. Dispersal in microbes: fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013;7:1262–1273. doi: 10.1038/ismej.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright M.B.N., Martiny J.B.H. Dispersal alters bacterial diversity and composition in a natural community. ISME J. 2018;12:296–299. doi: 10.1038/ismej.2017.161. https://europepmc.org/articles/PMC5739015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andam C.P., Doroghazi J.R., Campbell A.N., Kelly P.J., Choudoir M.J., Buckley D.H. A latitudinal diversity gradient in terrestrial bacteria of the genus streptomyces. MBio. 2016;7:e02200–e02215. doi: 10.1128/mBio.02200-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltrus D.A. Bacterial dispersal and biogeography as underappreciated influences on phytobiomes. Curr. Opin. Plant Biol. 2020;56:37–46. doi: 10.1016/j.pbi.2020.02.010. https://www.sciencedirect.com/science/article/pii/S1369526620300303 [DOI] [PubMed] [Google Scholar]
- Barberán A., Casamayor E.O., Fierer N. The microbial contribution to macroecology. Front. Microbiol. 2014;5:203. doi: 10.3389/fmicb.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberán A., Henley J., Fierer N., Casamayor E.O. Structure, inter-annual recurrence, and global-scale connectivity of airborne microbial communities. Sci. Total Environ. 2014;487:187–195. doi: 10.1016/j.scitotenv.2014.04.030. [DOI] [PubMed] [Google Scholar]
- Barberán A., Ladau J., Leff J.W., Pollard K.S., Menninger H.L., Dunn R.R., Fierer N. Continental-scale distributions of dust-associated bacteria and fungi. Proc. Natl. Acad. Sci. U S A. 2015;112:5756–5761. doi: 10.1073/pnas.1420815112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher P.G., Verschut V., Bibb M.J., Bush M.J., Molnár B.P., Barane E., Al-Bassam M.M., Chandra G., Song L., Challis G.L., et al. Developmentally regulated volatiles geosmin and 2-methylisoborneol attract a soil arthropod to streptomyces bacteria promoting spore dispersal. Nat. Microbiol. 2020;5:821–829. doi: 10.1038/s41564-020-0697-x. [DOI] [PubMed] [Google Scholar]
- Blazewicz S.J., Barnard R.L., Daly R.A., Firestone M.K. Evaluating rrna as an indicator of microbial activity in environmental communities: limitations and uses. ISME J. 2013;7:2061–2068. doi: 10.1038/ismej.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottos E.M., Kennedy D.W., Romero E.B., Fansler S.J., Brown J.M., Bramer L.M., Chu R.K., Tfaily M.M., Jansson J.K., Stegen J.C. Dispersal limitation and thermodynamic constraints govern spatial structure of permafrost microbial communities. FEMS Microbiol. Ecol. 2018;94:fiy110. doi: 10.1093/femsec/fiy110. [DOI] [PubMed] [Google Scholar]
- Brito I.L. Examining horizontal gene transfer in microbial communities. Nat. Rev. Microbiol. 2021;19:442–453. doi: 10.1038/s41579-021-00534-7. [DOI] [PubMed] [Google Scholar]
- Carini P. A “cultural” renaissance: genomics breathes new life into an old craft. mSystems. 2019;4 doi: 10.1128/mSystems.00092-19. e00092-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini P., Marsden P.J., Leff J.W., Morgan E.E., Strickland M.S., Fierer N. Relic dna is abundant in soil and obscures estimates of soil microbial diversity. Nat. Microbiol. 2016;2:1–6. doi: 10.1038/nmicrobiol.2016.242. [DOI] [PubMed] [Google Scholar]
- Caruso T., Chan Y., Lacap D.C., Lau M.C., McKay C.P., Pointing S.B. Stochastic and deterministic processes interact in the assembly of desert microbial communities on a global scale. ISME J. 2011;5:1406–1413. doi: 10.1038/ismej.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J.M. Drought mediates the importance of stochastic community assembly. Proc. Natl. Acad. Sci. U S A. 2007;104:17430–17434. doi: 10.1073/pnas.0704350104. https://www.pnas.org/content/104/44/17430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J.M., Leibold M.A. University of Chicago Press; 2003. Ecological Niches: Linking Classical and Contemporary Approaches. [Google Scholar]
- Chase J.M., Myers J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philosophical Trans. R. Soc. B: Biol. Sci. 2011;366:2351–2363. doi: 10.1098/rstb.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V.B., Nolimal S., Sosa-Hernández M.A., Egan C., Kastens J. Trait-based aerial dispersal of arbuscular mycorrhizal fungi. New Phytol. 2020;228:238–252. doi: 10.1111/nph.16667. [DOI] [PubMed] [Google Scholar]
- Chave J. Neutral theory and community ecology. Ecol. Lett. 2004;7:241–253. [Google Scholar]
- Chesson P.L., Warner R.R. Environmental variability promotes coexistence in lottery competitive systems. The Am. Naturalist. 1981;117:923–943. [Google Scholar]
- Choudoir M.J., Barberán A., Menninger H.L., Dunn R.R., Fierer N. Variation in range size and dispersal capabilities of microbial taxa. Ecology. 2018;99:322–334. doi: 10.1002/ecy.2094. [DOI] [PubMed] [Google Scholar]
- Choudoir M.J., Campbell A.N., Buckley D.H. Grappling with proteus: population level approaches to understanding microbial diversity. Front. Microbiol. 2012;3:336. doi: 10.3389/fmicb.2012.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A.M., Howard-Varona C., Coy S.R., Buchan A., Sullivan M.B., Weitz J.S. Revisiting the rules of life for viruses of microorganisms. Nat. Rev. Microbiol. 2021;19:501–513. doi: 10.1038/s41579-021-00530-x. [DOI] [PubMed] [Google Scholar]
- Correia M., Heleno R., da Silva L.P., Costa J.M., Rodríguez-Echeverría S. First evidence for the joint dispersal of mycorrhizal fungi and plant diaspores by birds. New Phytol. 2019;222:1054–1060. doi: 10.1111/nph.15571. [DOI] [PubMed] [Google Scholar]
- Crowther T.W., Maynard D.S., Crowther T.R., Peccia J., Smith J.R., Bradford M.A. Untangling the fungal niche: the trait-based approach. Front. Microbiol. 2014;5:579. doi: 10.3389/fmicb.2014.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didelot X., Maiden M.C. Impact of recombination on bacterial evolution. Trends Microbiology. 2010;18:315–322. doi: 10.1016/j.tim.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elphick C.S. How you count counts: the importance of methods research in applied ecology. J. Appl. Ecol. 2008;45:1313–1320. [Google Scholar]
- Emerson J.B., Roux S., Brum J.R., Bolduc B., Woodcroft B.J., Jang H.B., Singleton C.M., Solden L.M., Naas A.E., Boyd J.A., et al. Host-linked soil viral ecology along a permafrost thaw gradient. Nat. Microbiol. 2018;3:870–880. doi: 10.1038/s41564-018-0190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein S.S. Microbial awakenings. Nature. 2009;457:1083. doi: 10.1038/4571083a. [DOI] [PubMed] [Google Scholar]
- Eriksson N., Macpherson J.M., Tung J.Y., Hon L.S., Naughton B., Saxonov S., Avey L., Wojcicki A., Pe’er I., Mountain J. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet. 2010;6:e1000993. doi: 10.1371/journal.pgen.1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S.E., Bell-Dereske L.P., Dougherty K.M., Kittredge H.A. Dispersal alters soil microbial community response to drought. Environ. Microbiol. 2020;22:905–916. doi: 10.1111/1462-2920.14707. https://sfamjournals.onlinelibrary.wiley.com/doi/abs/10.1111/1462-2920.14707 [DOI] [PubMed] [Google Scholar]
- Evans S., Martiny J.B., Allison S.D. Effects of dispersal and selection on stochastic assembly in microbial communities. ISME J. 2017;11:176–185. doi: 10.1038/ismej.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer N., Leff J.W., Adams B.J., Nielsen U.N., Bates S.T., Lauber C.L., Owens S., Gilbert J.A., Wall D.H., Caporaso J.G. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. U S A. 2012;109:21390–21395. doi: 10.1073/pnas.1215210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B.J. Global dispersal of free-living microbial eukaryote species. science. 2002;296:1061–1063. doi: 10.1126/science.1070710. [DOI] [PubMed] [Google Scholar]
- Finlay B.J., Fenchel T. Cosmopolitan metapopulations of free-living microbial eukaryotes. Protist. 2004;155:237–244. doi: 10.1078/143446104774199619. [DOI] [PubMed] [Google Scholar]
- Fodelianakis S., Lorz A., Valenzuela-Cuevas A., Barozzi A., Booth J.M., Daffonchio D. Dispersal homogenizes communities via immigration even at low rates in a simplified synthetic bacterial metacommunity. Nat. Commun. 2019;10:1–12. doi: 10.1038/s41467-019-09306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami T. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annu. Rev. Ecol. Evol. Syst. 2015;46:1–23. [Google Scholar]
- Gogarten J.P., Doolittle W.F., Lawrence J.G. Prokaryotic evolution in light of gene transfer. Mol. Biol. Evol. 2002;19:2226–2238. doi: 10.1093/oxfordjournals.molbev.a004046. [DOI] [PubMed] [Google Scholar]
- Golan J.J., Pringle A. Long-distance dispersal of fungi. Microbiol. Spectr. 2017;5:4–5. doi: 10.1128/microbiolspec.funk-0047-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Bohannan B.J. Spatial scaling of microbial biodiversity. Trends Ecol. Evol. 2006;21:501–507. doi: 10.1016/j.tree.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Green J.L., Bohannan B.J., Whitaker R.J. Microbial biogeography: from taxonomy to traits. science. 2008;320:1039–1043. doi: 10.1126/science.1153475. [DOI] [PubMed] [Google Scholar]
- Grime J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. The Am. naturalist. 1977;111:1169–1194. [Google Scholar]
- Hanson C.A., Fuhrman J.A., Horner-Devine M.C., Martiny J.B.H. Beyond biogeographic patterns: processes shaping the microbial landscape. Nat. Rev. Microbiol. 2012;10:497–506. doi: 10.1038/nrmicro2795. https://app.dimensions.ai/details/publication/pub.1046290766 [DOI] [PubMed] [Google Scholar]
- Harshey R.M. Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 2003;57:249–273. doi: 10.1146/annurev.micro.57.030502.091014. [DOI] [PubMed] [Google Scholar]
- Hawkes C.V., Keitt T.H. Resilience vs. historical contingency in microbial responses to environmental change. Ecol. Lett. 2015;18:612–625. doi: 10.1111/ele.12451. [DOI] [PubMed] [Google Scholar]
- Hiscox J., Savoury M., Müller C.T., Lindahl B.D., Rogers H.J., Boddy L. Priority effects during fungal community establishment in beech wood. ISME J. 2015;9:2246–2260. doi: 10.1038/ismej.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R.D. Bringing the hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proc. Natl. Acad. Sci. U S A. 2009;106(Supplement 2):19659–19665. doi: 10.1073/pnas.0905137106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner-Devine M.C., Lage M., Hughes J.B., Bohannan B.J. A taxa–area relationship for bacteria. Nature. 2004;432:750–753. doi: 10.1038/nature03073. [DOI] [PubMed] [Google Scholar]
- Hubbell S.P. Vol. 32. Princeton University Press; 2001. (The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32)). [Google Scholar]
- Hungate B.A., Marks J.C., Power M.E., Schwartz E., van Groenigen K.J., Blazewicz S.J., Chuckran P., Dijkstra P., Finley B.K., Firestone M.K., et al. The functional significance of bacterial predators. mBio. 2021;12 doi: 10.1128/mBio.00466-21. e00466-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Rivera M.C., Moore J.E., Lake J.A. Horizontal gene transfer in microbial genome evolution. Theor. Popul. Biol. 2002;61:489–495. doi: 10.1006/tpbi.2002.1596. [DOI] [PubMed] [Google Scholar]
- Jarrell K.F., McBride M.J. The surprisingly diverse ways that prokaryotes move. Nat. Rev. Microbiol. 2008;6:466–476. doi: 10.1038/nrmicro1900. [DOI] [PubMed] [Google Scholar]
- Jones S.E., Elliot M.A. Streptomyces exploration: competition, volatile communication and new bacterial behaviours. Trends Microbiology. 2017;25:522–531. doi: 10.1016/j.tim.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Jones S.E., Ho L., Rees C.A., Hill J.E., Nodwell J.R., Elliot M.A. Streptomyces exploration is triggered by fungal interactions and volatile signals. Elife. 2017;6:e21738. doi: 10.7554/eLife.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.E., Lennon J.T. Dormancy contributes to the maintenance of microbial diversity. Proc. Natl. Acad. Sci. U S A. 2010;107:5881–5886. doi: 10.1073/pnas.0912765107. https://www.pnas.org/content/107/13/5881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judith K., Carolin P., Griebler C., Andreas K., Rainer K., Deng L. A rigorous assessment and comparison of enumeration methods for environmental viruses. Scientific Rep. 2020;10:18625. doi: 10.1038/s41598-020-75490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns P.J., Angell J.H., Howard E.M., Deegan L.A., Stanley R.H., Bowen J.L. Nutrient enrichment induces dormancy and decreases diversity of active bacteria in salt marsh sediments. Nat. Commun. 2016;7:1–9. doi: 10.1038/ncomms12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg C.A., Griffin D.W. Aerobiology and the global transport of desert dust. Trends Ecol. Evol. 2006;21:638–644. doi: 10.1016/j.tree.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Kivlin S.N. Global mycorrhizal fungal range sizes vary within and among mycorrhizal guilds but are not correlated with dispersal traits. J. Biogeogr. 2020;47:1994–2001. [Google Scholar]
- Kohlmeier S., Smits T.H., Ford R.M., Keel C., Harms H., Wick L.Y. Taking the fungal highway: mobilization of pollutant-degrading bacteria by fungi. Environ. Sci. Technol. 2005;39:4640–4646. doi: 10.1021/es047979z. [DOI] [PubMed] [Google Scholar]
- Koide R.T., Fernandez C., Malcolm G. Determining place and process: functional traits of ectomycorrhizal fungi that affect both community structure and ecosystem function. New Phytol. 2014;201:433–439. doi: 10.1111/nph.12538. [DOI] [PubMed] [Google Scholar]
- Kuo V., Lehmkuhl B.K., Lennon J.T. Resuscitation of the microbial seed bank alters plant-soil interactions. Mol. Ecol. 2021;30:2905–2914. doi: 10.1111/mec.15932. [DOI] [PubMed] [Google Scholar]
- Lavorel S., Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the holy grail. Funct. Ecol. 2002;16:545–556. [Google Scholar]
- Lawrence D., Bell T., Barraclough T.G. The effect of immigration on the adaptation of microbial communities to warming. Am. Naturalist. 2016;187:236–248. doi: 10.1086/684589. [DOI] [PubMed] [Google Scholar]
- Leibold M.A., Holyoak M., Mouquet N., Amarasekare P., Chase J.M., Hoopes M.F., Holt R.D., Shurin J.B., Law R., Tilman D., et al. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 2004;7:601–613. [Google Scholar]
- Lennon J., Muscarella M., Placella S., Lehmkuhl B. How, when, and where relic DNA affects microbial diversity. MBio. 2018;9 doi: 10.1128/mBio.00637-18. e00637-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon J.T., Jones S.E. Microbial seed banks: the ecological and evolutionary implications of dormancy. Nat. Rev. Microbiol. 2011;9:119–130. doi: 10.1038/nrmicro2504. [DOI] [PubMed] [Google Scholar]
- Liao J., Cao X., Zhao L., Wang J., Gao Z., Wang M.C., Huang Y. The importance of neutral and niche processes for bacterial community assembly differs between habitat generalists and specialists. FEMS Microbiol. Ecol. 2016;92:fiw174. doi: 10.1093/femsec/fiw174. [DOI] [PubMed] [Google Scholar]
- Locey K., Muscarella M., Larsen M., Bray S., Jones S., Lennon J.T. Dormancy dampens the microbial distance–decay relationship. Philosophical Trans. R. Soc. B. 2020;375:20190243. doi: 10.1098/rstb.2019.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeppmann S., Semenov M., Kuzyakov Y., Blagodatskaya E. Shift from dormancy to microbial growth revealed by RNA: DNA ratio. Ecol. Indicators. 2018;85:603–612. [Google Scholar]
- Lussenhop J. Mechanisms of microarthropod-microbial interactions in soil. Adv. Ecol. Res. 1992;23:1–33. [Google Scholar]
- Lynch M.D., Neufeld J.D. Ecology and exploration of the rare biosphere. Nat. Rev. Microbiol. 2015;13:217–229. doi: 10.1038/nrmicro3400. [DOI] [PubMed] [Google Scholar]
- Malik A.A., Martiny J.B., Brodie E.L., Martiny A.C., Treseder K.K., Allison S.D. Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change. ISME J. 2020;14:1–9. doi: 10.1038/s41396-019-0510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan S.A., Adler G.H. Consumption of arbuscular mycorrhizal fungi by terrestrial and arboreal small mammals in a panamanian cloud forest. J. Mammalogy. 2000;81:563–570. [Google Scholar]
- Martiny J.B.H., Bohannan B.J., Brown J.H., Colwell R.K., Fuhrman J.A., Green J.L., Horner-Devine M.C., Kane M., Krumins J.A., Kuske C.R., et al. Microbial biogeography: putting microorganisms on the map. Nat. Rev. Microbiol. 2006;4:102–112. doi: 10.1038/nrmicro1341. [DOI] [PubMed] [Google Scholar]
- Martiny J.B., Jones S.E., Lennon J.T., Martiny A.C. Microbiomes in light of traits: a phylogenetic perspective. Science. 2015;350:aac9323. doi: 10.1126/science.aac9323. [DOI] [PubMed] [Google Scholar]
- Mestre M., Höfer J. The microbial conveyor belt: connecting the globe through dispersion and dormancy. Trends Microbiol. 2020;29:482–492. doi: 10.1016/j.tim.2020.10.007. [DOI] [PubMed] [Google Scholar]
- Mitchell J.G., Kogure K. Bacterial motility: links to the environment and a driving force for microbial physics. FEMS Microbiol. Ecol. 2006;55:3–16. doi: 10.1111/j.1574-6941.2005.00003.x. [DOI] [PubMed] [Google Scholar]
- Müller A.L., Gu W., Patsalo V., Deutzmann J.S., Williamson J.R., Spormann A.M. An alternative resource allocation strategy in the chemolithoautotrophic archaeon methanococcus maripaludis. Proc. Natl. Acad. Sci. U S A. 2021;118 doi: 10.1073/pnas.2025854118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutshinda C.M., O’Hara R.B. Integrating the niche and neutral perspectives on community structure and dynamics. Oecologia. 2011;166:241–251. doi: 10.1007/s00442-010-1831-x. [DOI] [PubMed] [Google Scholar]
- Nelson M.B., Martiny A.C., Martiny J.B. Global biogeography of microbial nitrogen-cycling traits in soil. Proc. Natl. Acad. Sci. U S A. 2016;113:8033–8040. doi: 10.1073/pnas.1601070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemergut D.R., Schmidt S.K., Fukami T., O’Neill S.P., Bilinski T.M., Stanish L.F., Knelman J.E., Darcy J.L., Lynch R.C., Wickey P., Ferrenberg S. Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev. 2013;77:342–356. doi: 10.1128/MMBR.00051-12. https://mmbr.asm.org/content/77/3/342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofiţeru I.D., Lunn M., Curtis T.P., Wells G.F., Criddle C.S., Francis C.A., Sloan W.T. Combined niche and neutral effects in a microbial wastewater treatment community. Proc. Natl. Acad. Sci. U S A. 2010;107:15345–15350. doi: 10.1073/pnas.1000604107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley M.A. The nineteenth century roots of’everything is everywhere. Nat. Rev. Microbiol. 2007;5:647–651. doi: 10.1038/nrmicro1711. [DOI] [PubMed] [Google Scholar]
- Oneto D.L., Golan J., Mazzino A., Pringle A., Seminara A. Timing of fungal spore release dictates survival during atmospheric transport. Proc. Natl. Acad. Sci. U S A. 2020;117:5134–5143. doi: 10.1073/pnas.1913752117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Or D., Smets B., Wraith J., Dechesne A., Friedman S. Physical constraints affecting bacterial habitats and activity in unsaturated porous media – a review. Adv. Water Resour. 2007;30:1505–1527. https://www.sciencedirect.com/science/article/pii/S030917080600131X [Google Scholar]
- Paget E., Simonet P. On the track of natural transformation in soil. FEMS Microbiol. Ecol. 1994;15:109–117. [Google Scholar]
- Papke R.T., Ramsing N.B., Bateson M.M., Ward D.M. Geographical isolation in hot spring cyanobacteria. Environ. Microbiol. 2003;5:650–659. doi: 10.1046/j.1462-2920.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- Papp K., Mau R.L., Hayer M., Koch B.J., Hungate B.A., Schwartz E. Quantitative stable isotope probing with h 2 18 o reveals that most bacterial taxa in soil synthesize new ribosomal RNA. ISME J. 2018;12:3043–3045. doi: 10.1038/s41396-018-0233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peay K.G., Bruns T.D., Kennedy P.G., Bergemann S.E., Garbelotto M. A strong species–area relationship for eukaryotic soil microbes: island size matters for ectomycorrhizal fungi. Ecol. Lett. 2007;10:470–480. doi: 10.1111/j.1461-0248.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- Peay K.G., Garbelotto M., Bruns T.D. Evidence of dispersal limitation in soil microorganisms: isolation reduces species richness on mycorrhizal tree islands. Ecology. 2010;91:3631–3640. doi: 10.1890/09-2237.1. [DOI] [PubMed] [Google Scholar]
- Pirt S.J. The energetics of microbes at slow growth rates: maintenance energies and dormant organisms. J. Ferment. Technology. 1987;65:173–177. [Google Scholar]
- Placella S.A., Brodie E.L., Firestone M.K. Rainfall-induced carbon dioxide pulses result from sequential resuscitation of phylogenetically clustered microbial groups. Proc. Natl. Acad. Sci. U S A. 2012;109:10931–10936. doi: 10.1073/pnas.1204306109. https://www.pnas.org/content/109/27/10931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser J.I., Bohannan B.J., Curtis T.P., Ellis R.J., Firestone M.K., Freckleton R.P., Green J.L., Green L.E., Killham K., Lennon J.J., et al. The role of ecological theory in microbial ecology. Nat. Rev. Microbiol. 2007;5:384–392. doi: 10.1038/nrmicro1643. [DOI] [PubMed] [Google Scholar]
- Prosser J.I., Tough A.J. Growth mechanisms and growth kinetics of filamentous microorganisms. Crit. Rev. Biotechnol. 1991;10:253–274. doi: 10.3109/07388559109038211. [DOI] [PubMed] [Google Scholar]
- Prussin A.J., Garcia E.B., Marr L.C. Total concentrations of virus and bacteria in indoor and outdoor air. Environ. Sci. Technology Lett. 2015;2:84–88. doi: 10.1021/acs.estlett.5b00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin A.J., Marr L.C. Sources of airborne microorganisms in the built environment. Microbiome. 2015;3:1–10. doi: 10.1186/s40168-015-0144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno M.L., Held N.L., Fields C.J., Burke P.V., Whitaker R.J. Biogeography of the sulfolobus islandicus pan-genome. Proc. Natl. Acad. Sci. U S A. 2009;106:8605–8610. doi: 10.1073/pnas.0808945106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronce O. How does it feel to be like a rolling stone? ten questions about dispersal evolution. Annu. Rev. Ecol. Evol. Syst. 2007;38:231–253. [Google Scholar]
- Rosselló-Móra R., Amann R. Past and future species definitions for bacteria and archaea. Syst. Appl. Microbiol. 2015;38:209–216. doi: 10.1016/j.syapm.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Roszak D., Cowell R. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddick S., Williams S. Studies on the ecology of actinomycetes in soil v. some factors influencing the dispersal and adsorption of spores in soil. Soil Biol. Biochem. 1972;4:93–103. [Google Scholar]
- San J.E., Baichoo S., Kanzi A., Moosa Y., Lessells R., Fonseca V., Mogaka J., Power R., de Oliveira T. Current affairs of microbial genome-wide association studies: approaches, bottlenecks and analytical pitfalls. Front. Microbiol. 2020;10:3119. doi: 10.3389/fmicb.2019.03119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šantl-Temkiv T., Gosewinkel U., Starnawski P., Lever M., Finster K. Aeolian dispersal of bacteria in southwest Greenland: their sources, abundance, diversity and physiological states. FEMS Microbiol. Ecol. 2018;94:fiy031. doi: 10.1093/femsec/fiy031. [DOI] [PubMed] [Google Scholar]
- Shade A., Dunn R.R., Blowes S.A., Keil P., Bohannan B.J., Herrmann M., Küsel K., Lennon J.T., Sanders N.J., Storch D., Chase J. Macroecology to unite all life, large and small. Trends Ecol. Evol. 2018;33:731–744. doi: 10.1016/j.tree.2018.08.005. https://www.sciencedirect.com/science/article/pii/S0169534718301861 [DOI] [PubMed] [Google Scholar]
- Shoemaker W.R., Lennon J.T. Evolution with a seed bank: the population genetic consequences of microbial dormancy. Evol. Appl. 2018;11:60–75. doi: 10.1111/eva.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.J., Timonen H.J., Jaffe D.A., Griffin D.W., Birmele M.N., Perry K.D., Ward P.D., Roberts M.S. Intercontinental dispersal of bacteria and archaea by transpacific winds. Appl. Environ. Microbiol. 2013;79:1134–1139. doi: 10.1128/AEM.03029-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.R., Peay K.G. Multiple distinct, scale-dependent links between fungi and decomposition. Ecol. Lett. 2021;24:1352–1362. doi: 10.1111/ele.13749. [DOI] [PubMed] [Google Scholar]
- Smith G.R., Steidinger B.S., Bruns T.D., Peay K.G. Competition–colonization tradeoffs structure fungal diversity. ISME J. 2018;12:1758–1767. doi: 10.1038/s41396-018-0086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen J.W., Shade A. Dormancy dynamics and dispersal contribute to soil microbiome resilience. Philosophical Trans. R. Soc. B. 2020;375:20190255. doi: 10.1098/rstb.2019.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprockett D., Fukami T., Relman D.A. Role of priority effects in the early-life assembly of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2018;15:197–205. doi: 10.1038/nrgastro.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr E.P., Nuccio E.E., Pett-Ridge J., Banfield J.F., Firestone M.K. Metatranscriptomic reconstruction reveals rna viruses with the potential to shape carbon cycling in soil. Proc. Natl. Acad. Sci. U S A. 2019;116:25900–25908. doi: 10.1073/pnas.1908291116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegen J.C., Lin X., Fredrickson J.K., Chen X., Kennedy D.W., Murray C.J., Rockhold M.L., Konopka A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013;7:2069–2079. doi: 10.1038/ismej.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegen J.C., Lin X., Fredrickson J.K., Konopka A.E. Estimating and mapping ecological processes influencing microbial community assembly. Front. Microbiol. 2015;6:370. doi: 10.3389/fmicb.2015.00370. https://www.frontiersin.org/article/10.3389/fmicb.2015.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenström J., Svensson K., Johansson M. Reversible transition between active and dormant microbial states in soil. FEMS Microbiol. Ecol. 2001;36:93–104. doi: 10.1111/j.1574-6941.2001.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Stolpovsky K., Martinez-Lavanchy P., Heipieper H.J., Van Cappellen P., Thullner M. Incorporating dormancy in dynamic microbial community models. Ecol. Model. 2011;222:3092–3102. [Google Scholar]
- Svoboda P., Lindström E.S., Osman O.A., Langenheder S. Dispersal timing determines the importance of priority effects in bacterial communities. ISME J. 2018;12:644–646. doi: 10.1038/ismej.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamme R., Götzenberger L., Zobel M., Bullock J.M., Hooftman D.A., Kaasik A., Pärtel M. Predicting species’ maximum dispersal distances from simple plant traits. Ecology. 2014;95:505–513. doi: 10.1890/13-1000.1. [DOI] [PubMed] [Google Scholar]
- Telford R.J., Vandvik V., Birks H.J.B. Dispersal limitations matter for microbial morphospecies. Science. 2006;312:1015. doi: 10.1126/science.1125669. [DOI] [PubMed] [Google Scholar]
- Thompson P.L., Fronhofer E.A. The conflict between adaptation and dispersal for maintaining biodiversity in changing environments. Proc. Natl. Acad. Sci. U S A. 2019;116:21061–21067. doi: 10.1073/pnas.1911796116. https://www.pnas.org/content/116/42/21061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson F.J., Moles A.T., Auld T.D., Ramp D., Ren S., Kingsford R.T. Chasing the unknown: predicting seed dispersal mechanisms from plant traits. J. Ecol. 2010;98:1310–1318. [Google Scholar]
- Trubl G., Jang H.B., Roux S., Emerson J.B., Solonenko N., Vik D.R., Solden L., Ellenbogen J., Runyon A.T., Bolduc B., et al. Soil viruses are underexplored players in ecosystem carbon processing. MSystems. 2018;3 doi: 10.1128/mSystems.00076-18. e00076–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellend M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010;85:183–206. doi: 10.1086/652373. [DOI] [PubMed] [Google Scholar]
- Vos M., Velicer G.J. Isolation by distance in the spore-forming soil bacterium myxococcus xanthus. Curr. Biol. 2008;18:386–391. doi: 10.1016/j.cub.2008.02.050. [DOI] [PubMed] [Google Scholar]
- Wagner P.L., Waldor M.K. Bacteriophage control of bacterial virulence. Infect. Immun. 2002;70:3985–3993. doi: 10.1128/IAI.70.8.3985-3993.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.T., Tu B.P., Tang Y. Eight kinetically stable but thermodynamically activated molecules that power cell metabolism. Chem. Rev. 2018;118:1460–1494. doi: 10.1021/acs.chemrev.7b00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Jagadamma S., Mayes M.A., Schadt C.W., Steinweg J.M., Gu L., Post W.M. Microbial dormancy improves development and experimental validation of ecosystem model. ISME J. 2015;9:226–237. doi: 10.1038/ismej.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D.M., Cohan F.M., Bhaya D., Heidelberg J.F., Kühl M., Grossman A. Genomics, environmental genomics and the issue of microbial species. Heredity. 2008;100:207–219. doi: 10.1038/sj.hdy.6801011. [DOI] [PubMed] [Google Scholar]
- Waring B., Hawkes C.V. Ecological mechanisms underlying soil bacterial responses to rainfall along a steep natural precipitation gradient. FEMS Microbiol. Ecol. 2018;94:fiy001. doi: 10.1093/femsec/fiy001. [DOI] [PubMed] [Google Scholar]
- Warmink J., Nazir R., Corten B., van Elsas J. Hitchhikers on the fungal highway: the helper effect for bacterial migration via fungal hyphae. Soil Biol. Biochem. 2011;43:760–765. https://www.sciencedirect.com/science/article/pii/S0038071710004657 [Google Scholar]
- Warner R.R., Chesson P.L. Coexistence mediated by recruitment fluctuations: a field guide to the storage effect. Am. Naturalist. 1985;125:769–787. [Google Scholar]
- Webb C.T., Hoeting J.A., Ames G.M., Pyne M.I., LeRoy Poff N. A structured and dynamic framework to advance traits-based theory and prediction in ecology. Ecol. Lett. 2010;13:267–283. doi: 10.1111/j.1461-0248.2010.01444.x. [DOI] [PubMed] [Google Scholar]
- Wisnoski N.I., Leibold M.A., Lennon J.T. Dormancy in metacommunities. Am. Naturalist. 2019;194:135–151. doi: 10.1086/704168. [DOI] [PubMed] [Google Scholar]
- Wisnoski N.I., Shoemaker L.G. Seed banks alter metacommunity diversity: The interactive effects of competition, dispersal and dormancy. Ecol. Lett. 2021 doi: 10.1111/ele.13944. [DOI] [PubMed] [Google Scholar]
- Wright S. Isolation by distance. Genetics. 1943;28:114. doi: 10.1093/genetics/28.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Ning D. Stochastic community assembly: does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017;81 doi: 10.1128/MMBR.00002-17. https://mmbr.asm.org/content/81/4/e00002-17 e00002–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Wang H.-T., Zheng F., Yang X.-R., Christie P., Zhu Y.-G. Collembolans accelerate the dispersal of antibiotic resistance genes in the soil ecosystem. Soil Ecol. Lett. 2019;1:14–21. [Google Scholar]